Introduction
Erythema gyratum repens (EGR), considered one of the most specific cutaneous paraneoplastic phenomena, is characterized by a serpiginous morphology and a migrating scaly border. EGR is associated with malignancy, most commonly bronchial, esophageal, and breast, in more than 80% of cases.1, 2 Here we report 2 cases of patients with pityriasis rubra pilaris (PRP), whose eruption transformed to an EGR-like eruption without any evidence of malignancy.
Report of 2 cases
A 55-year-old man presented shortly after a diagnosis of biopsy-proven pityriasis rubra pilaris. Initially erythrodermic with palmoplantar keratoderma, the patient's condition improved on cyclosporine, but given disease persistence, other systemic medications, including acitretin, methotrexate, adalimumab, apremilast, and ustekinumab with acitretin were trialed, none providing significant improvement. The patient then started on ixekizumab, in addition to acitretin, with improvement. Eight months later, the patient presented with a dramatically altered eruption consistent with erythema gyratum repens (Fig 1, A). He reported having stopped his acitretin several months before the rash transformation. A thorough review of systems failed to disclose any history of fever, anorexia, weight loss, respiratory, or gastrointestinal symptoms. No lymphadenopathy was noted on physical examination. A complete blood count, complete metabolic panel, urinalysis, sedimentation rate, and chest radiograph were within normal limits.
Fig 1.
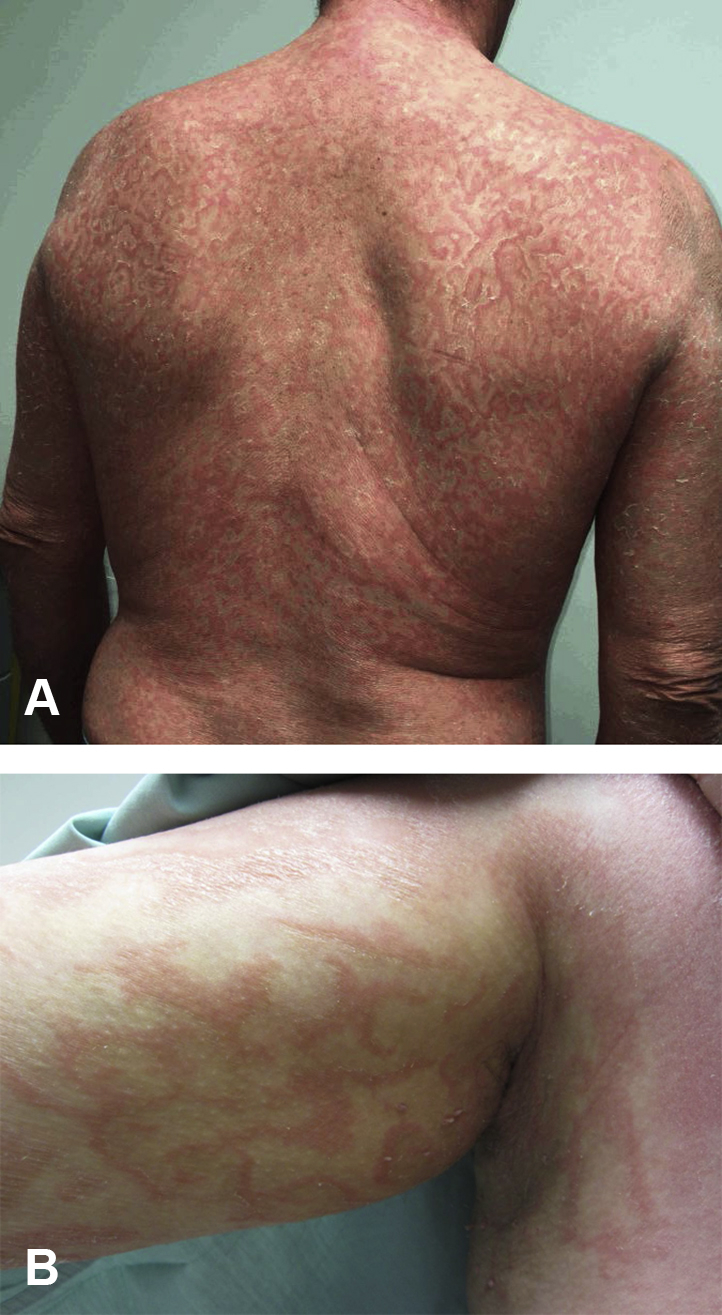
EGR-like eruption. Serpiginous erythematous plaques with fine scale trailing the leading edge, in a wood-grain polycyclic pattern on the back (A) and in the right axilla (B).
Similarly, a 50-year-old man initially presented to our clinic with a pruritic, erythematous scaly rash spreading in a cephalocaudal manner, with waxy keratoderma of the palms and soles. His biopsy was consistent with pityriasis rubra pilaris (Fig 2). Several months after acitretin initiation, rapid transformation to an EGR-like eruption occurred (Fig 1, B). In this patient as well, a malignancy workup (urinalysis, complete blood count, complete metabolic panel, stool guaiac) proved negative.
Fig 2.
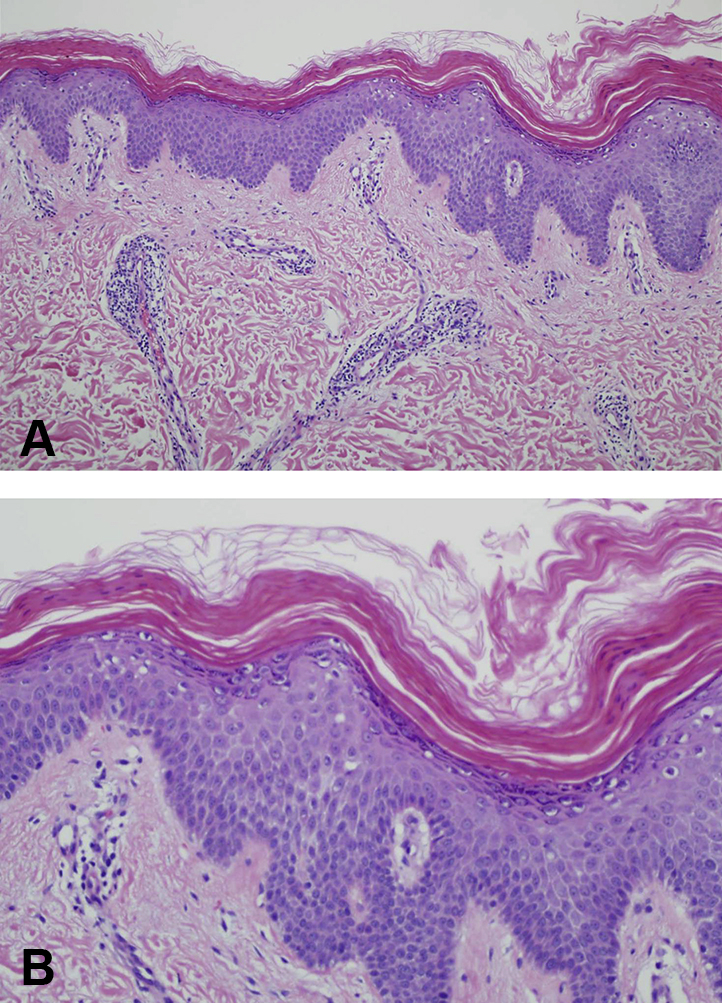
Histopathology of PRP. A, Perivascular infiltrate, predominantly lymphocytic in nature, with focal hypergranulosis and broad rete ridges. B, Alternating orthokeratosis and parakeratosis in both vertical and horizontal directions, with focal hypergranulosis.
Discussion
The transformation from PRP to an EGR-like eruption has been reported 8 times in the literature.1, 3, 4, 5, 6 In none of these cases was this transformation indicative of internal malignancy, despite thorough investigations in some cases.1 In all subjects, the eruption appeared during the resolving phase of PRP.1, 3, 4, 5, 6 What drives this transition remains unknown. Of our 2 patients reported above, one completely cleared the altered eruption, whereas the other still displays persistent EGR, now 8 months after initial transformation.
EGR affects men twice as much as women, with an average age of 63 years, whereas PRP has an equal male/female ratio and a bimodal age distribution.1, 2 Histologically, PRP is associated with hyperkeratosis, acanthosis, follicular plugging, and alternating parakeratosis and orthokeratosis, whereas EGR displays hyperkeratosis, parakeratosis, spongiosis, acanthosis, and a superficial perivascular inflammatory infiltrate.1 Of the patients with PRP to EGR-like transformation biopsied, the histology has been compatible with EGR.1, 2, 3
Among cases of reported nonparaneoplastic EGR, 8 cases were idiopathic, whereas 12 presented with a concomitant skin disease, including pityriasis rubra pilaris, psoriasis, ichthyosis, CREST (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia), rheumatoid arthritis, tuberculosis, bullous pemphigoid, linear IgA disease, and hypereosinophilic syndrome.7 Cases considered drug induced include a patient on azathioprine with type I autoimmune hepatitis and 2 patients with EGR triggered by interferon given for hepatitis C virus–related chronic hepatitis.7, 8
Interestingly, 4 reports of psoriasis vulgaris associated with EGR without malignancy highlight the fact that this transformation may not be limited to PRP.2 One young man presented with what was thought to be EGR-like psoriasis, as his repeated histology displayed features of abortive pustular psoriasis (therefore likely making his case an unusual clinical presentation of the primary dermatosis).2 One patient with resolving psoriasis had EGR after treatment with acitretin, whereas another presented with EGR after resolution of pustular psoriasis.2 The final patient was started on methotrexate for biopsy-proven plaque psoriasis; 8 weeks later, she had a different rash, the clinical and histologic features of which were consistent with EGR.2 A role of localized ground substance adaptive phenomenon may be a potential explanation for the transformation to an EGR-like eruption in diseases like PRP, bullous pemphigoid, psoriasis, and linear IgA disease.2
In reported cases of PRP to EGR-like transformation, all patients have been white, with the exception of one Afro-Caribbean man; all but 1 of the patients were men.1, 3, 4, 5, 6 As discussed above, EGR-like eruptions have been reported in immune-mediated conditions.1, 2 CARD14, a known activator of NF-κB signaling implicated in inflammatory disorders, is expressed in both familial PRP and familial psoriasis.9 Activation of NF-κB in mice leads to development of epidermal hyperplasia, hyperkeratosis, parakeratosis, hypogranulosis, T-cell infiltration, and microabscess formation, suggesting that CARD14 mutations lead to aberrant immunologic activation.9 The fact that tumor necrosis factor-α antagonists target this pathway may explain why these drugs are therapeutically beneficial in patients with PRP and why they potentially may trigger transformation to an EGR-like eruption, especially because response to these drugs is in part determined by polymorphisms in genes belonging to the NF-κB pathway.9 It is postulated that the underlying neoplasm in paraneoplastic EGR may trigger an immunologic mechanism.7
Retinoid treatment has also been proposed as playing a role in this transformation (presumably through alternation of keratinization), given treatment with acitretin (or etretinate) before transformation in many cases.1 This finding may be confounded by the fact that acitretin is a first-line treatment for PRP, which therefore makes it highly likely that many patients with PRP have been exposed to the medication at one point. In addition, the transformation has also occurred in association with use of a topical retinoid, methotrexate, infliximab, secukinumab, and now, ixekizumab.4, 5 One patient was treated with infliximab, followed by secukinumab, remaining on acitretin 25 to 50 mg/d throughout.6 Secukinumab was ceased after 9 months; however, as his PRP resolved, he had an EGR-like eruption.6 Five months later, the new eruption persisted (as in our patient), and he was continued on acitretin at a reduced dose of 10 mg/d.6
Conclusion
An eruption consistent with EGR does not necessarily indicate underlying malignancy. In the context of PRP, it appears the occurrence of this eruption could signal impending resolution of the primary dermatosis. Until more data become available, patients should undergo age-based cancer screening per national guidelines, with symptom-based malignancy evaluations only as clinically indicated.
Footnotes
Funding sources: none.
Conflicts of interest: None disclosed.
References
- 1.Almaani N., Robson A., Sarkany R., Griffiths W.A.D. Erythema gyratum repens associated with pityriasis rubra pilaris. Clin Exp Dermatol. 2011;36:161–164. doi: 10.1111/j.1365-2230.2010.03861.x. [DOI] [PubMed] [Google Scholar]
- 2.Singal A., Sonthalia S., Pandhi D. Erythema gyratum repens-like eruption occurring in resolving psoriasis during methotrexate therapy. Int J Dermatol. 2010;49:306–307. doi: 10.1111/j.1365-4632.2009.04256.x. [DOI] [PubMed] [Google Scholar]
- 3.Gebauer K., Singh G. Resolving pityriasis rubra pilaris resembling erythema gyratum repens. Arch Dermatol. 1993;129:917–918. [PubMed] [Google Scholar]
- 4.Cheesbrough M.J., Williamson D.M. Erythema gyratum repens, a stage in the resolution of pityriasis rubra pilaris? Clin Exp Dermatol. 1985;10:466–471. doi: 10.1111/j.1365-2230.1985.tb00606.x. [DOI] [PubMed] [Google Scholar]
- 5.Marchetti M., Greer K. Pityriasis rubra pilaris treated with methotrexate resolving with an erythema gyratum repens-like appearance. J Am Acad Dermatol. 2013;69:e32–e33. doi: 10.1016/j.jaad.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Davenport R., Le S.T., Gin A., Goh M.S., Foley P. Resolving classic pityriasis rubra pilaris, mimicker of erythema gyratum repens. Australas J Dermatol. 2017 doi: 10.1111/ajd.12752. [DOI] [PubMed] [Google Scholar]
- 7.Rongioletti F., Fausti V., Parodi A. Erythema gyratum repens is not an obligate paraneoplastic disease: a systematic review of the literature and personal experience. J Eur Acad Dermatol Venereol. 2014;28:112–115. doi: 10.1111/j.1468-3083.2012.04663.x. [DOI] [PubMed] [Google Scholar]
- 8.Günther R, Nasser S, Hinrichsen H, Fölsch UR. Erythema gyratum repens: drug reaction following azathioprine administration in a patient with type I autoimmune hepatitis. [DOI] [PubMed]
- 9.Fuchs-Telem D., Sarig O., van Steensel M.A. Familial pityriasis rubra pilaris is caused by mutations in CARD14. Am J Hum Genet. 2012;91(1):163–170. doi: 10.1016/j.ajhg.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


