Abstract
Thrombocytopenia is frequent among sick neonates. While most cases are transient, some neonates experience prolonged and severe thrombocytopenia. These infants often pose diagnostic and therapeutic challenges, and may receive large numbers of platelet transfusions. Romiplostim (ROM) is a thrombopoietin-receptor-agonist (TPO-RA) approved for treatment of adults with chronic immune thrombocytopenia (ITP). The Immature Platelet Fraction (IPF) is a novel measure of newly produced platelets, which could aid with the diagnostic evaluation of thrombocytopenic neonates. This study had the following two objectives: 1) Compare the response of newborn and adult mice to escalating doses of ROM in vivo; and 2) Assess the correlation between IPF and megakaryocyte (MK) mass in newborn and adult treated and untreated mice. In the first set of studies, newborn (day 1) and adult mice received a single subcutaneous (SC) dose of ROM ranging from 0 to 300 ng/g, and platelet counts were followed every other day for 14 days. Both sets of mice responded with dose-dependent platelet and IPF increases, peaking on days 5–7 post-treatment, but neonates had a blunted response (2.1-fold compared to 4.2-fold maximal increase in platelet counts, respectively). On day 5 post-treatment with 300 ng/g ROM, MKs in the bone marrow (BM) and spleen of adult mice were significantly increased in numbers and size (p<0.0001 for both) compared to controls. MKs in the spleen and BM (but not liver) of treated neonates also increased in number, but not in size. The immature platelet count (IPC, calculated as IPF x platelet count) was highly correlated with the MK number and size in neonatal and adult BM and spleen, but not neonatal liver. The lack of response of neonatal liver MKs was not due to a cell-intrinsic reduced responsiveness to TPO, since neonatal liver progenitors were more sensitive to mTPO in vitro than adult BM progenitor. In vivo treatment of newborn mice with high rTPO doses or with higher doses of ROM (900 ng/g) resulted in peak platelet counts approaching 3-fold of controls. Taken together, our data indicates that newborn mice are less responsive to ROM than adult mice in vivo, due to a combination of likely pharmacokinetic differences and developmental differences in the response of MKs to thrombopoietic stimulation, evidenced by neonatal MKs increasing in numbers but not in size. PK/PD studies in human infants treated with ROM are warranted.
Keywords: thrombocytopenia, newborn, immature platelet fraction (IPF), thrombopoietin mimetic
Introduction
Thrombocytopenia is a frequent problem among sick infants, affecting 18–35% of infants admitted to the Neonatal Intensive Care Unit (NICU) [1–3]. The incidence of thrombocytopenia is inversely proportional to the gestational age, and reaches 70% among the smallest, most premature infants (<1,000 g) [4]. Intra-uterine infection (bacterial or viral), chronic fetal hypoxia due to placental insufficiency, and transplacental passage of allo- or auto-immune anti-platelet antibodies from mother to fetus are the most common etiologies of fetal and early-onset neonatal thrombocytopenia. Neonatal thrombocytopenia associated with chronic intrauterine hypoxia and fetal growth restriction has been shown to be associated with reduced numbers of circulating megakaryocyte (MK) progenitors and bone marrow (BM) MKs [5, 6]. Recently, anti HPA-1a antiplatelet antibodies (which cause the majority of cases of fetal/neonatal alloimmune thrombocytopenia) were shown to inhibit megakaryopoiesis by inducing apoptosis of early MK progenitors, in a manner similar to anti-platelet antibodies found in idiopathic thrombocytopenia (ITP) [7, 8]. Thus, decreased platelet production is a contributing factor to these common varieties of early-onset neonatal thrombocytopenia. Late-onset neonatal thrombocytopenia, occurring later than the first 3 days of life, is most commonly associated with bacterial sepsis or necrotizing enterocolitis [9, 10]. Neonates with liver failure are particularly predisposed to prolonged thrombocytopenia [11].
While most cases of neonatal thrombocytopenia have a recognizable etiology and resolve within 7–14 days, some neonates experience prolonged thrombocytopenia lasting >4 weeks [11–14]. In many of these infants, the mechanisms underlying the thrombocytopenia are unclear, partly because of the difficulties in obtaining BM specimens to assess megakaryopoiesis in small sick neonates. These neonates present both diagnostic and therapeutic challenges and frequently receive large numbers of platelet transfusions, currently their only therapeutic option [12].
Two thrombopoietin (TPO) mimetics, romiplostim (ROM) and eltrombopag, have been approved for the treatment of adults with ITP. Eltrombopag was recently approved for pediatric patients with chronic ITP, but no studies have been conducted in neonates beyond sporadic reports [14]. Our prior in vitro studies demonstrated that human neonatal MK progenitors are significantly more sensitive to TPO than adult progenitors [15–17], but no in vivo studies have explored the response of neonates to ROM or eltrombopag.
This study was designed with two objectives: 1) To compare the in vivo responses of newborn versus adult mice to ROM. Eltrombopag was not evaluated, because it only binds to the TPO receptor in humans and chimpanzees, and thus has no thrombopoietic activity in mice; and 2) To evaluate the correlation between the immature platelet fraction (IPF), a novel automated measure of newly released platelets in the circulation, and the MK mass in the BM, spleen, and liver of ROM treated vs. control mice. We reasoned that, given the difficulties associated with obtaining BM samples in neonates, a small-volume blood test that accurately reflected megakaryopoietic activity would be particularly helpful for the evaluation of thrombocytopenia in this patient population.
Methods
Romiplostim Dose Response
Animals were housed in a pathogen-free environment and all experiments were approved by the Boston Children’s Hospital Animal Care and Use Committee. Healthy adult (6–8 weeks old) C57BL/6 mice (Jackson Labs or Charles River) were given a single subcutaneous (SC) injection of 0.1% bovine serum albumin (BSA; Sigma Aldrich, St. Louis, MO) or ROM (Amgen, Thousand Oaks, CA) at a dose of 10, 30, 100, or 300 ng/g body weight. Newborn mice on post-natal day 1 (P1) received a single SC injection of either 0.1% BSA (controls) or ROM at a dose of 30 or 300 ng/g body weight. In later experiments, newborn mice (P1) received a single ROM dose of 600 or 900 ng/g body weight.
Serial blood samples were obtained from adult mice by retro-orbital puncture, and from newborn mice using a 30-gauge needle to puncture the anterior facial vein. 5 μL of blood were collected immediately after puncture with a calibrated micro-pipette, and were diluted 1:20 in Cellpack (Sysmex Corporation, Kobe, Japan), supplemented with 5 mM EDTA (Invitrogen, Carlsbad, CA), and 500 nM prostaglandin E1 (Sigma Aldrich, St. Louis, MO) to prevent platelet activation.
Complete blood counts parameters, including platelet counts, were measured using a Sysmex XT-2000iV automated hematology analyzer (Sysmex Corporation, Kobe, Japan) prior to injection and then every other day. The manufacturer’s software package allows user-defined gates in specific plots. To identify the population of immature platelets, a first gate was set in the PLT-O dot plot around the platelet population to exclude debris, erythrocytes, and reticulocytes. A second gate was then placed within the first gate to separate the majority of the platelet population (95%) of healthy wild type adult C57BL/6 mice from the highly fluorescent and larger platelets, representing the immature platelet fraction (Figure 1). The immature platelet count (IPC) was calculated by multiplying the IPF x platelet count.
Figure 1.
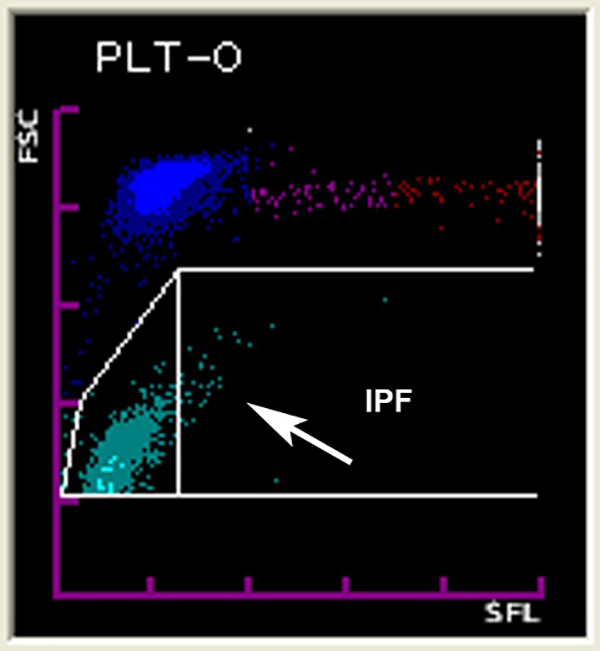
Screenshot of the gate in the Sysmex XT-2000iV hematology analyzer to detect the IPF. The larger and more intensely fluorescent platelets shown inside the gate represent the more immature, newly released platelets.
Megakaryopoiesis in Response to Romiplostim
To evaluate the effects of ROM on megakaryopoiesis, adult and newborn mice were treated with a one-time SC injection of 0.1% BSA (controls) or 300 ng/g ROM and euthanized on day 5 after injection. Liver, spleen, and BM samples were fixed in 4% paraformaldehyde. Bones from adult mice were decalcified with 300mM EDTA. Samples were subsequently embedded in paraffin and sectioned. MKs were immunohistochemically stained for von Willebrand factor (DAKO, Denmark) as described [18].
MKs in adult BM and spleen and in newborn BM, liver, and spleen were then quantified using an eyepiece reticle (Klarmann Rulings, Inc.). The MK concentration was expressed as the number of MKs in a standardized area of tissue measuring 250 × 250 μm. To assess MK size, immunohistochemically stained slides were scanned using a NanoZoomer microscope. The diameter of a minimum of 50 MKs per tissue type per animal was measured using NDP.view2 quantification software (Hamamatsu, Hamamatsu City, Japan).
In vitro Response to mTPO
To assess megakaryocyte progenitor numbers, murine MK colony assays were performed using MegaCult-C culture media (StemCell Technologies, Vancouver, B.C., Canada). Adult BM cells were flushed from femora, and newborn livers were obtained from P1 mice, and single cell suspensions were prepared by filtering through a 100-μm nylon strainer. Enriched hematopoietic progenitors (Sca1+) were isolated from BM and neonatal liver by immunomagnetic positive selection according to the manufacturer protocol (StemCell Technologies, Vancouver, B.C., Canada). Sca1+ cells were then cultured in MegaCult-C supplemented with 10, 50 or 100 ng/mL of murine TPO (mTPO, Peprotech) and/or 10 ng/mL mIL-3 (StemCell) ± 50 ng/mL mTPO as indicated, and plated into double-chamber culture slides at a concentration of 2.3×104 cells/chamber. After 8 days of culture, the slides were dehydrated, fixed with acetone, and stained for acetylcholinesterase to identify MKs. MK colonies were counted using an Olympus BX40 microscope under a 10× objective, and expressed as number of colonies/chamber. Colonies containing more than 3 megakaryocytes (AchE+ cells) were considered CFU-MKs, and those containing more than 50 MKs were counted as BFU-MKs.
In vivo Response to murine TPO (mTPO)
To evaluate whether the developmental differences observed with ROM were also present in response to mTPO, adult (6–8 weeks old) and newborn (P1) C57BL/6 mice were given a single SC injection of 0.1% bovine BSA or mTPO at a dose of 100 ng/g body weight. In a separate study, newborn mice received a daily mTPO dose of 100 ng/g for 3 consecutive days, starting on P1. Platelet counts were followed every other day as described above.
Statistical Analyses
To delineate the time course of platelet and IPF levels in adult and newborn mice in response to ROM and mTPO, we employed repeated-measures analysis of variance (ANOVA). Adults and newborns were analyzed separately. Fixed effects in the model were dose, day, and dose×day interaction. The interaction term served to test the hypothesis that the magnitude and timing of peak response differed according to dose. We used an autoregressive covariance structure to account for serial correlation of levels in successive samples from a given animal. Adjusted estimates of mean level at each time point were obtained from the fitted model, each with a standard error and 95% confidence limits.
To compare MK numbers and diameter among tissues in adult and newborn mice 5 days after ROM injection, we employed factorial ANOVA with ROM dose, tissue, and age as fixed factors. We tested all interactions among the fixed factors and retained those that were strongly significant (age×tissue, age×dose) in a final model. From the reduced model we obtained adjusted estimates for age- and tissue-specific mean levels and contrasts for dose response.
To describe the effect of mTPO and mIL3 on proliferation of MK in cell cultures from adult and newborn mice, we employed factorial ANOVA with concentration and age as fixed factors. Concentration×age interaction was included in the model to test whether the response differed between adult and newborn mice. We included a random factor to account for correlation among the multiple cultures that were developed from a given animal and treated with different doses of mTPO and mIL3.
All of the above endpoints showed markedly skewed distribution and were log10-transformed for use as dependent variables in ANOVA. For reporting, we retransformed each adjusted mean on the log scale (ӯ) to the original units (10ӯ) and used its standard error (SE) to obtain an asymptotic standard error ((10ӯ+SE–10ӯ–SE)/2). We retransformed each contrast on the log scale (Δ) to a -fold difference (10Δ) and used its standard error (SE) to construct 95% confidence limits (10Δ–1.96SE to 10Δ+1.96SE). We used the Spearman correlation coefficient to assess the association of MK measures with platelet and IPF levels. We replaced zero in vitro colony counts with a value of 0.5. SAS software (Version 9.4, Cary, NC) was used for all computations.
Results
Platelet Counts and IPF in Response to Romiplostim
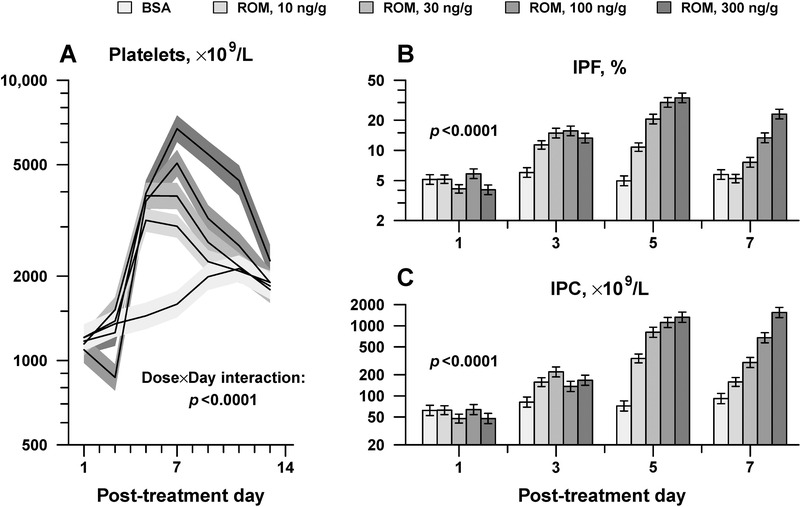
In these studies, the responses of adult and newborn C57BL/6 mice to ROM were compared. The baseline platelet count in adult mice was 1,184 ± 204 ×109/L (mean ± SD, n=17). Adult mice treated with ROM exhibited a dose-dependent increase in platelet counts, which peaked on day 5 in those receiving lower ROM doses (10 and 30 ng/g), and on day 7 in those receiving higher ROM doses (100 and 300 ng/g). On day 7, adult mice treated with 300 ng/g ROM exhibited a 4.2-fold increase (95% confidence interval 3.1 to 5.7-fold) in platelet counts, compared to BSA controls (6,720 ± 730 vs. 1,589 ± 172 ×109/L, respectively; p < 0.0001; Figure 2A). The rising platelet counts were accompanied by dose-dependent increases in the IPF (Figure 2B) and the IPC (Immature Platelet Count, the product of IPF x platelet count) (Figure 2C), indicating that the platelet count increment was caused by an up-regulation in platelet production, as expected.
Figure 2.
Adult mice dose-responses to ROM. Platelet counts (A), IPF (B), and IPC (C) were measured every other day after a single SC injection of ROM at the indicated doses (n≥3 mice per group). Data is presented as mean ± standard error. Displayed p-values are from repeated-measures analysis of variance, testing whether the magnitude and timing of peak response varied according to ROM dose (Dose x Day interaction).
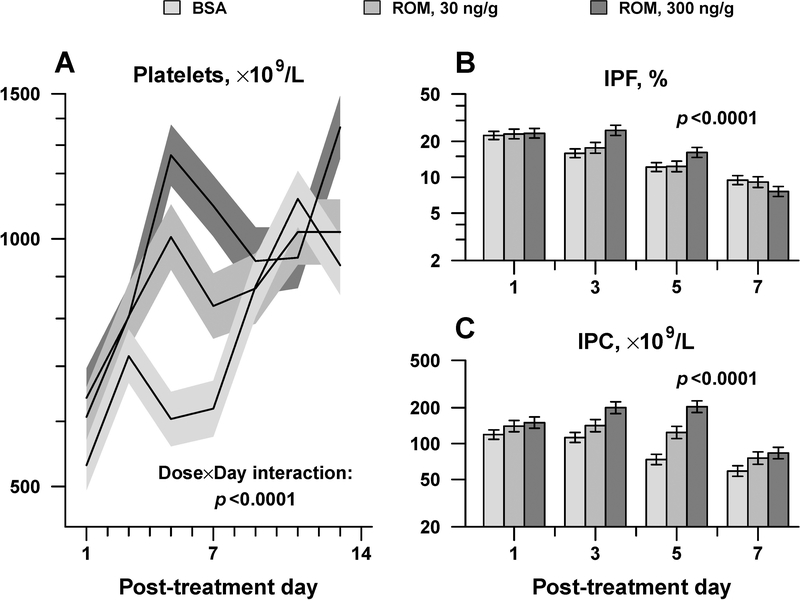
Newborn mice (P1) had significantly lower baseline platelet counts (614 ± 148 ×109/L, mean ± SD, n=53; p<0.0001) compared to adults, and similarly responded to single SC ROM injection with a dose-dependent increase in platelet count that peaked on day 5 (Figure 3A). Platelet counts on post-natal day 5 (P5) were 1,005 ± 92 ×109/L (adjusted mean ± standard error) for newborn mice treated with 30 ng/g ROM, and 1,263 ± 107 ×109/L for newborn mice treated with 300 ng/g ROM, representing a significant increase over BSA-treated pups (1.7- and 2.1-fold increase, respectively; p<0.0001 for both), but of lower magnitude than that seen in adults treated with the same ROM doses (Figure 3A). In neonates, as in adults, the IPF and IPC were higher on day 5 post-injection in animals treated with 300 ng/g of ROM compared to those who had received placebo (Figures 3B, C).
Figure 3.
Newborn mice dose-responses to ROM. Platelet counts (A), IPF (B), and IPC (C) were measured every other day after a single SC injection of ROM at the indicated doses (n=17 pups per group). Data is presented as mean ± standard error. Displayed p-values are from repeated-measures analysis of variance, testing whether the magnitude and timing of peak response varied according to ROM dose (Dose x Day interaction).
Neonatal and Adult Megakaryopoiesis in Response to ROM
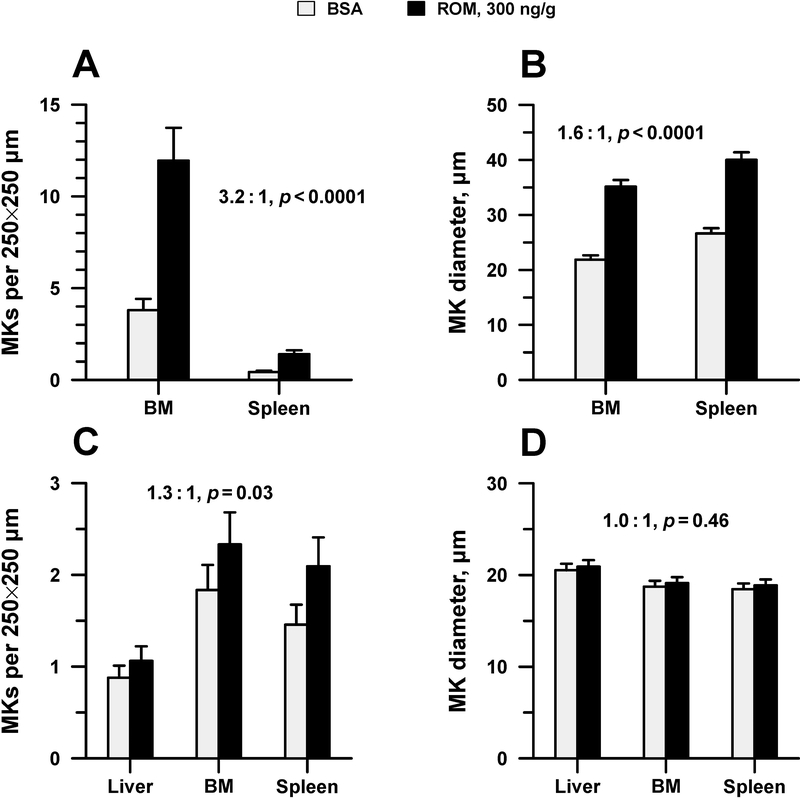
Next, MK number and size were evaluated in the BM and spleen of adult mice five days after treatment with 300 ng/g of ROM or BSA. Overall, ROM-treated adult mice had significantly increased numbers of MKs compared to controls in both BM and spleen (overall 3.2-fold increase; p<0.0001) (Figure 4A). MKs present in the BM and spleen of adult ROM treated mice were also 1.6-fold larger than controls (mean diameter of 35.2±1.2 vs. 21.9±0.8 μm in BM and 40.0±1.4 vs. 26.6±1.0 μm in spleen, adjusted mean ± standard error; p <0.0001) (Figure 4B).
Figure 4.
MK size and concentration in newborn and adult mice 5 days following the administration of a single SC dose of 300 ng/g ROM or placebo. A. MK concentration (in an area measuring 250 × 250 μm) in the BM and spleen of adult treated mice vs. controls. B. Mean diameter of MKs in the BM and spleen of adult treated mice vs. controls. C. MK concentration in the liver, BM, and spleen of newborn treated mice vs. controls. D. Mean diameter of MKs in the liver, BM and spleen of newborn treated mice vs. controls. Bars represent the mean ± standard error (n=7–8 mice per group). Displayed ROM:BSA ratios apply to both marrow and spleen for adult mice (A, B) and to all three tissues for newborn mice (C, D). Factorial analysis of variance showed no significant differences in this ratio across tissues, and thus a pooled estimate was used. Displayed p-values indicate whether the ROM:BSA ratio differed from 1.
Newborn mice treated with the same dose of ROM (300 ng/g) or BSA were also histologically evaluated 5 days after the administration of ROM, at the time of peak response. At that time point, ROM-treated mice exhibited a small but statistically significant increase in total MK number (1.3-fold; p=0.03), observed primarily in the BM and the spleen, but not in the liver (Figure 4C). Contrary to adult mice, neonatal mice did not increase the MK size in response to ROM in any of the organs studied (Figure 4D).
Correlation Between IPF and MK Mass
Next, we evaluated the association between the IPC in the blood and the MK concentration and size in the BM, spleen and liver of mice 5 days following the ROM or placebo injection. As shown in Table 1, 5 days following the ROM or placebo injection in newborn mice there was a highly significant correlation between IPC and MK number/size in the BM and spleen, but not in the liver. Importantly, the IPC exhibited a stronger correlation with both MK parameters than the platelet count. Since platelet production depends both on MK number and size, we then multiplied the MK number and diameter in each organ to obtain a surrogate measure in arbitrary units of the “MK mass” (as previously done) [18]. The IPC was strongly correlated with this measure in BM and spleen, but not in the newborn liver.
Table 1.
Correlation of MK measures with platelet count and IPC, 5 days after ROM or placebo injection in adult and newborn mice.*
| MK measure | Tissue | Correlation with platelet count |
Correlation with IPC |
||
|---|---|---|---|---|---|
| r | p | r | p | ||
| Number† | BM | 0.30 | 0.11 | 0.48 | 0.008 |
| Spleen | 0.43 | 0.02 | 0.58 | 0.0007 | |
| Liver | 0.21 | 0.43 | 0.26 | 0.34 | |
| Diameter | BM | 0.27 | 0.15 | 0.49 | 0.006 |
| Spleen | 0.32 | 0.09 | 0.38 | 0.04 | |
| Liver | –0.04 | 0.88 | –0.04 | 0.89 | |
| MK mass‡ | BM | 0.30 | 0.11 | 0.53 | 0.003 |
| Spleen | 0.46 | 0.01 | 0.68 | <0.0001 | |
| Liver | 0.06 | 0.83 | 0.14 | 0.60 | |
Spearman correlation coefficient; p tests for zero correlation. Values for marrow and spleen are partial correlations from a sample comprising 15 adult and 16 newborn mice, adjusted for age group to yield a tissue-specific within-age correlation. Values for liver are simple correlations from the sample of 16 newborn mice.
Per 250 × 250 μm field.
Number × mean diameter3.
Newborn Liver and Adult BM Response to murine TPO (mTPO) in vitro
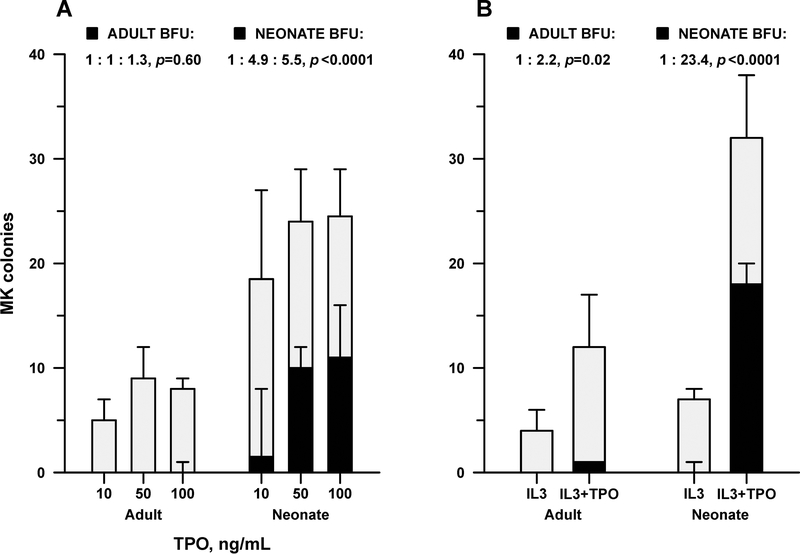
Given the lack of response of neonatal liver MKs to ROM, we sought to determine whether the in vivo findings reflected a cell-intrinsic hyporesponsiveness of newborn liver MK progenitors to TPO. Contrary to the in vivo observations, however, enriched hematopoietic progenitor (Sca1+) cells isolated from neonatal (P1) livers generated 2.8-fold more MK colonies in vitro than adult BM progenitors (p=0.0003). In addition, the percentage of large colonies, which represent a more primitive MK progenitor (the Burst-Forming Unit MK, or BFU-MK) capable of generating large numbers of MKs, was significantly higher in neonatal liver than in adult BM cultures (Figure 5). While the total number of MK colonies increased only marginally in response to rising TPO concentrations (10, 50, and 100 ng/mL) in both adult and neonatal samples, the number of BFU-MKs increased nearly 5-fold in neonatal cultures treated with TPO 50 vs. TPO 10 (p<0.0001), but this increased response was not seen in adult cultures (p=0.6) (Figure 5A). A similar increase in BFU-MKs was observed in neonatal liver cultures supplemented with IL3 + TPO, compared to IL3 alone (23-fold increase, p<0.0001). Adult BFU-MKs only increased 2-fold (p=0.02) under the same conditions (Figure 5B).
Figure 5.
In vitro dose-response of newborn liver and adult BM hematopoietic progenitor cells to mTPO. The full bars indicate the number of total MK progenitors and the black areas indicate the subset of more primitive progenitors, the Burst Forming Unit-MKs (BFU-MKs), cultured in each chamber from adult BM (n=5) and neonatal liver (n=6) in response to increasing concentrations of mTPO (10–100 ng/mL) (A), or to IL-3 ± 50 ng/mL of mTPO (B). Bars represent the median, with error bars reaching to the 75th percentile. Where a bar is not visible, median count was 0; where the error bar is not visible, the 75th percentile was equal to the median. Displayed ratios, corresponding to the solid bars below, are derived from mixed-model analysis of variance of log-transformed BFU counts. P values indicate whether any ratio differed from 1.
Neonatal Responses to mTPO in vivo
Next, we sought to determine whether newborn mice would be more responsive than adult mice to mTPO in vivo, resembling the in vitro findings. To accomplish this, the response of newborn and adult mice to a single SC dose of mTPO (100 ng/g, TPO 100) was evaluated in vivo. Adult animals receiving this dose increased their platelet counts to a peak of 3,064±174 ×109/L (on day 5 post-treatment, adjusted mean ± standard error), which represented a 1.7-fold increment compared to BSA controls (p<0.0001). Newborn mice receiving the same dose increased their platelet count to 1352±252 × 109/L, also equivalent to a 1.7-fold increase compared to age-matched controls (p=0.03).
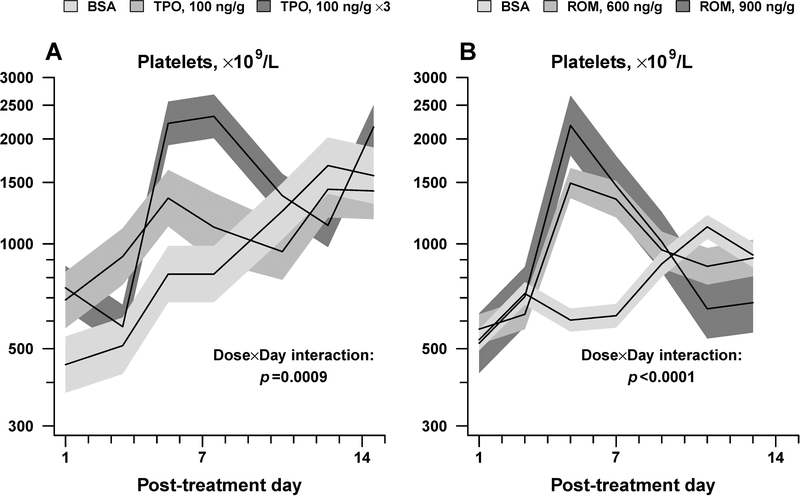
To determine if newborn mice could increase their platelet counts further with additional thrombopoietic stimulation, and reasoning that romiplostim was engineered to have a longer half-life than thrombopoietin, the response of newborn pups to 3 consecutive daily doses of TPO 100 was next evaluated. These mice exhibited peak platelet counts of 2322 ± 335 × 109/L, which represented a 2.8-fold increase over controls (p<0.0001) (Figure 6A).
Figure 6.
In vivo response of newborn mice to mTPO (100 ng/g) given daily for 1 day or 3 days (A) and to single ROM doses of 600 and 900 ng/g (B). Data is shown as mean platelet counts ± standard error (shaded areas). (n=3–6 mice per group). Displayed p-values are from repeated-measures analysis of variance, testing whether the magnitude and timing of peak response to TPO or ROM varied according to dose (Dose x Day interaction).
Neonatal Responses to Higher ROM doses
The responses of newborn mice to mTPO in vivo suggested that newborn mice were capable of increasing their platelet counts to levels higher than those achieved with 300 ng/g of ROM. To test whether similar responses could be achieved with higher ROM doses, newborn mice were treated with a single ROM dose of 600 or 900 ng/g given at P1. Animals receiving these doses reached peak platelet counts of 1,494±147×109/L and 2186±433 × 109/L on day 5 post-injection (adjusted mean ± standard error), comparable to those reached with 100 and 300 ng/g of TPO (Figure 6B).
Discussion
This is the first study to evaluate the in vivo responses of newborn animals to the TPO mimetic ROM. This study was undertaken in anticipation of the potential use of this TPO-mimetic in selected groups of thrombocytopenic neonates. Based on multiple prior studies by us and others showing the increased proliferative potential and increased sensitivity to TPO (and ROM) of human neonatal compared to adult MK progenitors [15, 16, 19] in vitro, we hypothesized that newborn pups would be more sensitive to ROM than adults in vivo. The more limited response of newborn mice treated with ROM was therefore a surprising finding.
The histological data in P5 mice treated with 300 ng/g of ROM, showing a modest increase in the number of MKs in the spleen and BM, but not the liver, raised the question of whether newborn liver MK progenitors are intrinsically not responsive to TPO. Contrary to the in vivo observations, in vitro cultures demonstrated that newborn liver MK progenitors are highly sensitive to TPO and have a predominance of primitive progenitors (Burst Forming Units-MK, or BFU-MK), which generate larger colonies and substantially more MKs than the more mature progenitors (Colony Forming Unit-MK, or CFU-MK) that predominate in the adult BM (Figure 4). These observations are highly consistent with the developmental differences that have been reported between human neonatal and adult BM MK progenitors in their responses to TPO in vitro.
The reasons why neonatal hepatic megakaryopoiesis is not stimulated by ROM in vivo are unclear, but are likely at least in part related to the fact that the liver is the main site of TPO production at all stages of development [20–22]. Because of this, TPO concentrations within the liver microenvironment are presumably very high, and could lead to most TPO receptors on neonatal MK progenitors being occupied. Since TPO and ROM bind to the same portion of the TPO receptor and compete for binding, a TPO-rich microenvironment would likely limit the effects of ROM.
In all treated animals, ROM increased platelet counts and MK numbers in the spleen and BM, but this increase was substantially smaller in the neonates. Although we did not measure ROM levels (due to severe blood volume limitations in newborn mice), higher ROM doses were required in neonates to achieve responses comparable to those of adults, suggesting that there may also be pharmacokinetic differences between neonatal and adult mice. Considering the rapid growth that mice experience during the first two weeks of post-natal life, it is likely that the volume of distribution is different in newborn compared to adult mice and this may contribute to these differences. Similar developmental pharmacokinetic differences exist for recombinant erythropoietin in humans, with neonates requiring substantially higher doses than adults.
In adult mice, the response to ROM was characterized by an increase in MK concentration and size, both factors that increase the MK mass and thrombopoiesis. The finding that neonatal splenic and BM MKs did not increase their size in response to ROM was consistent with our prior studies demonstrating the presence of small, low ploidy MKs in murine as well as human neonates [17, 23], and their limited or absent increase in size in response to thrombocytopenia [18, 24]. Human neonatal MK progenitors (cord blood-derived) treated in vitro with increasing concentrations of TPO or ROM in fact exhibited decreasing ploidy in response to higher concentrations of either compound [16, 25]. Our study is the first to show that this developmental limitation in the response to ROM is also present in vivo, and might limit the maximal neonatal response to thrombopoietic agents.
In that regard, an important question is the time when megakaryopoiesis transitions from a neonatal (hyperproliferative) to an adult phenotype (characterized by low proliferative rates, but large and highly polyploidy MKs), since adult-like responses would be expected after this transition. In mice, we previously reported that the MK size progressively increases over the first two weeks of life, so that BM MKs at P14 are almost as large as adult MKs [23]. In humans, Fuchs and collaborators measured MK diameters in 72 normal BM samples from patients aged 3 days to 80 years. This study revealed that neonates had MKs of uniform small sizes, which diverged into separate clusters of smaller and larger cells beginning at 2 years, and transitioned to larger (adult-like) MKs by 4 years [26]. These data suggested that infants and young children up to 2 years might have a “neonatal” pattern of megakaryopoiesis, characterized by small-low ploidy MKs, which depend on a very high proliferative rate to up-regulate platelet production.
An important aspect of this study was the demonstration that the IPF, a novel marker of newly released platelets (carrying increased RNA), increased with upregulated megakaryopoiesis, and that the IPC was highly correlated with the MK mass in the BM and the spleen of both neonates and adults. The availability of such a marker is particularly useful for the evaluation of neonates with thrombocytopenia of unclear etiology, because BM studies are technically difficult and are only rarely performed in this population. Prior studies in human adults and neonates have suggested that the IPF is a useful marker to differentiate clinical disorders associated with decreased platelet production vs. increased platelet consumption [9, 27, 31]. However, this is to our knowledge the first demonstration that the IPC is highly correlated with the MK mass in an experimental neonatal and adult murine model.
This study has limitations. First, while the liver is the main hematopoietic organ in newborn mice, the BM is the main hematopoietic site in human neonates born at any gestational age. As a consequence, the limitations in the response to ROM determined by the TPO-rich microenvironment in the liver in the first few days of murine life might not be a factor in human neonates or infants. Nevertheless, we postulate that awareness of species-related and developmental stage-specific differences is critical to correctly interpret the findings in experimental animal models.
The developmental pharmacokinetic differences suggested by our studies and the limited ability of neonatal MKs to increase their size in response to thrombopoietic stimulation, however, are factors likely to impact the response of human neonates and infants/young children to ROM. In support of this possibility, a recent randomized controlled trial of ROM in children with immune thrombocytopenia found that the youngest patients (ages 1 to <6 years) had the lowest rates of durable platelet responses despite being exposed to higher doses of ROM than the older children in the study (6 to 18 years) [33]. Taken together, these clinical observations and our experimental data suggest that there might be developmental differences in the response of neonatal and “neonatal/like” MKs to thrombopoietic stimulation. The possibility of ROM pharmacokinetic differences between human neonates/young children and adults should be explored in clinical studies. From the diagnostic perspective, our data also indicate that the IPC accurately reflects the MK mass and should be a particularly useful tool to evaluate mechanisms of thrombocytopenia in neonates, in whom bone marrow studies are technically difficult.
Acknowledgements
We would like to thank Dr. Jolanta Kunicka and Tiffany Ivers (Sysmex America, Inc.) for technical assistance setting the IPF gate in the Sysmex instrument, and Dr. James Bussel for his critical reading of the manuscript.
Declaration of Interest statement
This work was supported by NIH grants P01 HL046925 and R01 HL69990. The Sysmex XT-2000i veterinary hematology analyzer in Dr. Sola-Visner’s laboratory is a generous loan from Sysmex America Inc.
References
- 1.Andrew M, Castle V, Saigal S, Carter C, Kelton JG. Clinical impact of neonatal thrombocytopenia. The Journal of pediatrics 1987;110:457–64. [DOI] [PubMed] [Google Scholar]
- 2.Castle V, Andrew M, Kelton J, Giron D, Johnston M, Carter C. Frequency and mechanism of neonatal thrombocytopenia. The Journal of pediatrics 1986;108:749–55. [DOI] [PubMed] [Google Scholar]
- 3.Mehta P, Vasa R, Neumann L, Karpatkin M. Thrombocytopenia in the high-risk infant. The Journal of pediatrics 1980;97:791–4. [DOI] [PubMed] [Google Scholar]
- 4.Christensen RD, Henry E, Wiedmeier SE, et al. Thrombocytopenia among extremely low birth weight neonates: data from a multihospital healthcare system. Journal of perinatology : official journal of the California Perinatal Association 2006;26:348–53. [DOI] [PubMed] [Google Scholar]
- 5.Murray NA, Roberts IA. Circulating megakaryocytes and their progenitors in early thrombocytopenia in preterm neonates. Pediatric research 1996;40:112–9. [DOI] [PubMed] [Google Scholar]
- 6.Sola MC, Calhoun DA, Hutson AD, Christensen RD. Plasma thrombopoietin concentrations in thrombocytopenic and non-thrombocytopenic patients in a neonatal intensive care unit. Br J Haematol 1999;104:90–2. [DOI] [PubMed] [Google Scholar]
- 7.Liu ZJ, Bussel JB, Lakkaraja M, et al. Suppression of in vitro megakaryopoiesis by maternal sera containing anti-HPA-1a antibodies. Blood 2015;126:1234–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warwick RM, Vaughan J, Murray N, Lubenko A, Roberts I. In vitro culture of colony forming unit-megakaryocyte (CFU-MK) in fetal alloimmune thrombocytopenia. Br J Haematol 1994;88:874–7. [DOI] [PubMed] [Google Scholar]
- 9.Cremer M, Weimann A, Szekessy D, Hammer H, Buhrer C, Dame C. Low immature platelet fraction suggests decreased megakaryopoiesis in neonates with sepsis or necrotizing enterocolitis. Journal of perinatology : official journal of the California Perinatal Association 2013;33:622–6. [DOI] [PubMed] [Google Scholar]
- 10.Brown RE, Rimsza LM, Pastos K, et al. Effects of Sepsis on Neonatal Thrombopoiesis. Pediatric research 2008. [DOI] [PubMed] [Google Scholar]
- 11.Del Vecchio A, Sola MC, Theriaque DW, et al. Platelet transfusions in the neonatal intensive care unit:factors predicting which patients will require multiple transfusions. Transfusion 2001;41:803–8. [DOI] [PubMed] [Google Scholar]
- 12.Dohner ML, Wiedmeier SE, Stoddard RA, et al. Very high users of platelet transfusions in the neonatal intensive care unit. Transfusion 2009;49:869–72. [DOI] [PubMed] [Google Scholar]
- 13.Christensen RD, Baer VL, Henry E, Snow GL, Butler A, Sola-Visner MC. Thrombocytopenia in Small-for-Gestational-Age Infants. Pediatrics 2015;136:e361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen RD, Yaish HM, Leon EL, Sola-Visner MC, Agrawal PB. A de novo T73I Mutation in PTPN11 in a Neonate with Severe and Prolonged Congenital Thrombocytopenia and Noonan Syndrome. Neonatology 2013;104:1–5. [DOI] [PubMed] [Google Scholar]
- 15.Sola MC, Du Y, Hutson AD, Christensen RD. Dose-response relationship of megakaryocyte progenitors from the bone marrow of thrombocytopenic and non-thrombocytopenic neonates to recombinant thrombopoietin. Br J Haematol 2000;110:449–53. [DOI] [PubMed] [Google Scholar]
- 16.Pastos KM, Slayton WB, Rimsza LM, Young L, Sola-Visner MC. Differential effects of recombinant thrombopoietin and bone marrow stromal-conditioned media on neonatal versus adult megakaryocytes. Blood 2006;108:3360–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu ZJ, Italiano J Jr., Ferrer-Marin F, et al. Developmental differences in megakaryocytopoiesis are associated with up-regulated TPO signaling through mTOR and elevated GATA-1 levels in neonatal megakaryocytes. Blood 2011;117:4106–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu Z, Slayton WB, Rimsza LM, Bailey M, Sallmon H, Sola-Visner MC. Differences between newborn and adult mice in their response to immune thrombocytopenia. Neonatology 2010;98:100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murray NA, Watts TL, Roberts IA. Endogenous thrombopoietin levels and effect of recombinant human thrombopoietin on megakaryocyte precursors in term and preterm babies. Pediatric research 1998;43:148–51. [DOI] [PubMed] [Google Scholar]
- 20.Sola MC, Juul SE, Meng YG, et al. Thrombopoietin (Tpo) in the fetus and neonate: Tpo concentrations in preterm and term neonates, and organ distribution of Tpo and its receptor (c-mpl) during human fetal development. Early human development 1999;53:239–50. [DOI] [PubMed] [Google Scholar]
- 21.Dame C Developmental biology of thrombopoietin in the human fetus and neonate. Acta Paediatr Suppl 2002;91:54–65. [DOI] [PubMed] [Google Scholar]
- 22.Wolber EM, Dame C, Fahnenstich H, et al. Expression of the thrombopoietin gene in human fetal and neonatal tissues. Blood 1999;94:97–105. [PubMed] [Google Scholar]
- 23.Liu ZJ, Hoffmeister KM, Hu Z, et al. Expansion of the neonatal platelet mass is achieved via an extension of platelet lifespan. Blood 2014;123:3381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sola-Visner MC, Christensen RD, Hutson AD, Rimsza LM. Megakaryocyte size and concentration in the bone marrow of thrombocytopenic and nonthrombocytopenic neonates. Pediatric research 2007;61:479–84. [DOI] [PubMed] [Google Scholar]
- 25.Currao M, Balduini CL, Balduini A. High doses of romiplostim induce proliferation and reduce proplatelet formation by human megakaryocytes. PloS one 2013;8:e54723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuchs DA, McGinn SG, Cantu CL, Klein RR, Sola-Visner MC, Rimsza LM. Developmental differences in megakaryocyte size in infants and children. American journal of clinical pathology 2012;138:140–5. [DOI] [PubMed] [Google Scholar]
- 27.Cremer M, Paetzold J, Schmalisch G, et al. Immature platelet fraction as novel laboratory parameter predicting the course of neonatal thrombocytopenia. Br J Haematol 2009;144:619–21. [DOI] [PubMed] [Google Scholar]
- 28.Cremer M, Weimann A, Schmalisch G, Hammer H, Buhrer C, Dame C. Immature platelet values indicate impaired megakaryopoietic activity in neonatal early-onset thrombocytopenia. Thromb Haemost 2010;103:1016–21. [DOI] [PubMed] [Google Scholar]
- 29.Briggs C, Kunka S, Hart D, Oguni S, Machin SJ. Assessment of an immature platelet fraction (IPF) in peripheral thrombocytopenia. Br J Haematol 2004;126:93–9. [DOI] [PubMed] [Google Scholar]
- 30.Abe Y, Wada H, Tomatsu H, et al. A simple technique to determine thrombopoiesis level using immature platelet fraction (IPF). Thromb Res 2006;118:463–9. [DOI] [PubMed] [Google Scholar]
- 31.Sokolic R, Oden N, Candotti F. Assessment of Immature Platelet Fraction in the Diagnosis of Wiskott-Aldrich Syndrome. Front Pediatr 2015;3:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelemen E, Calvo W, Fliedner TM. Atlas of Human Hemopoietic Development: Springer-Verlag; 1979. [Google Scholar]
- 33.Tarantino MD, Bussel JB, Blanchette VS, et al. Romiplostim in children with immune thrombocytopenia: a phase 3, randomised, double-blind, placebo-controlled study. Lancet 2016. [DOI] [PubMed] [Google Scholar]