Abstract
Transcription factors regulate various developmental and functional aspects of B cells. T-bet is a recently appreciated transcription factor associated with “Age-associated B cells” or ABCs, the development of autoimmunity, and viral infections. T-bet expression is favored by nucleic acid-containing antigens and immune complexes and is regulated by interplay between various cytokines, notably, the TFH cytokines IL-21, IL-4 and IFNγ. Adaptive signals by themselves cannot upregulate T-bet; however, they have a synergistic effect on induction of T-bet by innate receptors. The functional role of T-bet + B cells is unclear, although it is known that T-bet promotes class switching to IgG2a/c. It is likely T-bet serves dichotomous roles in B cells, promoting pathogenic autoreactive antibodies on one hand but mediating microbial immunity on the other, making it a target of interest in both therapeutic and prophylactic settings.
Keywords: B cell, T-bet, TLR, IL21, IFN-gamma, Signalling
Host-pathogen interactions and environmental cues collectively shape the quality of primary adaptive immune responses by initiating circuits that enable effector and memory lymphocytes to provide protective immunity and react effectively to subsequent challenges. Inappropriate differentiation can result in a failure to protect the host, and can engender immune pathologies associated with autoimmunity, allergy, and chronic inflammatory disorders. Accordingly, a complete understanding of the signaling networks underlying the establishment of discrete effector and memory cell pools is key to developing effective vaccines and therapeutic strategies.
Shifts in transcriptional programs are fundamental to the direction of cell fate, and these shifts are determined by the aggregate of extrinsic signals received during activation. Herein, we briefly summarize key aspects of transcriptional regulation within the B lineage, followed by a more detailed consideration of the signals that drive antigen-experienced B cells to adopt fates associated with T-bet expression.
1. Transcription factors guide B cell differentiation and function
As in all cell lineages, B cell genesis and differentiation require turning on appropriate developmental programs and silencing those that foster other fates. Detailed reviews about the nature and interactions of transcription factors that orchestrate late B cell development can be found elsewhere [1-4], and are thus treated briefly here. The Pax5, EBF1 and E2A proteins are some of the earliest controllers that establish the transcriptional network responsible for promoting B cell development and suppressing other lineages. For example, Pax5 expression is critical for commitment to B cell fate; Pax5-deficient pro-B cells retain the potential to develop into non-B cell lineages [5,6]. Pax5 regulates the expression of many B cell surface molecules and receptors, including CD19, CD21, CD79a, as well as other relevant transcription factors like IRF4/8 and BACH2. All mature B cells continue to express Pax5, and deletion even at these mature stages yields reversion to a multipotent progenitor-like state, highlighting the role of this transcription factor in maintaining B cell character [7]. Exogenous signals that activate key transcriptional regulatory pathways also govern triage into different pre-immune B cell pools; for example, Notch-2 transcriptional activities are required for marginal zone B cell differentiation. Once a B cell is within the quiescent mature follicular (FO) or marginal zone (MZ) pools, these transcriptional programs are maintained at steady state unless activating signals are received.
2. Activation initiates transcriptional program shifts
Analogous to the differentiation of pre-immune B cells, the fates of activated B cells are also guided by shifts in transcription factor representation. In accord with the tenets of clonal selection, B cells require engagement of their antigen receptor – the BCR – to initiate activation. The immediate consequences of BCR signaling involve modification or further activation of pre-existing transcriptional regulatory systems, such as NF-κB, NFAT, and AP-1. The strength and duration of the BCR signal per se can impact eventual cell fate. For example, strong BCR signaling is associated with a higher propensity to rapidly adopt a plasma cell fate [8,9]. Despite the influence of BCR ligation on these relatively short-term outcomes, the ultimate fate of BCR-activated cells is also strongly influenced by additional exogenous signals. These signals include co-stimulation received during cognate T helper interactions, cytokines within the activating milieu, and signals from Pathogen Associated and Danger Associated Molecular Patterns (PAMPs and DAMPs) via innate receptors such as toll-like receptors (TLRs). The permutations, kinetics, and downstream integration of these signaling cues prompt the establishment of distinct transcription factor landscapes, which in turn drive fate choice and effector function. Two archetypical examples of this in antigen-activated B cells are Bcl6 and BLIMP1, transcription factors required for germinal center (GC) formation and GC B cell proliferation versus plasma cell (PC) differentiation, respectively. Thus, BCL6 expression is upregulated in response to cognate helper T cell interactions, and represses the activity of cell cycle regulators and molecules involved in DNA damage response. As a result, GC B cells are able to proliferate rapidly and undergo somatic hypermutation. In contrast, BLIMP1 promotes the development of plasma cells. BCL6 and BLIMP1 are reciprocally antagonistic – so BCL6/Blimp1 mutual repression is essential for B cells to commit exclusively to either GC or PC fate. These functions are clearly evidenced by the phenotype of Bcl6-deficient mice; GC development is blocked but plasma cells secreting low-affinity antibodies still develop [10].
These examples illustrate how fundamental and master transcriptional regulators act to govern major fate and differentiation choices within the pre-immune and antigen-experienced B cell pools, based on the aggregate of initiating upstream signals. While the existence of broad categories – such as GC versus plasma cell fates – have been appreciated for some time, recent findings indicate that further functional subsets exist among antigen-experienced B cells – and this diversification is similarly established through engagement of key transcriptional regulators. One such example is T-bet, encoded by the tbx21 gene. This transcription factor was first described in helper T cells – hence the moniker “T-Box Expressed in T cells” – in studies that showed T-bet promotes IFNγ production, but suppresses IL-4 and IL-5. Thus, T-bet skews the differentiation of naive CD4 cells to a Th1 profile while repressing the Th2 program [11,12]. Subsequent studies revealed that T-bet is required for differentiation and function of effector CD8 + T cells [13], and interactions between T-bet and other transcription factors play key roles in the development immune cell subsets. For example, the T-bet versus Eomes axis is critical to CD8 effector versus memory differentiation [14-16].
It is now clear that T-bet expression defines a unique, antigen-experienced B cell subset. Early studies suggested that T-bet played a role in inflammatory cytokine production and immunoglobulin isotype switching [17,18], and more recent observations have expanded these findings to show that T-bet is a key player in determining the nature and quality of effector and memory B cell subsets. Studies from Szabo et al. established a link between T-bet expression and IFNγ production in B cells. While these authors did not examine the exact signals driving T-bet expression, they laid the groundwork for other studies that went on to identify activation requirements and cytokine circuits that are instrumental in inducing T-bet expression in B cells. Subsequent work revealed that immunoglobulin isotype switching to IgG2 a/c is facilitated by T-bet [18–21], as are some instances of anti-viral and antibacterial immunity which, incidentally, rely on IgG2a/c-mediated protection [22–24]. More recently, T-bet was found to be important for the emergence of age-associated B cells (ABCs), and T-bet expressing B cells have been described in a variety of infections and autoimmune scenarios. While these at first glance these may seem disparate and poorly connected phenomena, they likely provide clues to common signals that initiate the T-bet transcriptional program in activated B cells.
3. Age-associated B cells, a T-bet driven subset
The discovery of a B cell subset that accumulates with age, and also arises in infection and autoimmunity, led to questions about what transcriptional programs direct its differentiation. Phenotypically, these naturally occurring ABCs express B220, CD19, and are negative for CD43 and CD93, indicating that they are mature B2 cells. However, they lack CD23 and CD21/35, canonical pre-immune B cell markers that discriminate between FO and MZ B cell subsets. Also distinct from FO and MZ subsets, roughly half of all ABCs defined by these criteria express T-bet, and among these, about one-third also express CD11c. ABCs do not proliferate (but still survive) in response to BCR ligation in vitro. Instead, ABCs proliferate in response to endosomal TLR signals, particularly from TLR7 and TLR9. In accord with increased T-bet expression, they tend to secrete antibodies of the IgG2a/c isotype when activated [25,26]. Since T-bet positive B cells are a subset of ABCs, it is pertinent to summarize what is known about development of ABCs prior to addressing factors inducing T-bet expression
4. The genesis of ABCs
The origin of naturally arising ABCs remains incompletely understood, although increasing evidence suggests that most, if not all, are the result of antigen-driven activation. It remains possible that age-related alterations in B cell lymphopoiesis foster the generation of a pre-immune B cell subset with these characteristics. However, sublethal irradiation and autoreconstitution of aged mice resulted in a splenic B cell profile similar to young mice, with a marked absence of ABCs [25]. Thus, the aged bone marrow microenvironment is not fundamentally predisposed to generating ABC-like cells. Nonetheless, increasing evidence suggests that ABCs themselves may dampen overall B lymphopoiesis ([27], Riley et al. this volume). Cell cycle analysis revealed that ABCs themselves are quiescent, leading to the conclusion that they accumulate with age, rather than self-renew [25]. To explore whether ABCs can be derived from existing mature B cell subsets, FO B cells were CFSE-labelled and adoptively transferred into young congenic hosts. A month later, some of the transferred cells had divided, and those that had undergone the most exhaustive division had also acquired an ABC phenotype. Thus, ABCs can arise from pre-immune pools such as FO B cells, consistent with the notion that they reflect antigen-driven differentiation, and accumulate over time. The observation that FO B cells underwent several divisions before giving rise to ABCs led to the question of what cell intrinsic and microenvironmental requisites were necessary for this process. To address this, Russell Knode et al. modified the adoptive transfer system described above, and used donor CD23+ B cells from either MHC II−/− or CD154−/− mice. While WT donor cells proliferated, and adopted ABC characteristics (CD23− and T-bet+), the knockout cells failed to do either. Additionally, aging CD154−/− mice did not develop ABCs [28]. Together, these observations indicate that the development of T-bet expressing ABCs from pre-immune B cells requires antigen presentation and cognate help.
These observations make it tempting to speculate that most ABCs are derived from antigen-driven events, and several further observations favor this possibility. First, our recent analyses of the Ig heavy and light chains from sorted, naturally occurring ABCs revealed a largely stochastic representation of VL and VH gene segment usage, suggesting that these ABCs reflect an aggregate of immune experiences over the life of the individual, thus drawing from the full repertoire of BCRs. Second, these analyses revealed clear evidence of somatic hypermutation among ABCs, strengthening the case for a germinal center origin [29]. Nonetheless, it is worth remembering that T-bet expression is a characteristic of only about half of CD23−CD21− B cells. It is as yet unknown what prompts the dichotomy of T-bet expression in the mature CD23−CD21− pool. Perhaps the overall ‘natural’ CD23−CD21− ABC population includes both naive and antigen experienced cells, the latter being characterized by T-bet expression (see Swain et al., this volume, for a discussion of this idea). Little is known about whether and how the T-bet positive and negative fractions differ functionally, or if T-bet expression is more easily induced in the T-bet negative fraction of these cells as compared to FO or MZ subsets. As more insights are gained, we will have a better picture of how the T-bet positive ABC fraction differs from the negative one in terms of antigen experience and downstream signals.
5. Signals driving T-bet in B cells
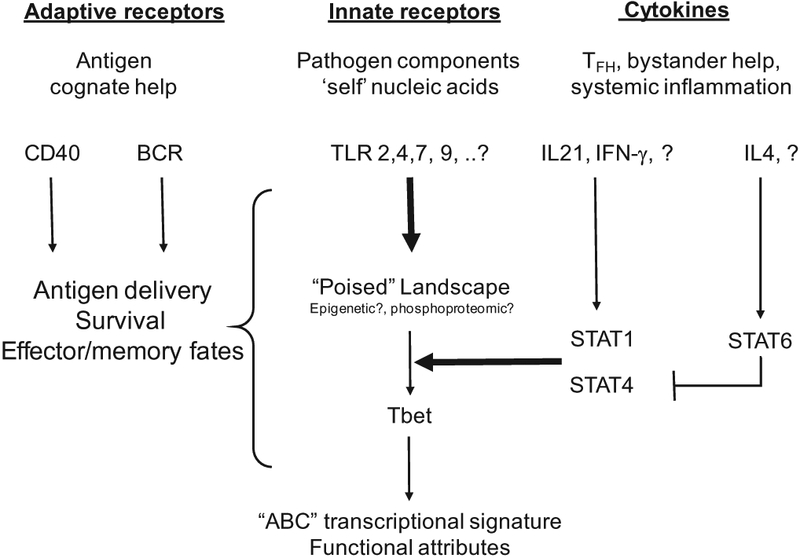
Observations to date suggest that T-bet expression in B cells is governed primarily by innate receptor signals and prevailing cytokine milieu, either alone or superimposed on activation via adaptive receptors and co-stimulation. This triumvirate of signaling systems, as well as their downstream impact on T-bet expression, are schematized in Fig. 1.
Fig. 1.
Signals driving T-bet expression in B cells. The three signaling systems that govern the initiation of Tbet expression and subsequent differentiative fates in B cells are shown. Necessary signals are shown with bold arrows. Question marks indicate that further analogous signals may remain undiscovered. Pathogen-associated or self ligands that engage innate receptors, like TLRs, are required to poise B cells for T-bet expression. TLRs that have been directly implicated in T-bet expression are listed, although other DAMPs and PAMPs may also provide such signals. Actual transcription of T-bet requires the appropriate cytokine milieu and associated STAT signaling. To date, IL-21 and IFN-γ singly and synergistically induce T-bet expression in poised cells. In contrast, IL-4 can block IL21-driven T-bet expression. Other cytokines signaling through similar Jak/STAT pathways have not been investigated; they could shed light on how T-bet expression is regulated in different infections, autoimmunity or inflammation. Adaptive signaling- BCR and co-stimulation – are not sufficient to induce T-bet expression. However, BCR-mediated delivery of nucleic acids containing antigens amplifies TLR-driven T-bet expression. Cognate CD4, while not sufficient, appears to be essential for T-bet expression, but can foster a GC versus plasma cell fate decisions among T-bet+ cells.
T-bet expression in murine FO B cells is driven by TLR but not BCR signaling. Liu et al. were the first to show that CpG and LPS could induce T-bet at the mRNA level as early as three hours after stimulation [20]. Rubtsova et al. subsequently determined that TLR7 signaling was crucial for the emergence of CD11c+ ABCs, most of which also have high T-bet gene expression [22]. These observations were confirmed and extended by Naradikian et al., where T-bet expression was induced in response to TLR9 or TLR7 signaling, but not to BCR and/or anti-CD40 stimulation alone [30]. These data bear interesting parallels to the original findings of Hao et al. and Rubtsova et al., who reported that ABCs proliferate only when stimulated via TLR9 or TLR7. Nonetheless, several observations suggest that BCR signals and co-stimulation via CD40 are often associated with the generation of Tbet+ ABC-like cells. First, simultaneous ligation of the BCR and TLR has a synergistic effect on of ABC proliferation [25], suggesting that while BCR ligation is dispensable for ABC activation, it is nonetheless capable of amplifying the response once TLR signals are received. Moreover, Russel-Knode et al. recently reported that ABCs fail to arise in aged CD154-deficient mice, and that neither MHC II-deficient nor CD40 knockout B cells acquire the ABC phenotype following adoptive transfer [28]. Thus, T-bet expression in B cells seems to require TLR-mediated signals, but – at least in the case of naturally arising T-bet+ ABCs – probably occurs in the context of cognate CD4T cell help. This led to the question of whether canonical T follicular helper (TFH) cytokines – which would be received concomitant with cognate helper interactions – also play roles in adoption of the T-bet+ fate.
Cytokine milieu modulates transcriptional profile of immune cells, and T-bet expression is regulated by interplay between the TFH cytokines IL-21, IL-4 and IFNγ. Both IFNγ and IL-21 drive T-bet expression, but while IL-4 enhances IFNγ-induced T-bet, it has a suppressive effect on IL-21-induced expression. This points towards a complex regulatory circuit that modulates immune responses in the context of cytokine availability. This can be observed even in uninfected/unimmunized mice: the prototypical TH2-skewed mouse strain BALB/c lacks spontaneous T-bet+ GC B cells and has reduced T-bet expression in memory B cells (Bmem) compared to C57BL/6, which is TH1-skewed [30]. Pathways downstream of cytokine signaling that lead to T-bet expression in ABCs are as yet unknown. Nonetheless, as might be predicted by the roles for common gamma-chain cytokines, the existing literature points to a role for the JAK-STAT pathways. Thus, STAT1−/− knockout splenocytes do not upregulate T-bet when stimulated in vitro with IFNγ, whereas STAT4−/− splenocytes have T-bet levels comparable to wild type controls [31]. Earlier studies reported a reduction in T-bet expression in STAT4−/− mice [32], which was presumably due to a deficiency of IFNγ. These reports show that a signaling circuitry involving STATs and cytokines stimulates T-bet expression in an antigen driven manner. Similar STAT-mediated regulatory networks apparently exist in B cells. Naradikian et al., showed that IL-4 suppressed T-bet expression induced by IL-21 in a STAT6 dependent manner. Interestingly, IL-4 synergistically enhanced IFNγ driven T-bet expression, indicating that STAT-mediated signaling is complex and context-dependent [30]. There is evidence of a feedback loop amongst T-bet, STAT and cytokines. Targeted deletion of T-bet in B cells leads to reduction in STAT1 gene transcription [18]. Since STAT1 regulates IFNγ signaling, T-bet’s impact on STAT-1 transcription could be a positive regulatory mechanism to maintain its own transcription (via IFNγ).
The association between STAT signaling and T-bet expression in ABCs requires deeper research. Furthermore, only a few cytokines have been tested for their ability to induce/suppress T-bet. As T-bet expression is examined in various pathological conditions, more cytokines of interest will be revealed, hopefully providing a tool to interrogate the STAT pathway in the context of T-bet expression and function.
Cytokine-mediated regulation of T-bet expression is secondary to whether the B cell was stimulated via TLRs. In in vitro experiments, IL-21 induced T-bet only in B cells activated via TLR9 or TLR7, and not in those activated via BCR and CD40 ligation alone. This indicates that TLR signaling must poise B cells to express T-bet upon receiving appropriate cytokine cues. Thus, mode of activation by an antigen, coupled with available cytokines yields a tripartite regulatory network that modulates T-bet expression. For instance, a Th2 type pathogen (e.g.; Helminth infection) would lead to increased IL-4, which would suppress IL-21′s ability to direct B cells towards a T-bet- driven transcriptional profile. Similarly, viral infections, or nucleic acid-based antigens would elicit a Th1 response, where IFNγ and IL-21 together would promote increased T-bet expression in a TLR-dependent manner, enabling class switching to IgG2a/c and other T-bet-associated characteristics. Indeed, an increasing body of literature implicates T-bet expressing B cells in a variety of infections, including influenza [30], LCMV [33], HCV [34], and HIV [35][36].
It is also important to address the role of cognate T cell help in the context of infections. CD154-deficient mice do not acquire naturally arising ABCs with age, leading to the conclusion that cognate help is important for T-bet expression. However, we do not know if the effects are direct or indirect: is CD40-CD40L signaling inducing T-bet expression at a cell intrinsic level, or is it merely necessary for GC formation, which in turn leads to the development of T-bet expressing B cells? The latter possibility would imply a bystander effect of CD4 help. Based on existing data, one would propose a model wherein cognate help following TCR activation would lead to GC formation and T-bet expression. However, cognate help might well be an event which is parallel – not precursor- to induction of T-bet expression. To address this, one needs to closely monitor when GC and T-bet expression programs are switched on in a cell, and whether T-bet+ B cells always arise from GC B cells. Further insights can be gained from a carefully controlled experiment in which GC reaction is blocked but cognate interactions occur; would T-bet+ B cells still be induced? Deconstructing the events leading to induction of T-bet expression is an important next step in ABC research.
Simultaneous ligation of BCR and TLR dampens cytokine-mediated T-bet expression [30]. On the other hand, delivery of TLR agonist via BCR has a synergistic effect, and this axis may be relevant to autoimmunity. Leadbetter et al., showed that BCR-mediated delivery of TLR antigens is a potential mechanism for delivery of autoantigens (chromatin) that can activate autoreactive B cells [37]. Data from our lab provides further mechanistic insights into this phenomenon; when a B cell is stimulated via this route and survives, it expresses T-bet in the presence of IL-21 or IFNγ and promotes class-switching to antibodies of the IgG2a/c isotype [38]. This points to the existence of a TLR-driven checkpoint to regulate survival of autoreactive clones and the implication of T-bet in promoting detrimental autoimmune responses.
The need for TLR signaling in promoting T-bet expression, and that of BCR (along with co-stimulation) in repressing the same, points to distinct signaling outcomes downstream of NF-KB and MYD88. Moreover, the observation that relevant cytokines upregulate T-bet in an exclusively TLR-dependent manner adds a further layer of complexity of involving Jak/STAT signaling. Is TLR signaling facilitating STAT binding to the Tbx21 locus? How these signals integrate is still a mystery- its resolution will likely shed some more light on B cell differentiation.
6. Outcome of T-bet driven signaling in B cells
While the complexity of signals driving T-bet expression in B cells is still being probed, a parallel avenue of investigation is the implication and functional consequence of signaling downstream of T-bet. Viral infections provide an excellent model to track the development and properties of antigen-specific ABCs, since they are nucleic-acid associated antigens that can trigger TLR signaling. Indeed, T-bet expression in B cells can be critical for effective pathogen clearance, since B lineage-restricted T-bet deficiency yielded inability to control LCMV infection, which could be ameliorated by adoptive transfer of immune serum [33]. Whether this solely reflects the effector function of IgG2a/c, or involves other T-bet driven mediators produced by B cells, such as IFN-γ, for full effect remains unclear. In addition to these incisive findings, reports in several different viral infection systems are consistent with a central role for Tbet+ B cells in immune protection [22,29,33,34,36]. In influenza infection, antigen-specific T-bet+ B cells can be detected as late as 100 days post infection where they make up about 20–30% of the total antigen-specific B cell pool [28]. Does this mean that T-bet+ B cells are a subset of memory B cells? A recent report by Chang et al. describes T-bet B cells in HCV-infected patients [34]. They found that T-bet+ B cells were increased only in patients with HCV infection, whereas the non-HCV cirrhotic cohort was similar to healthy controls. This supports the notion that T-bet expression requires nucleic-acid containing antigen. This population also expressed markers of tissue-resident memory as well as naturally arising murine ABCs (CD27−CD21−CD95+CD11c+CXCR3+). Viral clearance was accompanied by reduction in circulating T-bet+ B cells, whereas in vitro stimulation of B cells (already exposed to HCV once) with HCV antigens lead to increased T-bet expression. Thus, T-bet+ expression could be a hallmark of certain memory B cells, which are most likely tissue resident.
There is a growing appreciation for specialization even amongst effector and memory immune subsets. T lymphocytes, for example, can diverge into central, effector, stem cell memory and tissue resident memory depending on antigen, cytokine and even metabolic cues. These subsets differ not just phenotypically but also functionally. This would lead one to question, if T-bet expressing B cells are indeed a novel variety of memory B cells, what is their functional relevance? Clearly, memory subsets can impact the nature of secondary responses.
Their association with autoimmunity would indicate a pathological role. Clues regarding T-bet expressing B cells also come to us from autoimmune models. An abundance of nucleic acid containing autoantigens, accompanied by cytokine imbalance makes for a conducing environment for increased T-bet expression. Indeed, ABCs accumulated in young mice prone to autoimmunity and T-bet+ B cells have been described in autoimmune patients. These cells secrete autoantibodies upon in vitro stimulation, pointing towards a role in autoimmune pathology [22,26]. B cell-specific conditional deletion of T-bet in a spontaneous murine model of SLE resulted in reduced kidney damage, mortality and lowered serum levels of anti-chromatin autoantibodies [39]. Interestingly, the authors report that the difference in serum autoantibody levels between conditional knockout mice, and their T-bet−sufficient littermates decreased over time, suggesting that T-bet may not be the only factor regulating development of autoimmune phenotype. How T-bet interacts with other B cell-intrinsic factors to shape favorable and detrimental immune responses is an avenue for future research.
The role of T-bet+ B cells in models of infection is a bit less clear, particularly with reference to secondary immune responses. ABCs are critical components of the primary immune response [30,33] and persist long after infection has cleared [28]. They may rapidly produce antibodies in response to a secondary infection by the same pathogen, which is definitely an advantage to the host. However, it is unknown how these cells react to infection with a heterosubtypic virus. Lung-resident memory cells could mediate original antigenic sin by rapidly producing IgG2a/c antibodies specific to the primary antigen and reducing the efficacy of response to the secondary virus. It is also vital to understand difference between T-bet positive and negative cells. T-bet is a transcriptional regulator; hence it would be expected to fundamentally alter downstream signaling outcomes, with likely changes in function as well. Since T-bet+ B cells are detected several weeks post infection, their survival requirements are definitely different from most B cells. However, are they the same or different from the surviving T-bet negative pool?
T-bet expression is critical for isotype switching to IgG2a/c, which connects ABCs to plasma cell development and differentiation. Their role in humoral immunity could be associated with formation of plasma cells. In this regard, it is necessary to investigate the relationship of T-bet expressing B cells with different plasma cell populations. Switching to IgA, for example, is enabled by RORα, not T-bet [18]. Whether T-bet and RORα exert their effects independently is yet unknown. T-bet, being a regulator of lineage determination, could be guiding the development of one kind of plasma cell subset over another, perhaps modulating the process by interacting with classical plasma cell transcription factors. If this is indeed the case, then it would mean that T-bet expression is playing a major role in shaping secondary immune responses to infections, response to vaccines and development of autoimmunity. Addressing these questions requires deeper investigation into nuances of signals driving T-bet expression in B cells.
References
- [1].Nutt SL, Hodgkin PD, Tarlinton DM, Corcoran LM, The generation of antibody-secreting plasma cells, Nat. Rev. Immunol. 15 (2015) 160–171. [DOI] [PubMed] [Google Scholar]
- [2].Bartholdy B, Matthias P, Transcriptional control of B cell development and function, Gene 327 (2004) 1–23. [DOI] [PubMed] [Google Scholar]
- [3].Busslinger M, Transcriptional control of early B cell development, Annu. Rev. Immunol. 22 (2004) 55–79. [DOI] [PubMed] [Google Scholar]
- [4].Schebesta M, Heavey B, Busslinger M, Transcriptional control of B-cell development, Curr. Opin. Immunol. 14 (2002) 216–223. [DOI] [PubMed] [Google Scholar]
- [5].Nutt SL, Heavey B, Rolink AG, Busslinger M, Commitment to the B-lymphoid lineage depends on the transcription factor Pax5, Nature 401 (1999) 556–562. [DOI] [PubMed] [Google Scholar]
- [6].Rolink AG, Nutt SL, Melchers F, Busslinger M, Long-term in vivo reconstitution of T-cell development by Pax5-deficient B-cell progenitors, Nature 401 (1999) 603–606. [DOI] [PubMed] [Google Scholar]
- [7].Cobaleda C, Jochum W, Busslinger M, Conversion of mature B cells into T cells by dedifferentiation to uncommitted progenitors, Nature 449 (2007) 473–477. [DOI] [PubMed] [Google Scholar]
- [8].Krautler NJ, Suan D, Butt D, Bourne K, Hermes JR, Chan TD, Sundling C, Kaplan W, Schofield P, Jackson J, Basten A, Christ D, Brink R, Differentiation of germinal center B cells into plasma cells is initiated by high-affinity antigen and completed by Tfh cells, J. Exp. Med 214 (2017) 1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Phan TG, Paus D, Chan TD, Turner ML, Nutt SL, Basten A, Brink R, High affinity germinal center B cells are actively selected into the plasma cell compartment, J. Exp. Med. 203 (2006) 2419–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fukuda T, Yoshida T, Okada S, Hatano M, Miki T, Ishibashi K, Okabe S, Koseki H, Hirosawa S, Taniguchi M, Miyasaka N, Tokuhisa T, Disruption of the Bcl6 gene results in an impaired germinal center formation, J. Exp. Med. 186 (1997) 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH, A novel transcription factor, T-bet, directs Th1 lineage commitment, Cell 100 (2000) 655–669. [DOI] [PubMed] [Google Scholar]
- [12].Glimcher LH, Murphy KM, Lineage commitment in the immune system: the T helper lymphocyte grows up, Genes Dev. 14 (2000) 1693–1711. [PubMed] [Google Scholar]
- [13].Sullivan BM, Juedes A, Szabo SJ, von Herrath M, Glimcher LH, Antigen-driven effector CD8 T cell function regulated by T-bet, Proc. Natl. Acad. Sci. U.S.A. 100 (2003) 15818–15823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL, Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin, Nat. Immunol. 6 (2005) 1236–1244. [DOI] [PubMed] [Google Scholar]
- [15].Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, Shen H, Cereb N, Yang SY, Lindsten T, Rossant J, Hunter CA, Reiner SL, Control of effector CD8+ T cell function by the transcription factor Eomesodermin, Science 302 (2003) 1041–1043. [DOI] [PubMed] [Google Scholar]
- [16].Kaech SM, Cui W, Transcriptional control of effector and memory CD8+ T cell differentiation, Nat. Rev. Immunol. 12 (2012) 749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Peng SL, Szabo SJ, Glimcher LH, T-bet regulates IgG class switching and pathogenic autoantibody production, Proc. Natl. Acad. Sci. U.S.A. 99 (2002) 5545–5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang NS, McHeyzer-Williams LJ, Okitsu SL, Burris TP, Reiner SL, McHeyzer-Williams MG, Divergent transcriptional programming of class-specific B cell memory by T-bet and RORalpha, Nat. Immunol. 13 (2012) 604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gerth AJ, Lin L, Peng SL, T-bet regulates T-independent IgG2a class switching, Int. Immunol. 15 (2003) 937–944. [DOI] [PubMed] [Google Scholar]
- [20].Liu N, Ohnishi N, Ni L, Akira S, Bacon KB, CpG directly induces T-bet expression and inhibits IgG1 and IgE switching in B cells, Nat. Immunol. 4 (2003) 687–693. [DOI] [PubMed] [Google Scholar]
- [21].Peng SL, T-bet regulates IgG class switching and pathogenic 99 (2002) 5545–5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rubtsova K, Rubtsov AV, van Dyk LF, Kappler JW, Marrack P, T-box transcription factor T-bet, a key player in a unique type of B-cell activation essential for effective viral clearance, Proc. Natl. Acad. Sci. U.S.A 110 (2013) E3216–3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Coutelier JP, van der Logt JT, Heessen FW, Warnier G, Van Snick J, IgG2a restriction of murine antibodies elicited by viral infections, J. Exp. Med. 165 (1987) 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Markine-Goriaynoff D, Coutelier JP, Increased efficacy of the immunoglobulin G2a subclass in antibody-mediated protection against lactate dehydrogenase-elevating virus-induced polioencephalomyelitis revealed with switch mutants, J. Virol. 76 (2002) 432–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hao Y, O'Neill P, Naradikian MS, Scholz JL, Cancro MP, A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice, Blood 118 (2011) 1294–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, Marrack P, Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c(+) B-cell population is important for the development of autoimmunity, Blood 118 (2011) 1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ratliff M, Alter S, Frasca D, Blomberg BB, Riley RL, In senescence, age-associated B cells secrete TNFalpha and inhibit survival of B-cell precursors, Aging Cell 12 (2013) 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Russell Knode LM, Naradikian MS, Myles A, Scholz JL, Hao Y, Liu D, Ford ML, Tobias JW, Cancro MP, Gearhart PJ, Age-associated B cells express a diverse repertoire of VH and Vkappa genes with somatic hypermutation, J. Immunol. (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Russell Knode LM, Naradikian MS, Myles A, Scholz JL, Hao Y, Liu D, Ford ML, Tobias JW, Cancro MP, Gearhart PJ, Age-Associated B Cells Express a Diverse Repertoire of VH and Vkappa Genes with Somatic Hypermutation, J. Immunol. 198 (2017) 1921–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Naradikian MS, Myles A, Beiting DP, Roberts KJ, Dawson L, Herati RS, Bengsch B, Linderman SL, Stelekati E, Spolski R, Wherry EJ, Hunter C, Hensley SE, Leonard WJ, Cancro MP, Cutting Edge: IL-4, IL-21, and IFN-gamma Interact To Govern T-bet and CD11c Expression in TLR-Activated B Cells, J. Immunol. 197 (2016) 1023–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lighvani AA, Frucht DM, Jankovic D, Yamane H, Aliberti J, Hissong BD, Nguyen BV, Gadina M, Sher A, Paul WE, O'Shea JJ, T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells, Proc. Natl. Acad. Sci. U.S.A. 98 (2001) 15137–15142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Grogan JL, Mohrs M, Harmon B, Lacy DA, Sedat JW, Locksley RM, Early transcription and silencing of cytokine genes underlie polarization of T helper cell subsets, Immunity 14 (2001) 205–215. [DOI] [PubMed] [Google Scholar]
- [33].Barnett BE, Staupe RP, Odorizzi PM, Palko O, Tomov VT, Mahan AE, Gunn B, Chen D, Paley MA, Alter G, Reiner SL, Lauer GM, Teijaro JR, Wherry EJ, Cutting edge: B cell-intrinsic T-bet expression is required to control chronic viral infection, J. Immunol. 197 (2016) 1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chang LY, Li Y, Kaplan DE, Hepatitis C viraemia reversibly maintains subset of antigen-specific T-bet+ tissue-like memory B cells, J. Viral Hepat. 24 (2017) 389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Knox JJ, Buggert M, Kardava L, Seaton KE, Eller MA, Canaday DH, Robb ML, Ostrowski MA, Deeks SG, Slifka MK, Tomaras GD, Moir S, Moody MA, Betts MR, T-bet+ B cells are induced by human viral infections and dominate the HIV gp140 response, JCI Insight 2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Knox JJ, Kaplan DE, Betts MR, T-bet-expressing B cells during HIV and HCV infections, Cell. Immunol. (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A, Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors, Nature 416 (2002) 603–607. [DOI] [PubMed] [Google Scholar]
- [38].Sindhava VJ, Oropallo MA, Moody K, Naradikian M, Higdon LE, Zhou L, Myles A, Green N, Nundel K, Stohl W, Schmidt AM, Cao W, Dorta-Estremera S, Kambayashi T, Marshak-Rothstein A, Cancro MP, A TLR9-dependent checkpoint governs B cell responses to DNA-containing antigens, J. Clin. Invest. 127 (2017) 1651–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rubtsova K, Rubtsov AV, Thurman JM, Mennona JM, Kappler JW, Marrack P, B cells expressing the transcription factor T-bet drive lupus-like autoimmunity, J. Clin. Invest. 127 (2017) 1392–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]