SUMMARY
Late in their maturation, nascent small (40S) ribosomal subunits bind 60S subunits to produce 80S-like ribosomes. Due to the analogy of this translation-like cycle to actual translation, and because 80S-like ribosomes do not produce any protein, it was suggested that this represents a quality-control mechanism for subunit functionality. Here, we use genetic and biochemical experiments to show that the essential ATPase Fap7 promotes formation of the rotated state, a key intermediate in translocation, thereby releasing the essential assembly factor Dim1 from pre-40S subunits. Bypassing this quality control step produces defects in reading frame maintenance. These results show how progress in the maturation cascade is linked to a test for a key functionality of 40S ribosomes, their ability to translocate the mRNA•tRNA pair. Furthermore, our data demonstrate for the first time that the translation-like cycle is a quality control mechanism that ensures the fidelity of the cellular ribosome pool.
Keywords: 80S-like ribosome, translocation, Fap7, Dim1, quality control, ribosome assembly
(Introduction)
The two ribosomal subunits cooperate to ensure the faithful translation of mRNAs into protein. While the peptide bond is formed on the large subunit (60S in eukaryotes), the small subunit (40S in eukaryotes) safeguards the fidelity of the process. This is done by inspection of the mRNA•tRNA match during decoding, as well as by maintenance of the reading frame during translocation. Translocation, the movement of the mRNA•tRNA pairs from the ribosomal A and P sites to the P and E sites, respectively, resets the ribosome for acceptance of a new tRNA after every round of amino acid addition. Translocation is catalyzed by the binding and subsequent GTP hydrolysis of eEF2 (EF-G in bacteria), and involves a series of concerted conformational changes largely in the small subunit (Belardinelli et al., 2016; Frank et al., 2007; Munro et al., 2010; Ratje et al., 2010; Zhou et al., 2014). First, rotation of the body of the small subunit relative to the large subunit, and swiveling of the head leads to the formation of the so-called hybrid or rotated state, which is thermodynamically stabilized by peptidyl-tRNA and binding of eEF2 (Munro et al., 2009). In this state, the tRNA acceptor arms have moved on the large ribosomal subunit, while their anticodon loops remain at the same place on the small subunit (Frank and Agrawal, 2000; Moazed and Noller, 1989). Back rotation, coupled with back-swiveling of the head, completes translocation by moving the anticodon stem loops of the tRNAs on the 40S subunit. GTP hydrolysis by eEF2 links movement of the body and head and ensures directionality (Ermolenko and Noller, 2011; Holtkamp et al., 2014; Ling and Ermolenko, 2016; Taylor et al., 2007). This process is particularly complex, as it requires plasticity on the ribosome, while maintaining the reading frame. Careful kinetic and thermodynamic measurements have demonstrated a near-equilibrium for the different ribosome conformations (Munro et al., 2009). Interestingly, programmed frameshifting occurs by manipulation of the translocation process (Caliskan et al., 2014; Chen et al., 2014). Both of these observations underscore the importance and delicacy of this process.
Defects in translation fidelity can have deleterious effects on cellular protein homeostasis. Many clinically relevant antibiotics target the ribosome, and induce errors in all stages of the decoding process. Furthermore, while programmed ribosomal frameshift (PRF) is exploited by some viruses (and their host cells) to control the amount of their structural and enzymatic proteins available for viral particle assembly (Brierley, 1995; Caliskan et al., 2015; Dinman, 1995; Dunkle and Dunham, 2015), slippage of ribosomes in either direction (5’ (−1) or 3’ (+1)) will produce a truncated protein, or lead to mRNA degradation via nonsense-mediated decay (Belew and Dinman, 2015).
During the late cytoplasmic steps of their assembly, nascent small ribosomal subunits enter a translation-like cycle where they are joined by mature 60S, in an eIF5B-dependent manner (Lebaron et al., 2012; Strunk et al., 2012). Because assembly factors (AFs) block the binding of mRNA and tRNA, the resulting 80S-like ribosomes do not produce any polypeptide. After maturation is completed, 80S-like complexes are dissociated by the translation termination factor Rli1, thereby further extending the similarities of this translation-like cycle to translation.
80S-like ribosomes accumulate in the absence of the essential ATPase Fap7 (Strunk et al., 2012), which also interacts with, and is activated by the small ribosomal protein Rps14 (Granneman et al., 2005; Hellmich et al., 2013; Loc’h et al., 2014). Furthermore, Fap7 was crosslinked to the AF Dim1 in vivo (Strunk et al., 2012). Dim1 is located on the subunit interface of the 40S (Johnson et al., 2017; Strunk et al., 2011), thereby outlining the Fap7 binding site between Dim1 (near the P-site) and Rps14 (on the platform).
The Dim1 binding site overlaps with that of translation initiation factor 1 (eIF1) (Strunk et al., 2011), which is required for mRNA recruitment and proper start site selection. How Dim1 dissociates from nascent 40S remains unknown. Nonetheless, the observation that Dim1 is present in the 80S-like intermediates that accumulate in the absence of Fap7, but absent in a slightly later intermediate stalled by depletion of Rli1, indicates that it dissociates from 80S-like ribosomes after Fap7 binds (Strunk et al., 2012).
Here, we have used a combination of genetic and biochemical experiments to show that Fap7 induces formation of the rotated state in 80S-like ribosomes, thereby releasing Dim1 from pre-40S subunits. Using luciferase reporter assays we also demonstrate that bypass of the Fap7 step increases the rate of −1 frameshifting, showing that this assembly phase is critical for quality control. Together, these data indicate that the ATPase Fap7 tests the ability of 40S ribosomes to carry out the conformational steps required for translocation, thus ensuring the fidelity of the cellular ribosome pool.
RESULTS:
Stabilization of the Rotated State Rescues Fap7 Depletion
We previously showed that 80S-like ribosomes, which accumulate in the absence of Fap7, contain Tsr1 (Strunk et al., 2012). Thus, we used TAP-tagged Tsr1 in a yeast strain where Fap7 could be depleted by growth in glucose (Gal::Fap7; Tsr1-TAP) to purify 80S-like ribosomes. SDS-PAGE and mass spectrometry analysis of the resulting complexes reveals that in addition to assembly factors they contain the translation elongation factor eEF2 (Fig. S1). During translation eEF2 promotes translocation by favoring the rotated state (Spiegel et al., 2007; Taylor et al., 2007) and by blocking reverse translocation upon reverse rotation. We hypothesized that eEF2 might be part of the translation-like cycle, utilized to quality check the ability of nascent small subunits to carry out the conformational changes required for eEF2-dependent translocation during translation elongation. To test this hypothesis, we used sordarin as a probe for eEF2 function. Sordarin blocks release of eEF2 from the ribosome, thereby stabilizing the rotated state (Figure 1A, (Spahn et al., 2004)). The data in Figure 1B show a positive chemical genetic interaction between sordarin and depletion of Fap7: While deletion of Fap7 increases the doubling time in the absence of sordarin (comparing the two light columns), it decreases the doubling time in the presence of sordarin (dark columns). Furthermore, addition of sordarin also reduces the accumulation of 20S rRNA, the 18S rRNA precursor, observed in the absence of Fap7 (Fig. 1C). To test if sordarin also rescued the accumulation of 80S-like ribosomes, we carried out sucrose gradient analysis of cell lysates produced from cells lacking Fap7 and grown in the presence or absence of sordarin. As expected, addition of sordarin to cells grown in the absence of Fap7 reduces the accumulation of pre-18S rRNA (20S pre-rRNA) in the 80S fraction (from 63 to 50% of 20S in the 80S-like fraction, Fig. 1D-E), demonstrating that addition of sublethal concentrations of sordarin can partially substitute for the absence of Fap7 during maturation of 80S-like ribosomes. Since sordarin stabilizes eEF2 on ribosomes in the rotated state (Spahn et al., 2004), thereby thermodynamically stabilizing the rotated state, these data indicate that one function of Fap7 is to promote the rotated state during 40S maturation.
Figure 1.
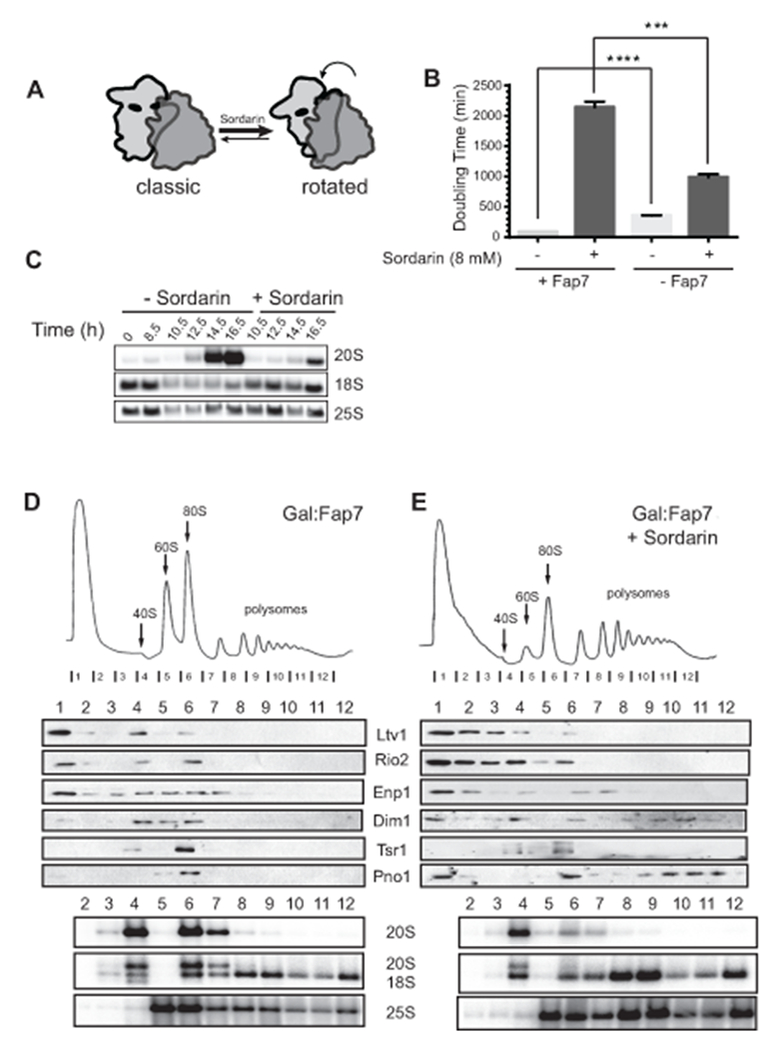
Sordarin rescues the phenotypes from Fap7 depletion. (A) Sordarin stabilizes the rotated state. (B) Doubling times of Gal::Fap7 cells in YPD media in presence (right) or absence (left) of 8 μg/mL sordarin. Data are the average of three biological replicates and error bars indicate SEM. Unpaired t test was used for statistical analysis; **** p <0.0001, *** P=0.0004. (C) Northern blot analyses from total cellular RNA from Gal::Fap7 cells grown in YPD with or without sordarin for up to 16.5 hours. Pre-18S rRNA (20S) and mature 18S and 25S rRNAs are detected. (D) 10-50% sucrose gradients from cell lysates of Gal::Fap7 cells grown in glucose for 16 hours in the absence (D) or presence (E) of sordarin. Shown below the absorbance profile at 254 nm are Western blots of assembly factors and Northern blots of 20S, 18S and 25S rRNAs. 63% and 50% of 20S rRNA are in the 80S fraction without and with sordarin, respectively. See also Supplemental Figure S1.
To further test if Fap7-induced formation of the rotated state plays a role during 40S maturation, we took advantage of a previously described mutant in the large subunit ribosomal protein Rpl3, Rpl3_H256A. The H256A mutation stabilizes the rotated state, leading to stronger binding of eEF2 and weaker binding of P-site tRNA (Fig. 2A, (Meskauskas and Dinman, 2007)). If Fap7 helps to induce the rotated state during 40S ribosome maturation, then the H256A mutation in Rpl3 might rescue the Fap7 dependence of 40S maturation akin to sordarin. To test this prediction, we produced a strain where Rpl3 and Fap7 can be co-depleted by growth in glucose, and supplemented this strain with plasmids encoding wild type or mutant Rpl3. As previously observed (Meskauskas and Dinman, 2007), the Rpl3_H256A mutation produces a slow-growth phenotype when Fap7 is present (Fig. 2B-C). In contrast, in the absence of Fap7, cells containing the Rpl3_H256A mutation grow slightly better than cells containing wild type Rpl3, producing a strongly epistatic effect (Fig. 2B). This was even more obvious when the doubling times of these cells were compared. In the presence of Fap7, Rps3_H256A show a nearly 50% increase in the doubling time (growing more slowly), while in the absence of Fap7, they show a 25% decrease (growing faster, Fig. 2C). Furthermore, in presence of Rpl3_H256A the accumulation of 20S pre-rRNA observed from the absence of Fap7 was strongly reduced (Fig. 2D). Finally, sucrose gradient sedimentation analysis indicated that the accumulation of 80S-like ribosomes observed in the absence of Fap7 in cells containing wild type Rpl3 was also strongly reduced when Rpl3_H256A was expressed (from 41% to 21% of 20S in 80S-like ribosomes, Fig. 2E-F). Together, these data demonstrate that the H256A mutation in Rpl3, which stabilizes the rotated state, rescues all effects from the depletion of Fap7.
Figure 2.
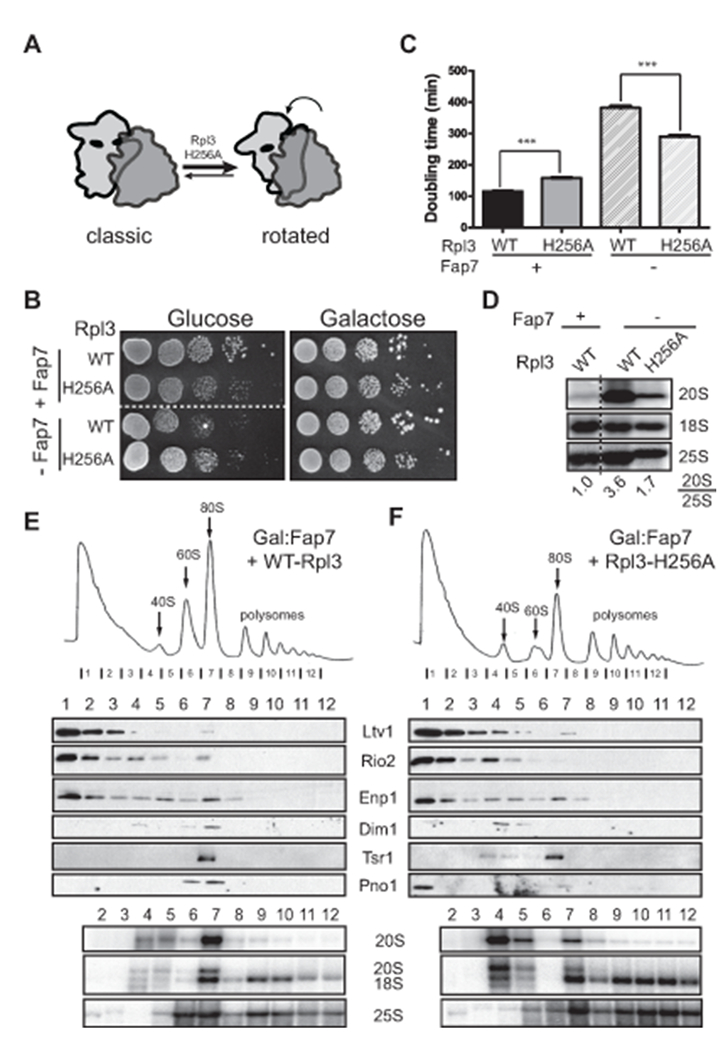
The Rpl3_H256A mutation rescues the phenotypes from Fap7 depletion. (A) The Rpl3_H256A mutation stabilizes the rotated state (Meskauskas and Dinman, 2007). (B) Growth of cells with wild-type Rpl3 or Rpl3-H256A in the presence or absence of Fap7, was compared by 10-fold serial dilutions on YPD or YPgal plates (C) Doubling times of wild-type or Rpl3-H256A cells in the presence of absence of Fap7 in minimal media containing glucose. The data are the average of three biological replicates and error bars indicate SEM. Dunnett’s Multiple Comparison Test was used for statistical analysis; ****= p <0.0001. (D) Northern blots of total RNA from cells with wild-type Rpl3 or the Rpl3_H256A mutant, grown in the presence or absence of Fap7 and probed for 20S, 18S and 25S rRNAs. Numbers indicate the relative amount of 20S pre-rRNA to 25S rRNA. Sucrose gradients of whole cell extracts from Fap7-depleted cells with wild-type Rpl3 (E) or Rpl3_H256A (F). Shown below the absorbance profile at 254 nm are Western blots of assembly factors and Northern blots of 20S, 18S and 25S rRNAs. 41% or 21% of 20S rRNA are in the 80S fraction for WT and H256A, respectively.
Weakening the Rotated State Phenocopies Fap7 Depletion.
In contrast to the Rpl3_H256A mutation, the Rpl3_W255C mutation induces the classical state by promoting the binding of aa-tRNA to the P-site and weakening the interaction of eEF2 and ribosome (Fig. 3A, (Meskauskas and Dinman, 2007)). Thus, this mutation should phenocopy Fap7 depletion, albeit in a milder form reflecting the mild growth defect from this mutation (Fig. 3B). Indeed, cells lacking endogenous Rpl3 and carrying plasmids encoding Rpl3_W255C show 20S rRNA accumulation relative to wild type cells (Fig. 3C). To test if these mutant cells accumulate 80S-like ribosomes, we analyzed the sucrose gradient sedimentation of their total cell lysates. As predicted, 20S pre-rRNA accumulated in the 80S fraction of the gradient (8 % vs 12% of 20S rRNA) and was accompanied by assembly factors found in 80S-like ribosomes (Fig. 3D-E). Thus, the Rpl3_W255C mutation mimics the effects from the depletion of Fap7 and leads to accumulation of 80S-like ribosomes.
Figure 3.
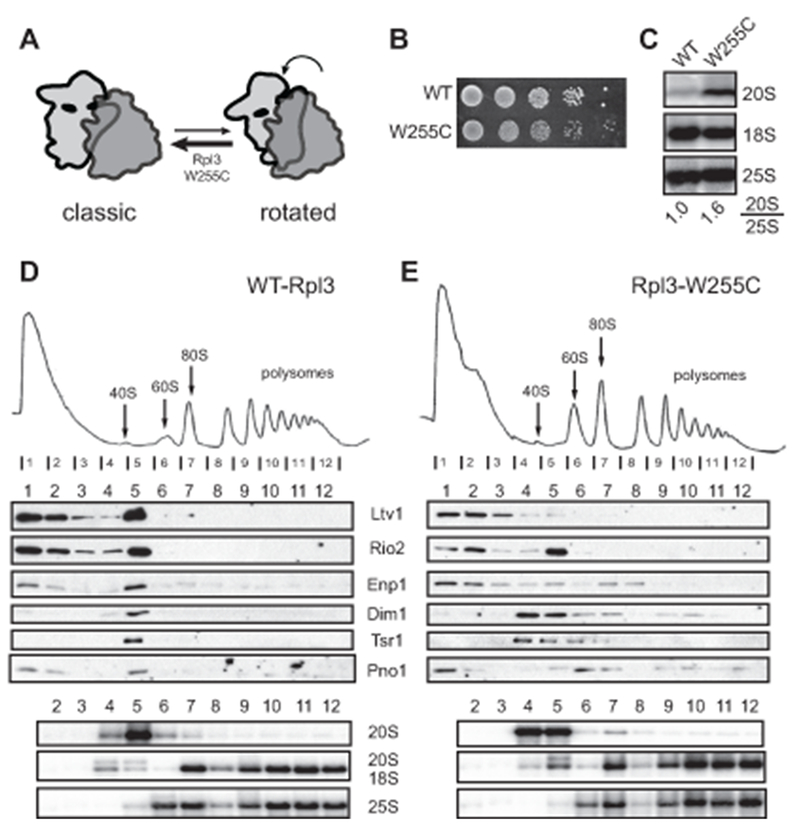
The Rpl3_W255C mutation confers a phenotype similar to that of Fap7 depletion. (A) The W255C mutation stabilizes the classic state (Meskauskas and Dinman, 2007). (B) Growth analysis of cells containing wild type or W255C Rpl3. (C) Northern blot of pre-18S rRNA (20S) and mature rRNAs (25S and 18S) demonstrate a block in 40S maturation in the Rpl3_W255C mutant. Sucrose gradient and Western and Northern analysis of the fractions from whole cell extracts of parent (D) and Rpl3_W255C strains (E). 8% or 12% of 20S rRNA are in the 80S fractions for WT and W255C, respectively.
Fap7 Addition to 80S-Like Ribosomes Stabilizes the Rotated State.
The genetic and biochemical data above indicate that the role of Fap7 is to induce the rotated state during 40S maturation. A corollary of this finding would be that 80S-like ribosomes, in contrast to mature yeast 80S ribosomes, are in the classic state. To test this prediction, and confirm that Fap7 addition induces the rotated state, we used RNA structure probing to test the differential accessibility of residues in the 40S subunit, similar to previous experiments (Sulima et al., 2014). The previously mapped differences between classic and rotated 80S complexes were mainly mapped to the 60S subunits, therefore we screened for additional changes on the 40S subunits that lead to changes in their DMS reactivity. This was done by assembling 80S ribosomes from 40S and 60S subunits as previously described (Acker et al., 2007). Yeast 80S ribosomes are in the rotated state (Agirrezabala et al., 2008; Ben-Shem et al., 2011; Sulima et al., 2014). To produce ribosomes largely in the classical state, we used 60S subunits from Rpl3_W255C yeast (Meskauskas and Dinman, 2007; Sulima et al., 2014). After surveying most of the subunit interface, the most reproducible differences we were able to observe were located at residues A869 and A892, which both become protected in the rotated state (Fig. 4).
Figure 4.
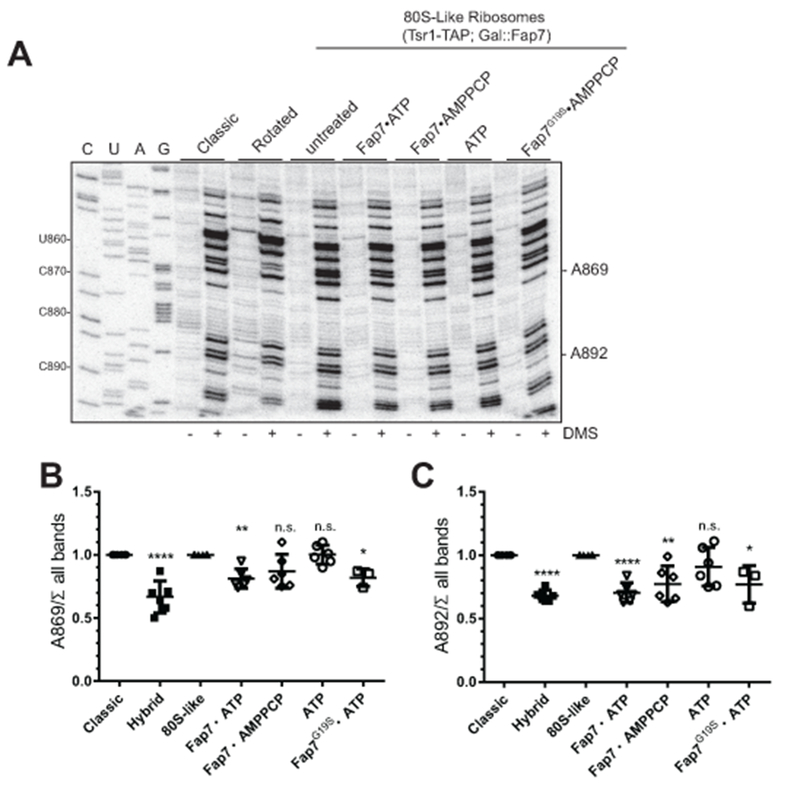
Fap7 induces the rotated state in 80S-like ribosomes. (A) 80S-like ribosomes purified from Gal::Fap7 cells via Tsr1-TAP were mixed with recombinant proteins and nucleotides as indicated and treated with DMS (plus) or solvent (minus). DMS modification is detected by reverse transcription. Classic and rotated mature 80S complexes were used as controls. (B-C) Quantitation of data shown in A. The data is the average of 2 biological replicates (one for G19S) with 3 technical replicates each, and error bars indicate SEM. Significance for each column was analyzed relative to the 80S-like column with Dunnett’s Multiple Comparison Test. n.s.: non-significant. For B: **** P< 0.0001, ** P = 0.0037, * P = 0.028. For C: **** P< 0.0001, ** P = 0.0017 and * P = 0.01.
We next purified 80S-like ribosomes from Gal::Fap7 cells, depleted of Fap7 by growth in glucose, using a TAP-tag on Tsr1. These ribosomes were either left untreated or Fap7 was added in the presence of ATP, the non-hydrolyzable ATP analog AMPPCP, or with ATP alone. After incubation with these reagents, DMS was added before RNA was extracted for reverse transcription and PAGE-analysis. To quantify accessibility and account for loading differences, we normalized the reverse transcription stops at A869 and A892 to all bands on the gel, and then normalized the data to the reactivity of the classic state.
The data in Figure 4 show that in 80S-like ribosomes alone A869 and A892 are at least as accessible to DMS as classic ribosomes, suggesting that 80S-like ribosomes are in the classic state. Addition of Fap7•ATP or the ATPase-compromised Fap7_G19S•ATP, but not ATP alone, leads to protection of A869 and A892. Fap7•AMPPCP provides intermediate level of protection. Similar compromised activity from the non-hydrolyzable ATP analog has been observed in other systems (Ortiz et al., 2013; Yount et al., 1971). Together, these data strongly suggest that 80S-like ribosomes are in the classic conformation, and that addition of Fap7•ATP promotes formation of the rotated state. Furthermore, the data also indicate that ATP hydrolysis is not needed for induction of the rotated state, as Fap7_G19S•ATP is as effective as Fap7•ATP.
Fap7 Binds Dim1 and Promotes its Release during 40S Maturation.
Fap7 interacts with the small subunit ribosomal protein Rps14 (Granneman et al., 2005). This interaction stimulates the ATPase activity of Fap7 (Hellmich et al., 2013), which is essential for its function in the translation-like cycle (Strunk et al., 2012). We have previously shown that the AF Dim1, a universally conserved methyltransferase, crosslinks to Fap7 in vivo. No other AFs were found in this analysis (Strunk et al., 2012). This was particularly interesting, as Dim1 is bound near Rps14 on the subunit interface (Johnson et al., 2017; Strunk et al., 2011), raising the possibility that this interaction is functional.
Protein binding experiments using recombinant purified components confirmed a direct interaction between Dim1 and Fap7 (Fig. 5A). Furthermore, addition of Rps14 resulted in formation of a ternary complex of Fap7•Rps14 and Dim1 (Fig. S2A). Surprisingly, when Dim1 was added to Rps14•Fap7, which were co-expressed and co-purified, no ternary complex was observed (Figure S2B), indicating that the Rps14•Fap7 co-purified complex is in a highly stable, alternative conformation. The biological relevance of this conformation remains unknown.
Figure 5.
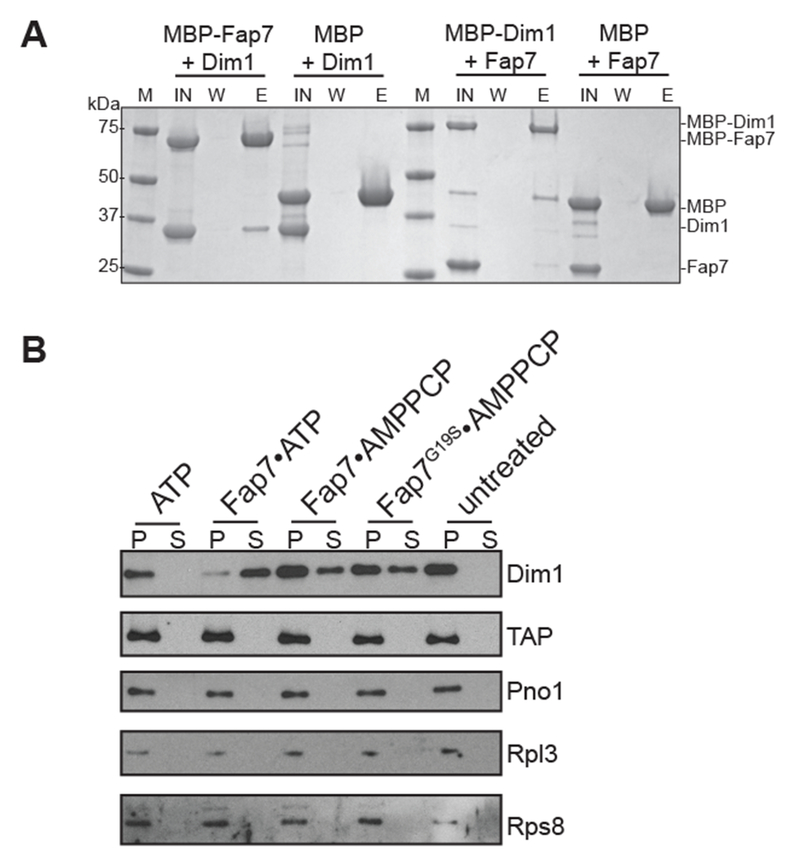
Fap7 is required for release of Dim1 from 80S-like ribosomes. (A) Fap7 binds Dim1. Coomassie-stained SDS–PAGE of protein binding assays on amylose beads. IN, input; W, final wash; E, elution. Note that the MBP-tag on Dim1 appears to weaken the Fap7•Dim1 interaction. (B) Co-sedimentation assay to study release of Dim1. Western blots of Dim1, Tsr1-TAP, Pno1, Rpl3 and Rps8 in the bound (pellet [P]) and released (supernatant [S]) fractions of purified 80S-like ribosomes after no or the indicated treatments. See also Supplemental Figure S2.
Dim1 blocks binding of eIF1, and is released during the translation-like cycle, after Fap7 functions (Strunk et al., 2011; Strunk et al., 2012). Given the direct interaction between Dim1 and Fap7, and given that Dim1 is released around the time Fap7 normally functions (Strunk, 2012), we wondered if the Fap7-induced rotated state is used to release Dim1 from nascent 40S ribosomes. To test this hypothesis we purified 80S-like ribosomes as described above, incubated these ribosomes with Fap7•ATP, Fap7•AMPPCP, Fap7_G19S•ATP, or ATP alone, and then used a pelleting assay (Ghalei et al., 2015) to measure release of Dim1. Addition of Fap7•ATP, Fap7•AMPPCP, or Fap7_G19S•ATP, but not ATP alone leads to release of Dim1 from the ribosome-bound pellet into the supernatant, while not affecting the binding of the assembly factors Tsr1-TAP and Pno1 (Figure 5B), or the integrity of the 80S-like ribosomes (Rpl3 and Rps8). These data show that Fap7•ATP binding leads to Dim1 release from 80S-like ribosomes.
To further test this model in vivo, we hypothesized that Dim1 mutants that bind weakly to the ribosome could at least partially rescue the slow growth phenotype from Fap7-deficient mutants, as these might release independently of Fap7. To test this hypothesis, we constructed a yeast strain in which both Fap7 and Dim1 were galactose-inducible and could be simultaneously depleted by growth in glucose. These cells were supplemented with plasmids encoding wild type or mutant Fap7 and Dim1. Screening of Dim1 mutants that were designed to bind weekly to the ribosome, identified a triple mutant (Dim1_EKR), where three conserved residues contacting the tip of the helix 44 in 20S pre-rRNA (Figure S3A) were mutated to opposite charged amino acids (E93R, K96D and R97E). The Dim1_EKR mutant bound more weakly to the ribosome in vivo as judged by the amount of free protein in the sucrose gradient analysis of lysates produced from cells expressing wild type or mutant Dim1 (Fig. S3B-C). Importantly, the weakly-binding Dim1_EKR mutation slows growth in the context of wild type Fap7, but increases the growth rate in the case of the ATPase inactive Fap7 mutants G19S and K20R (Fig. 6A). Furthermore, analysis of rRNA processing shows that weakening Dim1 binding to pre-40S ribosomes via the Dim1_EKR mutation reduces the accumulation of 20S pre-rRNA observed upon inactivation of Fap7 (Fig. 6B). To test if Dim1_EKR also rescued the accumulation of Dim1 in 80S-like ribosomes, we compared sucrose gradients of lysates from cells with Fap7_G19S and wild type Dim1 or Dim1_EKR. As expected, cells expressing Fap7_G19S and Dim1_EKR showed less Dim1 in the 80S fraction compared to cells expressing Fap7_G19S and wild type Dim1 (Fig. 6C-D). Instead Dim1 was found in the free fraction in these cells. A similar effect was observed when Fap7_K20R was used (Fig. S4A-B). Collectively, these data demonstrate that Fap7 interacts with Dim1 and strongly suggest that induction of the rotated state by Fap7 is used to release Dim1 from pre-40S ribosomes.
Figure 6.
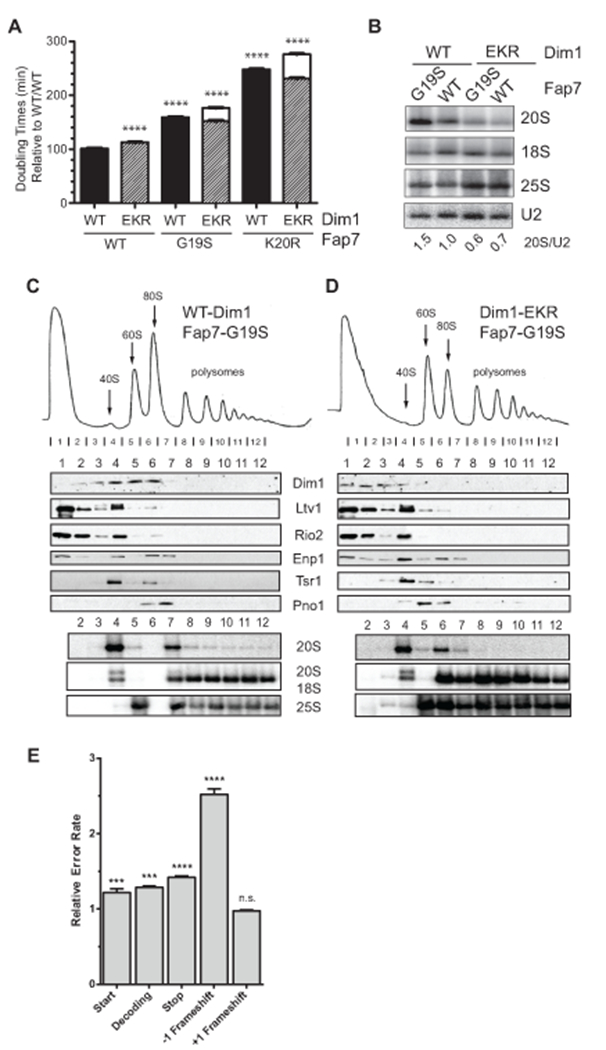
Fap7-independent release of Dim1 leads to translational errors. (A) Comparison of doubling times of wild-type (WT, black column) Dim1 or Dim1_EKR (EKR, hatched column) in presence of WT or mutant Fap7 (G19S and K20R) in minimal media containing glucose. The data is the average of three biological replicates and error bars show SEM. Each column was analyzed relative to the WT/WT column with Dunnett’s Multiple Comparison Test; ****= p <0.0001. The white columns show the expected doubling times if there were no rescue of the G19S/K20R mutations by Dim1_EKR. The height of these columns is calculated by multiplying the observed fold differences for each single mutation (G19S or K20R with EKR). (B) Northern blots of total RNA from cells with wild-type (WT) or mutant Dim1 (EKR) proteins, grown in presence of wild-type Fap7 (WT) or mutant Fap7 (G19S) and probed for 20S, 18S and 25S rRNAs. Numbers indicate the relative amount of 20S pre-rRNA to 25S rRNA. Scrose gradients of whole cell extracts from Fap7-K20R cells with either wild-type Dim1 (C) or Dim1_EKR (D). Shown below the absorbance profile at 254 nm are Western blots of assembly factors and Northern blots of 20S, 18S and 25S rRNAs. (E) The effects from the Dim1_EKR mutation on the fidelity of start codon recognition, decoding, stop codon recognition, and frame shifting (−1 and +1) was assayed using dual-luciferase reporters (Figure S5) in a Gal::Dim1 strain supplemented with plasmids encoding wild type or mutant Dim1. Shown are the relative error rates of the Dim1_EKR samples relative to Dim1_wild type samples. The data is the average of 3–6 biological replicates and error bars indicate the SEM. *** p <0.001 and **** p<0.0001 by unpaired t-test. See also Supplemental Figure S3–S6.
Interestingly, while ATP hydrolysis does not appear to be required for subunit rotation or Dim1 release in vivo, it is clearly required for ribosome assembly in vivo, suggesting that ATP hydrolysis is required for release of Fap7 after Dim1 release.
Fap7-Independent Release of Dim1 Leads to Translational Errors
80S-like ribosomes resemble mature translating ribosomes, but are not able to translate as they lack mRNA or tRNA. Hence they are ideal to test the quality of the nascent 40S subunit. We hypothesized that if one function of the Fap7-regulated release of Dim1 is to test the ability of ribosomes to translocate, bypassing this step should result in production of ribosomes that might be defective in maintaining the mRNA reading frame during translocation. We therefore assayed the effect from circumventing the Fap7-regulated step on the quality of cellular ribosomes using dual luciferase reporter assays. In these experiments we used a series of previously published dual luciferase reporter plasmids (Fig. S5, (Cheung et al., 2007; Harger and Dinman, 2003; Keeling et al., 2004; Salas-Marco and Bedwell, 2005)) in yeast strains expressing either wild type Dim1 or Dim1_EKR. In these plasmids, firefly luciferase activity is dependent on a translational error, while renilla luciferase is constitutive, and used for normalization to account for sample-to-sample differences arising from differences in lysis, plasmid retention, etc.
Cells were tested for the fidelity of their ribosomes in start-site selection, stop codon read-through, frameshifting and codon selection. While Dim1_EKR cells did not show an effect on +1 frameshifting, they showed small (<1.5 fold), but significant differences in start and stop codon selection, as well as miscoding. Moreover, they showed a nearly three-fold increase in their propensity to carry out −1 frameshifting (Figure 6E). Control experiments demonstrate that ribosomes from the Dim1_EKR strain are nearly fully methylated at A1779/1780 (Fig. S6A). Furthermore, entirely abolishing methylation using the Dim1_E85A mutation (Pulicherla et al., 2009), has small effects on stop-codon selection and miscoding, but not on −1 frameshifting (Fig. S6B). Thus, while it appears likely that the small effects on stop-codon selection and misreading observed in the Dim1_EKR strain might arise from incomplete methylation of A1779/1780, the observed defect on frameshifting is independent of the Dim1 function in rRNA methylation. This suggests strongly that the frameshifting errors observed in the Dim1_EKR strain arise from assembly defects that precede the Dim1 step, which bypass the Fap7-dependent proofreading step, and are thus not filtered out. These data show that Fap7-mediated changes in 40S structure, and the associated release of Dim1 from 80S-like ribosomes is a functional test for translocation and illustrate the importance of quality control during ribosome assembly.
DISCUSSION:
Fap7 Induces the Rotated State in 80S-like Ribosomes and Releases Dim1.
During translation 40S subunits carry out two key functions: decoding of the mRNA and translocation of the mRNA•tRNA pair after each round of peptide bond formation to reset the ribosome for the next round. Both are critical to the fidelity of translation, as mistakes would either lead to point mutations in the polypeptide or frameshifts. The importance of safeguarding the fidelity of these steps is illustrated by the cytotoxic effects from antibiotics, many of which function by increasing the error rates of these processes.
Translocation of tRNA•mRNA through the ribosome requires concerted conformational changes within the 40S subunit, and relative to the 60S subunit (Ling and Ermolenko, 2016; Munro et al., 2009; Rodnina and Wintermeyer, 2011; Voorhees and Ramakrishnan, 2013). A large body of work indicates that peptide-bond formation, as well as binding of the translocation GTPase eEF2 (EF-G in bacteria), promote the formation of the so-called hybrid or rotated state, where the small subunit has undergone a counter-clockwise rotation with respect to the large subunit and the head has swiveled, thereby translocating the tRNA on the large subunit (Agirrezabala et al., 2008; Ermolenko et al., 2007; Frank and Agrawal, 2000; Julian et al., 2008; Moazed and Noller, 1989; Rodnina et al., 1997; Spiegel et al., 2007). Subsequent back-rotation as well as back-swiveling complete translocation by movement of the tRNA on the small subunit. These conformational changes are discrete and carefully energetically balanced to ensure maintenance of the reading frame. Conversely, small structural and energetic perturbations dramatically increase the probability to frameshift, further demonstrating the importance of getting the energetics of all interactions right.
Here we show that the ability to carry out the conformational changes that lead to translocation is probed during maturation of the small ribosomal subunit. Our data show that the absence of the late cytoplasmic 40S assembly factor Fap7 can be partially suppressed by stabilizing the binding of eEF2 or by ribosome mutations that stabilize the rotated state directly. Furthermore, addition of Fap7•ATP to purified 80S-like ribosomes induces the rotated state, suggesting strongly that one role of Fap7 is to stabilize the rotated state during 40S maturation (Fig. 7A). The release of Dim1 from 80S-like ribosomes by addition of Fap7•ATP, together with the partial suppression of Fap7 inactivation by mutations that weaken binding of Dim1, strongly suggest that formation of the rotated state is used to release Dim1 from 80S-like ribosomes. Furthermore, our data show that ATP-hydrolysis is not required for Dim1 release, as AMPPCP is functional, as is the ATPase-deficient G19S mutant. We therefore suggest that ATP hydrolysis is required to release Fap7 from 80S-like ribosomes, consistent with release of Rps14 from Fap7 in an ATP hydrolysis-dependent manner (Loc’h et al., 2014).
Figure 7.
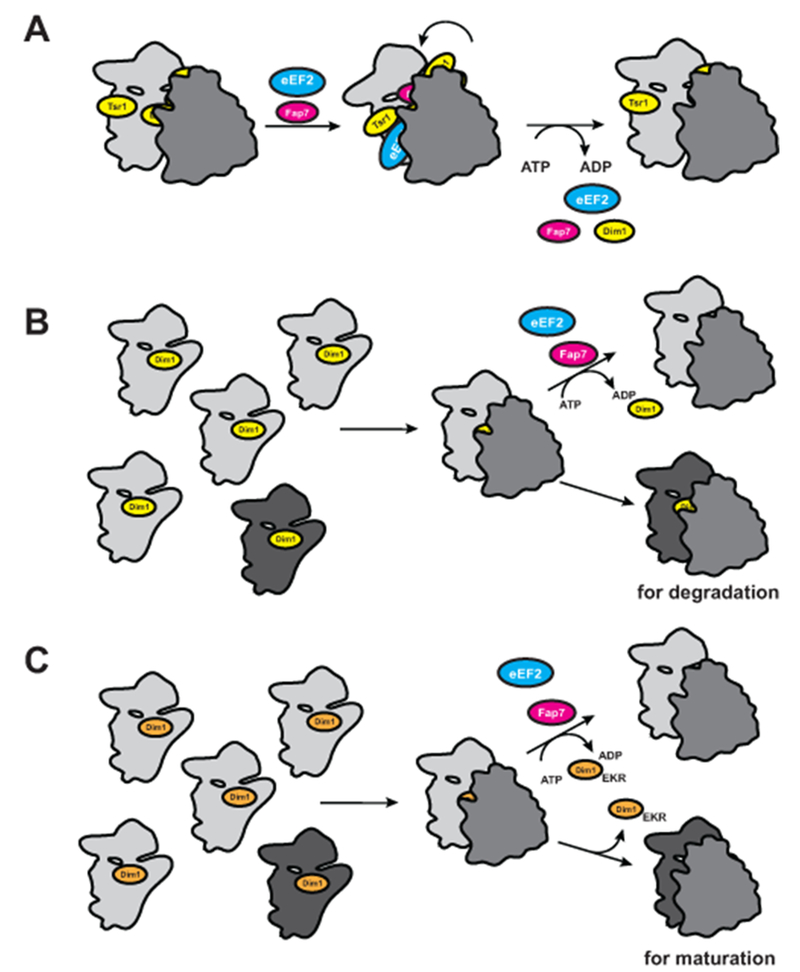
Model for Fap7-dependent probing of the ability to adopt the hybrid state during 40S maturation. (A) Model for the role of Fap7 and eEF2 in 40S ribosome assembly. (B) Interplay between Fap7-mediated Dim1 release and quality control. The assembly cascade produces pre-40S ribosomes that are correctly assembled (light grey), and those defective for subunit rotation (dark grey). The defective 40S retain Dim1, as they are deficient in the Fap7-dependent release of Dim1, and will be degraded. (C) Dim1_EKR allows for bypass of the Fap7-mediated step, as Dim1 can be released Fap7-independently, thereby allowing the defective ribosomes to evade degradation and continue maturation. See also Figure S7.
It has recently been suggested that Fap7 also plays a role in the nuclear incorporation of Rps14 (Pena et al., 2016), consistent with previously reported nuclear and cytoplasmic localization of Fap7 (Huh et al., 2003). This role could be in addition to the role in promoting the rotated state described herein, and is therefore fully consistent with this report, although alternative explanations for the genetic interaction between Fap7 and Tsr2 have been described (Ferretti et al., 2017).
Intriguingly, Dim1 is located at the subunit interface partially overlapping P-site tRNA (Fig. S7), suggesting that the 40S motions that relocate P-site tRNA to the E-site during translation could release Dim1 during maturation. The 3’-end of 18S rRNA is positioned differently near the endonuclease Nob1 in the presence and absence of Dim1 (Johnson et al., 2017), suggesting that dissociation of Dim1 regulates Nob1 by repositioning pre-18S rRNA. Thus, the Fap7•ATP-dependent release of Dim1 gates 18S rRNA formation, consistent with the previously observed ATP-dependence of 18S rRNA formation, which also requires the presence of Fap7 (Loc’h et al., 2014).
While translocation does not strictly require eEF2/EF-G, binding of eEF2/EF-G stabilizes the rotated state (Spiegel et al., 2007), while GTP hydrolysis leads to mRNA/tRNA movement on the small subunit (Wilden et al., 2006). We show that eEF2 is bound to 80S-like ribosomes and that sordarin, which acts via eEF2, partially rescues the effect from depletion of Fap7. These data show that eEF2 can promote translocation in 80S-like ribosomes in the absence of Fap7 and suggest that eEF2 is also involved in 40S maturation in the presence of Fap7. Nevertheless, we were unable to demonstrate this conclusively, as depletion of eEF2 leads to rapid accumulation of early 40S assembly intermediates (data not shown). Presumably, this is because the depletion of eEF2 inhibits translation, rapidly depleting the stores of free ribosomal proteins, which blocks nucleolar 40S maturation.
Such a non-canonical role for eEF2 would not be unique as eEF2 translocates initiator tRNA from the A-site to the P-site during translation initiation from the cricket paralysis virus IRES (Fernandez et al., 2014; Koh et al., 2014; Murray et al., 2016).
Why Is Fap7 Required for the Translocation-like Event During 40S Maturation?
The data herein suggest strongly that one role of Fap7 during 40S maturation is the stabilization of a rotated-state-like structure. We presume that Fap7 is necessary for stabilization of the rotated state during 40S maturation but not during translation, because other elements that typically stabilize the rotated state are absent during maturation. E.g., deacylated tRNA in the P-site, an A-site tRNA, as well as interactions between the tRNA elbow in the E-site and the L1 stalk, all stabilize the formation of the rotated state (Munro et al., 2009). Yet, our previous data strongly indicates that 80S-like ribosomes do not contain an initiator tRNA (Strunk et al., 2012), which would be in the P-site. We thus suspect that Fap7 makes interactions with the 80S-like ribosome that stabilize the rotated state. Given its likely location near the E-site (as deduced from its simultaneous binding to Dim1 and Rps14), it is tempting to speculate that Fap7 might interact with the L1-stalk akin to E-site tRNA.
The Translation-like Cycle is a Quality Control Cycle.
We have previously shown that nascent 40S subunits enter a translation-like cycle, whereby the translation factors eIF5B and Rli1 promote the joining and subsequent dissociation of 60S subunits, respectively (Strunk et al., 2012). Because of the analogy of this process to the actual translational cycle, and because this cycle did not produce protein, but was able to serve as a dry-run to test the ability of nascent subunits to bind 60S subunits and translation factors, we speculated that it was a quality control pathway. This hypothesis was furthermore consistent with our observation that assembly intermediates blocked from maturation were actively degraded, a hallmark of quality control mechanisms. Here we have both extended the analogy to the translational cycle, and provide the first evidence that this cycle is functional in quality control, as previously hypothesized.
While we observe a nearly three-fold increase in −1 frameshifting from bypass of Fap7, the defects on other steps in translation are small and likely not to arise from Fap7 bypass but defects in Dim1. Previous work has shown that −1 frameshifting occurs during translocation, and is the result of uncoupling of translocation and back-rotation of the small subunit, resulting in an unusual rotated state (Caliskan et al., 2014; Chen et al., 2014). Thus, our data strongly suggest that the Fap7-regulated release of Dim1 is a functional test for correct adoption of the rotated state by nascent 40S subunits and further emphasize the importance of quality control during ribosome assembly.
Importantly, the magnitude of the translational defects is large relative to others described in the literature, including that of mutations in Rpl3, which affect the equilibrium between the rotated and non-rotated states (Al-Hadid et al., 2016; Meskauskas et al., 2003; Peltz et al., 1999), or point mutations in rRNA (Baudin-Baillieu et al., 2009; Piekna-Przybylska et al., 2008). This is even more impressive, given that in contrast to point mutations, bypass of quality control is unlikely to lead to homogeneous populations of defective ribosomes. This is because even though all ribosomes might bypass quality control, many, perhaps most, are expected not to need it because they are correctly assembled (Fig 7B-C).
How does the Dim1_EKR mutation lead to increased frameshifting? As illustrated in Figure 7B the assembly cascade likely produces mostly ribosomes that are proficient for frameshifting, while only a small percentage are deficient. These defective ribosomes cannot proceed in the assembly cascade, leading to the persistence of Dim1, and ultimately their degradation (Strunk et al., 2012). If, however, these defective ribosomes have Dim1_EKR bound, they can release Dim1, even if they are deficient in the formation of the rotated state (Fig. 5&S6), thereby allowing for their continued maturation (Fig. 7C). Thus, the translational defects observed in the Dim1_EKR strain do not arise from defects in Dim1, but from unknown prior assembly mistakes, which persist because the resulting ribosomes can bypass the Fap7-dependent release of Dim1. Future experiments will be required to determine if the population of defective ribosomes is uniform (i.e. they all have the same or similar defects), or not. Insight into the nature of such defects would likely provide novel insights into the mechanism of translocation.
STAR METHODS:
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Katrin Karbstein (kkarbst@scripps.edu).
METHOD DETAILS
Yeast strains and cloning.
All yeast strains (Table S1) were made using PCR-based recombination (Longtine et al., 1998), and confirmed by PCR and Western blotting when antibodies were available. Plasmids used in this study are listed in Table S2. All mutations were introduced by site-directed mutagenesis and confirmed by sequencing.
Protein expression and purification.
Dim1 was purified as previously described (Campbell and Karbstein, 2011). Expression of Fap7 was induced in Rosetta DE3 cells (EMD Millipore) by addition of 1 mM IPTG after transferring cells to 30°C for 4h. Cells were lysed by sonication in lysis buffer (50 mM Tris, pH 8.0, 100 mM KCl, 2 mM EDTA) supplemented with 0.5 mM PMSF and 5 mM β-mercaptoethanol (β-ME). The cleared lysate was loaded onto a HiTrap Q FF column (GE Healthcare), washed, and proteins were eluted with a linear gradient of 200–700 mM KCl over 20 column volumes. Fractions containing Fap7 were pooled and precipitated by addition of ammonium sulfate. The pellet was resuspended in lysis buffer and dialyzed overnight at 4ºC in 50 mM Tris pH 8.0, 250 mM KCl and 2 mM EDTA. The protein was further purified on a MonoQ column and a Superdex75 size-exclusion column equilibrated with 25 mM HEPES, pH 7.5, 200 mM KCl, 1mM DTT and 1mM TCEP.
Fap7 and SUMO-Rps14 were co-expressed in Rosetta DE3 cells, induced at 16°C by addition of 1 mM IPTG and harvested after 16 h. For purification, cells were sonicated in 50 mM HEPES, pH 7.5, 150 mM NaCl, 10 mM imidazole, 5 mM β-mercaptoethanol, 5% (vol/vol) glycerol, 0.5 mM PMSF and 10 μg/mL lysozyme. The complex was captured on Ni2+-NTA resin (QIAGEN), washed with 50 bed volumes of buffer and eluted with lysis buffer supplemented with 200 mM imidazole. The eluted complex was dialyzed against 50 mM HEPES, pH 7.5, 150 mM NaCl, and 2 mM DTT and was further purified using a Mono Q column equilibrated in dialysis buffer. The complex was eluted with a linear gradient from 0.2 to 1 M NaCl and further purified by gel filtration on a Superdex 75 column equilibrated in 20 mM HEPES, pH 7.5, 150 mM NaCl, 2 mM DTT and 5% glycerol.
MBP-Fap7 was overexpressed as described above for untagged Fap7. Cells were lysed by sonication in Ni-lysis buffer and purified on Ni-NTA resin according to the manufacturer’s instructions. The protein was dialyzed overnight at 4°C against 50 mM Tris, pH 7.5, 200 mM NaCl, and 2 mM DTT and further purified on a Mono Q column. Proteins were eluted with a linear gradient of 200–500 mM NaCl over 30 column volumes, pooled and concentrated for size-exclusion chromatography (Superdex 200; GE Healthcare) equilibrated with 25 mM Tris, pH 7.5, 150 mM NaCl, 5 mM glycerol, 1 mM DTT and 1 mM TCEP.
Sucrose density gradient analysis.
Sucrose gradient fractionation of whole cell lysates and subsequent Northern blot and Western blot analyses were carried out as described before (Strunk et al., 2012). To calculate the amount of 20S in the 80S-like fraction, we quantified the signal in the 40S fraction (fractions 3 and 4) and the 80S fraction (fractions 6 and 7), and then divided the 80S signal by the sum of the 40S and 80S signal.
Preparation of 80S-like ribosomes.
Tsr1TAP;Gal::Fap7 cells were grown in YPgal overnight and then depleted for 16 h in YPD in a 10 L fermenter. Cells were harvested at an OD600 of 1 and cryoground in IgG binding buffer (30 mM Hepes, pH 7.4, 100 mM NaCl, 10 mM MgCl2, and 0.075% NP-40). Clarified lysate was applied to IgG resin (GE Healthcare) equilibrated in IgG binding buffer and washed with 130 column volumes of binding buffer. 80S-like ribosomes were collected after TEV cleavage in cleavage buffer (IgG buffer, 0.5 mM EDTA, 2 mM DTT).
DMS footprinting of pre-ribosomes.
2 µM Fap7 and 1 mM ATP or AMPPCP were added to purified 80S-like ribosomes in a buffer containing 80 mM Hepes, pH 7.4, 50 mM NaCl, 5 mM Mg(OAc)2, and 0.075% NP-40. The reactions were incubated for 5 min at 30°C. Each reaction was divided into two tubes, mixed with either DMS or an equal volume of ethanol, and incubated for 10 min at 30̊ C before ethanol precipitation. RNAs were purified with the High Pure RNA isolation kit (Roche) before reverse transcription was performed using Superscript III (ThermoFisher) according to the manufacturer’s protocol. To quantify the footprinting intensity, we performed a line scan, subtracted background using the Rolling Disk calculation method, and then divided the intensity in the bands for A892 and A869 (separately) by the intensity in the entire lane. This accounts for loading differences.
Dim1 release assay.
~2 pmol of purified 80S-like ribosomes from Tsr1TAP; Gal:Fap7 cells were incubated with 100 pmol of recombinant Fap7 or Fap7-G19S in 50 µl RB buffer (50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 10 mM MgCl2, 0.075% NP-40, and 2mM DTT). ATP or AMPPCP were added to a final concentration of 1 mM. The mixture was incubated for 5 min at 30°C, placed on 400 µl of a 20% sucrose cushion, and centrifuged for 4 h at 400,000 g in a TLA 100.1 rotor. The supernatant was TCA precipitated and the pellet was resuspended in SDS-loading dye. Supernatants and pellets were analyzed by SDS-PAGE followed by Western blotting.
Protein binding assays.
3 µM MBP, MBP-Dim1 or MBP-Fap7 were mixed with 5 µM Fap7 or Dim1 in binding buffer (50 mM Tris, pH 7.5, 150 mM NaCl, and 2 mM DTT) and incubated for 15 min at 4°C. The mixture was applied to 25 µl of amylose resin (New England Biolabs) pre-equilibrated in binding buffer and incubated on a rotating platform at 4°C for 30 min. Resin was washed four times with 200 µl binding buffer and bound proteins were eluted with 25 µl of binding buffer supplemented with 20 mM maltose.
Yeast growth measurements.
Cells grown to mid-log phase in minimal media were diluted into fresh media and growth rates were measured in the Bioscreen C Automated Microbiology Growth Curve Analysis System (Growth Curves USA) by measuring OD600 every 20 min.
Assaying the translational effects of Dim1_EKR using the dual-luciferase reporter system.
1 mL of cells in mid-log phase were pelleted, washed, and flash frozen for storage at −80°C. Control experiments demonstrated that luciferase activity is not affected by storage (data not shown). Luciferase activities were measured using the Promega Dual-Luciferase kit by resuspending cells in 200 µL of Passive Lysis Buffer and incubating for 5 min. 5 µL lysate were mixed with 15 µL Luciferase Assay Reagent II, followed by addition of 15 µL Stop & Glo® Reagent to measure firefly and Renilla activities, respectively, in black Fluotrac™ 600 96W Microplates (Greiner Bio-One), using a 2104 EnVision Multilabel plate reader (Perkin Elmer). For each sample, firefly luciferase activity was normalized against Renilla activity, and then values observed for Dim1_EKR were normalized against those for wild type Dim1.
Antibodies.
Antibodies against recombinant Dim1, Tsr1, Pno1 and Rio2 were raised in rabbits by Josman LLP, and tested against purified recombinant proteins and yeast lysates. The anti-TAP antibody (CAB1001) was from ThermoFisher Scientific. The eEF2 antibody (a gift from T. Goss Kinzy, Rutgers University) and Rps8 antibody (gift from G. Dieci, Università degli Studi di Parma (Dieci et al., 2005)) were also raised in rabbits. The Rpl3 antibody, developed by Warner J.R., was obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242. The HRP-conjugated anti–rabbit secondary antibody was obtained from Rockland Immunochemicals.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical analyses were performed using GraphPad Prism version 6.0 (GraphPad Software, La Jolla, California). Two different statistical tests were used as appropriate, and as indicated in the respective figure legends. One-way ANOVA followed by Dunnett’s multiple comparisons test was used where there were three or more independent groups of samples. Unpaired, two-tailed t-test was used on datasets with less than three groups of samples.
Supplementary Material
ACKNOWLEDGEMENTS:
This work was supported by NIH grant R01-GM086451 and an HHMI Faculty Scholar grant (to K.K.). H.G. was supported in part by a PGA National postdoctoral fellowship. We thank T. Goss Kinzy for the gift of the anti-eEF2 antibody, J. Dinman for the gift of the galactose-inducible Rpl3 strain, and the plasmids encoding Rpl3_H256A and Rpl3-W255C. The dual luciferase assay plasmids were kindly provided by J. Dinman (frameshifting), D. Bedwell (stop codon readthrough and miscoding) and J. Lorsch (start site recognition). We thank J. Blozinski for technical assistance and members of the Karbstein lab for comments on the manuscript.
REFERENCES:
- Acker MG, Kolitz SE, Mitchell SF, Nanda JS, and Lorsch JR (2007). Reconstitution of yeast translation initiation. Methods Enzymol 430, 111–145. [DOI] [PubMed] [Google Scholar]
- Agirrezabala X, Lei J, Brunelle JL, Ortiz-Meoz RF, Green R, and Frank J (2008). Visualization of the hybrid state of tRNA binding promoted by spontaneous ratcheting of the ribosome. Mol Cell 32, 190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hadid Q, Roy K, Chanfreau G, and Clarke SG (2016). Methylation of yeast ribosomal protein Rpl3 promotes translational elongation fidelity. RNA 22, 489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudin-Baillieu A, Fabret C, Liang XH, Piekna-Przybylska D, Fournier MJ, and Rousset JP (2009). Nucleotide modifications in three functionally important regions of the Saccharomyces cerevisiae ribosome affect translation accuracy. Nucleic Acids Res 37, 7665–7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belardinelli R, Sharma H, Caliskan N, Cunha CE, Peske F, Wintermeyer W, and Rodnina MV (2016). Choreography of molecular movements during ribosome progression along mRNA. Nat Struct Mol Biol 23, 342–348. [DOI] [PubMed] [Google Scholar]
- Belew AT, and Dinman JD (2015). Cell cycle control (and more) by programmed −1 ribosomal frameshifting: implications for disease and therapeutics. Cell Cycle 14, 172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shem A, Garreau de Loubresse N, Melnikov S, Jenner L, Yusupova G, and Yusupov M (2011). The structure of the eukaryotic ribosome at 3.0 A resolution. Science 334, 1524–1529. [DOI] [PubMed] [Google Scholar]
- Brierley I (1995). Ribosomal frameshifting viral RNAs. The Journal of general virology 76 ( Pt 8), 1885–1892. [DOI] [PubMed] [Google Scholar]
- Caliskan N, Katunin VI, Belardinelli R, Peske F, and Rodnina MV (2014). Programmed −1 frameshifting by kinetic partitioning during impeded translocation. Cell 157, 1619–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliskan N, Peske F, and Rodnina MV (2015). Changed in translation: mRNA recoding by −1 programmed ribosomal frameshifting. Trends Biochem Sci 40, 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell MG, and Karbstein K (2011). Protein-Protein Interactions within Late Pre-40S Ribosomes. PLoS One 6, e16194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Petrov A, Johansson M, Tsai A, O’Leary SE, and Puglisi JD (2014). Dynamic pathways of −1 translational frameshifting. Nature 512, 328–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung YN, Maag D, Mitchell SF, Fekete CA, Algire MA, Takacs JE, Shirokikh N, Pestova T, Lorsch JR, and Hinnebusch AG (2007). Dissociation of eIF1 from the 40S ribosomal subunit is a key step in start codon selection in vivo. Genes Dev 21, 1217–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieci G, Bottarelli L, and Ottonello S (2005). A general procedure for the production of antibody reagents against eukaryotic ribosomal proteins. Protein and peptide letters 12, 555–560. [DOI] [PubMed] [Google Scholar]
- Dinman JD (1995). Ribosomal frameshifting in yeast viruses. Yeast 11, 1115–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkle JA, and Dunham CM (2015). Mechanisms of mRNA frame maintenance and its subversion during translation of the genetic code. Biochimie 114, 90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermolenko DN, Majumdar ZK, Hickerson RP, Spiegel PC, Clegg RM, and Noller HF (2007). Observation of intersubunit movement of the ribosome in solution using FRET. J Mol Biol 370, 530–540. [DOI] [PubMed] [Google Scholar]
- Ermolenko DN, and Noller HF (2011). mRNA translocation occurs during the second step of ribosomal intersubunit rotation. Nat Struct Mol Biol 18, 457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez IS, Bai XC, Murshudov G, Scheres SH, and Ramakrishnan V (2014). Initiation of translation by cricket paralysis virus IRES requires its translocation in the ribosome. Cell 157, 823–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti MB, Ghalei H, Ward EA, Potts EL, and Karbstein K (2017). Rps26 directs mRNA-specific translation by recognition of Kozak sequence elements. Nat Struct Mol Biol In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J, and Agrawal RK (2000). A ratchet-like inter-subunit reorganization of the ribosome during translocation. Nature 406, 318–322. [DOI] [PubMed] [Google Scholar]
- Frank J, Gao H, Sengupta J, Gao N, and Taylor DJ (2007). The process of mRNA-tRNA translocation. Proc Natl Acad Sci U S A 104, 19671–19678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghalei H, Schaub FX, Doherty JR, Noguchi Y, Roush WR, Cleveland JL, Stroupe ME, and Karbstein K (2015). Hrr25/CK1delta-directed release of Ltv1 from pre-40S ribosomes is necessary for ribosome assembly and cell growth. J Cell Biol 208, 745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granneman S, Nandineni MR, and Baserga SJ (2005). The putative NTPase Fap7 mediates cytoplasmic 20S pre-rRNA processing through a direct interaction with Rps14. Mol. Cell Biol. 25, 10352–10364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harger JW, and Dinman JD (2003). An in vivo dual-luciferase assay system for studying translational recoding in the yeast Saccharomyces cerevisiae. RNA 9, 1019–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmich UA, Weis BL, Lioutikov A, Wurm JP, Kaiser M, Christ NA, Hantke K, Kotter P, Entian KD, Schleiff E, et al. (2013). Essential ribosome assembly factor Fap7 regulates a hierarchy of RNA-protein interactions during small ribosomal subunit biogenesis. Proc Natl Acad Sci U S A 110, 15253–15258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtkamp W, Cunha CE, Peske F, Konevega AL, Wintermeyer W, and Rodnina MV (2014). GTP hydrolysis by EF-G synchronizes tRNA movement on small and large ribosomal subunits. EMBO J 33, 1073–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh WK, Falvo JV, Gerke LC, Carroll AS, Howson RW, Weissman JS, and O’Shea EK (2003). Global analysis of protein localization in budding yeast. Nature 425, 686–691. [DOI] [PubMed] [Google Scholar]
- Johnson MC, Ghalei H, Doxtader KA, Karbstein K, and Stroupe ME (2017). Structural Heterogeneity in Pre-40S Ribosomes. Structure 25, 329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian P, Konevega AL, Scheres SH, Lazaro M, Gil D, Wintermeyer W, Rodnina MV, and Valle M (2008). Structure of ratcheted ribosomes with tRNAs in hybrid states. Proc Natl Acad Sci U S A 105, 16924–16927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling KM, Lanier J, Du M, Salas-Marco J, Gao L, Kaenjak-Angeletti A, and Bedwell DM (2004). Leaky termination at premature stop codons antagonizes nonsense-mediated mRNA decay in S. cerevisiae. Rna 10, 691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh CS, Brilot AF, Grigorieff N, and Korostelev AA (2014). Taura syndrome virus IRES initiates translation by binding its tRNA-mRNA-like structural element in the ribosomal decoding center. Proc Natl Acad Sci U S A 111, 9139–9144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebaron S, Schneider C, van Nues RW, Swiatkowska A, Walsh D, Bottcher B, Granneman S, Watkins NJ, and Tollervey D (2012). Proofreading of pre-40S ribosome maturation by a translation initiation factor and 60S subunits. Nat Struct Mol Biol 19, 744–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling C, and Ermolenko DN (2016). Structural insights into ribosome translocation. Wiley Interdiscip Rev RNA 7, 620–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loc’h J, Blaud M, Rety S, Lebaron S, Deschamps P, Bareille J, Jombart J, Robert-Paganin J, Delbos L, Chardon F, et al. (2014). RNA mimicry by the fap7 adenylate kinase in ribosome biogenesis. PLoS Biol 12, e1001860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine MS, McKenzie A 3rd, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, and Pringle JR (1998). Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953–961. [DOI] [PubMed] [Google Scholar]
- Meskauskas A, and Dinman JD (2007). Ribosomal protein L3: gatekeeper to the A site. Mol Cell 25, 877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meskauskas A, Harger JW, Jacobs KL, and Dinman JD (2003). Decreased peptidyltransferase activity correlates with increased programmed −1 ribosomal frameshifting and viral maintenance defects in the yeast Saccharomyces cerevisiae. RNA 9, 982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed D, and Noller HF (1989). Intermediate states in the movement of transfer RNA in the ribosome. Nature 342, 142–148. [DOI] [PubMed] [Google Scholar]
- Munro JB, Sanbonmatsu KY, Spahn CM, and Blanchard SC (2009). Navigating the ribosome’s metastable energy landscape. Trends Biochem Sci 34, 390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro JB, Wasserman MR, Altman RB, Wang L, and Blanchard SC (2010). Correlated conformational events in EF-G and the ribosome regulate translocation. Nat Struct Mol Biol 17, 1470–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J, Savva CG, Shin BS, Dever TE, Ramakrishnan V, and Fernandez IS (2016). Structural characterization of ribosome recruitment and translocation by type IV IRES. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz D, Gossack L, Quast U, and Bryan J (2013). Reinterpreting the action of ATP analogs on K(ATP) channels. J Biol Chem 288, 18894–18902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltz SW, Hammell AB, Cui Y, Yasenchak J, Puljanowski L, and Dinman JD (1999). Ribosomal protein L3 mutants alter translational fidelity and promote rapid loss of the yeast killer virus. Mol Cell Biol 19, 384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena C, Schutz S, Fischer U, Chang Y, and Panse VG (2016). Prefabrication of a ribosomal protein subcomplex essential for eukaryotic ribosome formation. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekna-Przybylska D, Przybylski P, Baudin-Baillieu A, Rousset JP, and Fournier MJ (2008). Ribosome performance is enhanced by a rich cluster of pseudouridines in the A-site finger region of the large subunit. J Biol Chem 283, 26026–26036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulicherla N, Pogorzala LA, Xu Z, HC OF, Musayev FN, Scarsdale JN, Sia EA, Culver GM, and Rife JP (2009). Structural and functional divergence within the Dim1/KsgA family of rRNA methyltransferases. J Mol Biol 391, 884–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratje AH, Loerke J, Mikolajka A, Brunner M, Hildebrand PW, Starosta AL, Donhofer A, Connell SR, Fucini P, Mielke T, et al. (2010). Head swivel on the ribosome facilitates translocation by means of intra-subunit tRNA hybrid sites. Nature 468, 713–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodnina MV, Savelsbergh A, Katunin VI, and Wintermeyer W (1997). Hydrolysis of GTP by elongation factor G drives tRNA movement on the ribosome. Nature 385, 37–41. [DOI] [PubMed] [Google Scholar]
- Rodnina MV, and Wintermeyer W (2011). The ribosome as a molecular machine: the mechanism of tRNA-mRNA movement in translocation. Biochem Soc Trans 39, 658–662. [DOI] [PubMed] [Google Scholar]
- Salas-Marco J, and Bedwell DM (2005). Discrimination between defects in elongation fidelity and termination efficiency provides mechanistic insights into translational readthrough. J Mol Biol 348, 801–815. [DOI] [PubMed] [Google Scholar]
- Spahn CM, Gomez-Lorenzo MG, Grassucci RA, Jorgensen R, Andersen GR, Beckmann R, Penczek PA, Ballesta JP, and Frank J (2004). Domain movements of elongation factor eEF2 and the eukaryotic 80S ribosome facilitate tRNA translocation. EMBO J 23, 1008–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel PC, Ermolenko DN, and Noller HF (2007). Elongation factor G stabilizes the hybrid-state conformation of the 70S ribosome. RNA 13, 1473–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunk BS, Loucks CR, Su M, Vashisth H, Cheng S, Schilling J, Brooks CL 3rd, Karbstein K, and Skiniotis G (2011). Ribosome assembly factors prevent premature translation initiation by 40S assembly intermediates. Science 333, 1449–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunk BS, Novak MN, Young CL, and Karbstein K (2012). A translation-like cycle is a quality control checkpoint for maturing 40S ribosome subunits. Cell 150, 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulima SO, Gulay SP, Anjos M, Patchett S, Meskauskas A, Johnson AW, and Dinman JD (2014). Eukaryotic rpL10 drives ribosomal rotation. Nucleic Acids Res 42, 2049–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DJ, Nilsson J, Merrill AR, Andersen GR, Nissen P, and Frank J (2007). Structures of modified eEF2 80S ribosome complexes reveal the role of GTP hydrolysis in translocation. EMBO J 26, 2421–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorhees RM, and Ramakrishnan V (2013). Structural basis of the translational elongation cycle. Annu Rev Biochem 82, 203–236. [DOI] [PubMed] [Google Scholar]
- Wilden B, Savelsbergh A, Rodnina MV, and Wintermeyer W (2006). Role and timing of GTP binding and hydrolysis during EF-G-dependent tRNA translocation on the ribosome. Proc. Natl. Acad. Sci. U. S. A. 103, 13670–13675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yount RG, Ojala D, and Babcock D (1971). Interaction of P--N--P and P--C--P analogs of adenosine triphosphate with heavy meromyosin, myosin, and actomyosin. Biochemistry 10, 2490–2496. [DOI] [PubMed] [Google Scholar]
- Zhou J, Lancaster L, Donohue JP, and Noller HF (2014). How the ribosome hands the A-site tRNA to the P site during EF-G-catalyzed translocation. Science 345, 1188–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


