Abstract
Objective:
We aimed to evaluate the contribution of acinar-to-ductal metaplasia (ADM) to the accumulation of cells with a ductal phenotype in cultured human exocrine pancreatic tissues and reveal the underlying mechanism.
Method:
We sorted and cultured viable cell populations in human exocrine pancreatic tissues with a flow cytometry-based lineage tracing method to evaluate possible mechanisms of ADM. Cell surface markers, gene expression pattern and sphere formation assay were used to examine ADM.
Results:
A large proportion of acinar cells gained CD133 expression during the 2D culture and showed down-regulation of acinar markers and up-regulation of ductal markers, assuming an ADM phenotype. In a serum free culture condition, ADM induction was mainly dependent on TGF-β secreted from cultured ductal cells. Human acinar cells when cultured alone for a week in a serum free condition do not undergo ADM. However, serum may contain other factors besides TGF-β to induce ADM in human acinar cells. In addition, we found that TGF-β cannot induce ADM of murine acinar cells.
Conclusion:
Ductal cells are the major source of TGF-β that induces ADM in cultured human exocrine pancreatic tissues. This culture system might be a useful model to investigate the mechanism of ADM in human cells.
Keywords: acinar cells, metaplasia, TGF-β, human
Introduction
The exocrine pancreas consists mainly of two types of epithelial cells, acinar and ductal cells. Both acinar and ductal cells are differentiated from the same progenitor cells during development.1,2 Under normal physical conditions, ductal cells form the afferent system that delivers digestive enzymes produced by acinar cells. Acinar to ductal metaplasia (ADM) has frequently been observed in pancreatitis and pancreatic ductal adenocarcinoma (PDAC), which is characterized by the replacement of acinar tissues with newly generated duct structures in the pancreas.3,4 Recent studies suggest that these duct structures are composed of ductal-like cells transdifferentiated from acinar cells.5,6 Moreover, ADM has been proposed to play an important role in tissue regeneration after pancreatic injury, as well as in PDAC initiation,7,8,9 raising the interest in revealing the underlying molecular mechanism promoting ADM and the unique features of ADM cells.
While the ADM process can be monitored in vivo with mouse models,5,10,11 studies on human ADM still largely depend on in vitro culture models.12,13,14 It has long been known that cells with ductal-like phenotypes are enriched during the conventional 2D culture of primary human exocrine pancreas tissues.12,13,15,16 We and others have demonstrated the cellular plasticity of primary human pancreatic acinar cells.12,17 However, the contribution of the ADM process to this culture-induced “enrichment” has not been determined, limiting the usage of this model to study the molecular mechanism inducing human ADM. We have recently developed a flow cytometry-based high resolution lineage tracing method for human primary exocrine pancreatic tissues,17 which can readily identify and purify viable acinar, ductal and ductal-like cells derived from acinar cells (AD cells). We showed that human exocrine tissue clusters cultured in a 3D condition can undergo ADM with TGF-β treatment. In this study, we focused on using this method to evaluate the ADM of human primary tissues during 2D culture and reveal the underlying mechanisms.
MATERIALS AND METHODS
Cell Preparation and Cell Culture
Human islet-depleted cell fractions were obtained from healthy, non-diabetic organ donors deceased due to acute traumatic or anoxic death by the Surgery Department, University of Pennsylvania School of Medicine and by Prodo Laboratories, Inc, (Aliso Viejo, Calif) that were then shipped overnight to our laboratory. Total donor tissues used in this study were 26. Tissues were maintained as previously described17. The cell fractions were washed with PBS and incubated for 30 minutes with 100 μg/mL fluorescein isothiocyanate (FITC)-conjugated UEA-1 (Sigma-Aldrich, L9006, St. Louis, Mo) in CMRL media without serum. The tissue culture plates were pre-coated with 5% Matrigel (Corning, 354230, Bradford, Mass) to improve attachment. Tissues stained by UEA-1 were cultured in a serum-free advanced DMEM/F-12 medium supplemented with 10 µM Y27632 (Rock inhibitor). TGF-β1 and Activin A were used at final concentrations of 5 ng/ml and 200ng/ml respectively. To inhibit the activation of signalling pathways, tissues were treated with indicated inhibitors. All inhibitors were purchased from Selleckchem (Houston, Texas). SB431542 (S1067) and LY2157299 (S2230) were used at a final concentration of 1 µM. The media was changed daily for all experiments.
Isolation of Mouse Primary Pancreatic Acinar Cells, 3D Culture, and Quantification
Our protocol for isolation of mouse primary pancreatic acinar cells has been previously described in detail.18 For 3D culture, acinar cells were seeded in a mixture of rat tail collagen I/Waymouth media without supplements on cell culture plates coated with rat tail collagen I (BD Biosciences, San Jose, Calif). TGF-α (R&D Systems, Minneapolis, Minn) or TGF-β1 (Preprotech, Rocky Hill, NJ) were added to the media on top at 50 ng/ml. At the endpoint viability of cells was confirmed using Hoechst 33342 (Invitrogen, Carlsbad, Calif). All samples/experimental conditions were performed in triplicates and, after five days of 3D culture, numbers of ducts per field (whole well) for each condition were determined and photos were taken to document cellular structures. The experiment was performed in three replicates using pancreata of different individual mice.
Flow Cytometry Analysis and Cell Sorting
Flow cytometry assays were performed with BD LSRII (BD Biosciences) and cell sorting experiments were performed with BD Accuri (BD Biosciences).17 The cells were washed with PBS and trypsinised with 0.05% Trypsin-EDTA solution (Life Technologies, Grand Island, NY) for five minutes. Cells were washed with FACS buffer (10 mM EGTA, 2% FBS in PBS) and collected by centrifugation. The pellet was then digested in a 1 U/ml dispase solution (Life Technologies) containing 0.1 mg/ml DNaseI in PBS at 37 °C for five minutes. Cells were washed and suspended in FACS buffer for further subsequent antibody staining for flow cytometry. The data was analysed with FlowJo software (V9.9.5, FLOWJO, LLC, Ashland, Ore).
For cell sorting, trypsin digestion was stopped by adding serum. Dissociated cells were passed through a 40 µm cell strainer and stained with biotin-conjugated CD133 antibodies (clone AC133 and 293C3, Miltenyi Biotec, Auburn, Calif) for 15 minutes at 4 °C. After washing, cells were stained with APC-conjugated Streptavidin, Pacific blue-conjugated CLA, and 7AAD (BioLegend, San Diego, Calif) for 15 minutes at 4°C. Cell pellets were collected by centrifugation and washed with PBS after each staining step. The cells were sorted using a FACS Aria II (BD Biosciences) and collected in 100% FBS. After sorting, cells were washed with serum-free Advanced DMEM/F-12 media (Life Technologies), and re-suspended in ice-cold Advanced DMEM/F-12 media.
For sphere formation, 30 µl of growth factor-reduced Matrigel was then added to a 30 µl cell suspension (8,000 cells), and the mixture was placed around the bottom rim of each well in 48-well plates. After solidification at 37°C for 60 minutes, each well was overlaid with 300 µl of Advanced DMEM/F-12 media supplemented with recombinant human (rh) EGF (50 ng/ml, Sigma-Aldrich), rhR-spondin I (500 ng/ml, R&D systems), rhFGF10 (50 ng/ml, R&D systems), recombinant mouse Noggin (100 ng/ml, R&D systems), and 10 mM Nicotinamide. Media was changed twice per week. For the 2D culture, 96-well plates were coated with 5% Matrigel. The sorted cells were suspended in Advanced DMEM/F-12 media supplemented with 5% serum and 10 µM Y27632 for 24 hours. Thereafter, cells were cultured in serum-free Advanced DMEM/F-12 media supplemented with 10 µM Y27632. For the 3D-on-top culture, 96-well plates were coated with 50% Matrigel to form a thick layer on which cells were seeded.
cDNA Preparation and qRT-PCR Analyses
Total RNA was prepared using a Direct-zol RNA miniprep kit (Zymo Research, Irvine, Calif), and used for cDNA synthesis using a high capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, Calif), according to the manufacturer’s protocol. Relative mRNA levels were measured by qRT-PCR for each cDNA in duplicate with iTaq Universal SYBR Green Supermix (Bio-Rad, Hercules, Calif) and the CFX96 Real-Time system (Bio-Rad). Normalizations across samples were performed using 18s RNA primers. Information regarding the primer and probe sets is available upon request.
Statistical Analysis
Data is presented as mean (SD). P-values were acquired with the Student’s t or one-way ANOVA test using Prism (V6, GraphPad Software, La Jolla, Calif), and P < 0.05 is considered statistically significant.
RESULTS
Characterization of Acinar to Ductal Metaplasia in Human Pancreatic Exocrine Tissue in 2D In Vitro Culture
Culture-induced “enrichment” of cells with a ductal phenotype in the primary human exocrine pancreas has been well documented.12,15,16 Acinar to ductal metaplasia (ADM) has been proposed to contribute to this “enrichment”, however, the underlying mechanism is not clear.12 Taking advantage of our recently developed method,17 we monitor ADM during 2D culture and investigate the underlying mechanisms.
A Lectin UEA-1 was found to specifically and stably bind to human acinar cells.12,17 We pre-stained human exocrine pancreatic tissue with FITC-conjugated UEA-1 and cultured these tissues on plates pre-coated with 5% Matrigel in a serum-free medium. We analysed the UEA-1 expression by flow cytometry over a period of six days, and found that the UEA-1 staining intensity remained unchanged, suggesting that UEA-1 stably binds to acinar cells and that acinar cells do not proliferate. However, we observed that acinar cells gradually gained CD133 expression when cultured (Figure 1A). More specifically, almost all UEA-1high cells were CD133 negative on day one, consistent with their acinar origin. On day three, only a few UEA-1highCD133+ cells could be detected by flow cytometry analysis. However, by day five, a large population of UEA-1high cells had gained expression of CD133, indicating that a significant portion of the acinar cells acquired ductal-like features during the culture (Figure 1A). However, the UEA-1highCD133+ cells were negative for CLA staining, a ductal marker that we identified previously, suggesting these cells are different from ductal cells (Figure 1A). The cell surface staining of ductal cells remained unchanged during the culture. Although we found that all samples analysed had undergone ADM, the frequencies of this conversion varied among the samples from 59% to 88% of the acinar population (n = 8), possibly due to heterogeneity among the donors from whom the tissues were derived.
FIGURE 1. Characterization of ADM of primary human pancreatic acinar cells in vitro.
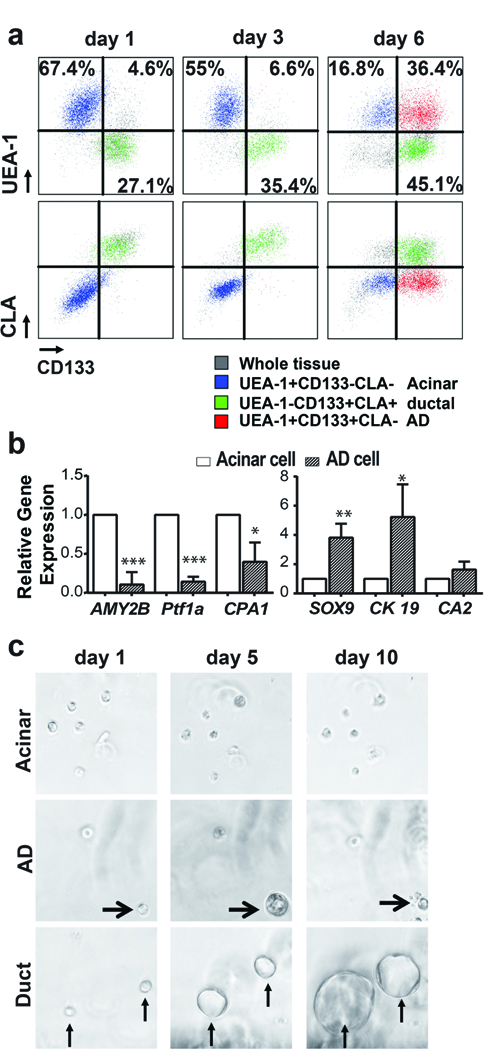
a) Cells of acinar origin (blue, UEAhighCD133−) gained CD133 expression during culture to become CD133+ cells (red, UEAhighCD133+), but continued to be CLA negative. Cells of ductal origin (green, UEAlowCD133+) showed no significant change in CD133 and CLA expression during culture (n=8). b) AD cells expressed significantly higher levels of ductal-specific genes (CK19 and SOX9) and lower levels of acinar-specific genes (AMY2B and PTF1a) than acinar cells (n=3). Data represents mean (SD) from three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001. c) Sphere formation assay for cells sorted from 2D culture. Ductal cells proliferated continuously to form large spheres and acinar cells could not form spheres, AD cells acquired the ability to proliferate transiently to form small spheres (arrowhead).
Next, we compared the gene expression of acinar and ductal markers for UEA-1highCLA−CD133− acinar cells and UEA-1highCLA−CD133+ AD cells sorted from tissues cultured for five days by qRT-PCR (n = 3). Consistent with the observation of ADM, the UEA-1highCLA−CD133+ cells showed a dramatic down-regulation of acinar specific genes AMY2B and PTF1a, with a concomitant significant up-regulation of ductal specific genes SOX9 and CK19 (Figure 1B). However, carbonic anhydrase II (CA2), a ductal specific enzyme, did not show a significant up-regulation, further indicating that AD cells are different from ductal cells.
We have previously shown that TGF-β treatment in 3D culture induced ADM and transiently activated the proliferation of AD cells.17 To test whether 2D culture-induced ADM also activated the proliferation of AD cells, we sorted AD cells and acinar cells from the tissues cultured under 2D conditions for five days (n = 3). Similar to a previous study,17,19 while acinar cells did not form spheres, AD cells could form small spheres by day seven and began to die after 10 days in culture. In contrast, ductal cells continuously proliferated to form big ring-like spheres (Figure 1C).
TGF-β Signalling Pathway Mediates ADM in 2D Cultured Cells
We have previously demonstrated that TGF-β1 treatment induced ADM of human primary pancreatic acinar cells in 3D culture. Thus, we tested whether the ADM occurring in 2D culture was mainly due to the activation of the TGF-β signalling pathway. Primary tissues were stained with UEA-1-FITC and cultured under 2D conditions for five days in the absence or presence of the TGF-β receptor 1 inhibitors, SB431542 (1 uM) or Ly2157299 (1 uM). As shown in Figure 2a (n = 3), both inhibitors almost completely abolished the conversion of acinar cells to CD133+ ductal-like cells induced by 2D culture, similar to a previous study.14 We also treated the primary tissues with TGF-β1 in 2D culture for three days (n = 3). While the majority of acinar cells in the untreated groups remained to be CD133 negative at this time point, a large population of acinar cells gained the CD133 expression after TGF-β1 treatment (Figure 2B). Taken together, this data suggested that activation of this pathway was essential for 2D culture to induce ADM in a serum-free condition.
FIGURE 2. Activation of TGF-β signalling pathways mediates ADM in cultured human tissues.
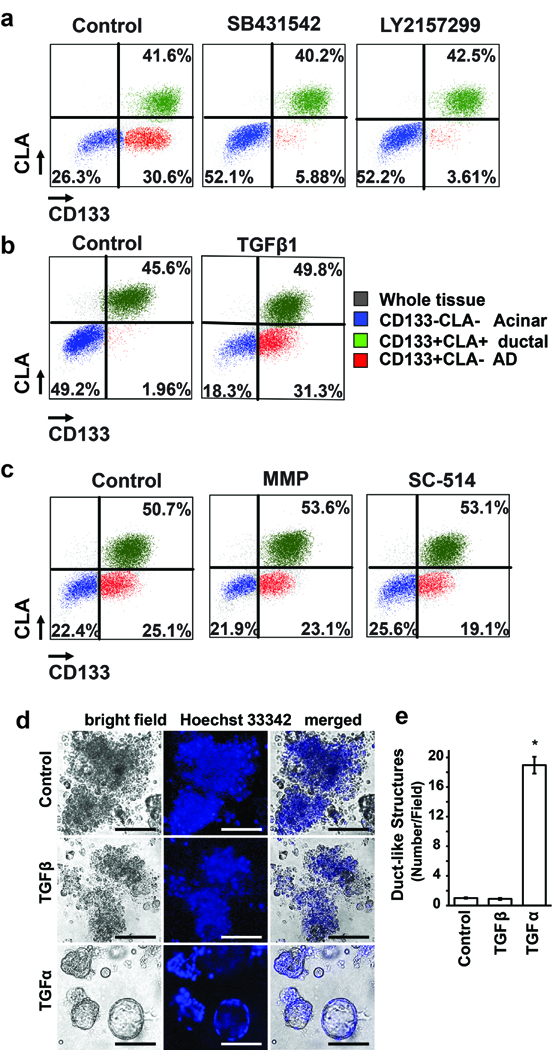
A, Five days after culture, a significant proportion of CLA−CD133− cells (blue, acinar cells) gained CD133 expression to become CLA−CD133+ cells (red, AD cells). SB431542 and LY2157299 treatment strongly inhibited this conversion. (n =5 ). B, Compared with an untreated control culture, Activin A and TGF-β1 treatment for three days strongly induced the conversion of CLA−CD133− cells (blue, acinar cells) to CLA−CD133+ cells (red, AD cells) (n = 3). C, Five days after culture, a significant proportion of CLA−CD133− cells (blue, acinar cells) gained CD133 expression to become CLA−CD133+ cells (red, AD cells) in the presence of MMP and NF- NF-κb inhibitors (n = 3). D, Primary mouse pancreatic acinar cells were isolated from wildtype mice and seeded in 3D collagen explant culture in presence of TGF-α (50 ng/ml) or TGF-β1 (50 ng/ml) as indicated. At day 5, bright field pictures were taken. e) The numbers of duct-like structures were counted. Data represents mean (SD) from three independent experiments. *P < 0.05
Activation of the NF-κB and MMP pathways has been shown to play an important role in ADM induction in murine acinar cells.18 We tested whether these two pathways are also required for human ADM induction. The human exocrine pancreatic fractions were cultured in the presence of NF-κB or MMP inhibitors for five days (n = 3). We found that these inhibitors showed no significant effect on ADM induction in human pancreatic acinar cells (Figure 2C), suggesting the minor role of these pathways in 2D culture-induced ADM of human cells.
It has been reported that mouse acinar cells that undergo ADM mainly depend on the EGF/TGF-α signalling pathway.3,20 Although data has hinted that mouse acinar cells may not respond to TGF-β, no published data shows whether mouse ADM can be induced by TGF-β. Thus, we tested whether TGF-β signaling promotes ADM of mouse cells. Mouse pancreatic acinar clusters were embedded in three-dimensional (3D) fibrillary collagen18 and treated with TGF-β1 for five days. Cells treated with TGF-α were used as a positive control. As shown in Figure 2D, while TGF-α treatment induced ADM in mouse acinar cells to form ductal like structures, TGF-β1 treatment did not. This data confirmed that mouse acinar cells do not undergo ADM by the TGF-β signaling pathway, further emphasizing the difference in inductive signals of ADM in human and mouse cells.
Ductal Cells Secrete TGF-β to Induce ADM in 2D Cultured Cells
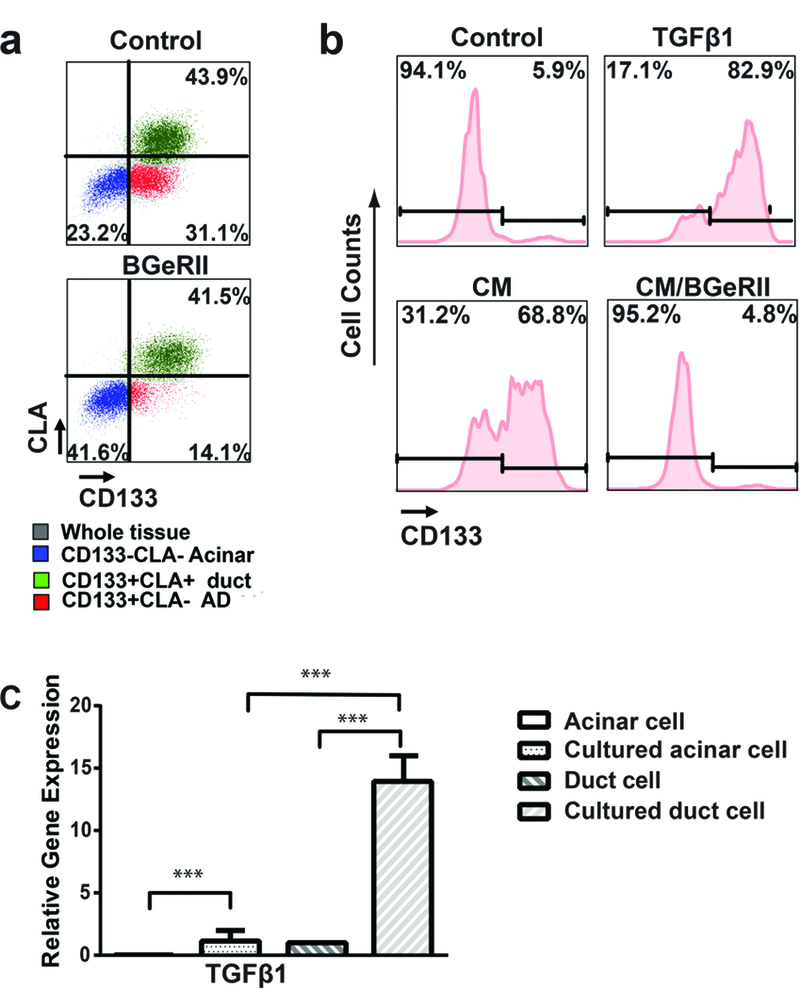
Our 2D culture conditions were serum-free and had no exogenous TGF-β, thus, we speculated that exocrine tissues might secrete these factors to induce ADM during the culture. We first tested whether TGF-β neutralization protein BGeRII21 could block culture-induced ADM in 2D cultured cells (n = 3). As shown in Figure 3A, the addition of BGeRII strongly attenuated ADM in 2D culture, indicating the presence of TGF-β in 2D culture.
FIGURE 3. Paracrine TGF-β from ductal cells promotes ADM of primary human pancreatic acinar cells in vitro.
A, Five days after culture, a significant proportion of CLA−CD133− cells (blue, acinar cells) gained CD133 expression to become CLA−CD133+ cells (red, AD cells). The TGF-β binding protein BGeRII (depletion of secreted TGF-βs) treatment inhibited CD133 up-regulation in acinar cells (n = 3). B, Sorted acinar cells did not gain CD133 expression when cultured alone in the 2D system. c) Both TGF-β1 and conditioned media from cultured ductal cells induced the conversion of acinar cells to AD cells. BGeRII treatment abolished the inductive effects of condition media (n = 3). C, Five days after culture, TGF-β1 mRNA was up-regulated in both acinar and ductal cells. However, the expression of TGF-β1 in acinar cells was significantly lower than that in ductal cells (n = 3). Data represent mean (SD) from three independent experiments, ***P < 0.001.
We then questioned whether the source of TGF-β was acinar or ductal cells. To address this we sorted acinar and ductal cells from fresh human exocrine tissues by flow cytometry and cultured them in the same serum free medium for six days (n = 3). We found that sorted acinar cells underwent ADM much slower than unsorted cells when cultured in 2D conditions (Figure 3B). We reasoned that either the ductal cells secreted factors, or that direct contact with ductal cells induced acinar cells to make TGF-β. To test whether ductal cells secretes TGF-β to induce ADM of acinar cells, we sorted ductal cells and cultured them for six days in serum-free media (n = 3). We then collected conditioned media from sorted ductal cells and used it to culture freshly sorted acinar cells in a 2D condition. Interestingly, the conditioned media promoted the transition of acinar cells to CD133 positive AD cells (Figure 3B). Moreover, adding BGeRII to the conditioned media diminished the ADM inductive effect (Figure 3B), suggesting that TGF-β was in the ductal cells conditioned media. To test whether 2D culture could induce TGF-β1 in human ductal cells, we measured the TGF-β1 mRNA levels in the acinar and ductal cells sorted before and after culture. As shown in Figure 3C, cultured ductal cells expressed significantly higher levels of TGF-β1 than other cells (n = 3). This data together suggests that TGF-β secreted by ductal cells acts as a paracrine factor that induces acinar cells to undergo ADM in 2D culture.
TGF-β-independent ADM Inductive Effect of Serum
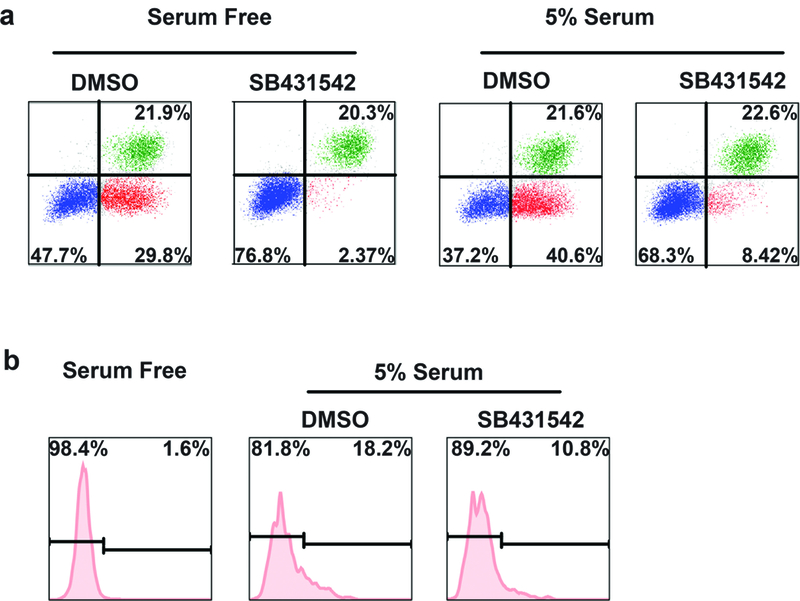
Serum has been used for cell culture and can maintain cells healthier. We examined whether serum could induce ADM in human tissues. As shown in Figure 4A, adding serum induced a modest but reproducible ADM in tissues cultured for five days. We further tested whether blocking TGF-β signalling pathway can abolish this effect of serum by treating the culture with TGF-β receptor I inhibitor SB431542. Interestingly, although SB431542 treatment almost completely blocked ADM in tissues cultured in a serum free condition, we found a fraction of cells underwent ADM in tissues cultured in serum with the treatment of SB431542. To further confirm the TGF-β-independent effect of serum in the induction of ADM in human acinar cells, we sorted acinar cells to culture for five days in a serum containing medium with and without SB431542. As shown in Figure 4B, the acinar cells did not gain CD133 expression when cultured with a serum free medium. However, CD133 expression was induced in a fraction of acinar cells cultured with a serum containing medium. Moreover, this induction effect could not be completely blocked by SB431542 treatment, supporting the TGF-β-independent effect of serum to induce ADM in human cells. This data suggests that TGF-β signalling pathway remains the major mechanism promoting ADM in human tissues cultured with a serum containing medium, but serum can induce ADM independent of the TGF-β pathway.
FIGURE 4. TGF-β independent ADM inductive effect of serum on primary human pancreatic acinar cells.
A, Serum enhanced the ADM induction in primary human tissues cultured in vitro. TGF-β receptor inhibitor did not completely block the ADM of human tissues cultured with serum containing medium. B, A fraction of sorted acinar cells gained CD133 expression when cultured with serum containing medium. This inductive effect could not be completely blocked by TGF-β receptor inhibitor treatment.
DISCUSSION
Using the flow cytometry-based lineage tracing method, we showed that the frequency of CD133 positive cells increased in human primary exocrine pancreatic tissues during 2D culture. This is consistent with the well-known observation that culturing cells can induce the “enrichment” of cells with a ductal-like phenotype in human primary exocrine pancreatic tissues.15,16 Although we and others have demonstrated that primary human pancreatic acinar cells could undergo ADM to become ductal-like cells,12,17 whether ADM is one of the major reasons for this culture-induced “enrichment” is controversial.13,15,16 Previous studies have proposed that the enrichment of cells with a ductal phenotype was due to the selective survival and expansion of ductal cells.13,16 However, our data showed a large proportion of CD133 positive cells in cultured primary human exocrine pancreatic cells were derived from acinar cells. Moreover, qRT-PRC analysis revealed the up-regulation of ductal markers (SOX9 and CK19) and down-regulation of acinar markers (AMY2B and PTF1a) in these acinar-derived CD133 positive cells. Taken together, this data strongly suggested that ADM is one of the major contributors to 2D culture-induced “enrichment” of ductal-like cells.
Consistent with the previous studies,14 we found that SB431542 or LY2157299 strongly blocked ADM induced by 2D culture, indicating ADM induction is mediated by the activation of the TGF-β signalling pathway. We found TGF-β1 treatment dramatically accelerated ADM in the 2D culture condition. Nevertheless, we found that depletion of TGF-β by neutralizing proteins blocked 2D culture-induced ADM, suggesting TGF-β is the major factor for 2D culture-induced ADM. Although TGF-β1 could efficiently induce ADM of human cells, it failed to promote ADM formation of mouse acinar cells, highlighting the presence of the difference between human and mouse.
We further demonstrated that TGF-β secreted by ductal cells is the major paracrine factor for ADM induction in our culture systems. Interestingly, ductal epithelia are also the main cellular sources of TGF-β1 in chronic obstructive pancreatitis,22 increasing the possibility that ductal cell secretion of TGF-β1 might play an important role in ADM induction during pancreatitis. Nevertheless, we cannot exclude the possibility that other cells, such as infiltrating immune cells and activated stromal cells,23,24 also secrete TGF-β in pancreatitis.25 Our data suggested that TGF-β is a potent ADM inducer for primary human pancreatic acinar cells, at least under in vitro culture conditions. The pathological relevance of this observation needs to be further tested in future studies. Indeed, we found that tissues cultured with serum containing medium showed enhanced ADM induction when compared to human tissues cultured under serum free conditions. Importantly, although inhibiting the activation of the TGF-β pathway dramatically suppressed ADM in human tissues during in vitro culture, it did not affect the modest but reproducible ADM inductive effects of serum. This data indicates that additional unidentified pathways can promote ADM in human cells, which may also have important pathological functions in human disease development. Further studies are needed to reveal these pathways.
ACKNOWLEDGMENTS
We thank the members of the Wang research laboratory and Junhua Yang for materials and assistance. We thank Benjamin J. Daniel and Karla M. Gorena for their help in flow cytometry. Data was generated in the Flow Cytometry Shared Resource Facility, which is supported by UTHSCSA, NIH-NCI P30 CA054174-20 (CTRC at UTHSCSA) and UL1 TR001120 (CTSA grant).
Source of financial support: This work is supported by the Cancer Prevention and Research Institute of Texas (P. Wang) and NIH grant CA200572 (P. Storz). Pei Wang is a CPRIT scholar. Jun Liu is supported by a training grant from the Cancer Prevention and Research Institute of Texas (RP140105).
Footnotes
Disclosure: The authors declare no conflict of interest.
REFERENCE
- 1.Guney MA, Gannon M Pancreas Cell Fate. Birth Defects Res. Part C Embryo Today Rev. 2009;87:232–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kopp JL, Grompe M, Sander M. Stem cells versus plasticity in liver and pancreas regeneration. Nat Cell Biol. 2017;18:238–245. [DOI] [PubMed] [Google Scholar]
- 3.Morris JP, Wang SC, Hebrok M. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat Rev Cancer. 2010;10:683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey JM, DelGiorno KE, Crawford HC. The secret origins and surprising fates of pancreas tumors. Carcinogenesis. 2014;35:1436–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kopp JL, von Figura G, Mayes E, et al. Identification of Sox9-Dependent Acinar-to-Ductal Reprogramming as the Principal Mechanism for Initiation of Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2012;22:737–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie VK, Maitra A. Krüppel-Like Factor 4 Promotes Pancreatic Acinar-to-Ductal Metaplasia and Tumor Initiation. Pancreas. 2017;46:139–142. [DOI] [PubMed] [Google Scholar]
- 7.Habbe N, Shi G, Meguid RA, Fendrich V, et al. Spontaneous induction of murine pancreatic intraepithelial neoplasia (mPanIN) by acinar cell targeting of oncogenic Kras in adult mice. Proc Natl Acad Sci USA. 2008;105:18913–18918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raimondi S, Lowenfels AB, Morselli-Labate AM, et al. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract Res Clin Gastroenterol. 2010;4:349–358. [DOI] [PubMed] [Google Scholar]
- 9.Bailey JM, Hendley AM, Maitra A. New insights into plasticity of pancreatic cancer: cancer to acinar cell reprogramming by the basic helix-loop-helix transcription factor E47. Pancreas. 2015;44:683–685. [DOI] [PubMed] [Google Scholar]
- 10.Means AL, Meszoely IM, Suzuki K, et al. Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development. 2005;32:3767–3776. [DOI] [PubMed] [Google Scholar]
- 11.Collins MA, Bednar F, Zhang Y, et al. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J Clin Invest. 2012;122:639–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houbracken I, de Waele E, Lardon J, et al. Lineage Tracing Evidence for Transdifferentiation of Acinar to Duct Cells and Plasticity of Human Pancreas. Gastroenterology. 2011;141:731–741. [DOI] [PubMed] [Google Scholar]
- 13.Klein T, Heremans Y, Heimberg H, et al. Investigation and Characterization of the Duct Cell-Enriching Process During Serum-Free Suspension and Monolayer Culture Using the Human Exocrine Pancreas Fraction. Pancreas. 2009;38:36–48. [DOI] [PubMed] [Google Scholar]
- 14.De Waele E, Wauters E, Ling Z. Conversion of human pancreatic acinar cells toward a ductal-mesenchymal phenotype and the role of transforming growth factor β and activin signaling. Pancreas. 2014;43:1083–1092. [DOI] [PubMed] [Google Scholar]
- 15.Hall PA, Lemoine NR. Rapid acinar to ductal transdifferentiation in cultured human exocrine pancreas. J Pathol. 1992;166:97–103. [DOI] [PubMed] [Google Scholar]
- 16.Street CN, Lakey JR, Rajotte RV, et al. Enriched Human Pancreatic Ductal Cultures Obtained from Selective Death of Acinar Cells Express Pancreatic and Duodenal Homeobox Gene-1 Age-Dependently. Rev Diabet Stud. 2004;1:66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Akanuma N, Liu C, et al. TGF-β1 promotes acinar to ductal metaplasia of human pancreatic acinar cells. Sci Rep. 2016;6:30904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liou GY, Döppler H, Necela B, et al. Macrophage-secreted cytokines drive pancreatic acinar-to-ductal metaplasia through NF-κB and MMPs. J Cell Biol. 2013;202:563–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J, Sugiyama T, Liu Y, et al. Expansion and conversion of human pancreatic ductal cells into insulin-secreting endocrine cells. eLife. 2013;2:e00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siveke JT, Crawford HC. KRAS above and beyond - EGFR in pancreatic cancer. Oncotarget. 2012;3:1262–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verona EV, Tang YP, Millstead TK, et al. Expression, purification and characterization of BGERII: a novel pan-TGF-β inhibitor. Protein Eng Des Sel. 2008;21:463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukumura Y, Suda K, Mitani K, et al. Expression of Transforming Growth Factor β by Small Duct Epithelium in Chronic, Cancer-Associated, Obstructive Pancreatitis: An In Situ Hybridization Study and Review of the Literature. Pancreas. 2007;35:353–357. [DOI] [PubMed] [Google Scholar]
- 23.Aoki H, Ohnishi H, Hama K, et al. Autocrine loop between TGF-beta1 and IL-1beta through Smad3- and ERK-dependent pathways in rat pancreatic stellate cells. Am J Physiol Cell Physiol. 2006;290:C1100–1108. [DOI] [PubMed] [Google Scholar]
- 24.Shek FW, Benyon RC, Walker FM, et al. Expression of transforming growth factor-beta 1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis. Am J Pathol. 2002;160:1787–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xue J, Sharma V, Hsieh MH, et al. Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis. Nat Commun. 2015;6:7158. [DOI] [PMC free article] [PubMed] [Google Scholar]


