Abstract
Tetrabenazine (TBZ) ((±)-1) and dihydrotetrabenazines (DHTBZ) are potent inhibitors of VMAT2. Herein, a practical chemical resolution of (±)-1 and stereoselective synthesis of all eight DHTBZ stereoisomers are described. The result of VMAT2 binding assay revealed that (+)-1 (Ki = 4.47 nM) was 8000-fold more potent than (−)-1 (Ki = 36,400 nM). Among all eight DHTBZ stereoisomers, (2R,3R,11bR)-DHTBZ ((+)-2: Ki = 3.96 nM) showed the greatest affinity for VMAT2. The (3R,11bR)-configuration appeared to play a key role for VMAT2 binding. In summary, (+)-1, (+)-2, and their derivatives warrant further studies in order to develop more potent and safer drugs for the treatment of chorea associated with Huntington’s disease and other hyperkinetic disorders.
Keywords: Tetrabenazine enantiomers, Dihydrotetrabenazine stereoisomers, Resolution, Huntington’s chorea, VMAT2, Hyperkinetic disorders
1. Introduction
Tetrabenazine (TBZ, Fig. 1) is a drug used for the treatment of Huntington’s chorea and other hyperkinetic movement disorders [1–3]. TBZ ((±)-1) was first approved in Europe in 1971 for the treatment of Huntington’s disease (HD), and in 2008, TBZ gained FDA approval for the treatment of chorea associated with HD. TBZ has two chiral centers, and hence can theoretically exist in four isomeric forms, however, the marketed drug TBZ (Nitoman, Xena-zine) is only a racemic mixture of (+)-(3R,11bR)-TBZ ((+)-1) and (−)-(3S,11bS)-TBZ ((−)-1) due to the thermodynamic instability of the cis-isomer of TBZ.
Fig. 1.
Structures of tetrabenazine (TBZ, (±)-1) and tetrabenazine enantiomers.
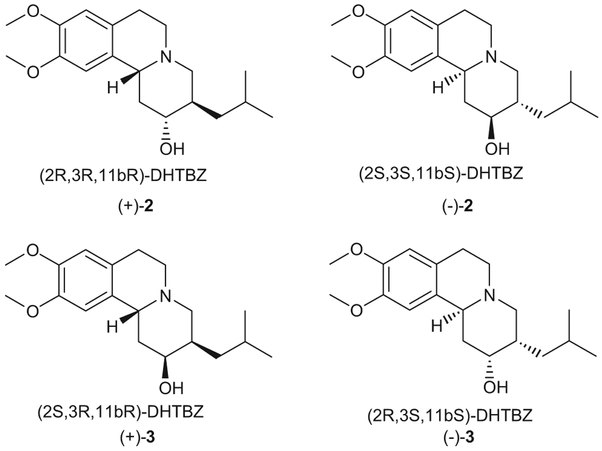
In vivo, (±)-1 is rapidly and extensively metabolized by first-pass metabolic reduction of the 2-keto group, providing four isomers of dihydrotetrabenazines (DHTBZ), which include (2R,3R,11bR)- DHTBZ ((+)-2), (2S,3S,11bS)-DHTBZ ((−)-2), (2S,3R,11bR)-DHTBZ ((+)-3), and (2R,3S,11bS)-DHTBZ ((−)-3) (Fig. 2) [4–6]. The four TBZ metabolites are likely the major pharmacologically active substances in vivo. The primary pharmacological action of TBZ and its active metabolites is to deplete the levels of monoamines (e.g. dopamine, serotonin, and norepinephrine) within the central nervous system by inhibiting the human vesicular monoamine transporter 2 (VMAT2) [7–9]. This transporter is predominantly expressed in the brain, and translocates monoamines from cytoplasm into synaptic vesicles, where they are both stored and protected from metabolism prior to their synaptic release. Multiple lines of evidence indicate that the binding of the TBZ metabolites to VMAT2 is stereospecific [10–12]. In the VMAT2 binding assay, (+)-2 was more potent (Ki = 1.9 nM) [13] than (−)-2 (Ki = 202 nM) or (−)-3 (Ki = 714 nM). On the other hand, besides the four DHTBZ metabolites with relative trans-configuration at C-11b vs. C-3 (Fig. 2), there should exist four DHTBZ stereoisomers with relative cis-configuration at C-11b vs. C-3 (Fig. 3) as three chiral centers exist in the core structure of DHTBZ. The four isomers of 3,11b-cis-DHTBZ include (2R,3S,11bR)-DHTBZ ((+)-4), (2S,3R,11bS)-DHTBZ ((−)-4), (2S,3S,11bR)-DHTBZ ((+)-5), and (2R,3R,11bS)-DHTBZ ((−)-5) (Fig. 3). In this regard, Clarke et al. claimed the preparation of the four isomers of 3,11b-cis-DHTBZ by using Mosher’s ester methodology; however, the absolute configuration and optical purity of the four isomers were not reported [14]. Interestingly, two of the four 3,11b-cis-DHTBZ isomers exhibited high affinity for VMAT2 but negligible binding at dopamine receptors, indicating that they might be unlikely to give rise to the dopaminergic side effects encountered with (±)-1 [14]. Moreover, they also lacked the unwanted sedative effects associated with (±)-1 [14]. The stereospecific binding profile of DHTBZ indicates that TBZ enantiomers and DHTBZ stereoisomers may have different pharmacological and/or toxicological profiles, which still remain to be determined. Therefore, it is highly desirable to develop a practical access to optically pure TBZ enantiomers and DHTBZ stereoisomers to support the development of more potent and safer drugs for the treatment of Huntington’s disease and hyperkinetic disorders.
Fig. 2.
Structures of the TBZ metabolites (DHTBZ) with trans-configuration at C-3 vs. C-11b.
Fig. 3.
Structures of DHTBZ stereoisomers with cis-configuration at C-3 vs. C-11b.
Rishel et al. completed the first asymmetric synthesis of (+)-1 by the use of Sodeoka’s palladium-catalyzed asymmetric malonate addition as the key step [15]. High optical purity (ee >97%) and reasonable yields were achieved in this eight-step synthesis. However, this method suffers from several practical limitations, such as the use of an expensive chiral catalyst, long reaction sequences, and a low total yield, and thus is not suitable for industrial production. Recently, Boldt et al. developed a synthesis of (+)-1 from the resolution of a-DHTBZ, followed by Swern oxidation of the resulting (+)-α-DHTBZ [16]. This indirect methodology suffers from a low atom-economy due to additional reduction/oxidation steps. Another access to optically pure TBZ employs the resolution of (±)-1 with preparative chiral HPLC that, similarly, is not suitable for large-scale preparation [13]. Surprisingly, since the initial launch of (±)-1 in 1971, direct chemical resolution of (±)-TBZ has seen little success [16]. Recently, Yu et al. [17] reported the preparation of (+)-1 and (−)-1 by a chemical resolution procedure that is similar to ours [18]. This publication prompts us to report our results describing a practical route to TBZ enantiomers via a chemical resolution and the efficient synthesis of all eight DHTBZ stereoisomers. This allowed our assay of the VMAT2 binding affinity and SAR analysis of (±)-TBZ, TBZ enantiomers and all eight DHTBZ stereoisomers.
2. Results and discussion
2.1. Chemistry
2.1.1. Chemical resolution of tetrabenazine
In our search for an efficient method to resolve TBZ, which was readily prepared according to literature procedures [1,19], we investigated several variations of conditions, including the resolving agents, the solvents and reflux time, crystallization temperature, and seed crystals, etc. After extensive experimentation, resolution of (±)-1 was achieved by using (1S)-(+)-10-camphorsulfonic acid ((+)-CSA) as a resolving agent (Scheme 1) [18]. Other chiral acids, including (+)-tartaric acid, (+)-dibenzoyl-D-tartaric acid, and (2R,3R)-2’-nitrotartranilic acid, gave disappointing results. Consequently, (+)-CSA was chosen as the resolving agent for further optimization studies, which subsequently showed that the molar equivalents of the resolving agent, the resolving solvents and crystallization temperature were key factors to accomplish successful resolution of (±)-1. Specifically, the equivalents of (+)-CSA played a key role in the efficient resolution of (±)-1. When 1 equivalent of (+)-CSA was used, only moderate ee (approximately 40%) was obtained after one-time crystallization of the (+)-CSA(+)-1 salt from acetone, even though a high ee could be achieved by repeated recrystallization of this salt in acetone. When the amount of (+)-CSA was reduced to 0.5 equivalent, the initial batch of crystals of the (+)-CSA(+)-1 salt precipitating from acetone possessed up to a 96% ee upon the release of (+)-1 as a free base. One-time recrystallization of this salt in acetone led to more than a 98% ee (see Supporting information). Among the various resolving solvents, acetone gave the best results in terms of yield and high ee value. The proportion of acetone vs. (±)-1 was found to be critical for successful resolution, and the best result was obtained when the ratio of (±)-1 to acetone was in the order of 1 g:13 mL. Crystallization temperature also played an important function. When crystallization temperature was kept between 15 and 20 °C, high ee values and high yields could be achieved. In the event that the crystallization temperature was below 10 °C, the enantiomeric excess dropped significantly. Using (−)-CSA in place of (+)-CSA as a resolving agent, (−)-1 was obtained by the same methodology (Scheme 1).
Scheme 1.
Resolution of tetrabenazine ((±)-1). Reagents and conditions: (a) (1S)-(+)-10-camphorsulfonic acid, acetone; (b) (1R)-(−)-10-camphorsulfonic acid, acetone; (c) NH4OH, MeOH.
Based on the premise of the existence of an interconversion between benzo[a]quinolizine and isoquinolinium upon exposure to an acid [20], we hypothesized that (±)-1 could be recovered from the resolution mother liquid upon acid-catalyzed racemization, and then resolved again. As expected, after adding additional (+)-CSA to the resolution mother liquid and refluxing overnight, complete racemization took place, and when the reaction mixture was cooled to 15 °C with stirring, the camphorsulfonate salt of (+)-1 was formed as crystals with a moderate ee value. The resulting salt was further purified by repeated recrystallization with acetone until more than 98% ee was reached upon release of the (+)-1 as a free base. This racemization—resolution process could be repeated for several times leading to a high-resolution yield.
2.1.2. Synthesis of eight DHTBZ stereoisomers
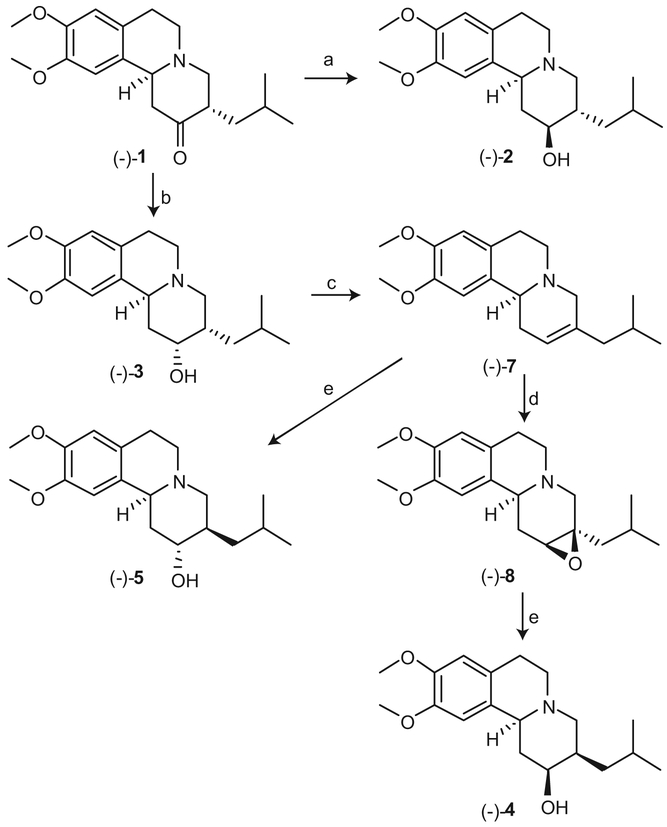
Synthesis of (2R,3R,11bR)-DHTBZ ((+)-2), (2S,3R,11bR)-DHTBZ ((+)-3), (2R,3S,11bR)-DHTBZ ((+)-4), and (2S,3S,11bR)-DHTBZ ((+)-5) are shown in Scheme 2. Reduction of (+)-1 with NaBH4 [21] yielded a mixture of (2R,3R,11bR)-DHTBZ ((+)-2) and (2S,3R,11bR)-DHTBZ ((+)-3) in a 4:1 ratio. It proved extremely difficult to purify either (+)-2 or (+)-3 by column chromatography or recrystallization, although (±)-2 could be separated from (±)-3 by recrystallization of the crude product resulting from NaBH4 reduction of (±)-1 [21]. When borane was used as a reducing reagent and the reaction temperature was maintained at −20 °C, good stereo-selectivity was achieved to provide a 19:1 mixture of (+)-2 and (+)-3, which was readily purified by recrystallization from acetone—water to afford pure (+)-2 (see Supporting information).
Scheme 2.
Synthesis of (+)-DHTBZ stereoisomers (+)-2, (+)-3, (+)-4 and (+)-5. Reagents and conditions: (a) BH3—Me2S, THF, −20 °C; (b) L-Selectride®, EtOH/THF, 0 °C; (c) PCl5, CH2Cl2; (d) HClO4, mCPBA, MeOH; (e) i. BH3, THF; ii. NaOH, H2O2.
Following Clarke’s methodology [14] for the generation of (±)-3, (±)-4 and (±)-5 by starting with (±)-1, stereoselective preparation of (+)-3, (+)-4 and (+)-5 was achieved from (+)-1 (Scheme 2). Stereoselective reduction of (+)-1 with L-selectride® at 0 °C furnished (+)-3 in 43% yield. Dehydration of (+)-3 with PCl5 at 0 °C afforded alkene (+)-7 in 57% yield. Thereafter, epoxidation of (+)-7 with m-chloroperoxybenzoic acid (mCPBA) in the presence of perchloric acid gave epoxide (+)-8. The relative configuration of (+)-8 was confirmed by a 2D-NMR (NOESY) study (see Supporting information), and demonstrated that the hydrogen atoms at the C-2 and C-11b positions both had nuclear overhauser effects (NOE) with the same hydrogen atom at the C-1 position. Regioselective and stereoselective reductive ring-opening of (+)-8 with borane [22,23], followed by oxidation with hydrogen peroxide in the presence of NaOH, afforded (+)-4 in 31% yield. Regioselective and stereoselective hydroboration—oxidation of (+)-7 provided alcohol (+)-5 in 41% yield. In a similar fashion, (2S,3S,11bS)-DHTBZ ((−)-2), (2R,3S,11bS)-DHTBZ ((−)-3), (2S,3R,11bS)-DHTBZ ((−)-4), and (2R,3R,11bS)-DHTBZ ((−)-5) were synthesized starting with (−)-1 (Scheme 3).
Scheme 3.
Synthesis of (−)-DHTBZ stereoisomers (−)-2, (−)-3, (−)-4 and (−)-5. Reagents and conditions: (a) BH3Me2S, THF, −20 °C; (b) L-Selectride®, EtOH/THF, 0 °C; (c) PCl5, CH2Cl2; (d) HClO4, mCPBA, MeOH; (e) i. BH3, THF; ii. NaOH, H2O2.
2.2. VMAT2 binding assay and SAR analysis
Eleven compounds, including (±)-TBZ, (+)-TBZ, (−)-TBZ, and eight DHTBZ stereoisomers, were evaluated for VMAT2 binding affinity by quantifying their ability to displace [3H]dihydrotetrabenazine ([3H]DHTBZ) from rat striatum [17]. The assay results are summarized in Table 1, and VMAT2 binding affinity is described as a dissociation constant (Ki) value.
Table 1.
VMAT2 binding affinity of TBZ, TBZ enantiomers, and DHTBZ stereoisomers.
| No. | Compound | Ki ± SEM (nM)a |
|---|---|---|
| (±)−1 | (±)-TBZ | 7.62 ± 0.20 |
| (+)−1 | (+)-TBZ | 4.47 ± 0.21 |
| (−)−1 | (−)-TBZ | 36,400 ± 4560 |
| (+)−2 | (2R,3R,11bR)-DHTBZ | 3.96 ± 0.40 |
| (−)−2 | (2S,3S,11bS)-DHTBZ | 23,700 ± 2350 |
| (+)−3 | (2S,3R,11bR)-DHTBZ | 13.4 ± 1.36 |
| (−)−3 | (2R,3S,11bS)-DHTBZ | 2460 ± 333 |
| (+)−4 | (2R,3S,11bR)-DHTBZ | 71.1 ± 6.66 |
| (−)−4 | (2S,3R,11bS)-DHTBZ | 4630 ±350 |
| (+)−5 | (2S,3S,11bR)-DHTBZ | 593 ± 69.7 |
| (−)−5 | (2R,3R,11bS)-DHTBZ | 1253 ± 314 |
Ki values represent experimental n = 3 or greater (p < 0.05). All experiments were undertaken in triplicate.
As expected, (±)-TBZ ((±)-1) exhibited a high affinity (Ki = 7.62 ± 0.20 nM) for VMAT2, and this value was in the range of the literature value [17]. Surprisingly, whereas (+)-TBZ ((+)-1) possessed a high affinity (Ki = 4.47 ± 0.21 nM) for VMAT2, the (−)-form of TBZ ((−)-1) (Ki = 36,400 ± 4560 nM) proved to be 8000-fold less potent than its (+)-enantiomer ((+)-1). In contrast, in the previous study [17], (−)-TBZ proved only 3-fold less potent than (+)-TBZ. In this prior study, the VMAT2 affinity of (−)-TBZ and (+)-TBZ was assessed in the form of their camphorsulfonate salts rather than their corresponding free bases, and although X-ray crystallographic analysis and optical rotation were provided and matched the literature [16], their optical purity was not reported.
There is discrepancy in the VMAT2 Ki, values of TBZ metabolites. Kilbourn et al. reported that (2R,3R,11bR)-DHTBZ ((+)-2) (Ki=0.97 ± 0.48 nM) was 2000-fold more potent than (2S,3S,11bS)-DHTBZ ((−)-2) (Ki = 2.2 ± 0.3 mM) in their VMAT2 binding assay [10], whereas Gano claimed that (+)-2 (Ki = 1.9 nM) showed only 100-fold more potent binding affinity than (−)-2 (Ki = 202 nM) [13]. In this study, (+)-2 (Ki = 3.96 ± 0.40 nM) proved to be 6000-fold more potent than (−)-2 (Ki = 23.70 ± 2.35 mM), which is in line with Kilbourn’s report [10]. In Gano’s study [13], (2S,3R,11bR)-DHTBZ ((+)-3) exhibited a potent VMAT2 affinity (Ki = 13 nM) that is similar to our value (Ki = 13.4 ± 1.36 nM). In contrast with Gano’s result on (2R,3S,11bS)-DHTBZ ((−)-3) (Ki = 714 nM), in our hands ((−)-3) had Ki value of 2460 ± 333 nM. Among the four DHTBZ isomers with a cis-configuration at C-3 vs. C-11b, (2R,3S,11bR)-DHTBZ ((+)-4) (Ki = 71.1 ± 6.66 nM) showed the most potent binding affinity, whereas (2S,3R,11bS)-DHTBZ ((−)-4) (Ki = 4630 ± 350 nM) was the least potent.
SAR analysis revealed that the (3R,11bR)-configuration made the most important contribution to VMAT2 binding affinity, and all the corresponding compounds ((+)-1: Ki = 4.47 ± 0.21 nM; (+)-2: Ki = 3.96 ± 0.40 nM; (+)-3: Ki = 13.4 ± 1.36 nM) exhibited very high affinity. By contrast, compounds with a (3S,11bR) configuration demonstrated moderate to weak potency (e.g. (+)-4: Ki = 71.1 ± 6.66 nM; (+)-5: Ki = 593 ± 69.7 nM). Interestingly, all the compounds with an 11bS configuration (e.g. (−)-1 Ki = 36,400 ± 4560 nM; (−)-2: Ki = 23,700 ± 2350 nM; (−)-3: Ki = 2460 ± 333 nM; (−)-4: Ki = 4630 ± 350nM;(−)-5: Ki = 1253 ± 314nM)were essentially inactive. In summary, all dextrorotatory enantiomers exhibited dramatically more potent VMAT2 binding affinity than their corresponding levorotatory isomers.
3. Conclusion
In the current study, a practical chemical resolution of tetrabenazine with camphorsulfonic acids has been carried out to produce optically pure tetrabenazine enantiomers in high yields. With both tetrabenazine enantiomers in hand, all eight stereoisomers of dihydrotetrabenazines were synthesized with high stereoselectivity. Analysis of VMAT2 binding revealed that (+)-tetrabenazine ((+)-1) was 8000-fold more potent than (−)-tetrabenazine ((−)-1). Among all eight dihydrotetrabenazine stereoisomers, (2R,3R,11bR)-dihydrotetrabenazine ((+)-2: Ki = 3.96 nM) showed the highest affinity for VMAT2, and proved slightly more potent than (+)-tetrabenazine ((+)-1: Ki = 4.47 nM). The (3R,11bR)-configuration played a key role in delineating the affinity of tetrabenazine and dihydrotetrabenazines binding to VMAT2. In summary, (+)-1, (+)-2, and their derivatives warrant further studies in order to develop more potent and safer drugs to ameliorate chorea associated with Huntington’s disease and other hyperkinetic disorders.
4. Experimental
4.1. Chemistry
All commercially available solvents and reagents were used without further purification. Melting points were determined with a Buchi capillary apparatus and were not corrected. 1H and 13C NMR spectra were recorded on an ACF* 300Q Bruker, spectrometer in CDCl3, with Me4Si as the internal reference, or in d6-DMSO. Low-and high-resolution mass spectra (LRMS and HRMS) were recorded in electron impact mode. Reactions were monitored by TLC on Silica Gel 60 F254 plates (Qingdao Ocean Chemical Company, China). Column chromatography was carried out on silica gel (200–300 mesh, Qingdao Ocean Chemical Company, China).
4.1.1. (3R,11bR)-Tetrabenazine ((+)-1)
A warm solution of tetrabenazine ((±)-1) (17 g, 53.6 mmol) in acetone (230 mL) was treated with (1S)-(+)-10-camphorsulfonic acid (6.2 g, 26.7 mmol). After cooling to room temperature with stirring, the mixture was left at room temperature for 48 h and the resulting crystals were collected (8 g, ee 96.5% for released free base). These crystals were then recrystallized from acetone (80 mL) to give (1S)-(+)-10-camphorsulfonic acid salt of (+)-(3R,11bR)-tetrabenazine (6.5 g, ee 98.9% for released free base). This salt was dissolved in MeOH (26 mL) and neutralized with NH4OH to pH = 8. Thereafter water (190 mL) was added, and the solids were collected to afford (+)-1 as a white solid (3.4 g), ee 98.7% (Chiral HPLC analytical conditions: Chiralpak IC column, 4.6 mm × 250 mm, eluting with 100% EtOH + 0.1% Et2NH, flow rate 0.5 mL/min, oven temperature 35 °C, detection UV 220 nm). [α]D22 = +66.3° (c 1.04, MeOH). 1H NMR (300 MHz, CDCl3): δ 6.62 (s, 1H), 6.55 (s, 1H), 3.85 (s, 3H), 3.83 (s, 3H), 3.51 (d, 1H, J = 10.0 Hz), 3.28 (dd, 1H, J = 6.2, 11.5 Hz), 3.14–3.09 (m, 2H), 2.89 (dd, 1H, J = 3.2,13.7 Hz), 2.75–2.49 (m, 4H), 2.35 (t, 1H, J = 11.6 Hz), 1.85–1.66 (m, 2H), 1.07–1.01 (m, 1H), 0.90 (m, 6H); 13C NMR (75 MHz, CDCl3): δ 210.0, 147.8, 147.5, 128.6, 126.0, 111.5, 107.9, 62.5, 61.5, 56.0, 55.9, 50.5, 47.6, 47.5, 35.0, 29.3, 25.4, 23.2, 22.1; ESI-MS m/z 318.2 [M + H]+, 340.2 [M + Na]+.
The acetone mother liquid from the above resolution was evaporated to dryness to give a yellow solid (14 g) that was again dissolved in acetone (240 mL), and (1S)-(+)-10-camphorsulfonic acid (7 g, 30.1 mmol) was added. After stirring at reflux overnight, the mixture was cooled with stirring and left at room temperature for 48 h. The resulting crystals were collected (8.6 g, ee 70.8% for released free base), and recrystallized twice from acetone to give optically pure (1S)-(+)-10-camphorsulfonic acid salt of (+)-(3R,11bR)-tetrabenazine as a white solid (4.1 g, ee 98.7% for released free base). The salts were dissolved in MeOH (17 mL) and neutralized with NH4OH to pH = 8. Water (100 mL) was then added, and the solids were collected to afford (+)-1 as a white solid (2 g), ee >99%, [α]D22 = +66.5° (c 0.39, MeOH).
The above racemization-resolution process was repeated three times, and collectively 10.27 g of (+)-1 (ee >98%) was obtained from the resolution of 17 g of tetrabenazine.
4.1.2. (3S,11bS)-Tetrabenazine ((−)-1)
Resolution of tetrabenazine with (1R)-(−)-10-camphorsulfonic acid was carried out following the procedure for preparation of (3R,11bR)-tetrabenazine to give (−)-1 as a white solid, ee 99.0% (Chiral HPLC analytical conditions:Chiralpak IC column, 4.6mm × 250 mm, eluting with 100% EtOH + 0.1% Et2NH, flow rate0.5 mL/min, oven temperature 35 °C, detection UV 220 nm). [α]D25 = −63.7° (c 0.27, MeOH), 1H NMR(300 MHz, CDCl3): δ 6.62 (s, 1H), 6.55 (s, 1H), 3.85 (s, 3H), 3.82 (s, 3H), 3.52 (m, 1H), 3.28 (dd, 1H, J = 6.3,11.5 Hz), 3.20–3.05 (m, 2H), 2.89 (dd, 1H, J = 3.0, 13.6 Hz), 2.79–2.65 (m, 2H), 2.48–2.63 (m, 2H), 2.35 (t, 1H, J = 11.6 Hz), 1.85–1.76 (m, 1H), 1.72–1.57 (m, 1H), 1.13–0.96 (m, 1H,), 0.90 (m, 6H); 13C NMR (75 MHz, CDCl3): δ 210.0, 147.8, 147.5, 128.6, 111.5, 107.9, 62.4, 61.5, 56.0, 55.9, 50.5, 47.6, 47.5, 35.0, 29.3, 25.4, 23.2, 22.1; ESI-MS m/z 318.2 [M + H]+, 340.2 [M + Na]+.
4.1.3. (2R,3R,11bR)-3-Isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-ol ((+)-2)
To a solution of (3R,11bR)-tetrabenazine (1.0 g, 3.2 mmol) in THF (11 mL) was added dropwise 2 M borane—Me2S—THF (3.2 mL, 6.4 mmol) at −20 °C. After stirring at this temperature for 2 h, ammonia water (11 mL) was added, and the mixture was warmed to 35 °C and stirred overnight. The mixture was then diluted with brine, and extracted with ether. The organic layer was washed with brine, dried over Na2SO4, and concentrated under vacuum to give a white solid. This crude product was recrystallized twice from acetone-water to afford (+)-2 as a white solid (0.64 g, 64%), mp: 100–102 °C, ee >99% (Chiral HPLC analytical conditions: Chiralpak IC column, 4.6 mm × 250 mm, eluting with 100% MeOH + 0.1% Et2NH, flow rate 0.5 mL/min, oven temperature 35 °C, detection UV 220 nm). [α]D21 = +58.93° (c 0.6, MeOH). 1H NMR (300 MHz, CDCl3): δ 6.68 (s,1H), 6.58 (s,1H), 3.84 (s, 6H), 3.39 (ddd, 1H, J = 4.6, 10.6, 10.6 Hz), 3.15–2.97 (m, 4H), 2.66–2.55 (m, 2H), 2.45 (ddd, 1H, J = 3.6, 11.0, 11.0 Hz), 1.98 (t, 1H, J = 11.3 Hz), 1.78–1.43 (m, 5H), 1.14–1.01 (m, 1H), 0.94 (d, 3H, J = 6.5 Hz), 0.92 (d, 3H, J = 6.5 Hz); 13C NMR (75 MHz, CDCl3): δ 147.6, 147.3, 129.4, 126.5, 111.6, 108.1, 74.6, 60.9, 60.1, 56.0, 55.9, 51.9, 41.7, 40.6, 39.7, 29.2, 25.4, 24.1, 21.8; ESI-MS m/z 320.3 [M + H]+; HRMS calcd for C19H30NO3 [M + H]+ m/z 320.2226, found 320.2240.
4.1.4. (2S,3S,11bS)-3-Isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-ol ((−)-2)
Reduction of (3S,11bS)-tetrabenazine with borane-methyl sulfide in THF was carried out following the procedure for preparation of (2R,3R,11bR)-dihydrotetrabenazine to afford (−)-2 as a white solid, mp: 98–100 °C, ee >99% (Chiral HPLC analytical conditions: Chiralpak IC column, 4.6 mm × 250 mm, eluting with 100% MeOH + 0.1% Et2NH, flow rate 0.5 mL/min, oven temperature 35 °C, detection UV 220 nm). [α]D25 = −56.1° (c 0.23, MeOH). 1H NMR (300 MHz, CDCl3): δ 6.67 (s, 1H), 6.58 (s, 1H), 3.87 (s, 6H), 3.43–3.34 (m, 1H), 3.14–2.96 (m, 4H), 2.65–2.54 (m, 2H), 2.53 (ddd, 1H, J = 11.1, 11.1, 3.6 Hz), 2.01–1.94 (m, 1H), 1.77–1.64 (m, 2H), 1.62–1.36 (m, 2H), 1.10–0.99 (m, 1H), 0.95–0.70 (m, 6H); 13C NMR (75 MHz, CDCl3): δ 147.50, 147.23, 129.36, 126.42, 111.49, 107.96, 74.63, 60.88, 60.08, 55.94, 55.84, 51.89, 41.65, 40.58, 39.70, 29.18, 25.35, 24.12, 21.76; ESI-MS m/z 320.2 [M + H]+, 342.2 [M + Na]+; HRMS calcl3 for C19H30NO3 m/z 320.2226, found 320.2242.
4.1.5. (2S,3R,11bR)-3-Isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-ol ((+)-3)
L-Selectride® (1 M, 126 mL) was added dropwise to a solution of (3R,11bR)-tetrabenazine ((+)-1) (14 g, 44.2 mmol, ee >99%) in EtOH (70 mL) and THF (70 mL) at 0 °C. After stirring at 0 °C for 40 min, the mixture was poured into 400 mL of ice-water and extracted with ether. The organic layer was washed with brine, dried over Na2SO4, and concentrated under vacuum (<25 °C, protected from light) to give a yellow oil. To a solution of the crude product in EtOH (25 mL) was added 1 equivalent of methanesulfonic acid, and the resulting mixture was stirred overnight at room temperature to give a crude methanesulfonic acid salt (9.2 g), which was recrystallized from EtOH to provide methanesulfonic acid salt of (2S,3R,11bR)-dihydrotetrabenazine (8.14 g). This salt was dissociated with NH4OH to afford (+)-3 as a white solid (6.05 g, 42.9%), ee 100% (Chiral HPLC analytical conditions: Chiralpak IC column, 4.6 mm × 250 mm, eluting with 25% EtOH + 75% n-hexane, flow rate 0.5 mL/min, oven temperature 35 °C, detection UV 220 nm). [α]D25 = +98.6° (c 0.27, MeOH). 1HNMR(300 MHz, CDCl3): δ 6.66 (s,1H), 6.56 (s, 1H), 4.08 (d, 1H, J = 2.7 Hz), 3.83 (s, 6H), 3.48 (d, 1H, J = 11.6 Hz), 3.16–3.05 (m, 1H), 2.97–2.92 (m, 1H), 2.67 (dd, 1H, J = 4.2, 11.3 Hz), 2.60–2.36 (m, 7H), 2.00–1.94 (m, 1H), 1.73–1.62 (m, 2H), 1.31–1.10(m, 2H), 0.93 (d, 3H, J = 5.5 Hz), 0.91 (d, 3H, J = 5.5 Hz); 13C NMR (75 MHz, CDCl3) δ: 147.4, 147.1, 130.0, 126.9, 111.6, 108.1, 68.0, 56.4, 56.3, 56.0, 55.8, 52.5, 39.2, 38.9, 37.8, 29.2, 24.9, 23.0, 22.9; ESI-MS m/z 320.3 [M + H]+. HRMS calcd for C19H30NO3 [M + H]+ m/z 320.2226, found 320.2242.
4.1.6. (2R,3S,11bS)-3-Isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-ol ((−)-3)
Reduction of (3S,11bS)-tetrabenazine with L-Selectride® was carried out following the procedure for preparation of (+)-3 to afford (−)-3 as a white solid, ee 100% (Chiral HPLC analytical conditions: Chiralpak IC column, 4.6 mm × 250 mm, eluting with 25% EtOH + 75% n-hexane, flow rate 0.5 mL/min, oven temperature 35 °C, detection UV 220 nm). [α]D25 = −93.8° (c 0.23, MeOH). 1H NMR (300 MHz, CDCl3): δ 6.68 (s, 1H), 6.59 (s, 1H), 4.09 (bs, 1H), 3.85 (s, 6H), 3.50 (d, 1H, J = 12 Hz), 3.12 (m, 1H), 2.99–2.96 (m, 1H), 2.71–2.53 (m, 3H), 2.46–2.38 (m, 2H), 2.00 (m, 1H), 1.76–1.66 (m, 2H), 1.31–1.15 (m, 2H), 0.96–0.92 (m, 6H); 13C NMR (75 MHz, CDCl3) δ: 147.39, 147.13, 130.07, 126.94, 111.56, 108.10, 68.03, 56.39, 56.35, 56.00, 55.83, 52.48, 39.20, 38.86, 37.81, 29.22, 24.87, 22.95; ESI-MS m/z 320.2 [M + H]+; HRMS calcl3 for C19H30NO3 m/z 318.2069, found 320.2246.
4.1.7. (11bR)-1,6,7,11b-Tetrahydro-9,10-dimethoxy-3-(2-methylpropyl)-4H-benzo[a]quinolizine ((+)-7)
PCl5 (8.2 g, 39.2 mmol) was added in portions over 30 min to a solution of (+)-3 (5 g, 15.7 mmol, ee 100%) in CH2Cl2 (50 mL) at 0 °C. The mixture was stirred at 0 °C for 30 min and then poured into 200 mL of ice-water. The mixture was neutralized with NH4OH to pH = 8, and extracted with CH2Cl2. The organic layer was washed with brine, dried over Na2SO4, and concentrated under vacuum to give a yellow oil (5.2 g). The crude product was purified by column chromatography (SiO2, CH2Cl2:CH3OH = 250:1) to give (+)-7 as a yellow oil (2.48 g, 57%), [×]D23 = +250.9° (c 0.53, MeOH). 1H NMR (300 MHz, CDCl3): δ 6.65 (s, 1H), 6.57 (s, 1H), 5.50 (m, 1H), 3.85 (s, 3H), 3.84 (s, 3H), 3.35 (m, 1H), 3.22 (d, 1H, J = 15.6 Hz), 3.14–3.02 (m, 2H), 2.92 (m, 1H), 2.65–2.45 (m, 3H), 2.20–2.12 (m, 1H), 1.94–1.70 (m, 3H), 0.91 (d, 3H, J = 5.3 Hz), 0.89 (d, 3H, J = 5.3 Hz); 13C NMR (75 MHz, CDCl3): δ 147.4, 135.5, 130.2, 126.6, 120.2, 111.3, 108.6, 58.8, 58.6, 56.0, 55.8, 51.5, 45.0, 33.4, 29.0, 26.2, 22.9, 22.2; ESI-MS m/z 302.2 [M + H]+; HRMS calcd for C19H28NO2 m/z 302.2120, found 302.2138.
4.1.8. (11bS)-1,6,7,11b-Tetrahydro-9,10-dimethoxy-3-(2-methylpropyl)-4H-benzo[a]quinolizine ((−)-7)
Dehydration of (−)-3 with PCl5 was carried out following the procedure for preparation of (+)-7 to give (−)-7 as a yellow oil (62.5%), [α]D22 = −263.3° (c 0.20, MeOH).1H NMR (300 MHz, CDCl3) δ 6.66 (s, 1H), 6.59 (s, 1H), 5.53 (s, 1H), 3.86 (s, 3H), 3.85 (s, 3H), 3.43–3.41 (m, 1H), 3.31–3.09 (m, 4H), 2.70–2.58 (m, 3H), 2.27–2.18 (m, 1H), 1.93–1.71 (m, 3H), 0.91 (d, 6H, J = 5.8 Hz), 0.89 (d, 6H, J = 5.3 Hz); 13C NMR (75 MHz, CDCl3) δ 147.5, 147.4, 135.6, 130.4, 126.8, 120.3, 111.4, 108.7, 59.0, 58.8, 56.1, 55.9, 51.6, 45.2, 33.2, 29.1, 26.3, 23.0, 22.4; ESI-MS m/z 302.2 [M + H]+; HRMS calcd for C19H28NO2 [M + H]+ m/z 302.2120, found 302.2138.
4.1.9. (2S,3S,11bR)-3-Isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-ol ((+)-5)
To a solution of (+)-7 (100 mg, 0.33 mmol) in dry THF was added dropwise 2 M borane–Me2S–THF (0.73 mL, 1.46 mmol) at 0 °C. The reaction mixture was stirred at below 15 °C for 48 h, and then 0.2 mL of water was added and the mixture was basified to pH = 12 with 30% aqueous NaOH solution. 30% Hydrogen peroxide solution (0.28 mL) was added and the mixture was stirred at reflux for 1.5 h. The reaction mixture was then cooled and 10 mL of water was added, and extracted with EtOAc. The organic layer was washed with brine, dried over Na2SO4, and concentrated under vacuum to give a yellow oil (110 mg). The crude product was purified by preparative TLC to give (+)-5 (43 mg, 41%) as a yellow oil, ee 99.0% (Chiral HPLC analytical conditions: Chiralpak IC column, 4.76mm × 250 mm, eluting with 100% i-PrOH + 0.1% Et2NH, flow rate 0.5 mL/min, oven temperature 35 °C, detection UV 220 nm). [α]D23 = +120.6° (c 0.11, MeOH). 1H NMR (300 MHz, CDCl3): δ 6.68 (s, 1H), 6.57 (s, 1H), 3.84 (s, 6H), 3.54 (m, 1H), 3.08 (m, 1H), 2.90 (m, 1H), 2.76 (dd, 1H, J = 3.4, 11.6 Hz), 2.62–2.53 (m, 3H), 2.20–2.15 (m, 1H), 1.92–1.45 (m, 5H), 1.27–1.18 (m, 1H), 0.90 (d, 3H, J = 6.7 Hz), 0.88 (d, 3H, J = 6.7 Hz); 13C NMR (75 MHz, CDCl3) δ: 147.2, 130.1, 127.2, 111.7, 108.1, 70.0, 57.0, 56.0, 55.9, 54.3, 52.7, 40.0, 39.6, 35.5, 28.4,25.7, 23.2, 22.4; ESI-MS m/z 320.2 [M + H]+; HRMS calcd for C19H30NO3 m/z 320.2226, found 320.2248.
4.1.10. (2R,3R, 11bS)-3-Isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-ol ((−)-5)
Hydroboration-oxidation of (−)-7 was carried out following the procedure for preparation of (+)-5 to afford (−)-5 as a yellow oil, ee 100% (Chiral HPLC analytical conditions: Chiralpak IC column, 4.6mm × 250 mm, eluting with 100% i-PrOH + 0.1% Et2NH, flow rate 0.5 mL/min, oven temperature 35 °C, detection UV 220 nm). [α]D23 = −113.33° (c 0.15, MeOH). 1H NMR (300 MHz, CDCl3) δ 6.68 (s, 1H), 6.58 (s, 1H), 3.84 (s, 6H), 3.60 (s, 1H), 3.16–3.02 (m, 1H), 2.91–2.76 (m, 2H), 2.63–2.53 (m, 3H), 2.21–2.17 (m, 1H), 1.96–1.42 (m, 5H), 1.29–1.19 (m, 1H), 0.89 (d, 6H, J = 7.0 Hz); 13C NMR (75 MHz, CDCl3) δ 147.3, 147.2, 130.0, 127.1, 111.7, 108.0, 70.0, 57.0, 56.0, 55.9, 54.3, 52.7, 40.0, 39.6, 35.5, 28.4, 25.7, 23.2, 22.4; ESI-MS m/z 320.2 [M + H]+; HRMS calcd for C19H30NO3 [M + H]+ m/z 320.2226, found 320.2248.
4.1.11. (8aS,9aR,10aR)-5,8,8a,9a,10,10a-Hexahydro-2,3-dimethoxy-8a-(2-methylpropyl)-6H-benz[a]oxireno[g]quinolizine ((+)-8)
To a solution of (+)-7 (100 mg, 0.33 mmol) in MeOH (1.7 mL) was added a solution of 70% perchloric acid (28 mL, 0.33 mmol) in MeOH (1.7 mL) and, then, 85% mCPBA (103 mg, 0.876 mmol) was added. The reaction mixture was stirred in the dark at room temperature for 38 h. Thereafter, the mixture was poured into saturated aqueous sodium sulfite solution (16 mL), then water (16 mL) and CH2Cl2 (40 mL) were added. The mixture was basified with saturated aqueous NaHCO3, and extracted with CH2Cl2. The organic layer was washed with brine, dried over Na2SO4, and concentrated under vacuum to give a yellow oil (160 mg). The crude product was purified by column chromatography (SiO2, petroleum ether:EtOAc: NH4OH = 70:10:1.4) to give (+)-8 (49 mg, 46.7%). [α]D23 = +189.1° (c 0.4, MeOH). IR (KBr, cm−1) 3416, 2954, 2762, 1609, 1514, 1458, 1385, 1369, 1333, 1260, 1230, 1210, 1142, 1103, 1022, 877, 781; 1H NMR (300 MHz, CDCl3) δ 6.57 (s, 1H), 6.54 (s,1H), 3.86 (s, 6H), 3.27 (d, 1H, J = 12.9 Hz), 3.16–2.92 (m, 4H), 2.60–2.49 (m, 3H), 2.40 (ddd, 1H, J = 3.3, 11.4, 11.4 Hz), 2.00–1.82 (m, 2H), 1.63–1.41 (m, 2H), 0.99 (d, 3H, J = 6.5 Hz), 0.97 (d, 3H, J = 6.5 Hz); 13C NMR (75 MHz, CDCl3) δ 147.5, 147.3, 129.3, 126.9, 111.3, 108.4, 58.4, 57.8, 57.6, 56.6, 56.0, 55.8, 51.7, 43.8, 32.4, 29.0, 25.2, 23.1, 22.9; ESI-MS m/z 318.2 [M + H]+; HRMS calcd for C19H28NO3 [M + H]+ m/z 318.2069, found 318.2088.
4.1.12. (8aR,9aS,10aS)-5,8,8a,9a,10,10a-Hexahydro-2,3-dimethoxy-8a-(2-methylpropyl)-6H-benz[a]oxireno[g]quinolizine ((−)-8)
Epoxidation of (−)-7 was carried out following the procedure for preparation of (+)-8 to afford (−)-8, [α]D22 = −190.5° (c 0.21, MeOH).1H NMR (300 MHz, CDCl3) δ 6.57 (s, 1H), 6.55 (s, 1H), 3.84 (s, 6H), 3.29 (d, 1H, J = 13.1 Hz), 3.17–2.98 (m, 4H), 2.60–2.40 (m, 4H), 2.00–1.81 (m, 2H), 1.64–1.42 (m, 3H), 0.99 (d, 6H, J = 7.4 Hz); 13C NMR (75 MHz, CDCl3) δ 147.5, 147.3, 129.3, 126.9, 111.2, 108.4, 58.3, 57.8, 57.6, 56.6, 56.0, 55.8, 51.7, 43.8, 32.3, 28.9, 25.1, 23.1, 22.9; ESI-MS m/z 318.2 [M + H]+; HRMS calcd for C19H28NO3 [M + H]+ m/z 318.2069, found 318.2076.
4.1.13. (2R,3S,11bR)-3-Isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-ol ((+)-4)
To a solution of (+)-8 (100 mg, 0.33 mmol) in dry THF was added dropwise 2 M borane–Me2S–THF (0.73 mL, 1.46 mmol) at 0 °C. The reaction mixture was stirred at below 15 °C for 48 h, and then 0.5 mL of water was added. The mixture was heated at reflux for 30 min. Then 30% aqueous NaOH solution (0.67 mL) and aqueous 30% hydrogen peroxide solution (0.28 mL) were added. The resulting mixture was heated at reflux for an additional 1.5 h. After cooling, 10 mL of water was added, and the mixture was extracted with EtOAc. The organic layer was washed with brine, dried over Na2SO4, and concentrated under vacuum to give a yellow oil. The crude product was purified by preparative TLC to afford (+)-4 as a white solid (32 mg, 31%), ee 100% (Chiral HPLC analytical conditions: Chiralpak IC column, 4.6 mm × 250 mm, eluting with 100% i-PrOH + 0.1% Et2NH, flow rate 0.5 mL/min, oven temperature 35 °C, detection UV 220 nm). [α]D21 = +148.2° (c 0.16, MeOH). 1H NMR (300 MHz, CDCl3) δ 6.68 (s, 1H), 6.58 (s, 1H), 3.95–3.92 (m, 1H), 3.84 (s, 6H), 3.11–3.07 (m, 2H), 2.86–2.83 (m, 2H), 2.60–2.55 (m, 1H), 2.44–2.29 (m, 3H), 1.92 (brs, 1H), 1.64–1.61 (m, 4H), 1.26–1.22 (m, 1H), 0.93 (d, 3H, J = 6.3 Hz), 0.89 (d, 3H, J = 6.4 Hz); 13C NMR (75 MHz, CDCl3) δ 147.4, 147.3, 129.9, 126.7, 111.6, 108.0, 71.8, 60.6, 57.8, 56.0, 55.9, 52.2, 38.1, 36.2, 34.0, 29.2, 26.1, 23.8, 22.0; ESI-MS m/z 320.2 [M + H]+; HRMS calcd for C19H30NO3 [M + H]+ m/z 320.2226, found 320.2248.
4.1.14. (2S,3R,11bS)-3-Isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-ol ((−)-4)
Reductive ring-opening of (−)-8 was carried out following the procedure for preparation of (+)-4 to give (−)-4 as a white solid, ee 100% (Chiral HPLC analytical conditions: Chiralpak IC column, 4.6mm × 250 mm, eluting with 100% i-PrOH + 0.1% Et2NH, flow rate 0.5 mL/min, oven temperature 35 °C, detection UV 220 nm). [α]D22 = −143.6° (c 0.23, MeOH). 1H NMR (300 MHz, CDCl3) δ 6.68 (s, 1 H), 6.58 (s, 1H), 3.95–3.92 (m, 1H), 3.84 (s, 6H), 3.13–3.02 (m, 2H), 2.91–2.82 (m, 2H), 2.60–2.55 (m, 1H), 2.48–2.29 (m, 3H), 1.92 (brs, 1H), 1.67–1.58 (m, 4H), 1.26–1.19 (m, 1H), 0.94 (d, 3H, J = 6.4 Hz), 0.90 (d, 3H, J = 6.4 Hz); 13C NMR (75 MHz, CDCl3) δ 147.4, 147.3, 129.9, 126.7, 111.6, 108.0, 71.9, 60.7, 57.8, 56.0, 55.9, 52.2, 38.1, 36.3, 34.0, 29.2, 26.1, 23.8, 22.0; ESI-MS m/z 320.2 [M + H]+; HRMS calcd for C19H30NO3 [M + H]+ m/z 320.2226, found 320.2236.
4.2. VMAT2 binding
The brains from male Sprague–Dawley rats (approximately 200–225 g weight) (Taconic Labs) were removed, placed on ice, and the striatum was rapidly removed and frozen (−80 °C). Thereafter, membranes were prepared by homogenizing striatum samples in 20 volumes (w/v) of ice cold HEPES-sucrose buffer (50 mM HEPES, 0.32 M sucrose, pH adjusted to 8.0) using a Brinkman Polytron (setting 6 for 20 s), and centrifuging them at 20,000×g for 10 min at 4 °C. The resulting pellet was resuspended in buffer, recentrifuged and again resuspended in buffer to provide a concentration of 10 mg/mL. Ligand binding experiments were conducted in polystyrene assay tubes containing 0.5 mL HEPES-sucrose buffer for 60 min at room temperature. Each tube contained 2 nM [3H]DHTBZ (specific activity 20 Ci/mmol) (American Radiolabeled Chemicals) and 1.0 mg striatal tissue (original wet weight). Nonspecific binding was determined using 20 μM (±)-TBZ (Sigma Chemical Co., St Louis, MO). Incubations were terminated by rapid filtration through Whatman GF/B filters, presoaked in 0.5% PEI (polyethyleneimine), using a Brandel R48 filtering manifold (Brandel Instruments Gaithersburg, MD). These filters were then washed twice with 5 mL cold buffer and placed in scintillation vials. Beckman Ready Safe (3.0 mL) was added and the vials were counted the next day using a Beckman 6000 liquid scintillation counter (Beckman Coulter Instruments, Fullerton, CA). Data were analyzed by using GraphPad Prism software (San Diego, CA) from a 13 point dose-response curve that spanned 1 × 10(−10) to 1 × 10(−4) M.
Supplementary Material
Acknowledgment
This work was supported in part by the “111 Project” from the Ministry of Education of China and the State Administration of Foreign Expert Affairs of China (No. 111-2-07). It was additionally supported in part by the Intramural Research Programs of the National Institute on Aging and National Institute on Drug Abuse, NIH.
Footnotes
Appendix. Supporting information
Supplementary data related to this article can be found online at doi:10.1016/j.ejmech.2011.02.046.
References
- [1].Brossi A, Lindlar H, Walter M, Schnider O, Helv. Chim. Acta 41 (1958) 119–139. [Google Scholar]
- [2].Jankovic J, Lancet Neurol 8 (2009) 844–856. [DOI] [PubMed] [Google Scholar]
- [3].Silay YS, Jankovic J, Expert Opin. Emerg. Drugs 10 (2005) 365–380. [DOI] [PubMed] [Google Scholar]
- [4].Schwartz DE, Bruderer H, Rieder J, Brossi A, Biochem. Pharmacol 15 (1966) 645–655. [DOI] [PubMed] [Google Scholar]
- [5].Roberts MS, McLean S, Millingen KS, Galloway HM, Eur. J. Clin. Pharmacol 29 (1986) 703–708. [DOI] [PubMed] [Google Scholar]
- [6].Fasano A, Bentivoglio AR, Expert Opin. Pharmacother 10 (2009) 2883–2896. [DOI] [PubMed] [Google Scholar]
- [7].Scherman D, Gasnier B, Jaudon P, Henry JP, Mol. Pharmacol 33 (1988) 72–77. [PubMed] [Google Scholar]
- [8].Pletscher A, Brossi A, Gey KF, Int. Rev. Neurobiol 4 (1962) 275–306. [Google Scholar]
- [9].Vartak AP, Nickell JR, Chagkutip J, Dwoskin LP, Crooks PA, J. Med. Chem 52 (2009) 7878–7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kilbourn M, Lee L, Borght TV, Jewett DM, Frey K, Eur. J. Pharmacol 278 (1995) 249–252. [DOI] [PubMed] [Google Scholar]
- [11].Kilbourn MR, Lee LC, Heeg MJ, Jewett DM, Chirality 9 (1997) 59–62. [DOI] [PubMed] [Google Scholar]
- [12].Kilbourn MR, Lee LC, Jewett DM, Koeppe RA, Frey KA, J. Cereb. Blood Flow Metab 15 (1995) S650. [DOI] [PubMed] [Google Scholar]
- [13].Gano KW, PCT patent application: WO2008058261 (2008).
- [14].Clarke I, Turtle R, Johnston G, PCT patent application: WO2005077946 (2005).
- [15].Rishel MJ, Amarasinghe KKD, Dinn SR, Johnson BF, J. Org. Chem 74 (2009) 4001–4004. [DOI] [PubMed] [Google Scholar]
- [16].Boldt KG, Biggers MS, Phifer SS, Brine GA, Rehder KS, Synth. Commun 39 (2009) 3574–3585. [Google Scholar]
- [17].Yu QS, Luo WM, Deschamps J, Holloway HW, Kopajtic T, Katz JL, Brossi A, Greig NH, Med. Chem. Lett 1 (2010) 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sun HB, Yao ZY, Chinese patent application: 2009100318475 (2009).
- [19].Openshaw HT, Whittaker N, J. Chem. Soc (1963) 1449–1460. [Google Scholar]
- [20].Openshaw HT, Whittaker N, J. Chem. Soc (1963) 1461–1471. [Google Scholar]
- [21].Lee LC, Borght TV, Sherman PS, Frey KA, Kilbourn MR, J. Med. Chem 39 (1996) 191–196. [DOI] [PubMed] [Google Scholar]
- [22].Brown HC, Moon NM, J. Am. Chem. Soc 90 (1968) 2686–2688. [Google Scholar]
- [23].Smith WB, J. Org. Chem 49 (1984) 3219–3220. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.