Abstract
Background.
Heart failure is a common long term condition affecting around 900 000 people in the UK and patients commonly present to primary care. The prognosis of patients with a code of heart failure in their primary care record is unknown.
Objective.
The study sought to determine the overall survival rates for patients with heart failure in a primary care population from the time of diagnosis.
Methods.
Survival analysis was carried out using UK primary care records from The Health Improvement Network (THIN) between 1 January 1998 and 31 December 2012. Patients age 45 or over with a first diagnostic label of heart failure were matched by age, sex and practice to people without heart failure. Outcome was death in the heart failure and no heart failure cohorts. Kaplan-Meier curves were used to compare survival. Age-specific survival rates at 1, 5 and 10 years were determined for men and women with heart failure. Survival rates by year of diagnosis and case definition were also calculated.
Results.
During the study period, 54313 patients had a first diagnostic code of heart failure. Overall survival rates for the heart failure group were 81.3% (95%CI 80.9–81.6), 51.5% (95%CI 51.0–52.0) and 29.5% (95%CI 28.9–30.2) at 1, 5 and 10 years respectively and did not change over time.
Conclusions.
In a primary care population, the survival of patients diagnosed with heart failure did not improved over time. Further research is needed to explain these trends and to find strategies to improve outlook.
Keywords: Community, heart failure, prognosis, primary care, survival.
Introduction
Heart failure is an important public health problem associated with significant morbidity and mortality for patients and high costs for healthcare systems (1). An estimated one to two in every 100 adults in Western populations are living with heart failure (2). In the UK, the costs of heart failure to the National Health Service (NHS) are second highest for any disease after stroke (3). Accurate estimates of heart failure prognosis are vital to healthcare commissioners to allow appropriate allocation of resources, to physicians in making management decisions and, perhaps most importantly, to patients to allow informed choices about treatments and end of life care (4).
Heart failure prognosis has previously been reported as worse than many cancers although the outcome of patients with heart failure is not always reliably recorded (5). Cancer registries help to identify patterns in both incidence and survival of common cancers and have reported significant improvements in prognosis over time (6). Global heart failure registries are lacking so outcomes have been established from hospital data and screening studies.
Analysis of hospital records from a cohort of 5.1 million patients in Scotland reported median survival rates of 1.33 years (95%CI 1.17–1.50) in men following a first hospitalisation for heart failure in 1986. With an increase in prescribing of evidence-based treatments (angiotensin converting enzyme inhibitors (ACE-I), B blockers and mineralocorticoid receptor antagonists) survival at 1 year increased to 2.34 years (95%CI 2.15–2.56) by 2003 (7). More recent analysis of Medicare billing data in the US found that following an admission with heart failure, 67.4% of patients were readmitted to hospital within a year and 35.8% died within the same period (8). Heart failure requiring hospital admission therefore continues to have a poor outcome for patients and is also associated with significant healthcare costs arising from multiple admissions.
The outlook for participants in community screening studies is less bleak. In the Olmstead County population in the US, 1-year mortality was 21% for men and 17% for women and at 5 years was 50% and 46% for men and women, respectively (9). The Echocardiographic Heart Of England Screening (ECHOES) study, which screened a general population cohort in the UK over the age of 45, found a 5-year survival rate of 53% in participants with heart failure due to left ventricular systolic dysfunction (LVSD) (10).
Hospital data and screening studies however fail to explore the survival of patients who are diagnosed in a community setting and not necessarily admitted to hospital nor actively involved with screening for research purposes. Electronic primary care records provide a valuable source of data directly relevant to community populations (11). The computerisation of primary care in the UK and increasingly robust coding of medical information has led to the creation of large datasets which, following anonymization, can be used to explore epidemiological trends, including survival rates. The Health Improvement Network (THIN) is one of the largest databases of general practice records in the world; it currently includes data from 587 practices in the UK, approximately 6% of the whole population (12).
The aim of this study was to determine the 1, 5 and 10-year survival rates of patients with heart failure in a primary care population and examine whether prognosis has improved over time.
Methods
Design
Survival analysis was carried out using an open matched retrospective cohort from THIN database for the period from 1 January 1998 to 31 December 2012.
Setting
THIN is a primary care database containing routinely collected electronic patient records in the UK. At each consultation, the GP records details of the medical encounter, including diagnosis. Demographic details such as age, sex and linked deprivation scores also form part of the electronic record.
Practices that contributed at least 1 year of clinical data were included in the study. In addition, because there may be under recording of mortality in earlier years, data were only included after each practice’s acceptable mortality reporting date (13). This is the date after which recorded mortality in the practice is consistent with predicted mortality. This was to ensure that the main outcome of interest was being accurately recorded by the participating practices.
Population
The dataset extracted from THIN database included all persons aged 45 years and over, registered at a practice for at least 12 months during the study period. This age cut-off was chosen because the types of heart failure affecting children and younger people are pathologically distinct from those found in older adults. Eligible cases had a clinical code of heart failure. The index date was the first date of a recorded heart failure code. Patients with a previous diagnosis of heart failure, either prior to age 45 or the study start date, were excluded. Cases were matched with up to five comparator patients who were registered in the same practice on the index date and did not have a diagnosis of heart failure on that date (but could become a case later). Comparators were also matched on sex and age ±5 years.
Clinical codes
Patients with a diagnosis of heart failure were identified using clinical codes input by GPs to record new diagnoses in the medical record. The NHS Clinical Terminology Browser and Quality and Outcomes Framework (QOF) guidelines were used to generate a comprehensive list of clinical codes used to record a diagnosis (Supplementary Table S1). Heart failure is a clinical syndrome and the diagnosis requires the presence of symptoms and objective evidence of a structural or functional abnormality of the heart. Patients with a clinical code of heart failure and either an echocardiograph report or a hospital letter were classified as being a confirmed case of heart failure and those with just a clinical code alone as an unconfirmed case.
Baseline variables
Demographic variables including age, sex, ethnicity, area deprivation quintile, cardiovascular risk factors and co-morbidities were extracted. The latest deprivation quintile prior to the index date was used or, if unavailable, the most recently recorded after the index date. Cardiovascular risk factors (smoking, blood pressure, cholesterol, body mass index (BMI)) were the most recently recorded prior to the index date. Cardiovascular co-morbidities (angina, myocardial infarction (MI), ischaemic heart disease, diabetes, hypertension, stroke, atrial fibrillation (AF), valve disease), were defined by the presence of a clinical code at any time prior to the index date.
Outcome measure
The outcome measure was overall survival rate for the cohort which was determined using the date of death for patients who had died from any cause (all-cause mortality).
Statistical analyses
Data were extracted directly from THIN database using clinical codes. Analysis was carried out using Stata versions 10 and 11. The absolute number of heart failure cases, and overall incidence rate by age and sex, was calculated.
Heart failure patients and matched patients without heart failure, included in the survival analyses were followed until the earliest of the following dates: patient died, patient left (de-registered from) their practice, the practice ceased contributing data to THIN, or the study ended. Kaplan-Meier curves were used to compare survival in the heart failure and no heart failure cohorts. Log rank tests were used to compare survival between groups.
One-, five- and ten-year survival rates for the heart failure cohort were calculated by 10-year age band from the age of 45. For comparison, survival rates of the no heart failure cohort were also determined. To examine trends over time, age-sex specific and overall survival rates were calculated by year of diagnosis. Case definition was also explored by calculating overall survival rates by year of diagnosis for the confirmed and unconfirmed heart failure groups separately.
Results
Five hundred and sixty four practices contributed at least 1 year of data between 1 January 1998 and 31 December 2012. A total of 2728841 patient records were included in the dataset; 54313 patients had a new clinical code of heart failure during the study period. Overall heart failure incidence was 3.00 (95%CI 2.98–3.03) per 1000 person-years, increasing with age and higher in men than women in every age band. Incidence of heart failure in age group 65 and older was 6.78 (95% CI 6.72–6.84) per 1000 person-years and in the age group 75 years and older was 10.86 (95%CI 10.74–10.97) per 1000 person-years.
Survival in heart failure and no heart failure cohorts
The characteristics of patients with and without heart failure are shown in Table 1. All five Townsend deprivation quintiles were similarly represented except for the most deprived group which had around one-third less cases than the other four groups. BMI was similar but there were 1.2% more smokers in the heart failure group. Cardiovascular co-morbidities, particularly ischaemic heart disease, AF and valvular disease were more common in patients with heart failure.
Table 1.
Characteristics of participants with heart failure and matched participants without heart failure from The Health Improvement Network (THIN) database between 1 January 1998 and 31 December 2012
| Characteristic | Heart failure (n = 54313) | No heart failure (n = 254809) |
|---|---|---|
| Age (years) | ||
| 45–54 | 1738 (3.2%) | 8685 (3.4%) |
| 55–64 | 5268 (9.7%) | 26323 (10.3%) |
| 65–74 | 12127 (22.3%) | 60308 (23.7%) |
| 75–84 | 20942 (38.6%) | 100184 (39.3%) |
| 85–94 | 13021 (24.0%) | 55311 (21.7%) |
| ≥95 | 1217 (2.2%) | 3998 (1.6%) |
| Male | 27983 (51.5%) | 130413 (51.2%) |
| Townsend score | ||
| 1 | 10935 (20.1%) | 59001 (23.2%) |
| 2 | 11156 (20.5%) | 55974 (22.0%) |
| 3 | 11089 (20.4%) | 50893 (20.0%) |
| 4 | 10864 (20.0%) | 45959 (18.0%) |
| 5 | 7940 (14.6%) | 31956 (12.5%) |
| Not known | 2329 (4.3%) | 11026 (4.3%) |
| BMI | ||
| Mean (SD) | 27.9 (5.9) | 26.4 (4.7) |
| Missing | 8664 (16.0%) | 51692 (20.3%) |
| Smoking status | ||
| Smoker | 7656 (14.1%) | 32803 (12.9%) |
| Not current smoker | 43198 (79.5%) | 196407 (77.1%) |
| Missing | 3459 (6.4%) | 25599 (10.1%) |
| Diabetes | 10729 (19.8%) | 27483 (10.8%) |
| Hypertension | 28820 (53.1%) | 106661 (41.9%) |
| Angina | 12522 (23.1%) | 28656 (11.3%) |
| Myocardial infarction | 12282 (22.6%) | 16339 (6.4%) |
| Ischaemic heart disease | 23308 (42.9%) | 43803 (17.2%) |
| Stroke | 4969 (9.2%) | 14983 (5.9%) |
| Atrial fibrillation | 15473 (28.5%) | 17969 (7.1%) |
| Valvular disease | 5522 (10.2%) | 6281 (2.5%) |
Overall, heart failure cases had a significantly worse prognosis than comparators (log rank test, chi-square (1) 12732.59, 1, P < 0.0001). The survival rates for the heart failure cohort, and age, sex and practice matched cohort without heart failure, are shown in Table 2. Overall survival rates in the heart failure group were 81.3% (95%CI 80.9–81.6) at 1 year from date of diagnosis, 51.5% (95%CI 51.0–52.0) at 5 years and 29.5% (95%CI 28.9–30.2) at 10 years.
Table 2.
One-, five- and ten-year survival rates in participants with heart failure and matched participants without heart failure from The Health Improvement Network (THIN) database between 1 January 1998 and 31 December 2012
| Age (years) | Survival (%) | |||||
|---|---|---|---|---|---|---|
| 1 year (95% CI) | 5 years (95% CI) | 10 years (95% CI) | ||||
| Heart failure | No heart failure | Heart failure | No heart failure | Heart failure | No heart failure | |
| 45–54 | 94.0 (92.7–95.0) | 100.0 (99.5–99.8) | 80.9 (78.6–83.0) | 97.8 (97.4–98.2) | 68.2 (64.4–71.6) | 95.4 (94.6–96.1) |
| 55–64 | 91.5 (90.7–92.3) | 99.1 (99.0–99.3) | 75.0 (73.6–76.4) | 94.7 (94.4–95.0) | 58.2 (56.0–60.3) | 87.8 (87.1–88.5) |
| 65–74 | 87.6 (87.0–88.2) | 97.5 (97.4–97.7) | 64.5 (63.4–65.4) | 86.6 (86.3–86.9) | 40.4 (39.0–41.8) | 71.4 (70.8–71.9) |
| 75–84 | 81.9 (81.3–82.4) | 94.2 (94.0–94.3) | 49.5 (48.7–50.3) | 71.9 (71.6–72.2) | 23.8 (22.8–24.9) | 46.0 (45.5–46.6) |
| 85–94 | 70.7 (69.8–71.5) | 87.5 (87.2–87.8) | 28.5 (27.5–29.6) | 49.6 (49.0–50.1) | 7.9 (6.8–9.0) | 19.2 (18.4–19.9) |
| >=95 | 53.8 (50.7–56.7) | 77.5 (76.0–78.8) | 9.0 (6.5–12.0) | 24.3 (22.3–26.3) | 0 — | 0 — |
| Overall | 81.3 (80.9–81.6) | 94.0 (93.9–94.1) | 51.5 (51.0–52.0) | 74.0 (73.8–74.3) | 29.5 (28.9–30.2) | 54.1 (53.8–54.4) |
Survival over time
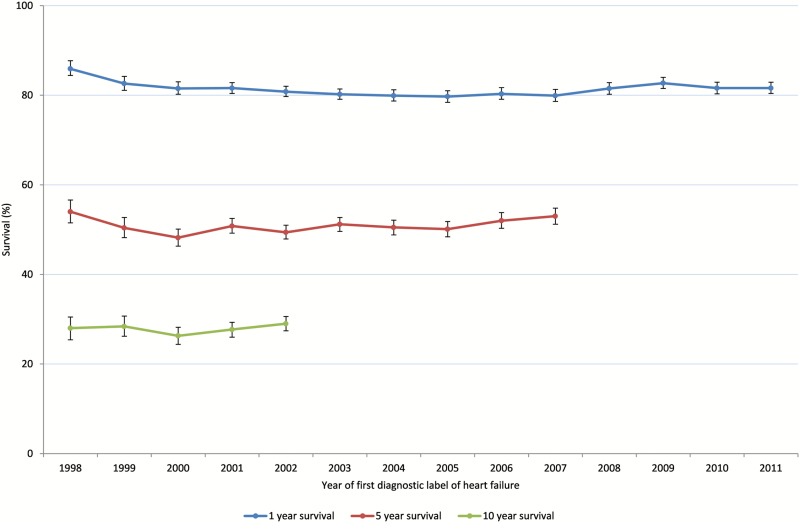
The survival rates of all heart failure cases by year of diagnosis are shown in Figure 1. One- and five-year survival rates remained stable during the study period at 80% and 50%, respectively. Ten-year survival rates for those diagnosed between 1998 and 2002 were 28%.
Figure 1.
One-, five- and ten-year survival in heart failure cohort by year of diagnosis.
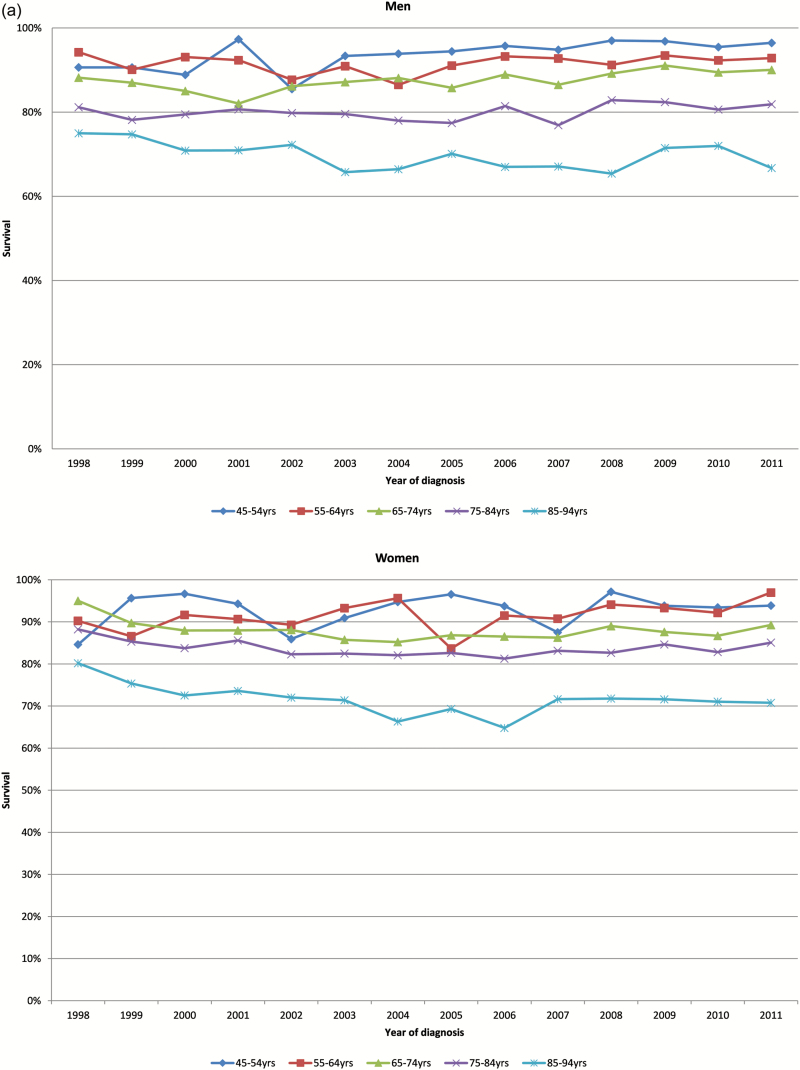
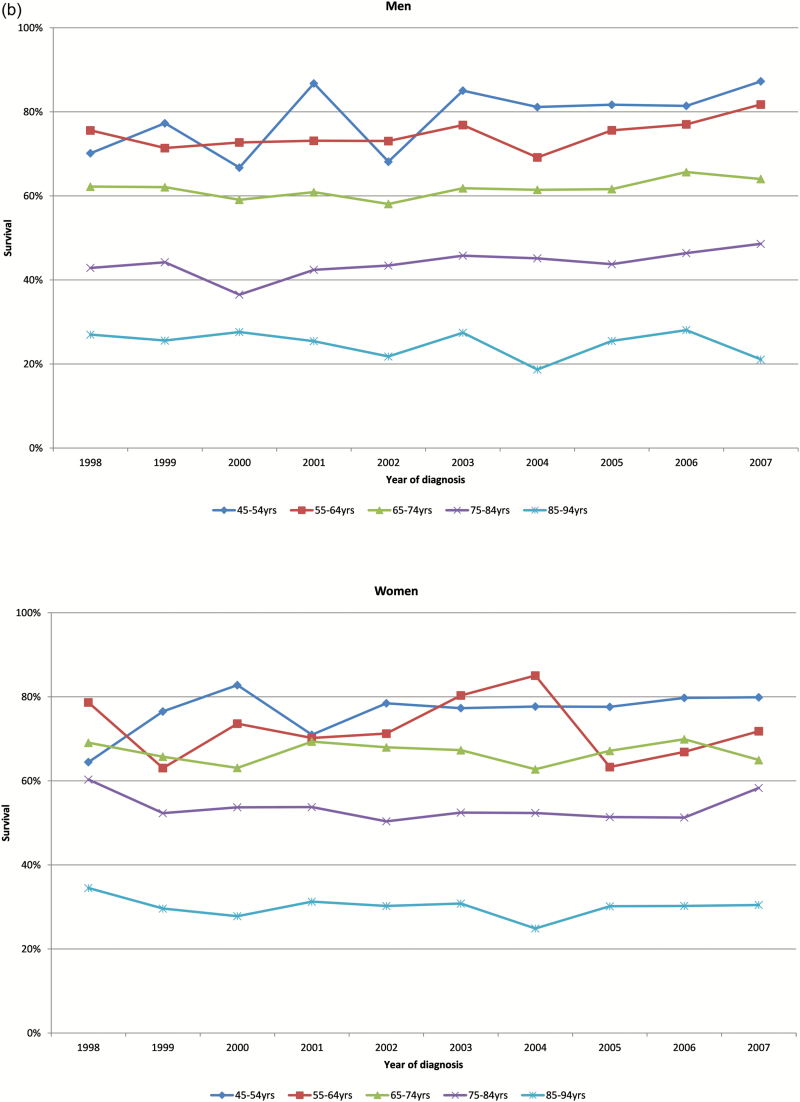
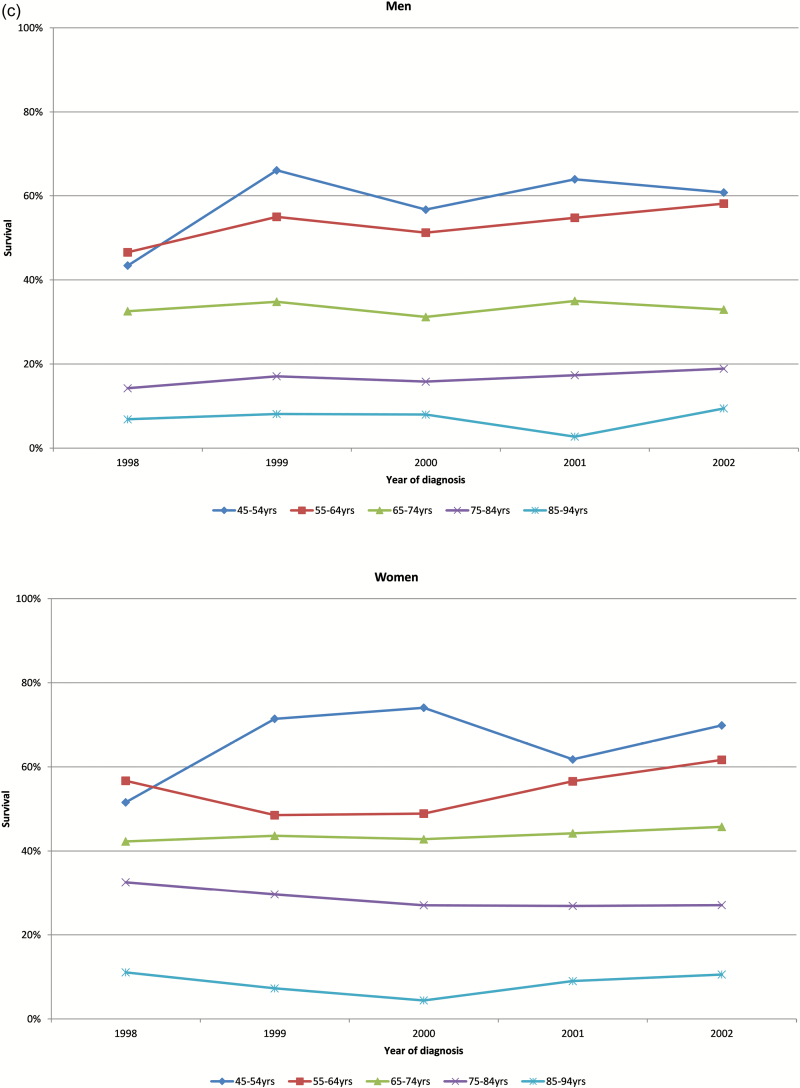
One-, five- and ten-year survival rates for men and women by age group are shown in Figure 2a–c, respectively. Survival rates for all groups did not change significantly over time.
Figure 2.
(a) Age-specific 1-year survival of men and women in heart failure cohort by year of diagnosis. (b) Age-specific 5-year survival of men and women in heart failure cohort by year of diagnosis. (c) Age-specific 10-year survival of men and women in heart failure cohort by year of diagnosis.
Survival by case definition
The number of confirmed cases (13773) made up just over a quarter (26.5%) of the total number of heart failure cases (54313) in the dataset overall and did not increase significantly over a 10-year period. Cases with confirmed heart failure were younger and 56% were male compared to 50% in the unconfirmed group. The proportion of patients in each Townsend quintile was similar for confirmed and unconfirmed cases. There were 2% more smokers in the confirmed compared to the unconfirmed heart failure group. Ischaemic heart disease, angina and MI were all more common in the confirmed group.
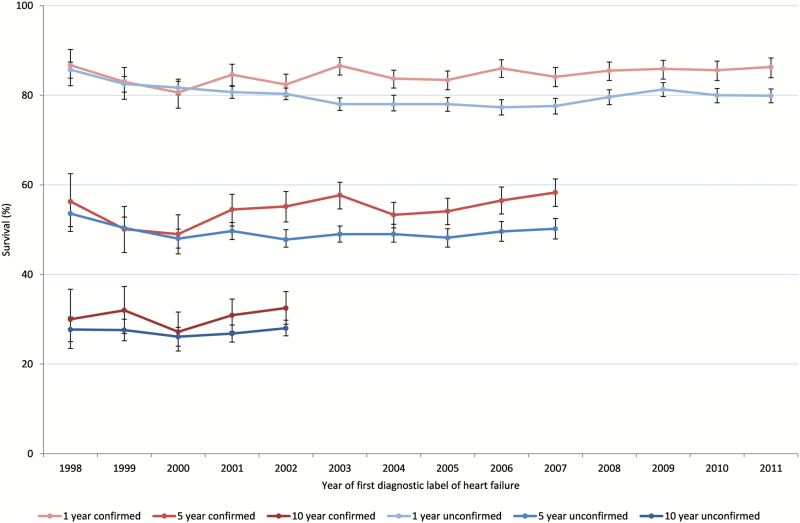
The overall survival rates were lower in the unconfirmed heart failure group than in the confirmed heart failure group (log rank test, chi-square (1) 170.37, 1, P < 0.0001). Figure 3 shows 1-, 5- and 10-year survival rates in the confirmed and unconfirmed heart failure groups over time. The unconfirmed heart failure group had an overall 5% lower survival rate at 1, 5 and 10 years.
Figure 3.
One-, five- and ten-year survival rates of people with confirmed and unconfirmed heart failure.
Conclusions
This study provides contemporary survival rate estimates for patients with a first diagnostic code of heart failure in their GP record. The outlook for patients with heart failure did not improve over time. Patients with a confirmed diagnosis had a slightly better outcome than those that were unconfirmed.
The cohort in this analysis included 54313 patients with heart failure, much larger than most prospective studies, and the follow-up period was over a 15-year period. The NHS provides healthcare to the entire population of the UK, free at the point of access and most patients are registered with a primary care provider. Unlike screening cohort studies, primary care databases do not rely on participants volunteering to take part in the study rather they represent a cross-section of the entire population. These results are therefore likely to be generalizable to the community population as a whole (14). The large number of patients in each age and sex category also improves the accuracy of the survival rate estimates.
The main limitation of the study is the reliability of GP coding. Research using general practice databases is reliant on the accuracy of clinical coding input by GPs during the consultation. Heart failure is a chronic condition which is often insidious in onset and can masquerade as other conditions making early and accurate diagnosis difficult. Over time the use and accuracy of clinical coding has improved, particularly since the introduction of the QOF in 2004 (15). The definition of heart failure has also changed over time leading to dysynchrony between epidemiological studies, making comparison difficult, and the meaning of a code for heart failure in an individual’s medical record may be based on different criteria depending on the accepted definition at the time of diagnosis. The benefit of THIN and similar primary care databases are that they provide an insight into real-life general practice and the survival rates of patients with a clinical code of heart failure in their record is likely to be an important statistic for practising GPs.
There is a paucity of contemporary epidemiological information on heart failure from a primary care setting. Annual QOF data shows a prevalence of 0.8–0.9% each year since the introduction of the heart failure indicator in 2006 but the number of new cases per year is not recorded (16). National heart statistics include heart failure incidence and mortality within an umbrella term of ‘cardiovascular disease’ so estimates for heart failure alone are lacking. Survival rate estimates have therefore come from screened cohorts and hospital databases. The ECHOES study is one of the few studies to report 10-year, as well as 5-year, survival rates (10). All deaths in the cohort were collated from routinely collected mortality data. Overall the mortality rate for all-cause heart failure was 9% per year; survival was 53% at 5 years and 27% at 10 years. The overall 10-year survival rate of 29.5% for the heart failure group in the THIN study is therefore similar to the screened population in ECHOES.
Sweden has well-developed, high-quality health registries which allow more detailed examination of the epidemiology of diseases. A cross-sectional study of 2.1 million residents of the Stockholm region was carried out using an administrative health data register (17). Between 2006 and 2010, the incidence of heart failure was 3.8 per 1000 person-years. The overall 5-year survival rate in the Swedish study was 48%, similar to the THIN study results, however between 2006 and 2010 there was a 19% decrease in mortality in both men and women (P < 0.001). A study of the Framingham cohort in the US also found an overall improvement in survival of 12% per decade between 1950 and 2000 (18).
Cancer survival rates in the UK have doubled in the last 40 years (19), yet in this study the outlook for patients with a first diagnostic label of heart failure has not improved over time. More research is needed into the disease trajectory and management of heart failure in order to explain the lack of improvement in survival over time despite the availability of evidence-based therapies shown to improve outcomes in clinical trials. This study did not examine medication use following a heart failure diagnosis and this is an area of planned further work. The treatment used in heart failure with reduced ejection fraction have not shown the same benefit in patients with heart failure with preserved ejection fraction which may partly explain lack of survival improvement. It was not possible to differentiate the type of heart failure due to lack of specific coding based on ejection fraction. However, as heart failure with preserved ejection fraction is increasingly recognised, and therefore more likely to be coded, further analysis by subtype may become possible.
The results of this study are estimates at a population level but discussions with the individual patient require tailored statistics and information in a form that patients and their carers can understand. Prognostic estimates vary depending on age and sex but also other co-morbidities, treatments and variables such as blood pressure and ejection fraction (20). Prognostic modelling to include other variables from the THIN database to more accurately predict survival for individual patients is also an area where further research is planned.
Supplementary Material
Supplementary data are available at Family Practice online.
Declaration
Funding: the study was funded by the National Institute for Health Research (NIHR) School for Primary Care Research. This paper presents independent research funded by the NIHR. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. CJT was funded by an NIHR Doctoral Research Fellowship (DRF-2012-05-407) and is now an NIHR Academic Clinical Lecturer. TM was partly funded by the NIHR through the Collaborations for Leadership in Applied Health Research and Care for West Midlands (CLAHRC-WM). FDRH is part funded, through supervision sessions, by the NIHR School for Primary Care Research, NIHR Oxford Biomedical Research Centre, NIHR Collaboration for Leadership in Applied Health Research and Care Oxford and Harris Manchester College, University of Oxford.
Ethical approval: the Health Improvement Network Scientific Review Committee (Ref No. 13-010).
Conflict of interest: over the past decade, FDRH has received occasional research support or subsidy with assays from Roche Diagnostics and occasional speaker or consultancy fees from Novartis and Pfizer.
Supplementary Material
References
- 1. Braunwald E. The war against heart failure: the Lancet lecture. Lancet 2015; 385: 812–24. [DOI] [PubMed] [Google Scholar]
- 2. Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart 2007; 93: 1137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stewart S, Jenkins A, Buchan S, et al. The current cost of heart failure to the National Health Service in the UK. Eur J Heart Fail 2002; 4(3): 361–71. [DOI] [PubMed] [Google Scholar]
- 4. Shipman C, Gysels M, White P, et al. Improving generalist end of life care: national consultation with practitioners, commissioners, academics, and service user groups. BMJ 2008; 337: a1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stewart S, MacIntyre K, Hole DJ, et al. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail 2001; 3(3): 315–22. [DOI] [PubMed] [Google Scholar]
- 6. Askoxylakis V, Thieke C, Pleger ST, et al. Long-term survival of cancer patients compared to heart failure and stroke: a systematic review. BMC Cancer 2010; 10: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jhund PS, Macintyre K, Simpson CR, et al. Long-term trends in first hospitalization for heart failure and subsequent survival between 1986 and 2003: a population study of 5.1 million people. Circulation 2009; 119: 515–23. [DOI] [PubMed] [Google Scholar]
- 8. Dharmarajan K, Hsieh AF, Kulkarni VT, et al. Trajectories of risk after hospitalization for heart failure, acute myocardial infarction, or pneumonia: retrospective cohort study. BMJ 2015; 350: h411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Redfield M, Jacobsen SJ, Burnett JC, et al. Burden of systolic and diastolic ventricular dysfunction in the community. JAMA 2003; 289: 194– 202. [DOI] [PubMed] [Google Scholar]
- 10. Hobbs FD, Roalfe AK, Davis RC, et al. Prognosis of all-cause heart failure and borderline left ventricular systolic dysfunction: 5 year mortality follow-up of the Echocardiographic Heart of England Screening Study (ECHOES). Eur Heart J 2007; 28(9):1128–34. [DOI] [PubMed] [Google Scholar]
- 11. Muller S. Electronic medical records: the way forward for primary care research? Fam Pract 2014; 31: 127–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cegedim Strategic Data Medical Research UK. THIN Data Statistics http://csdmruk.cegedim.com/ (accessed on 22 August 2016).
- 13. Maguire A, Blak BT, Thompson M. The importance of defining periods of complete mortality reporting for research using automated data from primary care. Pharmacoepidemiol Drug Saf 2009; 18: 76–83. [DOI] [PubMed] [Google Scholar]
- 14. Blak BT, Thompson M, Dattani H, Bourke A. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care 2011; 19: 251–5. [DOI] [PubMed] [Google Scholar]
- 15. Doran T, Kontopantelis E, Valderas JM, et al. Effect of financial incentives on incentivised and non-incentivised clinical activities: longitudinal analysis of data from the UK Quality and Outcomes Framework. BMJ 2011; 342: d3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Quality and Outcomes Framework. Statistics and Research https://www.health-ni.gov.uk/topics/doh-statistics-and-research/quality-outcomes-framework-qof (accessed on 03 January 2017).
- 17. Zarrinkoub R, Wettermark B, Wändell P, et al. The epidemiology of heart failure, based on data for 2.1 million inhabitants in Sweden. Eur J Heart Fail 2013; 15: 995–1002. [DOI] [PubMed] [Google Scholar]
- 18. Levy D, Kenchaiah S, Larson MG, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med 2002; 347: 1397–402. [DOI] [PubMed] [Google Scholar]
- 19. Cancer Research UK. Cancer survival statistics http://www.cancerresearchuk.org/health-professional/cancer-statistics/survival (accessed on 03 January 2017).
- 20. Pocock SJ, Ariti CA, McMurray JJ, et al. Predicting Survival in Heart Failure: a risk score based on 39372 patients from 30 studies. Eur Heart J 2013; 34(19): 1404–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.