Abstract
A general method for the enantioselective synthesis of carbo- and heterocyclic carbonyl compounds bearing fluorinated α-tetrasubstituted stereocenters using palladium-catalyzed decarboxylative allylic alkylation is described. The stereoselective Csp3 –Csp3 cross-coupling reaction delivers five- and six-membered ketone and lactam products bearing (poly)fluorinated tetrasubstituted chiral centers in high yields and enantioselectivities. These fluorinated, stereochemically rich building blocks hold potential value in medicinal chemistry and are prepared using an orthogonal and enantioselective approach into such chiral moieties compared to traditional approaches, often without the use of electrophilic fluorinating reagents.
Graphical Abstract

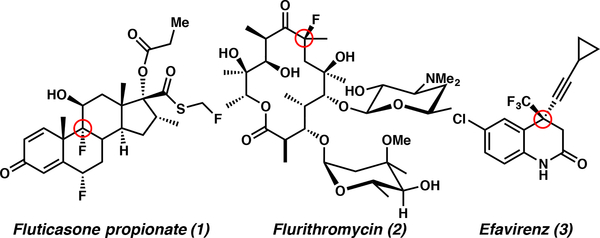
Organofluorine compounds often play a critical role in the lead optimization phase of drug discovery, due to their impact on various physico-chemical properties such as absorption, distribution, metabolitic stability, and excretion. Consequently, more than 20% of marketed pharmaceuticals contain C–F motifs, despite the fact that organofluorinated compounds are exceedingly rare in nature.1 Recently, molecules with tetrasubstituted stereocenters have attracted the interest of medicinal chemists aiming to incorporate three-dimensionality and added novelty.2 Importantly, there are many successful marketed pharmaceuticals bearing fluorinated tetrasubstituted stereocenters (1–3, Figure 1). For these reasons, there has been renewed interest in the synthesis of fluorinated tetrasubstituted stereocenters for use in drug discovery, and in particular, access to new classes of fluorinated analogs. Therefore, we believe that a general method for the construction of fluorine-containing tetrasubstituted stereocenters will be of particular interest to chemists in the area of drug discovery and development.
Figure 1.
Marketed Active Pharmaceutical Ingredients Bearing Fluorinated Tetrasubstitued Stereocenters.
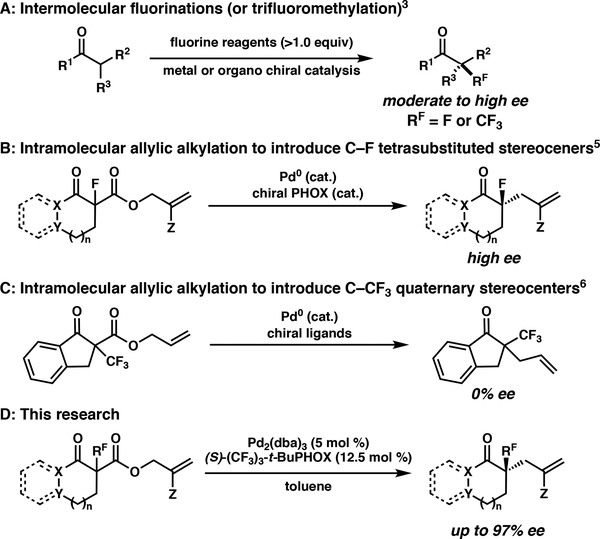
Methods to construct fluorine-containing α-tetrasubstituted ketones have been the subject of intense investigation over the past decade. The most prevalent strategy for fluorine incorporation is intermolecular catalytic asymmetric electrophilic fluorination (or trifluoromethylation) of enolates (Scheme 1A).3 Despite their potential utility in organic synthesis, the relatively low abundance of cheap, commercially available electrophilic fluorinating and trifluoromethylating reagents prohibit their widespread usage. As far back as 2005, the Stoltz and Nakamura groups independently reported the intramolecular asymmetric allylic alkylation of prochiral enolates derived from the decarboxylation of 1,3-dicarbonyl substrates (Scheme 1B).4,5 Using this strategy, several optically active α-fluoro α-tetrasubstitued cyclic carbonyl derivatives have been synthesized in high yield and enantioselectivity.
Scheme 1:
Asymmetric Construction of Fluorine- Containing α-Tetrasubstitued Ketones
While stereogenic C–F moieties have been previously investigated, the compatibility of fluoroalkyl groups in palladiumcatalyzed asymmetric allylic alkylation has remained unknown until recently. In 2011, Shibata and coworkers reported the first example of the construction of trifluoromethyl-bearing quaternary centers by intramolecular decarboxylative allylic alkylation of α-trifluoromethyl β-ketoesters (Scheme 1C).6 Unfortunately, attempts to render their reaction enantioselective were unsuccessful. Due to our interest in the field of asymmetric allylic alkylation, we endeavored to build on these previous reports and investigate a number of fluoroalkyl and fluoroallyl derivatives in asymmetric allylic alkylation reactions. Herein, we report the first general method for the construction of carbo- and heterocyclic carbonyl derivatives bearing α-fluoro-, α-fluoroalkyl-, or α-(2-fluoro)allyl substituents using palladium-catalyzed enantioselective decarboxylative allylic alkylation (Scheme 1D).
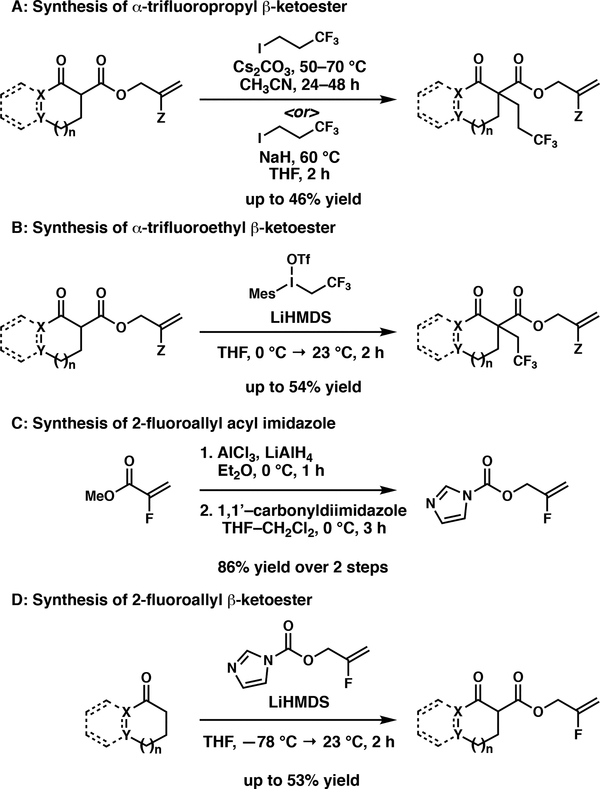
Importantly, with this strategy, a number of fluorinated alkyl and allyl groups are introduced into the substrate via standard 1,3-dicarbonyl chemistry (thermal, acidic or basic conditions) to produce racemic mixtures of compounds that serve as substrates for the mild and neutral asymmetric allylic alkylation reaction. In some cases, these fluorinated substrates are synthesized without the use of electrophilic fluorinating reagents. Furthermore, this allows for the non-asymmetric formation of the C–F or C–CF3 bonds, which are significantly more developed than their asymmetric equivalents. For example, 1,1,1,trifluoropropyl groups can be installed using standard β-keto ester alkylation conditions utilizing 1,1,1-trifluoropropyl iodide and base in moderate yields (Scheme 2A). The synthesis of 1,1,1-trifluoroethyl substituted β-keto esters proceeded smoothly with the use of 2,2,2-Trifluoroethyl (mesityl)iodonium trifluoromethanesulfonate7 (available in 2 steps from commercial materials) in the presence of LiHMDS. (Scheme 2B) During the preparation of this manuscript, a report using 2,2,2-Trifluoroethyl(mesityl) iodonium trifluoromethanesulfonate for the alkylation of 1,3-dicarbonyls was disclosed using similar conditions.8
Scheme 2:
Synthesis of Fluorinated β-Ketoesters
In addition to α-fluoroalkyl groups, a number of 2-fluoro allyl substrates were prepared without the use of electrophilic fluorinating reagents. Starting from commercially available Methyl 2-fluoroacrylate, reduction of the ester to the alcohol, followed by treatment with 1,1’-carbonyldiimidazole resulting in the formation of an acylating reagent (Scheme 2C). This reagent could then be used as previously reported9 to form a β-keto ester (Scheme 2D), which can be subsequently alkylated or fluorinated.4,5 Additionally, using known chemistry, α-fluoro β-keto esters can be synthesized using Selectfluor5d and α trifluoromethyl β-keto esters can be synthesized using Umemoto’s Reagent10, both of which are commercially available.
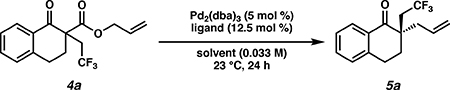
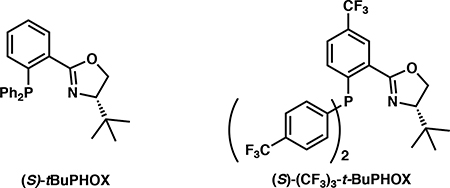
Initial reaction optimization started with trifluoroethyl substituted β-ketoester 4a using catalytic Pd2(dba)3 at 23 °C in diethylether in the presence of a chiral PHOX ligand toward the synthesis of ketone 5a. (Table 1).11 Employing the classic (S)-t-BuPHOX ligand, the desired product was formed in 88% yield and 85% ee (entry 1). Switching to the electron deficient (S)-(CF3)3-t-BuPHOX ligand, the desired product was furnished in an improved 99% yield and 90% ee (entry 2). Solvent effects were not very significant (entries 3–5), however THF gave a decreased ee of 86% (entry 3), while the less polar TBME and non-polar toluene performed similarly to diethyl ether. Based on these results, we determined that using Pd2(dba)3 (5.0 mol %) with (S)-(CF3)3-t-BuPHOX in toluene (0.033 M) at room temperature proved optimal. 12,13
Table 1:
Optimization of Conditions for Enantioselective Palladium-Catalyzed Allyllic Alkylationa
 | ||||
|---|---|---|---|---|
| entry | ligand | solvent | yield (%) | ee (%)b |
| 1 | (S)-t-BuPHOX | Et2O | 88 | 85 |
| 2 | (S)-(CF3)3-t-BuPHOX | Et2O | 99 | 90 |
| 3 | (S)-(CF3)3-t-BuPHOX | THF | 93 | 86 |
| 4 | (S)-(CF3)3-t-BuPHOX | TBME | 95 | 90 |
| 5 | (S)-(CF3)3-t-BuPHOX | toluene | 99 | 90 |
 | ||||
Conditions: β-ketoester 4a (0.1 mmol), Pd2(dba)3 (5.0 mol %), ligand (12.5 mol %), toluene (3 mL).
Determined by analytical chiral SFC.
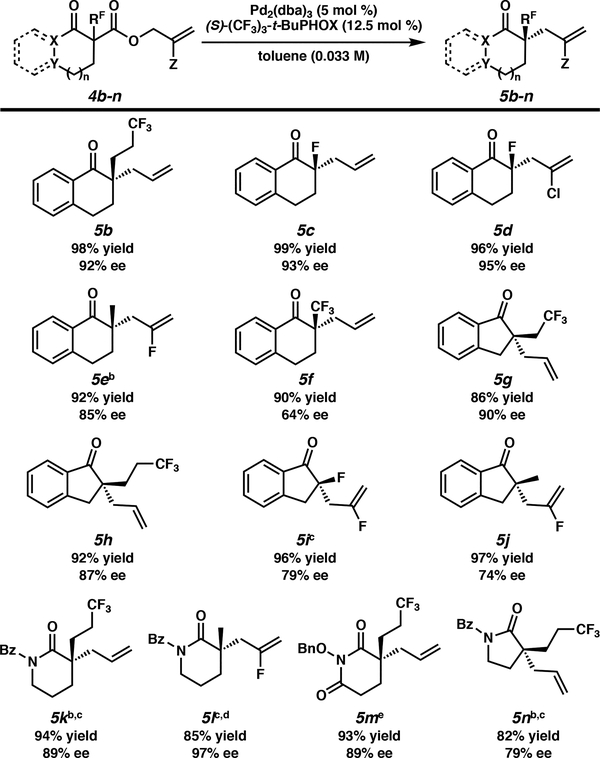
Subsequently, we explored the substrate scope of the enantioselective allylic alkylation of fluorine-containing 1,3-dicarbonyl compounds (Scheme 3). We found that our reaction was tolerant of a variety of α-fluoro-, α-fluoroalkyl-, and α-fluoroallyl substituents to deliver five- and six-membered ketone and lactam products bearing fluorinated tetrasubstituted stereocenters in high yields and enantioselectivities. Trifluoropropyl substituted 4b exhibited similar enantioinduction as 4a to furnish 5b in 92% ee and an extremely high yield. α-Fluoro tetrasubstituted compounds, which are usually introduced by direct fluorination with fluorine reagents and chiral catalysts,3 were prepared in a very efficient manner with high enantioselectivity (5c, 5d), even in the presence of a chloroallyl substituent (5d). Surprisingly, 2-fluoroallyl groups survived the palladium-catalyzed allylic alkylation even at elevated temperatures (40 °C),3 albeit in a slightly decreased enantioselectivity (5e). Recently, Shibata and coworkers described that enantioenriched indanone α-trifluoromethyl β-ketoesters lost their optical activity under the palladiumcatalyzed allylic alkylation reaction conditions in the presence of achiral ligands to deliver a racemic α-quaternary ketone, and when they tried to render the transformation enantioselective, they were unsuccessful.6 However, we were pleased to see that β-trifluoromethyl substituted tetralone substrate 4f reacted to furnish 5f with a moderate level of enantioselectivity. Generally, five membered cyclic β-ketoesters have performed worse than the corresponding 6-membered ring substrates, often providing the α-tetrasubstituted ketone products in comparatively low ee.4k Under these conditions, alkylation of the fivemembered indanone substrates 4g and 4h occurred with levels of enantioinduction similar to those observed for the tetralone substrates, with only a slightly diminished 87% ee for trifluoropropyl-substituted indanone 5h. Indanone substrates bearing a 2-fluoroallyl substituent proceeded in high yield, but only moderate enantioselectivity, to form products 5i and 5j, following the trend of the 2-fluoroallyl tetralone substrates. Gratifyingly, lactam substrates were also well tolerated in the reaction. Trifluoropropyl-substituted N-benzoyl δ-valero-lactam (5k) was obtained in 94% yield, and 89% ee. Surprisingly, in contrast to the negative influence of the 2-fluoroallyl substituent on substrates 5e, 5i, and 5j, the fluorine on the allyl group of N-benzoyl δ-valero-lactam 4l enhanced the enantioselectivity, providing 5l in 97% ee. Additionally, trifluoropropyl-substituted N-benzyloxy glutarimide was furnished in 89% ee with high yield. Finally, N-benzoyl pyrrolidinone 5n was obtained in diminished yield and ee.
Scheme 3:
Substrate Scope of Fluorine-Containing compounds in Enantioselective Allylic Alkylation a Unless otherwise noted, all reported yields are isolated yields. Enantiomeric excess (ee) was determined by chiral SFC. Standard conditions: β-ketoester 5 (0.1 mmol), Pd2(dba)3 (5 mol %), (S)-(CF3)3-t-BuPHOX (12.5 mol %), toluene (3 mL), 23 °C, 24 h. bReaction performed at 40 °C. cReaction performed in the presence of Pd2(pmdba)3 instead of Pd2(dba)3. dReaction performed at 60 °C. e Reaction performed at rt for 70 h.
In conclusion, we have developed a general method to construct fluorine-containing tetrasubstitued stereocenters by enantioselective palladium-catalyzed decarboxylative allylic alkylation. A strategy was adopted with the pre-introduction of fluorine on racemic substrates, which could be used as an orthogonal approach to the traditional fluorination and trifluoromethylation strategies. The reaction manifold demonstrated significant substitution tolerance to furnish a wide range of five- and six-membered ketone and lactam products bearing fluorinated tetrasubstituted stereocenters in high yields and enantioselectivities. Furthermore, we provide the first examples demonstrating that 2-fluoroallyl substituents can survive in the presence of certain palladium sources, and deliver related fluoroalkylated products in elevated enantiopurity.
Supplementary Material
ACKNOWLEDGEMENTS
The NIH-NIGMS (R01GM080269) and Caltech are thanked for support of our research program. E.L.G recognizes the NSF for a predoctoral research fellowship (No. DGE-1745301). Y.L. thanks the Program of Leading Graduate Schools: IGER Program in Green Natural Sciences (MEXT) at Nagoya University for financial support.
Footnotes
ASSOCIATED CONTENT
Supporting Information.
The Supporting Information is available free of charge on the ACS Publications website.
Experimental procedures and compound characterization (PDF)
Supporting Information Placeholder
REFERENCES
- 1.(a) For reviews on fluorine in medicinal chemistry, see:Böhm H-J; Banner D; Bendels S; Kansy M; Kuhn B; Müller K; Ob- stSander U; Stahl M ChemBioChem 2004, 5, 637. [DOI] [PubMed] [Google Scholar]; (b) Isanbor C; O’Hagan DJ Fluorine Chem. 2006, 127, 303. [Google Scholar]; (c) Kirk KL J. Fluorine Chem 2006, 127, 1013. [Google Scholar]; (d) Müller K; Faeh C; Diederich F Science 2007, 317, 1881. [DOI] [PubMed] [Google Scholar]; (e) Kirk KL Org. Process Res. Dev. 2008, 12, 305. [Google Scholar]; (f) O’Hagan D Chem. Soc. Rev 2008, 37, 308.18197347 [Google Scholar]; (g) Purser S; Moore PR; Swallow S; Gouverneur V Chem. Soc. Rev. 2008, 37, 320. [DOI] [PubMed] [Google Scholar]; (h) Hagmann WK J. Med. Chem. 2008, 51, 4359. [DOI] [PubMed] [Google Scholar]; (i) Qiu X-L; Xu X-H; Qing F-L Tetrahedron 2010, 66, 789. [Google Scholar]; (j) Hunter L Beilstein J. Org. Chem. 2010, 6, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Vulpettil A; Dalvit C Drug Discovery Today 2012, 17, 890. [DOI] [PubMed] [Google Scholar]; (l) Wang J; Sánchez-Roselló M; Aceña JL; del Pozo C; Sorochinsky AE; Fustero S; Soloshonok VA; Liu H Chem. Rev. 2013, 114, 2432. [DOI] [PubMed] [Google Scholar]; (m) Gillis Eric P., Eastman Kyle J., Hill Matthew D., Donnelly David J., and Meanwell Nicholas A. J. Med. Chem. 2015, 58, 8315. [DOI] [PubMed] [Google Scholar]
- 2.Lovering F; Bikker J; Humblet CJ Med. Chem. 2009, 52, 6752. [DOI] [PubMed] [Google Scholar]
- 3.(a) For reviews on fluorination and trifluoromethylation to construct α-tetrasubstituted stereocenters on ketones, see: Babbio C; Gouverneur V . Org. Biomol. Chem 2006, 4, 2065. [DOI] [PubMed] [Google Scholar]; (b) Prakash GKS; Beier P Angew. Chem., Int. Ed. 2006, 45, 2172. [DOI] [PubMed] [Google Scholar]; (c) Pihko PM Angew. Chem., Int. Ed. 2006, 45, 544. [DOI] [PubMed] [Google Scholar]; (d) Hamashima Y; Sodeoka M Synlett 2006, 1467. [Google Scholar]; (e) Shibata N; Ishimaru T; Nakamura S; Toru TJ Fluorine Chem. 2007, 128, 469. [Google Scholar]; (f) Ma J-A; Cahard D Chem. Rev. 2008, 108, PR1. [DOI] [PubMed] [Google Scholar]; (g) Shibata N; Mizuta S; Kawai H Tetrahedron: Asymmetry 2008, 19, 2633. [Google Scholar]; (h) Brunet VA; O’Hagan D Angew. Chem. Int. Ed. 2008, 47, 1179. [DOI] [PubMed] [Google Scholar]; (i) Ueda M; Kano T; Maruoka K Org. Biomol. Chem. 2009, 7, 2005. [DOI] [PubMed] [Google Scholar]; (j) Lectard S; Hamashima Y; Sodeoka M Adv. Synth. Catal. 2010, 352, 2708. [Google Scholar]; (k) Cahard D; Xu X; Couve-Bonnaire C; Pannecoucke X Chem. Soc. Rev. 2010, 39, 558. [DOI] [PubMed] [Google Scholar]; (l) Zheng Y; Ma J-A Adv. Synth. Catal. 2010, 352, 2745. [Google Scholar]; (m) Shibata N; Matsnev A; Cahard D Beilstein J. Org. Chem. 2010, 6, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Furuya T; Kamlet AS; Ritter T Nature 2011, 473, 470. [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) Valero G; Companyó X; Rios R Chem. Eur. J. 2011, 17, 2018. [DOI] [PubMed] [Google Scholar]; (p) Hollingworth C; Gouverneur V Chem. Commun. 2012, 48, 2929. [DOI] [PubMed] [Google Scholar]; (q) Macé Y; Magnier E Eur. J. Org. Chem. 2012, 2479. [Google Scholar]; (r) Liang T; Neumann CN; Ritter T Angew. Chem. Int. Ed. 2013, 52, 8214. [DOI] [PubMed] [Google Scholar]; (s) Yang X; Wu T; Phipps RJ; Toste DF Chem. Rev. 2015, 115, 826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) For accounts and reviews on palladium-catalyzed allylic alkylation chemistry, see: Trost BM; Vranken Van D. L. Chem. Rev. 1996, 96, 395. [DOI] [PubMed] [Google Scholar]; (b) Trost BM Acc. Chem. Res. 1996, 29, 355 [Google Scholar]; (c) Pfaltz A; Lautens M “Comprehensive Asymmetric Catalysis” III, Vol. 2 (Eds.: Jacobsen EN; Pfaltz A; Yamamoto H), Springer: New York, 1999, pp. 833–884. [Google Scholar]; (d) Helmchen GJ Organomet. Chem. 1999, 576, 203. [Google Scholar]; (e) Trost BM; Lee C “Catalytic Asymmetric Synthesis ”, 2nd ed (Ed.: Ojima I), Wiley: New York, 2000, pp. 593–649. [Google Scholar]; (f) Trost BM Chem. Pharm. Bull. 2002, 50, 1. [DOI] [PubMed] [Google Scholar]; (g) Graening T; Schmalz H-G Angew. Chem 2003, 115, 2684; Angew. Chem. Int. Ed. 2003, 42, 2580. [DOI] [PubMed] [Google Scholar]; (h) Trost BM J. Org. Chem. 2004, 69, 5813. [DOI] [PubMed] [Google Scholar]; (i) Lu Z; Ma S Angew. Chem 2008, 120, 264; Angew. Chem. Int. Ed. 2008, 47, 258. [Google Scholar]; (j) Behenna DC; Mohr JT; Sherden NH; Marinescu SC; Harned AW; Tani K; Seto M; Ma S; Novák Z; Krout MR; McFadden RM; Roizen JL; Enquist JA Jr.; White DE; Levine SR; Petrova KV; Iwashita A; Virgil SC;Stoltz BM Liu Y; Han S; Liu W; Stoltz BM Acc. Chem. Res. 2015, 48, 740.25715056 [Google Scholar]
- 5.(a) Mohr JT; Behenna DC; Harned AM; Stoltz BM Angew. Chem., Int. Ed. 2005, 44, 6924. [DOI] [PubMed] [Google Scholar]; (b) Nakamura M; Hajra A; Endo K; Nakamura E Angew. Chem 2005, 117, 7414; Angew. Chem. Int. Ed. 2005, 44, 7248. [DOI] [PubMed] [Google Scholar]; (c) Behenna DC; Liu Y; Yurino T; Kim J; White DE; Virgil SC; Stoltz BM Nat. Chem. 2012, 4, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Mohr JT; Nishimata T; Behenna DC; Stoltz BM J. Am. Chem. Soc. 2006, 128, 11348. [DOI] [PubMed] [Google Scholar]
- 6.Shibata N; Suzuki S; Furukawa T; Kawai H;K Adv. Synth. Catal. 2011, 353, 2037. [Google Scholar]
- 7.Tolnai GL; Szekely A; Mako Z; Gati T; Daru J; Bihari T; Stirling A; Novak Z Chem. Commun , 2015, 51, 4488. [DOI] [PubMed] [Google Scholar]
- 8.Zhao C-L; Yang J; Han Z-Z; Zheng C-PJ Fluor. Chem , 2017, 204, 23. [Google Scholar]
- 9.Trost BM; Xu JJ Org. Chem. 2007, 72, 9372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shibata N; Suzuki S; Furukawa T; Kawai H; Tokunaga E; Yuan Z; Cahardb D Adv. Synth. Catal. 2011, 353, 2037. [Google Scholar]
- 11.Absolute configuration of 5c was determined by comparison of the optical rotation of the same compound to the known literature value, see: Nakamura M; Hajra A; Endo K; Nakamura E. Angew. Chem 2005, 117, 7414; Angew. Chem. Int. Ed. 2005, 44, 7248 The absolute configuration of all other products generated herein was assigned by analogy to the absolute configuration of 5c. For full details, see the Supporting Information. [DOI] [PubMed] [Google Scholar]
- 12.With the consideration of heating requirements for some less reactive substrates, toluene was assigned as the best solvent.
- 13.A de-fluorinated side product was obtained in the presence of Pd(PPh3)4 for preparing racemic standards. Therefore, racemic samples were prepared in the presence of Pd2(dba)3 (or Pd2(pmdba)3) and achiral Gly-PHOX for fluoro-allyl products. For full details, see the Supporting Information.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.