Abstract
Developing efficacious treatments for alcohol use disorder (AUD) has proven difficult. The insidious nature of the disease necessitates a deep understanding of its underlying biology as well as innovative approaches to ameliorate ethanol-related patho-physiology. Excessive ethanol seeking and relapse are generated by long-term changes to membrane properties, synaptic physiology, and plasticity throughout the limbic system and associated brain structures. Each of these factors can be modulated by metabotropic glutamate (mGlu) receptors, a diverse set of G protein-coupled receptors highly expressed throughout the central nervous system. Here, we discuss how different components of the mGlu receptor family modulate neurotransmission in the limbic system and other brain regions involved in AUD etiology. We then describe how these processes are dysregulated following ethanol exposure and speculate about how mGlu receptor modulation might restore such pathophysiological changes. To that end, we detail the current understanding of the behavioral pharmacology of mGlu receptor-directed drug-like molecules in animal models of AUD. Together, this review highlights the prominent position of the mGlu receptor system in the pathophysiology of AUD and provides encouragement that several classes of mGlu receptor modulators may be translated as viable treatment options.
Keywords: Alcohol use disorder, metabotropic glutamate receptor, synaptic plasticity, prefrontal cortex, nucleus accumbens, bed nucleus of the stria terminalis
Graphical abstract
BACKGROUND
Alcohol use disorder (AUD) persists as a major societal burden. A recent epidemiological survey reported the United States 12 month and lifetime prevalence as 13.9 and 29.1%,1 respectively. These staggering statistics suggest that AUD is an insidious disease with poor availability of satisfactory treatment options. Developing an efficacious treatment for AUD is a remarkably complex undertaking, as primary risk factors for relapse—alcohol and related cues—are ubiquitous. In addition, relapse is readily instigated by social, economic, and physical stressors, which cannot be eliminated from a modern lifestyle. To overcome the pervasive nature of these hazards, medication development necessitates a deeper understanding of the underlying biology of AUD.
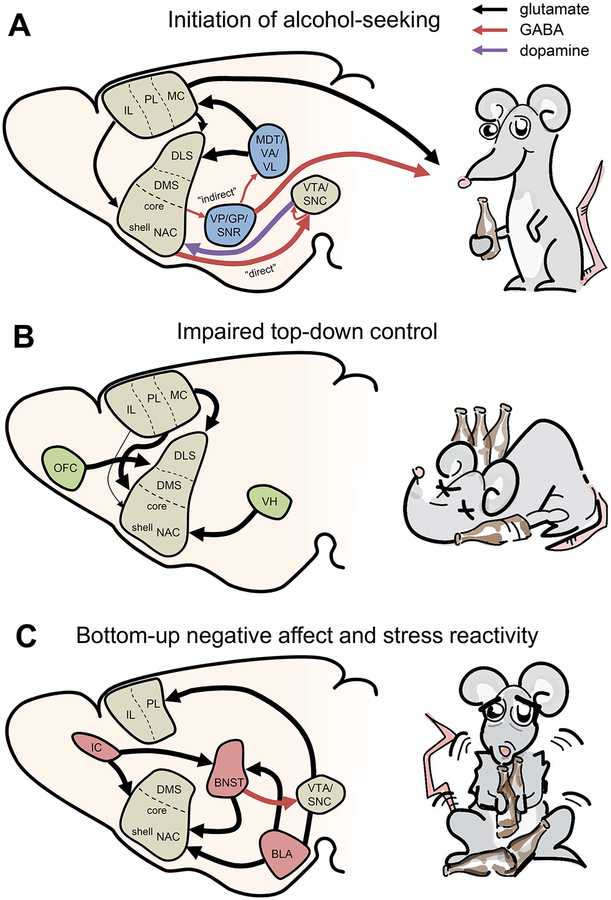
The acute actions of ethanol are complex and have been the subject of recent extensive reviews.2,3 In brief, ethanol acts primarily by modulating a variety of ion channels, including voltage-gated potassium channels and ionotropic receptors for the major neurotransmitters glutamate and γ-aminobutyric acid (GABA). Collectively, these molecular actions alter the function of meso-corticolimbic neurotransmission, which, over time, can generate a disease state that promotes excessive, persistent, and maladaptive ethanol seeking. AUD is a chronic disorder, characterized by repeated cycles of binging, withdrawal, preoccupation, and relapse.4 Distinct but overlapping brain circuits are thought to mediate the motivational and affective states across these periods (Box 1 and Figure 1), and we refer the reader to other thorough reviews for additional information.2,5
Box 1. Neurocircuitry Underlying AUD.
The mesocorticolimbic system lies at the heart of AUD. Dopa-mine neurons from the ventral tegmental area (VTA) send extensive projections to the nucleus accumbens (NAC) shell and core in the ventral striatum. In a simplified sense, dopa-mine is released during rewarding experiences and assists in the integration of excitatory transmission from a variety of brain areas. Like other reinforcing experiences, ethanol exposure increases the rate of extracellular NAC dopamine release, generally enhancing glutamatergic drive and modulating excitability of NAC projection neurons. Through direct and indirect projections, these actions lead to a disinhibition of dopa-mine neurons in the VTA and substantia nigra pars compacta (SNC). This feedforward circuit promotes ethanol seeking, and its dysregulation is often described as the final common pathway of relapse. Over time, ethanol seeking behaviors are thought to be mediated by more dorsal and lateral structures within mesostriatal pathways; analogous loops among the NAC core, SNC, and dorsomedial and dorsolateral striatum (DMS and DLS, respectively) underlie the development of habitual, compulsive-like behaviors.205 Throughout the ventral and dorsal striatum, projection neurons rest at hyperpolarized membrane potentials; therefore, glutamatergic input from cortical and limbic brain areas is essential to activate the striatum and promote subsequent ethanol-driven behaviors. These glutamatergic inputs to the striatum arise from a diverse set of structures. The cortices, notably including the prefrontal cortex (PFC), are thought to exert top-down control onto the striatum. Dysregulation of the PFC, as well as the orbitofrontal and cingulate cortices, is believed to underlie excessive preoccupation and out-of-control ethanol seeking. As mentioned, stressors and ethanol-related cues are the primary events that instigate relapse. These emotional triggers enhance the activity of the basolateral amygdala (BLA), which sends relapse-promoting excitatory projections to the NAC and DMS. In addition, nuclei within the extended amygdala modulate the BLA, mid-brain nuclei, and other stress-related circuits. Notably, the bed nucleus of the stria terminalis (BNST) interfaces with the stress and reward systems and contributes to stress-induced reinstatement as well as negative symptomology during ethanol withdrawal. In summary, animal models of AUD and patients alike display alterations to the striatal dopamine system, impaired cortical top-down control over that system, and heightened stress-related reactivity of the extended amygdala neurocircuitry.
Figure 1.
Simplified neurocircuitry underlying normal and pathophysiological ethanol seeking. (A) Basic motor circuit supporting initiation of ethanol seeking. Dopaminergic midbrain areas project to the dorsal and ventral striatum to promote motivated behaviors. Dopamine promotes the activation of dopamine receptor subtype 1 (D1)-expressing “direct pathway” medium spiny neurons (MSNs). D1 MSNs send inhibitory afferents back to the VTA and SNC, where they inhibit local GABAergic neurons and disinhibit dopamine neurons. In contrast, striatal dopamine release inhibits D2-MSNs, thereby relieving inhibition on the pallidum. Decreased output from the pallidum disinhibits the thalamus, the striatum, and the MC. The MC and pallidum provide the major outputs of this circuit to the brainstem to promote the initiation of ethanol seeking. (B) Impaired top-down control and excessive compulsive ethanol seeking. Dysregulation of the cortical inputs onto the striatum is believed to underlie the loss of control observed in AUD. Decreased function of the IL PFC inputs to the NAC shell and enhanced activity of the VH and PL PFC inputs are thought to mediate excessive motivation to seek ethanol as well as reinstatement. Alterations in the OFC projections to the dorsal striatum are also believed to play a part in habitual, compulsive-like ethanol seeking. (C) Enhanced bottom-up stress reactivity in AUD. The extended amygdala plays a key role in the regulation of emotions and stress response, particularly in the context of ethanol seeking. Excitatory projections from the BLA to the PFC, NAC, and BNST may each be important in promoting stress-induced reinstatement. The BNST is composed of both excitatory and inhibitory projection neurons that modulate the function of the NAC and also the dopaminergic midbrain areas. Finally, the enhanced activity of the IC is thought to be involved in stress-related ethanol seeking, and its projections to the NAC and BNST may be particularly important for these effects.
The persistent ethanol-induced changes to circuit function proceed through a diverse set of pathophysiological adaptations. Ethanol usurps several modes of signaling to alter behavior. Changes in membrane properties, excitatory and inhibitory synaptic strength, and synaptic plasticity are each important to consider, as each of these factors modulates neurotransmission and therefore ethanol seeking, negative affect, and vulnerability to relapse. Collectively, the metabotropic glutamate (mGlu) receptor family modulates each of the physiological parameters mentioned above, suggesting that modulating mGlu receptor signaling may provide mechanisms for ameliorating ethanol-induced physiological and behavioral disruptions. Here, we will extensively describe the physiology and pharmacology of the mGlu receptors, a diverse family of receptors that regulate physiology and plasticity throughout the central nervous system (CNS). Afterward, we will delve into behavioral pharmacology. We discuss what is currently known about how individual mGlu receptor subtypes contribute to ethanol seeking and how these exciting targets might be exploited to develop treatments for AUD.
BASIC MGLU RECEPTOR PHYSIOLOGY
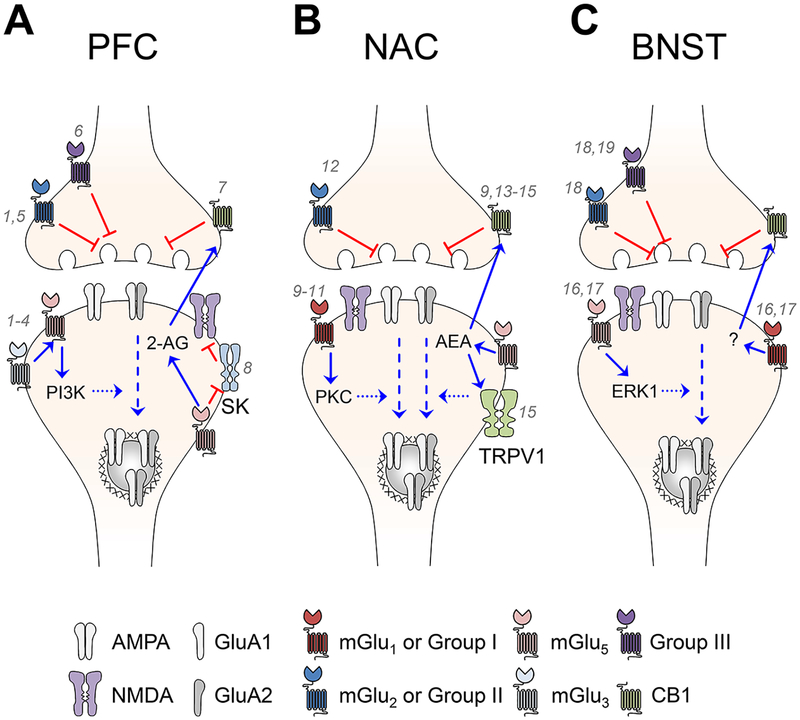
The mGlu receptor family is composed of eight receptor subtypes, which are divided into three groups based on genetics, pharmacology, and function.6 The group I subtypes, mGlu1 and mGlu5, are generally expressed at postsynaptic sites where canonical downstream signaling proceeds through Gq proteins and related effectors. In contrast, the group II (mGlu2 and mGlu3) and group III (mGlu4 and mGlu6−mGlu8) sub-types couple with Gi proteins and are enriched at presynaptic locations in many parts of the CNS. mGlu6 expression is limited to the retina and is thought not to play a role in the CNS (for a review, see ref 7). All mGlu receptor subtypes are members of the class C family of G protein-coupled receptors (GPCRs) and are characterized by large, extracellular, ligand-binding domains at the N-terminus. Like other class C GPCRs, mGlu receptors assemble as obligate dimers. Both homodimerization and heterodimerization occur, although only a few receptor pairs in groups II and III have been shown toform dimers with receptors outside their own group. We refer the reader elsewhere for more information about mGlu receptor structural characteristics8 and expression of mGlu receptors in the periphery.9 Here, we will extensively describe mGlu receptor functions and plasticity mechanisms across the limbic system (Figure 2 and Table 1), along with the currently known dysfunctions following ethanol exposure.
Figure 2.
Contrasting mechanisms of mGlu receptor plasticity across excitatory synapses in the limbic system. (A) Plasticity mechanisms in the PFC. mGlu2 and mGlu4 each inhibit glutamate release in the PFC. Whether mGlu7 and mGlu8 function similarly at these synapses has not been determined. On the postsynaptic side, activation of mGlu3 initiates a cascade of signaling events that culminates with AMPA receptor internalization. This process depends on the activation of mGlu5 and PI3K. mGlu5 activation here has also been shown to promote the production of 2-AG, retrograde activation of CB1, and inhibition of glutamate release probability. Furthermore, mGlu5 inhibits SK channels, relieving an inhibitory constraint on NMDA receptors and promoting additional mechanisms of plasticity not depicted here. (B) Plasticity mechanisms in the NAC. Activation of mGlu2/3 decreases glutamate release probability, but the subtype(s) involved has not been determined conclusively. In normal animals, activation of mGlu leads to a presynaptic LTD through the activation of CB1. In the core subregion, this phenomenon is specific to D2 MSNs and coincides with an AEA- and TRPV1-dependent internalization of AMPA receptors. However, in animals conditioned with chronic cocaine self-administration, postsynaptic LTD mediated by mGlu1 can emerge. This depotentiation requires the activation of PKC and internalization of GluA2-lacking AMPA receptors, and it is unknown whether similar alterations to plasticity may emerge following ethanol exposure. (C) Plasticity mechanisms in the BNST. Activation of presynaptic group II or group III mGlu receptors transiently inhibits glutamate release probability. mGlu8 activation is sufficient for this effect, but the contribution of the other subtypes is not well understood. Postsynaptic mGlus induces ERK1-dependent AMPA receptor internalization. Activation of group I mGlu receptors also induces CB1-dependent LTD; however, the subtype and specific endocannabinoid species involved have not been determined.
Table 1.
| region | no. | first author (ref) | summary |
|---|---|---|---|
| PFC | 1 | Joffe (88) | Activation of mGlu3 promotes AMPA receptor internalization on pyramidal cells. LTD occurs at long-range inputs from BLA, but not VH, and does not occur on fast-spiking interneurons. |
| 2 | Di Menna/Joffe (84) | mGlu3 enhances mGlu5-mediated signaling, including phosphoinositide production and Ca2+ mobilization. mGlu5 activity is also required for the induction of mGlu LTD. | |
| 3 | Walker (99) | Genetic deletion of mGlu3, but not mGlu2, blocks LTD induced by group II agonists. Inhibition of mGlu3 disrupts extinction of cued fear. | |
| 4 | Joffe (100) | mGlu3 LTD does not require the mobilization of intracellular Ca2+ stores. Instead, mGlu3 LTD proceeds through activation of PI3K and Akt. mGlu3 LTD is impaired by acute restraint stress. | |
| 5 | Joffe (97) | Glutamate release is enhanced by acute application of a group II mGlu receptor antagonist or mGlu2 NAM. Acute application of an mGlu3 NAM does not affect basal release probability. | |
| 6 | Zhang (104) | Application of a group III mGlu receptor agonist reduces glutamate released by serotonin. The specific mGlu receptor subtype mediating this effect is not known. | |
| 7 | Lafourcade (44) | Synaptic or pharmacologic stimulation of mGlu5 induces the production of 2-AG. 2-AG activates presynaptic CB1 receptors to induce LTD. | |
| 8 | Cannady (21) | mGlu5 activation inhibits SK channels, which normally act to inhibit NMDA receptor function. This mechanism promotes the induction of NMDA receptor LTP and extinciton of alcohol seeking. | |
| NAC | 9 | McCutcheon (50) | mGlu5 induces eCB LTD. Following extended cocaine exposure, mGlu5 function is impaired, and mGlu1 induces postsynaptic PKC-dependent LTD instead. |
| 10 | Loweth (61) | Abstinence from cocaine exposure upregulates GluA2-lacking AMPA receptors. Activating mGlu1 depotentiates these synapses and reduces incubation of cocaine craving. | |
| 11 | Turner (51) | In the NAC shell, mGlu5 induces LTD at MDT inputs onto D1-expressing neurons. In contrast, mGlu1 activation generates LTD at PFC inputs onto both D1(+) and D1(−) MSNs. | |
| 12 | Robbe (86) | Group II mGlu receptor agonists induce presynaptic LTD. The mechanism involves cAMP, PKA, and the inhibition of P/Q type Ca2+ channels. | |
| 13 | Robbe (48) | mGlu5 induces canonical eCB LTD through intracellular Ca2+ mobilization and activation of presynaptic CB1 receptors. | |
| 14 | Fourgeaud (98) | A single administration of cocaine impairs mGlu5 LTD. This impairment requires in vivo activation of D1 and is associated with enhanced Homer expression and mGlu5 internalization. | |
| 15 | Grueter (47) | In the NAC core, mGlu5 LTD occurs specifically on D2-expressing neurons. AEA is released to activate CB1 as well as postsynaptic TRPV1, which promotes AMPA receptor internalization. | |
| BNST | 16 | Grueter (28, 29) | Activation of mGlu5 promotes the internalization of AMPA receptors. The mechanism requires the activation of ERK1 and is disrupted by repeated cocaine administration. |
| 17 | McElligott (30) | mGlu5 LTD is distinct from LTD induced by aradrenergic receptor activation. mGlu5 LTD does not involve GluA2-lacking AMPA receptors and is not impaired by stress. | |
| 18 | Grueter (113) | Group II and group III mGlu receptor agonists depress excitatory transmission. Prolonged activation of group II mGu receptors induces LTD. | |
| 19 | Gosnell (112) | An mGlu8−specific agonist induces a transient depression of excitatory transmission, and mGlu4 does not appear to modulate presynaptic release probability. |
Group I mGlu Receptor Physiology.
mGlu1 and mGlu5 are perhaps the best-studied mGlu receptor subtypes. The two receptors perform a diverse array of functions involving the regulation of neuronal excitability, excitatory and inhibitory synaptic strength, and synaptic plasticity. In addition to canonical Gq−coupled signaling [i.e., phospholipase C, protein kinase C (PKC), and mobilization of intracellular Ca2+ stores10], activation of the group I mGlu receptor subtypes can promote signaling through phosphoinositide 3-kinase (PI3K),11 casein kinase,12 Ca2+/calmodulin-dependent kinase II (CaMKII),13,14 Src-family kinases,15,16 the mechanistic target of rapamycin (mTOR),17 protein kinase A (PKA),18 phospholipase D,19 and a variety of voltage-sensitive ion channels.10,20 Moreover, mGlu5 activation modulates the function of NM DA receptors through second-messenger-mediated posttranslational modifications10,21,22 and physical interactions with scaffolding proteins like Homer.23 Activation of these signaling pathways, as well as the endocannabinoid (eCB) system,24 places the group I mGlu receptors in a key position to regulate synaptic plasticity in many different cell types and circuits throughout the CNS.
Group I mGlu Receptors and Synaptic Plasticity.
The group I mGlu receptor subtypes have been intimately linked with several forms of synaptic plasticity, including long-term potentiation (LTP) and its primary counterpart, long-term depression (LTD).25,26 mGlu1 and mGlu5 have been shown to promote LTP and LTD in different brain regions and even at the same synapse under different circumstances.25,27 Mammalian research on synaptic plasticity has its roots in the Schaeffer collateral (SC) CA1 synapses in the hippocampus. Here, mGlu1 and mGlu5 both contribute to a postsynaptic form of LTD involving PI3K-Akt signaling and the dephosphorylation and subsequent internalization of AMPA receptors.25,26 This LTD can be induced pharmacologically or through low-frequency stimulation of SC inputs. Similar postsynaptic forms of plasticity have been observed in other brain regions, including mGlu5-mediated LTD in the BNST,28–30 as well as m Glu1−mediated depotentiation in the VTA.31,32 In many of these brain regions, m Glu1/5 LTD requires mTOR activity, the rapid initiation of protein translation, and the local synthesis of important signaling proteins;32–34 however, notable exceptions in specific disease states have been reported.35–37 In any case, the rapid translation of cytoskeletal association proteins,38,39 tyrosine phosphatases,36 and small-conductance AMPA receptor subunits32 have been shown to occur during m Glu1/5 LTD.
Interestingly, the underlying mechanisms of group I mGlu receptor-mediated LTD can vary following in vivo experience. For example, the extinction of fear conditioning induces meta-plastic changes such that strong activation of mGlu5 promotes CA1 LTP instead of LTD.40 Accordingly, in preparations from control animals, modest activation of mGlu5 promotes the induction of NMDA receptor-mediated LTP in animals.41,42 This effect, termed “priming”, occurs through eCB production and the inhibition of the release of GABA from nearby inter-neurons. In addition to its effects on inhibitory transmission, group I mGlu/eCB synaptic plasticity of excitatory transmission has been observed in several components of the limbic system, including the prefrontal cortex (PFC),43,44 dorsolateral striatum (DLS),45,46 nucleus accumbens (NAC),47–51 and lateral habenula.52 mGlu1 has also been extensively linked with the eCB system in the cerebellum.53 Heterogeneous eCB-driven effects occur through multiple lipid species [e.g., anandamide (AEA) and 2-arachidonoylglycerol (2-AG)] and receptor targets [e.g., CB1, CB2, and transient receptor potential vanilloid 1 (TRPVl) receptors], varying based on the brain region, cell type, and stimulation parameters.24 CB1 and CB2 are generally thought to inhibit neurotransmitter release probability through the translation-dependent54,55 modulation of presynaptic Ca2+ and K+ channels,56 whereas TRPV1 has been shown to induce LTD with both presynaptic57 and postsynaptic47 loci of action. Finally, the eCB system is increasingly being recognized as modulating dopaminergic transmission,58,59 and these actions are thought to guide goal-directed behaviors, including ethanol seeking.
As mentioned, mGlu5 activation can facilitate NM DA receptor-dependent LTP at SC CA1 through GABAergic disinhibition.41,42 Similar phenomena have been observed in other brain regions; however, some underlying mechanistic differences can occur. In the PFC, for example, activation of mGlu5 inhibits the function of small-conductance Ca2+−activated K+ (SK) channels.21 SK channel inhibition reduces synaptic K+ currents and enhances postsynaptic depolarization and NMDA receptor activation. Through this disinhibitory pathway, PFC mGlu5 activation thereby promotes NMDA receptor-dependent signaling and LTP. Alternatively, activation of group I mGlu receptors has been linked with direct phosphorylation of NMDA receptors by Src-family kinases.15,16 These posttranslational modifications, along with physical interactions via Homers and associated scaffolding proteins,23,60 contribute to enhanced NMDA receptor-dependent plasticity. Finally, activation of mGlu1 (but not mGlu5) has been linked to a unique form of LTD that proceeds through the internalization of Ca2+−permeable, GluA2-lacking AMPA receptors.32,61 Each of these molecular cascades is important for modulating synaptic strength and is important to consider in the development and expression of ethanol-related behaviors.
Group I mGlu Receptor Dysfunction following Ethanol Exposure.
Throughout many brain regions, acute ethanol exposure disrupts NM DA receptor-dependent LTP, in a manner consistent with direct antagonism or blockade of the NMDA receptor itself.62–67 However, several findings also point toward ethanol disrupting m Glu1/5 function and interactions with NMDA receptor signaling. In the NAC, application of acute ethanol (50 mM) disrupts the ability of mGlu1/5 activation to potentiate NMDA receptor function68 and also disrupts a form of LTP that requires activation of group I mGlu receptors.69 Moreover, acute ethanol disrupts mGlu5-stimulated eCB mobilization in the NAC (40 mM)70 as well as m Glu1−and eCB-dependent LTD in the cerebellum (50 mM).71,72 These effects may stem from an induced posttranslational modification, as ethanol has been shown to promote PKC phosphorylation of mGlu5 at an inhibitory site (30–200 mM).73 In addition to these acute actions, exposure to chronic intermittent ethanol (CIE) dysregulates mGlul/5 LTD in both the DLS74,75 and CAl.76 Together, these data indicate that acute ethanol generally disrupts mGlu1/5 function and/or interactions between group I mGlu receptors and NMDA receptors.
In accordance with the functional effect on SC CAl LTD, CIE decreases the level of association between NMDA receptors and several proteins that interact with mGlu1/5, such as Homer.76 Conversely, changes in the response to ethanol exposure have been observed by manipulating Homer protein expression and its interactions with mGlu1/5. Through interactions with the mGlul/5 C-terminal EVHl domain, Homers link group I mGlu receptors to intracellular scaffolding proteins and effector systems.60 Three genes encode Homer proteins, and multiple splice variants exist for Homerl and Homer2. Genetic deletion of Homer2 leads to a behavioral phenotype that avoids high concentrations of ethanol and remains relatively insensitive to its rewarding properties.77 This pheno-type is reversed by restoration of the Homer2b splice variant in the NAC, suggesting that Homer2b–mGlul/5 interactions are essential for the expression of ethanol-related reward. Conversely, overexpression of Homer2 in the NAC facilitates the rewarding properties of ethanol exposure.78 Consistent with these genetic manipulations, repeated periods of binge drinking enhance NAC Homer2 expression, and ethanol intake is inhibited by NAC knockdown of Homer2 or pharmacological inhibition of mGlu5.79–81 Similarly, chronic mild stress has been shown to elevate NAC levels of Homer2 and mGlul and, in turn, enhance the psychomotor and rewarding properties of ethanol.82 Homer-mGlul/5 interactions within the central nucleus of the amygdala (CEA) have also been implicated in the behavioral effects of withdrawal,83 demonstrating the diversity of Homer-mGlul/5 involvement in ethanol-related behaviors across multiple brain areas. Clearly, this literature provides strong evidence that dysregulation of group I mGlu receptor function is intimately linked with several behavioral com ponents of ethanol consumption. Modulating these signaling pathways may provide a means to develop novel approaches for treating AUD.
Group II and Group III mGlu Receptor Physiology.
mGlu2−mGlu4, mGlu7, and mGlu8 are enriched at presynaptic sites in many brain regions, where they generally function as autoreceptors or heteroreceptors to reduce neurotransmitter release probability. Group II and group III mGlu receptors signal through Gi/o−coupled pathways,6 notably including a decreased rate of accumulation of cyclic adenosine m ono-phosphate (cAMP) and subsequent inhibition of PKA, Ca2+ channels, and hyperpolarization-activated cyclic nucleotide-gated channels. Liberation of the Gβγ, subunit can also act on intracellular signaling cascades,84 G protein-gated K+ channels,85 and presynaptic neurotransmitter release machinery.86
Unique among the Gi/o−coupled mGlu receptors, mGlu3 is highly expressed at perisynaptic areas,87 where its activation can modulate Ca2+ mobilization and the function of AMPA and NMDA receptors.84,88,89 Interestingly, we have shown that some of these functions occur, at least in part, through a functional interaction between mGlu3 and the group I subtype, mGlu5.84 mGlu3 is also the only mGlu receptor subtype expressed on astrocytes in the adult CNS.90 While its specific functions remain to be fully characterized, glial mGlu3 is believed to exert neuroprotective effects and may modulate interactions between glutamate signaling and other neurotransm itter systems. In addition, mGlu3 and other mGlu receptor subtypes are thought to be expressed on microglia and dendritic cells and are currently being investigated for their involvement in neuro-inflammation.91
Group II and Group III mGlu Receptors and Synaptic Plasticity.
Consistent with their predominant presynaptic localization, group II mGlu receptor agonists generally depress glutamate release probability. Indeed, mGlu2/3 agonists generate presynaptic LTD in the NAC,86 DLS,92,93 basolateral amygdala (BLA),94 and lateral amygdala95 and at the mossy fiber synapse.96 In the PFC, autoreceptor function has been linked with mGlu2 specifically, as selectively inhibiting mGlu2, but not mGlu3, increases the rate of basal glutamate release.97 Similar to the effects of CB1 cannabinoid receptor activation, the mechanism involved in these presynaptic actions is thought to involve inhibition of P/Q-type Ca2+ channels.86,98
In contrast to this presynaptic mechanism, but in accordance with its cellular localization, mGlu3 induces postsynaptic forms of plasticity in the PFC and area CA1 of the hippocampus. In the PFC, activation of mGlu3 induces LTD through the internalization of AMPA receptors and reduction of the number of functional synapses.84,88,99 Similar to group I LTD at the SC-CA1 synapse, interactions with mGlu5, Homer proteins, and PI3K/Akt signaling are involved in this form of LTD. However, PFC mGlu3 LTD does not require activation of mTOR or protein translation.100 At the SC-CA1 synapse, on the other hand, prolonged activation of mGlu3 induces NMDA receptor-dependent LTP.89 Remarkably, this LTP also requires the activity of mGlu5 and related downstream effectors, including CaMKII.14 Together, these recent findings suggest that neuronal mGlu3 exerts a major impact on synaptic strength and raise the possibility that mGlu3 may regulate other forms of plasticity and neuromodulation that involve mGlu5. In addition, astrocytic mGlu3 is likely involved in several forms of plasticity, including regulation of synaptic plasticity through interactions with the adrenergic system.101,102 While research on astrocytic mGlu3 has historically relied on glial toxins and correlative immunohistochemistry, the development of cell-type-specific mouse models may soon provide conclusive evidence of the separable functions of neuronal and glial mGlu3 populations.
The group III mGlu receptor subtypes also attenuate pre-synaptic release probability. mGlu4 transiently depresses synaptic transmission from the thalamus103,104 and olfactory tract;105 however, persistent mGlu4−mediated LTD has only been shown to occur in the cerebellum.106 Expression of mGlu7 has been detected within the hippocampus, cortex, and basal ganglia.107 mGlu7 is unique among mGlu receptor subtypes in that its affinity for glutamate is extremely low,108 such that receptor activation is thought to occur only under conditions of excessive glutamate release.109,110 Accordingly, mGlu7 is involved in short- and long-term plasticity following tetanic stimulation at SC CA1.110 Activation of mGlu7 on parvalbumin-expressing interneurons decreases GABA release probability, disinhibiting NMDA receptors and thereby promoting LTP. Finally, mGlu8 exhibits the lowest level of expression of the group III mGlu receptor subtypes and can be detected in the cortex, amygdala, olfactory bulb, and reticular nucleus of the thalamus.111 mGlu8 transiently depresses excitatory synaptic transmission at multiple synapses, including the BNST,112,113 lateral amygdala,109 olfactory tract,105 and lateral performant path;114 however, no effects on long-term plasticity have been identified. Ultimately, while relatively little is known about how group III mGlu receptor subtypes modulate synaptic plasticity in the limbic system, their expression patterns suggest that these targets merit further study in the context of AUD.
Group II and Group III mGlu Receptor Dysfunction following Ethanol Exposure.
In addition to changes in post-synaptic mGlu receptor function in the NAC, disruptions in glutamate homeostasis have been observed following chronic ethanol exposure. Both CIE and voluntary access to ethanol increase basal glutamate levels in the NAC core,115,116 and such increases can promote excessive ethanol consumption.117 In these studies, the function of presynaptic mGlu2/3 autoreceptors was left intact;116 however, others have observed decreased infralimbic (IL) PFC Grm2 transcript and NAC shell mGlu2/3 autoreceptor function in ethanol-dependent rats.118 While at apparent odds with Pati et al., Meinhardt et al. found no change in the Grm2 transcript in the prelimbic PFC, the subregion from which the NAC core receives its major cortical input. These studies suggest that within the NAC, mGlu2/3 function in the shell subregion may be particularly sensitive to the effects of ethanol exposure. In addition, drinking-induced decreases in mGlu2/3 autoreceptor function have also been observed in the VTA.119 In contrast to these findings in the mesocorticolimbic system, enhanced sensitivity to mGlu2/3 agonists has been observed in the CEA and BNST of ethanol-dependent rats.120 Interestingly, these findings were associated with enhanced behavioral sensitivity to mGlu2/3 agonists in their ability to reduce ethanol-motivated behavior. Together, the physiological studies of group II mGlu receptor subtypes suggest that potentiating the function of mGlu2/3 could attenuate consumption under normal and disease-like conditions of ethanol intake. While group III mGlu receptor modulators do exert behavioral effects in behavioral models of ethanol seeking, little is known about corresponding ethanol-related changes in the physiology or function of mGlu4, mGlu7, or mGlu8.
MGLU RECEPTOR BEHAVIORAL PHARMACOLOGY
The development of selective ligands was instrumental to the field of mGlu receptor research: this innovation provides a means to isolate the function of distinct mGlu receptor subtypes. These initial selective compounds, many of which are still widely used today, were designed as rotationally constrained glutamate analogues and therefore act at the orthosteric (i.e., glutamate-binding) site.121 With few exceptions, orthosteric agonists and antagonists display similar potencies at all subtypes across a given mGlu receptor group;108 however, their high affinities, aqueous solubility, and occasional bioavailability make them invaluable tools in modern preclinical mGlu receptor research.
The highly conserved nature of the orthosteric binding site led to a steep structure—activity relationship and the inability to widely develop ligands selective for a single mGlu receptor subtype.6 An alternative approach, targeting allosteric (“other”) sites on the receptors, has been fruitful; positive allosteric modulators (PAMs) and negative allosteric modulators (NAMs) have been developed for most mGlu receptor subtypes. Moreover, many allosteric modulators are systemically active and “drug-like”, enabling researchers to probe how individual mGlu receptor subtypes modulate ethanol seeking behavior. Several common and/or essential orthosteric and allosteric ligands are listed in Table 2 (and reviewed in refs 7 and 108). In tandem with genetically modified mouse lines, these tools provided the ability to discover how specific mGlu receptor subtypes modify synaptic plasticity and behaviors related to ethanol intoxication and drug seeking.
Table 2.
| group | effector | agonist | antagonist | subtype | PAMs | NAMs |
|---|---|---|---|---|---|---|
| group I | Gq, PLC/PKC, PI3K/Akt, mTOR ERK, PLD, PKA, NMDAR, eCB | DHPG, CHPG, quisqualate | MCPG, AIDA | mGlu1 | Ro 67–7476 | JNJ16259685 |
| mGlu5 | CDPPB, VU29, VU0409551 | MPEP, MTEP, fenobam | ||||
| group II | Gi/o, inhibition of adenylyl cyclase, modulation of K+ and Ca2+ channels | DCG-IV, LY354740, LY379268 | LY341495 | mGlu2 | BINA, LY487379 | |
| mGlu3 | ML337 | |||||
| group III | Gi/o, inhibition of adenylyl cyclase, modulation of K+ and Ca2+ channels | L-AP4, LSP4–2022 | CPPG | mGlu4 | PHCCC | |
| mGlu7 | AMN082 (allosteric agonist) | ADX71743 | ||||
| mGlu8 | DCPG (orthosteric agonist) |
As mentioned in the introductory sections, AUD is composed of several symptom clusters, related to binging and intoxication, depressive symptoms and withdrawal, and excessive preoccupation with future alcohol use. As such, alcohol seeking, inebriation, and dependence are modeled by a diverse set of preclinical exposure paradigms and behaviors.122 Each model is intended to recapitulate only a specific facet of the disorder. By and large, these preclinical models are widely applied to investigate the motivational properties of most drugs of abuse and natural rewards. In this review, however, we will limit our discussion to alcohol research.
Reward and Reinforcement.
A reward is a stimulus that possesses innate positive value. These stimuli, including ethanol, often serve as reinforcers, items that prom ote learned behavior with an action—outcome contingency. Models of reward are designed to yield information about the hedonic valence of the stimulus, whether positive or negative. In contrast, models of reinforcement are used to assess how the stimulus engenders future behavior, typically by examining the rate, duration, magnitude, or latency of the appetitive and consummatory responses.
The most common model for assessing conditioned reward is place conditioning, commonly termed conditioned place preference (CPP). In the CPP assay, rodents are conditioned in an apparatus with at least two contextually distinct chambers. Ethanol is repeatedly administered to an animal while it is confined in one component of the chamber and vehicle treatments are administered in the other. Over time, a learned association is formed between the unconditioned stimulus (i.e., ethanol) and the conditioned stimulus (i.e., chamber). The propensity to spend more time in the ethanol-paired side is then taken as a measure of reward. Importantly, this assay can readily detect aversive properties of ethanol or withdrawal, in which case it is commonly termed conditioned place aversion.
Reinforcement, by contrast, is generally assessed through self-administration. These assays are run as a two-bottle choice procedure in the home cage or as an operant task in a separate chamber. Unlike CPP and classical models of reward, which involve experimenter-delivered ethanol, self-administration requires that the animal learn to generate an instrumental response that will result in ethanol delivery (e.g., drinking from the ethanol bottle or pressing a lever). A major advantage of the operant paradigm is that a variety of reinforcement schedules and technical parameters can be adjusted to model compulsive-or addiction-like ethanol seeking.123–125 Ethanol self-administration models, in either the home cage or an operant box, can be readily used to examine intake in both dependent and nondependent subjects.
Repeatedly and consistently, genetic or pharmacologic mGlu5 inhibition decreases ethanol seeking behavior in a wide variety of rodent models.126–140 These actions occur at least in part within the NAC, as local mGlu5 inhibition is sufficient to attenuate ethanol self-administration.128,140 Moreover, interactions among mGlu5, Homer2, and related downstream signaling pathways in the NAC appear to be particularly important for the efficacy of mGlu5 NAMs.79,141,142 Interestingly, mGlu5 antagonism may not be related to an attenuation of ethanol’s rewarding properties per se, as inhibiting mGlu5 has mixed effects on ethanol CPP.129,133,143–145 However, mGlu5 NAMs do disrupt the interoceptive cue following ethanol exposure (see below). While less is known about mGlu1 function in this context, mGlu1 inhibition appears to reduce ethanol intake133,139,146,147 and CPP,145 but these effects may be somewhat confounded by concomitant reductions in locomotion.
Group II receptor activation reduces ethanol seeking in several animal models.117,120,128,137,148,149 Activation of mGlu2/3 in the NAC recapitulates these effects,117,128 but other brain regions may also be involved in the effects observed following systemic agonist administration. Indeed, data obtained in other behavioral assays suggest that amygdalar regions may also be involved in the attenuation of ethanol’s behavioral effects by mGlu2/3 activation150 (see Interoception). Relatively little is known with regard to the role of each mGlu receptor subtype involved in the effects of group II agonists. Recent studies using an mGlu2−specific PAM have shown mixed effects on alcohol self-administration.151,152 Furthermore, genetic deletion of Grm2 leads to increased ethanol preference and intake in rodent models,153,154 consistent with the decreased IL PFC Grm2 transcript observed in ethanol-dependent rats.118 While specifically potentiating the function of mGlu2 appears to be a promising means to attenuate ethanol reinforcement, the effects of Grm3 ablation or pharmacological modulators specific for mGlu3 have not been reported. As the selective pharmacological and genetic tools continue to develop, teasing apart the mechanisms and circuits underlying group II efficacy in reducing ethanol seeking will be a crucial area of future research.
mGlu7 has been the best-studied group III subtype in animal models of AUD. Global deletion of Grm7 has been shown to enhance voluntary ethanol intake in mice, suggesting that mGlu7 may typically gate this process.155 Furthermore, regionally specific viral-mediated knockdown of NAC mGlu7 enhanced ethanol consumption in a two-bottle choice paradigm, without affecting alternative liquid intake.156 Consistent with an effect on ethanol reward, mGlu7 knockdown also increased rats’ ethanol sensitivity in CPP. Conversely, activation of mGlu7 with systemic delivery of AMN082 reduces ethanol consumption157 and preference.158 Additionally, a recently discovered mGlu4/7 orthosteric agonist, LSP2–9166, significantly reduced ethanol self-administration in the reacquisition phase of a rodent relapse model.159 Future studies using improved pharmacological tools and examining other neurocircuits and behaviors are warranted in efforts to translate mGlu7 as a potential treatment approach.
Very few studies have examined the other group III mGlu receptors in ethanol seeking contexts. One study reported that mGlu4 knockout mice exhibited an impairment of locomotor hyperactivity following ethanol administration.160 Further examination of the mGlu4 knockouts revealed no differences in models of consumption, withdrawal, and hypnosis, suggesting that mGlu4 may modulate some of ethanol’s stimulatory effects but likely does not substantially impact its addictive properties. In contrast, recent findings posit that activation of mGlu8 may exert therapeutic-like benefits in models of ethanol reward and reinforcement. Delivery of the agonist DCPG decreased ethanol intake, preference, and the acquisition of CPP.161 Studies using genetic manipulations are warranted as there are few pharmacological tools available to specifically modulate mGlu8 function.
Extinction and Reinstatement.
Increasingly, extinction and reinstatement paradigms have been used to model facets of ethanol seeking pertinent for the treatment of recovering patients. Extinction is often considered to be analogous to rehabilitation therapy. Ethanol is replaced with a neutral stimulus, usually water, and the animals learn to cease approach or instrumental behavior. Manipulations that enhance extinction learning are expected to benefit patients in active recovery. Reinstatement then occurs when a stimulus is applied to renew ethanol seeking. Like relapse, reinstatement can occur following a brief exposure to stress, a drinking-related cue, or ethanol itself.
As with reward and reinforcement, mGlu5 is perhaps the best-studied mGlu receptor subtype in extinction and reinstatement models. Recent research has shown that mGlu5 potentiation can accelerate the extinction of ethanol seeking. Administration of an mGlu5 PAM, either systemically or localized to the IL PFC, facilitates the extinction of ethanol seeking.21 These effects are dependent on the inhibition of SK channels and persist in a model of ethanol dependence.162 On the other hand, systemic administration of an mGlu5 NAM reduces ethanol-induced,144 cue-induced,148,163 and stress-induced137 reinstatement, and receptors located in the BLA and NAC appear to be particularly important for these effects. An agonist of mGlu2/3 receptors blocked both stress-and cue-induced reinstatement of ethanol seeking, and actions within the extended amygdala may be particularly relevant for these effects.164 Moreover, activation of group II mGlu receptors reduces stress-induced reinstatement in a manner that is more potent in dependent subjects.120,137 No studies have specifically addressed which specific mGlu receptor subtype mediates the effects of group II agonists on reinstatement, but some findings point toward mGlu2 enhancement as being sufficient. An mGlu2−specific PAM has been shown to inhibit stress- and cue-induced reinstatement,151 and lentiviral overexpression of mGlu2 in the IL PFC attenuated cue-induced reinstatement.118 To the best of our knowledge, no studies have directly assessed the involvement of mGlu1, mGlu3, or any group III mGlu receptor subtype in the extinction or reinstatement of ethanol seeking.
Interoception.
The interoceptive (i.e., subjective) effects produced by drugs regulate, in part, drug use and drug seeking.165 Drug discrimination techniques that utilize operant or Pavlovian procedures are useful in investigating ethanol’s discriminative stimulus/interoceptive effects. In drug discrimination procedures, the experimenter administers the ethanol training dose (e.g., intraperitoneal injection or intragastric gavage) and the presence or absence of interoceptive ethanol cues guides the behavior (i.e., lever press or head poke).166 Extensive research has demonstrated that the interoceptive effects of ethanol are mediated systemically and at specific central sites by ligands that reduce excitatory transmission.167–171 Preclinical studies utilizing mGlu receptor ligands have demonstrated a role for groups I and II in modulating the discriminative stimulus effects of ethanol. Specifically, the interoceptive effects of a moderate ethanol dose (1.0 g/kg, intragastric) require activation of mGlu5, as systemic administration of MPEP decreased the sensitivity to the discriminative stimulus effects.172 Furthermore, NAC-specific infusion of the mGlu5−preferring agonist, CHPG, enhanced the discriminative stimulus effects of ethanol in a manner that was blocked by MPEP.173 Additionally, systemic administration of the m Glu1 NAM JNJ16259685 had no effect on the ethanol discriminative stimulus, consistent with the specific involvement of mGlu5.
Albeit less clear, the group II mGlu receptor subtypes also appear to be involved in ethanol interoception. Systemic activation with LY379268 or inhibition with LY341495 decreased the sensitivity to the discriminative stimulus effects of ethanol.150,174 Moreover, these effects may occur at specific central sites, as infusion of LY379268 in the amygdala, but not NAC, recapitulated the decreased sensitivity to ethanol’s interoceptive cue.150 Given that mGlu2/3 modulation also alters the discriminative stimulus effects of hallucinogens,175,176 these findings raise the possibility that mGlu2/3 ligands may induce inter-oceptive effects of their own.174 To the best of our knowledge, this hypothesis has not yet been tested and studies have not been designed to tease apart the relative contribution of mGlu2 versus mGlu3 in modulating ethanol’s discriminative stimulus effects.
Taken together, these studies suggest that mGlu receptor signaling can modulate (but not mediate) the interoceptive effects of ethanol, as ligands for these receptors neither substitute nor abolish ethanol’s discriminative stimulus.177 In contrast, NMDA receptor antagonists and GABAa receptor agonists can completely substitute for the discriminative stimulus effects of ethanol.178 Interestingly, mGlu5 may modulate the discriminative stimulus effects of ethanol through downstream interactions with the GABAa receptor.172 The more modest effects of mGlu receptor ligands might therefore be attributable to the slower kinetics of GPCR signaling relative to ligand-gated ion channels. Finally, activation of mGlu2/3 or mGlu5 in the NAC core restores the discriminative stimulus effects of ethanol following stress horm one exposure,179 suggesting these targets merit further study in preclinical models of stress-induced changes in ethanol seeking.180
Binge/Intoxication.
Episodic heavy drinking (blood alcohol content of >80 mg/dL) occurring in a short time span (2 h) can be hazardous and lead to increased susceptibility to developing an AUD.181 Thus, animal models have been developed to mimic this behavior. In the limited-access drinking-in-the-dark (DID) procedure, ethanol replaces water for a limited time (2–4 h) during the dark photoperiod,182 while in the scheduled high-alcohol consumption (SHAC) procedure, mice have daily limited access to water that is replaced by ethanol every fourth day.183 Utilizing these procedures, group I mGlu receptors and their scaffolding signaling proteins (Homer2, PI3K, and PLC), particularly within the NAC, have been implicated in modulating binge drinking.79,141 Furthermore, mGlu5/Homer2/PI3K/PKCε signaling in the NAC shell is functionally important in maintaining binge-like ethanol intake, as transgenic or pharmacological interruptions reduce ethanol intake in both DID and SHAC procedures.79,141,142 Additionally, basal mGlu1 and Homer2 expression in the NAC shell correlates with high binge drinking in a selectively bred high-DID mouse line.79,141 Group I mGlu receptor activity has also been implicated in the CEA. Expression of mGlu1, Homer2a/b, and PLC is enhanced in the CEA following DID, and infusions of mGlu1, mGlu5, or PLC inhibitors were found to decrease binge ethanol intake.139 Concurrent inhibition of mGlu1 and PLC produced additive effects, whereas mGlu5 and PLC inhibition did not, suggesting that mGlu5 may be the more relevant group I subtype in the CEA. Thus, on the basis of these reports, group I mGlu receptors in the NAC shell and CEA are recruited following binge-drinking paradigms, through distinct site-dependent alterations in mGlu receptor scaffolding and signaling proteins.
Given the functional role of mGlu5 in modulating ethanol consumption and interoception, it is no surprise that mGlu5 has been shown to modulate ethanol intoxication, as well. Antagonism of mGlu5 with the NAM MPEP enhanced the sedative and hypnotic effects of acute ethanol, as measured with spontaneous locomotor activity and ethanol-induced loss of righting reflex, respectively.184 In contrast, the mGlu1 antagonist CPCCOEt had no effects. The mGlu2/3 antagonist LY341495 attenuated ethanol’s sedative—hypnotic effects; however, the effects of mGlu2/3 agonists have not been reported. These findings indicate that while some classes of mGlu receptor modulators attenuate ethanol seeking, these mechanisms may have the potential to exacerbate intoxication while the targets are engaged.
Binge drinking and intoxication have the potential to disrupt cognition, potentially through inflammation signaling processes. Relatively few studies have examined whether mGlu receptor signaling may modulate this phenomenon, but both have focused on the group II mGlu receptor subtypes. Activation of mGlu2/3 was shown to be neuroprotective in a rat model of binge-like exposure (experim enter-adm inistered), and the treatment also rescued a deficit in spatial reversal learning.185 Additionally, activation of group II mGlu receptors rescued an acute ethanol-induced deficit in novel object recognition memory,186 an effect that was recapitulated by enhancing extracellular levels of N−acetylaspartylglutamate, a putative endogenous agonist for mGlu3. Studies using knockout mice confirmed that these pro-cognitive effects were mediated by activation of mGlu3 and not mGlu2. These studies are consistent with the breadth of literature demonstrating that mGlu3 potentiation exerts pro-cognitive effects in a variety of animal models.187 Thus, mGlu3 PAMs may provide a means to attenuate ethanol seeking and ameliorate co-occurring cognitive disturbances in AUD patient populations.
Withdrawal and Negative Affect.
AUD and negative affective symptoms are highly comorbid, particularly during periods of withdrawal and extended abstinence. Withdrawn alcoholics commonly list stress and negative affective states as potent triggers of cravings and relapse. The severity of negative affect is strongly correlated with relapse susceptibility, thus implicating negative affective behaviors in both the initiation and abstinence phases of AUDs. Most rodent species do not voluntarily drink enough ethanol to develop dependence; therefore, modeling withdrawal symptoms often necessitates noncontingent ethanol delivery through forced liquid diets or CIE vapor inhalation.188,189 Depressive symptoms, on the other hand, can be readily modeled in rodents following abstinence from voluntary ethanol drinking.190 The diverse mechanisms through which mGlu receptors modulate neurotransmission provide opportunities for mitigating hyperactive brain circuits associated with ethanol abstinence and many affective disorders. Indeed, group I NAMs and group II and III agonists have been shown to effectively reduce anxiety and depression in a variety of preclinical models (for a review, see ref 191). However, the effectiveness of targeting the mGlu receptor system for treating withdrawal-induced negative affective symptoms remains unclear.
Despite their convincing efficacy in attenuating ethanol self-administration and reinstatement, group I NAMs produced somewhat conflicting results in treating withdrawal-induced seizures. Blocking mGlu5 receptors successfully reduced audio-genic seizures in early withdrawal.145 In contrast, others have shown that MPEP had minimal effects on handling-induced convulsions following repeated cycles of withdrawal.192 These discrepancies can likely be attributed to variations in ethanol exposure procedures as well as the complex circuitry beyond mGlu receptors involved in producing withdrawal-induced seizures in rodents. A more recent study demonstrated that an mGlu5 NAM was effective in reducing the overall number of withdrawal-induced affective behaviors.193 While induced seizures have long been considered the gold standard for testing early withdrawal, implementing a battery of tests to assess withdrawal-induced affective behaviors may prove to be a more effective strategy for testing mGlu receptor NAMs and antagonists in future ethanol withdrawal studies. Indeed, ethanol-induced increases in mGlu1 receptor expression in the CEA,81,139 a region classically associated with affective disturbances, suggest a group I NAM may display efficacy in models of AUD-induced negative affect. While group I mGlu receptors appear to be involved in ethanol withdrawal, future studies are necessary tofully elucidate their therapeutic potential. Unfortunately, little is known about whether modulating group II or group III mGlu receptors may confer therapeutic benefits for treating withdrawal-induced negative affect. Given their involvement in depressive-like behavior and ethanol reward and reinforcement, these mGlu receptor subtypes certainly merit further investigation in animal models during withdrawal.
CLINICAL FINDINGS AND LOOKING TO THE FUTURE
Here, we have provided an overview of the neurocircuitry underlying AUD and a thorough description of how the mGlu receptor system modulates normal physiology and plasticity. In addition, we described how ethanol exposure dysregulates mGlu receptor function and the corresponding understanding of how modulating various mGlu receptor subtypes affects ethanol-related behaviors. While this burgeoning area of preclinical research provides excitement about the translation of novel therapeutics, very little is known about how mGlu receptor modulators might act in clinical AUD populations. Notwithstanding, some important genetic studies do corroborate these preclinical findings and support further efforts toward the translation of mGlu receptor modulators for AUD.
Single-nucleotide polymorphisms (SNPs) within the genes encoding both group I mGlu receptor subtypes (GrmI and Grm5) have been associated with the frequency of alcohol consumption and alcohol-related problems.194,195 Moreover, an association with a network of genes involved in downstream, postsynaptic plasticity was also discovered. In addition to these genetic studies, enhanced expression of mGlu5 in the amygdala has been observed in recovering AUD patients relative to controls,196 and changes in mGlu5 expression have been previously reported in smokers197 and in major depressive disorder.198 Consistent with the rich preclinical literature relating group II mGlu receptor function to ethanol seeking in rodent models, SNPs for Grm3 have also been related to alcohol dependence199 as well as substance use disorder. Finally, a genome-wide association study revealed that variations in Grm7 and Grm8 are linked to alcohol response phenotype,200 corroborating findings that enhancing mGlu7 function may attenuate alcohol seeking behaviors and providing a rationale for continuing to investigate mGlu8 in preclinical models.
In addition to these findings in AUD, SNPs for Grm3 and GrmI have been linked with schizophrenia, a disease that carries a high risk for a comorbid AUD diagnosis. Modulators for mGlu3 and m Glu1 are being developed and evaluated as potential treatments for schizophrenia,201 and the clinical genetic findings are intriguing for the potential treatment of patients with co-occurring AUDs. Along these lines, modulators of mGlu5 and mGlu8 have been examined in models of anxiety, and mGlu5 and mGlu7 PAMs are being considered for the treatment of autism spectrum disorders.7,26 These mechanisms provide the potential to rescue two related sets of symptoms with a single molecular approach, and this opportunity warrants further preclinical research into the etiology of comorbid AUD diagnoses. While the results have been mixed, several mGlu-directed compounds (i.e., mGlu2/3 agonists, mGlu2/3 NAMs, and mGlu5 NAMs) have undergone clinical trials for the treatment of schizophrenia, major depressive disorder, Fragile × syndrome, and other psychiatric diseases.201–204 Despite some initial successes in small-scale early phase trials, these mechanisms ultimately failed because of a lack of efficacy in expanded patient populations. Concerns with prior medication treatment histories and strong placebo effects have obfuscated potentially beneficial results; however, minimal adverse effect liability was observed throughout these trials. Because all mGlu modulators tested have been well-tolerated overall, their future use in the treatment of AUD still holds promise.
In summary, modulating a variety of components within the mGlu receptor system holds promise for the treatment of AUD. In particular, extensive preclinical evidence supports the development of molecules that inhibit mGlu1 and mGlu5 signaling as agents to attenuate alcohol seeking, consumption, and relapse. Additionally, positive modulators for mGlu2, mGlu3, or both may be effective in reducing alcohol seeking as well as stress-induced relapse. Finally, while the least is known about mGlu7 and mGlu8, the available preclinical literature suggests that PAMs of those receptors may be viable mechanisms for the development of novel AUD treatments. Continued mechanistic and translational research, in tandem, will be vital for the development of novel medications for AUD. The mGlu receptor system contains several exciting targets that may one day be applied as effective pharmaco-therapies.
ACKNOWLEDGMENTS
The authors thank members of the Conn and Winder laboratories for thoughtful and stimulating discussions concerning the contents of this review and Kendra Oliver for assistance with figure design.
Funding
This work was supported by National Institutes of Health (NIH) Grants R01MH062646 (P.J.C.), R37NS031373 (P.J.C.), and R01AA019455 (D.G.W.). M.E.J. was supported by a postdoctoral fellowship through the Pharmaceutical Research and Manufacturers of America Foundation.
ABBREVIATIONS
General Terminology
- AUD
alcohol use disorder
- CIE
chronic intermittent ethanol
- CNS
central nervous system
- CPP
conditioned place preference
- GPCR
G protein-coupled receptor
- LTD
long-term depression
- LTP
long-term potentiation
- NAM
negative allosteric modulator
- PAM
positive allosteric modulator
- SNP
single-nucleotide polymorphism
Targets and Neurotransmitters
- 2-AG
2-arachidonoylglycerol
- AEA
anandamide
- Akt
protein kinase B
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepro-pionic acid
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- cAMP
cyclic adenosine monophosphate
- CB1/2
cannabinoid receptor subtype 1/2
- eCB
endocannabinoid
- GABA
γ-aminobutyric acid
- Grm
glutamate receptor, metab-otropic (gene/transcript)
- mGlu
metabotropic glutamate receptor (protein)
- mTOR
mechanistic target of rapamycin
- NMDA
N−methyl-D-aspartate
- PI3K
phosphatidylinositide 3-kinase
- PLC
phospholipase C
- PKA
protein kinase A
- PKC
protein kinase C
- SK
small-conductance Ca2+−activated K+ channels
- TRPV1
transient receptor potential vanilloid 1
Anatomy
- BLA
basolateral amygdala
- BNST
bed nucleus of the stria terminalis
- CEA
central nucleus of the amygdala
- DLS
dorsolateral striatum
- DMS
dorsomedial striatum
- GP
globus pallidus
- IC
insular cortex
- IL
infralimbic PFC
- MC
motor cortex
- MSN
medium spiny neuron
- NAC
nucleus accumbens
- PFC
prefrontal cortex
- PL
prelimbic PFC
- SC CA1
Schaeffer collateral Cornu Ammonis 1 synapse
- SNC
substantia nigra pars compacta
- SNR
substantia nigra pars reticulate
- VH
ventral hippocampus
- VP
ventral pallidum
- VTA
ventral tegmental area
Footnotes
The authors declare the following competing financial interest(s): P.J.C. has been funded by the National Institutes of Health, AstraZeneca, Bristol-Myers Squibb, the Michael J. Fox Foundation, the Dystonia Medical Research Foundation, the CHDI Foundation, and the Thome Memorial Foundation. Over the past three years, he has served on the Scientific Advisory Boards for the Michael J. Fox Foundation, the Stanley Center for Psychiatric Research Broad Institute, Karuna Pharmaceuticals, the Lieber Institute for Brain Development, Clinical Mechanism and Proof of Concept Consortium, and the Neurobiology Foundation for Schizophrenia and Bipolar Disorder. P.J.C. is an inventor on patents that protect different classes of mGlu receptor allosteric modulators. M.E.J., S.W.C., A.A.J., and D.G.W. declare no conflicts of interest.
REFERENCES
- (1).Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, and Hasin DS (2015) Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 72, 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Abrahao KP, Salinas AG, and Lovinger DM (2017) Alcohol and the Brain: Neuronal Molecular Targets, Synapses, and Circuits. Neuron 96, 1223–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Ron D, and Barak S (2016) Molecular mechanisms underlying alcohol-drinking behaviours. Nat. Rev. Neurosci 17, 576–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Koob GF, and Le Moal M (1997) Drug abuse: hedonic homeostatic dysregulation. Science 278, 52–58. [DOI] [PubMed] [Google Scholar]
- (5).Cui C, Noronha A, Morikawa H, Alvarez VA, Stuber GD, Szumlinski KK, Kash TL, Roberto M, and Wilcox MV (2013) New insights on neurobiological mechanisms underlying alcohol addiction. Neuropharmacology 67, 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Niswender CM, and Conn PJ (2010) Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol 50, 295–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Yin S, and Niswender CM (2014) Progress toward advanced understanding of metabotropic glutamate receptors: structure, signaling and therapeutic indications. Cell. Signalling 26, 2284–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Pin JP, and Bettler B (2016) Organization and functions of mGlu and GABAB receptor complexes. Nature 540, 60–68. [DOI] [PubMed] [Google Scholar]
- (9).Julio-Pieper M, Flor PJ, Dinan TG, and Cryan JF (2011) Exciting times beyond the brain: metabotropic glutamate receptors in peripheral and non-neural tissues. Pharm acol Rev. 63, 35–58. [DOI] [PubMed] [Google Scholar]
- (10).Mannaioni G, Marino MJ, Valenti O, Traynelis SF, and Conn PJ (2001) Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. J. Neurosci 21, 5925–5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Ronesi JA, and Huber KM (2008) Homer interactions are necessary for metabotropic glutamate receptor-induced long-term depression and translational activation. J. Neurosci 28, 543–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Chergui K, Svenningsson P, and Greengard P (2005) Physiological role for casein kinase 1 in glutamatergic synaptic transmission. J. Neurosci 25, 6601–6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Bortolotto ZA, and Collingridge GL (1998) Involvement of calcium/calmodulin-dependent protein kinases in the setting of a molecular switch involved in hippocampal LTP. Neuropharmacology 37, 535–544. [DOI] [PubMed] [Google Scholar]
- (14).Stansley BJ, Joffe ME, Nicoletti F, Lindsley CW, Niswender CM, and Conn PJ (2017) Modulation of mGlu3−induced LTP in the hippocampus by mGlu5. 9th International Conference on Metabotropic Glutamate Receptors, Taormina, Italy. [Google Scholar]
- (15).Benquet P, Gee CE, and Gerber U (2002) Two distinct signaling pathways upregulate NMDA receptor responses via two distinct metabotropic glutamate receptor subtypes. J. Neurosci 22, 9679–9686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Heidinger V, Manzerra P, Wang XQ, Strasser U, Yu SP, Choi DW, and Behrens MM (2002) Metabotropic glutamate receptor 1-induced upregulation of NMDA receptor current: mediation through the Pyk2/Src-family kinase pathway in cortical neurons. J. Neurosci 22, 5452–5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Hou L, and Klann E (2004) Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J. Neurosci 24, 6352–6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Chen Y, Granger AJ, Tran T, Saulnier JL, Kirkwood A, and Sabatini BL (2017) Endogenous Galphaq-Coupled Neuro-modulator Receptors Activate Protein Kinase A. Neuron 96, 1070–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Boss V, and Conn PJ (1992) Metabotropic excitatory amino acid receptor activation stimulates phospholipase D in hippocampal slices. J. Neurochem 59, 2340–2343. [DOI] [PubMed] [Google Scholar]
- (20).Saugstad JA, Marino MJ, Folk JA, Hepler JR, and Conn PJ (1998) RGS4 inhibits signaling by group I metabotropic glutamate receptors. J. Neurosci 18, 905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Cannady R, McGonigal JT, Newsom RJ, Woodward JJ, Mulholland PJ, and Gass JT (2017) Prefrontal Cortex KCa2 Channels Regulate mGlu5-Dependent Plasticity and Extinction of Alcohol-Seeking Behavior. J. Neurosci 37, 4359–4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Awad H, Hubert GW, Smith Y, Levey AI, and Conn PJ (2000) Activation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus. J. Neurosci 20, 7871–7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, and Worley PF (1999) Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron 23, 583–592. [DOI] [PubMed] [Google Scholar]
- (24).Castillo PE, Younts TJ, Chavez AE, and Hashimotodani Y (2012) Endocannabinoid signaling and synaptic function. Neuron 76, 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Luscher C, and Huber KM (2010) Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron 65, 445–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Senter RK, Ghoshal A, Walker AG, Xiang Z, Niswender CM, and Conn PJ (2016) The Role of mGlu Receptors in Hippocampal Plasticity Deficits in Neurological and Psychiatric Disorders: Implications for Allosteric Modulators as Novel Therapeutic Strategies,. Curr. N europharm acol 14, 455–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Ayala JE, Chen Y, Banko JL, Sheffler DJ, Williams R, Telk AN, Watson NL, Xiang Z, Zhang Y, Jones PJ, Lindsley CW, Olive MF, and Conn PJ (2009) mGluR5 positive allosteric modulators facilitate both hippocampal LTP and LTD and enhance spatial learning. Neuropsychopharmacology 34, 2057–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Grueter BA, Gosnell HB, Olsen CM, Schramm-Sapyta NL, Nekrasova T, Landreth GE, and Winder DG (2006) Extracellular-signal regulated kinase 1-dependent metabotropic gluta-mate receptor 5-induced long-term depression in the bed nucleus of the stria terminalis is disrupted by cocaine administration. J. Neurosci 26, 3210–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Grueter BA, McElligott ZA, Robison AJ, Mathews GC, and Winder DG (2008) In vivo metabotropic glutamate receptor 5 (mGluR5) antagonism prevents cocaine-induced disruption of postsynaptically maintained mGluR5-dependent long-term depression. J. Neurosci 28, 9261–9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).McElligott ZA, Klug JR, Nobis WP, Patel S, Grueter BA, Kash TL, and Winder DG (2010) Distinct forms of Gq-receptor-dependent plasticity of excitatory transmission in the BNST are differentially affected by stress. Proc. Natl. Acad. Sci. U. S. A 107, 2271–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Bellone C, and Luscher C (2006) Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat. Neurosci 9, 636–641. [DOI] [PubMed] [Google Scholar]
- (32).Mameli M, Balland B, Lujan R, and Luscher C (2007) Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science 317, 530–533. [DOI] [PubMed] [Google Scholar]
- (33).Huber KM, Kayser MS, and Bear MF (2000) Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science 288, 1254–1256. [DOI] [PubMed] [Google Scholar]
- (34).Karachot L, Shirai Y, Vigot R, Yamamori T, and Ito M (2001) Induction of long-term depression in cerebellar Purkinje cells requires a rapidly turned over protein. J. Neurophysiol 86, 280–289. [DOI] [PubMed] [Google Scholar]
- (35).Nosyreva ED, and Huber KM (2006) Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile × syndrome. J. Neurophysiol 95, 3291–3295. [DOI] [PubMed] [Google Scholar]
- (36).Moult PR, Correa SA, Collingridge GL, Fitzjohn SM, and Bashir ZI (2008) Co-activation of p38 mitogen-activated protein kinase and protein tyrosine phosphatase underlies metabo-tropic glutamate receptor-dependent long-term depression. J. Physiol 586, 2499–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Hou L, Antion MD, Hu D, Spencer CM, Paylor R, and Klann E (2006) Dynamic translational and proteasomal regulation of fragile × mental retardation protein controls mGluR-dependent long-term depression. Neuron 51, 441–454. [DOI] [PubMed] [Google Scholar]
- (38).Davidkova G, and Carroll RC (2007) Characterization of the role of microtubule-associated protein 1B in metabotropic glutamate receptor-mediated endocytosis of AMPA receptors in hippocampus. J. Neurosci 27, 13273–13278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, and Huber KM (2008) Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron 59, 84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Stansley BJ, Fisher NM, Gogliotti RG, Lindsley CW, Conn PJ, and Niswender CM (2017) Contextual Fear Extinction Induces Hippocampal Metaplasticity Mediated by Metabotropic Glutamate Receptor 5. Cereb Cortex, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Chevaleyre V, and Castillo PE (2004) Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron 43, 871–881. [DOI] [PubMed] [Google Scholar]
- (42).Xu J, Antion MD, Nomura T, Kraniotis S, Zhu Y, and Contractor A (2014) Hippocampal metaplasticity is required for the formation of temporal associative memories. J. Neurosci 34, 16762–16773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Martin HGS, Lassalle O, and Manzoni OJ (2017) Differential Adulthood Onset mGlu5 Signaling Saves Prefrontal Function in the Fragile × Mouse. Cereb. Cortex 27, 5592–5602. [DOI] [PubMed] [Google Scholar]
- (44).Lafourcade M, Elezgarai I, Mato S, Bakiri Y, Grandes P, and Manzoni OJ (2007) Molecular components and functions of the endocannabinoid system in mouse prefrontal cortex. PLoS One 2, No. e709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Gubellini P, Saulle E, Centonze D, Bonsi P, Pisani A, Bernardi G, Conquet F, and Calabresi P (2001) Selective involvement of mGlu1 receptors in corticostriatal LTD. Neuro-pharmacology 40, 839–846. [DOI] [PubMed] [Google Scholar]
- (46).Lerner TN, and Kreitzer AC (2012) RGS4 is required for dopaminergic control of striatal LTD and susceptibility to parkinsonian motor deficits. Neuron 73, 347–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Grueter BA, Brasnjo G, and Malenka RC (2010) Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat. Neurosci 13, 1519–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Robbe D, Kopf M, Remaury A, Bockaert J, and Manzoni OJ (2002) Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc. Natl. Acad. Sci. U. S. A 99, 8384–8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Fourgeaud L, Mato S, Bouchet D, Hemar A, Worley PF, and Manzoni OJ (2004) A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens,. J. Neurosci 24, 6939–6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).McCutcheon JE, Loweth JA, Ford KA, Marinelli M, Wolf ME, and Tseng KY (2011) Group I mGluR activation reverses cocaine-induced accumulation of calcium-permeable AMPA receptors in nucleus accumbens synapses via a protein kinase C-dependent mechanism. J. Neurosci 31, 14536–14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Turner BD, Rook JM, Lindsley CW, Conn PJ, and Grueter BA (2018) mGlu1 and mGlu5 modulate distinct excitatory inputs to the nucleus accumbens shell. Neuropsychopharmacology, n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Valentinova K, and Mameli M (2016) mGluR-LTD at Excitatory and Inhibitory Synapses in the Lateral Habenula Tunes Neuronal Output. Cell Rep. 16, 2298–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Kreitzer AC, and Regehr WG (2001) Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron 29, 717–727. [DOI] [PubMed] [Google Scholar]
- (54).Yin HH, Davis MI, Ronesi JA, and Lovinger DM (2006) The role of protein synthesis in striatal long-term depression,. J. Neurosci 26, 11811–11820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Younts TJ, Monday HR, Dudok B, Klein ME, Jordan BA, Katona I, and Castillo PE (2016) Presynaptic Protein Synthesis Is Required for Long-Term Plasticity of GABA Release. Neuron 92, 479–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Mato S, Lafourcade M, Robbe D, Bakiri Y, and Manzoni OJ (2008) Role of the cyclic-AMP/PKA cascade and of P/Q-type Ca++ channels in endocannabinoid-mediated long-term depression in the nucleus accumbens. Neuropharmacology 54, 87–94. [DOI] [PubMed] [Google Scholar]
- (57).Gibson HE, Edwards JG, Page RS, Van Hook MJ, and Kauer JA (2008) TRPV1 channels mediate long-term depression at synapses on hippocampal interneurons. Neuron 57, 746–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Foster DJ, Wilson JM, Remke DH, Mahmood MS, Uddin MJ, Wess J, Patel S, Marnett LJ, Niswender CM, Jones CK, Xiang Z, Lindsley CW, Rook JM, and Conn PJ (2016) Antipsychotic-like Effects of M4 Positive Allosteric Modulators Are Mediated by CB2 Receptor-Dependent Inhibition of Dopamine Release. Neuron 91, 1244–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Mateo Y, Johnson KA, Covey DP, Atwood BK, Wang HL, Zhang S, Gildish I, Cachope R, Bellocchio L, Guzman M, M orales M, Cheer JF, and Lovinger DM (2017) Endocannabinoid Actions on Cortical Terminals Orchestrate Local Modulation of Dopamine Release in the Nucleus Accumbens. Neuron 96, 1112–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Xiao B, Tu JC, Petralia RS, Yuan JP, Doan A, Breder CD, Ruggiero A, Lanahan AA, Wenthold RJ, and Worley PF (1998) Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron 21, 707–716. [DOI] [PubMed] [Google Scholar]
- (61).Loweth JA, Scheyer AF, Milovanovic M, LaCrosse AL, Flores-Barrera E, Werner CT, Li X, Ford KA, Le T, Olive MF, Szumlinski KK, Tseng KY, and Wolf ME (2014) Synaptic depression via mGluR1 positive allosteric modulation suppresses cue-induced cocaine craving. Nat. Neurosci 17, 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Yin HH, Park BS, Adermark L, and Lovinger DM (2007) Ethanol reverses the direction of long-term synaptic plasticity in the dorsomedial striatum. Eur. J. Neurosci 25, 3226–3232. [DOI] [PubMed] [Google Scholar]
- (63).Weitlauf C, Egli RE, Grueter BA, and Winder DG (2004) High-frequency stimulation induces ethanol-sensitive long-term potentiation at glutamatergic synapses in the dorsolateral bed nucleus of the stria terminalis. J. Neurosci 24, 5741–5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Sinclair JG, and Lo GF (1986) Ethanol blocks tetanic and calcium-induced long-term potentiation in the hippocampal slice. Gen. Pharmacol 17, 231–233. [DOI] [PubMed] [Google Scholar]
- (65).Nie Z, Madamba SG, and Siggins GR (1994) Ethanol inhibits glutamatergic neurotransmission in nucleus accumbens neurons by multiple mechanisms. J. Pharmacol. Exp. Ther 271, 1566–1573. [PubMed] [Google Scholar]
- (66).Pyapali GK, Turner DA, Wilson WA, and Swartzwelder HS (1999) Age and dose-dependent effects of ethanol on the induction of hippocampal long-term potentiation. Alcohol 19, 107–ill. [DOI] [PubMed] [Google Scholar]
- (67).Blitzer RD, Gil O, and Landau EM (1990) Long-term potentiation in rat hippocampus is inhibited by low concentrations of ethanol. Brain Res. 537, 203–208. [DOI] [PubMed] [Google Scholar]
- (68).Mishra D, Zhang X, and Chergui K (2012) Ethanol disrupts the mechanisms of induction of long-term potentiation in the mouse nucleus accumbens. Alcohol.: Clin. Exp. Res 36, 2117–2125. [DOI] [PubMed] [Google Scholar]
- (69).Schotanus SM, and Chergui K (2008) Dopamine Dl receptors and group I metabotropic glutamate receptors contribute to the induction of long-term potentiation in the nucleus accumbens. Neuropharmacology 54, 837–844. [DOI] [PubMed] [Google Scholar]
- (70).Renteria R, Jeanes ZM, and Morrisett RA (2014) Ethanol attenuation of long-term depression in the nucleus accumbens can be overcome by activation of TRPVl receptors. Alcohol.: Clin. Exp. Res 38, 2763–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Carta M, Mameli M, and Valenzuela CF (2006) Alcohol potently modulates climbing fiber-Purkinje neuron synapses: role of metabotropic glutamate receptors. J. Neurosci 26, 1906–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Su LD, Sun CL, and Shen Y (2010) Ethanol acutely modulates mGluRl-dependent long-term depression in cerebellum. Alcohol.: Clin. Exp. Res 34, 1140–1145. [DOI] [PubMed] [Google Scholar]
- (73).Minami K, Gereau R. W. t., Minami M, Heinemann SF, and Harris RA (1998) Effects of ethanol and anesthetics on type l and 5 metabotropic glutamate receptors expressed in Xenopus laevis oocytes. Mol. Pharmacol 53, 148–156. [DOI] [PubMed] [Google Scholar]
- (74).Xia JX, Li J, Zhou R, Zhang XH, Ge YB, and Ru Yuan X (2006) Alterations of rat corticostriatal synaptic plasticity after chronic ethanol exposure and withdrawal. Alcohol.: Clin. Exp. Res 30, 819–824. [DOI] [PubMed] [Google Scholar]
- (75).DePoy L, Daut R, Brigman JL, MacPherson K, Crowley N, Gunduz-Cinar O, Pickens CL, Cinar R, Saksida LM, Kunos G, Lovinger DM, Bussey TJ, Camp MC, and Holmes A (2013) Chronic alcohol produces neuroadaptations to prime dorsal striatal learning. Proc. Natl. Acad. Sci. U. S. A 110, 14783–14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Wills TA, Baucum AJ 2nd, Holleran KM, Chen Y, Pasek JG, Delpire E, Tabb DL, Colbran RJ, and Winder DG (2017) Chronic intermittent alcohol disrupts the GluN2B-associated proteome and specifically regulates group I mGlu receptor-dependent long-term depression. Addict Biol. 22, 275–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Szumlinski KK, Lominac KD, Oleson EB, Walker JK, Mason A, Dehoff MH, Klugmann M, Cagle S, Welt K, During M, Worley PF, Middaugh LD, and Kalivas PW (2005) Homer2 is necessary for EtOH-induced neuroplasticity. J. Neurosci 25, 7054–7061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Szumlinski KK, Ary AW, Lominac KD, Klugmann M, and Kippin TE (2008) Accumbens Homer2 overexpression facilitates alcohol-induced neuroplasticity in C 57BL/6J mice. Neuropsychopharmacology 33, 1365–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Cozzoli DK, Goulding SP, Zhang PW, Xiao B, Hu JH, Ary AW, Obara I, Rahn A, Abou-Ziab H, Tyrrel B, Marini C, Yoneyama N, Metten P, Snelling C, Dehoff MH, Crabbe JC, Finn DA, Klugmann M, Worley PF, and Szumlinski KK (2009) Binge drinking upregulates accumbens mGluR5-Homer2-PI3K signaling: functional implications for alcoholism. J. Neurosci 29, 8655–8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Haider A, Woodward NC, Lominac KD, Sacramento AD, Klugmann M, Bell RL, and Szumlinski KK (2015) Homer2 within the nucleus accumbens core bidirectionally regulates alcohol intake by both P and Wistar rats. Alcohol 49, 533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Obara I, Bell RL, Goulding SP, Reyes CM, Larson LA, Ary AW, Truitt WA, and Szumlinski KK (2009) Differential effects of chronic ethanol consumption and withdrawal on homer/glutamate receptor expression in subregions of the accumbens and amygdala of P rats. Alcohol.: Clin. Exp. Res 33, 1924–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Quadir SG, Santos JR, Campbell RR, Wroten MG, Singh N, Holloway JJ, Bal SK, Camarini R, and Szumlinski KK (2016) Homer2 regulates alcohol and stress cross-sensitization. Addict Biol. 21, 613–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Lee KM, Coelho MA, Class MA, and Szumlinski KK (2018) mGlu5-dependent modulation of anxiety during early withdrawal from binge-drinking in adult and adolescent male mice. Drug Alcohol Depend. 184, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Di Menna L, Joffe ME, Iacovelli L, Orlando R, Lindsley CW, Mairesse J, Gressens P, Cannella M, Caraci F, Copani A, Bruno V, Battaglia G, Conn PJ, and Nicoletti F (2018) Functional partnership between mGlu3 and mGlu5 metabotropic glutamate receptors in the central nervous system. Neuropharmacology 128, 301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Niswender CM, Johnson KA, Luo Q, Ayala JE, Kim C, Conn PJ, and Weaver CD (2008) A novel assay of Gi/o-linked G protein-coupled receptor coupling to potassium channels provides new insights into the pharmacology of the group III metabotropic glutamate receptors,. Mol. Pharmacol 73, 1213–1224. [DOI] [PubMed] [Google Scholar]
- (86).Robbe D, Alonso G, Chaumont S, Bockaert J, and Manzoni OJ (2002) Role of p/q-Ca2+ channels in metabotropic glutamate receptor 2/3-dependent presynaptic long-term depression at nucleus accumbens synapses. J. Neurosci 22, 4346–4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Jin LE, Wang M, Galvin VC, Lightbourne TC, Conn PJ, Arnsten AF, and Paspalas CD (2017) mGluR2 versus mGluR3Metabotropic Glutamate Receptors in Primate Dorsolateral Prefrontal Cortex: Postsynaptic mGluR3 Strengthen Working Memory Networks. Cereb. Cortex 28, 974–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Joffe ME, Santiago CI, Engers JL, Lindsley CW, and Conn PJ (2017) Metabotropic glutamate receptor subtype 3 gates acute stress-induced dysregulation of amygdalo-cortical function. Mol. Psychiatry, n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Rosenberg N, Gerber U, and Ster J (2016) Activation of Group II Metabotropic Glutamate Receptors Promotes LTP Induction at Schaffer Collateral-CAl Pyramidal Cell Synapses by Priming NMDA Receptors. J. Neurosci 36, 11521–11531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Sun W, McConnell E, Pare JF, Xu Q, Chen M, Peng W, Lovatt D, Han X, Smith Y, and Nedergaard M (2013) Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science 339, 197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Fazio F, Ulivieri M, Volpi C, Gargaro M, and Fallarino F (2018) Targeting metabotropic glutamate receptors for the treatment of neuroinflammation. Curr. Opin. Pharmacol 38, 16–23. [DOI] [PubMed] [Google Scholar]
- (92).Johnson KA, Mateo Y, and Lovinger DM (2017) Metabotropic glutamate receptor 2 inhibits thalamically-driven glutamate and dopamine release in the dorsal striatum. Neuro-pharmacology 117, 114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Lovinger DM, and McCool BA (1995) Metabotropic glutamate receptor-mediated presynaptic depression at corticostriatal synapses involves mGLuR2 or 3. J. Neurophysiol 73, 1076–1083. [DOI] [PubMed] [Google Scholar]
- (94).Li C, and Rainnie DG (2014) Bidirectional regulation of synaptic plasticity in the basolateral amygdala induced by the Dl-like family of dopamine receptors and group II metabotropic glutamate receptors. J. Physiol 592, 4329–4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Lucas SJ, Bortolotto ZA, Collingridge GL, and Lodge D (2013) Selective activation of either mGlu2 or mGlu3 receptors can induce LTD in the amygdala. Neuropharmacology 66, 196–201. [DOI] [PubMed] [Google Scholar]
- (96).Nicholls RE, Zhang XL, Bailey CP, Conklin BR, Kandel ER, and Stanton PK (2006) mGluR2 acts through inhibitory Galpha subunits to regulate transmission and long-term plasticity at hippocampal mossy fiber-CA3 synapses. Proc. Natl. Acad. Sci. U. S. A 103, 6380–6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Joffe ME, Santiago CI, Engers JL, Lindsley CW, and Conn PJ (2018) Efficacy of negative allosteric modulators of mGlu2 and mGlu3 in a rodent model of major depressive disorder. The American Society for Pharmacology and Experimental Therapeutics Annual Meeting, San Diego, CA. [Google Scholar]
- (98).Kupferschmidt DA, and Lovinger DM (2015) Inhibition of presynaptic calcium transients in cortical inputs to the dorsolateral striatum by metabotropic GABA(B) and mGlu2/3 receptors. J. Physiol 593, 2295–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Walker AG, Wenthur CJ, Xiang Z, Rook JM, Emmitte KA, Niswender CM, Lindsley CW, and Conn PJ (2015) Metabotropic glutamate receptor 3 activation is required for long-term depression in medial prefrontal cortex and fear extinction. Proc. Natl. Acad. Sci. U. S. A 112, 1196–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Joffe ME, Santiago CI, Stansley BJ, Gogliotti RG, Engers JL, Nicoletti F, Lindsley CW, and Conn PJ (2017) Metabotropic glutamate receptor subtype 3 gates acute stress-induced dysregulation of amygdalo-cortical function. 9th International Conference on Metabotropic Glutamate Receptors, Taormina, Italy. [Google Scholar]
- (101).Walker AG, Sheffler DJ, Lewis AS, Dickerson JW, Foster DJ, Senter RK, Moehle MS, Lv X, Stansley BJ, Xiang Z, Rook JM, Emmitte KA, Lindsley CW, and Conn PJ (2017) Co-Activation of Metabotropic Glutamate Receptor 3 and Beta-Adrenergic Receptors Modulates Cyclic-AMP and Long-Term Potentiation, and Disrupts Memory Reconsolidation. Neuropsycho-pharmacology 42, 2553–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Winder DG, Ritch PS, Gereau R W. t., and Conn, P. J. (1996) Novel glial-neuronal signalling by coactivation of metabotropic glutamate and beta-adrenergic receptors in rat hippocampus. J. Physiol 494 (Part 3), 743–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).Turner JP, and Salt TE (1999) Group III metabotropic glutamate receptors control corticothalamic synaptic transmission in the rat thalamus in vitro. J. Physiol 519, 481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (104).Zhang C, and Marek GJ (2006) Group III metabotropic glutamate receptor agonists selectively suppress excitatory synaptic currents in the rat prefrontal cortex induced by 5-hydroxytryptami-ne2A receptor activation. J. Pharmacol. Exp. Ther 320, 437–447. [DOI] [PubMed] [Google Scholar]
- (105).Jones PJ, Xiang Z, and Conn PJ (2008) Metabotropic glutamate receptors mGluR4 and mGluR8 regulate transmission in the lateral olfactory tract-piriform cortex synapse. Neuropharmacology 55, 440–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Chardonnet S, Bessiron T, Ramos CI, Dammak R, Richard MA, Boursier C, Cadilhac C, Coquelle FM, Bossi S, Ango F, Le Marechal P, Decottignies P, Berrier C, McLean H, and Daniel H (2017) Native metabotropic glutamate receptor 4 depresses synaptic transmission through an unusual Galphaq transduction pathway. Neuropharmacology 121, 247–260. [DOI] [PubMed] [Google Scholar]
- (107).Bradley SR, Rees HD, Yi H, Levey AI, and Conn PJ (1998) Distribution and developmental regulation of metabotropic glutamate receptor 7a in rat brain. J. Neurochem 71, 636–645. [DOI] [PubMed] [Google Scholar]
- (108).Schoepp DD, Jane DE, and Monn JA (1999) Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology 38, 1431–1476. [DOI] [PubMed] [Google Scholar]
- (109).Fendt M, Imobersteg S, Peterlik D, Chaperon F, Mattes C, Wittmann C, Olpe HR, Mosbacher J, Vranesic I, van der Putten H, McAllister KH, Flor PJ, and Gee CE (2013) Differential roles of mGlu(7) and mGlu(8) in amygdala-dependent behavior and physiology. Neuropharmacology 72, 215–223. [DOI] [PubMed] [Google Scholar]
- (110).Klar R, Walker AG, Ghose D, Grueter BA, Engers DW, Hopkins CR, Lindsley CW, Xiang Z, Conn PJ, and Niswender CM (2015) Activation of Metabotropic Glutamate Receptor 7 Is Required for Induction of Long-Term Potentiation at SC-CA1 Synapses in the Hippocampus. J. Neurosci 35, 7600–7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Saugstad JA, Kinzie JM, Shinohara MM, Segerson TP, and Westbrook GL (1997) Cloning and expression of rat metabotropic glutamate receptor 8 reveals a distinct pharmacological profile. Mol. Pharmacol 51, 119–125. [DOI] [PubMed] [Google Scholar]
- (112).Gosnell HB, Silberman Y, Grueter BA, Duvoisin RM, Raber J, and Winder DG (2011) mGluR8 modulates excitatory transmission in the bed nucleus of the stria terminalis in a stress-dependent manner. Neuropsychopharmacology 36, 1599–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Grueter BA, and Winder DG (2005) Group II and III metabotropic glutamate receptors suppress excitatory synaptic transmission in the dorsolateral bed nucleus of the stria terminalis. Neuropsychopharmacology 30, 1302–1311. [DOI] [PubMed] [Google Scholar]
- (114).Mercier MS, Lodge D, Fang G, Nicolas CS, Collett VJ, Jane DE, Collingridge GL, and Bortolotto ZA (2013) Characterisation of an mGlu8 receptor-selective agonist and antagonist in the lateral and medial perforant path inputs to the dentate gyrus. Neuropharmacology 67, 294–303. [DOI] [PubMed] [Google Scholar]
- (115).Griffin WC, Ramachandra VS, Knackstedt LA, and Becker HC (2015) Repeated cycles of chronic intermittent ethanol exposure increases basal glutamate in the nucleus accumbens of mice without affecting glutamate transport. Front. Pharmacol 6, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Pati D, Kelly K, Stennett B, Frazier CJ, and Knackstedt LA (2016) Alcohol consumption increases basal extracellular glutamate in the nucleus accumbens core of Sprague-Dawley rats without increasing spontaneous glutamate release. Eur. J. Neurosci 44, 1896–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (117).Griffin WC III, Haun HL, Hazelbaker CL, Ramachandra VS, and Becker HC (2014) Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice. Neuropsychopharmacology 39, 707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (118).Meinhardt MW, Hansson AC, Perreau-Lenz S, Bauder-Wenz C, Stahlin O, Heilig M, Harper C, Drescher KU, Spanagel R, and Sommer WH (2013) Rescue of infralimbic mGluR2 deficit restores control over drug-seeking behavior in alcohol dependence. J. Neurosci 33, 2794–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (119).Ding ZM, Ingraham CM, Hauser SR, Lasek AW, Bell RL, and McBride WJ (2017) Reduced Levels of mGlu2 Receptors within the Prelimbic Cortex Are Not Associated with Elevated Glutamate Transmission or High Alcohol Drinking. Alcohol.: Clin. Exp. Res 41, 1896–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (120).Kufahl PR, Martin-Fardon R, and Weiss F (2011) Enhanced sensitivity to attenuation of conditioned reinstatement by the mGluR 2/3 agonist LY379268 and increased functional activity of mGluR 2/3 in rats with a history of ethanol dependence. Neuropsychopharmacology 36, 2762–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (121).Conn PJ, Lindsley CW, Meiler J, and Niswender CM (2014) Opportunities and challenges in the discovery of allosteric modulators of GPCRs for treating CNS disorders. Nat. Rev. Drug Discovery 13, 692–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (122).Joffe ME, Grueter CA, and Grueter BA (2014) Biological substrates of addiction. Wiley Interdiscip Rev. Cogn Sci. 5, 151–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Giuliano C, Pena-Oliver Y, Goodlett CR, Cardinal RN, Robbins TW, Bullmore ET, Belin D, and Everitt BJ (2018) Evidence for a Long-Lasting Compulsive Alcohol Seeking Phenotype in Rats. Neuropsychopharmacology 43, 728–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (124).Hopf FW, and Lesscher HM (2014) Rodent models for compulsive alcohol intake. Alcohol 48, 253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (125).Radke AK, Jury NJ, Kocharian A, Marcinkiewcz CA, Lowery-Gionta EG, Pleil KE, McElligott ZA, McKlveen JM, Kash TL, and Holmes A (2017) Chronic EtOH effects on putative measures of compulsive behavior in mice. Addict Biol. 22, 423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (126).Backstrom P, Bachteler D, Koch S, Hyytia P, and Spanagel R (2004) mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology 29, 921–928. [DOI] [PubMed] [Google Scholar]
- (127).Besheer J, Faccidomo S, Grondin JJ, and Hodge CW (2008) Regulation of motivation to self-administer ethanol by mGluR5 in alcohol-preferring (P) rats. Alcohol.: Clin. Exp. Res 32, 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).Besheer J, Grondin JJ, Cannady R, Sharko AC, Faccidomo S, and Hodge CW (2010) Metabotropic glutamate receptor 5 activity in the nucleus accumbens is required for the maintenance of ethanol self-administration in a rat genetic model of high alcohol intake. Biol. Psychiatry 67, 812–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (129).Bird MK, Kirchhoff J, Djouma E, and Lawrence AJ (2008) Metabotropic glutamate 5 receptors regulate sensitivity to ethanol in mice. Int. J. Neuropsychopharmacol 11, 765–774. [DOI] [PubMed] [Google Scholar]
- (130).Cowen MS, Djouma E, and Lawrence AJ (2005) The metabotropic glutamate 5 receptor antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine reduces ethanol self-administration in multiple strains of alcohol-preferring rats and regulates olfactory glutamatergic systems,. J. Pharmacol. Exp. Ther 315, 590–600. [DOI] [PubMed] [Google Scholar]
- (131).Gupta T, Syed YM, Revis AA, Miller SA, Martinez M, Cohn KA, Demeyer MR, Patel KY, Brzezinska WJ, and Rhodes JS (2008) Acute effects of acamprosate and MPEP on ethanol Drinking-in-the-Dark in male C 57BL/6J mice. Alcohol.: Clin. Exp. Res 32, 1992–1998. [DOI] [PubMed] [Google Scholar]
- (132).Hodge CW, Miles MF, Sharko AC, Stevenson RA, Hillmann JR, Lepoutre V, Besheer J, and Schroeder JP (2006) The mGluR5 antagonist MPEP selectively inhibits the onset and maintenance of ethanol self-administration in C 57B L/6J mice,. Psychopharmacology (B erl) 183, 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (133).Lominac KD, Kapasova Z, Hannun RA, Patterson C, Middaugh LD, and Szumlinski KK (2006) Behavioral and neurochemical interactions between Group 1 mGluR antagonists and ethanol: potential insight into their anti-addictive properties. Drug Alcohol Depend. 85, 142–156. [DOI] [PubMed] [Google Scholar]
- (134).McMillen BA, Crawford MS, Kulers CM, and Williams HL (2005) Effects of a metabotropic, mglu5, glutamate receptor antagonist on ethanol consumption by genetic drinking rats. Alcohol Alcohol. 40, 494–497. [DOI] [PubMed] [Google Scholar]
- (135).Olive MF, McGeehan AJ, Kinder JR, McMahon T, Hodge CW, Janak PH, and Messing RO (2004) The mGluR5 antagonist 6-methyl-2-(phenylethynyl)pyridine decreases ethanol consumption via a protein kinase C epsilon-dependent mechanism,. Mol. Pharmacol 67, 349–355. [DOI] [PubMed] [Google Scholar]
- (136).Schroeder JP, Overstreet DH, and Hodge CW (2005) The mGluR5 antagonist MPEP decreases operant ethanol self-administration during maintenance and after repeated alcohol deprivations in alcohol-preferring (P) rats,. Psychopharmacology (Berl) 179, 262–270. [DOI] [PubMed] [Google Scholar]
- (137).Sidhpura N, Weiss F, and Martin-Fardon R (2010) Effects of the mGlu2/3 agonist LY379268 and the mGlu5 antagonist MTEP on ethanol seeking and reinforcement are differentially altered in rats with a history of ethanol dependence. Biol. Psychiatry 67, 804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (138).Cowen MS, Krstew E, and Lawrence AJ (2007) Assessing appetitive and consummatory phases of ethanol self-administration in C57BL/6J mice under operant conditions: regulation by mGlu5 receptor antagonism. Psychopharmacology (Berl) 190, 21–29. [DOI] [PubMed] [Google Scholar]
- (139).Cozzoli DK, Courson J, Wroten MG, Greentree DI, Lum EN, Campbell RR, Thompson AB, Maliniak D, Worley PF, Jonquieres G, Klugmann M, Finn DA, and Szumlinski KK (2014) Binge alcohol drinking by mice requires intact group 1 metabotropic glutamate receptor signaling within the central nucleus of the amygdala. Neuropsychopharmacology 39, 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (140).Gass JT, and Olive MF (2009) Role of protein kinase C epsilon (PKCvarepsilon) in the reduction of ethanol reinforcement due to mGluR5 antagonism in the nucleus accumbens shell. Psychopharmacology (Berl) 204, 587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (141).Cozzoli DK, Courson J, Caruana AL, Miller BW, Greentree DI, Thompson AB, Wroten MG, Zhang PW, Xiao B, Hu JH, Klugmann M, Metten P, Worley PF, Crabbe JC, and Szumlinski KK (2012) Nucleus accumbens mGluR5-associated signaling regulates binge alcohol drinking under drinking-in-the-dark procedures. Alcohol.: Clin. Exp. Res 36, 1623–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (142).Cozzoli DK, Strong-Kaufman MN, Tanchuck MA, Hashimoto JG, Wiren KM, and Finn DA (2014) The Effect of mGluR5 Antagonism During Binge Drinkingon Subsequent Ethanol Intake in C57BL/6J Mice: Sex- and Age-Induced Differences,. Alcohol.: Clin. Exp. Res 38, 730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (143).McGeehan AJ, and Olive MF (2003) The mGluR5 antagonist MPEP reduces the conditioned rewarding effects of cocaine but not other drugs of abuse,. Synapse 47, 240–242. [DOI] [PubMed] [Google Scholar]
- (144).Lee JY, Choe ES, Yang CH, Choi KH, Cheong JH, Jang CG, Seo JW, and Yoon SS (2016) The mGluR5 antagonist MPEP suppresses the expression and reinstatement, but not the acquisition, of the ethanol-conditioned place preference in mice,. Pharmacol., Biochem. Behav 140, 33–38. [DOI] [PubMed] [Google Scholar]
- (145).Kotlinska JH, Bochenski M, and Danysz W (2011) The role of group I mGlu receptors in the expression of ethanol-induced conditioned place preference and ethanol withdrawal seizures in rats,. Eur. J. Pharmacol 670, 154–161. [DOI] [PubMed] [Google Scholar]
- (146).Besheer J, Faccidomo S, Grondin JJ, and Hodge CW (2008) Effects of mGlu1-receptor blockade on ethanol self-administration in inbred alcohol-preferring rats. Alcohol 42, 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (147).Lum EN, Campbell RR, Rostock C, and Szumlinski KK (2014) mGluR1 within the nucleus accumbens regulates alcohol intake in mice under limited-access conditions. Neuropharmacology 79, 679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (148).Backstrom P, and Hyytia P (2005) Suppression of alcohol self-administration and cue-induced reinstatement of alcohol seeking by the mGlu2/3 receptor agonist LY379268 and the mGlu8 receptor agonist (S)-3,4-DCPG. Eur. J. Pharmacol 528, 110–118. [DOI] [PubMed] [Google Scholar]
- (149).Rodd ZA, McKinzie DL, Bell RL, McQueen VK, Murphy JM, Schoepp DD, and McBride WJ (2006) The metabotropic glutamate 2/3 receptor agonist LY404039 reduces alcohol-seeking but not alcohol self-administration in alcohol-preferring (P) rats,. Behav. Brain Res. 171, 207–215. [DOI] [PubMed] [Google Scholar]
- (150).Cannady R, Grondin JJ, Fisher KR, Hodge CW, and Besheer J (2011) Activation of group II metabotropic glutamate receptors inhibits the discriminative stimulus effects of alcohol via selective activity within the amygdala. Neuropsychopharmacology 36, 2328–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (151).Augier E, Dulman RS, Rauffenbart C, Augier G, Cross AJ, and Heilig M (2016) The mGluR2 Positive Allosteric Modulator, AZD8529, and Cue-Induced Relapse to Alcohol Seeking in Rats,. Neuropsychopharmacology 41, 2932–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (152).Windisch KA, and Czachowski CL (2018) Effects of group II metabotropic glutamate receptor modulation on ethanol- and sucrose-seeking and consumption in the rat. Alcohol 66, 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (153).Wood CM, Nicolas CS, Choi SL, Roman E, Nylander I, Fernandez-Teruel A, Kiianmaa K, Bienkowski P, de Jong TR, Colombo G, Chastagnier D, Wafford KA, Collingridge GL, Wildt SJ, Conway-Campbell BL, Robinson ES, and Lodge D (2017) Prevalence and influence of cys407* Grm2 mutation in Hannover-derived Wistar rats: mGlu2 receptor loss links to alcohol intake, risk taking and emotional behaviour. Neuropharmacology 115, 128–138. [DOI] [PubMed] [Google Scholar]
- (154).Zhou Z, Karlsson C, Liang T, Xiong W, Kimura M, Tapocik JD, Yuan Q, Barbier E, Feng A, Flanigan M, Augier E, Enoch MA, Hodgkinson CA, Shen PH, Lovinger DM, Edenberg HJ, Heilig M, and Goldman D (2013) Loss of metabotropic glutamate receptor 2 escalates alcohol consumption. Proc. Natl. Acad. Sci U. S. A 110, 16963–16968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (155).Gyetvai B, Simonyi A, Oros M, Saito M, Smiley J, and Vadasz C (2011) mGluR7 genetics and alcohol: intersection yields clues for addiction. Neurochem. Res 36, 1087–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (156).Bahi A (2013) Viral-mediated knockdown of mGluR7 in the nucleus accumbens mediates excessive alcohol drinking and increased ethanol-elicited conditioned place preference in rats. Neuropsycho-pharmacology 38, 2109–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (157).Salling MC, Faccidomo S, and Hodge CW (2008) Nonselective suppression of operant ethanol and sucrose self-administration by the mGluR7 positive allosteric modulator AMN082. Pharm acol. Biochem. Behav 91, 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (158).Bahi A, Fizia K, Dietz M, Gasparini F, and Flor PJ (2012) Pharmacological modulation of mGluR7 with AMN082 and MMPIP exerts specific influences on alcohol consumption and preference in rats. Addict Biol. 17, 235–247. [DOI] [PubMed] [Google Scholar]
- (159).Lebourgeois S, Vilpoux C, Jeanblanc J, Acher F, Marie N, Noble F, and Naassila M (2018) Pharmacological activation of mGlu4 and mGlu7 receptors, by LSP2–9166, reduces ethanol consumption and relapse in rat. Neuropharmacology 133, 163–170. [DOI] [PubMed] [Google Scholar]
- (160).Blednov YA, Walker D, Osterndorf-Kahanek E, and Harris RA (2004) Mice lacking metabotropic glutamate receptor 4 do not show the motor stimulatory effect of ethanol. Alcohol 34, 251–259. [DOI] [PubMed] [Google Scholar]
- (161).Bahi A (2017) Decreased anxiety, voluntary ethanol intake and ethanol-induced CPP acquisition following activation of the metabotropic glutamate receptor 8 “mGluR8”. Pharmacol., Biochem. Behav 155, 32–42. [DOI] [PubMed] [Google Scholar]
- (162).Gass JT, McGonigal JT, and Chandler LJ (2017) Deficits in the extinction of ethanol-seeking behavior following chronic intermittent ethanol exposure are attenuated with positive allosteric modulation of mGlu5. Neuropharmacology 113, 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (163).Sinclair CM, Cleva RM, Hood LE, Olive MF, and Gass JT (2012) mGluR5 receptors in the basolateral amygdala and nucleus accumbens regulate cue-induced reinstatement of ethanol-seeking behavior. Pharm acol., Biochem. Behav 101, 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (164).Zhao Y, Dayas CV, Aujla H, Baptista MA, Martin-Fardon R, and Weiss F (2006) Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. J. Neurosci 26, 9967–9974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (165).Verdejo-Garcia A, Clark L, and Dunn BD (2012) The role of interoception in addiction: a critical review,. Neurosci. Biobehav. Rev 36, 1857–1869. [DOI] [PubMed] [Google Scholar]
- (166).Solinas M, Panlilio LV, Justinova Z, Yasar S, and Goldberg SR (2006) Using drug-discrimination techniques to study the abuse-related effects of psychoactive drugs in rats. Nat. Protoc 1, 1194–1206. [DOI] [PubMed] [Google Scholar]
- (167).Kostowski W, and Bienkowski P (1999) Discriminative stimulus effects of ethanol: neuropharmacological characterization. Alcohol 17, 63–80. [DOI] [PubMed] [Google Scholar]
- (168).Grant KA (1999) Strategies for understanding the pharmacological effects of ethanol with drug discrimination procedures. Pharm acol., Biochem. Behav 64, 261–267. [DOI] [PubMed] [Google Scholar]
- (169).Hodge CW, and Cox AA (1998) The discriminative stimulus effects of ethanol are mediated by NMDA and GABA(A) receptors in specific limbic brain regions,. Psychopharmacology (Berl) 139, 95–107. [DOI] [PubMed] [Google Scholar]
- (170).Besheer J, Cox AA, and Hodge CW (2003) Coregulation of ethanol discrimination by the nucleus accumbens and amygdala. Alcohol.: Clin. Exp. Res 27, 450–456. [DOI] [PubMed] [Google Scholar]
- (171).Green KL, and Grant KA (1998) Evidence for overshadowing by components of the heterogeneous discriminative stimulus effects of ethanol. Drug Alcohol Depend. 52, 149–159. [DOI] [PubMed] [Google Scholar]
- (172).Besheer J, and Hodge CW (2005) Pharmacological and anatomical evidence for an interaction between mGluR5- and GABA(A) alpha1-containing receptors in the discriminative stimulus effects of ethanol. Neuropsychopharmacology 30, 747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (173).Besheer J, Grondin JJ, Salling MC, Spanos M, Stevenson RA, and Hodge CW (2009) Interoceptive effects of alcohol require mGlu5 receptor activity in the nucleus accumbens. J. Neurosci 29, 9582–9591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (174).Jaramillo AA, Randall PA, Frisbee S, Fisher KR, and Besheer J (2015) Activation of mGluR2/3 following stress hormone exposure restores sensitivity to alcohol in rats. Alcohol 49, 525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (175).Winter JC, Eckler JR, and Rabin R A. (2004) Serotonergic/glutamatergic interactions: the effects of mGlu2/3 receptor ligands in rats trained with LSD and PCP as discriminative stimuli. Psychopharmacology (Berl) 172, 233–240. [DOI] [PubMed] [Google Scholar]
- (176).Carbonaro TM, Eshleman AJ, Forster MJ, Cheng K, Rice KC, and Gatch MB (2015) The role of 5-HT2A, 5-HT 2C and mGlu2 receptors in the behavioral effects of tryptamine hallucinogens N,N-dimethyltryptamine and N,N-diisopropyltrypt-amine in rats and mice. Psychopharmacology (Berl) 232, 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (177).Allen DC, Ford MM, and Grant KA (2017) Cross-Species Translational Findings in the Discriminative Stimulus Effects of Ethanol. Curr. Top. Behav. Neurosci, n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (178).Shelton KL, and Balster RL (1994) Ethanol drug discrimination in rats: substitution with GABA agonists and NMDA antagonists. Behav. Pharmacol 5, 441–451. [PubMed] [Google Scholar]
- (179).Besheer J, Fisher KR, Jaramillo AA, Frisbee S, and Cannady R (2014) Stress hormone exposure reduces mGluR5 expression in the nucleus accumbens: functional implications for interoceptive sensitivity to alcohol. Neuropsychopharmacology 39, 2376–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (180).Besheer J, Fisher KR, Lindsay TG, and Cannady R (2013) Transient increase in alcohol self-administration following a period of chronic exposure to corticosterone. Neuropharmacology 72, 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (181).NIAAA Council approves definition of binge drinking (2004) NIAAA Newsletter, National Institute on Alcohol Abuse and Alcoholism, Bethesda, MD. [Google Scholar]
- (182).Thiele TE, Crabbe JC, and Boehm SL II (2014) “Drinking in the Dark” (DID): A simple mouse model of binge-like alcohol intake Current Protocols in Neuroscience, Vol. 68, pp 9.49.1–9.49.12, Wiley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (183).Finn DA, Belknap JK, Cronise K, Yoneyama N, Murillo A, and Crabbe JC (2005) A procedure to produce high alcohol intake in mice,. Psychopharmacology (Berl) 178, 471–480. [DOI] [PubMed] [Google Scholar]
- (184).Sharko AC, and Hodge CW (2008) Differential modulation of ethanol-induced sedation and hypnosis by metabotropic glutamate receptor antagonists in C57BL/6J mice. Alcohol.: Clin. Exp. Res 32, 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (185).Cippitelli A, Damadzic R, Frankola K, Goldstein A, Thorsell A, Singley E, Eskay RL, and Heilig M (2010) Alcohol-induced neurodegeneration, suppression of transforming growth factor-beta, and cognitive impairment in rats: prevention by group II metabotropic glutamate receptor activation. Biol. Psychiatry 67, 823–830. [DOI] [PubMed] [Google Scholar]
- (186).Olszewski RT, Janczura KJ, Bzdega T, Der EK, Venzor F, O’Rourke B, Hark TJ, Craddock KE, Balasubramanian S, Moussa C, and Neale JH (2017) NAAG Peptidase Inhibitors Act via mGluR3: Animal Models of Memory, Alzheimer’s, and Ethanol Intoxication. Neurochem. Res 42, 2646–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (187).Stansley BJ, and Conn PJ (2018) The therapeutic potential of metabotropic glutamate receptor modulation for schizophrenia,. Curr. Opin. Pharmacol 38, 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (188).Becker HC (2008) Alcohol dependence, withdrawal, and relapse. Alcohol Research & Health 31, 348–361. [PMC free article] [PubMed] [Google Scholar]
- (189).Griffin WC 3rd (2014) Alcohol dependence and free-choice drinking in mice. Alcohol 48, 287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (190).Holleran KM, and Winder DG (2017) Preclinical voluntary drinking models for alcohol abstinence-induced affective disturbances in mice. Genes Brain Behav 16, 8–14. [DOI] [PubMed] [Google Scholar]
- (191).Krystal JH, Mathew SJ, D’Souza DC, Garakani A, Gunduz-Bruce H, and Charney DS (2010) Potential psychiatric applications of metabotropic glutamate receptor agonists and antagonists. CNS Drugs 24, 669–693. [DOI] [PubMed] [Google Scholar]
- (192).Olive MF, and Becker HC (2008) Effects of the mGluR2/3 agonist LY379268 and the mGluR5 antagonist MPEP on handling-induced convulsions during ethanol withdrawal in mice. Alcohol 42, 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (193).Reynolds AR, Saunders MA, and Prendergast MA (2016) Ethanol Stimulates Endoplasmic Reticulum Inositol Triphosphate and Sigma Receptors to Promote Withdrawal-Associated Loss of Neuron-Specific Nuclear Protein/Fox-3. Alcohol.: Clin. Exp. Res 40, 1454–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (194).Schumann G, Johann M, Frank J, Preuss U, Dahmen N, Laucht M, Rietschel M, Rujescu D, Lourdusamy A, Clarke TK, Krause K, Dyer A, Depner M, Wellek S, Treutlein J, Szegedi A, Giegling I, Cichon S, Blomeyer D, Heinz A, Heath S, Lathrop M, Wodarz N, Soyka M, Spanagel R, and Mann K (2008) Systematic analysis of glutamatergic neurotransmission genes in alcohol dependence and adolescent risky drinking behavior. Arch. Gen. Psychiatry 65, 826–838. [DOI] [PubMed] [Google Scholar]
- (195).Meyers JL, Salling MC, Almli LM, Ratanatharathorn A, Uddin M, Galea S, Wildman DE, Aiello AE, Bradley B, Ressler K, and Koenen KC (2015) Frequency of alcohol consumption in humans; the role of metabotropic glutamate receptors and downstream signaling pathways. Transl. Psychiatry 5, No. e586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (196).Akkus F, Mihov Y, Treyer V, Ametamey SM, Johayem A, Senn S, Rosner S, Buck A, and Hasler G (2018) Metabotropic glutamate receptor 5 binding in male patients with alcohol use disorder. Transl. Psychiatry 8, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (197).Akkus F, Ametamey SM, Treyer V, Burger C, Johayem A, Umbricht D, Gomez Mancilla B, Sovago J, Buck A, and Hasler G (2013) Marked global reduction in mGluR5 receptor binding in smokers and ex-smokers determined by [11C]ABP688 positron emission tomography. Proc. Natl. Acad. Sci. U. S. A 110, 737–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (198).Deschwanden A, Karolewicz B, Feyissa AM, Treyer V, Ametamey SM, Johayem A, Burger C, Auberson YP, Sovago J, Stockmeier CA, Buck A, and Hasler G (2011) Reduced metabotropic glutamate receptor 5 density in major depression determined by [(11)C]A B P688 PET and postmortem study. Am. J. Psychiatry 168, 727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (199).Xia Y, Wu Z, Ma D, Tang C, Liu L, Xin F, Zhu D, and Hu J (2014) Association of single-nucleotide polymorphisms in a metabotropic glutamate receptor GRM3 gene subunit to alcohol-dependent male subjects. Alcohol Alcohol. 49, 256–260. [DOI] [PubMed] [Google Scholar]
- (200).Joslyn G, Ravindranathan A, Brush G, Schuckit M, and White RL (2010) Human variation in alcohol response is influenced by variation in neuronal signaling genes. Alcohol.: Clin. Exp. Res 34, 800–812. [DOI] [PubMed] [Google Scholar]
- (201).Maksymetz J, Moran SP, and Conn PJ (2017) Targeting metabotropic glutamate receptors for novel treatments of schizophrenia. Mol. Brain 10, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (202).Jeste SS, and Geschwind DH (2016) Clinical trials for neurodevelopmental disorders: At a therapeutic frontier. Sci. Transl. Med 8, 321fs1. [DOI] [PubMed] [Google Scholar]
- (203).Chaki S, and Fukumoto K (2018) mGlu receptors as potential targets for novel antidepressants. Curr. Opin. Pharmacol 38, 24–30. [DOI] [PubMed] [Google Scholar]
- (204).Barnes SA, Sheffler DJ, Semenova S, Cosford NDP, and Bespalov A (2018) Metabotropic Glutamate Receptor 5 as a Target for the Treatment of Depression and Smoking: Robust Preclinical Data but Inconclusive Clinical Efficacy. Biol. Psychiatry 83, 955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (205).Pignatelli M, and Bonci A (2018) Spiraling Connectivity of NAc-VTA Circuitry. Neuron 97, 261–262. [DOI] [PubMed] [Google Scholar]