Highlights
-
•
Innocuous thermal information is a key component of haptic exploration.
-
•
Thermal perception is immediate and robust from mouse to man.
-
•
Mouse thermosensory system is genetically and optically accessible.
-
•
Circuits and coding principles of thermal processing are starting to be elucidated.
Abstract
Thermal information about skin surface temperature is a key sense for the perception of object identity and valence. The identification of ion channels involved in the transduction of thermal changes has provided a genetic access point to the thermal system. However, from sensory specific ‘labeled-lines’ to multimodal interactive pathways, the functional organization and identity of the neural circuits mediating innocuous thermal perception have been debated for over 100 years. Here we highlight points in the system that require further attention and review recent advances using in vivo electrophysiology, cellular resolution calcium imaging, optogenetics and thermal perceptual tasks in behaving mice that have begun to uncover the anatomical principles and neural processing mechanisms underlying innocuous thermal perception.
Current Opinion in Neurobiology 2018, 52:98–106
This review comes from a themed issue on Systems neuroscience
Edited by Michael Long and Rosa Cossart
For a complete overview see the Issue and the Editorial
Available online 15th May 2018
https://doi.org/10.1016/j.conb.2018.04.006
0959-4388/© 2018 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
From the warmth of an open fire to the cold touch of a chilled beer bottle, thermal sensation is tightly woven into our everyday sensory experience. Subconscious monitoring of temperature is essential for core body temperature regulation and survival in an ever-changing thermal environment. Thermal information, however, can also evoke rapid motor and emotional responses and is tightly integrated with tactile information to generate a unified, coherent percept of an object during haptic exploration. Thermal stimuli can lead to the formation of highly acute percepts, with the threshold for detecting temperature changes by the human hand being <0.5 °C [1, 2]. Similarly, thermal stimuli can also trigger perceptual paradoxes such as Thunberg's ‘thermal grill’ illusion where painful, burning sensations can be evoked by touching alternating bars of innocuous cold and warm temperatures [3]; or Weber's phenomenon where an object appears heavier when it is cold than warm [4]. Taken together, these observations reveal that the thermal system has neural processing, wiring and perceptual repertoires reminiscent of better studied sensory pathways.
Despite its strong links to survival, emotion and behavior, the neural pathways and cellular mechanisms of thermal processing remain relatively poorly understood. In 1882, Blix used electrical current delivered via a pin, or water via a small cone, to study innocuous thermal sensations in humans [5]. Because the tiny stimulation spots evoked discrete cold or touch percepts, and more recent afferent ablation studies alter percepts of specific modalities, it has been suggested that the circuits carrying thermal information are anatomically distinct from touch, pain, and proprioception — a ‘labeled line’ system [5, 6, 7, 8, 9]. However, the perception of cold and warm co-varies in humans [10] and multi-modal (mostly touch and temperature) responses have been observed at the afferent [11, 12, 13, 14•], thalamic [15, 16, 17], and cortical [14•, 18] levels of the thermal system. These observations of functional and perceptual integration of somatosensory modalities has prompted models of sensory coding that combine specialized receptors pathways with temporal coding schemes [12, 13, 19, 20].
Here we summarize the current knowledge about the neural circuits underlying thermal perception (see also [21•]) and examine recent functional studies in the mouse. We highlight the mouse thermal system as amenable for integrating genetic, systems and behavioral analysis in the search for the neural mechanisms of sensory perception and principles of sensory wiring.
Thermal psychophysics
While psychophysical studies have revealed fundamental principles of thermal perception in humans [10, 21•], this is not true for mice. In part, this reflects the different questions asked in rodent studies, such as, how do mice avoid thermal stimuli or regulate body temperature? Classic thermal behaviors have used measurements of paw withdrawal latency to strong thermal stimuli, or assessments of dwell times in chamber systems where floor plates are set to different temperatures [22, 23, 24, 25, 26] (Figure 1a,b). These behavioral assessments are useful for monitoring reflexive movements and innate thermoregulatory behaviors like cold avoidance, but do not necessarily reflect the perception of a sensory stimulus. Moreover, the limited spatial and temporal control of the thermal stimulus in floor plate experiments is problematic. For example, floor plate experiments where rodents can gather thermal information using different body parts, have led to different conclusions about the cortical representation of thermal input, with some lesion studies suggesting that the primary somatosensory cortex is not required for thermal sensation while others have suggested that it is [27, 28, 29, 30].
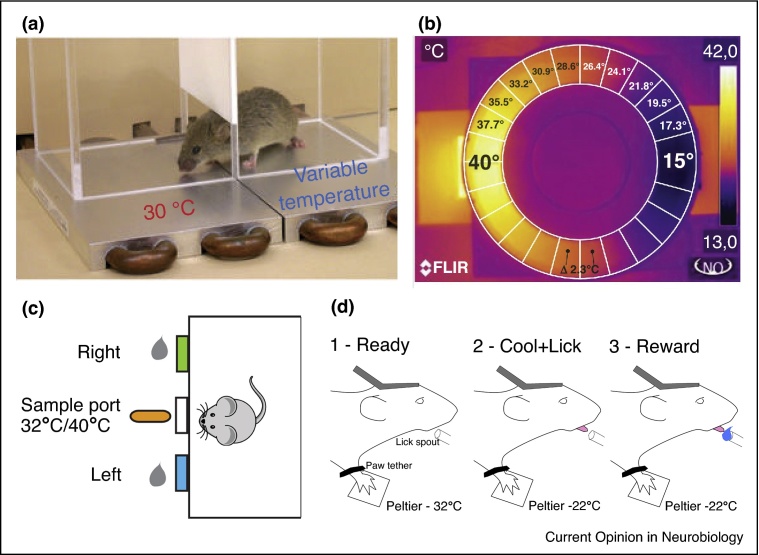
Figure 1.
Thermal behavioral tasks for rodents. (a) A 2-plate thermal avoidance task. Floor plates have different temperatures and experimenters monitor the time spent on either plate. (b) Similar task as in (a) but animals walk around within a ring-shaped disk (white circle on infrared image) with a gradient of floor temperatures. (c) A thermal discrimination task where freely moving mice are trained to discriminate between two temperatures of water droplets delivered to the central spout, mice report the temperature by moving to one of two reporting nose-poke ports. (d) Cartoon schematic showing different stages of thermal perception task in head-fixed paw-tethered mice based on task in [14•]. Mice are trained to report a thermal stimulus delivered to the glabrous skin of the right forepaw by licking a reward port. Following correct licking, mice are rewarded with water. Figure panels adapted with permission, from (a) [22], (b) [26], (c) [31••].
To address these problems, faster, goal-directed thermal perception behaviors have been recently developed for mice (Figure 1c,d). For example, Yarmolinksy et al. [31••] designed a warmth discrimination task where freely moving mice were trained to sample and report the temperature of drinking water using a three-port chamber consisting of a central sample port and a left or right reporting port. Mice could reach 90% discrimination accuracy within 2–3 weeks. To have stable access to the brain and improved stimulus control, Milenkovic et al. [14•] developed a head-fixed, paw-tethered task where mice are trained to report a thermal stimulus delivered to the glabrous skin of the right forepaw by licking a water reward with short latency. Mice learnt the behavior within a few days and can detect a <0.5 °C cooling stimulus (Paricio-Montesinos et al., unpublished observations), making their thermal perception abilities equivalent to that of humans. These approaches will make it possible to measure other fundamental aspects of thermal perception in mice (e.g. warming thresholds, the impact of baseline temperature, ramp speed, stimulus size, and somatotopic location) as well as the interaction between thermal and touch percepts. Moreover, head-fixation allows easier coupling of neuronal recordings and manipulations with behavior to investigate the neural mechanisms of thermal perception.
From skin to spinal cord
In recent years, the identification of the transient receptor potential (TRP) family of thermally sensitive ion channels in primary sensory afferent neurons [32, 33, 34], has provided a genetic access point to the thermal system. The thermal activation thresholds of TRP channels span the environmental temperature range and it is becoming increasingly evident they are co-expressed in adult primary sensory neurons [35, 36, 37]. TRPM8 [22, 23, 24] and TRPA1 [38, 39], for example, are thought to act in concert to transduce both innocuous and noxious cooling stimuli [40]. TRPM8 gene knockout leads to profound deficits in cooling avoidance [22, 23, 24] and innocuous cooling perception [14•]. The link between TRP channels and goal-directed warming perception is less clear. Recently, however, TRPM2 has been linked to warm thermo-regulation [41, 42] and warm preference behaviors [43]. Moreover, TRPV1 has been implicated in innocuous warming perception [31••, 44••]. However, TRPV1 is activated at noxious temperatures (>43 °C) and how TRPV1 activation leads to innocuous warming responses is unclear. Linking the TRP channels to goal-directed thermal perceptual tasks in mice is an important future goal.
Primary thermosensory neurons innervating the skin have cell bodies located in the dorsal root- and trigeminal ganglia. Classical work using single unit electrophysiological recordings has shown that there are two major groups of thermally sensitive afferent neurons; thinly myelinated Aδ fiber, and unmyelinated C-fibers (Figure 2). The degree of interplay between cooling, warming and tactile afferent input is still unclear. Thermal sensitive Aδ fibers were originally thought to be major temperature sensors due to the differential impact of myelinated nerve blocking on the perception of warmth in humans [45]. However, C-fibers can also respond to low threshold thermal stimuli [14•, 22, 40, 46], with recordings from the C-fibers in TRPM8−/− mice showing major deficits in cooling responsiveness with less impact on the thermal sensitivity of Aδ-fibers [14•, 40]. These experiments suggest that C-fibers, alongside their role in pain sensation, are also involved in innocuous thermal perception. To address this putative polymodal function, future experiments should combine population recordings of single cells [31••, 47, 48] and manipulations with perceptual tasks.
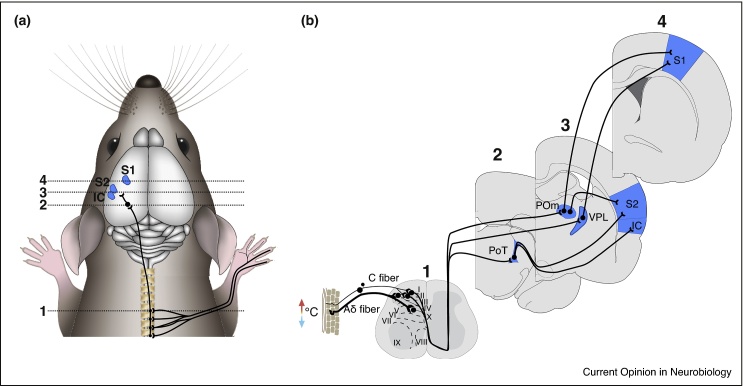
Figure 2.
Putative thermal pathways from paw to cortex in mice. (a) Cartoon mouse showing putative thermal pathways from skin to cortex via spinal cord and thalamus, primary somatosensory cortex (S1), secondary somatosensory cortex (S2), and insular cortex (IC). The thermal pathway via lateral parabrachial nucleus to hypothalamus is not included. (b) Schematic cross-sections of mouse nervous system taken at different levels with numbers corresponding to locations in (a). Thermal thalamic input to S1 is provided by ventral posterolateral (VPL) and posterior medial (POm), to S2 by POm and the posterior triangular nucleus (PoT), and IC by PoT.
Thermal stimulation using water or a Peltier element is relatively slow, difficult to perform in a spatially restricted manner and activates multiple subtypes of afferent neurons. However, recent advances in the molecular characterization of sensory neurons [31••, 35, 49, 50••, 51] coupled with the ease of accessing the skin with optical probes and its optical isolation from the brain, has prompted experimenters to perform optogenetic manipulations of selected subsets of sensory afferents [50••, 52, 53, 54]. Optogenetic stimulation with high temporal and spatial control will not only allow functional mapping of sensory responses at different stages of the pathway, but also the decoupling of different modalities of somatosensory input. Recently, this approach showed a cross-pathway impact of light touch on pinprick evoked pain [50••].
Histological analysis has shown that thermally sensitive afferent neurons project predominantly to laminae I and II (LI/II) of the dorsal horn of the spinal cord [55]. However, a minority of afferent fibers terminate in deeper layers and there is relatively little known about the identity and function of second order neurons contacted by thermal afferent neurons across spinal layers. Classical in vivo single unit recordings have shown responses of superficial layer neurons to thermal stimulation in anaesthetized animals [56, 57, 58, 59, 60, 61], suggesting that thermal afferents directly synapse with LI/II neurons. Confirmation of monosynaptic connectivity, however, will require restricted anatomical tracing in molecularly defined thermal afferent neurons. Here, advances in viral tracing techniques for anterograde, retrograde and trans-synaptic synaptic labeling of genetically defined subsets of somatosensory neurons [62, 63•, 64, 65] will be instrumental in revealing the wiring of the thermal system.
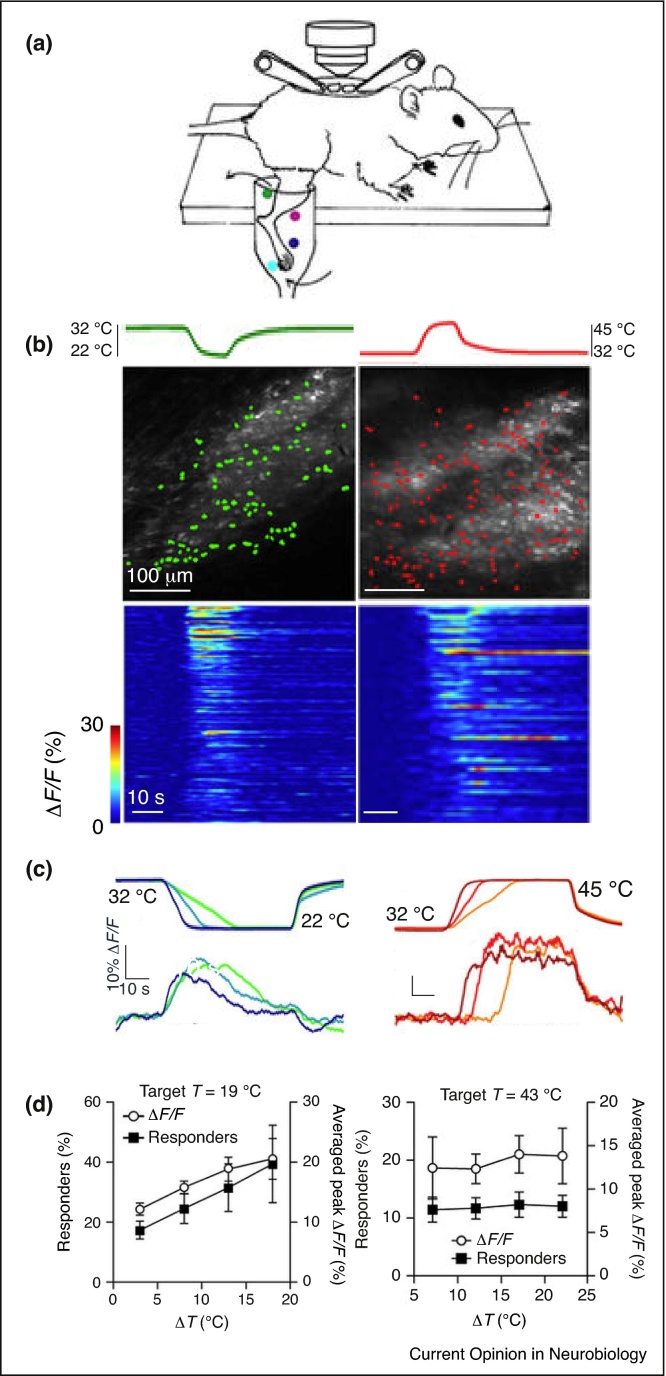
Recently, thermal processing in the superficial dorsal laminae of the mouse spinal cord has been addressed with in vivo single cell calcium imaging [44••]. In this study, Ran and colleagues immersed the hind limb in a water bath and monitored calcium responses of single neurons in LI/II of the lumbar spinal cord to changes in bath temperature (Figure 3a). The data revealed functional differences in the representation of warming and cooling. Cooling responsive neurons showed a broad distribution of activation thresholds with ∼70% being activated <6 °C cooling from skin temperature (Figure 3b). In contrast, <15% of warming responsive neurons responded at 5–8 °C from skin temperature and the vast majority (∼80%) were activated by warming stimuli over noxious thresholds (>42 °C). Moreover, of all thermosensitive spinal neurons, 7% responded to both innocuous cooling and warming, while 44% responded to both noxious cold and heat. The kinetics were also distinct with cooling responses peaking during the transient phase of the stimulus and subsequently adapting, whilst warming responses persisted at a similar level throughout the stimulus (Figure 3c). Moreover, warming responsive LI/II neurons appear to encode absolute temperatures whereas cooling neurons respond preferentially to changes in temperature (Figure 3d). In this study, tactile responses were not examined, but, while cooling specific LI/II neurons have been observed [61, 66], the majority of these neurons are likely to be multi-modal for combinations of cool, warm and/or touch. A major challenge is therefore to understand how thermal and tactile information are decoded and forwarded to cortical areas involved in perception.
Figure 3.
Imaging thermal processing in LI/II of the spinal cord. (a) Cartoon schematic of preparation for two-photon calcium imaging of the dorsal spinal cord during thermal stimulation of the hindpaw in anaesthetized mice. (b) Top: example in vivo images of LI/II with superimposed thermally responsive neurons (filled color) during either cooling (left) or warming (right) thermal stimulation. Bottom: calcium response dynamics from single neurons sorted by their maximum response amplitudes (n = 138 and 276 cold and warm responsive cells). (c) Example calcium responses (ΔF/F) from a single LI/II neuron to (left) 10 °C cooling (32–22 °C) and (right) 13 °C warming (32–45 °C) show different response dynamics, note the warming stimulus goes over thermal pain threshold (42 °C) while cooling does not. (d) Graphs showing the numbers of responding cells and the calcium responses (ΔF/F) in LI/II neurons in response to stimuli with a fixed peak temperature (left, cool to 19 °C, right, warming to 43 °C) and different baseline temperatures. LI/II neurons show a graded recruitment for different amplitude cooling stimuli but similar recruitment for different amplitude warming, implying that warming-responsive neurons code for absolute warming temperatures while the relative change in temperature is coded in cooling responsive neurons. Figure panels were adapted with permission from [44••].
From spinal cord to cortex
Classical anatomical tracing techniques and antidromic electrical stimulation during spinal cord recordings have shown that thermally responsive neurons project from LI/II travel, via the spino-thalamic tract, to the contralateral somatosensory nuclei of the thalamus [21•]. In the rat, superficial dorsal laminar spinal cord neurons project to restricted areas of the contralateral ventral posterolateral (VPL), posterior medial (POm), and the caudally positioned triangular posterior thalamic (PoT) nuclei [67, 68] (Figure 2). While there is some debate as to whether thalamo-cortical pathways supporting thermal processing are homologous in rodents and humans [69], caudal PoT has been suggested to be the rodent analog of the primate ventral medial nucleus (VMpo) [67] — a major target of spinal thermo-sensory LI/II neurons in primates [66, 70, 71, 72].
Single unit recordings of thermally responsive thalamic neurons have been made in humans [73], primates [66, 74, 75, 76] and cats [77, 78]. In anaesthetized rats, thalamic ventro-basal (VB, a structure encompassing both VPL and POm) neurons respond to innocuous cooling and warming of the scrotum and paw [15, 16]. Recently, lesions of the VPL in mice have been shown to have minimal effect on cool or warm avoidance behavior in a two-plate test [79]. However, LI/II neurons also project to the lateral parabrachial nucleus of the brainstem (which in turn projects to the preoptic area of the hypothalamus) [79, 80], and lesions of this pathway abolish cold avoidance [79], suggesting a major role for hypothalamic circuits in cold avoidance (see [81, 82] for a discussion on the circuits mediating thermoregulation).
VPL projects to primary somatosensory cortex (S1), POm to S1 and S2, primate VMpo to insular cortex (IC) [83], and rodent PoT [17] to IC and S2 (Figure 2). Reflecting this divergence of putative thermally sensitive thalamocortical pathways, evidence exists for cortical representations of thermal (mostly cooling) input in S1, S2 and IC.
-
(i)
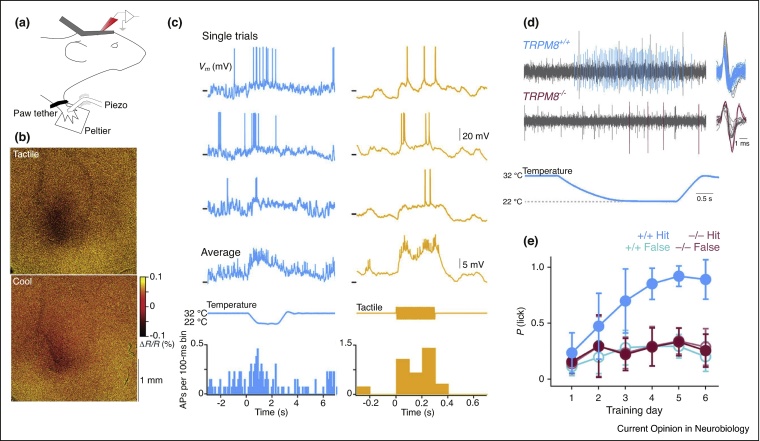
Primary somatosensory cortex. Responses of single neurons in primary somatosensory cortex to thermal stimulation been reported in the cat [18, 84, 85], rat [86, 87], and observed in human imaging [88, 89] and electroencephalography (EEG) studies [90]. Moreover, intracortical stimulation of S1 in awake humans can lead to thermal sensations [91]. In awake mice, Milenkovic et al. [14•] showed activation of S1 neurons to innocuous cooling and tactile stimulation of the glabrous forepaw skin (Figure 4) and that pharmacological silencing of forepaw S1 suppressed cooling perception.
-
(ii)
Secondary somatosensory cortex. Functional brain imaging in humans [92] and mice [93] has shown thermal responses in S2. In rodents, S2 is also a site for tactile processing [94] but, to our knowledge, innocuous thermal processing has not been examined at a cellular level in S2.
-
(iii)
Insular cortex. Thalamic wiring (see above), functional imaging [88, 95, 96], EEG [97], intra-cortical stimulation [98, 99] and lesion studies [100, 101] have linked primate and human IC to thermal processing. Broad scale mapping studies have shown tactile and noxious heat responses in rodent IC [93, 94, 102, 103]. Moreover, a recent activity-dependent immediate early gene (cfos) labeling study has shown strong activation of IC, as well as S1 and S2, following menthol (a TRPM8 agonist) application to the mouse forepaw [104].
Figure 4.
Innocuous cooling processing and perception in mice. (a) Cartoon schematic of head-fixed, paw-tethered preparation for sensory cortex recordings and thermo-tactile stimulation. (b) Intrinsic optical imaging shows overlapping response in primary somatosensory cortex (S1) to cooling and touch of the forepaw glabrous skin. (c) Example in vivo whole-cell membrane potential recording from the same layer 2/3 neuron in an awake mouse showing responses to thermal and tactile stimulation of the right forepaw. Forepaw is tethered to the thermal stimulating surface of a Peltier element. From top: single trial responses, averaged membrane potential (Vm), stimulus, peri-stimulus time histogram (PSTH) of action potential firing (n = 13 thermal, 12 tactile stimuli). (d) Example single unit afferent recordings from an in vitro skin-nerve preparation showing a response to thermal stimulation of the glabrous skin in TRPM8+/+ (cyan) and a reduced response in TRPM8−/− (magenta) mice. Colored action potentials depict individual spikes selected for analysis. (e) Learning curve in TRPM8+/+ (cyan) and TRPM8−/− (magenta) mice shows that TRPM8 is required for mice to learn to report a 10 °C cooling of the paw. Figure panels taken with permission from [14•].
Figure 2 summarizes putative thermal circuits from the forepaw to cortex in mice. We hypothesize that S1, S2 and IC act in concert during innocuous thermal sensation to ascribe modality identification with sensory features (e.g. somatotopic location or stimulus amplitude) and valence.
Summary and future directions
The thermal system is capable of generating rapid and acute percepts that are uniquely identifiable yet bound together with tactile inputs during object manipulation. It can evoke both innate and learned motor behaviors as well as strong emotional reactions from pleasure to pain. However, despite boasting such a rich perceptual repertoire and widespread influence on body function, our knowledge of innocuous thermal processing is heavily skewed toward the afferent and spinal level in anaesthetized animals and far less to thalamo-cortical processing during behavior. Novel solutions to this issue will stem from the genetic access to the periphery of the system and the ability to couple neuronal recordings with high-resolution perception tasks. Available data suggests that a number of neurons in the pathway are multi-modal, implying a degree of combinatorial coding and integrated wiring, but to understand the wiring principles and neural mechanisms of thermal processing, future experiments must aim to anatomically and functionally map thalamo-cortical circuits with single cell precision ultimately linking them to perception with recordings and manipulations in behavioral tasks. The mouse now offers a model system with the possibility of combining powerful genetic and synaptic tracing methods, with electrophysiological, optical and behavioral approaches, that make this wish list within thermal-touching distance.
Acknowledgements
We thank members of the Poulet lab for critical comments and discussion. This work was funded by the European Research Council (ERC-2015-CoG-682422, J.F.A.P.), the Deutsche Forschungsgemeinschaft (DFG, Exc 257 NeuroCure, FOR 1341, FOR 2143, J.F.A.P.), the Thyssen Foundation (J.F.A.P.), the Helmholtz Society (J.F.A.P.).
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
References
- 1.Frenzel H., Bohlender J., Pinsker K., Wohlleben B., Tank J., Lechner S.G., Schiska D., Jaijo T., Rüschendorf F., Saar K. A genetic basis for mechanosensory traits in humans. PLoS Biol. 2012;10:e1001318. doi: 10.1371/journal.pbio.1001318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens J.C., Choo K.K. Temperature sensitivity of the body surface over the life span. Somatosens Mot Res. 1998;15:13–28. doi: 10.1080/08990229870925. [DOI] [PubMed] [Google Scholar]
- 3.Thunberg T. Untersuchungen über die relative Tiefenlage der kälte-, wärme-, und schmerzpercipirenden Nervenenden in der Haut und über das Verhältniss der Kältenervenenden gegenüber Wärmereizen1. Skandinavisches Archiv Für Physiologie. 2012;11:382–435. [Google Scholar]
- 4.Stevens J.C., Green B.G. Temperature–touch interaction: Weber's phenomenon revisited. Sensory Processes. 1978;2:206–219. [PubMed] [Google Scholar]
- 5.Norrsell U., Finger S., Lajonchere C. Cutaneous sensory spots and the “law of specific nerve energies”: history and development of ideas. Brain Res Bull. 1999;48:457–465. doi: 10.1016/s0361-9230(98)00067-7. [DOI] [PubMed] [Google Scholar]
- 6.Craig A.D.B. Pain mechanisms: labeled lines versus convergence in central processing. Annu Rev Neurosci. 2003;26:1–30. doi: 10.1146/annurev.neuro.26.041002.131022. [DOI] [PubMed] [Google Scholar]
- 7.Knowlton W.M., Palkar R., Lippoldt E.K., McCoy D.D., Baluch F., Chen J., McKemy D.D. A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. J Neurosci. 2013;33:2837–2848. doi: 10.1523/JNEUROSCI.1943-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishra S.K., Hoon M.A. Ablation of TrpV1 neurons reveals their selective role in thermal pain sensation. Mol Cell Neurosci. 2010;43:157–163. doi: 10.1016/j.mcn.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavanaugh D.J., Lee H., Lo L., Shields S.D., Zylka M.J., Basbaum A.I., Anderson D.J. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green B.G., Akirav C. Individual differences in temperature perception: evidence of common processing of sensation intensity of warmth and cold. Somatosens Mot Res. 2007;24:71–84. doi: 10.1080/08990220701388117. [DOI] [PubMed] [Google Scholar]
- 11.Perl E.R. Ideas about pain, a historical view. Nat Rev Neurosci. 2007;8:71–80. doi: 10.1038/nrn2042. [DOI] [PubMed] [Google Scholar]
- 12.Green B.G. Temperature perception and nociception. J Neurobiol. 2004;61:13–29. doi: 10.1002/neu.20081. [DOI] [PubMed] [Google Scholar]
- 13.Ma Q. Labeled lines meet and talk: population coding of somatic sensations. J Clin Invest. 2010;120:3773–3778. doi: 10.1172/JCI43426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14•.Milenkovic N., Zhao W.-J., Walcher J., Albert T., Siemens J., Lewin G.R., Poulet J.F.A. A somatosensory circuit for cooling perception in mice. Nat Neurosci. 2014;17:1560–1566. doi: 10.1038/nn.3828. [DOI] [PubMed] [Google Scholar]; Primary somatosensory cortex is involved in innocuous cold processing and perception in mice. C-fibers are the likely mediators of afferent input underlying innocuous cold perception.
- 15.Hellon R.F., Misra N.K. Neurones in the ventrobasal complex of the rat thalamus responding to scrotal skin temperature changes. J Physiol (Lond) 1973;232:389–399. doi: 10.1113/jphysiol.1973.sp010276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schingnitz G., Werner J. Responses of thalamic neurons to thermal stimulation of the limbs, scrotum and tongue in the rat. J Therm Biol. 1980;5:53–61. [Google Scholar]
- 17.Gauriau C., Bernard J.-F. Posterior triangular thalamic neurons convey nociceptive messages to the secondary somatosensory and insular cortices in the rat. J Neurosci. 2004;24:752–761. doi: 10.1523/JNEUROSCI.3272-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuboi Y., Iwata K., Muramatsu H., Yagi J., Inomata Y., Sumino R. Response properties of primary somatosensory cortical neurons responsive to cold stimulation of the facial skin and oral mucous membrane. Brain Res. 1993;613:193–202. doi: 10.1016/0006-8993(93)90899-x. [DOI] [PubMed] [Google Scholar]
- 19.Prescott S.A., Ma Q., De Koninck Y. Normal and abnormal coding of somatosensory stimuli causing pain. Nat Neurosci. 2014;17:183–191. doi: 10.1038/nn.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doetsch G.S. Patterns in the brain. Physiol Behav. 2000;69:187–201. doi: 10.1016/s0031-9384(00)00201-8. [DOI] [PubMed] [Google Scholar]
- 21•.Filingeri D. John Wiley & Sons; 2011. Neurophysiology of Skin Thermal Sensations. [DOI] [PubMed] [Google Scholar]; A comprehensive review of thermal system.
- 22.Bautista D.M., Siemens J., Glazer J.M., Tsuruda P.R., Basbaum A.I., Stucky C.L., Jordt S.-E., Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- 23.Dhaka A., Murray A.N., Mathur J., Earley T.J., Petrus M.J., Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 24.Colburn R.W., Lubin M.L., Stone D.J., Jr., Wang Y., Lawrence D., D’Andrea M.R., Brandt M.R., Liu Y., Flores C.M., Qin N. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Almeida M.C., Vizin R.C.L., Carrettiero D.C. Current understanding on the neurophysiology of behavioral thermoregulation. Temperature. 2015;2:483–490. doi: 10.1080/23328940.2015.1095270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Touska F., Winter Z., Mueller A., Vlachova V., Larsen J., Zimmermann K. Comprehensive thermal preference phenotyping in mice using a novel automated circular gradient assay. Temperature. 2015;3:77–91. doi: 10.1080/23328940.2015.1135689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finger S., Frommer G.P. Effects of cortical and thalamic lesions on temperature discrimination and responsiveness to foot shock in the rat. Brain Res. 1970;24:69–89. doi: 10.1016/0006-8993(70)90274-x. [DOI] [PubMed] [Google Scholar]
- 28.Finger S., Scheff S., Warshaw I., Cohen K. Retention and acquisition of fine temperature discriminations following somatosensory cortical lesions in the rat. Exp Brain Res. 1970;10:340–346. doi: 10.1007/BF02324763. [DOI] [PubMed] [Google Scholar]
- 29.Downer J., Zubek J.P. Role of the cerebral cortex in temperature discrimination in the rat. J Comp Physiol Psychol. 1954;47:199–203. doi: 10.1037/h0053911. [DOI] [PubMed] [Google Scholar]
- 30.Porter L.H., Hecht G.S., Sheaffer R. Disturbances in the performance of thermal discrimination tasks following cortical ablations in rats. Brain Res. 1993;621:319–330. doi: 10.1016/0006-8993(93)90122-4. [DOI] [PubMed] [Google Scholar]
- 31••.Yarmolinsky D.A., Peng Y., Pogorzala L.A., Rutlin M., Hoon M.A., Zuker C.S. Coding and plasticity in the mammalian thermosensory system. Neuron. 2016;92:1079–1092. doi: 10.1016/j.neuron.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]; In vivo calcium imaging of trigeminal ganglion neurons during thermal stimulation of the mouse oral cavity shows sensory response properties and suggests a role for TRPV1 in warming responsiveness.
- 32.Palkar R., Lippoldt E.K., McKemy D.D. The molecular and cellular basis of thermosensation in mammals. Curr Opin Neurobiol. 2015;34:14–19. doi: 10.1016/j.conb.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vriens J., Nilius B., Voets T. Peripheral thermosensation in mammals. Nat Rev Neurosci. 2014;15:573–589. doi: 10.1038/nrn3784. [DOI] [PubMed] [Google Scholar]
- 34.Hoffstaetter L.J., Bagriantsev S.N., Gracheva E.O. TRPs et al.: a molecular toolkit for thermosensory adaptations. Pflugers Arch. 2018;69:15–44. doi: 10.1007/s00424-018-2120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Usoskin D., Furlan A., Islam S., Abdo H., Lönnerberg P., Lou D., Hjerling-Leffler J., Haeggström J., Kharchenko O., Kharchenko P.V. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. 2014;18:145–153. doi: 10.1038/nn.3881. [DOI] [PubMed] [Google Scholar]
- 36.Mishra S.K., Tisel S.M., Orestes P., Bhangoo S.K., Hoon M.A. TRPV1-lineage neurons are required for thermal sensation. EMBO J. 2011;30:582–593. doi: 10.1038/emboj.2010.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cavanaugh D.J., Chesler A.T., Jackson A.C., Sigal Y.M., Yamanaka H., Grant R., O’Donnell D., Nicoll R.A., Shah N.M., Julius D. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci. 2011;31:5067–5077. doi: 10.1523/JNEUROSCI.6451-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwan K.Y., Allchorne A.J., Vollrath M.A., Christensen A.P., Zhang D.-S., Woolf C.J., Corey D.P. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 39.Karashima Y., Talavera K., Everaerts W., Janssens A., Kwan K.Y., Vennekens R., Nilius B., Voets T. TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci U S A. 2009;106:1273–1278. doi: 10.1073/pnas.0808487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winter Z., Gruschwitz P., Eger S., Touska F., Zimmermann K. Cold temperature encoding by cutaneous TRPA1 and TRPM8-carrying fibers in the mouse. Front Mol Neurosci. 2017;10:204. doi: 10.3389/fnmol.2017.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan C.-H., McNaughton P.A. The TRPM2 ion channel is required for sensitivity to warmth. Nature. 2016;536:460–463. doi: 10.1038/nature19074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song K., Wang H., Kamm G.B., Pohle J., Reis F.D.C., Heppenstall P., Wende H., Siemens J. The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science. 2016;353:1393–1398. doi: 10.1126/science.aaf7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tan C.L., Cooke E.K., Leib D.E., Lin Y.-C., Daly G.E., Zimmerman C.A., Knight Z.A. Warm-sensitive neurons that control body temperature. Cell. 2016;167 doi: 10.1016/j.cell.2016.08.028. 47–59.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44••.Ran C., Hoon M.A., Chen X. The coding of cutaneous temperature in the spinal cord. Nat Neurosci. 2016;19:1201–1209. doi: 10.1038/nn.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]; In vivo calcium imaging shows distinct response properties of LI/II neurons to warming and cooling stimulation of the hind limb.
- 45.Mackenzie R.A., Burke D., Skuse N.F., Lethlean A.K. Fibre function and perception during cutaneous nerve block. J Neurol Neurosurg Psychiatr. 1975;38:865–873. doi: 10.1136/jnnp.38.9.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campero M., Baumann T.K., Bostock H., Ochoa J.L. Human cutaneous C fibres activated by cooling, heating and menthol. J Physiol (Lond) 2009;587:5633–5652. doi: 10.1113/jphysiol.2009.176040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim Y.S., Anderson M., Park K., Zheng Q., Agarwal A., Gong C., Saijilafu, Young L., He S., LaVinka P.C. Coupled activation of primary sensory neurons contributes to chronic pain. Neuron. 2016;91:1085–1096. doi: 10.1016/j.neuron.2016.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Emery E.C., Luiz A.P., Sikandar S., Magnusdottir R., Dong X., Wood J.N. In vivo characterization of distinct modality-specific subsets of somatosensory neurons using GCaMP. Sci Adv. 2016;2 doi: 10.1126/sciadv.1600990. e1600990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le Pichon C.E., Chesler A.T. The functional and anatomical dissection of somatosensory subpopulations using mouse genetics. Front Neuroanat. 2014;8:702. doi: 10.3389/fnana.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50••.Arcourt A., Gorham L., Dhandapani R., Prato V., Taberner F.J., Wende H., Gangadharan V., Birchmeier C., Heppenstall P.A., Lechner S.G. Touch receptor-derived sensory information alleviates acute pain signaling and fine-tunes nociceptive reflex coordination. Neuron. 2017;93:179–193. doi: 10.1016/j.neuron.2016.11.027. [DOI] [PubMed] [Google Scholar]; Optogenetic stimulation of subsets of primary afferent neurons shows a tactile mediated alleviation of pinprick evoked pain mediated by A-fibers.
- 51.Wende H., Lechner S.G., Cheret C., Bourane S., Kolanczyk M.E., Pattyn A., Reuter K., Munier F.L., Carroll P., Lewin G.R. The transcription factor c-Maf controls touch receptor development and function. Science. 2012;335:1373–1376. doi: 10.1126/science.1214314. [DOI] [PubMed] [Google Scholar]
- 52.Daou I., Tuttle A.H., Longo G., Wieskopf J.S., Bonin R.P., Ase A.R., Wood J.N., De Koninck Y., Ribeiro-da-Silva A., Mogil J.S. Remote optogenetic activation and sensitization of pain pathways in freely moving mice. J Neurosci. 2013;33:18631–18640. doi: 10.1523/JNEUROSCI.2424-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ji Z.-G., Ito S., Honjoh T., Ohta H., Ishizuka T., Fukazawa Y., Yawo H. Light-evoked somatosensory perception of transgenic rats that express channelrhodopsin-2 in dorsal root ganglion cells. PLoS One. 2012;7:e32699. doi: 10.1371/journal.pone.0032699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iyer S.M., Montgomery K.L., Towne C., Lee S.Y., Ramakrishnan C., Deisseroth K., Delp S.L. Virally mediated optogenetic excitation and inhibition of pain in freely moving nontransgenic mice. Nat Biotechnol. 2014;32:274–278. doi: 10.1038/nbt.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takashima Y., Ma L., McKemy D.D. The development of peripheral cold neural circuits based on TRPM8 expression. Neuroscience. 2010;169:828–842. doi: 10.1016/j.neuroscience.2010.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Christensen B.N., Perl E.R. Spinal neurons specifically excited by noxious or thermal stimuli: marginal zone of the dorsal horn. J Neurophysiol. 1970;33:293–307. doi: 10.1152/jn.1970.33.2.293. [DOI] [PubMed] [Google Scholar]
- 57.Craig A.D., Dostrovsky J.O. Differential projections of thermoreceptive and nociceptive lamina I trigeminothalamic and spinothalamic neurons in the cat. J Neurophysiol. 2001;86:856–870. doi: 10.1152/jn.2001.86.2.856. [DOI] [PubMed] [Google Scholar]
- 58.Burton H. Responses of spinal cord neurons to systematic changes in hindlimb skin temperatures in cats and primates. J Neurophysiol. 1975;38:1060–1079. doi: 10.1152/jn.1975.38.5.1060. [DOI] [PubMed] [Google Scholar]
- 59.Bester H., Chapman V., Besson J.-M., Bernard J.-F. Physiological properties of the lamina I spinoparabrachial neurons in the rat. J Neurophysiol. 2000;83:2239–2259. doi: 10.1152/jn.2000.83.4.2239. [DOI] [PubMed] [Google Scholar]
- 60.Andrew D., Craig A.D. Spinothalamic lamina I neurones selectively responsive to cutaneous warming in cats. J Physiol (Lond) 2001;537:489–495. doi: 10.1111/j.1469-7793.2001.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dostrovsky J.O., Craig A.D. Cooling-specific spinothalamic neurons in the monkey. J Neurophysiol. 1996;76:3656–3665. doi: 10.1152/jn.1996.76.6.3656. [DOI] [PubMed] [Google Scholar]
- 62.Wickersham I.R., Lyon D.C., Barnard R.J.O., Mori T., Finke S., Conzelmann K.-K., Young J.A.T., Callaway E.M. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron. 2007;53:639–647. doi: 10.1016/j.neuron.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63•.Zampieri N., Jessell T.M., Murray A.J. Mapping Sensory circuits by anterograde transsynaptic transfer of recombinant rabies virus. Neuron. 2014;81:766–778. doi: 10.1016/j.neuron.2013.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]; Monosynaptic anterograde tracing from primary somatosensory neurons in the forelimb to second order spinal neurons using a modified rabies virus.
- 64.Zingg B., Chou X.-L., Zhang Z.-G., Mesik L., Liang F., Tao H.W., Zhang L.I. AAV-mediated anterograde transsynaptic tagging: mapping corticocollicular input-defined neural pathways for defense behaviors. Neuron. 2017;93:33–47. doi: 10.1016/j.neuron.2016.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chan K.Y., Jang M.J., Yoo B.B., Greenbaum A., Ravi N., Wu W.-L., Sánchez-Guardado L., Lois C., Mazmanian S.K., Deverman B.E. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat Neurosci. 2017;20:1172–1179. doi: 10.1038/nn.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Craig A.D., Bushnell M.C., Zhang E.T., Blomqvist A. A thalamic nucleus specific for pain and temperature sensation. Nature. 1994;372:770–773. doi: 10.1038/372770a0. [DOI] [PubMed] [Google Scholar]
- 67.Gauriau C., Bernard J.-F. A comparative reappraisal of projections from the superficial laminae of the dorsal horn in the rat: the forebrain. J Comp Neurol. 2004;468:24–56. doi: 10.1002/cne.10873. [DOI] [PubMed] [Google Scholar]
- 68.Zhang X., Davidson S., Giesler G.J. Thermally identified subgroups of marginal zone neurons project to distinct regions of the ventral posterior lateral nucleus in rats. J Neurosci. 2006;26:5215–5223. doi: 10.1523/JNEUROSCI.0701-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Craig A.D. A rat is not a monkey is not a human: comment on Mogil (Nature Rev. Neurosci. 10, 283-294 (2009)) Nat Rev Neurosci. 2009;10:466. doi: 10.1038/nrn2606-c1. [DOI] [PubMed] [Google Scholar]
- 70.Blomqvist A., Zhang E.T., Craig A.D. Cytoarchitectonic and immunohistochemical characterization of a specific pain and temperature relay, the posterior portion of the ventral medial nucleus, in the human thalamus. Brain. 2000;123(Pt 3):601–619. doi: 10.1093/brain/123.3.601. [DOI] [PubMed] [Google Scholar]
- 71.Willis W.D., Zhang X., Honda C.N., Giesler G.J. A critical review of the role of the proposed VMpo nucleus in pain. J Pain. 2002;3:79–94. doi: 10.1054/jpai.2002.122949. [DOI] [PubMed] [Google Scholar]
- 72.Craig A.D., Blomqvist A. Is there a specific lamina I spinothalamocortical pathway for pain and temperature sensations in primates? J Pain. 2002;3:95–101. doi: 10.1054/jpai.2002.122953. (discussion 113–4) [DOI] [PubMed] [Google Scholar]
- 73.Davis K.D., Lozano R.M., Manduch M., Tasker R.R., Kiss Z.H., Dostrovsky J.O. Thalamic relay site for cold perception in humans. J Neurophysiol. 1999;81:1970–1973. doi: 10.1152/jn.1999.81.4.1970. [DOI] [PubMed] [Google Scholar]
- 74.POGGIO G.F., Mountcastle V.B. The functional properties of ventrobasal thalamus neurons studies in unanesthetized monkeys. J Neurophysiol. 1963;26:775–806. doi: 10.1152/jn.1963.26.5.775. [DOI] [PubMed] [Google Scholar]
- 75.Poulos D.A., Benjamin R.M. Response of thalamic neurons to thermal stimulation of the tongue. J Neurophysiol. 1968;31:28–43. doi: 10.1152/jn.1968.31.1.28. [DOI] [PubMed] [Google Scholar]
- 76.Burton H., Forbes D.J., Benjamin R.M. Thalamic neurons responsive to temperature changes of glabrous hand and foot skin in squirrel monkey. Brain Res. 1970;24:179–190. doi: 10.1016/0006-8993(70)90099-5. [DOI] [PubMed] [Google Scholar]
- 77.Auen E.L., Poulos D.A., Hirata H., Molt J.T. Location and organization of thalamic thermosensitive neurons responding to cooling the cat oral-facial regions. Brain Res. 1980;191:260–264. doi: 10.1016/0006-8993(80)90330-3. [DOI] [PubMed] [Google Scholar]
- 78.Landgren S. Thalamic neurones responding to cooling of the cat's tongue. Acta Physiol Scand. 1960;48:255–267. doi: 10.1111/j.1748-1716.1960.tb01860.x. [DOI] [PubMed] [Google Scholar]
- 79.Yahiro T., Kataoka N., Nakamura Y., Nakamura K. The lateral parabrachial nucleus, but not the thalamus, mediates thermosensory pathways for behavioural thermoregulation. Sci Rep. 2017;7:5031. doi: 10.1038/s41598-017-05327-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nakamura K., Morrison S.F. A thermosensory pathway that controls body temperature. Nat Neurosci. 2008;11:62–71. doi: 10.1038/nn2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nakamura K. Central circuitries for body temperature regulation and fever. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1207–R1228. doi: 10.1152/ajpregu.00109.2011. [DOI] [PubMed] [Google Scholar]
- 82.Clapham J.C. Central control of thermogenesis. Neuropharmacology. 2012;63:111–123. doi: 10.1016/j.neuropharm.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 83.Craig A.D., Paris ACFAIIPP . Supraspinal projections of lamina I neurons. In: Besson J.M., Guilbaud G., Ollat H., editors. Forebrain areas involved in pain processing. 1995. [Google Scholar]
- 84.Landgren S. Cortical reception of cold impulses from the tongue of the cat. Acta Physiol Scand. 1957;40:202–209. doi: 10.1111/j.1748-1716.1957.tb01489.x. [DOI] [PubMed] [Google Scholar]
- 85.Landgren S. Convergence of tactile, thermal, and gustatory impulses on single cortical cells. Acta Physiol Scand. 1957;40:210–221. doi: 10.1111/j.1748-1716.1957.tb01490.x. [DOI] [PubMed] [Google Scholar]
- 86.Hellon R.F., Misra N.K., Provins K.A. Neurones in the somatosensory cortex of the rat responding to scrotal skin temperature changes. J Physiol (Lond) 1973;232:401–411. doi: 10.1113/jphysiol.1973.sp010277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hellon R.F., Mitchell D. Convergence in a thermal afferent pathway in the rat. J Physiol (Lond) 1975;248:359–376. doi: 10.1113/jphysiol.1975.sp010979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Egan G.F., Johnson J., Farrell M., McAllen R., Zamarripa F., McKinley M.J., Lancaster J., Denton D., Fox P.T. Cortical, thalamic, and hypothalamic responses to cooling and warming the skin in awake humans: a positron-emission tomography study. Proc Natl Acad Sci U S A. 2005;102:5262–5267. doi: 10.1073/pnas.0409753102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rolls E.T., Grabenhorst F., Parris B.A. Warm pleasant feelings in the brain. Neuroimage. 2008;41:1504–1513. doi: 10.1016/j.neuroimage.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 90.Chatt A.B., Kenshalo D.R. Cerebral evoked responses to skin warming recorded from human scalp. Exp Brain Res. 1977;28:449–455. doi: 10.1007/BF00236469. [DOI] [PubMed] [Google Scholar]
- 91.Penfield W., Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- 92.Moulton E.A., Pendse G., Becerra L.R., Borsook D. BOLD responses in somatosensory cortices better reflect heat sensation than pain. J Neurosci. 2012;32:6024–6031. doi: 10.1523/JNEUROSCI.0006-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reimann H.M., Hentschel J., Marek J., Huelnhagen T., Todiras M., Kox S., Waiczies S., Hodge R., Bader M., Pohlmann A. Normothermic mouse functional MRI of acute focal thermostimulation for probing nociception. Sci Rep. 2016;6:1304. doi: 10.1038/srep17230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rodgers K.M., Benison A.M., Klein A., Barth D.S. Auditory, somatosensory, and multisensory insular cortex in the rat. Cereb Cortex. 2008;18:2941–2951. doi: 10.1093/cercor/bhn054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Craig A.D., Chen K., Bandy D., Reiman E.M. Thermosensory activation of insular cortex. Nat Neurosci. 2000;3:184–190. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- 96.Peltz E., Seifert F., DeCol R., Dörfler A., Schwab S., Maihöfner C. Functional connectivity of the human insular cortex during noxious and innocuous thermal stimulation. Neuroimage. 2011;54:1324–1335. doi: 10.1016/j.neuroimage.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 97.Greenspan J.D., Ohara S., Franaszczuk P., Veldhuijzen D.S., Lenz F.A. Cold stimuli evoke potentials that can be recorded directly from parasylvian cortex in humans. J Neurophysiol. 2008;100:2282–2286. doi: 10.1152/jn.90564.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Penfield W., Faulk M.E. The insula; further observations on its function. Brain. 1955;78:445–470. doi: 10.1093/brain/78.4.445. [DOI] [PubMed] [Google Scholar]
- 99.Mazzola L., Isnard J., Peyron R., Mauguière F. Stimulation of the human cortex and the experience of pain: Wilder Penfield's observations revisited. Brain. 2012;135:631–640. doi: 10.1093/brain/awr265. [DOI] [PubMed] [Google Scholar]
- 100.Birklein F., Rolke R., Müller-Forell W. Isolated insular infarction eliminates contralateral cold, cold pain, and pinprick perception. Neurology. 2005;65:1381. doi: 10.1212/01.wnl.0000181351.82772.b3. [DOI] [PubMed] [Google Scholar]
- 101.Veldhuijzen D.S., Greenspan J.D., Kim J.H., Lenz F.A. Altered pain and thermal sensation in subjects with isolated parietal and insular cortical lesions. Eur J Pain. 2010;14 doi: 10.1016/j.ejpain.2009.10.002. 535.e1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gogolla N., Takesian A.E., Feng G., Fagiolini M., Hensch T.K. Sensory integration in mouse insular cortex reflects GABA circuit maturation. Neuron. 2014;83:894–905. doi: 10.1016/j.neuron.2014.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Becerra L., Chang P.C., Bishop J., Borsook D. CNS activation maps in awake rats exposed to thermal stimuli to the dorsum of the hindpaw. Neuroimage. 2011;54:1355–1366. doi: 10.1016/j.neuroimage.2010.08.056. [DOI] [PubMed] [Google Scholar]
- 104.Beukema P., Cecil K.L., Peterson E., Mann V.R., Matsushita M., Takashima Y., Navlakha S., Barth A.L. TrpM8-mediated somatosensation in mouse neocortex. J Comp Neurol. 2018 doi: 10.1002/cne.24418. [DOI] [PMC free article] [PubMed] [Google Scholar]