Highlights
-
•
>50% of patients are willing to participate in clinical trials, only 18% are currently enrolled.
-
•
The top reason to participate in trials was to serve medical progress and cancer research.
-
•
Reasons for refusing were extensive travel time, no therapeutic advantage and too time-consuming.
-
•
Good information strategies need to be implemented, and doctors need to be aware of running trials.
-
•
Trial concepts must include patients’ needs, e.g. number of appointments, risk-benefit profile.
Keywords: Clinical trial, Clinical study, Patient recruitment, Patient participation, Surveys, Oncology
Abstract
Introduction
Prospective clinical trials are essential to translate new therapy concepts or rather any scientific development into the medical routine. Besides a sophisticated trial protocol, the success of clinical trials depends on patient recruitment and participation. Patient recruitment remains a challenge and depends on several factors. To get a current picture of the patients’ attitude, we conducted the present survey.
Methods
We designed a survey with seven questions, which was given to all oncological patients treated within a timeframe of three months between Mai and July 2017. Participation was voluntary and anonymous. The questionnaire mainly inquires patients’ participation in clinical trials in a university-based setting, their attitude towards clinical trials regarding risks and benefits, and their source of information in this context.
Results
771 patients (1:1 male/female) participated with a median age of 61 years (range 18–91 years) with a response rate of 71.5%. Of all, 17.8% (137/771) were participating in a clinical trial. The most mentioned reason was to serve medical progress and cancer research. Out of the patients not currently participating in a trial, 79 (12.7%, 79/623) refusers named the following main reasons: extensive travel time to the clinic, no therapeutic advantage, and too time-consuming. Out of the patients not offered to take part in a trial, 265 (51.0%, 265/520) would participate if offered. Of all patients, 8.3% (64/771) used the clinics' homepage as a source of information, of those 79.7% (51/64) were satisfied with its content. To enhance patient recruitment strategies, we asked how patients wish to be informed about possible trials: More than half (52.0%) of the questioned patients preferred an individual medical consultation with their physician.
We further analyzed the trial participation depending on age, gender, unit, and tumor entity. We could show a significant influence of age (p < 0.001) but not for gender (p = 0.724). The trial participation was also significantly associated with the treating unit (p < 0.001) and tumor entity (p = 0.001).
Conclusion
Patients are willing to participate in clinical trials. Better information strategies need to be implemented. Physicians need to be aware of running trials within their department and must counseling counsel patients effectively to improve recruitment. Trial concepts should keep in mind patients’ needs including an adequate number of appointments, positive risk-benefit profiles, and information material.
1. Introduction
Prospective clinical trials are essential to translate new therapy concepts or rather any scientific development into the medical routine. Back in 1753, James Lind conducted one of the first randomized controlled trials. He published data on the treatment of scurvy with lemons and oranges in his essay “A treatise of the scurvy” [1]. This marked the birth of clinical trials. A few years later, George Fordyce firstly brought up the idea of evidence-based medicine (EBM) [2]. To practice EBM, physicians must not only rely purely on their clinical expertise but on facts and numbers generated by trials of high quality. Since the days of Lind and Fordyce clinical trials have become an established instrument to evaluate new treatment strategies. The process of translating pre-clinical data into the daily medical routine is strictly regulated. Results must be proven in clinical trials from phase I (first-in-human testing) to phase IV (broad implementation).
Besides a sophisticated trial protocol, the success of clinical trials depends on patient recruitment and participation. Patient recruitment remains a challenge and depends on the following factors. Older and poorer patients tend to refuse participation more often, and patients with severe diseases, e.g. cancer [3], [4], [5], [6], [7]. However, new trends are emerging such as patient empowerment and taking an active role in influencing health [8]. It is our task to develop new attractive trial designs, use the factors which impact patients’ motivation, and ultimately encourage their participation in clinical trials. Eventually, patients benefit from trial participation by access to new and innovative therapies as well as the close affiliation with the treating department.
From the view of clinical personnel, we recently published a survey which asked for the obstacles that prevent recruiting patients into clinical trials [9]. In line with Khan et al. [10], limited human and technical resources and the enormous documentation effort were named as the most prominent issues. These are also factors that need to be optimized for future trial design to increase patient participation.
The awareness of factors influencing the patients’ motivation to participate in clinical trials are a relevant topic in teaching hospitals. The progress in oncology depends on clinical research and the constant improvement and development of innovative therapies. Especially in oncology centers in Germany, ≥5% of primary cases must be recruited into clinical trials to fulfill the certification requirements (pediatric oncology excluded, which requires ≥90% of primary cases) [11].
To get a current picture of the patients’ perspective, we conducted the present survey. Our evaluation analyses patient participation and attitude towards clinical trials in a large university-based Oncology Center with the aim to identify weak points in patient recruitment, information material, infrastructure, and the concerns and understanding of patients regarding clinical trials. Ultimately, optimized trial strategies are going be defined from the results to improve the general performance of clinical trials.
2. Methods
We designed a survey with seven questions, which was given to all oncological patients treated within a timeframe of three months. The questionnaire mainly inquires patients’ participation in clinical trials in a university-based setting, their attitude towards clinical trials regarding risks and benefits, and their source of information in this context. A team of experienced oncologists and medical computer scientists designed the questionnaire. The survey was tested on ten patients before broad initiation to ensure a patient-friendly format and wording. No changes needed to be made after the test run (Supplement file 1).
The survey was part of the yearly certification of Oncological Centers in Germany within the Oncology Center Munich (Onkologisches Zentrum (OZ) am RHCCC im MRI TU Munich (TUM)). The following eight units participated: dermatooncology (DERMA), breast center/gynecology (GYN), head-and-neck tumor center (HAN), hematooncology (HEM), neurooncology (NEURO), orthopedic surgery (ORTHO), radiation oncology (RADONC) and abdominal surgery (SUR). The evaluation was primarily based on following the certification criteria of the Deutsche Krebsgesellschaft (DKG). Each unit determined the number of distributed questionnaires itself. A total of 1220 questionnaires were distributed (Table 1).
Table 1.
Patient distribution for all and divided into the individual participating units.
| Unit | Questionnaires distributed | Questionnaires returned and filled out | Questionnaires not used | Rate |
|---|---|---|---|---|
| All | 1220 | 771 | 141 | 71.5% (771/1079) |
| DERMA | 50 | 37 | 0 | 74.0% (37/50) |
| GYN | 50 | 24 | 0 | 48.0% (24/50) |
| HAN | 250 | 208 | 0 | 83.2% (208/250) |
| HEM | 200 | 87 | 12 | 46.3% (87/188) |
| NEURO | 300 | 136 | 63 | 57.4% (136/237) |
| ORTHO | 50 | 24 | 12 | 63.2% (24/38) |
| RADONC | 170 | 128 | 33 | 93.4% (128/137) |
| SUR | 150 | 127 | 21 | 98.4% (127/129) |
Between May and July 2017, patients treated in one of the mentioned units were asked to participate. Inclusion criteria were: age older 18 years, German-speaking, and physical as well as mental health to fill out paper-based questionnaires. Participation was voluntary and anonymous; no written consent was required. The Ethics Committee of the Technical University of Munich (TUM) approved the nature and content of the study with the project number 167/17 S.
All statistic calculations were performed in a primarily descriptive way using SPSS v24 (IBM, USA). Dependencies of trial participation regarding the groups for gender, unit and tumor entity were calculated with Pearson Chi-Square tests; for age (continues variable) we used the Kolmogorov-Smirnov test.
3. Results
771 patients (1:1 male/female) participated in the survey with a median age of 61 years (range 18–91 years). Table 2 displays further patient characteristics.
Table 2.
Patient characteristic.
| Patients, n (%) | ||
|---|---|---|
| Gender | ||
| Female | 389 (50.5%) | |
| Male | 380 (49.3%) | |
| Unknown | 2 (0.2%) | |
| Received therapy (multiple answers possible) | ||
| Surgery | 284 (36.8%) | |
| Radiotherapy | 118 (15.3%) | |
| Systemic therapy | 271 (35.1%) | |
| No current treatment | 207 (26.8%) | |
| Unknown | 47 (6.1%) | |
| Tumor entity | ||
| Bone/spine cancer/metastases | 35 (4.5%) | |
| Brain tumors/metastases | 152 (19.7%) | |
| Breast cancer and gynecological tumors | 59 (7.7%) | |
| Head and neck cancer | 216 (28.0%) | |
| Hematological cancer | 76 (9.9%) | |
| Hepato-pancreato-biliary cancer | 48 (6.2%) | |
| Prostate cancer and urological tumors | 30 (3.9%) | |
| Skin cancer | 44 (5.7%) | |
| Upper and lower gastrointestinal cancer | 58 (7.5%) | |
| Other | 23 (3.0%) | |
| Unknown | 30 (3.9%) | |
| Trial participation, n = 771 | ||
| Yes | 137 (17.8%) | |
| No | 623 (80.8%) | |
| Unknown | 11 (1.4%) | |
| Currently not participating, but trial offered, n = 623 | ||
| Yes, but refused | 79 (12.7%) | |
| No | 520 (83.4%) | |
| Unknown | 24 (3.9%) | |
| No trial offered but would participate, n = 520 | ||
| Yes | 265 (51.0%) | |
| No | 207 (39.8%) | |
| Unknown | 48 (9.2%) | |
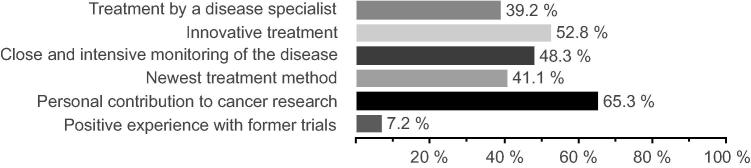
Of all patients questioned, 17.8% (137/771) were participating in a clinical trial. The most mentioned reason was to serve medical progress and cancer research; further reasons are listed in Fig. 1.
Fig. 1.
Reasons for participating in a clinical trial (n = 137, multiple-choice question).
Out of the patients not currently participating in a trial (80.8%, 623/771), 79 (12.7%, 79/623) refusers named the following main reasons: extensive travel time to the clinic (25.3%), no therapeutic advantage (24.1%), and too time-consuming (20.3%) (Fig. 2).
Fig. 2.
Reasons for refusing to participate in a clinical trial (n = 79, multiple-choice question).
Out of the patients not offered to take part in a trial (83.5%, 520/623), 265 (51.0%, 265/520) would participate if offered. The reasons are displayed in Fig. 3. As for the patients that are already in a trial, to serve medical progress and cancer research is mostly mentioned.
Fig. 3.
Reasons for participating in a clinical trial if offered (n = 265, multiple-choice question).
Of all, 22.7% (175/771) informed themselves about clinical trials via the internet (65.7%, 115/175), family physician/specialist (57.1%, 100/175), or in medical magazines (9.7%, 17/175).
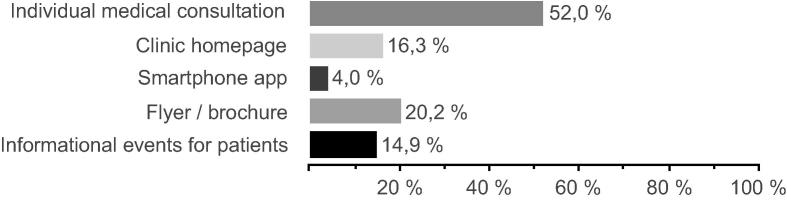
Of all patients, 8.3% (64/771) used the clinics’ homepage as a source of information, of that 79.7% (51/64) were satisfied with its content. Only ten patients mentioned that they did not find relevant information (6/10), did not find the right contact person (2/10) or could not understand the information on the homepage (4/10). To enhance patient recruitment strategies, we asked how patients wish to be informed about possible trials: More than half (52.0%) of the questioned patients preferred an individual medical consultation with their physician (Fig. 4).
Fig. 4.
Information sources/materials wished by patients (n = 771, multiple-choice question).
We further analyzed the trial participation depending on age, gender, unit, and tumor entity. We could show a significant influence of age (p < 0.001) but not for gender (p = 0.724). The younger the patient, the higher the probability that they participate in a trial. The trial participation was also significantly associated with the treating unit (p < 0.001) and tumor entity (p = 0.001) (Table 3). Combining patients who are already participating in a trial and patients who are willing to, showed a positive attitude towards clinical trials in 52.2% (405/771).
Table 3.
Trial participation according to unit and tumor entity. Percentages are calculated according to unit and tumor entity, respectively.
| Trial “no”, n (%) | Trial “yes”, n (%) | |||
|---|---|---|---|---|
| Unit | ||||
| DERMA | 34 | (94.4%) | 2 | (5.6%) |
| GYN | 12 | (50.0%) | 12 | (50.0%) |
| HAN | 189 | (91.3%) | 18 | (8.7%) |
| HEM | 70 | (81.4%) | 18 | (18.6) |
| NEURO | 102 | (77.3%) | 30 | (22.7%) |
| ORTHO | 20 | (83.3%) | 4 | (16.7%) |
| RADONC | 102 | (80.3%) | 25 | (19.7%) |
| SUR | 94 | (75.8%) | 30 | (24.2%) |
| Tumor entity | ||||
| Bone/spine cancer/metastases | 29 | (82.9%) | 6 | (17.1%) |
| Brain tumors/metastases | 115 | (77.7) | 33 | (22.3%) |
| Breast cancer and gynecological tumors | 40 | (67.8%) | 19 | (32.2%) |
| Head and neck cancer | 194 | (90.2%) | 21 | (9.8%) |
| Hematological cancer | 58 | (77.3%) | 17 | (22.7%) |
| Hepato-pancreato-biliary cancer | 37 | (80.4%) | 9 | (19.6%) |
| Prostate cancer and urological tumors | 27 | (93.1%) | 2 | (6.9%) |
| Skin cancer | 39 | (90.7%) | 4 | (9.3%) |
| Upper and lower gastrointestinal cancer | 40 | (70.2%) | 17 | (29.8%) |
Unknown answers for the question about trial participation (n = 11) were excluded.
4. Discussion
The purpose of our survey was to analyze the patient participation and attitude towards clinical trials in a large university-based Comprehensive Cancer Center (CCC), to identify problems, and as a consequence to define a first approach to improve the general performance of clinical trials.
Out of all patients, 52.2% are willing to participate in a clinical trial. However, only 17.8% were currently enrolled although treated at a university hospital. Clinical trials can be very different, e.g. ranging from simple observational studies to interventional trials and implying very different potential advantages or risk. The results are very likely driven by what patients consider as a clinical trial.
Patients refuse to take part in a clinical trial mainly because of the long commute to the clinic, the extra time required, and additional appointments. Some studies entail frequent clinical visits, e.g. in weekly periods. It might be a minor problem for patients living close to the treating clinic; however, patients from distant or rural areas are exposed to extensive travel time and costs if included in clinical trials. This is common, especially where large Oncology Centers are the leading research facilities. A solution is the integration of new and alternative strategies for clinical visits and aftercare. Outsourcing these to primary care physicians and rural medical centers reduces travel time and costs for patients [12], [13]. Here, data management and exchange are crucial so that the complete information is available in the leading clinic for scientific use [13], [14]. In previous works, we discussed that telemedicine and even app-accompanied clinical trials (smartRCTs) could reduce overall costs and duration of clinical trials [14]. Such new technologies can also be used to reduce personal presence during clinical visits [15]. Wallwiener et al. registered patient-reported outcomes electronically (ePRO) in the PRAEGNANT Real-Time Advanced and Metastatic Breast Cancer Registry [16], while Moon et al. used a wearable to monitor mobility of patients with multiple sclerosis [17].
Of all refusers, 24.1% questioned the therapeutic advantages, and 13.9% were scared of receiving an experimental treatment less effective than established treatment regimens. Participants in phase I or II studies may argue with such reasons. However, patients in phase III or IV studies resulting in broad implementation receive treatments methods which have been already tested in laboratory conditions, animals and healthy humans. Hence, it is essential to inform the patient sufficiently about the treatment, aims, and risks. Of all refusers, only 3.8% complained about insufficient information about the trial. This is a result and shows that physicians and study nurses explain the study and its aims detailed and complete. Patient education is important: Only a holistically informed patient can give informed consent. Mills and Campbell et al. argued similarly that educational, and team building strategies for the entire study team (physicians, patients, study nurses/managers) seem simple but improve participation rates [6], [7].
Cancer patients suffer from a severe illness, and often they are willing to do whatever it takes to support their recovery. Most patients named the close monitoring of their disease (34.3%) with an innovative treatment (26.3%) by specialists (21.2%), and their contribution to cancer research (75.5%) as motivating factors to participate. These positive reasons must be used and even intensified in the patient recruitment process. Physicians must be trained to use these factors to convince patients clearly and objectively about participating in a clinical trial. Therefore, it is essential to allow for in-depth patient counseling without time pressure. As also Chilton et al. [18] recently found out in their review article trust and Ambivalence are significant factors influencing trial participation. In busy hospitals where physicians rotate through different stages, it can be problematic. Physicians are often not aware of all the possible recruiting trials within the department or hospital. Therefore, involvement and teaching of all doctors are necessary, even though they hold no active role in the trial. Further, it must be a relevant part of young doctors’ education to coach them early in their career path about clinical trials the necessity and advances. It is known, that a good-doctor-patient relationship builds trust and satisfaction for the patient [5] and might influence their awareness and willingness for trial participation [19]. Our system continues to develop in more patient-centered healthcare where we must actively include the patient and focus on the individual needs. Personal benefits must be emphasized during patient counseling. The management of clinical trials is underestimated and requires a good investment from the hospital and if necessary even a release of the staff from everyday duty; it cannot be only a side job of personnel [9], [19].
In our survey, we could show significant differences in treating units and tumor entities. To find the causes and solutions we built a team that will analyze running clinical trials within the departments. We want to evaluate if clinical trials exist but are not recruiting, or if innovative trials for the most common diseases might be lacking and if this can be resolved by initiating or participating in multicenter trials. Further, we want to investigate how patients get information material about trials. Kiernan et al. [20] found out that with simple measures such as low-cost online infographics improve trial participation in trust in research. Hence, our focus will also be the Internet-based presentation. We will look at how the hospital and individual departments presenting their clinical trials and if the given information is up to date and easily accessible to the patient. Cowie et al. recently investigated new recruiting strategies for social media, e.g. Facebook, complementing traditional methods and could show that participation rates increased with advertising campaigns on this platforms [21]. In an oncological setting, this might be a more sensitive topic, but the online information structure is an efficient and cost-saving recruitment approach.
5. Conclusion
Patients are willing to participate in clinical trials. Better information strategies need to be implemented, and physicians need to be aware of running trials within their department and improve recruitment by counseling patients effectively. Trial concepts should keep in mind patients’ needs including an adequate number of appointments, positive risk-benefit profiles, and information material. Modern IT tools may help to solve some obstacles, such as the inclusion of patient-reported outcome (PRO) via the internet instead of in-person follow-up visits, telemedicine setups and digital information material on trial background and other relevant topics.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgement
This work was presented at an ESTRO 37 recommended session 2018.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2018.10.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Baron J.H. Evolution of clinical research: a history before and beyond James Lind. Perspect Clin Res. 2012;3:149. doi: 10.4103/2229-3485.103599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Almaie S.M., Al-Baghli N.A. Evidence based medicine: an overview. J Family Community Med. 2003;10:17–24. [PMC free article] [PubMed] [Google Scholar]
- 3.Blanch D.C., Rudd R.E., Wright E., Gall V., Katz J.N. Predictors of refusal during a multi-step recruitment process for a randomized controlled trial of arthritis education. Patient Educ Couns. 2008;73:280–285. doi: 10.1016/j.pec.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murthy V.H., Krumholz H.M., Gross C.P. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 5.Mopuru N.R., Jose S.P., Viswanath B., Kumar C.N., Math S.B., Thirthalli J. Factors influencing participation of psychiatry inpatients in clinical trials. Asian J Psychiatr. 2017;32:40–43. doi: 10.1016/j.ajp.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 6.Campbell M.K., Snowdon C., Francis D., Elbourne D., McDonald A.M., Knight R. Recruitment to randomised trials: strategies for trial enrollment and participation study. The STEPS study. Health Technol Assess. 2007;11 doi: 10.3310/hta11480. iii–ix–105. [DOI] [PubMed] [Google Scholar]
- 7.Mills E.J., Seely D., Rachlis B., Griffith L., Wu P., Wilson K. Barriers to participation in clinical trials of cancer: a meta-analysis and systematic review of patient-reported factors. Lancet Oncol. 2006;7:141–148. doi: 10.1016/S1470-2045(06)70576-9. [DOI] [PubMed] [Google Scholar]
- 8.Castro E.M., Van Regenmortel T., Vanhaecht K., Sermeus W., Van Hecke A. Patient empowerment, patient participation and patient-centeredness in hospital care: a concept analysis based on a literature review. Patient Educ Couns. 2016;99:1923–1939. doi: 10.1016/j.pec.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 9.Straube C., Herschbach P., Combs S.E. Which obstacles prevent us from recruiting into clinical trials: a survey about the environment for clinical studies at a German University Hospital in a Comprehensive Cancer Center. Front Oncol Frontiers. 2017;7:181. doi: 10.3389/fonc.2017.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan S.A., Payne P.R.O., Johnson S.B., Bigger J.T., Kukafka R. Modeling clinical trials workflow in community practice settings. AMIA Annu Symp Proc. American Medical Informatics Association. 2006, 2006,:419–423. [PMC free article] [PubMed] [Google Scholar]
- 11.DKG Krebsgesellschaft. Zertifizierung onkologischer Zentren, Erhebungsböge, Kennzahlen und Checklisten [Internet]. Available from: https://www.krebsgesellschaft.de/zertdokumente.html archived at http://www.webcitation.org/6wMninfR9.
- 12.Taylor-Gjevre R., Nair B., Bath B., Okpalauwaekwe U., Sharma M., Penz E. Addressing rural and remote access disparities for patients with inflammatory arthritis through video-conferencing and innovative inter-professional care models. Musculoskeletal Care. 2017;7:10. doi: 10.1002/msc.1215. [DOI] [PubMed] [Google Scholar]
- 13.Mathew A.S., Agarwal J.P., Munshi A., Laskar S.G., Pramesh C.S., Karimundackal G. A prospective study of telephonic contact and subsequent physical follow-up of radically treated lung cancer patients. Indian J Cancer. 2017;54:241–252. doi: 10.4103/0019-509X.219599. [DOI] [PubMed] [Google Scholar]
- 14.Vogel M.M.E., Combs S.E., Kessel K.A. mHealth and application technology supporting clinical trials: today's limitations and future perspective of smartRCTs. Front Oncol. 2017;7:37. doi: 10.3389/fonc.2017.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reider-Demer M., Raja P., Martin N., Schwinger M., Babayan D. Prospective and retrospective study of videoconference telemedicine follow-up after elective neurosurgery: results of a pilot program. Neurosurg Rev. Springer, Berlin Heidelberg. 2017;27:24–25. doi: 10.1007/s10143-017-0878-0. [DOI] [PubMed] [Google Scholar]
- 16.Wallwiener M., Heindl F., Brucker S.Y., Taran F.-A., Hartkopf A., Overkamp F. Implementation and feasibility of Electronic Patient-Reported Outcome (ePRO) data entry in the PRAEGNANT real-time advanced and metastatic breast cancer registry. Geburtsh Frauenheilk. 2017;77:870–878. doi: 10.1055/s-0043-116223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moon Y, McGinnis RS, Seagers K, Motl RW, Sheth N, Wright JA, et al. Monitoring gait in multiple sclerosis with novel wearable motion sensors. Thomas FP, editor. PLoS One. 2017;12:e0171346. [DOI] [PMC free article] [PubMed]
- 18.Chilton J.A., Rasmus M.L., Lytton J., Kaplan C.D., Jones L.A., Hurd T.C. Ambivalence: a key to clinical trial participation? Front Oncol. 2018;8:300. doi: 10.3389/fonc.2018.00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oude Rengerink K., Kalkman S., Collier S., Ciaglia A., Worsley S.D., Lightbourne A. Pragmatic trials and real world evidence: Paper 3. Patient selection challenges and consequences. J Clin Epidemiol. 2017;89:173–180. doi: 10.1016/j.jclinepi.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 20.Kiernan M., Oppezzo M.A., Resnicow K., Alexander G.L. Effects of a methodological infographic on research participants' knowledge, transparency, and trust. Health Psychol. 2018;37:782–786. doi: 10.1037/hea0000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cowie J.M., Gurney M.E. The use of facebook advertising to recruit healthy elderly people for a clinical trial: baseline metrics. JMIR Res Protocols. 2018;7 doi: 10.2196/resprot.7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.