Abstract
Objective
To determine the independent association of patient- and surgery-specific risk with receipt of outpatient preoperative testing.
Methods
Using administrative data from 2010–2013 (Marketscan® Commercial Claims and Encounters), we constructed a retrospective cohort of 678,368 privately-insured, non-elderly US adults who underwent one of ten operations, including one lower-risk and one higher-risk operation from five surgical specialties. Outcomes were receipt of nine outpatient tests in the 30 days before surgery and cost of those tests. Patient-specific risk was based on Revised Cardiac Risk Index (RCRI) and, alternatively, the Charlson Comorbidity Index (CCI). Surgery-specific risk was based on operation (higher- versus lower-risk within each specialty). Multivariable logistic regression models were constructed to measure the independent association of patient- and surgery-specific risk with the receipt of tests.
Results
Receipt of tests ranged from 0.9% (pulmonary function tests) to 46.8% (blood counts), and 65.2% of patients received at least one test. Mean cost per patient for all tests was $124.38. Higher RCRI was strongly associated (Odds Ratio (OR) >2) with receipt of stress tests and echocardiograms, and more modestly associated [OR <2] with receipt of most other tests. Undergoing higher-risk operations was strongly associated with receipt of most tests. Results were similar using the CCI for patient-specific risk.
Conclusion
Surgery-specific risk is strongly associated with receipt of most preoperative tests, which is consistent with preoperative testing protocols based as much or more on the planned operation as on patient-specific risk factors. Whether this pattern of preoperative testing represents optimal care is uncertain.
Keywords: Preoperative testing, non-cardiac surgery, overuse
1. INTRODUCTION
In an era of increased attention on overuse of medical services, preoperative testing has come under scrutiny.1–4 Evidence that preoperative testing improves outcomes is lacking5,6 and testing practices vary widely.7,8 Many have raised concerns that preoperative testing is overused.1,2 Choosing Wisely recommendations to perform fewer preoperative tests were made by numerous professional societies, including the American Society of Anesthesiologists and the American College of Surgeons.9
Recent clinical guidelines have recommended that preoperative testing not be performed “routinely.”10–12 The American Society of Anesthesiologists Practice Advisory for Preanesthesia Evaluation recommends that tests be ordered selectively “after consideration of specific information obtained from sources such as medical records, patient interview, physical examination, and the type or invasiveness of the planned procedure and anesthesia.”10 The American College of Cardiology/American Heart Association (ACC/AHA) guidelines on perioperative cardiovascular evaluation recommend against testing for coronary artery disease when the combined surgical and patient characteristics predict a risk of a major adverse cardiac event of less than 1%.11
While guidelines recommend that patient- and surgery-specific risk assessment should guide preoperative testing, how these factors affect testing in actual practice is unknown. Several recent studies have examined predictors of preoperative testing, but these studies were limited to a single test,13–15 or focused exclusively on low-risk operations and procedures,7,8,16,17 precluding the ability to assess the contribution of surgery-specific risk. In this study, we aimed to measure the independent association of patient- and surgery-specific risk with the receipt of preoperative tests in a range of operations using a nationwide data source.
2. MATERIALS AND METHODS
2.1 Data
We used MarketScan® Commercial Claims and Encounters (Truven Health Analytics) from 2010–2013. MarketScan® collects utilization and expenditure data for employees, retirees, and their dependents from more than 250 medium- and large-sized employers and health plans from across all 50 states and the District of Columbia. The database includes data from inpatient and outpatient visits for approximately 43 to 55 million beneficiaries in each of the years we examined, which represents approximately twenty percent of all privately insured individuals in the US. This study was exempted by the Johns Hopkins University Institutional Review Board.
2.2 Selection of Operations
To assess the effect of surgery-specific risk on preoperative testing, we chose two operations from five surgical specialties: general, vascular, orthopedic, urologic, and gynecologic. Our goal was to choose two common operations with gradient of surgery-specific risk (i.e., one “lower-risk” and one “higher-risk” operation in each specialty, not necessarily “low-risk” and “high-risk”). There are no universally accepted methods for classifying the intrinsic surgery-specific risk of different operations. We considered adopting the three-category classification system used in some versions of the ACC/AHA guidelines (i.e., of low-, intermediate-, and high-risk), but this schema is difficult to operationalize due to limited examples of operations within each category, and the ACC/AHA guidelines have moved away from this system in their most recent guidelines.11 Therefore, we opted to use the Johns Hopkins Surgical Classification System (JHSCS), which classifies operations into five risk categories based on physiologic factors such as expected blood loss and fluid shifts.18 While the JHSCS includes a fairly comprehensive list of operations in each category, we had to estimate the category for several operations that were not listed. However, we chose operations with high face-validity for having qualitatively different intrinsic risks (e.g., cholecystectomy has higher risk than hemorrhoidectomy, and total prostatectomy has higher risk than transurethral resection of the prostate). The higher-risk operations included laparoscopic cholecystectomy, carotid endarterectomy, total prostatectomy, total knee arthroplasty, and hysterectomy. The lower-risk operations included hemorrhoidectomy, peripheral artery angioplasty or stent, transurethral resection of the prostate, shoulder and knee arthroscopies, and tubal ligation. The Current Procedural Terminology codes and the JHSCS category for each operation are listed in Appendix Table 1.
Appendix Table 1.
Surgical procedures and their JHSCS Risk Category and CPT Codes
| Surgery Type | JHSCS Risk Level a | CPT Codes |
|---|---|---|
| General Surgery | ||
| Laparoscopic cholecystectomy b | 2 | 47562–7564 |
| Hemorrhoidectomy b | 1 c | 46083, 46221, 46250, 46255, 46260, 46320, 46500, 46930, 46945–46947 |
| Vascular Surgery | ||
| Carotid endarterectomy | 4 | 35301 |
| Peripheral artery angioplasty/stent b | 2 c | 37205, 37220, 37221, 37226, 37230, 37236 |
| Urology | ||
| Total Prostatectomy | 4 | 55810, 55812, 55815, 55840, 55842, 55845, 55866 |
| Transurethral resection of the prostate b | 2 c | 52450, 52601, 52612, 52614, 52630, 52647, 52648, 53850, 53852, 53853 |
| Orthopedic Surgery | ||
| Knee Arthroplasty | 3 | 27445–27447, 27486, 27487 |
| Arthroscopy (knee/shoulder) b | 2 | 29806, 29807, 29819–29828, 29866–29868, 29870, 29871,29873–29877, 29879–29887 |
| Gynecology | ||
| Hysterectomy | 3 | 58150; 58152; 58180, 58260, 58262, 58290, 58291, 58550, 58552–58554 |
| Tubal Ligation b | 2 | 58600, 58670, 58671 |
The JHSCS risk category ranges from 1 (low risk) to 5 (high risk)
include outpatient procedures only
estimated
Abbreviations: JHSCS – Johns Hopkins Surgical Classification System; CPT - Current Procedural Terminology
Our intent was to examine elective operations, as patients undergoing urgent or emergent operations may be less likely to undergo outpatient preoperative testing. Therefore, we included only operations performed on an outpatient basis for each of the lower-risk operations and laparoscopic cholecystectomy (the higher-risk general surgery operation). For the other higher-risk operations, we included those performed on an outpatient basis or on the first day of a hospital admission.
2.3 Study Population
We included beneficiaries aged 18 to 64 years who were continuously enrolled in a health plan for one year prior to their operation and had at least one outpatient visit with a primary care provider between one month and one year prior to their operation. If patients had more than one eligible operation, we only included the first they received.
To identify comorbid diagnoses, we searched for International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9) diagnostic codes in inpatient and outpatient records in the 12 months prior to the operation (included ICD-9 codes are listed in Appendix Table 2). As the measure of patient-specific risk, we calculated the revised cardiac risk index (RCRI), similar to previous studies using administrative data.19–21 Diabetes mellitus, ischemic heart disease, chronic kidney disease, congestive heart failure, and cerebrovascular disease were each assigned 1 point. For this study, we did not assign RCRI points for “high-risk” operations because we wanted to use a separate variable to account for surgery-specific risk, so the possible RCRI ranged from 0 to 5.
Appendix Table 2.
Comorbidity diagnosis codes
| Comorbidity | ICD-9-CM Codes |
|---|---|
| Diabetes | 250 |
| Ischemic Heart Disease | 410–413, 414.0, 414.12, 414.2–414.9 |
| Congestive Heart Failure | 398.91, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 425.4–425.9, 428 |
| Cerebrovascular Disease | 362.34, 430–438 |
| Chronic Kidney Disease | 403.01, 403.11, 403.91, 404.02, 404.03, 404.12, 404.13, 404.92, 404.93, 582, 583.0–583.7, 585, 586, 588.0, V42.0, V45.1, V56 |
| Chronic Pulmonary Disease | 416.8, 416.9, 490–505, 506.4, 508.1, 508.8 |
| Liver disease | 070.22, 070.23, 070.32, 070.33, 070.44, 070.54, 070.6, 070.9, 456.0–456.2, 570, 571, 572.2–572.8, 573.3, 573.4, 573.8, 573.9, V42.7 |
| Dementia | 290, 294.1, 331.2 |
| Hypertension | 362.11, 401–405 |
| Hyperlipidemia | 272.0–272.4 |
| Atrial Fibrillation | 427.31 |
| Anemia | 280–285 |
| Venous Thromboembolism | 415.1, 416.2, 451–453 |
Abbreviations: ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification
2.4 Outcomes
The primary outcome was receipt of nine tests performed as an outpatient during the 30 days before surgery. Included tests were blood counts, metabolic panels, coagulation tests, urinalyses, electrocardiograms, stress tests, echocardiograms, chest radiographs, and pulmonary function tests performed in an outpatient setting (Current Procedural Terminology codes are listed in Appendix Table 3).
Appendix Table 3.
Preoperative test CPT codes
| Test group | Test | CPT code |
|---|---|---|
| Metabolic panel | Basic metabolic panel | 80047, 80048 |
| Comprehensive metabolic panel | 80053 | |
| Renal function panel | 80069 | |
| Blood count | Complete blood count | 85025, 85027 |
| Hemoglobin | 85018 | |
| Hematocrit | 85014 | |
| Coagulation test | PTT | 85730 |
| PT w/INR | 85610 | |
| Urinalysis | Urinalysis | 81000, 81001, 81002, 81003, 81005 |
| ECG | 12-lead ECG | 93000, 93005, 93010 |
| Cardiac stress test | Stress ECG | 93015, 93016, 93017, 93018 |
| Stress ECHO | 93350, 93351 | |
| Myocardial Perfusion Imaging | 78451, 78452, 78453, 78454 | |
| Stress MRI | 75559, 75563 | |
| Echocardiography | Transthoracic Echocardiography | 93303, 93304, 93306, 93307, 93308 |
| Chest radiography | Chest radiograph | 71010, 71020 |
| PFT | Spirometry | 94010 |
| Spirometry pre- and post- | 94060 | |
| bronchodilator administration | ||
| Respiratory flow volume loop | 94375 |
Abbreviations: CPT – Current Procedural Terminology; ECG – electrocardiogram; PFT – Pulmonary Function Testing; MRI – magnetic resonance imaging; PTT – partial thromboplastin time; PT – prothrombin time; INR – international normalized ratio
The costs reported in the analysis are the total payments (not charges), which includes the payments by insurance and by patients. All costs were inflation-adjusted to 2013 dollars using the Consumer Price Index for medical care services.22 To deal with outliers, we replaced negative costs with $0, and we truncated high costs at the 99th percentile for each test.
Since we were not able to determine the indication for testing, we also assessed use of each test in the 180 days (six 30-day blocks) prior to the operation to compare use of tests in the preoperative month with the baseline use of each test in earlier months.
2.5 Statistical Analyses
We tabulated the percentage of patients who received each test prior to their operation. We tabulated the mean cost for each test for patients who received the test, as well as the mean cost per test distributed among all patients.
We used separate logistic regression models to estimate the odds of a patient receiving each preoperative test dependent on patient-specific risk (RCRI), surgery-specific risk (lower-risk or higher-risk surgery), and potential confounders (surgical specialty, age, and sex). Due to the small number of patients with RCRI scores greater than two, we collapsed the RCRI into a three-level categorical variable (0, 1, ≥ 2). We accounted for clustering of patients within surgical specialties when calculating the variance. Additionally, we performed two sensitivity analyses and an exploratory analysis. First, we repeated the analyses using the Charlson Comorbidity Index (CCI)23 as the measure of patient-specific risk instead of using the RCRI. Second, we repeated the analyses using the RCRI as the measure of patient-specific risk and also included indicator variables for each of the eight additional comorbidities (hypertension, hyperlipidemia, liver disease, thromboembolism, chronic pulmonary disease, atrial fibrillation, anemia, and dementia) that may affect the actual or perceived need for a preoperative testing. Finally, as an exploratory analysis, we repeated the main analyses including an interaction term between patient-specific and surgery-specific risk variables. We used STATA version 14.0 for all statistical analyses.
3. RESULTS
A total of 678,368 patients were in the final analysis (Table 1). The median age was 51 years, 58.9% were female, and 43.3% underwent the higher-risk operations. Hypertension (39.4%) and hyperlipidemia (37.9%) were the most common comorbidities, and diabetes was the most common RCRI comorbidity (14.0%). A majority of patients had no RCRI comorbidities (79.1%).
Table 1.
Patient Characteristics
| Total | Lower-Risk Operations | Higher-Risk Operations | |
|---|---|---|---|
| Patients | 678,368 | 384,896 (56.3%) | 293,472 (43.3%) |
| Age, median (IQR), y | 51 (42–58) | 51 (42–58) | 51 (42–58) |
| Female Sex, N (%) | 58.9 | 48.2 | 73.0 |
| Comorbid Disease, % | |||
| Hypertension | 39.4 | 37.1 | 42.6 |
| Hyperlipidemia | 37.9 | 38.0 | 37.7 |
| Diabetes | 14.0 | 13.2 | 15.1 |
| Chronic Pulmonary | 12.1 | 11.5 | 13.0 |
| Disease | |||
| Anemia | 9.8 | 7.2 | 13.3 |
| Ischemic Heart | 6.1 | 5.8 | 6.5 |
| Disease | |||
| Liver Disease | 5.7 | 3.0 | 9.3 |
| Cerebrovascular | 3.0 | 2.6 | 3.4 |
| Disease | |||
| Venous | 1.7 | 1.6 | 1.8 |
| Thromboembolism | |||
| Chronic Kidney | 1.6 | 1.4 | 1.8 |
| Disease | |||
| Congestive Heart | 1.5 | 1.4 | 1.7 |
| Failure | |||
| Atrial Fibrillation | 1.5 | 1.5 | 1.7 |
| Dementia | <0.1 | <0.1 | 0.1 |
| RCRI, % | |||
| 0 | 79.1 | 80.3 | 77.6 |
| 1 | 16.7 | 15.9 | 17.7 |
| ≥2 | 4.2 | 3.8 | 4.7 |
| CCI, % | |||
| 0 | 62.7 | 68.0 | 55.8 |
| 1 | 22.5 | 21.2 | 24.1 |
| ≥2 | 14.9 | 10.8 | 20.1 |
Abbreviations: RCRI – Revised Cardiac Risk Index; CCI – Charlson Comorbidity Index; IQR – interquartile range
The proportion of patients receiving each preoperative test and costs are shown in Table 2. The most common preoperative test received were blood counts (46.8% of patients), while the least common test were pulmonary function tests (0.9% of patients). Nearly two-thirds (65.2%) of patients received at least one test. Among patients who received the test, stress tests were the most expensive test ($932.65) and urinalyses were the least expensive ($13.60). Mean cost for all tests was $124.38 per patient; blood counts, metabolic panels, coagulation tests, electrocardiograms, and chest radiographs each contributed between $13 and $26 per patient, while the other four tests contributed less than $10 each.
Table 2.
Receipt of Preoperative Tests and Their Cost
| Blood Count | MP | Coag Test | UA | ECG | Stress Test | ECHO | CXR | PFT | |
|---|---|---|---|---|---|---|---|---|---|
| Receipt of Test, % | |||||||||
| Cholecystectomy (n=132,536) | 57.2 | 53.7 | 11.0 | 25.3 | 35.1 | 2.2 | 1.6 | 18.6 | 0.7 |
| Hemorrhoidectomy (n=23,591) | 24.4 | 21.4 | 5.2 | 9.4 | 15.8 | 0.9 | 0.7 | 7.3 | 0.5 |
| CEA (n=2,671) | 50.3 | 48.0 | 32.8 | 17.2 | 58.7 | 21.6 | 12.7 | 44.4 | 2.3 |
| Peripheral artery angioplasty/stent (n=3,367) | 51.5 | 48.4 | 36.8 | 11.7 | 28.9 | 4.3 | 3.1 | 17.2 | 1.3 |
| Prostatectomy (n=16,393) | 59.1 | 53.3 | 34.5 | 39.9 | 63.6 | 5.4 | 3.1 | 41.0 | 0.9 |
| TURP (n=10,753) | 49.6 | 48.3 | 19.7 | 55.6 | 45.3 | 2.6 | 2.0 | 19.6 | 1.2 |
| Knee arthroplasty (n=71,524) | 60.9 | 56.5 | 39.1 | 49.5 | 66.3 | 6.2 | 4.1 | 42.3 | 1.6 |
| Arthroscopy (knee/shoulder) (n=326,595) | 37.0 | 34.0 | 10.5 | 13.6 | 37.6 | 1.7 | 1.3 | 13.3 | 0.8 |
| Hysterectomy (n=70,348) | 60.0 | 37.9 | 12.9 | 29.1 | 34.1 | 1.6 | 1.4 | 20.4 | 0.8 |
| Tubal ligation (n=20,590) | 55.2 | 21.3 | 6.3 | 23.0 | 11.7 | 0.3 | 0.5 | 5.3 | 0.5 |
| Total | 46.8 | 40.6 | 14.5 | 22.8 | 39.0 | 2.4 | 1.7 | 18.6 | 0.9 |
|
| |||||||||
| Cost per patient, mean $ | |||||||||
| Received test | 28.85 | 50.02 | 31.85 | 13.60 | 66.62 | 932.65 | 533.51 | 85.72 | 72.38 |
| All patients | 13.51 | 20.33 | 4.62 | 3.09 | 26.00 | 22.22 | 9.16 | 15.92 | 0.62 |
Abbreviations: CEA –carotid endarterectomy; Coag – coagulation; CXR – chest radiograph; ECHO – echocardiogram; ECG – electrocardiogram; MP – metabolic panel; PFT – pulmonary function test; TURP – transurethral resection of the prostate
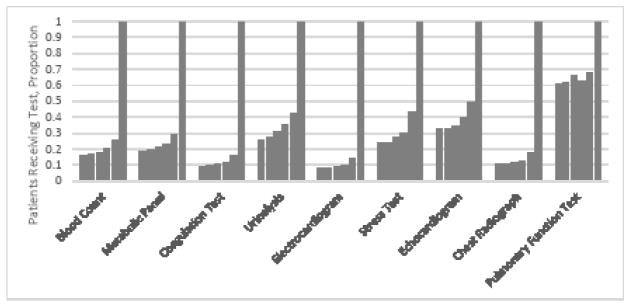
The receipt of each test during the six months before surgery is shown in Figure 1. For each test, use in the preoperative month was substantially higher than any of the preceding five months.
Figure 1.
Proportion of patients receiving each test during the six months prior to surgery. Proportions have been normalized so that the proportion receiving each test in preoperative month (the right-most column for each test) equals 1.
The association of patient- and surgery-specific risk with the receipt of preoperative tests is shown in Table 3. Higher RCRI was modestly associated [odds ratio (OR) greater than 1.0 and less than 2.0] with the receipt of most of the tests. The notable exceptions were stress tests and echocardiograms, where higher RCRI was strongly associated (OR greater than 2.0) with use. Undergoing the higher-risk operations was strongly associated with receipt of most of the tests.
Table 3.
Association of Patient- and Surgery-Specific Risk with Receipt of Preoperative Testing, Adjusted Analysis a
| Odds Ratio (95% CI) | |||
|---|---|---|---|
|
|
|||
| RCRI = 1 b | RCRI ≥ 2 b | Higher-Risk Operations c | |
| Blood Count | 1.28 (1.22, 1.34) | 1.47 (1.34, 1.62) | 2.15 (1.46, 3.17) |
| Metabolic Panel | 1.52 (1.43, 1.61) | 1.70 (1.51, 1.93) | 2.22 (1.50, 3.29) |
| Coagulation Test | 1.29 (1.21, 1.39) | 1.67 (1.42, 1.96) | 3.82 (2.61, 5.61) |
| Urinalysis | 1.23 (1.21, 1.27) | 1.40 (1.30, 1.51) | 3.53 (1.56, 7.99) |
| Electrocardiogram | 1.37 (1.28, 1.47) | 1.34 (1.20, 1.50) | 2.33 (1.81, 3.01) |
| Stress Test | 2.69 (2.46, 2.93) | 4.96 (4.14, 5.94) | 2.54 (2.28, 2.83) |
| Echocardiogram | 2.44 (2.31, 2.59) | 4.63 (4.19, 5.12) | 2.23 (2.13, 2.34 |
| Chest Radiograph | 1.29 (1.24, 1.35) | 1.49 (1.40, 1.58) | 3.50 (3.35, 3.66) |
| Pulmonary Function Test | 1.54 (1.47, 1.62) | 2.30 (2.21, 2.40) | 1.52 (1.27, 1.82) |
Abbreviations: CI – confidence interval; CCI – Charlson Comorbidity Index
Logistic regression models included RCRI score (0, 1, or ≥2), operation risk (higher-risk or lower-risk), and confounders (age, sex, and surgical specialty).
reference group is RCRI = 0
reference group is lower-risk operations
The results of the sensitivity analyses are shown in Appendix Tables 4 and 5. Using the CCI instead of the RCRI yielded similar results, with the notable exception that CCI had a strong association with receipt of pulmonary function tests (CCI = 0 reference; OR 4.64 (95% CI 4.37–4.93) for CCI = 1, OR 6.33 (95% CI 5.90–6.80) for CCI ≥2). Including the non-RCRI comorbidities in the models using RCRI as the measure of patient-specific risk yielded similar results for the association between surgery-specific risk and receipt of preoperative tests, but slightly attenuated the association between patient-specific risk and receipt of each preoperative test. The results of the exploratory analyses are shown in Appendix Tables 6 and 7. Including an interaction term between patient-specific and surgery-specific risk variables led to qualitatively similar results.
Appendix Table 4.
Association of Patient- and Surgery-Specific Risk with Receipt of Preoperative Testing, Adjusted Analysis Using CCI as Measure of Patient-Specific Risk a
| Odds Ratio (95% CI) | |||
|---|---|---|---|
|
|
|||
| CCI=1 b | CCI≥2 b | Higher-Risk Operations c | |
| Blood Count | 1.27 (1.19, 1.35) | 1.44 (1.27, 1.63) | 2.11 (1.42, 3.12) |
| Metabolic Panel | 1.39 (1.29, 1.50) | 1.56 (1.31, 1.84) | 2.17 (1.46, 3.21) |
| Coagulation Test | 1.28 (1.25, 1.31) | 1.54 (1.35, 1.76) | 3.75 (2.44, 5.76) |
| Urinalysis | 1.17 (1.11, 1.22) | 1.11 (0.76, 1.62) | 3.53 (1.58, 7.90) |
| Electrocardiogram | 1.29 (1.26, 1.32) | 1.33 (1.29, 1.38) | 2.30 (1.81, 2.91) |
| Stress Test | 1.70 (1.47, 1.97) | 2.05 (1.72, 2.44) | 2.59 (2.31, 2.90) |
| Echocardiogram | 1.75 (1.59, 1.93) | 2.56 (2.32, 2.81) | 2.24 (1.96, 2.55) |
| Chest Radiograph | 1.35 (1.30, 1.40) | 1.51 (1.43, 1.59) | 3.42 (3.13, 3.74) |
| Pulmonary Function Test | 4.64 (4.37, 4.93) | 6.33 (5.90, 6.80) | 1.36 (1.01, 1.81) |
Abbreviations: CI – confidence interval; CCI – Charlson Comorbidity Index
Logistic regression models included CCI score (0, 1, or ≥ 2), operation risk (higher-risk or lower-risk), and confounders (age, sex, and surgical specialty).
reference group is CCI = 0
reference group is lower-risk operations
Appendix Table 5.
Association of Patient- and Surgery-Specific Risk with Receipt of Preoperative Testing, Adjusted Analysis including non-RCRI Comorbidities a
| Odds Ratio (95% CI) | |||
|---|---|---|---|
|
|
|||
| RCRI=1 b | RCRI≥2 b | Higher-Risk Operations c | |
| Blood Count | 1.14 (1.11, 1.16) | 1.19 (1.09, 1.28) | 2.09 (1.42, 3.10) |
| Metabolic Panel | 1.28 (1.24, 1.32) | 1.29 (1.18, 1.40) | 2.14 (1.43, 3.21) |
| Coagulation Test | 1.14 (1.08, 1.21) | 1.26 (1.09, 1.44) | 3.76 (2.60, 5.44) |
| Urinalysis | 1.14 (1.10, 1.18) | 1.21 (1.11, 1.32) | 3.47 (1.55, 7.79) |
| Electrocardiogram | 1.16 (1.10, 1.22) | 1.02 (0.94, 1.11) | 2.26 (1.73, 2.94) |
| Stress Test | 2.27 (2.09, 2.48) | 3.88 (3.31, 4.55) | 2.48 (2.19, 2.80) |
| Echocardiogram | 2.02 (1.92, 2.12) | 3.28 (3.06, 3.51) | 2.13 (1.99, 2.29) |
| Chest Radiograph | 1.15 (1.11, 1.20) | 1.22 (1.15, 1.28) | 3.42 (3.31, 3.54) |
| Pulmonary Function Test | 1.22 (1.18, 1.25) | 1.32 (1.20, 1.44) | 1.47 (1.28, 1.68) |
Abbreviations: CI – confidence interval; RCRI – Revised Cardiac Risk Index
Logistic regression models included RCRI score (0, 1, or ≥ 2), operation risk (higher-risk or lower-risk), non-RCRI comorbidities (hypertension, hyperlipidemia, liver disease, thromboembolism, chronic pulmonary disease, atrial fibrillation, anemia, and dementia) and confounders (age, sex, surgical specialty).
reference group is RCRI = 0
reference group is lower-risk operations
Appendix Table 6.
Association of Patient- and Surgery-Specific Risk with Receipt of Preoperative Testing, Adjusted Analysis including an Interaction Term Between Patient- and Surgery-Specific Risk Variables, Stratified by Patient-Specific Riska
| Odds Ratio (95% CI) | |||
|---|---|---|---|
|
| |||
| RCRI=0 | RCRI=1 | RCRI≥2 | |
|
|
|||
| Higher-Risk Operations b | Higher-Risk Operations b | Higher-Risk Operations b | |
| Blood Count | 2.35 (1.55, 3.56) | 1.69 (1.21, 2.36) | 1.47 (1.13, 1.93) |
| Metabolic Panel | 2.51 (1.72, 3.66) | 1.62 (1.12, 2.35) | 1.34 (0.99, 1.82) |
| Coagulation Test | 4.13 (2.67, 6.40) | 3.44 (2.55, 4.65) | 2.68 (1.76, 4.08) |
| Urinalysis | 3.79 (1.60, 9.00) | 3.03 (1.39, 6.62) | 2.54 (1.27, 5.11) |
| Electrocardiogram | 2.47 (2.07, 2.95) | 2.05 (1.34, 3.14) | 1.84 (1.00, 3.39) |
| Stress Test | 2.91 (2.70, 3.14) | 2.38 (2.06, 2.75) | 2.07 (1.43, 2.99) |
| Echocardiogram | 2.49 (2.17, 2.87) | 2.09 (1.89, 2.31) | 1.88 (1.44, 2.45) |
| Chest Radiograph | 3.72 (3.34, 4.13) | 3.12 (2.91, 3.36) | 2.80 (2.31, 3.39) |
| Pulmonary Function Test | 1.59 (1.27, 2.00) | 1.42 (1.21, 1.68) | 1.38 (1.20, 1.58) |
Abbreviations: CI – confidence interval; RCRI – Revised Cardiac Risk Index
Logistic regression models included RCRI score (0, 1, or ≥2), operation risk (higher-risk or lower-risk), an interaction term between RCRI and operation risk, and confounders (age, sex, surgical specialty).
reference group is lower-risk operations
Appendix Table 7.
Association of Patient- and Surgery-Specific Risk with Receipt of Preoperative Testing, Adjusted Analysis including an Interaction Term Between Patient- and Surgery-Specific Risk Variables, Stratified by Surgery-Specific Risk a
| Odds Ratio (95% CI) | ||||
|---|---|---|---|---|
|
| ||||
| Lower-Risk Operations | Higher-Risk Operations | |||
|
|
||||
| RCRI=1 b | RCRI≥2 b | RCRI=1 b | RCRI≥2 b | |
| Blood Count | 1.48 (1.36, 1.61) | 1.82 (1.60, 2.06) | 1.07 (0.92, 1.24) | 1.14 (0.92, 1.41) |
| Metabolic Panel | 1.85 (1.67, 2.04) | 2.27 (1.97, 2.62) | 1.19 (0.97, 1.47) | 1.21 (1.00, 1.47) |
| Coagulation Test | 1.43 (1.37, 1.49) | 2.11 (2.01, 2.22) | 1.19 (0.96, 1.47) | 1.37 (0.89, 2.11) |
| Urinalysis | 1.40 (1.24, 1.57) | 1.75 (1.50, 2.03) | 1.12 (0.97, 1.28) | 1.17 (0.92, 1.48) |
| Electrocardiogram | 1.49 (1.43, 1.54) | 1.53 (1.39, 1.69) | 1.23 (0.92, 1.66) | 1.14 (0.73, 1.77) |
| Stress Test | 3.03 (2.80, 3.27) | 6.09 (4.40, 8.42) | 2.48 (2.17, 2.83) | 4.32 (3.86, 4.85) |
| Echocardiogram | 2.70 (2.61, 2.80) | 5.47 (4.67, 6.40) | 2.27 (1.87, 2.75) | 4.11 (3.06, 5.52) |
| Chest Radiograph | 1.42 (1.39, 1.45) | 1.74 (1.62, 1.86) | 1.19 (1.01, 1.41) | 1.31 (1.02, 1.69) |
| Pulmonary Function Test | 1.63 (1.54, 1.72) | 2.48 (2.36, 2.60) | 1.46 (1.24, 1.71) | 2.15 (1.90, 2.42) |
Abbreviations: CI – confidence interval; RCRI – Revised Cardiac Risk Index
Logistic regression models included RCRI score (0, 1, or ≥2), operation risk (higher-risk or lower-risk), an interaction term between RCRI and operation risk, and confounders (age, sex, surgical specialty).
reference group is RCRI=0
4. DISCUSSION
In this analysis of privately insured, non-elderly patients undergoing ten common operations, the most common preoperative tests were metabolic panels, blood counts, and electrocardiograms, which were performed before nearly half of the operations. Higher patient-specific risk was only modestly associated with receipt of most preoperative tests, while higher surgery-specific risk was strongly associated with receipt of most of the tests.
The prevalence of preoperative tests in the current study is generally consistent with the recent literature. For example, Kerr and colleagues found that 0.7% of VA patients and 2.1% of Medicare patients had stress testing prior to low-risk surgical procedures,13 and Benarroch-Gampel and colleagues found that 54% of patients underwent at least one test prior to hernia repair.16 However, our study provides a nationally representative estimate of the prevalence of preoperative tests across a wider range of elective operations and estimates the cost contributions of each test.
Our finding that undergoing one of the higher-risk operations was strongly associated with receipt of preoperative tests can be interpreted in several ways. It is possible that clinicians performing preoperative evaluation are incorporating the surgery-specific risk into an overall risk estimate that guides the intensity of preoperative testing, which would be consistent with current guideline recommendations. Alternatively, there may be a threshold effect for some clinicians or hospitals, where a standardized evaluation including routine testing is performed for all patients undergoing what is considered to be a major operation. For example, guidelines published by the National Institute for Health and Care Excellence (NICE) in the United Kingdom recommend that all adults undergoing “major surgery” have complete blood counts tested prior to surgery, regardless of age and comorbidities.24 Guidelines for preoperative testing may be established by institutions who take a similar approach to NICE and require routine tests for certain operations.18 A recent qualitative study of surgeons supports the notion that a large proportion of preoperative testing is driven by hospital and anesthesiology requirements, which is in turn determined by the what operation is planned.25
Our findings for the group of patients undergoing general surgery procedures—laparoscopic cholecystectomy and hemorrhoidectomy—are particularly illustrative. Over half of the patients undergoing laparoscopic cholecystectomy received metabolic panels and blood counts and over one-third received an ECG, more than double the corresponding rates among patients undergoing hemorrhoidectomy. Previous studies have shown that laparoscopic cholecystectomy is generally not a high-risk operation, with operative mortality rates much lower than one percent.26,27 However, laparoscopic cholecystectomy is typically performed in an operating room under general anesthesia, in contrast to hemorrhoidectomy, which is often performed in an office setting under local anesthesia. Widespread use of the RCRI may create confusion about the risk of laparoscopic cholecystectomy, since as an intraperitoneal operation, it would be classified as a high-risk operation by the RCRI.28 Alternatively, some hospitals may adhere to policies requiring all patients undergoing general anesthesia in an operating room setting to have certain preoperative tests, resulting in overuse of testing before low-risk operations like laparoscopic cholecystectomy.
Our findings need to be interpreted in light of the limited evidence regarding the effects of preoperative testing on surgical outcomes, which this study was not able to address. Some commentators have argued that preoperative testing is overused in many situations,1,2 largely based on observational studies showing that routine tests are infrequently abnormal and unlikely to change management. However, except for cataract surgery,29 high-quality evidence demonstrating the safety of forgoing preoperative testing has proven difficult to generate.30 Preoperative testing is also associated with potential harms, such as invasive procedures to follow-up false positive results or delaying the planned operation.3 Much of the recent attention on potential overuse of preoperative testing has focused on low-risk patients or those undergoing low-risk operations. While the benefit-risk ratio of preoperative testing may be particularly unfavorable in these situations, it may not be favorable for patients undergoing higher risk operations either. The fact that none of the tests were received by more than half of patients in our study suggests that a substantial number of clinicians may not believe that routine preoperative testing is helpful. More research into which patients actually benefit from preoperative testing is needed.
Despite concerns about potential overuse, the uncertainty about the effectiveness of preoperative tests and office visits means that there may be underuse of some preoperative tests as well. For example, some guidelines recommend (on the basis of expert opinion) performing a urinalysis prior to surgical implantation of foreign material or invasive urologic procedures.12 We found that only about half of patients undergoing knee arthroplasty or one of the urologic procedures had a urinalysis in the 30 days prior to surgery.
The cumulative cost of preoperative evaluation has been estimated to be between $3 billion31 and $40 billion.32 Our study was not designed to provide a precise estimate of the cumulative cost of preoperative testing, but there are approximately 27 million operations performed in the US annually33 and we found that the total cost of tests in the preoperative month to be over $120 per patient, so it supports an estimate of cumulative cost on the lower end of this range. Given this high cost, even a moderate amount of overuse could result in a significant amount of unnecessary spending. Our results highlight that only a small fraction of total spending results from the more expensive tests such as stress tests, which have received so much attention.13,14 Furthermore, solely focusing on potential overuse in low-risk operations overlooks the greater resources in preoperative testing before higher-risk operations, where overuse may also be present. Additional research is needed to determine whether the current state of preoperative testing represents overuse, underuse, or appropriate care.
One limitation of this research relates to identifying preoperative tests in claims data. We considered any outpatient test done within 30 days of surgery to be a preoperative test, but each of the tests we examined has numerous uses other than as preoperative testing, and some of the tests we identified may have been unrelated to the upcoming operation. However, the use of each test was lower in the months leading up to the preoperative month than in the preoperative month, suggesting that a majority were related to the upcoming operation. We were also unable to determine who ordered the tests. Dedicated preoperative clinics have been associated with decreased preoperative testing,34 but since many preoperative clinics do not bill those visits, they are not captured in claims data. Therefore, we were not able to determine whether the pattern of testing found in this study holds true for patients seen in preoperative clinics.
This study had several other limitations. Our primary analysis used the RCRI as the measure of patient-specific risk. The RCRI was developed as a measure of cardiac risk, so it may not be the ideal measure of more generic patient-specific risk. For example, cardiac death represents only about one-third of perioperative mortality,21 and the RCRI has not been shown to be highly predictive of total perioperative mortality.35 However, we obtained similar results when we used the CCI or included additional non-RCRI risk factors in our models, which suggests that our results are robust. Additionally, our population included only privately-insured, non-elderly patients in the US, so our findings may not apply to other populations. Finally, the nature of insurance claims data limits the ability to ascertain postoperative outcomes, particularly for outpatient operations where there is not even an opportunity to observe inhospital complications that might be captured by insurance claims, so we were unable assess the association of preoperative tests with postoperative outcomes.
5. CONCLUSIONS
This study found that patient-specific risk is modestly associated with receipt of most preoperative tests, while surgery-specific risk is more strongly associated with most of the tests. This pattern suggests that preoperative testing is based as much or more on the planned surgical procedure as it is on patient-specific risk factors. While this pattern of preoperative testing is generally adherent to guidelines that recommend that both patient-specific and surgery-specific factors guide the preoperative evaluation, more research examining the effect of preoperative testing on postoperative outcomes are needed to determine whether current practice represents optimal care.
Supplementary Material
Acknowledgments
Funding: This work was supported by the National Institutes of Health [grant numbers T32HL007180, K24AG049036] and the Agency for Healthcare Research and Quality [grant number K12HS023009]. Funding sources had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Financial support and conflict of interest disclosure:: Dr. Riggs’s work on this manuscript was funded by NIH grant T32 HL007180 and AHRQ grant K12 HS023009, and Dr. Segal’s work was funded by NIH grant K24 AG049036. The authors have no other conflicts of interest to disclose.
The authors appreciate assistance with statistical analysis from the Johns Hopkins Biostatistics Center, which is supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health through Grant Number 1UL1TR001079.
LIST OF ABBREVIATIONS
- ACC/AHA
American College of Cardiology/American Heart Association
- CCI
Charlson Comorbidity Index
- JHSCS
Johns Hopkins Surgical Classification System
- OR
Odds Ratio
- RCRI
Revised Cardiac Risk Index
Footnotes
Conflicts of Interest: The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brateanu A, Rothberg MB. Why do clinicians continue to order 'routine preoperative tests' despite the evidence? Cleve Clin J Med. 2015;82(10):667–670. doi: 10.3949/ccjm.82a.15118. [DOI] [PubMed] [Google Scholar]
- 2.Smetana GW. The Conundrum of Unnecessary Preoperative Testing. JAMA Intern Med. 2015;175(8):1359–1361. doi: 10.1001/jamainternmed.2015.2106. [DOI] [PubMed] [Google Scholar]
- 3.Baxi SM, Lakin JR. Preoperative Testing--A Bridge to Nowhere: A Teachable Moment. JAMA Intern Med. 2015;175(8):1272–1273. doi: 10.1001/jamainternmed.2015.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riggs KR, Segal JB. What is the rationale for preoperative medical evaluations? A closer look at surgical risk and common terminology. Br J Anaesth. 2016;117(6):681–684. doi: 10.1093/bja/aew302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balk EM, Earley A, Hadar N, Shah N, Trikalinos TA. Benefits and Harms of Routine Preoperative Testing: Comparative Effectiveness. Comparative Effectiveness Review No. 130. Rockville, MD: AHRQ; 2014. [PubMed] [Google Scholar]
- 6.Wijeysundera DN, Austin PC, Beattie W, Hux JE, Laupacis A. Outcomes and processes of care related to preoperative medical consultation. Arch Intern Med. 2010;170(15):1365–1374. doi: 10.1001/archinternmed.2010.204. [DOI] [PubMed] [Google Scholar]
- 7.Kirkham KR, Wijeysundera DN, Pendrith C, et al. Preoperative Laboratory Investigations: Rates and Variability Prior to Low-risk Surgical Procedures. Anesthesiology. 2016 doi: 10.1097/ALN.0000000000001013. [DOI] [PubMed] [Google Scholar]
- 8.Kirkham KR, Wijeysundera DN, Pendrith C, et al. Preoperative testing before low-risk surgical procedures. CMAJ. 2015;187(11):E349–358. doi: 10.1503/cmaj.150174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wijeysundera DN, Beattie WS, Karkouti K, Neuman MD, Austin PC, Laupacis A. Association of echocardiography before major elective non-cardiac surgery with postoperative survival and length of hospital stay: population based cohort study. BMJ. 2011:342. doi: 10.1136/bmj.d3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Apfelbaum JL, Connis RT, Nickinovich DG, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology. 2012;116(3):522–538. doi: 10.1097/ALN.0b013e31823c1067. [DOI] [PubMed] [Google Scholar]
- 11.Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(22):e77–e137. doi: 10.1016/j.jacc.2014.07.944. [DOI] [PubMed] [Google Scholar]
- 12.Feely MA, Collins CS, Daniels PR, Kebede EB, Jatoi A, Mauck KF. Preoperative testing before noncardiac surgery: guidelines and recommendations. Am Fam Physician. 2013;87(6):414–418. [PubMed] [Google Scholar]
- 13.Kerr EA, Chen J, Sussman JB, Klamerus ML, Nallamothu BK. Stress testing before low-risk surgery: so many recommendations, so little overuse. JAMA Intern Med. 2015;175(4):645–647. doi: 10.1001/jamainternmed.2014.7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheffield KM, McAdams PS, Benarroch-Gampel J, et al. Overuse of preoperative cardiac stress testing in medicare patients undergoing elective noncardiac surgery. Ann Surg. 2013;257(1):73–80. doi: 10.1097/SLA.0b013e31826bc2f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun LY, Gershon AS, Ko DT, et al. Trends in Pulmonary Function Testing Before Noncardiothoracic Surgery. JAMA Intern Med. 2015;175(8):1410–1412. doi: 10.1001/jamainternmed.2015.2087. [DOI] [PubMed] [Google Scholar]
- 16.Benarroch-Gampel J, Sheffield KM, Duncan CB, et al. Preoperative laboratory testing in patients undergoing elective, low-risk ambulatory surgery. Ann Surg. 2012;256(3):518–528. doi: 10.1097/SLA.0b013e318265bcdb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen CL, Lin GA, Bardach NS, et al. Preoperative medical testing in Medicare patients undergoing cataract surgery. N Engl J Med. 2015;372(16):1530–1538. doi: 10.1056/NEJMsa1410846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasternak RL. Preanesthesia Evaluation of the Surgical Patient. Refresher courses in Anesthesiology. 1996;24:205–219. [Google Scholar]
- 19.Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative Beta-Blocker Therapy and Mortality after Major Noncardiac Surgery. N Engl J Med. 2005;353(4):349–361. doi: 10.1056/NEJMoa041895. [DOI] [PubMed] [Google Scholar]
- 20.Lindenauer PK, Pekow P, Wang K, Gutierrez B, Benjamin EM. Lipid-lowering therapy and in-hospital mortality following major noncardiac surgery. JAMA. 2004;291(17):2092–2099. doi: 10.1001/jama.291.17.2092. [DOI] [PubMed] [Google Scholar]
- 21.Boersma E, Kertai MD, Schouten O, et al. Perioperative cardiovascular mortality in noncardiac surgery: validation of the Lee cardiac risk index. Am J Med. 2005;118(10):1134–1141. doi: 10.1016/j.amjmed.2005.01.064. [DOI] [PubMed] [Google Scholar]
- 22.Bureau of Labor Statistics. [Accessed February 20, 2016];Consumer price index for medical care services. http://www.bls.gov/data/
- 23.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 24.National Institute for Health and Care Excellence. [Accessed January 15, 2016];Preoperative tests for elective surgery. 2003 https://www.nice.org.uk/guidance/cg3. [PubMed]
- 25.Riggs KR, Berger ZD, Makary MA, Bass EB, Chander G. Surgeons’ views on preoperative medical evaluation: a qualitative study. Perioperative Medicine. 2017;6(1):16. doi: 10.1186/s13741-017-0072-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shea JA, Healey MJ, Berlin JA, et al. Mortality and complications associated with laparoscopic cholecystectomy. A meta-analysis. Annals of Surgery. 1996;224(5):609–620. doi: 10.1097/00000658-199611000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ingraham AM, Cohen ME, Ko CY, Hall BL. A Current Profile and Assessment of North American Cholecystectomy: Results from the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;211(2):176–186. doi: 10.1016/j.jamcollsurg.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation. 2011;124(4):381–387. doi: 10.1161/CIRCULATIONAHA.110.015701. [DOI] [PubMed] [Google Scholar]
- 29.Schein OD, Katz J, Bass EB, et al. The value of routine preoperative medical testing before cataract surgery. Study of Medical Testing for Cataract Surgery. N Engl J Med. 2000;342(3):168–175. doi: 10.1056/NEJM200001203420304. [DOI] [PubMed] [Google Scholar]
- 30.Chung F, Yuan H, Yin L, Vairavanathan S, Wong DT. Elimination of preoperative testing in ambulatory surgery. Anesth Analg. 2009;108(2):467–475. doi: 10.1213/ane.0b013e318176bc19. [DOI] [PubMed] [Google Scholar]
- 31.Fischer SP. Cost-effective preoperative evaluation and testing. Chest. 1999;115(suppl_2):96S–100S. doi: 10.1378/chest.115.suppl_2.96s. [DOI] [PubMed] [Google Scholar]
- 32.Roizen M. Preoperative patient evaluation. Can J Anaesth. 1989;36(1):S13–S19. doi: 10.1007/BF03005321. [DOI] [PubMed] [Google Scholar]
- 33.Wier L, Steiner C, Owens P. Surgeries in hospital-owned outpatient facilities, 2012. HCUP Statistical Brief #188. 2015 [Google Scholar]
- 34.Edwards AF, Slawski B. Preoperative Clinics. Anesthesiol Clin. 2016;34(1):1–15. doi: 10.1016/j.anclin.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Ford MK, Beattie WS, Wijeysundera DN. Systematic review: prediction of perioperative cardiac complications and mortality by the revised cardiac risk index. Ann Intern Med. 2010;152(1):26–35. doi: 10.7326/0003-4819-152-1-201001050-00007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.