Abstract
Background
Magnetic resonance imaging (MRI) is the standard neuroimaging technique to assess perinatal asphyxia-associated brain injury in full-term infants. Diffusion-weighted imaging (DWI) is most informative when assessed during the first week after the insult.
Objectives
To study the DWI abnormalities of the thalamus and basal ganglia in full-term infants with perinatal asphyxia.
Methods
Fifty-five (near) term infants (normothermia n = 23; hypothermia n = 32) with thalamus and/or basal ganglia injury were included. MRI findings were assessed visually and quantitatively calculating apparent diffusion coefficient (ADC) values. Thalamus/basal ganglia ADC ratios were calculated to analyze the differences between these areas. Infants with an early MRI (days 1–3) or later MRI (days 4–7) were compared.
Results
Isolated extensive thalamic injury was seen early, and focal thalamic and basal ganglia injury was seen later. On the early MRI, visual assessment underestimated abnormalities in the basal ganglia (59% abnormal vs. 90% abnormal on quantitative assessment; p = 0.015), suggesting the need for quantitative assessment. In infants treated with hypothermia, the thalamus/basal ganglia ADC ratio was lower.
Conclusions
Both visual analysis and quantitative evaluation of cerebral MRI after perinatal asphyxia are needed, especially during the first few days after birth. Timing of ADC changes is influenced by therapeutic hypothermia.
Keywords: Perinatal asphyxia, Hypoxic-ischemic encephalopathy, Diffusion-weighted imaging, Apparent diffusion coefficient, Magnetic resonance imaging
Introduction
Therapeutic hypothermia has become the standard of care for full-term infants with encephalopathy following perinatal asphyxia and has resulted in improved neurodevelopmental outcome in survivors [1]. Perinatal sentinel events are associated with magnetic resonance imaging (MRI)-detected injury in the thalamus, basal ganglia, hippocampus, cerebral peduncles, and perirolandic cortex [2, 3]. The basal ganglia and thalamus (BGT) pattern of injury is especially associated with motor impairment, while the watershed (WS) pattern of injury, seen after more prolonged and/or repetitive antenatal events, is more often associated with cognitive problems [4, 5, 6]. When using conventional T1- and T2-weighted sequences, the amount of injury may be underestimated during the first week after birth with limited interobserver agreement [7]. Diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) maps allow reliable detection of brain lesions shortly after birth, but may not reach their full extent for several days and will pseudonormalize after the first week after birth [8, 9, 10]. Abnormalities seen on early MRI, including DWI, often precede cystic or gliotic changes seen on a repeat MRI [11, 12]. Barkovich et al. [13] reported the evolution of DWI abnormalities on MRI performed during the first week after birth in full-term infants with perinatal asphyxia showing initial involvement of the thalami, followed by involvement of the basal ganglia as well. This study was, however, performed before the introduction of hypothermia.
In the present study, we addressed the following questions: (1) Is there a difference between visual assessment of the MRI and quantified ADC values? (2) What is the difference in ADC values between the thalamus and basal ganglia at the time of MRI?
Subjects and Methods
For this retrospective observational cohort study, patients with MRI-detected thalamus or basal ganglia injury on visual inspection and/or low ADC values following perinatal asphyxia were selected. All had been admitted to the level III NICU of the Wilhelmina Children's Hospital/University Medical Center in Utrecht between 2002 and 2016. Perinatal asphyxia was defined as described previously [14]. Therapeutic hypothermia was introduced in our NICU in 2008 [15]. Part of the infants in the present study were also described in previous studies [16, 17]. The study group consisted of 55 infants (gestational age ≥36.0 weeks) with perinatal asphyxia, including 2 infants with a postnatal collapse on the first day after birth. Infants with a first MRI beyond 7 days after birth, infants with severe congenital malformations or metabolic disorders, with a postmortem MRI only or with an MRI of a poor quality were excluded. As preterm birth will affect MRI findings, preterm infants with a gestational age below 36 weeks were excluded from the present study [18]. The Institutional Review Board (IRB) of the University Medical Center Utrecht, the Netherlands, gave approval for use of the clinically acquired data for study purposes. Since clinically obtained anonymized data were used, written informed parental consent for participation in the study was waived by our IRB.
MRI Protocol
MRI was performed using a 1.5-T (n = 22) or 3.0-T (n = 33) MR system (Philips Medical Systems, Best, The Netherlands). Vacuum pillows (MedVac, Kohlbrat and Bunz, Radstadt, Austria) were used to prevent infant movement during the examination. Two pairs of earmuffs were applied for hearing protection (Minimuffs, Natus Medical Inc., San Carlos, CA, USA; Em's 4 kids LLC, Culver City, CA, USA). Patients who did not receive intravenous morphine were sedated with either chloral hydrate (50 mg/kg orally) or an intramuscular injection of a combination of pethidine (2 mg/kg), chlorpromazine (0.5 mg/kg), and promethazine (0.5 mg/kg). Heart rate, respiratory rate, and transcutaneous oxygen saturation were monitored during examinations with an abdominal transducer (Philips Medical Systems) and a pulse oximeter (Nonin Medical Inc., Plymouth, MN, USA). A neonatologist or physician assistant was present throughout the examination and transport. MR images included T1- or T2-weighted sagittal sequence, T1- and T2-weighted axial sequences, and axial DWI (3 directions) with 3- to 4-mm-thick slice thickness, and b values of 0 and 800 (3T, until October 2013) or 1,000 s/mm2 (1.5 T, and 3.0 T after October 2013). An ADC map was generated by the Philips MR system. Details are presented in the online supplementary Appendix (see www.karger.com/doi/10.1159/000489159 for all online suppl. material).
Visual MRI Assessment
At the time of MRI assessment, the readers (F.G. and L.S.V.) were blinded to the clinical information. The pattern of injury was also based on the T1, T2, DWI or ADC abnormalities. The presence of signal intensity abnormalities was assessed in the thalami, basal ganglia (globus pallidus, putamen, and caudate nucleus), posterior limb of internal capsule (PLIC), cerebral peduncle, hippocampus, and central sulcus (Fig. 1). Lesions were scored as focal or extensive and unilateral or bilateral. Consensus was reached in all cases. MRIs were scored as (1) predominant BGT pattern, (2) WS pattern, and (3) near-total (NT) pattern [4]. Time at MRI was measured in hours after birth. In the 2 infants with postnatal collapse, time of MRI was calculated between the postnatal collapse and time of the MRI. The infants were divided into two groups: the early MRI group (days 1–3) and the later MRI group (days 4–7), and the groups were compared.
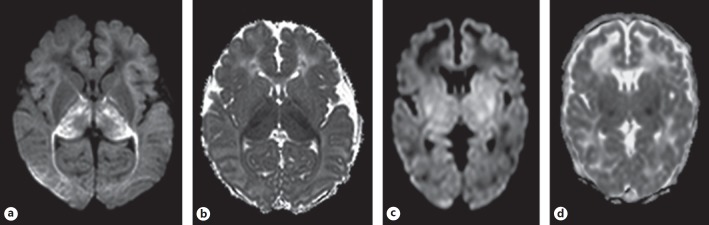
Fig. 1.
A representative case in the early group (a, b) and the later group (c, d). a, b The infant was born at 39+5 weeks. Birth weight was 3,200 g. Delivery was complicated by uterine rupture. Apgar score was 0 at 1 min and 0 at 5 min. Sarnat grade 3 and aEEG showed a flat trace. MRI was performed on day 2 and showed a “near-total” pattern of injury. Restricted diffusion was most marked in the thalami (apparent diffusion coefficient [ADC] value was 543 × 10–6 mm2/s), but was also present in the subcortical white matter, central sulcus, posterior limb of internal capsule, hippocampus, and peduncles (a). Restricted diffusion was not clearly seen in the basal ganglia; however, the mean ADC values were decreased (808 × 10–6 mm2/s) (b). The infant died following redirection of care. c, d The infant was born at 40+1 weeks. Birth weight was 4,200 g. Delivery was complicated by uterine rupture. Apgar score was 2 at 1 min and 4 at 5 min. Sarnat grade 2 and aEEG showed a burst suppression pattern. MRI was performed on day 4. The MRI showed a basal ganglia and thalamus pattern of injury. Focal areas of restricted diffusion are seen in the ventrolateral nuclei of thalami, posterior parts of basal ganglia, and cerebral peduncles (c). The mean ADC values were 861 × 10–6 mm2/s in the basal ganglia and 808 × 10–6 mm2/s in the thalamus (d).
Quantitative MRI Assessment
Following visual analysis, ADC values were calculated in the BGT, including the ventrolateral thalamus, as described previously [10]. Measurements were performed by one author (K.I.) who was blinded to the clinical information. The values were measured twice for each site and the mean value was calculated. The first 10 infants were measured twice in order to calculate the intrarater agreement which is used for continuous variables. This was more than 0.95. The mean ADC values of both sides were averaged for further analysis. For quantitative assessment, we used cutoff values of BGT, reported previously [10, 17]. For the basal ganglia, 974 and 1,031 × 10–6 mm2/s were used as cutoff values for hypothermia and normothermia, respectively, and for the thalami, 871 and 919 × 10–6 mm2/s for hypothermia and normothermia, respectively. Finally, we calculated the thalamus/basal ganglia ratio of the ADC values to investigate the association between these areas.
Clinical Data and Outcome
Clinical data are presented in Table 1. The severity of encephalopathy was graded as mild, moderate or severe based on the Sarnat classification. Neurodevelopmental outcome was assessed at regular intervals up to 24 months of age by a neonatologist and a special educator using the Griffith Mental Development Scales (GMDS) or Bayley Scales of Infant and Toddler Development (BSID), third edition, as described previously [10, 17]. An adverse outcome was defined as death during the neonatal period, cerebral palsy (CP), severe hearing or visual impairment, or a GMDS or BSID-III score of less than −1 SD at 18–24 months. Results of the most recent test were used for outcome assessment.
Table 1.
Clinical data of both groups
| Early (n = 29) | Later (n = 26) | p value | |
|---|---|---|---|
| Male, n (%) | 19 (66) | 13 (50) | 0.24 |
| Gestational age (mean ± SD), weeks | 40.1±1.6 | 40.3±1.2 | 0.59 |
| Birth weight (mean ± SD), g | 3,418±621 | 3,290±453 | 0.39 |
| Apgar score at 1 min, median (IQR)a | 1.5 (0–2) | 2 (1–32) | 0.46 |
| Apgar score at 5 min, median (IQR)b | 3 (1–54) | 4 (3–6) | 0.16 |
| Cord pH (mean ± SD)c | 6.91±0.23 | 6.97±0.20 | 0.35 |
| Sarnat classification, n (%) | 0.001 | ||
| Moderate | 12 (41) | 22 (85) | |
| Severe | 17 (59) | 4 (15) | |
| MRI device, n (%) | 0.74 | ||
| 1.5 T | 11 (38) | 11 (42) | |
| 3.0 T | 18 (62) | 15 (58) | |
| MRI pattern, n (%) | 0.07 | ||
| Near-total | 16 (55) | 8 (31) | |
| Basal ganglia/thalami | 13 (45) | 18 (69) | |
| Therapeutic hypothermia, n (%) | 17 (59) | 15 (58) | 0.94 |
| Died, n (%) | 25 (86) | 13 (50) | 0.004 |
Early group (n = 28), later group (n = 25).
Early group (n = 28).
Early group (n = 25), later group (n = 22).
Statistics Analysis
Student t test or Mann-Whitney U tests were used to compare continuous variables and Fisher exact tests or χ2 tests for categorical variables. To compare the relationship between the thalamus/basal ganglia ratio, time of MRI, Sarnat grading, and hypothermia, we used multivariable regression analysis. All statistical analyses were performed with SPSS version 21 (SPSS-IBM, Chicago, IL, USA).
Results
Clinical Characteristics and Neurodevelopmental Outcome
Thirty-two infants (58%) were born after January 2008 and treated with hypothermia and 23 infants were not; of these 55 infants, 38 (69%) died, mostly due to redirection of care. Early and later groups did not differ regarding gestational age, birth weight, and Apgar scores, but there were more infants with severe encephalopathy in the early MRI group and mortality was higher (Table 1, online supplementary Table). No infant had 2 scans during the first week.
One of the 19 surviving infants had not reached 2 years of age yet and therefore had no outcome data available. Among the remaining 18 infants, 9 infants were treated with hypothermia and 9 with normothermia. The mean cognitive composite score on the BSID-III in the 9 infants treated with hypothermia and tested at 24 months was 93 ± 17, of whom 4 had developed CP. All of the 9 normothermia infants had CP at 2 years of age. One of the 9 normothermia infants was tested with the BSID-III and had a cognitive composite score of 100, 5 were tested with the GMDS and their mean developmental quotient was 85 ± 20. The other 3 infants could not be tested due to their severe disabilities.
Magnetic Resonance Imaging
MRI was performed at a mean age of 3.5 days (SD 1.3). In the early group, the MRI showed an NT injury pattern in 16 infants (55%) compared to 8 (31%) in the later group. A BGT pattern was seen in 13 infants (45%), compared to 18 (69%) in the later group (p = 0.07). None of the infants had a WS pattern (Table 1).
Basal Ganglia and Thalamus
Visual assessment of the thalami often showed more extensive bilateral lesions on early MRIs (86%) than on later MRIs (38%, Fig. 2). Visual assessment of the basal ganglia showed fewer infants with DWI abnormalities of the basal ganglia on early MRIs (59%) than on later MRIs (84%).
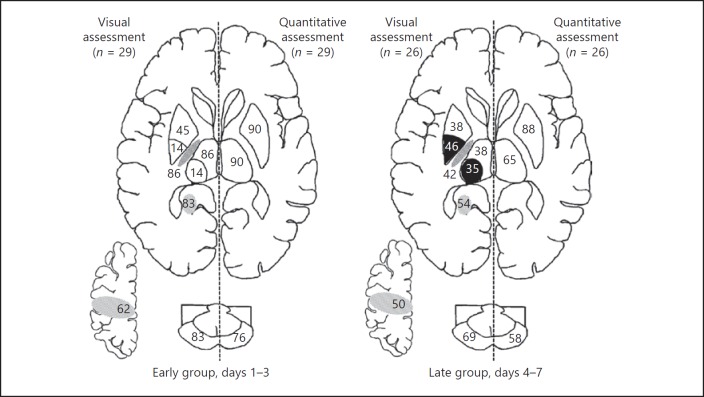
Fig. 2.
Percentage abnormalities per brain lesion in the early group (left) and the later group (right). In each image, the left side shows abnormalities on visual assessment, and the right side shows abnormalities using quantitative assessment with apparent diffusion coefficient (ADC) values. The numbers in the different brain areas represent the percentage of patients with brain lesions: basal ganglia with extensive lesion on visual assessment (top of the basal ganglia), basal ganglia with focal lesion on visual assessment (bottom of the basal ganglia), posterior limbs of the internal capsule, thalamus with extensive lesion on visual assessment (top of the thalamus), thalamus with focal lesion on visual assessment (bottom of the thalamus), hippocampus, central sulcus, and peduncles. The black areas represent higher percentage lesions in the later group compared to the early group. Abnormal patterns in basal ganglia and thalami are not graded/categorized further into extensive or focal in quantitative assessments.
When quantitative assessment was performed, abnormal ADC values were shown in 90% of infants in both the thalamus and basal ganglia in the early group. In infants with a late MRI, the percentages of abnormal ADC values were 65 and 88% in the thalamus and basal ganglia, respectively.
Comparison between Assessments in BGT
There were significant differences between qualitative and quantitative assessments (Fig. 2). In the early group, abnormalities in the basal ganglia were recognized visually in 59% of the infants, compared to 90% on quantitative assessment (p = 0.02). For both the early and the later group, the percentages of infants with restricted diffusion in the thalamus on visual assessment (100% early and 73% later) were comparable to using ADC values (90 and 65%, respectively).
ADC Values in Early and Later MRIs
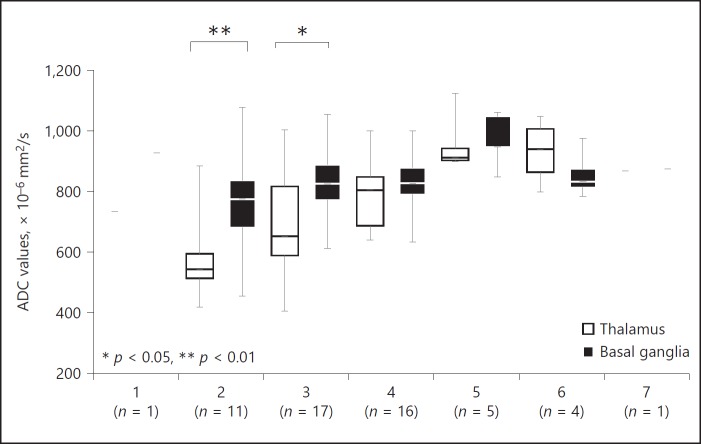
At the earliest time points (days 2 and 3, Fig. 3), ADC values were lower in the thalamus than in the basal ganglia, but not significantly different at later time points. Median ADC values in all structures were lowest on day 2: 542.5 in the thalamus and 775 × 10–6 mm2/s in the basal ganglia.
Fig. 3.
Time course of apparent diffusion coefficient (ADC) values. Time course of ADC values (×10–6 mm2/s) of the thalamus and basal ganglia. ADC values of the thalami were significantly lower on days 2 and 3.
Other Lesions
Using visual assessment, the percentage of infants with restricted diffusion in the peduncles, PLIC, and central sulcus tended to be higher in the early MRI group than in the later MRI group (Fig. 2).
Thalamus/Basal Ganglia Ratio
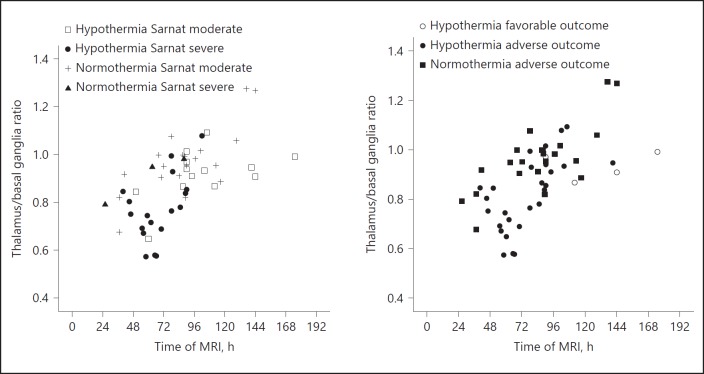
The thalamus/basal ganglia ADC ratio versus time of the MRI is shown in Figure 4. Infants with therapeutic hypothermia appeared to have lower ratios than infants with normothermia. There was no infant in the normothermia group with a favorable outcome. Multivariable regression analysis demonstrated that time of MRI after birth (0.0032/h; 95% CI 0.0022–0.0041) and therapeutic hypothermia (−0.11; 95% CI −0.17 to −0.05) were significantly (p < 0.001 for both) associated with the thalamus/basal ganglia ADC ratio, whereas Sarnat grading was not.
Fig. 4.
The relationship between thalamus/basal ganglia apparent diffusion coefficient (ADC) ratios and time of MRI based on hypothermia therapy and Sarnat classification (left) and based on hypothermia therapy and outcome (right). Infants with severe encephalopathy had lower thalamus/basal ganglia ADC ratios than infants with moderate encephalopathy (left). Furthermore, infants with therapeutic hypothermia had lower ratios, indicating a larger difference between basal ganglia and thalami (right).
Discussion
In the present study, we assessed the pattern of BGT changes on DWI performed within 7 days after birth in infants with perinatal asphyxia. ADC values were lower in the thalamus than in the basal ganglia, and were increased in both areas during the first week after birth. It has been shown that restricted diffusion corresponds to cytotoxic edema and therefore irreversible lesions with neuronal cell death [19].
Visual analysis underestimated injury to especially the basal ganglia on the early MRI, which only became apparent when performing ADC measurements. Visual assessment of the thalami and BGT may be more difficult in the NT pattern, because all structures show restricted diffusion. The time course of ADC abnormalities in thalami and basal ganglia was remarkably different. This could be explained in several ways. First of all, there may be a difference in vulnerability between the basal ganglia and thalami. Although the selective vulnerability in thalamus and striatal neurons after hypoxia-ischemia in full-term infants was suggested previously [20], the difference in vulnerability was not clearly seen on histology [16]. Secondly, the difference might be caused by the spreading of injury along axonal pathways from the cortex to the thalamus and further to the basal ganglia [21]. Thirdly, hypothermia itself reduces ADC values, but the effects are minor compared to the effects of severe hypoxia-ischemia (see below).
MRI is considered the method of choice to assess brain injury in full-term infants with perinatal asphyxia and this technique reliably predicts neurodevelopmental outcome [3, 5, 6, 17]. During the first week after birth, DWI has been shown to be more sensitive than conventional T1- and T2-weighted imaging [8, 9]. As reported previously, DWI may take a few days to reach the full extent of abnormalities [22]. An MRI including DWI is therefore recommended after rewarming (days 4–6) in infants receiving hypothermia [23, 24]. Beyond day 7, pseudonormalization of DWI is likely, and therefore infants examined after day 7 were excluded from the present study.
The abnormalities were mostly extensive in the early MRI group, and more focal in the later MRI group. This could be explained by the difference in severity of encephalopathy, with more infants with severe encephalopathy being scanned early allowing redirection of care in case of severe abnormalities [25].
The multivariable regression analysis demonstrated that not only time after the insult, but also the use of hypothermia influenced the thalamus/basal ganglia ADC ratios: infants treated with hypothermia had significantly lower ratios. A previous study showed that pseudonormalization of the ADC values of unspecified injured areas took longer in infants with therapeutic hypothermia, compared to normothermic infants, which is in line with our findings [26].
Hypothermia itself (from 37°C to a temperature of 33.5°C) may decrease ADC values by 5–7% [27]. Apparently, therapeutic hypothermia applied between 6 (start hypothermia) and 84 h (complete rewarming) after birth has a different effect on ADC values of the thalamus and basal ganglia, since thalamus/basal ganglia ADC ratios are different between hypothermic and normothermic infants both during hypothermia and rewarming.
In our multivariable model, the Sarnat classification did not have a significant effect, possibly because very few patients with Sarnat grade III were part of the later MRI group.
This study shows the importance of measuring ADC values. Especially in infants who are assessed early, the injury to the basal ganglia may be underestimated when only performing visual assessment. Measuring ADC values is easy and can be performed within minutes [10, 17].
Our study has several limitations. The main limitation of our study is that the infants were not scanned serially, and the more severely affected infants were scanned earlier. Early scans may be used in the decision to continue or redirect care [25]. Nevertheless, our cross-sectional findings are comparable to those of Bednarek et al. [26]. Moreover, although infants with Sarnat grade III were scanned earlier, Sarnat grading did not influence the time course of thalamus/basal ganglia ADC ratios in our multivariable analysis. As expected, the number of infants with a favorable outcome in our study was small, since only infants with lesions in the thalamus and basal ganglia were eligible for the study. The study by Rutherford et al. [8] demonstrated no significant ADC change in the BGT during the first week after birth in full-term infants with a good outcome.
In conclusion, using MRI we have demonstrated a difference between the thalami and basal ganglia in a relatively large group of infants with perinatal asphyxia and injury to those areas. The ADC values appeared to be modified by therapeutic hypothermia. When interpreting DWI changes after perinatal asphyxia early in the first week after birth, it is important to be aware that the full extent of abnormalities on visual assessment may not yet have been reached and that ADC values should be measured to assess the full extent of the injury.
Disclosure Statement
The authors declare no conflicts of interest.
Supplementary Material
Supplementary data
Acknowledgements
The authors thank the MR technicians, in particular Niels Blanken and Johan de Jong, for their dedicated help in the preparation of the MRI, and the NICU nurses for their help during scanning.
References
- 1.Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013;1 doi: 10.1002/14651858.CD003311.pub3. CD003311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller SP, Ramaswamy V, Michelson D, Barkovich AJ, Holshouser B, Wycliffe N, Glidden DV, Deming D, Partridge JC, Wu YW, Ashwal S, Ferriero DM. Patterns of brain injury in term neonatal encephalopathy. J Pediatr. 2005;146:453–460. doi: 10.1016/j.jpeds.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 3.Rutherford M, Ramenghi LA, Edwards AD, Brocklehurst P, Halliday H, Levene M, Strohm B, Thoresen M, Whitelaw A, Azzopardi D. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol. 2010;9:39–45. doi: 10.1016/S1474-4422(09)70295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Vries LS, Groenendaal F. Patterns of neonatal hypoxic-ischaemic brain injury. Neuroradiology. 2010;52:555–566. doi: 10.1007/s00234-010-0674-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez-Biarge M, Diez-Sebastian J, Rutherford MA, Cowan FM. Outcomes after central grey matter injury in term perinatal hypoxic-ischaemic encephalopathy. Early Hum Dev. 2010;86:675–682. doi: 10.1016/j.earlhumdev.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Biarge M, Bregant T, Wusthoff CJ, Chew AT, Diez-Sebastian J, Rutherford MA, Cowan FM. White matter and cortical injury in hypoxic-ischemic encephalopathy: antecedent factors and 2-year outcome. J Pediatr. 2012;161:799–807. doi: 10.1016/j.jpeds.2012.04.054. [DOI] [PubMed] [Google Scholar]
- 7.Jouvet P, Cowan FM, Cox P, Lazda E, Rutherford MA, Wigglesworth J, Mehmet H, Edwards AD. Reproducibility and accuracy of MR imaging of the brain after severe birth asphyxia. AJNR Am J Neuroradiol. 1999;20:1343–1348. [PMC free article] [PubMed] [Google Scholar]
- 8.Rutherford M, Counsell S, Allsop J, Boardman J, Kapellou O, Larkman D, Hajnal J, Edwards D, Cowan F. Diffusion-weighted magnetic resonance imaging in term perinatal brain injury: a comparison with site of lesion and time from birth. Pediatrics. 2004;114:1004–1014. doi: 10.1542/peds.2004-0222. [DOI] [PubMed] [Google Scholar]
- 9.Chau V, Poskitt KJ, Sargent MA, Lupton BA, Hill A, Roland E, Miller SP. Comparison of computer tomography and magnetic resonance imaging scans on the third day of life in term newborns with neonatal encephalopathy. Pediatrics. 2009;123:319–326. doi: 10.1542/peds.2008-0283. [DOI] [PubMed] [Google Scholar]
- 10.Alderliesten T, de Vries LS, Benders MJ, Koopman C, Groenendaal F. MR imaging and outcome of term neonates with perinatal asphyxia: value of diffusion-weighted MR imaging and (1)H MR spectroscopy. Radiology. 2011;261:235–242. doi: 10.1148/radiol.11110213. [DOI] [PubMed] [Google Scholar]
- 11.Wintermark P, Hansen A, Soul J, Labrecque M, Robertson RL, Warfield SK. Early versus late MRI in asphyxiated newborns treated with hypothermia. Arch Dis Child Fetal Neonatal Ed. 2011;96:F36–F44. doi: 10.1136/adc.2010.184291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakkarapani E, Poskitt KJ, Miller SP, Zwicker JG, Xu Q, Wong DS, Roland EH, Hill A, Chau V. Reliability of early magnetic resonance imaging (MRI) and necessity of repeating MRI in noncooled and cooled infants with neonatal encephalopathy. J Child Neurol. 2016;31:553–559. doi: 10.1177/0883073815600865. [DOI] [PubMed] [Google Scholar]
- 13.Barkovich AJ, Miller SP, Bartha A, Newton N, Hamrick SE, Mukherjee P, Glenn OA, Xu D, Partridge JC, Ferriero DM, Vigneron DB. MR imaging, MR spectroscopy, and diffusion tensor imaging of sequential studies in neonates with encephalopathy. AJNR Am J Neuroradiol. 2006;27:533–547. [PMC free article] [PubMed] [Google Scholar]
- 14.Cowan F, Rutherford M, Groenendaal F, Eken P, Mercuri E, Bydder GM, Meiners LC, Dubowitz LMS, de Vries LS. Origin and timing of brain lesions in term infants with neonatal encephalopathy. Lancet. 2003;361:736–742. doi: 10.1016/S0140-6736(03)12658-X. [DOI] [PubMed] [Google Scholar]
- 15.Groenendaal F, Casaer A, Dijkman KP, Gavilanes AW, de Haan TR, Ter Horst HJ, Laroche S, Naulaers G, Rijken M, van Straaten HL, Steiner K, Swarte RM, Zecic A, Zonnenberg IA. Introduction of hypothermia for neonates with perinatal asphyxia in the Netherlands and Flanders. Neonatology. 2013;104:15–21. doi: 10.1159/000348823. [DOI] [PubMed] [Google Scholar]
- 16.Alderliesten T, Nikkels PG, Benders MJ, de Vries LS, Groenendaal F. Antemortem cranial MRI compared with postmortem histopathologic examination of the brain in term infants with neonatal encephalopathy following perinatal asphyxia. Arch Dis Child Fetal Neonatal Ed. 2013;98:F304–F309. doi: 10.1136/archdischild-2012-301768. [DOI] [PubMed] [Google Scholar]
- 17.Alderliesten T, de Vries LS, Staats L, van Haastert IC, Weeke L, Benders MJ, Koopman-Esseboom C, Groenendaal F. MRI and spectroscopy in (near) term neonates with perinatal asphyxia and therapeutic hypothermia. Arch Dis Child Fetal Neonatal Ed. 2017;102:F147–f152. doi: 10.1136/archdischild-2016-310514. [DOI] [PubMed] [Google Scholar]
- 18.Schneider J, Kober T, Bickle Graz M, Meuli R, Huppi PS, Hagmann P, Truttmann AC. Evolution of T1 relaxation, ADC, and fractional anisotropy during early brain maturation: a serial imaging study on preterm infants. AJNR Am J Neuroradiol. 2016;37:155–162. doi: 10.3174/ajnr.A4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ginet V, Pittet MP, Rummel C, Osterheld MC, Meuli R, Clarke PG, Puyal J, Truttmann AC. Dying neurons in thalamus of asphyxiated term newborns and rats are autophagic. Ann Neurol. 2014;76:695–711. doi: 10.1002/ana.24257. [DOI] [PubMed] [Google Scholar]
- 20.McQuillen PS, Ferriero DM. Selective vulnerability in the developing central nervous system. Pediatr Neurol. 2004;30:227–235. doi: 10.1016/j.pediatrneurol.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Herrero MT, Barcia C, Navarro JM. Functional anatomy of thalamus and basal ganglia. Childs Nerv Syst. 2002;18:386–404. doi: 10.1007/s00381-002-0604-1. [DOI] [PubMed] [Google Scholar]
- 22.McKinstry RC, Miller JH, Snyder AZ, Mathur A, Schefft GL, Almli CR, Shimony JS, Shiran SI, Neil JJ. A prospective, longitudinal diffusion tensor imaging study of brain injury in newborns. Neurology. 2002;59:824–833. doi: 10.1212/wnl.59.6.824. [DOI] [PubMed] [Google Scholar]
- 23.Soul JS, Robertson RL, Tzika AA, du Plessis AJ, Volpe JJ. Time course of changes in diffusion-weighted magnetic resonance imaging in a case of neonatal encephalopathy with defined onset and duration of hypoxic-ischemic insult. Pediatrics. 2001;108:1211–1214. doi: 10.1542/peds.108.5.1211. [DOI] [PubMed] [Google Scholar]
- 24.Cowan FM, Pennock JM, Hanrahan JD, Manji KP, Edwards AD. Early detection of cerebra infarction and hypoxic ischemic encephalopathy in neonates using diffusion-weighted magnetic resonance imaging. Neuropediatrics. 1994;25:172–175. doi: 10.1055/s-2008-1073018. [DOI] [PubMed] [Google Scholar]
- 25.Bonifacio SL, deVries LS, Groenendaal F. Impact of hypothermia on predictors of poor outcome: how do we decide to redirect care? Semin Fetal Neonatal Med. 2015;20:122–127. doi: 10.1016/j.siny.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bednarek N, Mathur A, Inder T, Wilkinson J, Neil J, Shimony J. Impact of therapeutic hypothermia on MRI diffusion changes in neonatal encephalopathy. Neurology. 2012;78:1420–1427. doi: 10.1212/WNL.0b013e318253d589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hasegawa Y, Latour LL, Sotak CH, Dardzinski BJ, Fisher M. Temperature dependent change of apparent diffusion coefficient of water in normal and ischemic brain of rats. J Cereb Blood Flow Metab. 1994;14:383–390. doi: 10.1038/jcbfm.1994.49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data