Abstract
Background
Tolvaptan is a vasopressin type 2 receptor antagonist used in heart failure (HF) with refractory diuretic resistance. However, since tolvaptan is also ineffective in some HF patients with reduced ejection fraction (HFrEF), the identification of responders is important.
Methods
The study population consisted of 51 HFrEF patients who were administered tolvaptan (EF, 28 ± 7%). We defined responders as patients with a ≥50% increase in urine volume during the 24-hours after administration of tolvaptan. All patients underwent comprehensive transthoracic echocardiography before administration of tolvaptan. Patients were followed for 120 days to ascertain secondary events (cardiac death and rehospitalization for HF).
Results
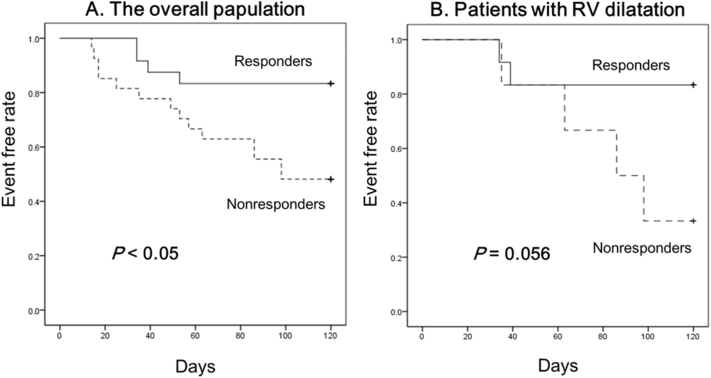
Multiple regression analysis indicated that right ventricular (RV) enlargement (defined as basal RV diameter > 41 mm and midlevel RV diameter > 35 mm, according to guidelines) remained a predictor of response after adjustment for age, sex, starting dosage of tolvaptan, and estimated glomerular filtration rate (odds ratio, 4.88; 95%-confidence interval, 1.26–18.9; P < 0.05), whereas left ventricular parameters and RV dysfunction were not. Kaplan-Meier curves indicated responsiveness to tolvaptan was associated with better prognosis among the overall population (P < 0.05); similar trends were observed among patients with RV dilatation (P = 0.056).
Conclusions
These findings suggest that RV enlargement, which represents right-sided volume overload, elevated filling pressure, and diastolic dysfunction similar to that seen in constrictive pericarditis, predicts responsiveness to tolvaptan in patients with HFrEF. Moreover, administration of tolvaptan may have the potential to improve the reportedly poor prognosis for HFrEF patients with RV dilatation.
Keywords: Heart failure, Echocardiography, Tolvaptan, Right ventricle, Drug responder
1. Introduction
Tolvaptan, an oral, selective vasopressin type 2 receptor antagonist, has become available for decompensated heart failure (HF) patients with refractory diuretic resistance, especially those with severe hyponatremia [[1], [2], [3], [4], [5]]. Tolvaptan therapy enables early improvement of dyspnea and increased urine volume without affecting blood electrolyte levels, activation of the renin-angiotensin-aldosterone system or the sympathetic nerve system, or worsening renal function [[6], [7], [8], [9], [10], [11]]. However, because tolvaptan is also ineffective in some HF patients with a reduced ejection fraction (HFrEF), the identification of responders is important. Although a decrease in urine osmolality after administration was associated with responsiveness to tolvaptan [12], we cannot predict responsiveness before administration using this method. Similarly, it is difficult to measure urine aquaporin-2, a reported novel predictor of response to tolvaptan, because of its cost [13,14].
An alternative method is transthoracic echocardiography (TTE). TTE plays an essential role in HF treatment because of its incomparable ability to provide noninvasive, repeatable, and less expensive assessment at the bedside. Moreover, TTE provides reliable assessment of right- and left-side hemodynamics, cardiac function, and cardiac structure, equivalent to catheter-based techniques [[15], [16], [17]]. However, the association between TTE parameters and responsiveness to tolvaptan has not been elucidated in HFrEF patients with volume overload.
The aim of the present study was, therefore, to assess the relationship between echocardiographic parameters (cardiac structure, cardiac function, and hemodynamics) and responsiveness to tolvaptan in patients with HFrEF.
2. Methods
2.1. Study population
This observational study conducted at Osaka City University Hospital was designed to clarify the echocardiographic findings associated with responsiveness to tolvaptan in patients with HFrEF. We defined reduced EF as left ventricular EF (LVEF) < 40%, according to published guidelines [4]. The study population comprised 51 consecutive inpatients with congestive HFrEF who received tolvaptan, with sustained excess body fluid that had not resolved despite receiving either of the following diuretic therapies with no change in dose: a loop diuretic of any dose, or combination therapy with a loop diuretic and an aldosterone antagonist of any dose. Concomitant use of the following drugs with no change in the dose was allowed: human atrial natriuretic peptide, catecholamines, and injected diuretics. The attending physician determined the administration, starting dosage, and duration of tolvaptan use according to each patient's condition. All patients had a New York Heart Association functional class of either III or IV. We excluded patients with any mechanical support, severe valvular stenosis, or a history of acute coronary syndrome within 30 days. Free water consumption was encouraged and daily salt intake was limited to 6 g/day for all patients. Urine output was measured for 24 h before and after administration of tolvaptan. Blood samples and vital signs were taken just before administration of tolvaptan. Informed consent was obtained from each patient and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's human research committee.
2.2. Definition of responder
We defined responders as patients with a ≥50% increase in urine volume during the 24 h after administration of tolvaptan [18].
2.3. Transthoracic echocardiography
TTE was performed prior to administration of tolvaptan using an iE33 (Philips Medical Systems, Andover, MA, USA), Aplio500 (Canon Medical Systems Corporation, Tochigi, Japan), or Vivid E9 (GE Healthcare, Milwaukee, WI, USA) machine equipped with a high-frequency transducer. A comprehensive examination was performed according to recommendations from the American society of Echocardiography and the European Association of Cardiovascular Imaging [19]. LVEF was calculated with the modified Simpson's method [19]. Inspiratory and expiratory inferior vena cava (IVC) diameters were measured from a subcostal view. IVC collapsibility index was calculated as [(expiratory IVC diameter – inspiratory IVC diameter)/expiratory IVC diameter] [20]. Right Ventricular (RV) diameter was measured from an RV-focused four-chamber view at the base level (D1) and midlevel (D2). RV dilatation was defined as D1 > 41 mm and D2 > 35 mm according to recommendations (Fig. 1) [19]. RV systolic function was evaluated using RV fractional area change and RV systolic dysfunction was defined as RV fractional area change <35% [19]. Estimated systolic pulmonary artery pressure was derived from RV systolic pressure by measuring the maximum tricuspid regurgitation jet velocity, and right atrial pressure was estimated by measuring the diameter and collapsibility of the IVC [21].
Fig. 1.
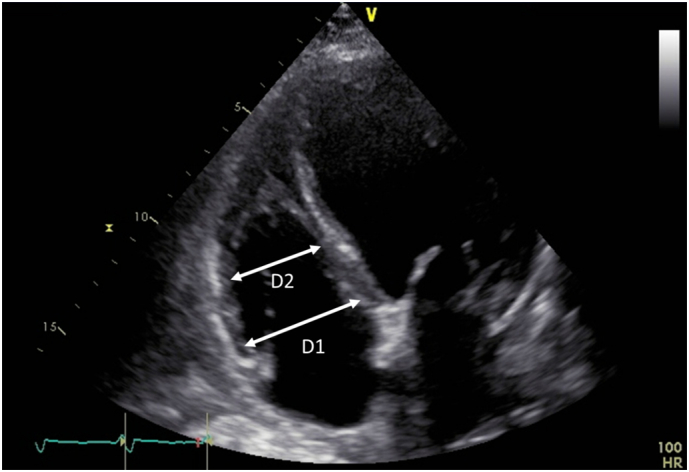
Echocardiographic assessment of RV dimensions from a, RV-focused apical four-chamber view.
D1 = maximum transverse dimension during the basal one-third of RV inflow at end diastole
D2 = transverse dimension during the middle third of RV inflow at end diastole.
RV, right ventricular.
2.4. Clinical outcomes
The study had complete outcome information (cardiac death or rehospitalization for HF) for all patients within 120 days.
2.5. Statistical analysis
Statistical analysis was performed using SPSS statistics version 24 software (IBM Corp, Armonk, NY, USA). Continuous variables were expressed as mean ± standard deviation, while categorical variables were expressed as percentages. The distribution of echocardiographic variables and potential covariates was evaluated in the overall population and among patients with and without responsiveness to tolvaptan. Comparisons between groups were performed by using the unpaired Student's t-test for continuous variables and the chi-square test for categorical variables. Multiple logistic regression analysis adjusted for age, sex, estimated glomerular filtration rate (eGFR), and starting dosage of tolvaptan was used to identify the echocardiographic variables associated with responsiveness to tolvaptan. Echocardiographic variables were entered into logistic regression analysis separately. Then, the survival rates were analyzed using Kaplan-Meier curves according to the responsiveness to tolvaptan therapy among the overall population and patients with RV dilatation, and significant differences were calculated using the log-rank test. We used the Kappa statistic to calculate inter and intra observer agreement of RV parameters. A P value of <0.05 was considered statistically significant for all tests.
3. Results
3.1. Patient characteristics
Among the 51 patients (mean age, 70 ± 12 years; LVEF, 28 ± 7%), 24 were responders to tolvaptan (47%). Clinical characteristics according to responsiveness to tolvaptan are shown in Table 1. The study population predominantly consisted of males (75%) with nonischemic cardiomyopathy (57%), chronic kidney disease (eGFR, 45 ± 24 mL/min/1.73 m2), and moderate furosemide dosage (54 ± 47 mg/day). Female gender (P = 0.06) and starting dose of tolvaptan (P = 0.07) tended to be associated with responsiveness to tolvaptan, whereas the other patient information was not significantly different between the 2 groups. Although there was no significant difference in urine volume during the 24 h before administration of tolvaptan (1216 ± 324 mL vs. 1464 ± 803 mL, P = 0.18) or increased water intake (195 ± 417 mL/day vs. 203 ± 408 mL/day, P = 0.95) during the 24 h after administration, urine volume significantly increased during the 24 h after administration in responders (2572 ± 856 mL vs. 1508 ± 814 mL, P < 0.01).
Table 1.
Baseline characteristics according to responsiveness to tolvaptan.
| Total |
Responder |
Nonresponder |
P | |
|---|---|---|---|---|
| N = 51 | N = 24 | N = 27 | ||
| Age (years) | 70 ± 12 | 67 ± 12 | 72 ± 12 | 0.19 |
| Sex (male, %) | 75 | 63 | 85 | 0.06 |
| Systolic blood pressure (mmHg) | 107 ± 20 | 106 ± 17 | 107 ± 22 | 0.74 |
| Diastolic blood pressure (mmHg) | 64 ± 15 | 65 ± 16 | 63 ± 14 | 0.54 |
| Pulse rate (bpm) | 86 ± 21 | 82 ± 17 | 90 ± 24 | 0.17 |
| Atrial fibrillation (%) | 28 | 25 | 31 | 0.65 |
| eGFR (mL/min/1.73 m2) | 45 ± 24 | 42 ± 21 | 48 ± 26 | 0.37 |
| Sodium (mEq/L) | 136 ± 6 | 136 ± 6 | 137 ± 5 | 0.57 |
| Albumin (g/dL) | 3.4 ± 0.5 | 3.5 ± 0.6 | 3.4 ± 0.4 | 0.47 |
| Furosemide (mg) | 54 ± 47 | 64 ± 38 | 46 ± 52 | 0.18 |
| β-Blocker (%) | 63 | 70 | 58 | 0.39 |
| ACEI/ARB (%) | 49 | 48 | 50 | 0.88 |
| Starting dosage of tolvaptan (mg) | 8.6 ± 3.4 | 9.5 ± 3.7 | 7.8 ± 2.9 | 0.07 |
| Days after admission (day) | 8.0 ± 7.0 | 7.6 ± 5.0 | 8.3 ± 8.5 | 0.73 |
| Pre-dose 24-h urine volume (mL) | 1347 ± 632 | 1216 ± 324 | 1464 ± 803 | 0.18 |
| Post-dose 24-h urine volume (mL) | 2008 ± 985 | 2572 ± 856 | 1508 ± 814 | <0.01 |
| Change in water intake from baseline (mL) | 199 ± 408 | 195 ± 417 | 203 ± 408 | 0.95 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; eGFR, estimated glomerular filtration rate.
3.2. Echocardiographic characteristics
The echocardiographic characteristics according to responsiveness to tolvaptan are shown in Table 2. The study population consisted of patients with severely decreased LVEF (28 ± 7%), dilated LV (LV end-diastolic dimension, 62 ± 14 mm), and dilated left atrium (left atrial dimension, 50 ± 9 mm), with coexistence of diastolic dysfunction or elevated filling pressure (E/e′, 27.1 ± 13.8), and mild pulmonary hypertension (estimated RV systolic pressure, 45 ± 17 mmHg). With regard to RV parameters, 18 patients (35%) had RV enlargement (defined as D1 > 41 mm and D2 > 35 mm), and 21 (41%) had RV dysfunction (defined as RV fractional area change <35%). The intra-observer agreement was excellent for basal RV diameter (κ = 0.85), midlevel RV diameter (κ = 0.80), and fractional area change (κ = 0.88). The inter-observer agreement was also excellent for basal RV diameter (κ = 0.95), midlevel RV diameter (κ = 0.91), and fractional area change (κ = 0.85). RV enlargement, but not LV dysfunction (P = 0.51) or RV dysfunction (P = 0.12), was significantly associated with responsiveness to tolvaptan (50% vs. 22%, P < 0.05). The other echocardiographic information was not significantly different between the 2 groups. Table 3 shows the echocardiographic variables associated with responsiveness to tolvaptan based on multiple logistic regression analysis. Only RV dilatation remained independently associated with responsiveness to tolvaptan after adjustment for age, sex, eGFR, and starting dosage (odds ratio, 4.88; 95% confidence interval, 1.26–18.9; P < 0.05).
Table 2.
Echocardiographic characteristics according to responsiveness to tolvaptan.
| Total |
Responder |
Nonresponder |
P | |
|---|---|---|---|---|
| N = 51 | N = 24 | N = 27 | ||
| Ejection fraction (%) | 28 ± 7 | 27 ± 7 | 29 ± 7 | 0.51 |
| LVDd (mm) | 62 ± 14 | 62 ± 15 | 61 ± 14 | 0.98 |
| LVDs (mm) | 52 ± 16 | 52 ± 17 | 51 ± 16 | 0.74 |
| Left atrial diameter (mm) | 50 ± 9 | 49 ± 8 | 51 ± 10 | 0.34 |
| E/e′ | 27.1 ± 13.8 | 26.7 ± 12.2 | 27.6 ± 15.6 | 0.83 |
| Maximum IVC (mm) | 18.9 ± 4.9 | 18.9 ± 5.7 | 18.9 ± 4.1 | 0.98 |
| IVC collapsibility index | 0.59 ± 0.20 | 0.57 ± 0.20 | 0.61 ± 0.20 | 0.60 |
| RV basal width (mm) | 44 ± 8 | 45 ± 10 | 43 ± 6 | 0.43 |
| RV mid width (mm) | 33 ± 7 | 35 ± 8 | 31 ± 7 | <0.05 |
| RV Dilatation (%) | 35 | 50 | 22 | <0.05 |
| Fractional area change (%) | 36 ± 11 | 34 ± 13 | 38 ± 9 | 0.12 |
| RV dysfunction (%) | 41 | 50 | 33 | 0.12 |
| TRPG (mmHg) | 36 ± 15 | 34 ± 17 | 38 ± 13 | 0.38 |
| Estimate RVP (mmHg) | 45 ± 17 | 44 ± 19 | 46 ± 15 | 0.64 |
Dd, end diastolic diameter; Ds, end systolic diameter; IVC, inferior vena cava; LV, left ventricular; PG, pressure gradient; RV, right ventricular; RVP, RV pressure; TR, tricuspid regurgitation.
Table 3.
Echocardiographic variables associated with responsiveness to tolvaptan: multivariable analysis.
| Variables | Responsiveness to tolvaptan |
||
|---|---|---|---|
| Odds ratio | 95% CI | P | |
| Ejection fraction (%) | 0.95 | 0.85–1.05 | 0.28 |
| LVDd (mm) | 1.00 | 0.96–1.06 | 0.87 |
| LVDs (mm) | 1.01 | 0.97–1.06 | 0.61 |
| E/e′ | 0.99 | 0.94–1.05 | 0.74 |
| RV dysfunction | 3.20 | 0.82–12.5 | 0.09 |
| RV dilatation | 4.88 | 1.26–18.9 | <0.05 |
Dd, end diastolic diameter; Ds, end systolic diameter; LV, left ventricular; RV, right ventricular.
3.3. Clinical outcomes
Fig. 2 shows the 120-day cardiac death-free and rehospitalization for HF-free rates in responders and non-responders among (A) the overall population and (B) patients with RV dilatation. Responsiveness to tolvaptan was associated with better prognosis among the overall population (P < 0.05); a similar trend was observed among patients with RV dilatation (P = 0.056).
Fig. 2.
120-day cardiac death-rate and rehospitalization for heart failure-free rate in responders and nonresponders among (A) the overall population and (B) patients with RV dilatation.
RV, right ventricular.
4. Discussion
The present study demonstrated that RV enlargement evaluated based on TTE was significantly associated with responsiveness to tolvaptan in patients with HFrEF. Moreover, responsiveness to tolvaptan showed a marked improvement in 120-day survival for patients with HFrEF. Furthermore, responsiveness to tolvaptan may improve the reportedly poor prognosis for HFrEF patients with RV dilatation [[22], [23], [24]]. To the best of our knowledge, this is the first study to demonstrate that RV enlargement evaluated by TTE is associated with responsiveness to tolvaptan in patients with HFrEF.
In our study, 24 patients (47%) were classified as responders. This finding is consistent with that from previous studies showing the importance of predicting responsiveness to tolvaptan (44–72%) in patients with HFrEF [[12], [13], [14],18,25]. Imamura et al. reported that a decrease in urine osmolality of >26% in patients with higher baseline urine osmolality (>352 mOsm/L) for the first 4–6 h after administration was associated with responsiveness to tolvaptan [12]. Similarly, they showed that increased urine aquaporin-2 relative to plasma arginine vasopressin is a predictor of response to tolvaptan [13,14]. Although these parameters are useful to distinguish responders from nonresponders, the percentage decrease in urine osmolality cannot predict responsiveness to tolvaptan before administration, and urine aquaporin-2 measurement is costly and is not generally available. An alternative method is TTE, which allows noninvasive and rapid bedside assessment for all patients before administration of tolvaptan. We revealed that RV parameters (i.e., RV dilatation), rather than LV parameters, offer incremental information for predicting responsiveness to tolvaptan in patients with HFrEF. This finding is consistent in part with a previous study that also failed to show the usefulness of LV parameters for prediction of responsiveness to tolvaptan [18]. However, this study was based on conventional LV assessment (i.e., LVEF and LV dimensions) and did not provide detailed information on the RV.
Although no studies to date have explored the impact of RV parameters on responsiveness to tolvaptan, our study revealed that RV dilatation is associated with responsiveness to tolvaptan in patients with HFrEF. Considering our results, we speculated that RV dilatation, but not LV parameters, plays an important role in the prediction of responsiveness. The mechanisms underlying our findings remain poorly understood. However, there are two considerable mechanisms that could explain the impact of RV dilatation on responsiveness to tolvaptan in HFrEF patients. Since tolvaptan can have a more favorable effect on patients with venous congestion [11,26,27], venous congestion may play a key role in responsiveness to tolvaptan. First, RV dilatation itself may be a reliable maker of venous congestion because RV dilatation was proven to represents right-sided volume overload and elevated filling pressure [28,29]. Second, patients with RV dilatation may tend to show RV diastolic dysfunction similar to that seen in constrictive pericarditis, and may have more severe venous congestion [24,[30], [31], [32], [33], [34], [35]]. Since our study population consisted of patients with RV dilatation with coexistence of LV and left atrial dilatation, the thin-walled RV is expected to result in less dilatation because of crowding within the limited pericardial space or diastolic ventricular interdependence.
The Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (the EVEREST outcome trial), which is a randomized, double-blind, placebo-controlled study, showed that tolvaptan therapy did not improve 2-year prognosis (all-cause mortality, cardiovascular death, and hospitalization for HF) in patients with HFrEF [33]. Similarly, the Targeting Acute Congestion with Tolvaptan in Congestive Heart Failure study (TACTICS-HF study), which is a randomized, double-blind, placebo-controlled study, also failed to show the ability of tolvaptan therapy to improve prognosis (30-day mortality or rehospitalization) in patients hospitalized with acute heart failure [36]. On the contrary, our study showed a positive association between responsiveness to tolvaptan and event-free survival in patients with HFrEF. These results indicate that efficacy of tolvaptan may not be superior to placebo in terms of long-term clinical outcome, but some potential beneficial effects might be left when focusing on responders. Moreover, responsiveness to tolvaptan also tended to be associated with better prognosis in HFrEF patients with RV dilatation. Although no studies to date have explored possible therapy to improve the prognosis of HFrEF patients with RV dilatation, tolvaptan may have the potential to improve prognosis in this population, when patients are appropriately selected (i.e., HFrEF patients with LV and RV dilatation).
5. Limitations
Our study has some limitations. First, because of the relatively small patient group, a large study is necessary to confirm whether RV dilatation indeed predicts responsiveness to tolvaptan in patients with HFrEF. Second, although we adjusted for the most pertinent variables that may affect responsiveness to tolvaptan, some confounding factors may have been incompletely adjusted for. Third, since administration or the starting dose of tolvaptan depends on the attending physician, these factors may cause selection bias and dose effects. Fourth, although we adjusted for the starting dosage of tolvaptan in multiple regression analysis, the starting dosage may affect the results. Fifth, although intra and inter observer agreement were excellent for RV dimension and function, because our data were obtained with TTE, cardiac magnetic resonance imaging (probably the best way to evaluate RV dimension and function) may be better for a more accurate analysis. Sixth, comparison of chronic outcomes may not be appropriate in a small number patients. Seventh, as this was a single-center study with no control group for comparison, the generalizability of the results about prognosis is limited.
6. Conclusions
RV enlargement, which represents right-sided volume overload, elevated filling pressure, and diastolic dysfunction similar to that seen in constrictive pericarditis, predicts responsiveness to tolvaptan in patients with HFrEF. Moreover, administration of tolvaptan may have the potential to improve the reportedly poor prognosis for HFrEF patients with RV dilatation.
Conflicts of interest
The authors report no relationships that could be construed as a conflict of interest.
Acknowledgments
None.
References
- 1.Inomata T., Izumi T., Matsuzaki M., Hori M., Hirayama A. Phase III clinical pharmacology study of tolvaptan. Cardiovasc. Drugs Ther. 2011;25(Suppl. 1):S57–S65. doi: 10.1007/s10557-011-6349-x. [DOI] [PubMed] [Google Scholar]
- 2.Matsuzaki M., Hori M., Izumi T., Fukunami M. Efficacy and safety of tolvaptan in heart failure patients with volume overload despite the standard treatment with conventional diuretics: a phase III, randomized, double-blind, placebo-controlled study (QUEST study) Cardiovasc. Drugs Ther. 2011;25(Suppl. 1):S33–S45. doi: 10.1007/s10557-011-6304-x. [DOI] [PubMed] [Google Scholar]
- 3.Kinugawa K., Sato N., Inomata T., Shimakawa T., Iwatake N., Mizuguchi K. Efficacy and safety of tolvaptan in heart failure patients with volume overload. Circ. J. 2014;78:844–852. [PubMed] [Google Scholar]
- 4.Ponikowski P., Voors A.A., Anker S.D. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the heart failure association (HFA) of the ESC. Eur. J. Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 5.Yancy C.W., Jessup M., Bozkurt B. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 6.Shirakabe A., Hata N., Yamamoto M. Immediate administration of tolvaptan prevents the exacerbation of acute kidney injury and improves the mid-term prognosis of patients with severely decompensated acute heart failure. Circ. J. 2014;78:911–921. doi: 10.1253/circj.cj-13-1255. [DOI] [PubMed] [Google Scholar]
- 7.Pang P.S., Gheorghiade M., Dihu J. Effects of tolvaptan on physician-assessed symptoms and signs in patients hospitalized with acute heart failure syndromes: analysis from the efficacy of vasopressin antagonism in heart failure outcome study with tolvaptan (EVEREST) trials. Am. Heart J. 2011;161:1067–1072. doi: 10.1016/j.ahj.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 8.Pang P.S., Konstam M.A., Krasa H.B. Effects of tolvaptan on dyspnoea relief from the EVEREST trials. Eur. Heart J. 2009;30:2233–2240. doi: 10.1093/eurheartj/ehp253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanatani A., Shibata A., Kitada R. Administration of tolvaptan with reduction of loop diuretics ameliorates congestion with improving renal dysfunction in patients with congestive heart failure and renal dysfunction. Heart Vessel. 2017;32:287–294. doi: 10.1007/s00380-016-0872-4. [DOI] [PubMed] [Google Scholar]
- 10.Gheorghiade M., Niazi I., Ouyang J. Vasopressin V2-receptor blockade with tolvaptan in patients with chronic heart failure: results from a double-blind, randomized trial. Circulation. 2003;107:2690–2696. doi: 10.1161/01.CIR.0000070422.41439.04. [DOI] [PubMed] [Google Scholar]
- 11.Costello-Boerrigter L.C., Smith W.B., Boerrigter G. Vasopressin-2-receptor antagonism augments water excretion without changes in renal hemodynamics or sodium and potassium excretion in human heart failure. Am. J. Physiol. Ren. Physiol. 2006;290:F273–F278. doi: 10.1152/ajprenal.00195.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imamura T., Kinugawa K., Shiga T. Novel criteria of urine osmolality effectively predict response to tolvaptan in decompensated heart failure patients–association between non-responders and chronic kidney disease. Circ. J. 2013;77:397–404. doi: 10.1253/circj.cj-12-0971. [DOI] [PubMed] [Google Scholar]
- 13.Imamura T., Kinugawa K., Fujino T. Increased urine aquaporin-2 relative to plasma arginine vasopressin is a novel marker of response to tolvaptan in patients with decompensated heart failure. Circ. J. 2014;78:2240–2249. doi: 10.1253/circj.cj-14-0244. [DOI] [PubMed] [Google Scholar]
- 14.Imamura T., Kinugawa K., Komuro I. Tolvaptan prolongs blockage of the vasopressin type II receptor over 24 hours in responders with stage D heart failure. Int. Heart J. 2016;57:41–46. doi: 10.1536/ihj.15-297. [DOI] [PubMed] [Google Scholar]
- 15.Nagueh S.F., Bhatt R., Vivo R.P. Echocardiographic evaluation of hemodynamics in patients with decompensated systolic heart failure. Circ. Cardiovasc. Imaging. 2011;4:220–227. doi: 10.1161/CIRCIMAGING.111.963496. [DOI] [PubMed] [Google Scholar]
- 16.Dokainish H., Nguyen J.S., Bobek J., Goswami R., Lakkis N.M. Assessment of the American Society of Echocardiography-European Association of Echocardiography guidelines for diastolic function in patients with depressed ejection fraction: an echocardiographic and invasive haemodynamic study. Eur. J. Echocardiogr. 2011;12:857–864. doi: 10.1093/ejechocard/jer157. [DOI] [PubMed] [Google Scholar]
- 17.Kirkpatrick J.N., Vannan M.A., Narula J., Lang R.M. Echocardiography in heart failure: applications, utility, and new horizons. J. Am. Coll. Cardiol. 2007;50:381–396. doi: 10.1016/j.jacc.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 18.Nakada Y., Okayama S., Nakano T. Echocardiographic characteristics of patients with acute heart failure requiring tolvaptan: a retrospective study. Cardiovasc. Ultrasound. 2015;13:27. doi: 10.1186/s12947-015-0022-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang R.M., Badano L.P., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015;28 doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Moreno F.L., Hagan A.D., Holmen J.R., Pryor T.A., Strickland R.D., Castle C.H. Evaluation of size and dynamics of the inferior vena cava as an index of right-sided cardiac function. Am. J. Cardiol. 1984;53:579–585. doi: 10.1016/0002-9149(84)90034-1. [DOI] [PubMed] [Google Scholar]
- 21.Bossone E., D'Andrea A., D'Alto M. Echocardiography in pulmonary arterial hypertension: from diagnosis to prognosis. J. Am. Soc. Echocardiogr. 2013;26:1–14. doi: 10.1016/j.echo.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Maekawa E., Inomata T., Watanabe I. Prognostic significance of right ventricular dimension on acute decompensation in chronic left-sided heart failure. Int. Heart J. 2011;52:119–126. doi: 10.1536/ihj.52.119. [DOI] [PubMed] [Google Scholar]
- 23.Lewis J.F., Webber J.D., Sutton L.L., Chesoni S., Curry C.L. Discordance in degree of right and left ventricular dilation in patients with dilated cardiomyopathy: recognition and clinical implications. J. Am. Coll. Cardiol. 1993;21:649–654. doi: 10.1016/0735-1097(93)90097-k. [DOI] [PubMed] [Google Scholar]
- 24.Sun J.P., James K.B., Yang X.S. Comparison of mortality rates and progression of left ventricular dysfunction in patients with idiopathic dilated cardiomyopathy and dilated versus nondilated right ventricular cavities. Am. J. Cardiol. 1997;80:1583–1587. doi: 10.1016/s0002-9149(97)00780-7. [DOI] [PubMed] [Google Scholar]
- 25.Niwa T., Waseda K., Mizuno T. Predictability of tricuspid annular plane systolic excursion for the effectiveness of tolvaptan in patients with heart failure. J. Echocardiogr. 2017;15:118–126. doi: 10.1007/s12574-017-0330-z. [DOI] [PubMed] [Google Scholar]
- 26.Udelson J.E., Orlandi C., Ouyang J. Acute hemodynamic effects of tolvaptan, a vasopressin V2 receptor blocker, in patients with symptomatic heart failure and systolic dysfunction: an international, multicenter, randomized, placebo-controlled trial. J. Am. Coll. Cardiol. 2008;52:1540–1545. doi: 10.1016/j.jacc.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 27.Udelson J.E., McGrew F.A., Flores E. Multicenter, randomized, double-blind, placebo-controlled study on the effect of oral tolvaptan on left ventricular dilation and function in patients with heart failure and systolic dysfunction. J. Am. Coll. Cardiol. 2007;49:2151–2159. doi: 10.1016/j.jacc.2007.01.091. [DOI] [PubMed] [Google Scholar]
- 28.López-Sendón J., Sá ELd. Ischemic right ventricular dysfunction. Cardiovasc. Drugs Ther. 1994;8:393–406. doi: 10.1007/BF00877324. [DOI] [PubMed] [Google Scholar]
- 29.Testani J.M., Khera A.V., St John Sutton M.G. Effect of right ventricular function and venous congestion on cardiorenal interactions during the treatment of decompensated heart failure. Am. J. Cardiol. 2010;105:511–516. doi: 10.1016/j.amjcard.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santamore W.P., Dell'Italia L.J. Ventricular interdependence: significant left ventricular contributions to right ventricular systolic function. Prog. Cardiovasc. Dis. 1998;40:289–308. doi: 10.1016/s0033-0620(98)80049-2. [DOI] [PubMed] [Google Scholar]
- 31.Feneley M.P., Gavaghan T.P., Baron D.W., Branson J.A., Roy P.R., Morgan J.J. Contribution of left ventricular contraction to the generation of right ventricular systolic pressure in the human heart. Circulation. 1985;71:473–480. doi: 10.1161/01.cir.71.3.473. [DOI] [PubMed] [Google Scholar]
- 32.Taylor R.R., Covell J.W., Sonnenblick E.H., Ross J. Dependence of ventricular distensibility on filling of the opposite ventricle. Am. J. Phys. 1967;213:711–718. doi: 10.1152/ajplegacy.1967.213.3.711. [DOI] [PubMed] [Google Scholar]
- 33.Konstam M.A., Gheorghiade M., Burnett J.C. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST outcome trial. JAMA. 2007;297:1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 34.Hunt S.A. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J. Am. Coll. Cardiol. 2005;46:e1–82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 35.Hines R. Right ventricular function and failure: a review. Yale J. Biol. Med. 1991;64:295–307. [PMC free article] [PubMed] [Google Scholar]
- 36.Felker G.M., Mentz R.J., Cole R.T. Efficacy and safety of tolvaptan in patients hospitalized with acute heart failure. J. Am. Coll. Cardiol. 2017;69:1399–1406. doi: 10.1016/j.jacc.2016.09.004. [DOI] [PubMed] [Google Scholar]