Abstract
Objectives
To explore phenotypic differences between individuals with sporadic inclusion body myositis (sIBM) who are seropositive for the NT5c1A antibody compared with those who are seronegative.
Methods
Cross-sectional clinical, serological and functional analysis in 25 consecutive participants with sIBM.
Results
All participants met criteria for clinically defined or probable sIBM. 18 of 25 participants with sIBM (72%) were seropositive for the NT5c1A antibody. No differences between median age and duration of illness between the two groups were seen. Females have higher odds of being seropositive (OR=2.30). Participants with seropositive sIBM took significantly longer to get up and stand (p=0.012). There were no significant differences between the two groups in terms of distance covered on a 6 min walk. Seropositive participants were more likely to require assistive devices such as a walker or wheelchair for mobility (OR=23.00; p=0.007). A number of secondary (exploratory) outcomes were assessed. NT5c1A seropositive sIBM cases had lower total Medical Research Council (MRC) sum score and MRC sum score on the right (p=0.03 and 0.02, respectively). Participants with the NT5c1A antibody were significantly more likely to have symptoms of dysphagia (OR=10.67; p=0.03) and reduced forced vital capacity (p=0.005). Facial weakness occurred in 50% of seropositive participants while it was only seen in 14% of seronegative participants.
Conclusions
Even though the small sample size limits definite conclusions, our cross-sectional study showed seropositivity to the NT5c1A antibody is associated with greater motor and functional disability in sIBM. The study also suggests more prominent bulbar, facial and respiratory involvement in individuals positive for NT5c1A antibodies.
BACKGROUND
Sporadic inclusion body myositis (sIBM) is a disabling disorder with male predominance, presenting at older ages with slowly progressive, asymmetric, weakness.1,2 Despite very prominent inflammatory changes in skeletal muscle, the disease is considered a degenerative disorder of the muscle3 that responds poorly to treatments such as immunosuppression.4,5
The debate remains regarding the pathogenesis of sIBM: there are two separate pathological processes in sIBM, a degenerative muscle process and an inflammatory process.6–10 It is unclear as to which is the cause of the disease or aetiology behind tissue damage. The evidence in favour of a primary inflammatory response keeps mounting. Muscle fibres could act as antigen presenting cells with upregulation of MHC-111 and expression of costimulatory molecules such as ICOS-L and BB1.12–15 Clonally expanded plasma cell infiltrates are present in muscle and clonally restricted populations of immunoglobulin transcripts have been identified in inflammatory nodules in muscle by laser capture and microarray.16 The incidence of sIBM is higher in immunodeficiency states, such as human T-cell lymphotropic virus (HTLV) and HIV.17
A serum antibody directed against a 43kD protein appears to be common in patients with sIBM;18 the autoantibody binds to the protein, NT5c1A, most abundant in skeletal muscle with a possible role in DNA repair.19,20 Subsequent work has shown presence of IgA and IgM isotypes in addition to the IgG isotype, anti-NT5c1A antibodies.21 The sensitivity of anti-NT5c1A antibodies is approximately 70% with specificity around 90%. Anti-NT5c1A antibodies are rare in the general population and have a low frequency in inflammatory myopathies other than sIBM.19,20 The pathogenic role of anti-NT5c1A antibodies is not clear.
In this cross-sectional analysis of patients with sIBM we asked whether patients with seropositive and seronegative sIBM have different disease phenotype. Our data suggests that seropositive sIBM with antibodies to NT5c1A may represent a more aggressive disease, with more severe motor and functional deficits and a higher incidence of bulbar and respiratory involvement.
MATERIALS AND METHODS
Participants characteristics
All consecutive new or established patients who met the European Neuromuscular Centre (ENMC) 2011 criteria22 for diagnosis of sIBM and seen in our neuromuscular clinic between 1 October 2013 and 15 April 2014 were included in this crosssectional study. All participants provided informed consent and HIPPA (Health Insurance Portability and Accountability Act of 1996) authorisation. All patients were seen at a specific study visit, during which investigators and evaluators blinded to the NT5c1A results performed all the assessments. All participants had NT5c1A antibodies assayed and serum creatine kinase (CK) measured. Demographic data was abstracted from the medical record; direct questions were asked about age of onset, duration of illness as well as presence of other immune disorders. History of bulbar dysfunction (swallow, chewing and speech difficulty) was obtained and facial muscles were directly examined for assessment of facial weakness.
Serological testing
Serological testing for the NT5c1A antibody was performed at the Neuromuscular Laboratory at Washington University in St. Louis. Presence of antibody was examined through western blot, and confirmed in positive samples with ELISA. For western blots, performed, as previously described,23 NT5c1A protein in 293 cell lysate was purchased from ABM (PL027845; Richmond, British Columbia, Canada), diluted in buffer (from 10X Tris /Glycine/SDS stock; 161-0732, BioRad), and used at 1 μg NT5c1A lysate per lane. Precast Criterion gels (345-0012; BioRad) were 10% Tris-HCl. Test serums were diluted to 1:100. Second antibodies were Biotinylated Anti-Human IgG (H+L; BA-3000, Vector), used at 1:500, followed by ABC (PK-4000, Vector). Serums were positive if they stained protein bands at the same molecular weight positions as a positive control serum tested on the same gel. None of 20 control serums showed serum IgG binding to NT5c1A.
For ELISA assays, performed as previously described using Immulon II plates,24 recombinant human NT5c1A protein was purchased from MyBioSource (MBS 70EP866304HU; San Diego, California, USA) and used at 150 ng recombinant NT5c1A per well. Serums were initially tested at dilutions of 1:3000. Second antibodies were peroxidase-conjugated Goat IgG Fraction to Human IgG FC (55226, MP Bio Medical) used at 1:75 000. Positives were diluted until the optical density signal was in the linear range. High values were more than 3 SDs above the mean of 50 control serums.
Motor testing
A blinded single investigator (TMC) performed all the motor testing. Manual muscle testing in the upper and lower extremities was performed and muscle strength was graded using the Medical Research Council (MRC) score. Quantitative MRC score was computed by summing the muscle grade score for the following muscles on each side: shoulder abductors, elbow flexors, wrist flexors, hip flexors, knee extensors and ankle dorsiflexors, with the maximum score on each side being 30 and maximum total score being 60. Handgrip strength was measured using a JAMAR grip meter (Sammons Preston Rolyan, Chicago, Illinois, USA) and pinch strength was measured with the thumb over index finger using a JAMAR pinch meter (Sammons Preston Rolyan).
A trained certified physiotherapist (PT) administered the IBM functional rating scale (IBMFRS). The IBMFRS has been validated in therapeutic trials of sIBM and is used widely clinically and in the research setting for monitoring disease progression in this disease.25 The same evaluator administered the timed get up and 6 min walk test. The modified Rankin Scale (mRS) to measure the degree of disability was also assessed. All of these are validated scales that have been used in a variety of neuromuscular disorders and in previous studies of IBM.26–28 Use of the armrest was allowed for the timed get up test and use of a cane or walker was allowed for the 6 min walk test. For statistical purposes any individual unable to get up from a chair, even with the use of arm rests, was scored at 20 s (maximal score) while for the 6 min walk test, a distance of 0 feet was scored for individuals who were unable to perform the test.
Pulmonary function testing
All participants underwent spirometry (forced vital capacity, FVC) measurements as well as inspiratory force measurements (maximal inspiratory pressure) and sniff nasal inspiratory pressure using a Renaissance II spirometer (Puritan Bennett, Pleasanton, California, USA) and MicroRPM handheld portable respiratory pressure meter (CareFusion, San Diego, California, USA), by a trained respiratory therapist. A facemask was used when significant facial weakness was detected.
Bulbar evaluation
Participants were asked directly if they had any problems with chewing or swallowing (a positive response, ie, difficulty with chewing or swallowing, was defined as dysphagia for the purposes of this study). A speech therapist (NA) blinded to the serological result and to symptoms of bulbar dysfunction, administered the modified oral bulbar facial respiratory scale (mOBFRS) to all participants. This scale was developed by Farrugia et al29 for use predominantly in myasthenia gravis and has been validated for use in that disease. The maximum score (severe dysfunction) is 17 while the minimum score possible (no dysfunction) is 0.
European quality of life 5D-5L and visual analogue scale score
European quality of life (EuroQOL) was developed for quantifying health burden on participants afflicted with chronic diseases (http://www.euroqol.org/home.html). There are two separate ways of administering it: individual domains affecting the quality of life, including effects on mobility, self-care, usual activities, pain and discomfort and anxiety and depression, can be assessed individually while the EuroQOL visual analogue scale score (EuroQOL VAS) can provide a global assessment of an individual’s quality of life. This tool has been used in multiple studies and provides a robust measurement of the health quality affected by the individual’s medical condition.30,31 A single individual (UA) performed all of the QOL assessments for our study.
Statistical analyses
The primary outcome measures for this study were two motor function assessments: 6 min walk test and timed get up and stand test. The secondary (exploratory) outcome measures were MRC sum score (total, and right and left sides), right and left hand grip, IBMFRS, mRS score, serum CK, FVC expressed as percentage predicted, negative inspiratory force (NIF), EuroQOL (VAS) and score on mOBFRS. For the primary outcomes, the non-parametric Mann-Whitney-Wilcoxon test was applied and the significance level was set at 0.025 for each of the tests. The continuous secondary outcomes were analysed similar to the primary outcomes. The categorical secondary outcomes, such as dysphagia, facial weakness, ambulation status, and mOBFRS level were evaluated using the Fisher’s exact test. The OR was calculated with corresponding 95% CI. All secondary outcome analysis was based on the significance level of 0.05. All analyses were performed on Graph Pad Prism V.5 (San Diego, California, USA). Data are presented with median and range (minimum, maximum) or frequency and percentage.
RESULTS
A total of 25 consecutive participants with sIBM were enrolled in this study: 19 participants fulfilled the ENMC 2011 criteria for clinically defined and 6 participants fulfilled the criteria for probable sIBM.22 The histopathological features for both these categories (clinically defined or probable) are the same and include: the presence of “one or more, but not all, of: endomysial inflammatory infiltrate, upregulation of MHC Class 1, rimmed vacuoles, protein accumulation or 15–18 nm filaments”. Since the muscle biopsy slides were unavailable to review for some patients, we based all pathological selection criteria on their muscle biopsy reports (which were reviewed for all patients). All patients had, at minimum, the presence of endomysial inflammatory infiltrate reported on the H&E stain.
NT5c1A antibodies were detected in 18 of the 25 participants with sIBM (72%, 13 clinically defined and 5 probable). This is similar to the sensitivity reported by Larman et al and is close to what has been seen at other centres (AL Mammen, personal communication). Antibody titres on ELISA ranged from 2000 to 100 000, with a mean titre of 34 483±5988 (N = 18).
Basic characteristics
Table 1 summarises the basic characteristics of the 25 participants. The median age of seropositive participants was 67 years while the median age of seronegative participants was 70 years. There were six females in this cohort; the majority (83%) of them were seropositive. The median age at onset for symptoms related to sIBM was 55.5 years for the seropositive participants and 54 years for the seronegative participants. Disease duration was 10 years in seropositive participants and 11 years in seronegative participants. Disease presentation in majority of seropositive participants was leg weakness (14 of 18; 77%); upper extremity onset was seen in two participants and another two seropositive participants presented with bulbar weakness. All of the seronegative participants had symptoms that started in the legs; however, two of them reported concomitant involvement of the arms. In the seropositive group, a history of hypothyroidism was reported in three participants; one each had a history of Hodgkin’s lymphoma, systemic lupus erythematosus and rheumatism. In the seronegative group, hypothyroidism and lymphoma was reported in two participants.
Table 1.
Basic demographic characteristics
| Seropositive sIBM Median (min, max) |
Seronegative sIBM Median (min, max) |
|
|---|---|---|
| Mean age | 67.0 (47.0, 77.0) | 70.0 (60.0, 85.0) |
| Mean age at onset | 55.5 (45.0, 71.0) | 54.0 (54.0, 78.0) |
| Duration of illness | 10.0 (3.0, 15.0) | 11.0 (4.0, 24.0) |
sIBM, sporadic inclusion body myositis.
Primary outcome measures (motor assessments)
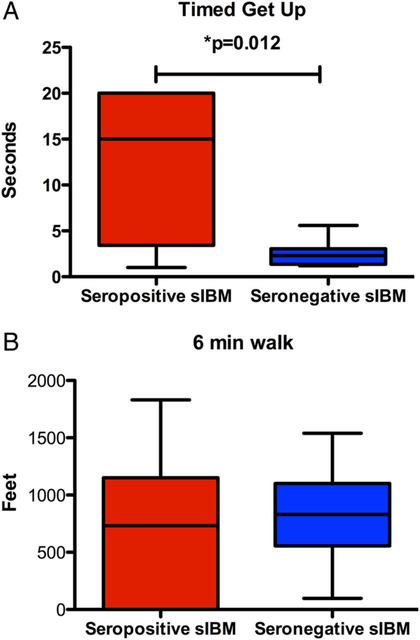
Figure 1 shows the median time to get up and stand test and the mean distance for 6 min walk test. The median time to get up from a standard chair was 15.0 s and was significantly longer (p = 0.012) in the seropositive group (n = 17, range: 1.01–20.0 s) than the seronegative group (n = 7, median 2.30 s; range: 2.30–5.60 s). The distance covered in the 6 min walk test was not different (p = 0.58) between the seropositive (n = 17, median 733.0; range 0–1831.0 feet) and seronegative group (n = 7, median 830.0; range 97.0–1539.0 feet).
Figure 1.
Box-Whiskers plot of primary outcome measures. (A) Showing the differences in timed get up and stand test between the seropositive and seronegative sporadic inclusion body myositis (sIBM) groups. Seropositive participants took significantly longer to get up and stand from a standard chair compared with seronegative participants (median time was 15.2 s (n=17) in the seropositive participants versus 2.3 s in the seronegative participants (n=7; p=0.12)). (B) Showing the distance covered on a 6 min walk test between the two groups. Seropositive participants covered a median distance of 733 feet (n=17) while the seronegative participants covered a distance of 830 feet (n=7). There was no significant difference between the two groups.
Secondary (exploratory) outcomes
A number of secondary measures were carried out as part of our assessments. Table 2 shows the results of the other exploratory measures. MRC sum score (total as well as on the right side) was significantly lower in the participants with seropositive sIBM compared with seronegative participants. Median total MRC sum score was 45.0 in the seropositive participants (n=18) versus 48.0 in the seronegative participants (n = 7; p=0.03) while the MRC sum score on the right was 22.0 in the seropositive participants (n = 18) versus 25.0 in the seronegative participants (n = 7; p = 0.02). MRC sum score on the left was not significantly different in the seropositive participants compared with the seronegative participants (p = 0.06).
Table 2.
Secondary (exploratory) outcome measures
| Seropositive sIBM Median (min, max) |
Seronegative sIBM Median (min, max) |
p Value | |
|---|---|---|---|
| MRC sum score (total) | 45.0 (16.0, 52.0) | 48.0 (46.0, 53.0) | 0.03 |
| MRC sum score (right) | 22.0 (8.0, 27.0) | 25.0 (23.0, 27.0) | 0.02 |
| MRC sum score (left) | 22.5 (8.0, 26.0) | 24.0 (22.0, 26.0) | 0.09 |
| Right hand grip (lbs) | 15.0 (0.0, 85.0) | 15.0 (5.0, 30.0) | 0.82 |
| left hand grip (lbs) | 5.0 (0.0, 100.0) | 20.0 (0.0, 30.0) | 0.31 |
| IBMFRS | 23.0 (17.0, 36.0) | 29.0 (22.0, 35.0) | 0.06 |
| Modified Rankin score | 3.0 (2.0, 4.0) | 3.0 (2.0, 4.0) | 0.80 |
| Serum creatine kinase (IU/L) | 435.0 (96.0, 3395.0) | 424.0 (109.0, 1450.0) | 0.76 |
| FVC (% predicted) |
81.5 (32.0, 97.0) | 92.0 (84.0, 102.0) | 0.005 |
| NIF (cm H2O) | −66.0 (−112, −24.0) | −102.0 (−150.0, −29.0) | 0.12 |
| EuroQOL (VAS) | 55.0 (25.0, 80.0) | 65.0 (50.0, 80.0) | 0.14 |
| Modified OBFRS | 4.0 (0, 9.0) | 2.0 (0, 4.0) | 0.06 |
Figures in bold signify a statistically significant value (p≤0.05).
EuroQOL, European quality of life; FVC, forced vital capacity; IBMFRS, inclusion body myositis functional rating scale; MRC, Medical Research Council; NIF, negative inspiratory force; OBFRS, oral bulbar facial respiratory scale; sIBM, sporadic inclusion body myositis; VAS, visual analogue scale score.
FVC in an erect posture was significantly lower in the seropositive participants compared with the seronegative participants. Median FVC predicted values in the seropositive participants were 81.5% (n=18) versus 92.0% in the seronegative participants (n=7; p=0.007). Median NIF values were also lower in the seropositive participants compared with the seronegative participants but did not reach statistical significance.
Two other parameters were of note. Median IBMFRS score was lower in the seropositive participants (23.0; n = 17) than in the seronegative participants (29.0; n=7; p = 0.06). Similarly, the median mOBFRS score was higher in the seropositive participants (4.0; n=17) compared with the seronegative participants (2.0; n=7; p=0.06).
There were no significant differences between the two groups when comparing handgrip and pinch strengths, mRS score, serum CK levels, and quality of life (measured by EuroQOL VAS).
Contingency analysis
Table 3 details the contingency analysis of select outcome measures. Ambulation was significantly more affected in seropositive participants; 11 seropositive participants (61%) required mobility assistive devices, either a walker or a wheelchair, while no seronegative participants (0%) required a walker or wheelchair. The odds of seropositive participants requiring assistance of walker or wheelchair were calculated at 23.00 (p = 0.007). Similarly odds of having dysphagia were significantly higher in the seropositive participants compared with the seronegative participants (OR=10.67; p=0.03). Seropositive participants were also more likely to have higher mOBFRS defined as score >5 (OR=10.71), facial weakness (OR=6.0), NIF<−60 cm H2O (OR=4.8) and FVC<50% (OR=2.93). Values of NIF<60cm and FVC<50% of predicted were chosen for the contingency analyses, as these values define thoracic respiratory insufficiency as per the Medicare criteria.
Table 3.
Contingency analyses
| Seropositive N (%) |
Seronegative N (%) |
p Value |
OR (95% CI) |
||
|---|---|---|---|---|---|
| Ambulation | Requiring no/minimal assistance Requiring walker/wheelchair |
7 (39) 11 (61) |
7 (100) 0 (0) |
0.008 | 23.00 (1.13 to 465.5) |
| Dysphagia | Yes No |
16 (89) 2(11) |
3 (43) 4 (57) |
0.03 | 10.67 (1.30 to 86.98) |
| mOBFRS>5 | Yes No |
7(41) 10 (59) |
0 (0) 7 (100) |
0.06 | 10.71 (0.52 to 218.0) |
| Facial weakness | Yes No |
9 (50) 9 (50) |
1 (14) 6 (86) |
0.17 | 6.00 (0.59 to 60.47) |
| NIF<−60 cm | Yes No |
8 (44) 10 (56) |
1 (14) 6 (86) |
0.35 | 4.80 (0.47 to 48.49) |
| FVC<50% | Yes No |
3 (17) 15 (83) |
0 (0) 6 (100) |
0.54 | 2.93 (0.13 to 65.31) |
| CK>500 IU/L | Yes No |
8 (50) 8 (50) |
2 (28) 5 (72) |
0.40 | 2.50 (0.36 to 16.90) |
| Female gender | Yes No |
5 (28) 13 (72) |
1 (14) 6 (86) |
0.63 | 2.30 (0.21 to 24.33) |
Figures in bold signify a statistically significant value (p≤0.05).
CK, creatine kinase; FVC, forced vital capacity; mOBFRS, modified oral bulbar facial respiratory scale; NIF, negative inspiratory force.
DISCUSSION
Given the small number of participants in our cohorts, these results should be interpreted with caution; however, this preliminary study demonstrates phenotypic differences between participants with sIBM who are seropositive for the NT5c1A antibody versus those who are seronegative. The participants with seropositive sIBM have more severe motor weakness (MRC sum score), significantly greater difficulty with proximal lower limb weakness (timed get up) and significantly higher odds of requiring walker or wheelchair for mobility. Additionally, the exploratory secondary outcome measures suggest seropositive individuals have significantly more bulbar and respiratory involvement and greater odds of having higher CK levels and facial weakness. Female participants were more likely to be seropositive.
Our data on overall motor disability is consistent with previous reports of severe disability in patients with sIBM.1 A 12-year follow-up study recently showed that at long term, 40% of patients with sIBM have severe disability (Barthel Index of <10) and another 20% are moderately disabled (Barthel Index of 10–15).1 In the same study, only 14% used a wheelchair at baseline but by the end of the study, the majority of surviving participants were wheelchair dependent.1 sIBM thus remains a very disabling disease; based on our data, it appears that a subset of these patients with sIBM who are seropositive have more severe disability (greater difficulty rising from a chair and are statistically more likely to require a walker or wheelchair).
The only other study to our knowledge that has looked at the correlation of antibody reactivity to clinical symptoms is the study by Larman et al,19 that found no correlation between antibody reactivity and age, duration of illness or strength of weakest finger flexors or knee extensors. In their study they did not evaluate for any differences between the seropositive and seronegative groups in terms of bulbar or respiratory functions nor did they assess for ambulatory status. We report higher prevalence of motor deficits, significantly more functional deficits (timed get up), and a much less likelihood to independently ambulate in the NT5c1A seropositive cases. One explanation could be that these seropositive participants have longer disease duration (and thus a higher burden of disease), but our data does not reflect that. There was no statistically significant difference between the two groups in terms of disease duration.
Dysphagia is a known complication in sIBM: reported in 10% of patients with sIBM at onset and in 40% at the time of diagnosis.5 Dysphagia in sIBM is not well characterised but appears to be related to cricopharyngeal muscle dysfunction or overcontraction of the pharyngeal muscles.32–35 A recent Japanese study showed that even in individuals with sIBM who did not report dysphagia, there was radiographic evidence of mild swallowing impairment.36 We did not do radiographic swallowing studies in all our participants and it is possible that there may be swallowing abnormalities that remain subclinical. However, our data suggests a significantly higher prevalence of dysphagia in the seropositive patients.
Respiratory dysfunction in sIBM has been reported previously in the context of aspiration pneumonia and primary respiratory failure.37–39 It remains the predominant cause of mortality in long-term follow-up of patients with sIBM.1 In our cohort we did not specifically evaluate for symptoms related to dyspnoea yet the FVC and NIF were significantly lower in the participants with seropositive sIBM than in the seronegative participants, both sensitive markers of diaphragmatic dysfunction.40 It is possible that these lower FVC values in the seropositive participants may be related to the presence of increased bifacial weakness; however, we used a facemask for all FVC evaluations to minimise the possibility that the reduced FVC could be secondary to facial weakness. We conclude that respiratory involvement in sIBM may be underappreciated and it would be important to serially follow respiratory functions in patients with sIBM and monitor for any related complications.
The evidence that sIBM represents an autoimmune disease where there is a chronic antigen-stimulated adaptive immune system (B cells) response with resultant repeated hypermutation of its receptors (immunoglobulins), has already been established.41 Discovery of the NT5c1A autoantibodies in sIBM may be further evidence in favour, but it is too early to tell. To further understand the pathogenicity of the NT5c1A antibodies, and its relationship to disease pathology, it would be important to create animal models to see if the antibodies are disease causing and if passive transfer or active immunisation with the NT5c1A antigen produces the disease. It is possible that the humoral immunity that develops in participants with sIBM is secondary to severe myodegenerative disease. More severe muscle fibre degeneration exposes this intracellular protein that leads to an antigenic response and generation of this antibody. It is thus conceivable that as muscle degeneration progresses in sIBM, more antibodies will be generated and thus the seronegative participants may at some point become seropositive. This has not been reported to date to our knowledge. However, if this were the case, then seropositivity to NT5c1A antibodies would have been a function of disease duration, which was not the case in our cohort. This issue of whether the generation of the antibody is related to more severe and longer degeneration needs to be explored further and long-term follow-up studies are required.
It also remains to be seen if the presence of this antibody predicts differences in muscle pathology (do seropositive patients have more or different inflammatory responses in muscle or have more or less vacuolated fibres), different rates of disease progression (do seropositive patients progress at a more rapid rate than seronegative patients), or a differential treatment response (are seropositive patients more likely to respond to immunosuppressive therapy vs seronegative patients). Such a study would be important to do and we are planning a larger prospective study to address these questions.
Acknowledgments
Funding This project was partially supported by grant UL1 TR000153 from the National Center for Advancing Translational Sciences through the Biostatistics, Epidemiology and Research Design Unit of UC Irvine Institute for Clinical Translational Science.
Footnotes
Competing interests NAG received clinical research support from ALSTDI, Alnylam, Cytokinetics, FDA, GSK, ISIS Pharmaceuticals, and Novartis. AP has received personal compensation for activities with Athena Diagnostics. AP has received royalty payments from Athena Diagnostics. AP holds stock and/or stock options in Johnson & Johnson. AP has received research support from Insmed, Knopp, Isis Pharmaceuticals, Sanofi-Aventis Pharmaceuticals Inc, and Prosensa. Mozaffar has received personal compensation from consulting activities to Baxter, Biogen idec, Biomarin, California Stem Cell Inc, Crescent (a Walgreens company) CSL Behring, Genzyme, Grifols, Idera, NuFactor, and Ultragenyx. Mozaffar is funded from NIH (#NS049203) and received clinical research support from ALSTDI, Alexion, Alnylam, Amicus, Biogen idec, Biomarin, CSL, Cytokinetics, FDA, Grifols, Genzyme, GSK, ISIS, Neuraltus, Novartis, and Ultragenyx.
Ethics approval The Institutional Review Board at University of California, Irvine, approved the study (HS# 2013-9521).
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Cox F, Titulaer M, Sont J, et al. A 12-year follow-up in sporadic inclusion body myositis: an end stage with major disabilities. Brain 2011;134:3167–75. [DOI] [PubMed] [Google Scholar]
- 2.Benveniste O, Guiguet M, Freebody J, et al. Long-term observational study of sporadic inclusion body myositis. Brain 2011;134:3176–84. [DOI] [PubMed] [Google Scholar]
- 3.Engel W, Askanas V. Inclusion-body myositis: clinical, diagnostic, and pathologic aspects. Neurology 2006;66:S20–9. [DOI] [PubMed] [Google Scholar]
- 4.Breithaupt M, Schmidt J. Update on treatment of inclusion body myositis. Curr Rheumatol Rep 2013;15:329. [DOI] [PubMed] [Google Scholar]
- 5.Lotz B, Engel A, Nishino H, et al. Inclusion body myositis. Observations in 40 patients. Brain 1989;112:727–47. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg S. Pathogenesis and therapy of inclusion body myositis. Curr Opin Neurol 2012;25:630–9. [DOI] [PubMed] [Google Scholar]
- 7.Karpati G, O’Ferrall E. Sporadic inclusion body myositis: pathogenic considerations. Ann Neurol 2009;65:7–11. [DOI] [PubMed] [Google Scholar]
- 8.Needham M, Mastaglia F. Inclusion body myositis: current pathogenetic concepts and diagnostic and therapeutic approaches. Lancet Neurol 2007;6:620–31. [DOI] [PubMed] [Google Scholar]
- 9.Dalakas M Muscle biopsy findings in inflammatory myopathies. Rheum Dis Clin North Am 2002;28:779–98. [DOI] [PubMed] [Google Scholar]
- 10.Pruitt J, Showalter C, Engel A. Sporadic inclusion body myositis: counts of different types of abnormal fibers. Ann Neurol 1996;39:139–43. [DOI] [PubMed] [Google Scholar]
- 11.Emslie-Smith A, Arahata K, Engel A. Major histocompatibility complex class I antigen expression, immunolocalization of interferon subtypes, and T cell-mediated cytotoxicity in myopathies. Hum Pathol 1989;20:224–31. [DOI] [PubMed] [Google Scholar]
- 12.Arahata K, Engel A. Monoclonal antibody analysis of mononuclear cells in myopathies. I: quantitation of subsets according to diagnosis and sites of accumulation and demonstration and counts of muscle fibers invaded by T cells. Ann Neurol 1984;16:193–208. [DOI] [PubMed] [Google Scholar]
- 13.Arahata K, Engel A. Monoclonal antibody analysis of mononuclear cells in myopathies. V: identification and quantitation of T8+cytotoxic and T8+suppressor cells. Ann Neurol 1988;23:493–9. [DOI] [PubMed] [Google Scholar]
- 14.Karpati G, Pouliot Y, Carpenter S. Expression of immunoreactive major histocompatibility complex products in human skeletal muscles. Ann Neurol 1988;23:64–72. [DOI] [PubMed] [Google Scholar]
- 15.Wiendl H, Hohlfeld R, Kieseier BC. Immunobiology of muscle: advances in understanding an immunological microenvironment. Trends Immunol 2005;26:373–80. [DOI] [PubMed] [Google Scholar]
- 16.Salajegheh M, Pinkus J, Amato A, et al. Permissive environment for B-cell maturation in myositis muscle in the absence of B-cell follicles. Muscle Nerve 2010;42:576–83. [DOI] [PubMed] [Google Scholar]
- 17.Cupler E, Leon-Monzon M, Miller J, et al. Inclusion body myositis in HIV-1 and HTLV-1 infected patients. Brain 1996;119:1887–93. [DOI] [PubMed] [Google Scholar]
- 18.Salajegheh M, Lam T, Greenberg S. Autoantibodies against a 43 KDa muscle protein in inclusion body myositis. PLoS ONE 2011;6:e20266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larman H, Salajegheh M, Nazareno R, et al. Cytosolic 5’-nucieotidase 1A autoimmunity in sporadic inclusion body myositis. Ann Neurol 2013;73:408–18. [DOI] [PubMed] [Google Scholar]
- 20.Pluk H, van Hoeve B, van Dooren S, et al. Autoantibodies to cytosolic 5’-nucleotidase 1A in inclusion body myositis. Ann Neurol 2013;73:397–407. [DOI] [PubMed] [Google Scholar]
- 21.Greenberg SA. Cytoplasmic 5’-nucleotidase autoantibodies in inclusion body myositis: isotypes and diagnostic utility. Muscle Nerve 2014;50:488–92. [DOI] [PubMed] [Google Scholar]
- 22.Rose MR ENMC IBM Working Group. 188th ENMC International Workshop: inclusion body myositis, 2–4 December 2011, Naarden, The Netherlands. Neuromuscul Disord 2013;23:1044–55. [DOI] [PubMed] [Google Scholar]
- 23.Pestronk A, Li F, Bieser K, et al. Anti-MAG antibodies: major effects of antigen purity and antibody cross-reactivity on ELISA results and clinical correlation. Neurology 1994;44:1131–7. [DOI] [PubMed] [Google Scholar]
- 24.Pestronk A, Li F, Griffin J, et al. Polyneuropathy syndromes associated with serum antibodies to sulfatide and myelin-associated glycoprotein. Neurology 1991;41:357–62. [DOI] [PubMed] [Google Scholar]
- 25.Jackson C, Barohn R, Gronseth G, et al. Muscle Study G. Inclusion body myositis functional rating scale: a reliable and valid measure of disease severity. Muscle Nerve 2008;37:473–6. [DOI] [PubMed] [Google Scholar]
- 26.Beauchet O, Fantino B, Allali G, et al. Timed up and go test and risk of falls in older adults: a systematic review. J Nutr Health Aging 2011;15:933–8. [DOI] [PubMed] [Google Scholar]
- 27.van der Ploeg A, Clemens P, Corzo D, et al. A randomized study of alglucosidase alfa in late-onset Pompe’s disease. N Engl J Med 2010;362:1396–406. [DOI] [PubMed] [Google Scholar]
- 28.Querol L, Rojas-Garcia R, Casasnovas C, et al. Long-term outcome in chronic inflammatory demyelinating polyneuropathy patients treated with intravenous immunoglobulin: a retrospective study. Muscle Nerve 2013;48:870–6. [DOI] [PubMed] [Google Scholar]
- 29.Farrugia M, Harle H, Carmichael C, et al. The Oculobulbar Facial Respiratory score is a tool to assess bulbar function in myasthenia gravis patients. Muscle Nerve 2011;43:329–34. [DOI] [PubMed] [Google Scholar]
- 30.Brusselle G, Michils A, Louis R, et al. “Real-life” effectiveness of omalizumab in patients with severe persistent allergic asthma: the PERSIST study. Respir Med 2009;103:1633–42. [DOI] [PubMed] [Google Scholar]
- 31.Hallert E, Husberg M, Skogh T. 28-Joint count disease activity score at 3months after diagnosis of early rheumatoid arthritis is strongly associated with direct and indirect costs over the following 4 years: the Swedish TIRA project. Rheumatology 2011;50:1259–67. [DOI] [PubMed] [Google Scholar]
- 32.Danon M, Friedman M. Inclusion body myositis associated with progressive dysphagia: treatment with cricopharyngeal myotomy. Can J Neurol Sci 1989;16:436–8. [DOI] [PubMed] [Google Scholar]
- 33.Danon M, Friedman M. Inclusion body myositis with cricopharyngeus muscle involvement and severe dysphagia. Muscle Nerve 1992;15:115. [PubMed] [Google Scholar]
- 34.Verma A, Bradley W, Soule N, et al. Quantitative morphometric study of muscle in inclusion body myositis. J Neurol Sci 1992;112:192–8. [DOI] [PubMed] [Google Scholar]
- 35.Wintzen A, Bots G, de Bakker H, et al. Dysphagia in inclusion body myositis. J Neurol Neurosurg Psychiatry 1988;51:1542–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murata KY, Kouda K, Tajima F, et al. A dysphagia study in patients with sporadic inclusion body myositis (s-IBM). Neurol Sci 2012;33:765–70. [DOI] [PubMed] [Google Scholar]
- 37.Littleton E, Man W, Holton J, et al. Human T cell leukaemia virus type I associated neuromuscular disease causing respiratory failure. J Neurol Neurosurg Psychiatry 2002;72:650–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen R, Lipper S, Dantzker D. Inclusion body myositis as a cause of respiratory failure. Chest 1993;104:975–7. [DOI] [PubMed] [Google Scholar]
- 39.Voermans N, Vaneker M, Hengstman G, et al. Primary respiratory failure in inclusion body myositis. Neurology 2004;63:2191–2. [DOI] [PubMed] [Google Scholar]
- 40.Lyall RA, Donaldson N, Polkey MI, et al. Respiratory muscle strength and ventilatory failure in amyotrophic lateral sclerosis. Brain 2001;124:2000–13. [DOI] [PubMed] [Google Scholar]
- 41.Bradshaw E, Orihuela A, McArdel S, et al. A local antigen-driven humoral response is present in the inflammatory myopathies. J Immunol 2007;178:547–56. [DOI] [PubMed] [Google Scholar]