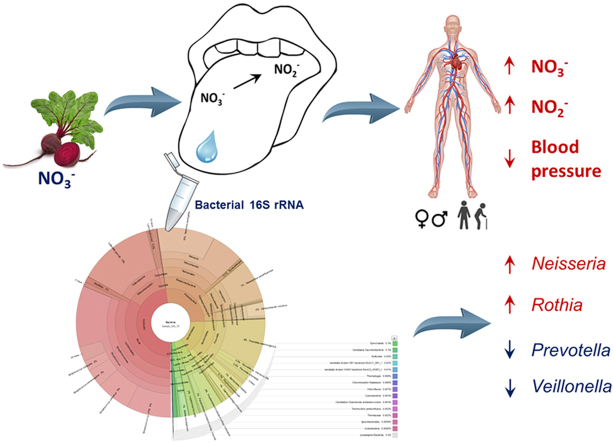
Abstract
Imbalances in the oral microbial community have been associated with reduced cardiovascular and metabolic health. A possible mechanism linking the oral microbiota to health is the nitrate (NO3-)-nitrite (NO2-)-nitric oxide (NO) pathway, which relies on oral bacteria to reduce NO3- to NO2-. NO (generated from both NO2- and L-arginine) regulates vascular endothelial function and therefore blood pressure (BP). By sequencing bacterial 16S rRNA genes we examined the relationships between the oral microbiome and physiological indices of NO bioavailability and possible changes in these variables following 10 days of NO3- (12 mmol/d) and placebo supplementation in young (18–22 yrs) and old (70–79 yrs) normotensive humans (n = 18). NO3- supplementation altered the salivary microbiome compared to placebo by increasing the relative abundance of Proteobacteria (+225%) and decreasing the relative abundance of Bacteroidetes (−46%; P < 0.05). After NO3-supplementation the relative abundances of Rothia (+127%) and Neisseria (+351%) were greater, and Prevotella (−60%) and Veillonella (−65%) were lower than in the placebo condition (all P < 0.05). NO3- supplementation increased plasma concentration of NO2- and reduced systemic blood pressure in old (70–79 yrs), but not young (18–22 yrs), participants. High abundances of Rothia and Neisseria and low abundances of Prevotella and Veillonella were correlated with greater increases in plasma [NO2-] in response to NO3- supplementation. The current findings indicate that the oral microbiome is malleable to change with increased dietary intake of inorganic NO3-, and that diet-induced changes in the oral microbial community are related to indices of NO homeostasis and vascular health in vivo.
Keywords: Ageing, Cardiovascular health, Oral nitrate reduction, Nitrite, 16S rRNA sequencing, Host-microbe symbiosis, Prebiotic
Graphical abstract
Highlights
-
•
Dietary nitrate alters the salivary microbiome of healthy young and old adults.
-
•
Nitrate supplementation decreases relative abundances of Prevotella and Veillonella.
-
•
Nitrate supplementation increases relative abundances of Rothia and Neisseria.
-
•
The salivary microbiome modulates nitric oxide bioavailability and blood pressure.
1. Introduction
Inorganic nitrate (NO3-) is a natural part of the human diet that is found in high concentrations in many vegetables. NO3- itself is biologically inert and human cells are believed to lack NO3--reductase capability. However, commensal bacteria in the oral cavity can use nitrate as a terminal electron acceptor for ATP synthesis, reducing NO3- to nitrite (NO2-; which can be vasoactive in low-oxygen and low-pH conditions) and this NO2- can be further reduced to the potent vasodilator, nitric oxide (NO) [9]. The NO3-- NO2--NO reduction pathway underpins the discovery that dietary NO3- supplementation through consumption of NO3- salts [27] or vegetable products such as beetroot juice [23], [40] reduces blood pressure (BP) in healthy young and old humans.
The importance of a functional oral microbiome for the NO3-- NO2--NO reduction pathway is highlighted in cases where use of antibacterial mouthwash markedly blunts the increase in plasma and saliva NO2- concentrations and associated decrease in BP following ingestion of a standardised NO3- dose [14], [22], [31]. Epidemiological studies also indicate that dysbiosis of the oral microbial community is associated with poor cardiovascular health [5]. Conversely, a diet rich in vegetables, which contain high concentrations of inorganic NO3-, significantly protects against both coronary heart disease and stroke [16], [2], [20]. Ageing has been associated with reduced salivary flow rate (‘dry mouth’) and altered oral bacterial colonisation [33], [42], but it is not known whether the abundances of NO3- reducing oral bacteria decline with age. Dietary NO3- intake, and the abundance of NO3--reducing oral bacteria, therefore represent routes to lower blood pressure and maintain and improve cardiovascular health across the human lifespan.
It is possible that dietary NO3- as a prebiotic treatment might promote proliferation of NO3- reducing bacteria. In a rodent model, a NO3--rich diet over 7 days increased abundance of oral bacteria (Streptococcus and Haemophilus) that contain NO3- reductase genes [17]. In saliva samples of hypercholesterolaemic humans, 6 weeks of NO3- supplementation with beetroot juice significantly increased the abundance of Neisseria flavescens and tended to increase Rothia mucilaginosa which are known NO3- reducers [39]. These studies indicate that increased dietary NO3- intake may alter the oral microbiome in a way which enhances an individual's ability to reduce ingested NO3-, resulting in greater plasma NO2- concentration and a greater reduction in systemic blood pressure. However, characterisation of potential changes in the oral microbiome of healthy young and old humans in response to NO3- supplementation is lacking.
In the present study, we used 16S rRNA gene sequencing to investigate whether abundances of NO3--reducing bacteria on the surface of the human tongue modulate an individual's response to NO3- supplementation in young (18–22 years) and old (70–79 years) normotensive adults. We hypothesised that at baseline, abundances of known NO3--reducing bacteria (including Neisseria, Prevotella, Rothia, Veillonella and Actinomycetales) would be greater in young compared to old participants, and that high abundances of these bacteria at baseline would be associated with higher plasma NO2- concentrations, and greater changes in blood pressure and arterial stiffness in response to NO3- supplementation. We also investigated whether 10 days of regular dietary NO3- ingestion altered the oral microbiome compared with placebo supplementation. It was hypothesised that oral microbiomes would be different between placebo and NO3- conditions, and specifically that the relative abundances of bacteria capable of NO3- reduction would be greater after NO3- compared to placebo supplementation.
2. Methods
2.1. Ethical approval
The study was approved by the institutional Ethics Committee (Sport and Health Sciences, University of Exeter) and conducted in accordance with the code of the ethical principles of the World Medical Association (Declaration of Helsinki). All participants gave their written, informed consent before the commencement of the study, once the experimental procedures, associated risks, and potential benefits of participation had been explained.
2.2. Study participants
Nine old adults including six females (mean ± SD, age 75 ± 3 yrs, age range 70–79 yrs, height 162 ± 6 cm, body mass 61.8 ± 14.0 kg) and three males (age 73 ± 5 yrs, age range 70–78 yrs, height 172 ± 4 cm, body mass 77.7 ± 11.6 kg) and nine young adults including five females (age 20 ± 1 yrs, age range 19–22 yrs, height 168 ± 7 cm, body mass 67.9 ± 10.3 kg) and four males (age 20 ± 2 yrs, age range 18–22 yrs, height 180 ± 4 cm, body mass 73.4 ± 12.9 kg) volunteered to participate in this study (Table 1). All participants were of Caucasian ethnicity. The nine old adults represented a subsample of a larger cohort tested for a Dunhill Medical Trust funded project (R269/1112) from which the microbiome of the tongue and saliva were retrospectively analysed. Participants were screened prior to participation to ensure suitability for the study. All participants were ostensibly healthy and were not taking medication or dietary supplements. None of the participants were tobacco smokers and all reported having no oral diseases. Participants were instructed to arrive at the laboratory in a rested and fully hydrated state, at least 3 h postprandial, and to avoid strenuous physical exertion in the 24 h preceding each laboratory visit. Participants were also asked to refrain from caffeine and alcohol intake 6 and 24 h before each test, respectively. All tests were performed at approximately the same time of day ( ± 2 h) for each participant.
Table 1.
Participant characteristics, nitrate (NO3-) dose and plasma [NO2-] responsiveness to supplementation, and salivary flow rate questionnaire (SFR-Q) results. The young and old participants were similar in terms of body mass and BMI. The NO3- dose was similar in both groups but old participants had a greater increase than the young in plasma [NO2-] in response to supplementation. The young reported feeling more frequent symptoms of low salivary flow rate than the old participants.
| Sex | Age (yrs) | Body mass (kg) | BMI (kg/m2) | NO3- dose (mmol/kg/d) | Δ[NO2-]/NO3- dose (nM/mmol/kg/d) | SFR-Q mean score | |
|---|---|---|---|---|---|---|---|
| OLD | |||||||
| 1 | F | 77 | 88.0 | 33.1 | 0.14 | 7543 | 1.2 |
| 2 | F | 79 | 66.2 | 22.1 | 0.19 | 2325 | 1.3 |
| 3 | F | 70 | 60.0 | 24.3 | 0.21 | 2962 | 1.7 |
| 4 | F | 76 | 53.1 | 20.7 | 0.23 | 3942 | 1.1 |
| 5 | F | 72 | 51.4 | 20.1 | 0.24 | 4819 | 1.7 |
| 6 | F | 74 | 52.3 | 20.2 | 0.24 | 5428 | 1.9 |
| 7 | M | 78 | 88.8 | 31.1 | 0.14 | 812 | 2.0 |
| 8 | M | 70 | 78.6 | 25.4 | 0.16 | 5461 | 1.0 |
| 9 | M | 70 | 65.6 | 22.7 | 0.19 | 4829 | 1.5 |
| Mean | 74.0 | 67.1 | 24.4 | 0.19 | 4236 | 1.4 | |
| SD | 3.6 | 14.8 | 4.7 | 0.04 | 1988 | 0.4 | |
| YOUNG | |||||||
| 10 | F | 22 | 71.5 | 29.4 | 0.17 | 803 | 1.9 |
| 11 | F | 19 | 60.8 | 22.9 | 0.20 | 2202 | 2.5 |
| 12 | F | 19 | 64.5 | 22.6 | 0.19 | 880 | 1.6 |
| 13 | F | 20 | 69.7 | 24.4 | 0.18 | 1604 | 2.7 |
| 14 | F | 19 | 85.2 | 28.5 | 0.15 | 999 | 1.5 |
| 15 | M | 18 | 55.7 | 18.0 | 0.22 | 2371 | 1.5 |
| 16 | M | 22 | 81.2 | 23.7 | 0.15 | 3254 | 1.6 |
| 17 | M | 19 | 72.3 | 22.3 | 0.17 | 1127 | 2.1 |
| 18 | M | 19 | 84.4 | 26.0 | 0.15 | 70 | 2.1 |
| Mean | 19.7* | 71.7 | 24.2 | 0.18 | 1479* | 2.0* | |
| SD | 1.4 | 10.4 | 3.5 | 0.03 | 977 | 0.4 | |
| OVERALL (OLD + YOUNG) | |||||||
| Mean | 46.8 | 69.4 | 24.3 | 0.18 | 2857 | 1.7 | |
| SD | 28.1 | 12.6 | 4.0 | 0.03 | 2079 | 0.5 | |
F, female; M, male; BMI, body mass index; Δ[NO2-]/NO3- dose, change in plasma [NO2-] relative to dose of NO3- ingested per kg body mass; SFR-Q, salivary flow rate questionnaire.
Different from old, P < 0.05.
2.3. Experimental design
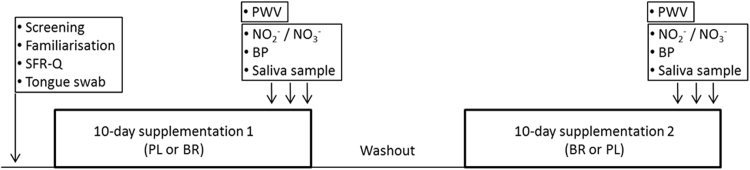
Prior to commencing dietary supplementation, participants visited the laboratory for health screening and familiarisation to test protocols. Participants completed a salivary flow rate questionnaire (SFR-Q; [11]). The SFR-Q included eleven questions which asked the participant to rate the frequency of various symptoms of low salivary flow rate on a scale of 1 (‘never’) to 5 (‘very often’). Participants then underwent two 10-day dietary supplementation periods with NO3- and placebo in a randomised, double-blind, cross-over design (Fig. 1). On days 8, 9 and 10 of each supplementation period, participants returned to the laboratory. Upon arrival at the laboratory on days 8, 9 and 10 venous blood samples were collected for the measurement of plasma [NO2-] and [NO3-] and resting BP was measured. The mean values of three measurements of [NO2-], [NO3-] and BP were used for further analyses. Saliva samples were also collected on each visit and these three samples were pooled for analysis of the salivary microbiome. Arterial stiffness was assessed on one occasion (day 8, 9 or 10) using radial-femoral pulse wave velocity (PWV).
Fig. 1.
Participants underwent 10-day supplementation periods with nitrate (~ 12.4 mmol/d) and placebo in a balanced cross-over design. Screening, protocol familiarisation and Salivary Flow Rate Questionnaires (SFR-Q) were completed at baseline. Measurements of plasma nitrite (NO2-) and nitrate (NO3-) concentrations, blood pressure (BP) of the brachial artery, arterial stiffness as carotid-femoral pulse wave velocity (PWV), and the collection of saliva samples for microbiome analysis were undertaken on days 8, 9 and 10 of each supplementation period.
2.4. Supplementation
The supplements were NO3- -rich concentrated beetroot juice (BR) (2 × 70 ml d−1, each 70 ml containing ~ 6.2 mmol NO3-; Beet It, James White Drinks, Ipswich, UK) and NO3- -depleted concentrated beetroot juice placebo (PL) (2 × 70 ml d−1, each 70 ml containing ~ 0.01 mmol NO3-; Beet It, James White Drinks, Ipswich, UK). The PL was indistinguishable from the BR supplement in appearance, taste and smell. Participants were instructed to consume one 70-ml beverage in the morning and one in the afternoon. On testing days, participants were asked to ingest one 70-ml beverage in the morning and one 2.5 h prior to their laboratory visit. A washout period of at least three days and up to 47 days (18 ± 14 days) separated the supplementation periods. Participants were instructed to maintain their normal daily activities, food intake and oral hygiene regime throughout the study. However, participants were instructed to refrain from using antibacterial mouthwash during the study period. Participants were advised that supplementation may cause beeturia (red urine) and red stools temporarily, but that such side effects were harmless.
2.5. Oral bacteria
Oral swabs of the tongue dorsum were collected at baseline. Saliva samples (~ 1 ml) were collected by expectoration, without stimulation, over a period of 5 min on three occasions following PL and BR supplementation periods. Oral swab and saliva samples were stored at − 80 °C until analysis. Genomic DNA was isolated from tongue swabs using a Gentra Puregene Buccal Cell Kit (Qiagen, Germantown, MD), and from saliva samples following the methods of Goode et al. [13]. Double-stranded DNA concentration was fluorometrically quantified (Qubit 3.0 high-sensitivity fluorescence detection, ThermoFisher Scientific, Waltham, MA). Library preparation employed a NEXTflex 16 S V1-V3 Amplicon-Seq Kit (Bioo Scientific, Austin, USA). The 16 S V1-V3 rDNA region was amplified using 5 ng of dsDNA and subjected to 8 thermal cycles of 30 s at 98°, 30 s at 60° and 30 s at 72° with primers A and B (Table S1). Following AMPure® XP bead cleanup (Becton Dickinson, Franklin Lakes, NJ), a subsequent PCR with indexing primers to identify individual samples, containing Illumina flow cell binding sites, was performed.
The samples were sequenced using paired-end 300 base pair (bp) MiSeq Illumina platform (Illumina, San Diego, CA) using v3 MiSeq reagents. For each sample, the nucleotide sequence data in FASTQ format was trimmed using Trim-Galore! (Krueger F. Trim-Galore!, accessible at http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/). Quality trimming was performed by removing low-quality bases from the 3’ read ends. The adapter sequences were subsequently removed from the 3’ end (the first 13 base pairs). Trim-Galore! paired-end validation was performed to remove short sequences once the trimming was complete, where the minimum specified length was 20 bp. The bacterial taxonomies and abundance were assigned using Kraken standard build, which uses the genomes in Refseq and NCBI taxonomic information (Kraken manual, accessible at: http://ccb.jhu.edu/software/kraken/MANUAL.html#kraken-databases). The paired read sequences were classified and processed by the Kraken Taxonomic Sequence Classification System [41]. Variations in the V1-V3 regions enabled NCBI taxonomic identification, and kraken-translate was used to translate the NCBI Identifiers to taxonomy identifiers. A Kraken report was generated for each sample, which was visualised using Krona bioinformatics pie charts [32].
2.6. Plasma [NO3-] and [NO2-]
Blood samples for determination of plasma [NO2-] and [NO3-] were collected from an antecubital vein into lithium heparin tubes and centrifuged for 8 min at 3000g and 4 °C within 2 min of collection. Plasma was extracted and samples stored at − 80 °C for later determination of [NO3-] and [NO2-] using a modified chemiluminescence technique as previously described [23].
2.7. Blood pressure and arterial stiffness
Blood pressure of the brachial artery was measured following 10 min of seated rest in a quiet room using an automated sphygmomanometer (Dinamap Pro, GE Medical Systems, Tampa, USA). A total of four measurements were taken, with the mean of the final three measurements recorded. The mean of the systolic (SBP), diastolic (DBP) and mean arterial pressure (MAP) measurements made over three laboratory visits in each condition were calculated for each individual and used for subsequent analyses. Arterial stiffness was estimated via pulse-wave velocity (PWV) (Complior SP; Alam Medical, Vincennes, Paris, France). Electrodes were placed on the carotid, femoral, and radial arteries, and the pulse transit time was calculated and recorded. A mean of three measurements was calculated and used for subsequent analyses. The position of each electrode was measured in relation to the nearest bony landmark to enable precise reproduction of the position of the electrodes in each condition.
2.8. Statistical analyses
The Kraken raw data output of phylogenetic data were analysed using R-script (R Development Core Team 2008), SPSS V20 and Microsoft Excel. Non-metric multidimensional scaling (NMDS) was used to assess the level of microbiome similarity between young and old, and PL and BR conditions using non-parametric relationships, and analysed using ADONIS (Vegan R Software). Differences between PL and BR conditions were assessed using paired t-tests with Bonferroni-Hoechberg correction on bacteria that made up > 0.01% of bacteria (R statistical software). The Shannon-Wiener diversity index (H’) was used to explore differences in diversity (Vegan R Software). Paired-samples t-tests were used to assess differences between BR and PL conditions in plasma [NO2-] and [NO3-], BP and arterial stiffness. Relationships between plasma NO biomarkers, oral microbiome and physiological responses to supplementation were assessed using Pearson's correlation coefficients. Statistical significance was accepted when P < 0.05 and statistical trend was defined as P < 0.10. Data were expressed as mean ± SD.
3. Results
The young and old participants were similar in terms of body mass and BMI (Table 1). The young participants had a greater mean score in SFR-Q than the old participants (Table 1), indicative of more frequent self-reported symptoms of low salivary flow rate. The young participants reported greater frequency of sensations associated with dry mouth (old 1.6 ± 1.0, young 2.7 ± 0.5; P < 0.05) and having difficulty eating dry foods (old 1.1 ± 0.3, young 1.8 ± 0.4; P < 0.05).
3.1. NO biomarkers, blood pressure and arterial stiffness
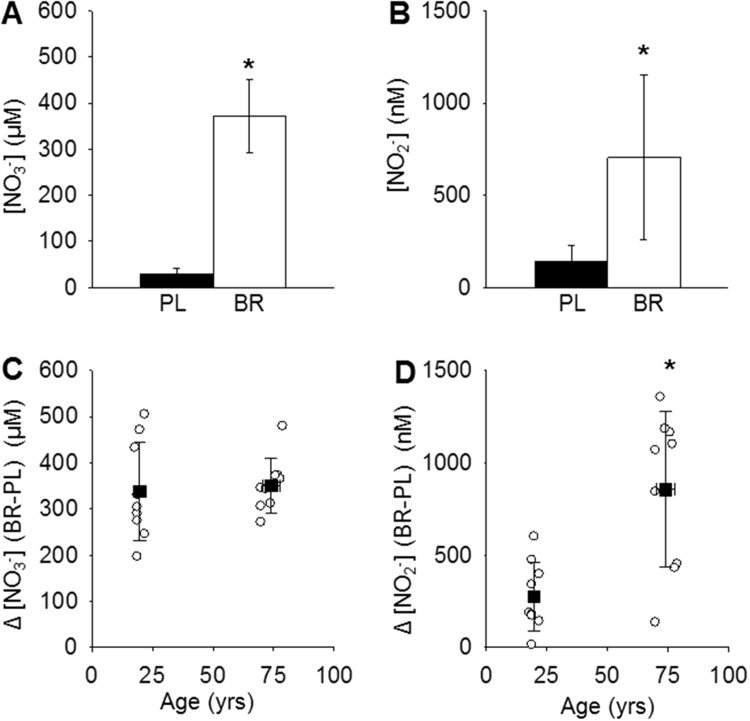
The NO3- dose relative to body mass was not different between young and old participants, but the latter had a greater increase in plasma [NO2-] in response to BR supplementation (Table 1). Plasma [NO3-] and [NO2-] were significantly higher following BR supplementation compared with PL (all P < 0.05; Fig. 2, panels A and B). BR supplementation increased plasma [NO3-] relative to PL by a similar amount in old (1509 ± 744%) and young participants (1481 ± 909%) (Fig. 2C), but the increase in [NO2-] was greater in old (648 ± 477%) compared to young participants (365 ± 249%, P < 0.05; Fig. 2D). There were no differences between young and old participants in plasma [NO3-] in PL (old: 28 ± 12 μM, young: 28 ± 14 μM; P > 0.05) or BR conditions (old: 379 ± 55 μM, young: 366 ± 101 μM; P > 0.05). Plasma [NO2-] tended to be greater in the old in PL (old: 173 ± 97 nM, young: 104 ± 63 nM; P = 0.09) and was significantly greater in the old than young participants in the BR condition (old: 1029 ± 393 nM, young: 380 ± 175 nM; P < 0.05).
Fig. 2.
Plasma [NO3-] (panel A) and [NO2-] (panel B) were significantly greater after nitrate supplementation (white bars) compared to placebo (black bars) (n = 18). The change (Δ) in plasma [NO3-] between nitrate and placebo conditions was similar in young (n = 9) and old participants (n = 9) (panel C), but Δ[NO2-] was significantly greater in the old compared to young participants (panel D). Error bars indicate standard deviations and black squares (panels C and D) indicate means for young and old participants. *P < 0.05.
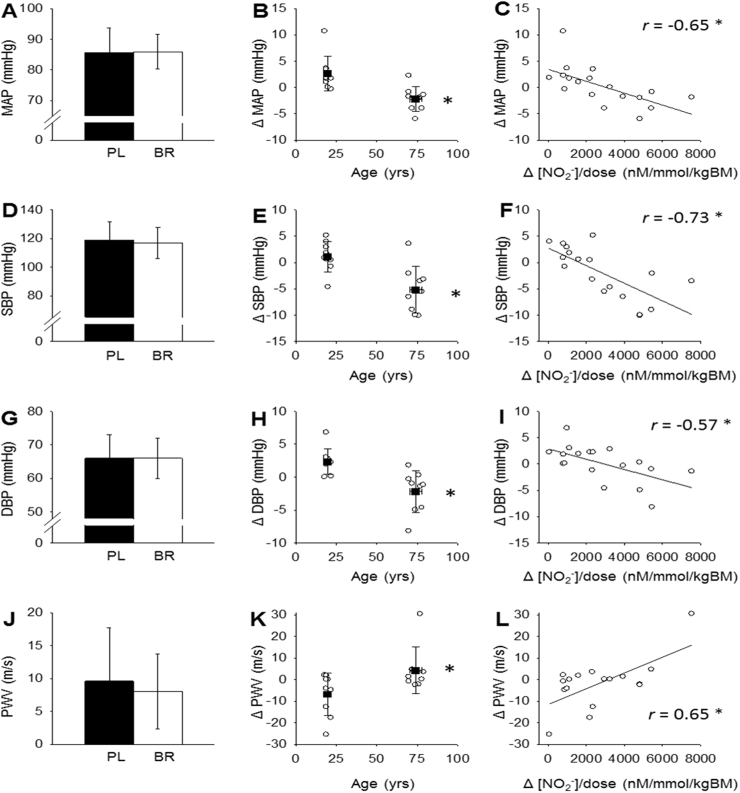
MAP, SPB, DBP and PWV were not different between PL and BR conditions across all participants (n = 18, P > 0.05; Fig. 3, panels A, D, G and J). In old participants (n = 9), SBP and MAP were significantly lower following BR supplementation compared with PL (Fig. 3, panels B and E). Changes between PL and BR (Δ) in MAP, SBP, and DBP between PL and BR conditions inversely correlated with the change in plasma [NO2-] relative to NO3- dose per kg body mass (Δ[NO2-]/NO3 dose; Fig. 3, panels C, F and I). BR supplementation did not significantly alter radial-femoral PWV (Fig. 3, panels J and K) across all participants, but ΔPWV between BR and PL in the old participants (increase of 4.3 ± 10.9 m s−1; n = 9) was different (P < 0.05) from ΔPWV in the young participants (decrease of −6.8 ± 9.8 m s−1; n = 9). ΔPWV positively correlated with Δ[NO2-]/NO3 dose (Fig. 3L). Absolute plasma [NO2-] or [NO3-] measured in the PL condition were not correlated with Δ[NO2-], ΔDBP, ΔSBP, ΔMAP or Δ PWV. One old male participant did not wish to undertake PWV measurement and therefore all PWV data are derived from 17 participants.
Fig. 3.
Mean arterial pressure (MAP; panel A), systolic blood pressure (SBP; panel D), diastolic blood pressure (DBP; panel G) and pulse wave velocity (PWV; panel J) were not different between placebo and nitrate conditions across all participants (n = 18). The old participants (n = 9) showed greater reductions (Δ) in MAP, SBP and DBP between placebo and nitrate conditions than the young participants (n = 9; panels B, E and H), as well as a greater increase in PWV (young n = 9, old n = 8; panel K). ΔMAP, ΔSBP and ΔDBP inversely correlated with the change in plasma [NO2-] relative to nitrate dose (Δ[NO2-]/NO3- dose) (panels C, F and I) and ΔPWV positively correlated with Δ[NO2-]/NO3- dose (panel L). *P < 0.05.
3.2. Tongue microbiome at baseline
Relative abundances of the five main oral bacterial phyla in tongue swab samples collected at baseline were Bacteroidetes 32 ± 9%, Fusobacteriales 27 ± 11%, Proteobacteria 20 ± 7%, Firmicutes 17 ± 5% and Actinobacteria 1 ± 1%. The most abundant bacterial species found on the tongue were Fusobacterium nucleatum subsp. nucleatum (16 ± 8%), Prevotella melaninogenica (14 ± 7%), Campylobacter concisus (13 ± 8%), Leptorichia buccalis, (7 ± 6%) Veillonella parvula (5 ± 3%), Prevotella intermedia (4 ± 2%), Fusobacterium nucleatum subsp. vincentii (3 ± 3%) and Neisseria meningitidis (3 ± 3%). Correlations between relative abundances of selected taxonomic units of tongue bacteria and physiological responses to BR supplementation are shown in Table 2. The greatest decreases in BP were associated with high abundances at baseline of Fusobacterium nucleatum subsp. vincentii and nucleatum, and order Actinomycetales, whereas a high relative abundance of Prevotella melaninogenica was associated with a greater mean score in SFR-Q, and smaller changes in plasma [NO2-], SBP and PWV in response to BR supplementation (Table 2).
Table 2.
Correlation coefficients for relationships between selected taxonomic units of the tongue microbiome (% of total bacteria) at baseline and subsequent changes between placebo and NO3- supplementation in plasma [NO2-], blood pressure and arterial stiffness. PWV data were not available for one old male participant, such that ΔPWV correlations are for n = 17.
| Δ[NO2-]/NO3- dose (nM/mmol/kg/D) | ΔDBP (mmHg) | ΔSBP (mmHg) | ΔMAP (mmHg) | ΔPWV (m/s) | SFR-Q mean score | ||
|---|---|---|---|---|---|---|---|
| Δ[NO2-]/NO3- dose | − 0.57* | − 0.73** | − 0.65** | 0.65** | − 0.52* | ||
| Actinobacteria | Actinomycetales (order) | 0.40 | − 0.39 | − 0.46# | − 0.46# | 0.47# | − 0.004 |
| Micrococcales (order) | − 0.41 | 0.25 | 0.36 | 0.40 | − 0.21 | − 0.04 | |
| Rothia (genus) | − 0.20 | 0.10 | 0.32 | 0.37 | − 0.07 | 0.20 | |
| Rothia mucilaginosa | − 0.22 | 0.17 | 0.38 | 0.44# | − 0.08 | 0.18 | |
| Proteobacteria | Neisseria (genus) | 0.21 | − 0.06 | 0.06 | 0.29 | 0.59* | − 0.39 |
| Neisseria meningitidis | − 0.09 | − 0.08 | 0.044 | 0.36 | 0.13 | − 0.19 | |
| Campylobacter concisus | − 0.55* | 0.43# | 0.65** | 0.34 | − 0.62** | 0.30 | |
| Bacteroidetes | Prevotella (genus) | − 0.49* | 0.19 | 0.43# | 0.41# | − 0.52* | 0.51* |
| Prevotella melaninogenica | − 0.57* | 0.16 | 0.53* | 0.37 | − 0.68** | 0.49* | |
| Firmicutes | Veillonella (genus) | − 0.22 | 0.22 | 0.35 | 0.09 | − 0.02 | 0.46# |
| Veillonella parvula | − 0.19 | 0.22 | 0.35 | 0.10 | 0.02 | 0.41# | |
| Fusobacteria | Fusobacterium nucleatum subsp. nucleatum | 0.44# | − 0.17 | − 0.56* | − 0.30 | 0.32 | − 0.29 |
| Fusobacterium nucleatum subsp. vincentii | 0.55* | − 0.43# | − 0.60** | − 0.63** | 0.45# | − 0.36 |
Δ = change between placebo and NO3-; [NO2-]/NO3- dose = plasma [nitrite] relative to nitrate dose per kg body mass ingested; DBP=diastolic blood pressure; SBP= systolic blood pressure; MAP= mean arterial pressure; PWV= pulse wave velocity; SFR-Q= salivary flow rate questionnaire.
P < 0.01.
P < 0.05.
P < 0.10.
3.3. Saliva microbiome after PL and BR supplementation
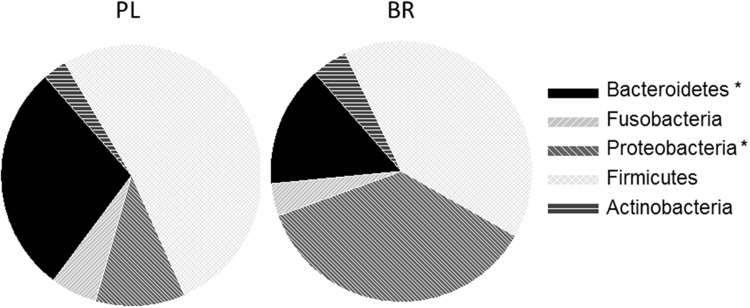
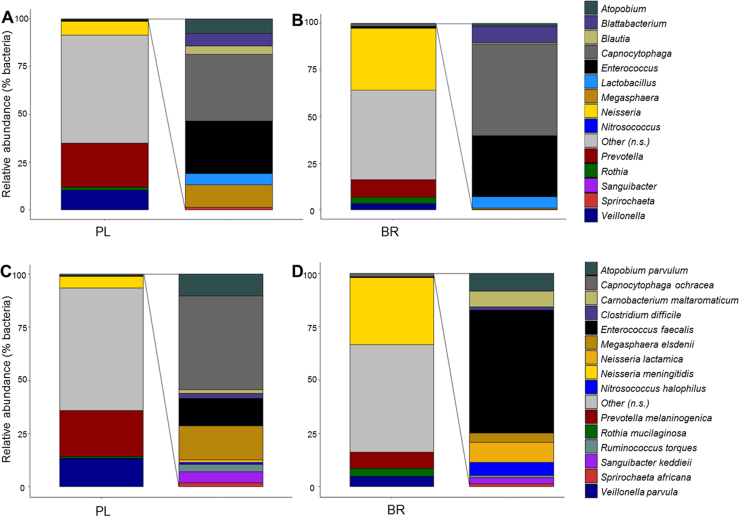
Relative abundances of the main phyla of oral bacteria differed between PL and BR conditions (Fig. 4). Relative abundance of Proteobacteria was greater and Bacteroidetes was lower following BR compared with PL (P < 0.05), while abundances of Firmicutes and Fusobacteria tended to be lower following BR supplementation compared with PL (P < 0.10). NMDS plots revealed that oral microbial communities differed significantly between PL and BR supplemented conditions (Fig. 5A), but there were no differences between young and old participants (Fig. 5B). A Shannon diversity index revealed no significant differences in species diversity between PL and BR conditions (Fig. 6). Overall, 52 taxonomic units were significantly different between BR and PL conditions. Fig. 7 illustrates statistically significant differences at genera and species levels between PL and BR. The trend for reduction in the relative abundance of Firmicutes following BR was primarily due to a decrease in Veillonella (− 65%), including a 65% decrease in Veillonella parvula species (both P < 0.05), while the order Lactobacilliales and genus Streptococcus were not affected by BR (P > 0.05). Within the phylum Bacteroidetes, BR resulted in a reduction in the genus Prevotella (− 60%), and specifically P. melaninogenica (− 67%) compared to PL (both P < 0.05). The increase in Proteobacteria after BR stemmed from an increase in the order Neisseriales (+348%), containing the genus Neisseria (+351%) and N. meningitidis (+439%) (all P < 0.05), while there were no statistically significant changes in the genera Campylobacter or Haemophilus. Proportions of the phylum Actinobacteria were not significantly different between PL and BR, but there was an increase in the genus Rothia (+127%, P < 0.05) and R. mucilaginosa (+234%, P < 0.05) after BR supplementation relative to PL. There was insufficient microbial DNA in a saliva sample of one young male participant and therefore the saliva microbiome data were for 17 participants.
Fig. 4.
The proportions of five main phyla of oral bacteria identified in the saliva samples following 10 days of placebo (PL) and NO3- supplementation (BR). *Difference between PL and BR (P < 0.05).
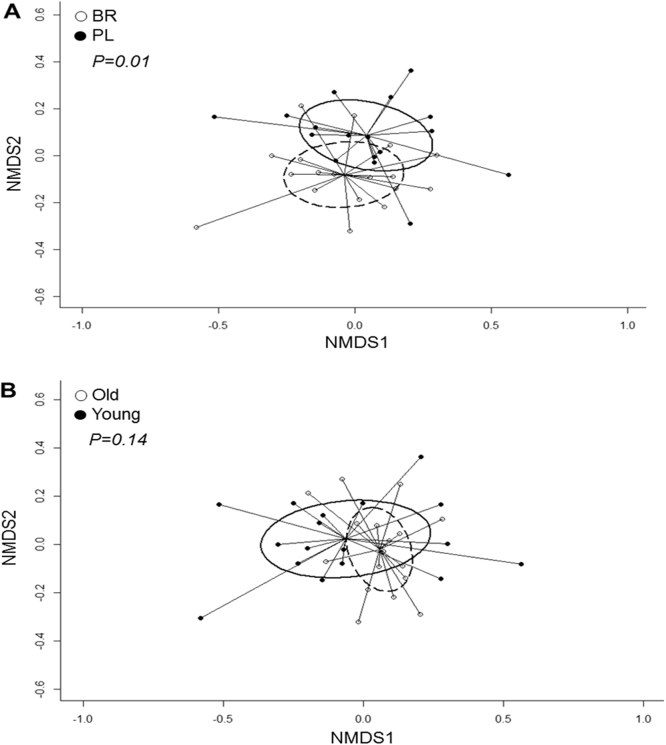
Fig. 5.
Overall salivary microbiome composition illustrated by non-metric multidimensional scaling (NMDS) analysis. The salivary microbiome composition was different between nitrate (BR) and placebo (PL) conditions (P < 0.05; panel A) but not between young and old participants (P > 0.05; panel B).
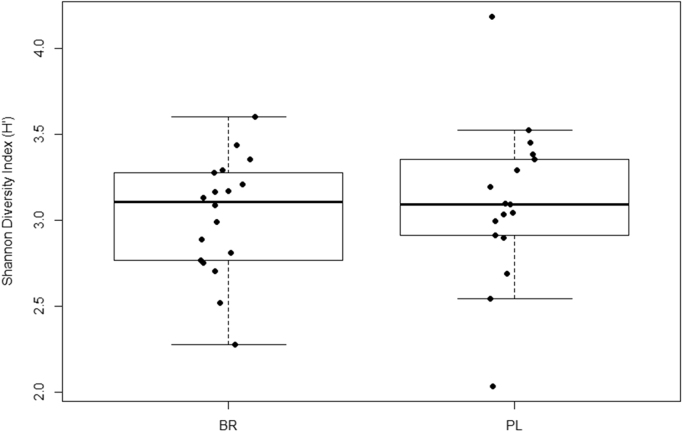
Fig. 6.
The Shannon diversity index indicated no statistically significant difference in species diversity between nitrate (BR) and placebo (PL) conditions.
Fig. 7.
The genera (panels A and B) and species (panels C and D) that comprised > 0.01% of all bacteria and showed significant differences (P < 0.05) between nitrate (BR) and placebo (PL) conditions.
Correlations across PL and BR conditions showed that plasma [NO3-] and [NO2-] positively correlated with relative abundances of Rothia and Neisseria and inversely correlated with Prevotella and Veillonella (Table 3). PWV positively correlated with relative abundance of Rothia and R. mucilaginosa (Table 3).
Table 3.
Correlation coefficients (r) for relationships between relative abundances of selected taxonomic units of saliva microbiome (% of total bacteria) and plasma nitrate ([NO3-]) and nitrite ([NO2-]); diastolic (DBP), systolic (SBP) and mean arterial (MAP) blood pressure; and pulse wave velocity (PWV) across placebo and nitrate conditions. Microbiome data were not available for one young male subject and PWV data were not available for one older male subject, such that [NO3-], [NO2-] and BP correlations are for n = 34 and PWV correlations are for n = 32.
| [NO3-] (μM) | [NO2-] (nM) | DBP (mmHg) | SBP (mmHg) | MAP (mmHg) | PWV (m/s) | ||
|---|---|---|---|---|---|---|---|
| Actinobacteria | Actinomycetales (order) | 0.25 | 0.09 | − 0.01 | − 0.09 | − 0.05 | 0.06 |
| Micrococcales (order) | 0.37* | 0.23 | − 0.03 | 0.02 | 0.00 | 0.48** | |
| Rothia (genus) | 0.45** | 0.30# | 0.04 | 0.03 | 0.04 | 0.45* | |
| Rothia mucilaginosa | 0.46** | 0.37* | 0.06 | − 0.03 | − 0.01 | 0.41* | |
| Proteobacteria | Neisseria (genus) | 0.61** | 0.64** | 0.02 | 0.15 | 0.14 | − 0.13 |
| Neisseria meningitidis | 0.54** | 0.53** | − 0.00 | 0.07 | 0.08 | − 0.23 | |
| Campylobacter concisus | − 0.26 | − 0.17 | − 0.17 | 0.08 | 0.02 | 0.42* | |
| Bacteroi-detes | Prevotella (genus) | − 0.47** | − 0.35* | 0.11 | 0.21 | 0.18 | − 0.18 |
| Prevotella melaninogenica | − 0.47** | − 0.35* | 0.06 | 0.17 | 0.12 | − 0.09 | |
| Firmicutes | Veillonella (genus) | − 0.62** | − 0.50** | − 0.10 | − 0.01 | − 0.22 | − 0.07 |
| Veillonella parvula | − 0.60** | − 0.49** | − 0.10 | − 0.03 | − 0.23 | − 0.08 | |
| Fuso-bacteria | Fusobacterium nucleatum subsp. nucleatum | − 0.28 | − 0.18 | 0.00 | 0.08 | 0.05 | − 0.10 |
| Fusobacterium nucleatum subsp. vincentii | − 0.16 | − 0.01 | − 0.03 | 0.12 | 0.07 | − 0.17 |
DBP=diastolic blood pressure; SBP= systolic blood pressure; MAP= mean arterial pressure; PWV= pulse wave velocity.
P < 0.01.
P < 0.05.
P < 0.10.
4. Discussion
We used an in vivo experimental model and bacterial 16S rRNA gene sequencing to examine relationships between the oral microbiome and physiological indices of NO bioavailability in humans and changes in these variables following NO3- supplementation. The principal finding of this study was that dietary NO3- supplementation altered the salivary microbiome in young (~ 20 yrs) and old (~ 74 yrs) normotensive humans, such that it increased relative abundances of some bacteria capable of NO3-reduction (Rothia and Neisseria) while reducing the abundances of other NO3- reducers (Prevotella and Veillonella). NO3- supplementation increased NO bioavailability in all participants, as indicated by plasma concentrations of NO3- and NO2-, and reduced systemic blood pressure in the old, but not young, participants. Across placebo and NO3- supplemented conditions, high abundances of Rothia and Neisseria and low abundances of Prevotella and Veillonella were associated with high NO bioavailability. The current findings indicate that the oral microbial community was malleable to change with increased dietary intake of inorganic NO3-, and, importantly, that the oral microbiome was related to indices of NO homeostasis and vascular health in vivo.
4.1. Relationships between the tongue microbiome at baseline and responsiveness to NO3- supplementation
It has been proposed that the oral microbiome modulates the magnitude of plasma [NO2-] increase, as well as changes in associated physiological indices, in response to NO3- supplementation [6], [17], [18], [25]. A recent study indicated that a composite relative abundance of seven species (including Prevotella melaninogenica, Veillonella parvula, and Rothia mucilaginosa) was positively correlated with the rise in salivary [NO2-], but not plasma [NO2-], in response to acute ingestion of a single NO3- bolus [7]. We found that individuals who had high proportions of P. melaninogenica and Campylobacter concisus at baseline were less responsive to chronic NO3- supplementation, i.e. had smaller increases in plasma [NO2-] and smaller or no reductions in BP, than those individuals who had low abundances of P. melaninogenica and C. concisus. In addition, a high mean SFR-Q score at baseline, which indicated more frequent self-reported symptoms of dry mouth, was associated with greater abundance of Prevotella and a smaller increase in plasma [NO2-] in response to NO3- supplementation. Campylobacter concisus is believed to express dissimilatory NO3- reduction to NH3 and its main physiological function is NO2- reduction [36], while P. melaninogenica has been shown to encode NO2-, but not NO3-, reductase genes [18]. It may, therefore, be speculated that during subsequent NO3- supplementation both C. concisus and P. melaninogenica, which were dominant species in tongue swab samples at baseline, acted as net consumers of NO2- in the oral cavity. In contrast, high abundances of Fusobacterium nucleatum subspecies and Actinomycetales at baseline were associated with greater increases in plasma [NO2-] and greater reductions in blood pressure in response to NO3- supplementation (Table 2). Actinomycetales are generally obligate anaerobes, and includes several species such as Actinomyces odontolyticus and Actinomyces naeslundii that have been identified as effective NO3- reducers [10]. Fusobacterium nucleatum can reduce NO2-, but does not possess NO3--reductase genes. However, F. nucleatum provides ‘scaffolding’ in biofilms, enabling microbial attachments [26], and it is possible that these bacteria may have facilitated attachment and proliferation of key NO3- reducing bacteria during dietary intervention.
4.2. Changes in the salivary microbiome after NO3- supplementation
We showed that, relative to placebo, NO3- supplementation altered the proportions of the main oral microbial phyla by decreasing the Bacteroidetes and increasing the Proteobacteria. At the genus level, NO3- supplementation significantly increased relative abundances of the previously identified NO3- reducers Neisseria [18] and Rothia [10] in saliva. This was consistent with a report of a significant increase in N. flavescens and a trend for an increase in R. mucilaginosa in saliva samples of hypercholesterolaemic patients after 6 weeks of NO3- supplementation [39]. A novel finding in the present study was that a high abundance of the facultative anaerobe R. mucilaginosa was associated with faster pulse wave velocity, indicative of lower arterial stiffness. These data suggest that high relative abundances of bacteria belonging to Neisseria and Rothia were related to high NO bioavailability and may promote vascular health.
We also found that compared to placebo, NO3- supplementation decreased relative abundances of the obligate anaerobic bacteria Prevotella and Veillonella in saliva. This finding appears to contradict studies that have used in vitro approaches to identify key oral NO3- reducing taxa. Hyde et al. [18] categorised biofilms prepared from tongue swab samples of six healthy humans as best, intermediate and worst NO3- reducers and found greater abundances of both Prevotella and Veillonella in the best versus worst NO3- reducing biofilms. Using tongue swab samples from ten healthy humans, which were incubated on solid medium under aerobic and anaerobic conditions and analysed by 16 S rDNA sequencing, Doel et al. [10] concluded that Veillonella were the most prevalent oral NO3- reducers and were major contributors to net NO2- production. Intricate metabolic interactions among the oral microbiota might mean that increased NO3- availability in the oral cavity may not facilitate the growth of all bacteria capable of NO3- reduction. It is not directly apparent why NO3- supplementation resulted in a decline in Veillonella. One factor that may have contributed to proliferation of some taxa and inhibition of others is the oral pH, which is a powerful modulator of the oral microbial community. Beetroot juice supplementation has been shown to increase oral pH from 7.0 to 7.5 [15], and notably, a pH of 8 is optimal for NO3- reductase activity [38]. Monitoring of salivary pH alongside alterations in the oral microbiome during NO3- supplementation should be undertaken in future studies to address the possible effects of pH.
Given that many oral bacteria with ability to reduce NO3- are also capable of downstream metabolism of the produced NO2-, it is important to differentiate between NO3-reducing bacteria in general and NO2- accumulating bacteria specifically. NO3- reducing oral bacteria have been identified in vitro from human samples, including Veillonella, Actinomyces, Rothia, Staphylococcus and Propionibacterium [10]. More recently Hyde et al. [18] added Neisseria, Haemophilus parainfluenzae, Prevotalla (including P. melaninogenica) and Granulicatella to the list of candidate species for most potent contributors to oral NO2- production. We showed that some of the oral bacteria that have been proposed as key NO2- accumulators on the basis of in vitro experiments, such as Veillonella and Prevotella, do not thrive under high NO3- availability in vivo. Indeed, we found no significant changes in relative abundances of Actinomyces, Staphylococcus, Propionibacterium, Granulicatella or Haemophilus after NO3- supplementation. In order to contribute significantly to the amount of NO2- that is swallowed from the oral cavity, the bacteria need to reduce NO3- at a faster rate than they reduce NO2-, or not undertake downstream metabolism of NO2- at all. Overall, to maximise NO bioavailability from a NO3- -rich diet, the composition of the oral microbial community needs to be such that it contains a greater quantity of net NO2- accumulators than net NO2- consumers. Further research is needed to establish whether the observed microbiome changes following chronic NO3- supplementation in the present cohort, are replicated in different populations and whether such changes are associated with an increased capacity for acute NO3- reduction in the oral cavity.
The present data suggest that the chronic (10-day) NO3- supplementation serves to change the relative abundance of a few, but not all, NO3- reducing taxa and that these changes are correlated with beneficial changes in NO bioavailability and indices of cardiovascular health. It should be noted that elevated NO bioavailability may have further beneficial effects on aspects of healthy ageing, including maintenance of a strong immune response. A recent study showed that tongue microbiomes that had high abundances of Prevotella and Veillonella species were associated with elevated risks of all-cause mortality and mortality from pneumonia in frail elderly nursing home residents [21]. NO3- supplementation in older people, which reduces the relative abundances of Prevotella and Veillonella, may therefore have potential to enhance the NO-mediated immune response in this high-risk population.
4.3. Differences in [NO2-] and blood pressure responses between young and old participants
Previous studies have shown appreciable inter-individual variability in the plasma [NO2-] and blood pressure responses to NO3- ingestion in both young and older populations (e.g. [8], [19], [23]). We found that despite ingesting the same dose of NO3- the old adults showed a greater plasma [NO2-] increase than the young adults, and also exhibited a reduction in blood pressure which was absent in the young participants. The magnitude of the BP response to NO3-supplementation is correlated with baseline blood pressure [22], [40], such that in the present study the scope for a BP decrease in the young adults, who had a BP of ~ 112/63 mmHg in placebo, was likely small. Although the balance of evidence indicates that NO3- represents an potent dietary means for reducing systemic BP [22], [25], [27], [40], [6], it is important to note that there are several studies that concur with the present finding of not showing a significant change in resting systolic or diastolic BP (or both) in young, healthy adults (e.g. [24], [28], [31], [37]).
Since the human vascular response to NO3- supplementation is dependent on efficient bacterial reduction of NO3- to NO2-, it is possible that at least some of this variability is linked to differences between individuals in the oral microbiota and therefore oral NO3- reducing capacity. NMDS analysis revealed no overall differences in the salivary microbiomes between young and old participants, or following placebo or NO3- supplementation. The greater responsiveness to supplementation in the old compared to young participants was surprising, given that ageing is typically associated with reduced salivary flow rate and altered oral bacterial colonisation [33]. It is important to note that although the self-reported frequency of dry mouth symptoms was greater in the young adults, we did not directly quantify salivary flow rate in this study, and agreement between self-reported xerostomia questionnaire data and measured saliva secretion rate varies [11], [29], [30]. We are therefore unable to determine whether the higher occurrence of self-reported symptoms of dry mouth in the young adults was in fact attributed to significantly lower salivary flow in comparison to the old adults. Whether possible differences in salivary flow rate and/or NO3- uptake into the enterosalivary circulation via sialin 2NO3-/H+ transporters [34] contribute to inter-individual variability in responsiveness to NO3- supplementation irrespective of age warrants further investigation with large cross-sectional cohorts across the human lifespan.
Physical exercise and diet are emerging as powerful modulators of the gut microbiota [1] and it is intuitive that the microbiota in the oral cavity, the uppermost section of the alimentary canal, may also vary according to age, diet, and with physical activity levels. There is evidence to suggest that the gut microbiome of healthy older people may be remarkably similar to that of young adults [4], and that age-related alterations in the gut microbiome may be related to advancing frailty and development of disease [3]. Whether the maintenance of a ‘young’ gut microbiome into older age is a cause or consequence of healthy ageing is unknown. Our data suggest that the oral microbiome of individuals who have reached their 8th decade of life without chronic disease is indistinguishable from oral microbiome of young adults. The inclusion criteria in the present study meant that the enrolled old participants were in exceptionally good health for their age and therefore not representative of an ageing population with poor cardiovascular and/or metabolic health, who may be less responsive to NO3- supplementation due to impairments in NO bioactivity [35]. Indeed, we found that there was no difference in blood pressure between young and old participants following NO3- supplementation. Longitudinal studies are necessary to identify possible changes that may occur in the oral microbiome, and NO bioavailability, at the onset of chronic disease.
The dietary interventions in the present study consisted of twice daily ingestion of NO3--rich and NO3--depleted beetroot juice concentrate that were matched for carbohydrate and polyphenol content [12]. It should be noted that the alterations we observed in the salivary microbiome following NO3--rich beetroot juice supplementation may be due to NO3- alone or an additive effect of NO3- and other nutritional components of the beetroot supplement. Elevated carbohydrate intake may have favoured the growth of those microbes that use carbohydrate as an energy substrate at the expense of proteolytic bacteria, such as Prevotella. Further studies using supplementation with salts of NO3- (KNO3, NaNO3) are warranted to ascertain the effect of NO3- alone on the oral microbiome.
4.4. Methodological considerations
Microbial analysis was performed on tongue swab samples at baseline and on saliva samples following placebo and NO3- supplementation periods. Saliva samples represent a composite of bacteria from all oral sites, while tongue swab samples target the tongue dorsum which was shown to have the highest NO3- reductase activity by Doel et al. [10]. The quantities and relative abundances of bacteria vary between different oral sites, such that potential changes in microbiome assessed from tongue swabs following NO3- supplementation may differ from those observed in the saliva samples in the present study. However, given the similarities of findings on Neisseria and Rothia between the present study and that of Velmurugan et al. [39], we are confident that the salivary microbiome analyses in the present study captured representative and meaningful differences between conditions in the NO3- reducing oral microbiome. A limitation of the current study was that saliva samples could not be included at the beginning of each supplementation period, and the wash-out period required for NO3- -induced changes in the oral microbiome to return to baseline following cessation of supplementation is not known. Future work should include in vivo tests of oral NO3- reduction capacity to ascertain whether the changes in oral microbiome following chronic NO3- supplementation are associated with enhanced oral NO3- reduction. In the present study, we inferred enhanced oral NO3- reduction from plasma [NO2-]. Finally, further investigation should target the optimisation of the dose and duration of prebiotic NO3- supplementation, and its possible interactions with the macronutrient content of the diet, to provide maximal functional effects of NO3- supplementation.
4.5. Conclusions
Imbalances in the oral microbial community and poor dental health have been associated with reduced cardiovascular and metabolic health. We showed that ageing, per se, in the absence of chronic disease, does not impair an individual's ability to reduce dietary NO3- and increase plasma [NO2-] in response to NO3- supplementation. Using 16S rRNA gene sequencing of oral bacteria in an in vivo experimental model, we showed that high abundances of oral bacteria belonging to genera Prevotella and Veillonella were likely detrimental, while high abundances of the genera Rothia and Neisseria were likely beneficial for the maintenance of NO homeostasis and associated indices of cardiovascular health. The symbiotic relationship between the oral microbiome and its human host is a fast evolving field of research with significant implications for development of prebiotic and probiotic interventions to improve cardiovascular and metabolic health. Our results identify dietary NO3- as a modulator of the oral NO2--producing microbiome in healthy humans and highlight the potential of oral microbiota-targeted therapies for ameliorating conditions related to low NO bioavailability.
Acknowledgement
The Exeter Sequencing Service and Computational Centre are core facilities at the University of Exeter. We are grateful for funding from a Medical Research Council Clinical Infrastructure award (MR/M008924/1), the Wellcome Trust Institutional Strategic Support Fund (WT097835MF), a Wellcome Trust Multi User Equipment Award (WT101650MA) and a BBSRC LOLA award (BB/K003240/1). Data collection on the nine older adults included in this study was supported by the Dunhill Medical Trust (R269/1112).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.freeradbiomed.2018.05.078.
Appendix A. Supplementary material
Supplementary material
References
- 1.Barton W., Penney N.C., Cronin O., Garcia-Perez I., Molloy M.G., Holmes E., Shanahan F., Cotter P.D., O'Sullivan O. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut. 2017 doi: 10.1136/gutjnl-2016-313627. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 2.Bondonno C.P., Blekkenhorst L.C., Prince R.L., Ivey K.L., Lewis J.R., Devine A., Woodman R.J., Lundberg J.O., Croft K.D., Thompson P.L., Hodgson J.M. Association of vegetable nitrate intake with carotid atherosclerosis and ischemic cerebrovascular disease in older women. Stroke. 2017;48:1724–1729. doi: 10.1161/STROKEAHA.117.016844. [DOI] [PubMed] [Google Scholar]
- 3.Biagi E., Nylund L., Candela M., Ostan R., Bucci L., Pini E., Nikkïla J., Monti D., Satokari R., Franceschi C., Brigidi P., De Vos W. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One. 2010;5(5):e10667. doi: 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bian G., Gloor G.B., Gong A., Jia C., Zhang W., Hu J., Zhang H., Zhang Y., Zhou Z., Zhang J., Burton J.P., Reid G., Xiao Y., Zeng Q., Yang K., Li J. The gut microbiota of healthy aged chinese is similar to that of the healthy young. mSphere. 2017;2(5) doi: 10.1128/mSphere.00327-17. (eCollection) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briskey D., Tucker P.S., Johnson D.W., Coombes J.S. Microbiota and the nitrogen cycle: Implications in the development and progression of CVD and CKD. Nitric Oxide. 2016;57:64–70. doi: 10.1016/j.niox.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Bryan N.S., Tribble G., Angelov N. Oral Microbiome and Nitric Oxide: the Missing Link in the Management of Blood Pressure. Curr. Hypertens. Rep. 2017;19(4):33. doi: 10.1007/s11906-017-0725-2. [DOI] [PubMed] [Google Scholar]
- 7.Burleigh M.C., Liddle L., Monaghan C., Muggeridge D.J., Sculthorpe N., Butcher J.P., Henriquez F.L., Allen J.D., Easton C. Salivary nitrite production is elevated in individuals with a higher abundance of oral nitrate-reducing bacteria. Free Radic. Biol. Med. 2018;120:80–88. doi: 10.1016/j.freeradbiomed.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 8.Casey D.P., Treichler D.P., Ganger C.T., 4th, Schneider A.C., Ueda K. Acute dietary nitrate supplementation enhances compensatory vasodilation during hypoxic exercise in older adults. J. Appl. Physiol. (1985) 2015;118:178–186. doi: 10.1152/japplphysiol.00662.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dejam A., Hunter C.J., Schechter A.N., Gladwin M.T. Emerging role of nitrite in human biology. Blood Cells Mol. Dis. 2004;32:423–429. doi: 10.1016/j.bcmd.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Doel J., Benjamin N., Hector M., Rogers M., Allaker R. Evaluation of bacterial nitrate reduction in the human oral cavity. Eur. J. Oral. Sci. 2005;113:14–19. doi: 10.1111/j.1600-0722.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- 11.Fox P.C., Busch K.A., Baum B.J. Subjective reports of xerostomia and objective measures of salivary gland performance. J. Am. Dent. Assoc. 1987;115:581–584. doi: 10.1016/s0002-8177(87)54012-0. [DOI] [PubMed] [Google Scholar]
- 12.Gilchrist M., Winyard P.G., Aizawa K., Anning C., Shore A., Benjamin N. Effect of dietary nitrate on blood pressure, endothelial function, and insulin sensitivity in type 2 diabetes. Free Radic. Biol. Med. 2013;60:89–97. doi: 10.1016/j.freeradbiomed.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 13.Goode M.R., Cheong S.Y., Li N., Ray W.C., Bartlett C.W. Collection and extraction of saliva DNA for next generation sequencing. J. Vis. Exp. 2014;(90):e51697. doi: 10.3791/51697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Govoni M., Jansson E.A., Weitzberg E., Lundberg J.O. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide. 2008;19:333–337. doi: 10.1016/j.niox.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Hohensinn B., Haselgrübler R., Müller U., Stadlbauer V., Lanzerstorfer P., Lirk G., Höglinger O., Weghuber J. Sustaining elevated levels of nitrite in the oral cavity through consumption of nitrate-rich beetroot juice in young healthy adults reduces salivary pH. Nitric Oxide. 2016;60:10–15. doi: 10.1016/j.niox.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Hung H.C., Joshipura K.J., Jiang R., Hu F.B., Hunter D., Smith-Warner S.A., Colditz G.A., Rosner B., Spiegelman D., Willett W.C. Fruit and vegetable intake and risk of major chronic disease. J. Natl. Cancer Inst. 2004;96:1577–1584. doi: 10.1093/jnci/djh296. [DOI] [PubMed] [Google Scholar]
- 17.Hyde E.R., Luk B., Cron S., Kusic L., McCue T., Bauch T., Kaplan H., Tribble G., Petrosino J.F., Bryan N.S. Characterization of the rat oral microbiome and the effects of dietary nitrate. Free Radic. Biol. Med. 2014;77:249–257. doi: 10.1016/j.freeradbiomed.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Hyde E.R., Andrade F., Vaksman Z., Parthasarathy K., Jiang H., Parthasarathy D.K., Torregrossa A.C., Tribble G., Kaplan H.B., Petrosino J.F., Bryan N.S. Metagenomic analysis of nitrate-reducing bacteria in the oral cavity: implications for nitric oxide homeostasis. PLoS One. 2014;9(3):e88645. doi: 10.1371/journal.pone.0088645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jajja A., Sutyarjoko A., Lara J., Rennie K., Brandt K., Qadir O., Siervo M. Beetroot supplementation lowers daily systolic blood pressure in older, overweight subjects. Nutr. Res. 2014;34:868–875. doi: 10.1016/j.nutres.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Joshipura K.J., Hung H.C., Li T.Y., Hu F.B., Rimm E.B., Stampfer M.J., Colditz G., Willett W.C. Intakes of fruits, vegetables and carbohydrate and the risk of CVD. Public Health Nutr. 2009;12:115–121. doi: 10.1017/S1368980008002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kageyama S., Takeshita T., Furuta M., Tomioka M., Asakawa M., Suma S., Takeuchi K., Shibata Y., Iwasa Y., Yamashita Y. Relationships of variations in the tongue microbiota and pneumonia mortality in nursing home residents. J. Gerontol. Biol. Sci. Med. Sci. 2017 doi: 10.1093/gerona/glx205. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 22.Kapil V., Haydar S.M., Pearl V., Lundberg J.O., Weitzberg E., Ahluwalia A. Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Radic. Biol. Med. 2013;55:93–100. doi: 10.1016/j.freeradbiomed.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly J., Fulford J., Vanhatalo A., Blackwell J.R., French O., Bailey S.J., Gilchrist M., Winyard P.G., Jones A.M. Effects of short-term dietary nitrate supplementation on blood pressure, O2 uptake kinetics, and muscle and cognitive function in older adults. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;304:R73–R83. doi: 10.1152/ajpregu.00406.2012. [DOI] [PubMed] [Google Scholar]
- 24.Kelly J., Vanhatalo A., Wilkerson D.P., Wylie L.J., Jones A.M. Effects of nitrate on the power-duration relationship for severe-intensity exercise. Med. Sci. Sports Exerc. 2013;45:1798–1806. doi: 10.1249/MSS.0b013e31828e885c. [DOI] [PubMed] [Google Scholar]
- 25.Koch C.D., Gladwin M.T., Freeman B.A., Lundberg J.O., Weitzberg E., Morris A. Enterosalivary nitrate metabolism and the microbiome: intersection of microbial metabolism, nitric oxide and diet in cardiac and pulmonary vascular health. Free Radic. Biol. Med. 2017;105:48–67. doi: 10.1016/j.freeradbiomed.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolenbrander P.E., Andersen R.N., Blehert D.S., Egland P.G., Foster J.S., Palmer R.J., Jr. Communication among oral bacteria. Microbiol. Mol. Biol. Rev. 2002;66:486–505. doi: 10.1128/MMBR.66.3.486-505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsen F.J., Ekblom B., Sahlin K., Lundberg J.O., Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N. Engl. J. Med. 2006;355:2792–2793. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 28.Larsen F.J., Weitzberg E., Lundberg J.O., Ekblom B. Dietary nitrate reduces maximal oxygen consumption while maintaining work performance in maximal exercise. Free Radic. Biol. Med. 2010;48:342–347. doi: 10.1016/j.freeradbiomed.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Lima D.L.F., Carneiro S.D.R.M., Barbosa F.T.S., Saintrain M.V.L., Moizan J.A.H., Doucet J. Salivary flow and xerostomia in older patients with type 2 diabetes mellitus. PLoS One. 2017;12(8):e0180891. doi: 10.1371/journal.pone.0180891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malicka B., Kaczmarek U., Skośkiewicz-Malinowska K. Prevalence of xerostomia and the salivary flow rate in diabetic patients. Adv. Clin. Exp. Med. 2014;23:225–233. doi: 10.17219/acem/37067. [DOI] [PubMed] [Google Scholar]
- 31.McDonagh S.T., Wylie L.J., Winyard P.G., Vanhatalo A., Jones A.M. The effects of chronic nitrate supplementation and the use of strong and weak antibacterial agents on plasma nitrite concentration and exercise blood pressure. Int. J. Sports Med. 2015;36:1177–1185. doi: 10.1055/s-0035-1554700. [DOI] [PubMed] [Google Scholar]
- 32.Ondov B.D., Bergman N.H., Phillippy A.M. Interactive metagenomic visualization in a Web browser. BMC Bioinform. 2011;12(1):385. doi: 10.1186/1471-2105-12-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Percival R.S., Challacombe S.J., Marsh P.D. Age-related microbiological changes in the salivary and plaque microflora of healthy adults. J. Med. Microbiol. 1991;35:5–11. doi: 10.1099/00222615-35-1-5. [DOI] [PubMed] [Google Scholar]
- 34.Qu X.M., Wu Z.F., Pang B.X., Jin L.Y., Qin L.Z., Wang S.L. From nitrate to nitric oxide: the role of salivary glands and oral bacteria. J. Dent. Res. 2016;95:1452–1456. doi: 10.1177/0022034516673019. [DOI] [PubMed] [Google Scholar]
- 35.Siervo M., Lara J., Jajja A., Sutyarjoko A., Ashor A.W., Brandt K., Qadir O., Mathers J.C., Benjamin N., Winyard P.G., Anning C., Shore A., Gilchrist M. Ageing modifies the effects of beetroot juice supplementation on 24-hour blood pressure variability: an individual participant meta-analysis. Nitric Oxide. 2015;47:97–105. doi: 10.1016/j.niox.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 36.Simon J., Klotz M.G. Diversity and evolution of bioenergetic systems involved in microbial nitrogen compound transformations. Biochim. Biophys. Acta. 2013;1827:114–135. doi: 10.1016/j.bbabio.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Vanhatalo A., Jones A.M., Blackwell J.R., Winyard P.G., Fulford J. Dietary nitrate accelerates postexercise muscle metabolic recovery and O2 delivery in hypoxia. J. Appl. Physiol. (1985) 2014;117:1460–1470. doi: 10.1152/japplphysiol.00096.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Maanen J.M., van Geel A.A., Kleinjans J.C. Modulation of nitrate-nitrite conversion in the oral cavity. Cancer Detect. Prev. 1996;20:590–596. [PubMed] [Google Scholar]
- 39.Velmurugan S., Gan J.M., Rathod K.S., Khambata R.S., Ghosh S.M., Hartley A., Van Eijl S., Sagi-Kiss V., Chowdhury T.A., Curtis M., Kuhnle G.G., Wade W.G., Ahluwalia A. Dietary nitrate improves vascular function in patients with hypercholesterolemia: a randomized, double-blind, placebo-controlled study. Am. J. Clin. Nutr. 2016;103:25–38. doi: 10.3945/ajcn.115.116244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Webb A.J., Patel N., Loukogeorgakis S., Okorie M., Aboud Z., Misra S., Rashid R., Miall P., Deanfield J., Benjamin N., MacAllister R., Hobbs A.J., Ahluwalia A. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wood D.E., Salzberg S.L. Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol. 2014;15:R46. doi: 10.1186/gb-2014-15-3-r46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu X., He J., Xue J., Wang Y., Li K., Zhang K., Guo Q., Liu X., Zhou Y., Cheng L., Li M., Li Y., Li Y., Shi W., Zhou X. Oral cavity contains distinct niches with dynamic microbial communities. Environ. Microbiol. 2015;17:699–710. doi: 10.1111/1462-2920.12502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material