Abstract
Background
Post-transplant anastomotic biliary strictures remain refractory to endoscopic therapy in a considerable number of cases. The aim of this meta-analysis was to compare fully-covered self-expandable metal and plastic stents in the management of post-transplant biliary strictures.
Methods
A meta-analysis was performed using a random effects model; results were expressed as odds ratio (OR) and mean standardized difference. The primary outcome was stricture resolution, while recurrence rate after stent placement, treatment time, and safety of the procedure were the secondary outcomes.
Results
Through a systematic literature review until October 2017, we identified 7 studies, of which 4 were randomized controlled trials. Stricture resolution was slightly higher with metal stents, with no statistical difference between the two procedures (OR 1.38, 95% confidence interval [CI] 0.60-3.15; P=0.45) and low heterogeneity (I2=6%). Stricture recurrence showed a non-significant trend in favor of plastic stents (OR 1.82, 95%CI 0.52-6.31, P=0.35). Endoscopic retrograde cholangiopancreatography with placement of metal stents offered a significant improvement in terms of reduced treatment time (mean standardized difference: -3.58 months, 95%CI -6.23 to -0.93; P=0.008), but with more frequent complications, although not significantly so (OR 2.34, 95%CI 0.75-7.25; P=0.14). Sensitivity analysis confirmed all the findings.
Conclusion
Metal stents appear to be a promising tool that can decrease treatment time, although there is still no clear evidence of their superiority over plastic stents in terms of efficacy.
Keywords: Fully-covered self-expandable metal stents, plastic stents, orthotopic liver transplantation, endoscopic retrograde cholangiopancreatography, meta-analysis
Introduction
Biliary stricture represents one of the most frequently observed complications after orthotopic liver transplantation (OLT), occurring in 40% of patients who undergo OLT, particularly those receiving an organ from a living donor [1,2]. After OLT, anastomotic strictures (80%) are more common than non-anastomotic strictures, which result mainly from hepatic ischemia and are less responsive to endoscopic therapy, requiring a longer duration of endoscopic intervention and sometimes even re-transplantation [3,4].
While recent years have seen significant improvement in the management of anastomotic biliary strictures (ABSs) using balloon dilation and multiple plastic stents, ABSs remain refractory to endoscopic therapy in a non-negligible number of cases, with relatively high rates of recurrence, particularly in the case of late ABSs (presenting later than 1 month post-OLT) [5-10]. Balloon dilation with stent placement is more effective than balloon dilation alone, and progressively increasing the number of stents placed during subsequent endoscopic retrograde cholangiopancreatography (ERCP) procedures seems to be the most effective treatment approach [11,12]. However, although serial balloon dilation and plastic stent exchanges (usually 3 months apart over an extended period) have been used worldwide for several years, the optimal strategy for managing ABSs still needs to be defined [11,12].
Fully-covered self-expandable metal stents (FCSEMS) are already used in clinical practice to treat malignant strictures, and in recent years they have also demonstrated interesting results in the management of benign conditions, including post-OLT ABS [13]. The main potential benefit of FCSEMS is their large caliber and longer duration of patency, allowing them to be left in place longer than plastic stents, thus reducing the need for procedures for serial dilations and stent placement [12].
There is currently limited comparative evidence on the use of FCSEMS with respect to plastic stents in the management of post-OLT ABS. In this systematic review, we performed a pairwise meta-analysis of studies comparing the efficacy of FCSEMS and plastic stents in the resolution of post-transplant ABSs. In addition, we evaluated as secondary outcomes the ABS recurrence rate after stent placement, the treatment time and the safety of the procedure.
Materials and methods
This meta-analysis was performed in accordance with indications described in the Cochrane Handbook [14] and was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines [15].
Search strategy and selection criteria
Figure 1 presents the search strategy followed in the meta-analysis. A computerized bibliographic search was performed on PubMed/Medline, Embase, Google Scholar and Cochrane library databases, independently by two authors (AF, ECR), using the following key words: “anastomotic biliary stricture”, “metal stent”, “plastic stent” and “ERCP”. A complementary manual search was performed by checking the references of all the main review articles on this topic, to identify possible additional studies.
Figure 1.
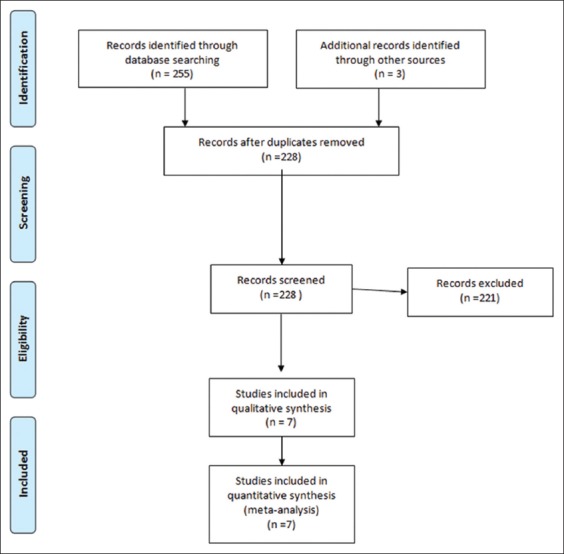
Study selection flow chart
Eligible studies were randomized-controlled trials (RCTs) and retrospective studies published until October 2017 that met the following inclusion criteria: (a) patients: adults undergoing ERCP and with radiologically confirmed diagnosis of post-OLT anastomotic biliary stricture; (b) intervention: ERCP with placement of FCSEMS; (c) comparator: ERCP with placement of multiple plastic stents; and (d) outcomes: stricture resolution as a primary outcome, stricture recurrence, treatment time (defined as time lapse between first and last therapeutic ERCP session), and complication rate as secondary outcomes.
We excluded narrative and systematic reviews [16], studies that did not report any of the main outcomes, and any studies not written in English. When incomplete information was available, attempts were made to contact the corresponding authors for additional data. Disagreements were resolved by discussion and following a third opinion (NM).
The quality of the included studies was assessed by two authors independently (AF, ECR), according to the Cochrane Collaboration's tool for assessing the risk of bias [17] for RCTs and the Newcastle-Ottawa scale [18] for observational studies. Any disagreements were addressed by reevaluation and following a third opinion (NM).
Statistical analysis
Pairwise meta-analysis was performed using a random-effects model to estimate the pooled odds ratio (OR) and 95% confidence interval (CI) [19]. We assessed statistical heterogeneity using the I2 statistic, with values over 50% indicating substantial heterogeneity, while small study effects were assessed by examining funnel plot asymmetry. Multiple sensitivity analyses were performed to assess the robustness of our findings. These were based on: (a) study design (RCT vs. retrospective), and (b) study quality (moderate vs. low). All calculations were performed using Review Manager 5.3 (the Cochrane Collaboration, Copenhagen, Denmark) and R 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria), “metafor” package.
Results
Included studies
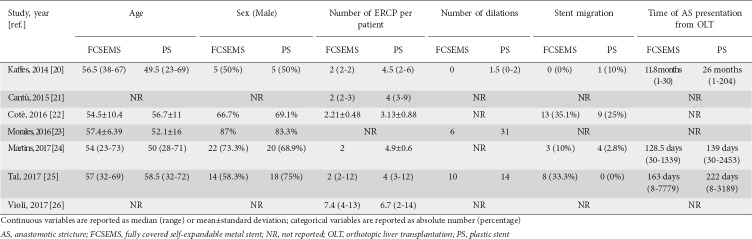
From 258 unique studies identified using the search strategy, we included 7, 4 RCTs [20,22,24,25] and 3 retrospective studies [21,23,26], whose characteristics are summarized in Table 1. All the retrospective studies were published as congress abstracts [21,23,26], while RCTs were available as full-text papers. Overall, 379 patients were included, of whom 148 underwent ERCP with FCSEMS and 231 were treated with plastic stents. The recruitment period ranged from 2006-2015.
Table 1.
Characteristics of included studies comparing metal and plastic stents
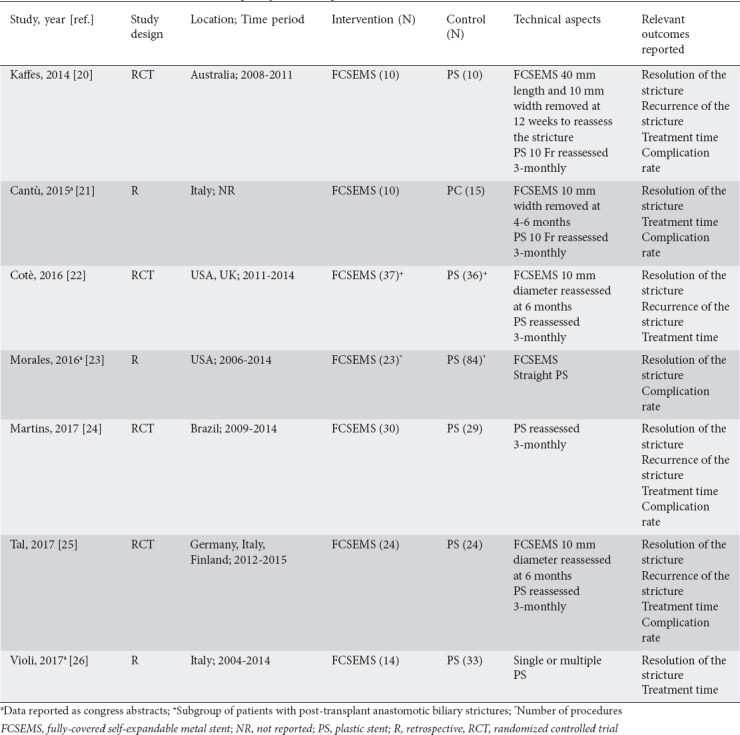
In the FCSEMS studies, the stents were removed at 4-6 months to reassess the stricture and were eventually replaced by new stents, whereas plastic stents were removed and replaced with a new stent at 3 months. The primary outcome, resolution of the stricture, was reported in all of the included studies.
In all the studies the two arms were well-balanced at baseline in terms of main demographic characteristics, as reported in Table 2. The number of procedures per patient and the need for balloon dilation were considerably higher in patients treated with plastic stents, whereas stent migration was usually more frequent in subjects undergoing ERCP with FCSEMS placement (Table 2). Notably, all strictures occurred >1 month after OLT and were thus classified as late strictures.
Table 2.
Baseline characteristics of patients enrolled in the studies comparing metal and plastic stents
Quality assessment was performed in the context of the primary outcomes, and two retrospective studies [21,26] and 3 RCTs [22,24,25] were considered to be of moderate quality, mainly because of the high risk of performance bias. Overall and study-level quality assessments are summarized in Supplementary Table 1.
Supplementary Table 1.
Risk of bias assessment and quality of included studies
Stricture resolution
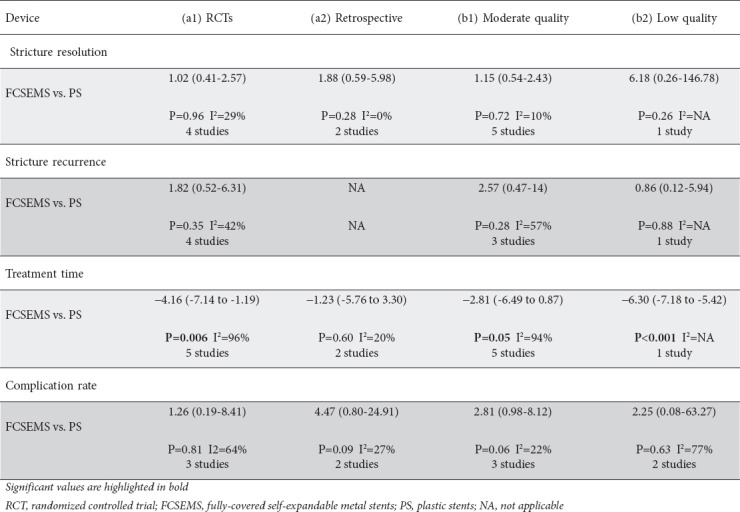
Data from six studies with 272 patients were pooled for the analysis of stricture resolution. The study by Morales et al was excluded because it did not report the number of patients, but only the number of procedures [23]. As depicted in Fig. 2, pooled OR was 1.38 (95%CI 0.60-3.15), with no statistical difference between the two procedures (P=0.45). A low level of heterogeneity was found (χ2=5.34, d.f.=5 [P=0.38], I2=6%) and no evidence of publication bias was detected by visual examination of a funnel plot (Supplementary Fig. 1A) or with Begg and Mazumdar's test (P=0.34). A sensitivity analysis based on both study design and quality confirmed the main summary estimate (Supplementary Table 2).
Figure 2.
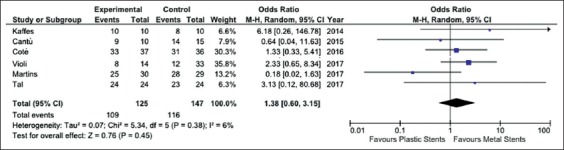
Meta-analysis of stricture resolution between fully-covered self-expandable metal stents and plastic stents
Supplementary Figure 1.
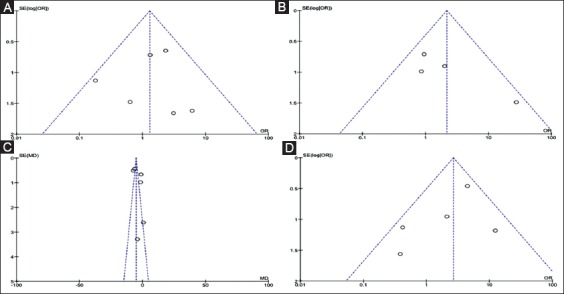
Funnel plots for detection of publication bias. (A) Stricture resolution; (B) Stricture recurrence; (C) Treatment time; (D) Complication rate
Supplementary Table 2.
Sensitivity analysis based on (a) study design (RCT vs. retrospective), and (b) quality of studies (moderate vs. low quality)
Stricture recurrence and treatment time
Stricture recurrence was reported in 4 studies [20,22,24,25] and showed a non-significant trend in favor of plastic stents (OR 1.82, 95%CI 0.52-6.31, P=0.35) with moderate heterogeneity (χ2=5.17, d.f.=3, I2=42%; P=0.16) (Fig. 3). No evidence of publication bias was found (Supplementary Fig. 1B). Sensitivity analysis confirmed these results across all the subgroups explored (Supplementary Table 2).
Figure 3.
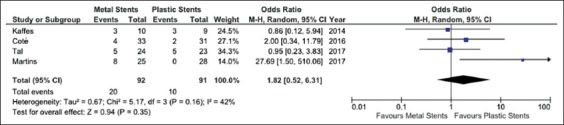
Meta-analysis of stricture recurrence between fully-covered self-expandable metal stents and plastic stents
Six studies enrolling 272 patients reported data on the comparison of treatment time between FCSEMS and plastic stents (Fig. 4). ERCP with placement of FCSEMS involved a significantly shorter treatment time (mean standardized difference: -3.58 months, 95%CI -6.23 to -0.93; P=0.008). It should be noted, though, that the robustness of this finding was impaired by high heterogeneity (χ2=80.20, d.f.=5, I2=94%; P<0.001; Fig. 4).
Figure 4.
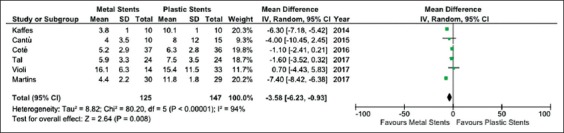
Meta-analysis of treatment time between fully-covered self-expandable metal stents and plastic stents
A funnel plot did not show any evidence of publication bias (Supplementary Fig. 1C) and sensitivity analysis confirmed the above reported findings, except for the subgroup of retrospective studies (Supplementary Table 2).
Complication rate
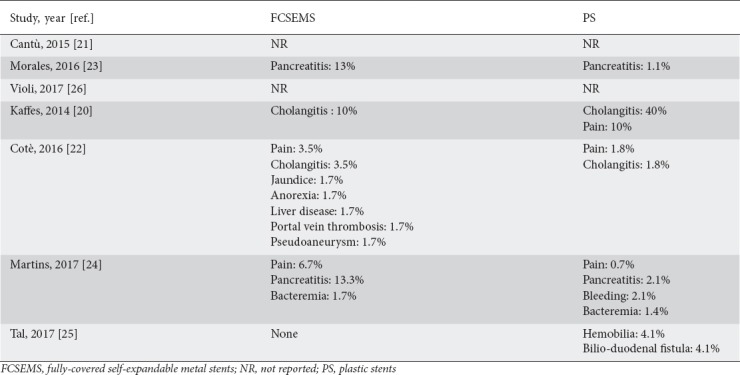
Five studies reported data on adverse events rate [20,21,23-25]. Complications were more frequent in the FCSEMS group, although not significantly so (OR 2.34, 95%CI 0.75-7.25; P=0.14) and with moderate heterogeneity (I2=44%, P=0.13; Supplementary Fig. 2). No evidence of publication bias was found (Supplementary Fig. 1D). A detailed list of the complications observed in the two groups is given in Supplementary Table 3. In particular, pancreatitis and post-procedural pain were observed more frequently after FCSEMS. Sensitivity analysis confirmed these results across all the subgroups explored (Supplementary Table 2).
Supplementary Figure 2.
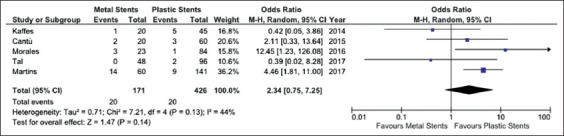
Meta-analysis of complication rate comparing fully-covered self-expandable metal stent and plastic stent
Supplementary Table 3.
Detailed list of complications observed in the two groups
Discussion
Balloon dilation and plastic stents have significantly improved the endoscopic management of post-OLT ABSs over the past decade, with successful stricture resolution in more than 60% of cases [16]. However, ABSs after living donor liver transplantation remain refractory to endoscopic therapy in most case series [5,8]. Therefore, to overcome the major drawbacks of serial balloon dilations with plastic stent exchanges (need to repeat the procedure over an extended period, considerable rate of recurrence), covered self-expandable metal stents already used for treating malignant strictures have been successfully introduced into clinical practice. In 2013, a systematic review of small case series showed promising results with metal stents [16], but a direct comparison between the two procedures was not feasible because of the lack of head-to-head RCTs.
Through a meta-analysis of 7 studies (including 4 RCTs), to the best of our knowledge the first ever published in this field, we made several key observations. First, the two procedures do not differ in terms of stricture resolution and recurrence, although FCSEMS showed more favorable results concerning ABS resolution and higher recurrence rates; the robustness of these findings is supported by the low level of heterogeneity and their stability in the sensitivity analysis. Second, ERCP with placement of FCSEMS is associated with significantly shorter treatment time, although this result should be interpreted with caution because of the high heterogeneity particularly in terms of pancreatitis and post-procedural pain, not reaching the significance threshold but sounding a note of caution for physicians using this device. Our findings confirm that the main potential benefit of FCSEMS is their large caliber and longer duration of patency, allowing them to be left in place longer than plastic stents and resulting in fewer procedures for serial dilations and placement of multiple plastic stents.
In the past, the use of SEMS (mainly uncovered) in benign biliary strictures was limited by the difficulty of their removal [27]. In recent years, small series have demonstrated the successful use of covered SEMS in the treatment of benign biliary and pancreatic strictures [27-29]. In all these studies, covered SEMS were successfully removed in the majority (>95%) of patients. Even in the absence of a clear benefit in terms of higher efficacy with FCSEMS, the lower need for repeated procedures and reduced treatment time constitute important points of strength to be considered when defining the therapeutic strategy in these patients. In this aspect, our results are in keeping with the current literature in the field of either benign or malignant strictures [30].
Some concerns were raised in the past about the increased migration rate of FCSEMS compared with uncovered SEMS [27]. Unfortunately, since this adverse event was inconsistently reported across the included studies, a reliable comparative assessment of the migration rate between the two procedures was not feasible. Nevertheless, as reported in Table 2, the occurrence of this adverse event was rather similar in the two treatment groups. Other adverse events were mostly mild and easily manageable, with pancreatitis and mild bleeding being the most frequently observed complications.
Our study had certain limitations. First, the relatively low number of studies, in particular RCTs, and the inclusion of both RCTs and retrospective studies necessitate particular caution when interpreting our results, especially in the case of sensitivity analyses, in which generally no more than three studies could be included. Second, there were also limitations in the individual studies, in particular regarding the limited sample size in most of the RCTs, the presence of low-quality studies, or publications in abstract form. Third, some of the main outcomes were unevenly reported across the included RCTs, mainly because of the different study design. Furthermore, there was significant heterogeneity among the studies with respect to the secondary outcomes.
On the other hand, our meta-analysis has several strengths. In fact, all the included studies presented a similar treatment strategy in terms of stents used and therapeutic schedule. Moreover, the absence of heterogeneity in the primary outcomes and the confirmation of our findings in the sensitivity analysis represent further strengths of our study. Finally, all the relevant outcomes were explored, including procedural time, which constitutes an important aspect in daily clinical practice.
In conclusion, despite the aforementioned weaknesses, our meta-analysis represents the first systematic review of the literature published in this field and describes an accurate comparison between the two techniques exploring the main clinical endpoints. Based on our results, FCSEMS appear to be a promising and reliable tool that can significantly decrease treatment time, although there is still no clear evidence of their superiority over plastic stents in terms of better stricture resolution. Large RCTs are needed in order to further confirm our results.
Summary Box.
What is already known:
Biliary stricture represents one of the most frequently observed complications after orthotopic liver transplantation
While significant improvement in the management of anastomotic biliary strictures (ABSs) has been achieved in the last years by using balloon dilation and multiple plastic stents, ABSs remain refractory to endoscopic therapy in a non-negligible number of cases
What the new findings are:
Fully-covered self-expandable metal stents appear to be a promising and reliable tool that can significantly decrease the treatment time, although there is still no clear evidence of their superiority over plastic stents in terms of better stricture resolution
Further large prospective randomized trials are warranted to confirm the results of our analysis
Biography
University of Foggia, Italy; University of Medicine and Pharmacy “Victor Babes” Timisoara, Romania; Federal Medical Centre Birnin Kudu, Jigawa State, Nigeria; University of Port Harcourt, Nigeria; Guru Gobind Singh Indraprastha University, New Delhi, India; University of Sao Paulo, Brazil
Footnotes
Conflict of Interest: None
References
- 1.Graziadei IW, Schwaighofer H, Koch R, et al. Long-term outcome of endoscopic treatment of biliary strictures after liver transplantation. Liver Transpl. 2006;12:718–725. doi: 10.1002/lt.20644. [DOI] [PubMed] [Google Scholar]
- 2.Lee YY, Gwak GY, Lee KH, et al. Predictors of the feasibility of primary endoscopic management of biliary strictures after adult living donor liver transplantation. Liver Transpl. 2011;17:1467–1473. doi: 10.1002/lt.22432. [DOI] [PubMed] [Google Scholar]
- 3.Koneru B, Sterling MJ, Bahramipour PF. Bile duct strictures after liver transplantation: a changing landscape of the Achilles'heel. Liver Transpl. 2006;12:702–704. doi: 10.1002/lt.20753. [DOI] [PubMed] [Google Scholar]
- 4.Guichelaar MM, Benson JT, Malinchoc M, Krom RA, Wiesner RH, Charlton MR. Risk factors for and clinical course of non-anastomotic biliary strictures after liver transplantation. Am J Transplant. 2003;3:885–890. doi: 10.1034/j.1600-6143.2003.00165.x. [DOI] [PubMed] [Google Scholar]
- 5.Seo JK, Ryu JK, Lee SH, et al. Endoscopic treatment for biliary stricture after adult living donor liver transplantation. Liver Transpl. 2009;15:369–380. doi: 10.1002/lt.21700. [DOI] [PubMed] [Google Scholar]
- 6.Chang JH, Lee IS, Choi JY, et al. Biliary stricture after adult right-lobe living-donor liver transplantation with duct-to-duct anastomosis: long-term outcome and its related factors after endoscopic treatment. Gut Liver. 2010;4:226–233. doi: 10.5009/gnl.2010.4.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsujino T, Isayama H, Kogure H, Sato T, Nakai Y, Koike K. Endoscopic management of biliary strictures after living donor liver transplantation. Clin J Gastroenterol. 2017;10:297–311. doi: 10.1007/s12328-017-0754-z. [DOI] [PubMed] [Google Scholar]
- 8.Kato H, Kawamoto H, Tsutsumi K, et al. Long-term outcomes of endoscopic management for biliary strictures after living donor liver transplantation with duct-to-duct reconstruction. Transpl Int. 2009;22:914–921. doi: 10.1111/j.1432-2277.2009.00895.x. [DOI] [PubMed] [Google Scholar]
- 9.Kim TH, Lee SK, Han JH, et al. The role of endoscopic retrograde cholangiography for biliary stricture after adult living donor liver transplantation: technical aspect and outcome. Scand J Gastroenterol. 2011;46:188–196. doi: 10.3109/00365521.2010.522722. [DOI] [PubMed] [Google Scholar]
- 10.Costamagna G, Tringali A, Mutignani M, et al. Endotherapy of postoperative biliary strictures with multiple stents: results after more than 10 years of follow-up. Gastrointest Endosc. 2010;72:551–557. doi: 10.1016/j.gie.2010.04.052. [DOI] [PubMed] [Google Scholar]
- 11.Dumonceau JM, Tringali A, Blero D, et al. European Society of Gastrointestinal Endoscopy. Biliary stenting: indications, choice of stents and results: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. 2012;44:277–298. doi: 10.1055/s-0031-1291633. [DOI] [PubMed] [Google Scholar]
- 12.Chathadi KV, Chandrasekhara V, Acosta RD, et al. ASGE Standards of Practice Committee. The role of ERCP in benign diseases of the biliary tract. Gastrointest Endosc. 2015;81:795–803. doi: 10.1016/j.gie.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 13.Cantù P, Parzanese I, Balassone V, et al. Management of biliary anastomotic strictures after liver transplantation (BASALT study): a nationwide Italian survey. Liver Transpl. 2017;23:257–261. doi: 10.1002/lt.24701. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration. 2011. [Accessed on October 30, 2017]. Available from http://handbook-5-1.cochrane.org .
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. W64. [DOI] [PubMed] [Google Scholar]
- 16.Kao D, Zepeda-Gomez S, Tandon P, Bain VG. Managing the post-liver transplantation anastomotic biliary stricture: multiple plastic versus metal stents: a systematic review. Gastrointest Endosc. 2013;77:679–691. doi: 10.1016/j.gie.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Altman DG, Gøtzsche PC, et al. Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers'to authors'assessments. BMC Med Res Methodol. 2014;14:45. doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Kaffes A, Griffin S, Vaughan R, et al. A randomized trial of a fully covered self-expandable metallic stent versus plastic stents in anastomotic biliary strictures after liver transplantation. Therap Adv Gastroenterol. 2014;7:64–71. doi: 10.1177/1756283X13503614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CantùP Parzanese I, Rosa R, Tenca A, Conte D, Penagini R. Fully covered metal stents vs multiple plastic stenting in the treatment of biliary stricture after liver transplantation: cost-effectiveness analysis. United European Gastroenterol J. 2015;3:A387. [Google Scholar]
- 22.Coté GA, Slivka A, Tarnasky P, et al. Effect of covered metallic stents compared with plastic stents on benign biliary stricture resolution: a randomized clinical trial. JAMA. 2016;315:1250–1257. doi: 10.1001/jama.2016.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morales S, Chalhoub WM, Almario JA, et al. Fully covered self-expandable metal stents outperform straight plastic stents in management of benign anastomotic biliary strictures following orthotopic liver transplantation. Gastrointest Endosc. 2016;83:AB611. [Google Scholar]
- 24.Martins FP, De Paulo GA, Contini MLC, Ferrari AP. Metal versus plastic stents for anastomotic biliary strictures after liver transplantation: a randomized controlled trial. Gastrointest Endosc. 2018;87:e1–e131.ne13. doi: 10.1016/j.gie.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 25.Tal AO, Finkelmeier F, Filmann N, et al. Multiple plastic stents versus covered metal stent for treatment of anastomotic biliary strictures after liver transplantation: a prospective, randomized, multicenter trial. Gastrointest Endosc. 2017;86:1038–1045. doi: 10.1016/j.gie.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Violi P, Bortolasi L, Carraro A, et al. Endoscopic treatment of biliary stricture after orthotopic liver transplantation. Gastroenterology. 2017;152(5):S1276. [Google Scholar]
- 27.Pfau PR, Pleskow DK, Banerjee S, et al. ASGE Technology Assessment Committee. Pancreatic and biliary stents. Gastrointest Endosc. 2013;77:319–327. doi: 10.1016/j.gie.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 28.Neri V, Ambrosi A, Fersini A, Tartaglia N, Lapolla F, Forlano I. Severe acute pancreatitis: clinical forms of different gravity. Ann Ital Chir. 2013;84:47–53. [PubMed] [Google Scholar]
- 29.Neri V, Fersini A, Ambrosi A, Tartaglia N, Valentino TP. Mild-moderate acute biliary pancreatitis: role of magnetic resonance cholangiopancreatography in preparation of cholecystectomy. Pancreas. 2009;38:717. doi: 10.1097/MPA.0b013e3181a83087. [DOI] [PubMed] [Google Scholar]
- 30.Moss AC, Morris E, Leyden J, MacMathuna P. Do the benefits of metal stents justify the costs?A systematic review and meta-analysis of trials comparing endoscopic stents for malignant biliary obstruction. Eur J Gastroenterol Hepatol. 2007;19:1119–1124. doi: 10.1097/MEG.0b013e3282f16206. [DOI] [PubMed] [Google Scholar]