Abstract
Neurotransmitter containing synaptic vesicles (SVs) form tight clusters at synapses. These clusters act as a reservoir from which SVs are drawn for exocytosis during sustained activity. Several components associated with synaptic vesicles likely to help forming such clusters have been reported, including synapsin. Here we found that synapsin can form a distinct liquid phase in an aqueous environment. Other scaffolding proteins could co-assemble into this condensate, but were not necessary for its formation. Importantly, the synapsin phase could capture small lipid vesicles. The synapsin phase rapidly disassembled upon phosphorylation by calcium/calmodulin-dependent protein kinase II (CaMKII), mimicking the dispersion of synapsin 1 that occurs at presynaptic sites upon stimulation. Thus, principles of liquid-liquid phase separation may apply to the clustering of SVs at synapses.
One Sentence Summary:
Synapsin forms liquid biomolecular condensates that can trap lipid vesicles.
The presence of synaptic vesicle (SV) clusters is a defining feature of nerve terminals. SVs are tightly packed in these structures, which are well-distinct from the surrounding cytoplasm, although there is no evidence for a restraining boundary (1). Vesicles intermix within the clusters (2) and can be exchanged between them (3). While SV clusters present at synapses are anchored to active zones of secretion, active zone proteins are not required for their formation (4, 5) and small clusters also occur in developing axons prior to synapse formation (6). How motility of SVs within clusters is compatible with their spatial confinement remains unknown. Recently, liquid-liquid phase separation has been shown to be a mechanism through which components of the cytoplasm (proteins and RNAs) can assemble into distinct compartments (biomolecular condensates) not delimited by a membrane (7–9). A key feature of proteins that can undergo liquid-liquid phase separation is their ability to engage in multivalent, low-affinity interactions, either through intrinsically disordered regions (IDRs) (10, 11), or through association with binding partners (8, 12). A major constituent of the matrix that connects SVs is synapsin (13–15), whose abundance in nerve terminals is several fold higher than the abundance of any other protein specifically localized in this matrix (16). Synapsin comprises an ATP binding module of unknown physiological function (15, 17), flanked by an N-terminal short region that partially penetrates membranes (18) and a C-terminal IDR with multiple SH3 domain binding motifs (15) (Fig. S1). This prompted us to hypothesize (1) that synapsin may be a key constituent of a biomolecular condensate that includes SVs.
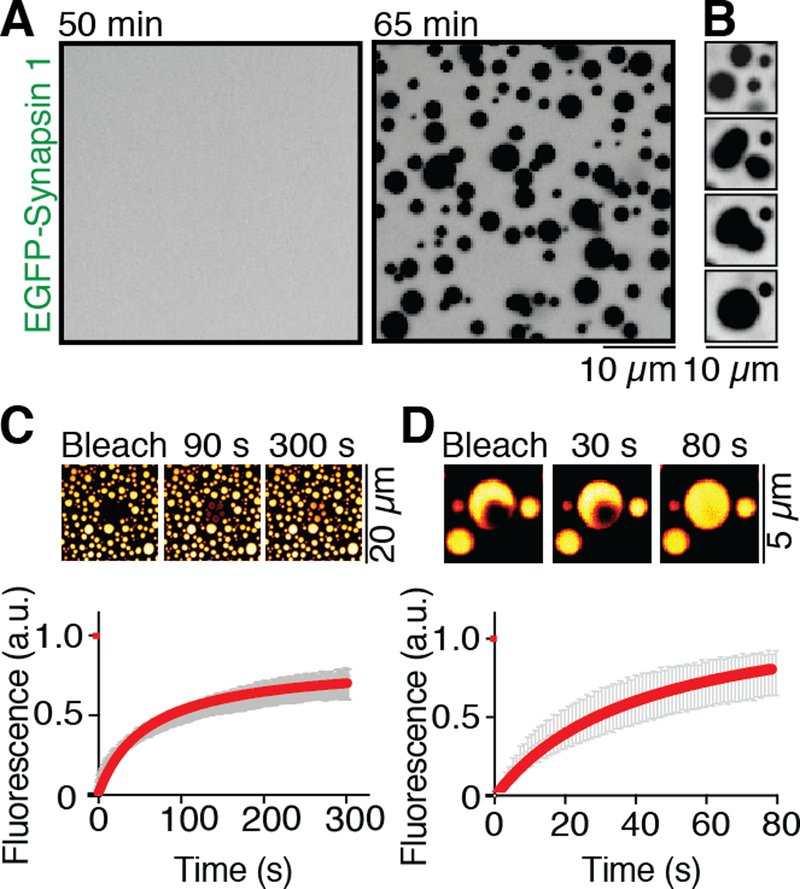
To assess whether synapsin, which forms homo and heterodimers, can phase separate through interactions of its IDR, we incubated EGFP-tagged synapsin 1 in a buffer of physiological salt concentration and pH on a glass bottom dish at room temperature (see Methods). After a lag of tens of minutes, synapsin 1 alone formed micrometer-size droplets (Fig. 1A and B, see also fig. S4 and movie S1) with the size of droplets correlating with its concentration (0.5–20 μM concentrations tested) (Figs. S2 and S3). The coalescence of synapsin 1 into droplets was confirmed by performing the incubation in suspension and measuring turbidity (Fig. S4). These concentrations were not above the physiological range, as synapsin 1 is estimated to reach concentrations above 100 μM in nerve terminals (16). Droplets of synapsin 1 had the expected properties of a liquid phase (9): they fused with each other (Fig. 1B) and bleaching of several droplets revealed that synapsin 1 molecules swiftly exchanged into and out of synapsin 1 droplets (t1/2 = 65 s; Fig. 1C and movie S2). Additionally, fluorescence recovery after photobleaching (FRAP) of a small area within the droplet was followed by rapid recovery (t1/2 = 40 s) of fluorescence, reflecting local rearrangement of synapsin 1 molecules (Fig. 1D, movie S3). Analysis of two purified fragments of synapsin 1 confirmed that its IDR (aa 421–706), but not its folded central ATP binding module (aa 113–420), which is known to dimerize (17), formed droplets (Fig. S1B). Droplet formation by the IDR alone was as efficient as droplet formation by the full length protein (Fig. S5). Synapsin 2, a paralogue of synapsin 1 that can heterodimerize with synapsin 1 (15, 17), also contains a C-terminal IDR, albeit shorter than the IDR of synapsin 1. Accordingly, also synapsin 2 phase separated (Fig. S6). Increasing the salt concentration above the physiological range impaired droplet formation implicating charge-dependent interactions in their formation (Fig. S7).
Fig. 1. Synapsin 1 undergoes liquid-liquid phase separation.
(A) EGFP-Synapsin 1 (10 μM) forms droplets when incubated for one hour in a buffer of physiological salt concentration at RT (B) Droplets of synapsin 1 show liquid behavior by fusing with each other and relaxing into a round-shaped structure, minimizing surface tension. (C) Photobleaching of several synapsin 1-droplets with subsequent recovery of fluorescence, as shown by micrograph and by quantification of the fluorescence recovery in the bleached region. Error bars represent s.e.m., and red is the fit with hyperbolic function. (D) Fluorescence recovery of synapsin 1 after photobleaching a region within a droplet (see inset). Error bars represent s.e.m., red is the fit with a hyperbolic function.
Polyvalent interactions between SH3 domain containing proteins and proteins harboring cognate proline rich motifs (PRM) can also generate distinct liquid phases (8, 12). Synapsin 1 interacts with several SH3 domain containing proteins via its IDR (19–22). One such protein, intersectin, is a component of a network of protein-protein interactions that serve for the clustering of SVs in conjuction with synapsin (22, 23). Thus, we examined whether upon incubation with SH3 domain containing binding partners, such as intersectin (22) and Grb2 (19), synapsin 1 phase separated together with them. Grb2 and intersectin contain two and five SH3 domains, respectively (22).
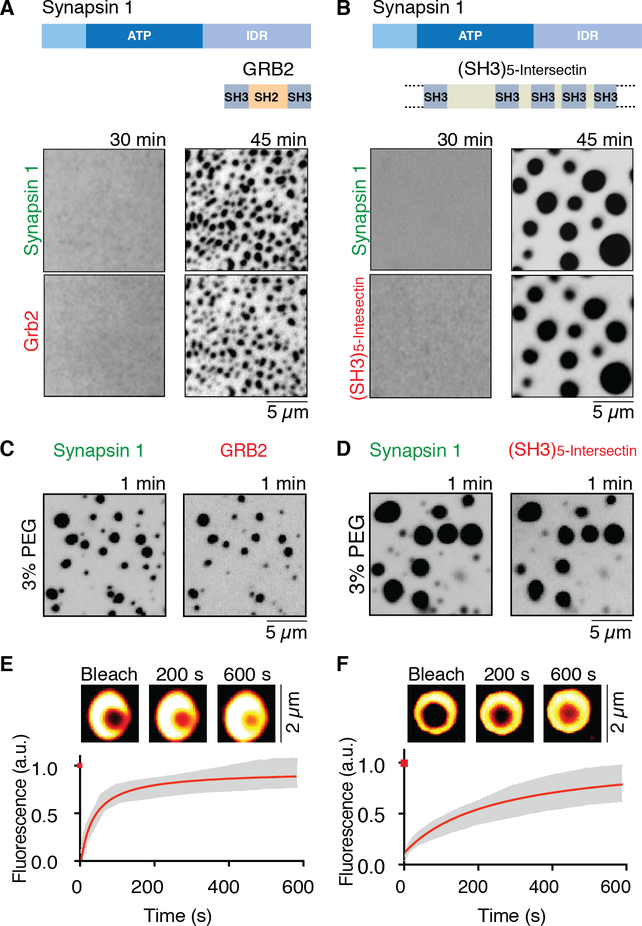
Synapsin 1 was mixed with either Grb2 (Fig 2A, movie S4) or a fragment from human intersectin comprising its five SH3 domains [(SH3)5-intersectin] (21) (all proteins at 10 μM and fused to fluorescent proteins) and incubated at room temperature in physiological salt concentration. After some delay droplets appeared containing both synapsin and its binding partners (Fig. 2B, movie S5). Droplet growth, after the initial nucleation, was faster than with synapsin 1 alone (Fig. S4). As before, droplets grew progressively or by fusing with each other, revealing a liquid state. The same concentration (10 μM) was used for synapsin 1 and its partners, although synapsin is thought to be the most abundant matrix protein within SV clusters (16). This was meant to reflect the presence at synapses of multiple SH3 domain containing synapsin 1 ligands, and thus a higher collective concentration of these proteins, than the concentration of any one of them. The synapsin 1:(SH3)5-intersectin droplets were larger, which could be explained by the higher valence of the intersectin fragment (five SH3 domains, although with different affinities for synapsin) relative to Grb2 (two SH3 domains) (22, 24).
Fig. 2. Synapsin 1 drives phase separation of SH3 domain containing binding partners.
(A) and (B) Full-length synapsin 1 (10 μM) and either Grb2 (10 μM), or the SH3 domain containing region of intersectin (10 μM) form droplets under physiological conditions. Top: domain organization of the proteins. Bottom: fluorescence images of the protein mixtures at 30 and 45min. (C) and (D) Fluorescence images of the solution immediately (within one min) after mixing of the two proteins in the presence of the crowding reagent (3 % PEG 8,000). (E) and (F) Fluorescence recovery of synapsin 1 after photobleaching a region within a synapsin 1-Grb2 droplet, or a synapsin 1-(SH3)5-intersectin droplet (see insets). Error bars represent s.e.m., red is the fit with a hyperbolic function.
The cytoplasm of a synaptic bouton is a crowded environment filled with organelles and macromolecules. To mimic this environment, in subsequent experiments we added poly-ethylene glycol (PEG), a crowding reagent, to the buffer. In the presence of 3% PEG, droplets of synapsin 1 alone (Fig. S3), or of synapsin 1 and its binding partners (Fig. 2C and D), formed immediately, with no lag phase. FRAP confirmed that, even under these conditions, proteins were mobile within the droplets, with a faster recovery time for synapsin 1 in droplets generated with Grb2 (t1/2 at 1.5 min) than in those generated with (SH3)5-intersectin (t1/2 at 3.2 min) (Fig. 2E and F). This again possibly reflects the higher valence of this protein relative to Grb2. Recovery of fluorescence was observed both when a region within a droplet (Fig. 2E and F) and when the entire droplet was bleached (Fig. S8), indicating that recovery results from both molecular rearrangements within the droplet and the exchange of molecules with the dilute phase. Presence of a synapsin 1 binding partner, (SH3)5-intersectin, had a biphasic effect on droplet formation. It enhanced this process at low to moderate excess stoichiometric ratio, but inhibited it when added in large excess (Fig. S9). Thus, (SH3)5-intersectin is not only a “client” of synapsin, but also an active player in the formation of the liquid condensate. The negative effect at high stoichiometric ratio may be due to the masking of sites within the synapsin IDR that interact with each other. Recruitment of synapsin interactors into the droplets was specific and did not occur with proteins that do not bind synapsin (Fig. S10).
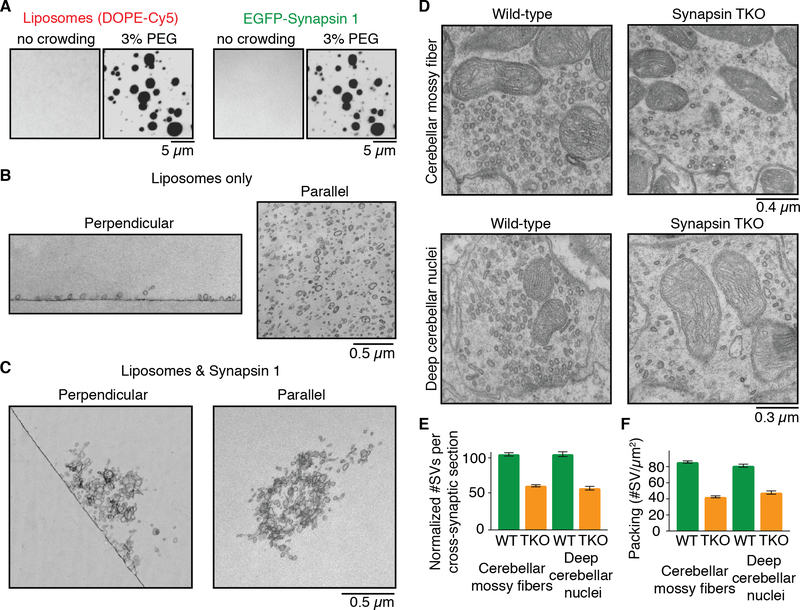
Synapsin binds synaptic vesicles (15, 18, 25). If its phase separating properties are involved in SV cluster formation, synapsin 1 should be capable of capturing vesicles into such phase. We thus incubated synapsin 1 with small lipid vesicles (~50–150 nm diameter) mimicking SVs in lipid composition supplemented with a fluorescently labeled lipid, Cy5-DOPE. Formation of synapsin droplets correlated with the appearance of droplets positive for the labeled lipid, while no droplets positive for the lipid were observed in the absence of synapsin 1 (Fig. 3A). Synapsin 1 condensates did not recruit vesicles lacking negatively charged phospholipids (Fig. S11), which is expected given that negatively charged lipids are necessary for synapsin binding to vesicles (26). Other well characterized protein liquid condensates that do not bind lipid membranes, such as droplets composed of NCK and a fragment of N-WASP that does not include the phospholipid-binding region (8), did not sequester lipid vesicles (Fig. S12). Lipid vesicles were mobile within the synapsin phase as indicated by FRAP (Fig. S13). Furthermore, lipid vesicles and SH3-domain synapsin 1 interactors co-assembled with synapsin (Fig. S14). EM analysis showed that these droplets were represented by clusters of small vesicles, while in the absence of synapsin 1, vesicles remained dispersed (Fig. 3B and C).
Fig. 3. Synapsin 1 condensates are reaction centers able to sequester lipid vesicles.
(A) Fluorescence images of mixture of liposome (left, Cy5-DOPE) and synapsin 1 (right, EGFPSynapsin 1) without or with crowding agent (3% PEG). (B) EM images of liposomes incubated without synapsin 1 in the same buffer conditions used for A. Left: section perpendicular to the liposome-glass interface. Right: section comprising the layer of liposomes absorbed to the glass surface. (C) Same as in B, but showing liposomes incubated with synapsin 1. The field shown at right is from a section parallel to the glass surface but above the glass interface. (D) EM images of synapses from cerebellar mossy fibers (top) and deep cerebellar nuclei (bottom) obtained from adult wild-type (WT, left) and synapsin triple knockout (TKO, right) mice. (E) SV number in synaptic cross-sections of wild-type and synapsin TKO mice, normalized to wild-type. (F) Synaptic vesicles number per unit area of synaptic section in wild-type and synapsin TKO mice. For each condition, fifty sections from three independent animals were examined. Error bars represent s.e.m.
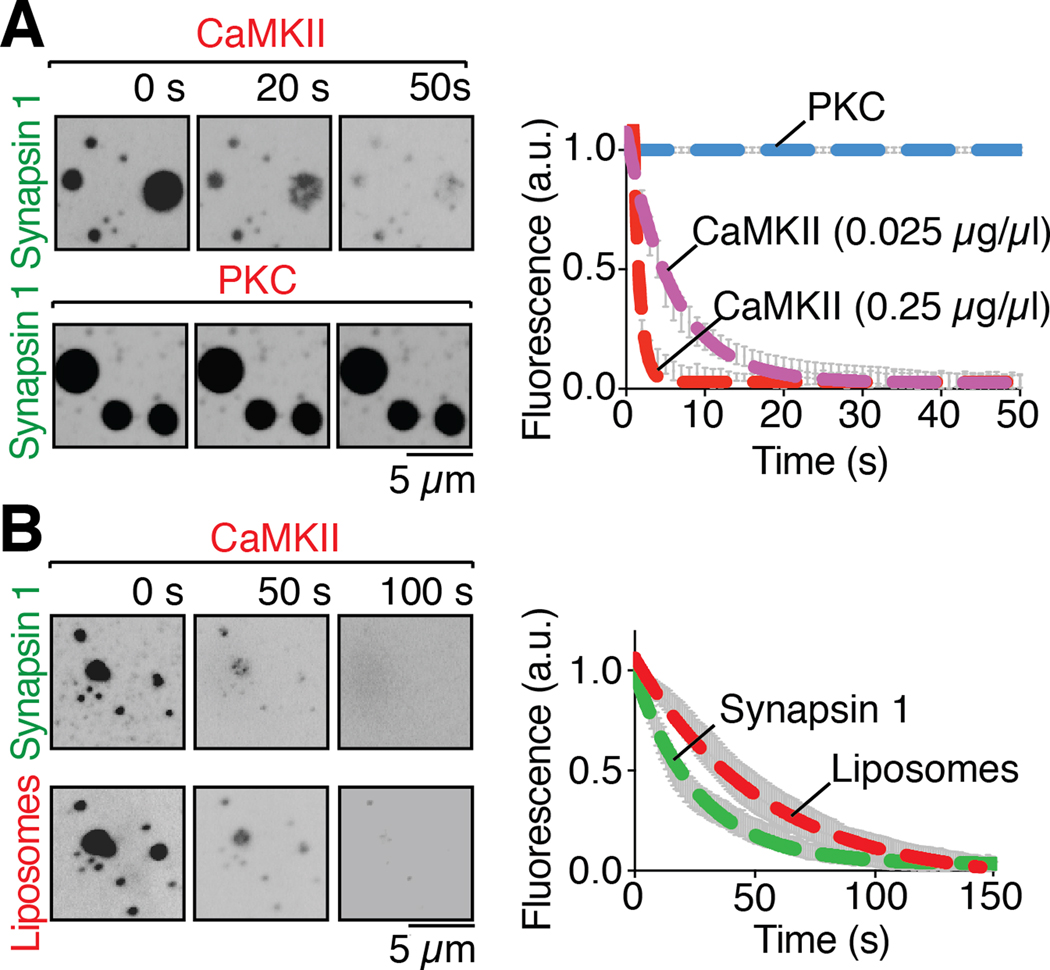
Synapsin 1 is a major presynaptic phosphoprotein that undergoes multisite phosphorylation (14). Sustained nerve terminal stimulation to trigger massive neurotransmitter release also induces the calcium-dependent phosphorylation of synapsin 1 (27). This results in its dissociation from SVs and dispersion within the nerve terminal cytosol (28, 29), as SVs are consumed by exocytosis. If the formation of a biomolecular condensate by synapsin has a physiological significance in its co-assembly with SVs, one would expect synapsin 1 droplets to disassemble upon calcium-dependent phosphorylation. Two prominent phosphorylation sites for calcium/calmodulin-dependent protein kinase II (CaMKII), called site 2 and 3, are present in its IDR (14, 15). Addition of CaMKII, calcium and calmodulin to synapsin 1 containing samples did not disperse droplets. In fact, CaMKII, which binds synapsin 1 (29), was recruited into the droplets (Fig. 4A). However, further addition of ATP (200 μM) to induce synapsin 1 phosphorylation caused rapid dispersal of both synapsin 1 and CaMKII (τ of 5.9 s) (Figs. 4A and S15, movie S6). Importantly, CaMKII also disassembled synapsin 1-liposome droplets (Fig. 4B, movie S7). As a control, we added to droplets protein kinase C, for which synapsin 1 is not a substrate (30), but neither addition of the kinase, nor the subsequent addition of ATP (200 μM), affected the droplets (Fig. 4A). The lack of effect of ATP in the latter experiment also rules out that droplet dispersion may be explained by a hydrotrope action of this nucleotide (31). Such an action, as reported for liquid droplets generated by other proteins, occurs only at much higher ATP concentration (Fig. S16) than the one used in our phosphorylation assay (see also Fig. S4).
Fig. 4. Phosphorylation of the intrinsically disordered region of synapsin 1 disperses condensates of either synapsin 1 alone or synapsin 1 and liposomes.
(A) Left: fluorescence images of EGFP-Synapsin 1 condensates preincubated with either CaMKII (0.025 μg/μl), calmodulin and calcium, or PKC, PS, DAG and calcium, upon addition, at 0 seconds, of ATP (200 μM), demonstrating dispersion of synapsin by CaMKII, but not by PKC. Right: time course of the effect of the kinases on the condensates, as assessed by the decrease of florescence on ROIs corresponding to randomly selected droplets (B) Left: Fluorescence images of liposome-synapsin condensates preincubated with CaMKII (0.25 μg/μl), calmodulin and calcium, upon addition, at 0 seconds, of ATP (200 μM), demonstrating dispersion of both synapsin and liposomes. Right: Time-course of the effect of CaMKII on liposome-synapsin droplets dispersion. Error bars represent s.e.m.; dashed lines represent the fit with a single exponential function. Incubations were carried out at RT in a buffer of physiological salt concentration supplemented with 3% PEG 8,000.
Strong evidence points to a physiological master role of synapsin in the clustering of SVs at living synapses (25, 32–34). In neuronal cultures from mice that lack all three synapsins the number of SVs at synapses is lower than in WT and this decrease is selective for SVs away from active zones (33). We extended these results. Even at synapses in situ – both excitatory (cerebellar mossy fibers) and inhibitory (deep cerebellar nuclei) nerve terminals – not only the total number, but also the packing of SVs was strikingly lower in synapsin triple KO (TKO) mice than in wild-type (WT) mice (Fig 3D to F).
Collectively, these findings demonstrate that synapsin can form a separate liquid biomolecular condensate either alone, or together with binding partners for its IDR, with lipid vesicles, or with both. Interactions occurring at the presynapse in situ are expected to be more complex than the interactions of synapsin with two SH3 domains containing proteins and artificial lipid membranes described here. Because SVs are membranous organelles selectively recruited into clusters, there must be additional factors that help provide specificity. However, the minimal systems used here provide some insight into the mechanisms responsible for the properties of SV clusters. Clusters of other membranous organelles may self-organize according to similar principles without the need for a surrounding membrane or protein-based structure to confine them.
Supplementary Material
Acknowledgments:
We thank Angus C. Nairn for providing CaMKII and for discussion; HHMI Summer Institute at the Marine Biology Laboratory for providing NCK and N-WASP.
Funding:
P.D.C. was supported by grants from the NIH (R37NS036251 and DA018343), and D.M. and X.B. were supported by a long-term postdoctoral fellowship from the Human Frontier Science Program.
Footnotes
Competing interests:
Authors declare no competing interests.
Data and materials availability:
All data is available in the main text or the supplementary materials.
References and Notes
- 1.Milovanovic D, De Camilli P, Synaptic Vesicle Clusters at Synapses: A Distinct Liquid Phase? Neuron. 93, 995–1002 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rizzoli SO, Betz WJ, The structural organization of the readily releasable pool of synaptic vesicles. Science. 303, 2037–2039 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Darcy KJ, Staras K, Collinson LM, Goda Y, Constitutive sharing of recycling synaptic vesicles between presynaptic boutons. Nat. Neurosci 9, 315–321 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Acuna C, Liu X, Südhof TC, How to Make an Active Zone: Unexpected Universal Functional Redundancy between RIMs and RIM-BPs. Neuron. 91, 792–807 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Wang SSH et al. , Fusion Competent Synaptic Vesicles Persist upon Active Zone Disruption and Loss of Vesicle Docking. Neuron. 91, 777–791 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kraszewski K et al. , Synaptic vesicle dynamics in living cultured hippocampal neurons visualized with CY3-conjugated antibodies directed against the lumenal domain of synaptotagmin. J. Neurosci 15, 4328–4342 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brangwynne CP et al. , Germline P Granules Are Liquid Droplets That Localize by Controlled Dissolution/Condensation. Science. 324, 1729–1732 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Li P et al. , Phase transitions in the assembly of multivalent signaling proteins. Nature. 483, 336–340 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banani SF, Lee HO, Hyman AA, Rosen MK, Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel A et al. , A Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation. Cell. 162, 1066–1077 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Woodruff JB et al. , The Centrosome Is a Selective Condensate that Nucleates Microtubules by Concentrating Tubulin. Cell. 169, 1066–1077.e10 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Banani SF et al. , Compositional Control of Phase-Separated Cellular Bodies. Cell. 166, 651–663 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Camilli P, Harris SM, Huttner WB, Greengard P, Synapsin I (Protein I), a nerve terminal-specific phosphoprotein. II. Its specific association with synaptic vesicles demonstrated by immunocytochemistry in agarose-embedded synaptosomes. J. Cell Biol. 96, 1355–1373 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huttner WB, Schiebler W, Greengard P, De Camilli P, Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J. Cell Biol. 96, 1374–1388 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Südhof TC et al. , Synapsins: mosaics of shared and individual domains in a family of synaptic vesicle phosphoproteins. Science. 245, 1474–1480 (1989). [DOI] [PubMed] [Google Scholar]
- 16.Wilhelm BG et al. , Composition of isolated synaptic boutons reveals the amounts of vesicle trafficking proteins. Science. 344, 1023–1028 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Hosaka M, Südhof TC, Homo- and heterodimerization of synapsins. J. Biol. Chem 274, 16747–16753 (1999). [DOI] [PubMed] [Google Scholar]
- 18.Benfenati F, Bähler M, Jahn R, Greengard P, Interactions of synapsin I with small synaptic vesicles: distinct sites in synapsin I bind to vesicle phospholipids and vesicle proteins. J. Cell Biol. 108, 1863–1872 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McPherson PS et al. , Interaction of Grb2 via its Src homology 3 domains with synaptic proteins including synapsin I. Proc. Natl. Acad. Sci. U.S.A 91, 6486–6490 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onofri F et al. , Specificity of the binding of synapsin I to Src homology 3 domains. J. Biol. Chem 275, 29857–29867 (2000). [DOI] [PubMed] [Google Scholar]
- 21.Yamabhai M et al. , Intersectin, a novel adaptor protein with two Eps15 homology and five Src homology 3 domains. J. Biol. Chem 273, 31401–31407 (1998). [DOI] [PubMed] [Google Scholar]
- 22.Gerth F et al. , Intersectin associates with synapsin and regulates its nanoscale localization and function. Proc. Natl. Acad. Sci. U.S.A 114, 12057–12062 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winther ÅME et al. , An Endocytic Scaffolding Protein together with Synapsin Regulates Synaptic Vesicle Clustering in the Drosophila Neuromuscular Junction. J. Neurosci 35, 14756–14770 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pechstein A et al. , Vesicle uncoating regulated by SH3-SH3 domain-mediated complex formation between endophilin and intersectin at synapses. EMBO Rep. 16, 232–239 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pietro De Camilli Benfenati F, Valtorta F, Greengard P, The Synapsins. Annu. Rev. Cell Biol 6, 433–460 (1990). [DOI] [PubMed] [Google Scholar]
- 26.Benfenati F, Greengard P, Brunner J, Bähler M, Electrostatic and hydrophobic interactions of synapsin I and synapsin I fragments with phospholipid bilayers. J. Cell Biol 108, 1851–1862 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huttner WB, DeGennaro LJ, Greengard P, Differential phosphorylation of multiple sites in purified protein I by cyclic AMP-dependent and calcium-dependent protein kinases. J. Biol. Chem 256, 1482–1488 (1981). [PubMed] [Google Scholar]
- 28.Chi P, Greengard P, Ryan TA, Synapsin dispersion and reclustering during synaptic systems. J. Neurosci 8, 281–288 (1988). [DOI] [PubMed] [Google Scholar]
- 29.Benfenati F et al. , Synaptic vesicle-associated Ca2+/calmodulin-dependent protein kinase II is a binding protein for synapsin I. Nature. 359, 417–420 (1992). [DOI] [PubMed] [Google Scholar]
- 30.Wang JK, Walaas SI, Greengard P, Protein phosphorylation in nerve terminals: comparison of calcium/calmodulin-dependent and calcium/diacylglycerol-dependentsystems. J. Neurosci 8, 281–288 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel A et al. , ATP as a biological hydrotrope. Science. 356, 753–756 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Pieribone VA et al. , Distinct pools of synaptic vesicles in neurotransmitter release. Nature. 375, 493–497 (1995). [DOI] [PubMed] [Google Scholar]
- 33.Gitler D et al. , Different presynaptic roles of synapsins at excitatory and inhibitory synapses. J. Neurosci 24, 11368–11380 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siksou L et al. , Three-dimensional architecture of presynaptic terminal cytomatrix. J. Neurosci 27, 6868–6877 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takamori S et al. , Molecular anatomy of a trafficking organelle. Cell. 127, 831–846 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Kennedy MB, McGuinness T, Greengard P, A calcium/calmodulin-dependent protein kinase from mammalian brain that phosphorylates Synapsin I: partial purification and characterization. J. Neurosci 3, 818–831 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahn J-H, Kim Y, Kim H-S, Greengard P, Nairn AC, Protein kinase C-dependent dephosphorylation of tyrosine hydroxylase requires the B56δ heterotrimeric form of protein phosphatase 2A. PLoS ONE. 6, e26292 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishida T, Kinoshita K, PrDOS: prediction of disordered protein regions from amino acid sequence. Nucleic Acids Res. 35, W460–4 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.