Abstract
Context
The role of antiangiogenic agents in advanced renal cell carcinoma (RCC) is well established. However, it is still not clear whether this benefit can be extrapolated to the adjuvant setting.
Objective
To determine the efficacy and safety of antiangiogenic agents in patients with RCC and a high risk of relapse after nephrectomy.
Evidence acquisition
We searched PubMed, Embase, and the Cochrane Central Register of Controlled Trials for randomized controlled trials (RCTs) evaluating the use of any oral antiangiogenic agent compared to placebo in post-nephrectomy RCC patients. Prespecified data elements were extracted from each trial. Outcomes of interest included overall survival (OS) and disease-free survival (DFS). The overall effect was pooled using the DerSimonian and Laird random-effects models.
Evidence synthesis
Three RCTs comparing antiangiogenics to placebo among 3693 patients met our inclusion criteria and were used in meta-analyses. Overall, antiangiogenics did not improve DFS (hazard ratio [HR] 0.92, 95% confidence interval [CI] 0.78–1.07) or OS (HR 0.99, 95% CI 0.79–1.25). These results persisted when restricting the analysis to patients with clear cell carcinoma and patients with highest risk of relapse. Similarly, sunitinib did not show any improvement in the entire cohort for either DFS (HR 0.89, 95% CI 0.67–1.19) or OS (HR 1.11, 95% CI 0.90–1.37).
Conclusions
In this meta-analysis, antiangiogenics did not improve OS and DFS over placebo in high-risk RCC after nephrectomy. Further studies are needed to identify the patient population that might derive a benefit from antiangiogenics in the adjuvant setting.
Patient summary
In this article, we found that there is currently insufficient evidence to support the use of oral antiangiogenics in nonmetastatic renal cell carcinoma after nephrectomy. In addition, the use of oral antiangiogenics was associated with a 2.7-fold higher rate of significant side effects compared to placebo.
Keywords: Adjuvant chemotherapy, Renal cell carcinoma, Pazopanib, Sunitinib, Sorafenib, Tyrosine kinase inhibitors
1. Introduction
Each year, there are approximately 64 000 new cases of renal cell carcinoma (RCC) in the USA, and 14 000 deaths [1]. Clear-cell histology is the most common histology, accounting for 75–80% of all RCC cases. Surgical resection with nephrectomy has been the standard of care for nonmetastatic RCC, with close surveillance afterwards. However, despite surgical resection, approximately one-third of patients experience relapse [2]. Given their efficacy and survival benefit in the metastatic RCC setting, antiangiogenics, also known as VEGF tyrosine kinase inhibitors (TKIs), have been studied in the adjuvant setting to evaluate their efficacy in potentially decreasing the rate of relapse and enhancing cure. Several trials utilizing different VEGF TKIs have been completed and reported conflicting results [3–7].
In this meta-analysis, we sought to determine the efficacy and safety of adjuvant VEGF TKIs in patients with RCC who are at high risk of relapse after nephrectomy.
2. Evidence acquisition
The reporting of this systematic review follows the Preferred Reporting Items for Systematic Review and Meta-Analyses statement [8].
2.1. Study eligibility
We included randomized controlled trials (RCTs). The search was not limited by language, sample size, or date of publication. We searched for studies that included patients with nonmetastatic RCC who underwent nephrectomy and afterwards received either a VEGF TKI or placebo in the adjuvant setting. Outcomes of interest were disease-free survival (DFS), overall survival (OS), and grade ≥3 toxicities.
2.2. Information sources and search methods
A comprehensive literature search was conducted from database inception through January 1, 2018 for the electronic databases MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) for relevant abstracts and titles. The detailed search strategy is described in the Supplementary material. Two individual reviewers (M.B.S. and T.H.) identified articles that were eligible for further review by screening the available abstracts and titles. If a study was deemed relevant, then it was obtained and reviewed. Disagreements were harmonized via consensus and through arbitration by a third reviewer if consensus was not possible. The final search identified five articles reporting three RCTs (Supplementary Fig. 1).
2.3. Data collection and extraction
Prespecified data elements were extracted from each trial, including baseline characteristics, study design, sample size, interventions used, outcome measures, funding sources, pathological features, and adverse events (Table 1) [3–7]. Two reviewers extracted the data from the included studies, and disagreements were resolved by referring to a third reviewer. The number of events in each trial was extracted, when available, based on the intention-to-treat approach.
Table 1.
Studies included in our meta-analysis
| Trial | Regimen | Pts (n) |
Study sites | Funding | Pathological inclusion criteria | Median age, yr (range) | DFS | OS |
|---|---|---|---|---|---|---|---|---|
| ASSURE 2016 [7] | Sunitinib Sorafenib Placebo |
647 649 647 |
226 centers; USA and Canada | US NCI, ECOG-ACRIN, Pfizer, and Bayer | CC and NCC RCC; pT1b G3-4 N0 M0 or T(any) G(any) N+ (resected) M0 | 56 (49-64) 55(48-63) 57(46-64) |
70 mo 73.4 mo 79.6 mo |
5-yr 77.9% 5-yr 80.5% 5-yr 80.3% |
| ASSURE high risk 2017 [4] | Sunitinib Sorafenib Placebo |
358 355 356 |
Same as ASSURE 2016 | Only CC RCC; pT3/pT4 or N+ disease | 59 (31-83) 56 (20-84) 58 (19-84) |
5-yr 47.7% 5-yr 49.9% 5-yr 50% |
5-yr 75.2% 80.20% 76.50% |
|
| S-TRAC 2016 [5] | Sunitinib Placebo |
309 306 |
99 centers; 21 countries | Pfizer | CC RCC; T3/T4, no or undetermined nodal involvement, or any T stage with local nodal involvement; any Fuhrman grade | 57 (25-83) 58 (21-82) |
6.8 yr 5.6 yr |
HR 1.01 (0.72-1.44; P=0.94) |
| S-TRAC 2017 [6] | Sunitinib Placebo |
194 194 |
Pfizer | T3, no or undetermined nodal involvement, Fuhrman grade ≥2, and ECOG PS ≥1; T4 and/or nodal involvement | NR NR |
6.2 yr 4 yr |
Median NR (HR 0.92, 95% CI 0.66-1.28; p = 0.6) Median NR | |
| PROTECT [3] | Pazopanib Placebo |
571 564 |
263 centers in 26 countries |
Novartis | Nonmetastatic CC RCC; pT2 G3-4 N0, pT3-4 G any N0, or pT any G any N1 | 58 (22-83) 58 (21-82) |
3-yr 67% 3-yr 64% |
HR 0.79, 95% CI 0.57-1.09; p = 0.16 |
ACRIN = American College of Radiology Imaging Network; CC = clear cell; DFS = disease-free survival; ECOG = Eastern Cooperative Oncology Group; NCC = non–clear cell; NCI = National Cancer Institute; NR = not reached; OS = overall-survival; PS = performance score; Pts = patients; RCC = renal cell carcinoma.
2.4. Risk of bias assessment and quality of evidence
We used the Cochrane Collaboration risk of bias assessment tool for randomized trials, focusing on randomization methods, allocation concealment, blinding, and attrition [9].
2.5. Statistical analysis
Analyses were conducted using features on RevMan version 5.3 (The Nordic Cochrane Center, Copenhagen, Denmark). We used the hazard ratio (HR) provided by the included trials to conduct a pooled HR for survival outcomes. We conducted random-effects meta-analyses using the Der-Simonian and Laird method to pool treatment effects from included studies [10]. We used the I2 statistic to assess for heterogeneity across the included studies. An I2 value >50% suggests substantial heterogeneity between studies. Two-sided p values <0.05 suggest statistical significance. We conducted sensitivity analyses using leave-one-out meta-analyses to assess the influence of each study on the overall results.
3. Evidence synthesis
3.1. Search strategy
In total, 1251 titles and abstracts were identified via the screening electronic strategy, of which five articles describing three RCTs met the inclusion criteria evaluating the use of VEGF TKIs versus placebo in post-nephrectomy RCC patients (Supplementary Fig. 1). The main reasons for exclusions were: the use of different medications in the adjuvant setting; the use of adjuvant VEGF TKIs in other malignancies; and nonrandomized controlled trials (mainly reviews). The three RCTs included a total of 3693 enrolled patients (Table 1).
3.2. Trial characteristics
The first study was the ASSURE trial (we will refer to this in the text as ASSURE 2016), which is a multicenter, phase 3 clinical trial in the USA and Canada, led by the Eastern Cooperative Oncology Group (ECOG-ACRIN) with participation by multiple other cooperative groups [7]. The study assessed the use of sunitinib or sorafenib compared to placebo in patients with nonmetastatic RCC post-nephrectomy (n = 1943) with node-positive (N+) or pT1b G3–4 N0 M0 disease (Table 1). Both clear cell and non-clear cell histologies were included. Patients received 1 yr of adjuvant sunitinib (50 mg), sorafenib (800 mg), or placebo. However, the study was later amended because of patient intolerance with a reduction in dose to 37.5 mg and 400 mg for sunitinib and sorafenib, respectively. Dose reductions to as low as 25 mg for sunitinib and 400 mg every other day for sorafenib were allowed. The ASSURE trial did not show any DFS or OS benefit of adjuvant use of VEGF TKIs for either sorafenib nor sunitinib. A separate article (referred to as ASSURE 2017) was recently published, reporting a post hoc analysis for high-risk clear cell carcinoma patients defined as pT3, pT4, or node-positive disease. Similarly, adjuvant use of VEGF TKI did not improve DFS or OS in this high-risk group [4].
The S-TRAC trial was a multicenter, international (21 countries) phase 3 trial assessing the efficacy of sunitinib in post-nephrectomy clear cell carcinoma of stage 3 or higher or with regional lymph-node metastasis or both (referred to as S-TRAC 2016) [5]. Patients (n = 615) were randomized to receive either sunitinib (50 mg) or placebo for 1 yr. Dose interruptions or dose reductions to 37.5 mg were allowed (25 mg was not allowed). A follow-up article (referred to as S-TRAC 2017) recently updated the S-TRAC OS data and further examined treatment outcomes in the group with highest risk in an exploratory analysis (defined as T3, no or undetermined nodal involvement, Fuhrman grade ≥2, and ECOG performance status ≥1; or T4 and/or nodal involvement) [6]. When assessing outcomes by blinded independent reviewers, DFS was found to be favorable in patients receiving adjuvant sunitinib compared to those on placebo in both the overall population (HR 0.76, 95% confidence interval [CI] 0.59–0.98; p = 0.03) and the group with highest risk (HR 0.74, 95% CI 0.55–0.99; p = 0.04). However, OS was not different between the two groups according to the longest follow-up period reported (HR 0.92, 95% CI 0.66–1.28; p = 0.6). Currently, it is unclear whether the DFS benefit will translate into an OS benefit on further follow up.
We only found one RCT (PROTECT trial), examining the effect of adjuvant use of pazopanib in RCC after nephrectomy [3]. PROTECT was a phase 3 RCT comparing the efficacy of pazopanib versus placebo for 1 yr in nonmetastatic clear-cell or predominant clear-cell RCC after nephrectomy (Table 1). Although the study was initially planned for pazopanib 800 mg, this was later reduced to 600 mg/d because of toxicities and treatment discontinuation. The primary endpoint of DFS for pazopanib 600 mg was not met and there was no added benefit of pazopanib 600 mg over placebo (HR 0.86, 95% CI 0.70–1.06; p = 0.165). In a secondary analysis evaluating DFS among patients who received pazopanib 800 mg, DFS was significantly better in the pazopanib group compared to placebo. However, there was no OS benefit of pazopanib in the adjuvant setting when compared to placebo for either pazopanib 600 mg (HR 0.79, 95% CI 0.57–1.09; p = 0.16) or pazopanib 800 mg (HR 0.89, 95% CI 0.54–1.46; p = 0.65; Table 1).
3.3. VEGF TKI versus placebo: meta-analysis
Overall, antiangiogenics did not improve DFS (HR 0.92, 95% CI 0.78–1.07) or OS (HR 0.99, 95% CI 0.79–1.25) when compared to placebo in post-nephrectomy patients with nonmetastatic RCC (Fig. 1). These results were robust when restricting the analysis to patients with clear cell carcinoma (ASSURE 2017 cohort) [4] for which neither DFS (HR 0.89, 95% CI 0.78–1.01) nor OS (HR 0.95, 95% CI 0.79–1.15) significantly differed between the VEGF TKI and placebo groups (Supplementary Fig. 2). Similarly, DFS was similar between the two groups (HR 0.89, 95% CI 0.78–1.02) when examining the effect of VEGF TKIs in the subsets of patients with the highest risk of relapse as reported by individual trials (PROTECT: pT2 G3–4 N0, pT3–T4 G any N0, or pT any G any N1; ASSURE 2017: pT3, pT4, or node-positive disease; STRAC 2017: T3, no or undetermined nodal involvement, Fuhrman grade ≥2, and ECOG performance score ≥1; T4 and/or nodal involvement; Supplementary Fig. 3).
Fig. 1.
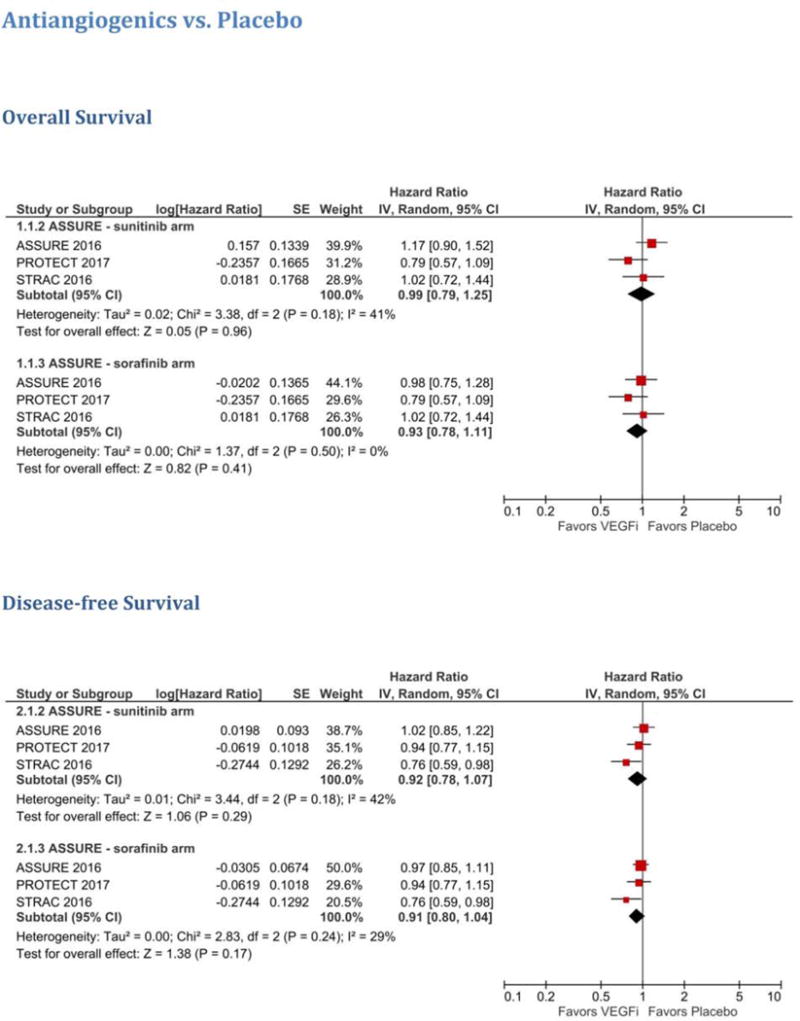
Meta-analysis of antiangiogenics versus placebo for the overall cohort. VEGFi = VEGF inhibitor.
3.4. Sunitinib versus placebo
Two studies evaluated the efficacy of sunitinib versus placebo in RCC patients in the adjuvant setting (Table 1). In a meta-analysis of these two trials (ASSURE 2016 and S-TRAC 2016), sunitinib did not show any improvement in the overall cohort for either DFS (HR 0.89, 95% CI 0.67–1.19) or OS (HR 1.11, 95% CI 0.90–1.37; Fig. 2). Similarly, in the clear-cell RCC population, sunitinib did not show benefit in terms of DFS (HR 0.85, 95% CI 0.69–1.05) or OS (HR 1.04, 95% CI 0.83–1.31; Supplementary Fig. 4). In addition, similar results were seen for the patients with the highest risk, with both groups having comparable DFS (HR 0.85, 95% 0.67–1.07; Supplementary Fig. 5). A meta-analysis of OS for the group with the highest risk was not carried out as OS data for that group were not reported in S-TRAC 2017.
Fig. 2.
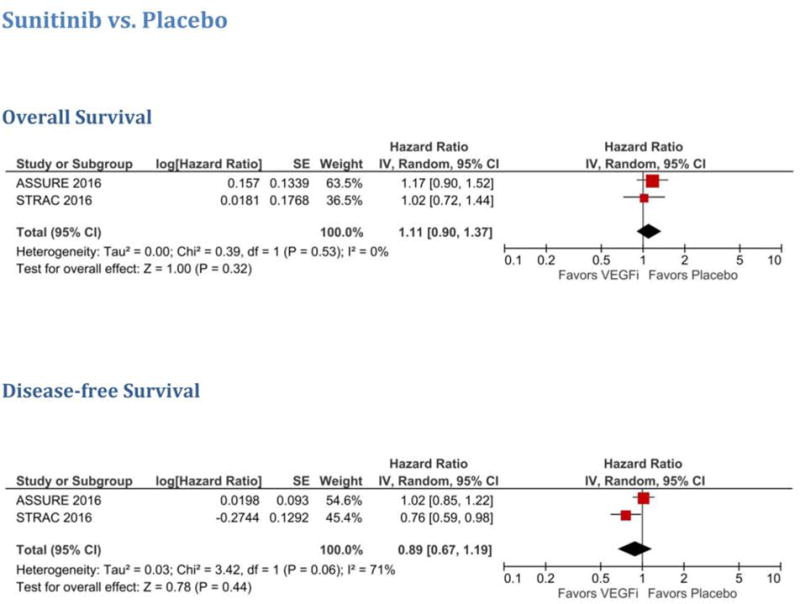
Meta-analysis of sunitinib versus placebo for the overall cohort. VEGFi = VEGF inhibitor.
3.5. Adverse events
Overall, VEGF TKIs were associated with a significantly higher risk of grade 3/4 toxicities compared to placebo (64.3% vs 22.7%; HR 2.74, 95% CI 2.49–3.03; Supplementary Table 1 and Fig. 3). These grade 3/4 toxicities led to discontinuation in 36.3% of patients in the VEGF TKI group versus 7% in the placebo group (HR 5.01, 95% CI 3.45–7.27). Compared to S-TRAC, there were similar numbers of grade 3/4 adverse events (AEs) in ASSURE, but higher discontinuation rates for both the placebo (10.3% vs 5%) and VEGF TKI groups (41.2% vs 28.1%). The most common grade 3/4 AEs in the sunitinib group compared to placebo were: hand-foot skin reaction (15.5% vs 0.7%), diarrhea (6.9% vs 0.3%), hypertension (12.3% vs 2.7%), and fatigue (11.2% vs 2.1%). The most common grade 3/4 AEs for pazopanib compared to placebo were: transaminitis (22% vs 1%), hypertension (24.8% vs 6.6%), and diarrhea (6.6% vs 0.7%). There were more grade 5 AEs reported in the VEGF TKI group, with 13 deaths compared to seven in the placebo group (0.7% vs 0.63%). However, this difference was not statistically significant (HR 1.34, 95% CI 0.52–3.49). Not all of these deaths were confirmed to be treatment-related. In ASSURE, there were five deaths “related to treatment or occurring within 30 days of the end of treatment”: one patient receiving sorafenib died from infectious colitis while on treatment and four patients receiving sunitinib died (1 neurological sequelae, 1 sequelae of gastric perforation, 1 pulmonary embolus, and 1 disease progression” [7]. In PROTECT, two grade 5 AEs occurred in the placebo group and four in the pazopanib group during the follow-up period. Three of the pazopanib deaths occurred in the safety 600 mg group and were considered unrelated to treatment (central nervous system hemorrhage, cerebral hypoxia, and renal failure), and one in the safety 800 mg group considered to be related to treatment (cardiomyopathy) [3]. In S-TRAC, there were five grade 5 AEs in each group, with no further information on treatment attribution.
Fig. 3.
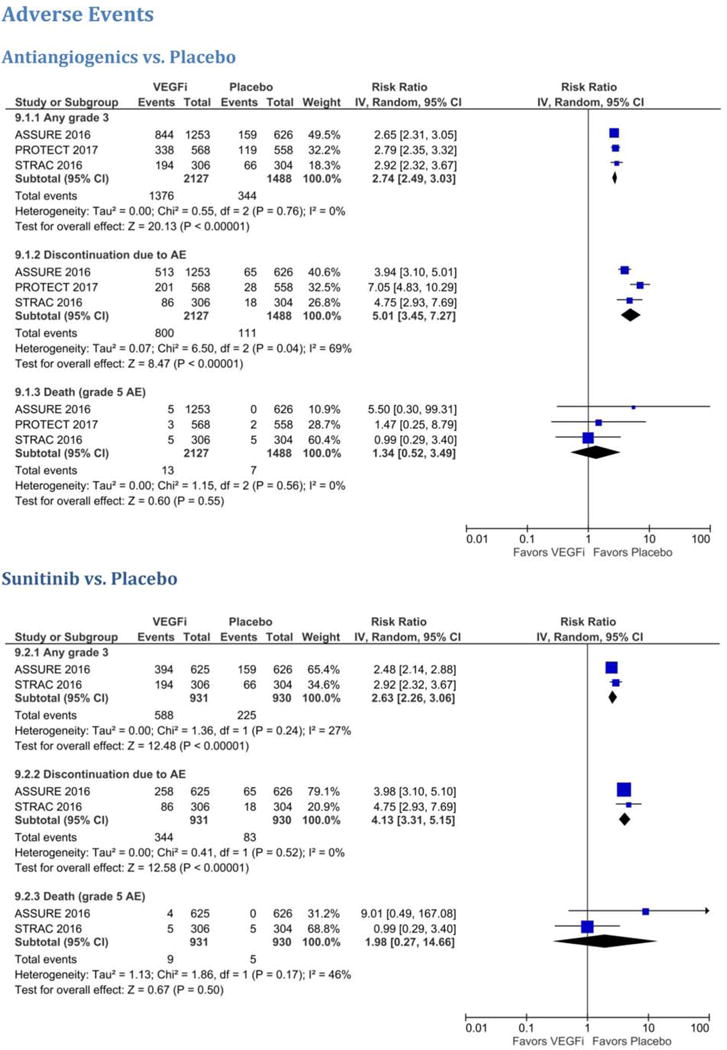
Meta-analysis of (A) antiangiogenics versus placebo and (B) sunitinib versus placebo for any grade ≥3 AEs, discontinuations due to AEs, and grade 5 AEs (death). AE = adverse event; VEGFi = VEGF inhibitor.
3.6. Risk of bias assessment
A qualitative assessment was performed by assessing various indicators for each individual study using the aforementioned tools for risk of bias and quality assessment. Overall, the studies were deemed to be at low risk of bias (Supplementary Table 2 and Supplementary Fig. 6). All three studies received at least partial funding from industry.
3.7. Discussion
In this systematic review and meta-analysis, we found no additional benefit in terms of DFS or OS to support the use of VEGF TKIs in post-nephrectomy patients with high-risk nonmetastatic RCC. Furthermore, use of VEGF TKIs was associated with dose reductions and a 2.7-fold higher risk of grade ≥3 AEs. These findings held true in an analysis for only clear-cell histology and for the groups with the highest risks as reported by individual studies. Similarly, when restricting the analysis to sunitinib, no OS benefit was seen in RCC patients. Multiple agents have been studied in the adjuvant RCC setting in attempts to improve survival and decrease the rate of recurrence after nephrectomy. However, all these trials failed to show a benefit [11]. VEGF TKIs, with their antiangiogenic activity, have significantly improved survival in advanced RCC. Therefore, studying these agents in the adjuvant setting was warranted. Both the ASSURE and S-TRAC trials examined the use of sunitinib compared to placebo in this patient population. While there was a DFS benefit in S-TRAC on blinded independent review (but not on investigator review), there was no DFS benefit detected in ASSURE. Importantly, both trials did not show an OS benefit. Our meta-analysis is consistent with the ASSURE trial, showing no benefit of sunitinib in the adjuvant setting in terms of DFS and OS. However, it is important to note the high heterogeneity in the DFS analysis (I2 = 71%), which is most likely secondary to the differences in design and study populations between ASSURE and S-TRAC (Table 1), mainly the inclusion of less “high-risk” patients in ASSURE (T1b) compared to S-TRAC (≥T3). In addition, approximately 20% of the patients included in ASSURE had non–clear cell histology compared to mainly clear-cell RCC in S-TRAC. However, our meta-analysis did not show a DFS benefit even when restricting the analysis to the population with the highest risk of relapse (ASSURE 2017 and S-TRAC 2017) or only clear-cell histology. Another difference between ASSURE and S-TRAC is the dosages. While ASSURE allowed a starting dose of 50 or 37.5 mg with dose reductions down to 25 mg when needed, the starting dose in S-TRAC was 50 mg and dose reductions were only allowed down to 37.5 mg. With the known limitations of exploratory analyses, the post hoc analysis of the ASSURE trial showed no difference in DFS based on dose intensity [4].
Our results are in accordance with a recent meta-analysis examining the use of adjuvant therapy (chemotherapy, vaccine immune therapy, and targeted therapy) in patients with RCC after nephrectomy [12] and the meta-analysis performed by Bex et al in the 2017 updated European Association of Urology guidelines [13]. Our study focused on VEGF TKI use and included three additional articles (S-TRAC 2017, ASSURE 2017, and PROTECT) with updated OS analysis. Furthermore, we found significant toxicity associated with the use of VEGEF TKIs compared to placebo (64.3% vs 22.7%). The S-TRAC trial reported the effect of these toxicities on quality of life; compared to placebo, patients on sunitinib had lower scores for quality-of-life questionnaires, which were only clinically meaningful for diarrhea and loss of appetite.
The US Food and Drug Administration recently approved sunitinib for adjuvant treatment of patients with a high risk of recurrent RCC following nephrectomy. This was based on the DFS benefit found in the S-TRAC trial, as discussed above. While recurrence-free survival and DFS are considered established surrogates for OS in the adjuvant setting for a few malignancies (colon and gastric cancers and melanoma), this has not been established for other cancers such as pancreatic cancer and RCC [14,15]. In a meta-analysis to evaluate whether DFS can be an early clinical surrogate for OS in the adjuvant setting for localized RCC, 13 trials were assessed and no strong correlation was noted between DFS and OS at 5 yr [14]. Taking this into consideration, along with the results of our meta-analysis and the significant toxicity of VEGF TKIs, there is currently insufficient evidence that VEGF TKI use in the adjuvant setting would enhance OS.
Future studies should potentially focus on identifying patients at higher risk of relapse on the basis of clinicopathological and molecular biomarkers. For instance, in a recent study, higher EZH2 expression was associated with a higher risk of death after adjusting for SSIGN (stage, size, grade and necrosis) score, even in patients with low risk according to SSIGN, a population that is typically excluded in the adjuvant trials [16].
The main limitation of our meta-analysis is the small number of RCTs available for analysis (n = 3), with differences in patient populations as acknowledged above; this led to more heterogeneity, which was mostly manifest in the DFS analysis, but not the OS analysis. We await the results of other ongoing clinical trials examining similar questions on the efficacy of different agents in the adjuvant setting: the SORCE trial (NCT00492258; sorafenib vs placebo), the ATLAS study (NCT01599754; axitinib vs placebo), and the EVEREST trial (NCT01120249) examining the role of the mTOR inhibitor everolimus versus placebo. Until then, there seems to be insufficient evidence to support the use of VEGF TKIs in the adjuvant setting in patients with RCC after nephrectomy.
In addition to adjuvant TKI and mTOR inhibitor trials, there are currently several ongoing studies looking at the efficacy of different checkpoint inhibitors after nephrectomy in RCC: the IMMotion010 trial (atezolizumab vs placebo; NCT03024996), KEYNOTE-564 (pembrolizumab vs placebo; NCT03142334), and Checkmate-914 (nivolumab + ipilimumab vs placebo; NCT03138512).
4. Conclusions
Antiangiogenics do not improve overall survival over placebo in high-risk RCC after nephrectomy. Other adjuvant clinical trials are currently ongoing and will further help to delineate any potential efficacy. Further studies are needed to identify the patient population that might derive a benefit from antiangiogenics in the adjuvant setting.
Supplementary Material
Acknowledgments
We acknowledge the support provided by the Gloria A. and Thomas J. Dutson Jr. Kidney Research Endowment. This work was supported by a Gerstner Family Career Development Award (T.H.H.), NCI R01 CA224917 (T.H.H.), and Department of Defense grant W81XWH-17-1-0546 (T.H.H.). The opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense.
Funding/Support and role of the sponsor: None.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Thai H. Ho had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Sonbol, Ho, Firwana.
Acquisition of data: Sonbol, Hilal, Almader-Douglas.
Analysis and interpretation of data: Sonbol, Firwana, Ho, Wang.
Drafting of the manuscript: Sonbol, Hilal, Firwana, Wang, Joseph, Ho.
Critical revision of the manuscript for important intellectual content: Sonbol, Hilal, Joseph, Ho.
Statistical analysis: Wang, Firwana.
Obtaining funding: None.
Administrative, technical, or material support: Sonbol, Ho.
Supervision: Ho.
Other: None.
Financial disclosures: Thai H. Ho certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Thai H. Ho has served on advisory boards for Pfizer, Genentech, and Exelixis. Richard W. Joseph has acted as a consultant for BMS, Exelixis, Novartis, Merck, and Incyte. The remaining authors have nothing to disclose.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Stewart SB, Thompson RH, Psutka SP, et al. Evaluation of the National Comprehensive Cancer Network and American Urological Association renal cell carcinoma surveillance guidelines. J Clin Oncol. 2014;32:4059–65. doi: 10.1200/JCO.2014.56.5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motzer RJ, Haas NB, Donskov F, et al. Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with localized or locally advanced renal cell carcinoma. J Clin Oncol. 2017;35:3916–23. doi: 10.1200/JCO.2017.73.5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haas NB, Manola J, Dutcher JP, et al. Adjuvant treatment for high-risk clear cell renal cancer: updated results of a high-risk subset of the ASSURE randomized trial. JAMA Oncol. 2017;3:1249–52. doi: 10.1001/jamaoncol.2017.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ravaud A, Motzer RJ, Pandha HS, et al. Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N Engl J Med. 2016;375:2246–54. doi: 10.1056/NEJMoa1611406. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Ravaud A, Patard JJ, et al. Adjuvant sunitinib for high-risk renal cell carcinoma after nephrectomy: subgroup analyses and updated overall survival results. Eur Urol. 2018;73:62–8. doi: 10.1016/j.eururo.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for high-risk, nonmetastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet. 2016;387:2008–16. doi: 10.1016/S0140-6736(16)00559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glenny AM, Altman DG, Song F, et al. Indirect comparisons of competing interventions. Health Technol Assess. 2005;9:1–134. doi: 10.3310/hta9260. [DOI] [PubMed] [Google Scholar]
- 11.Janowitz T, Welsh SJ, Zaki K, Mulders P, Eisen T. Adjuvant therapy in renal cell carcinoma—past, present, and future. Semin Oncol. 2013;40:482–91. doi: 10.1053/j.seminoncol.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai Y, Li S, Jia Z, Ding Y, Gu C, Yang J. Adjuvant therapy for locally advanced renal cell carcinoma: A meta-analysis and systematic review. Urol Oncol. 2018;36:79e1–10. doi: 10.1016/j.urolonc.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Bex A, Albiges L, Ljungberg B, et al. Updated European Association of Urology guidelines regarding adjuvant therapy for renal cell carcinoma. Eur Urol. 2017;71:719–22. doi: 10.1016/j.eururo.2016.11.034. [DOI] [PubMed] [Google Scholar]
- 14.Harshman LC, Xie W, Moreira RB, Gustavo R, Sweeney C, Choueiri TK. Meta-analysis of disease free survival (DFS) as a surrogate for overall survival (OS) in localized renal cell carcinoma (RCC) J Clin Oncol. 2017;35(6 Suppl):459. [Google Scholar]
- 15.Petrelli F, Tomasello G, Ghidini M, Lonati V, Passalacqua R, Barni S. Disease-free survival is not a surrogate endpoint for overall survival in adjuvant trials of pancreatic cancer: a systematic review of randomized trials. HPB (Oxford) 2017;19:944–50. doi: 10.1016/j.hpb.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Ho TH, Kapur P, Eckel-Passow JE, et al. Multicenter validation of enhancer of zeste homolog 2 expression as an independent prognostic marker in localized clear cell renal cell carcinoma. J Clin Oncol. 2017;35:3706–13. doi: 10.1200/JCO.2017.73.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


