Abstract
Background
Today, the plant Prosopis farcta is frequently used for traditional medicinal purposes. The aim of this study was the identification of luteolin in P. farcta extract (PFE) and to evaluate its effect on morphine discontinuation syndrome in rats.
Material/Methods
Using high-performance liquid chromatography (HPCL), luteolin was evaluated in PFE. The frequency of behavioral symptoms of morphine withdrawal (jumping, rearing, and teeth chattering) induced by naloxone challenge were illustrated in morphine-dependent rats receiving PFE, luteolin, saline, or clonidine. LD50 of PFE and luteolin was 540 mg/kg and 150 mg/kg, respectively. Signs of behavioral morphine withdrawal in rats were significantly inhibited by chronic co-administration of PFE, luteolin, or clonidine with morphine.
Results
This study showed that PFE was less effective than clonidine at a dose of 100 mg/kg, and at doses of 200 mg/kg and 300 mg/kg it was comparable to clonidine, and did not show a significant difference in the reduction of morphine withdrawal symptoms. Luteolin was comparable in 30 mg/kg, 60 mg/kg, and 90 mg/kg with clonidine to reduce the frequency of morphine withdrawal symptoms. PFE can be used as a source of luteolin.
Conclusions
The study findings suggest that PFE and luteolin might reduce the signs of narcotic withdrawal. Due to a similar effect to clonidine, its mechanism of action might be through the protein kinase A pathway and might have human therapeutic potential.
MeSH Keywords: Chromatography, Liquid; Clonidine; Luteolin; Product Recalls and Withdrawals; Prosopis; Safety-Based Drug Withdrawals
Background
Opioid dependence is a global health problem that creates many social, individual, and economic problems. There are 3 main pharmacological therapies for opium dependence, including opioid detoxification, agonist maintenance, and antagonist maintenance. Methadone, buprenorphine, α2-adrenoceptor agonists, and adjunct medications are used for opioid detoxification. In antagonist maintenance, naltrexone (an opioid antagonist) is also used. Pharmacological drugs with proven effects agonist maintenance include buprenorphine, buprenorphine/naloxone, and methadone [1,2]. The pharmacologic agents used in the treatment of opioid dependence have important limitations in efficacy and safety. Novel pharmacologic options with more efficacy and better safety profiles are needed for opioid dependence treatment [3,4]. Plants and natural products are a potential source of novel and safer compounds to use for drug withdrawal management [5,6]. It has previously been reported that medicinal plants such as Avena sativa, Hypericum perforatu, Passiflora incarnate, and Valeriana officinalis have antispasmodic, analgesic, antianxiety, and hypnotic effects and can improve the symptoms of morphine withdrawal [7]. The genus Prosopis L. belongs to the family Fabaceae (Leguminosae) that is widespread in different dry and semi-dry regions worldwide including Africa, Australia, America, and Asia. This genus comprises from 44 to 50 species [8]. Prosopis farcta (Banks & Sol.) is a little prickly spiny shrub that is native to the United States, Kuwait, Turkey, Iraq, Iran, Northern Africa, and South Western Asia [9]. A literature survey revealed that P. farcta has been used in the treatment of neurological disorders. Also, there is a long history of traditional use of the extraction of this plant for treatment of pain in Iran [10]. Some of the phytochemicals existing in P. farcta include quercetin, tryptamine, apigenin, 5-hydroxytryptamine, L-arabinose, and lectin; and it has been shown for the aforementioned, that one of the major components is luteolin [11]. Luteolin [2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-chromanone], a bioflavonoid which is known to be present in many types of plants and plant products, possesses diverse biological properties including antioxidant, anti-inflammatory, and anticancer activities [12]. As suggested by these properties, growing evidence indicates neuroprotective actions of luteolin against a number of insults. Also, luteolin was been shown to exhibit neuroprotection against kainic acid-induced damage in rats, and to improve learning and memory [13]. Moreover, it has been demonstrated that luteolin exerted antiamnesic effects against amyloid beta (Aβ)-induced toxicity [14]. However, the behavioral effects of luteolin and plant extracts of P. farcta on the withdrawal syndrome are not clearly understood. Therefore, PFE and its luteolin were hypothesized to reduce the intensity of opioid withdrawal syndrome in rats. The jumping, rearing, and teeth chattering behaviors during the withdrawal period were measured as indicative data to represent the intensity of morphine withdrawal symptoms in rats.
Material and Methods
Plant material and extraction
The seeds of P. farcta were collected from Ilam Province, Iran at the fruiting stage (November 2015). The voucher number of the plant specimen (herbarium code 74/1525) was deposited in the Birjand University, Iran. The specimens were powdered after drying and 20 grams were used for extraction. Initially, the powder was degreased by hexane and then extracted by a Soxhlet extraction method in a water-methanol solvent. After extraction, the solvents were removed by rotary (ILK HB 10) method and the rest of the extract, having a yield of 6.94%, was lyophilized and kept stored at −20°C. The lyophilized samples were dissolved in methanol and filtered through a 0.22 μm syringe filter [15].
Isolation and identification of the luteolin by high-performance liquid chromatography (HPLC)
High-performance liquid chromatography (HPLC) method was performed according to the previously described procedure [16]. A simple and reproducible reversed-phase HPLC with a Knauer liquid chromatography (Smart line; Knauer, Berlin, Germany) equipped with an ultraviolet detector (Well chrome, K-2600; Knauer) and a reverse-phase C18 column (Nucleosil H.P.; 25×0.46 cm internal diameter, pore size mm; Knauer) using isocratic elution with UV absorbance detection was developed and validated for the determination of luteolin. In the interval from zero to 60 minutes, solvent of 0.1% formic acid in water (solution B) was maintained at a range of 5% to 70% and solvent methanol (solution A) was fixed. Column temperature, mobile phase flow rate, injection volume, and detection wavelength were set at 25°C, 1 mL/min, 1 μL, and 348 nm, respectively. In the same condition, luteolin standard solution (dissolved in methanol) was run. Two 150 mg of dried extracts were dissolved in 10 mL HPLC-grade methanol, sonicated for 15 minutes, filtered through a 0.22 μm syringe filter and further diluted to 5 mg/mL. The peaks obtained from the PFE were compared with a luteolin standard. A stock solution of luteolin standard was prepared at 0.1 mg/mL in HPLC-grade methanol, filtered through a 0.22 μm syringe filter and further diluted in the same solvent to obtain 0.1, 0.2, 0.4, 0.8, 1.6, 3.2, 6.4, 12.5, 25, and 50 μg/mL.
Drugs
Morphine sulfate was purchased from the Darou Pakhsh pharmaceutical company (Iran). Naloxone hydrochloride was obtained from the Sigma-Aldrich Company (USA). All drugs and extracts were dissolved in normal saline. The drugs and extracts were prepared immediately prior to use and injected subcutaneously. The doses of total PFE, luteolin fraction, and morphine were as follows: total extract at 100, 200, and 300 mg/kg; luteolin fraction at 30, 60, and 90 mg/kg; and morphine at 50 mg/kg.
Animals
Male albino rats weighing 190–250 g from our own breeding colony (Institute of Medicinal Plants, ACECR, Karaj, Iran) were used. The rats were maintained at a temperature of 22–25ºC on a 12-hour dark-light cycle. The animals had access to standard rodent feed and water. Ten animals were used for each dose of the extracts or drugs, and the experiment was repeated 3 times. All animals were used only once. The grouping of the method used to study morphine withdrawal syndrome is shown in Table 1.
Table 1.
Grouping of animals and drugs used to create dependence and morphine withdrawal syndrome.
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | Group 7 | Group 8 | Group 9 |
|---|---|---|---|---|---|---|---|---|
| Morphine (control) | Morphine & clonidine (positive control) | Morphine & PFE 100 mg/kg | Morphine & PFE 200 mg/kg | Morphine & PFE 300 mg/kg | Morphine & luteolin 30 mg/kg | Morphine & luteolin 60 mg/kg | Morphine & luteolin 90 mg/kg | Nondependent rats (saline) |
LD50 (median lethal dose) in rats
To determine toxicity, different doses of the PFE and luteolin dissolved in normal saline were gavaged in the groups, each consisting of 20 rats. The administered doses were 50, 100, 200, 300, 400, 500, 600, and 700 mg/kg for PFE and 10, 20, 30, 60, 90, 120, 150, and 180 mg/kg for luteolin. Afterward, the numbers of dead animals within 72 hours after the gavage were counted and the LD50 was calculated. Mortality was noted, and graphs were plotted between log-concentration versus percent mortality and log-concentration versus probit mortality, and the LD50 was determined [17,18]. Therefore, PFE with doses of 100, 200, and 300 mg/kg and luteolin with doses of 30, 60, and 90 mg/kg were selected for continuing the experiments.
Morphine dependency method
For this experiment, for 7 days, morphine was injected subcutaneously into the rats twice daily. A dose of 2.5 mg/kg of morphine was used on days 1 and 2 (twice daily) and these doses doubled every day until the 6th day, reaching 40 mg/kg. On the 7th day, rats received a last dose of 50 mg/kg [19]. To produce the same stress, rats were injected in group 9 with normal saline.
Method of withdrawal syndrome
In all groups, rats received 3 mg/kg naloxone by intraperitoneal (i.p.) injection 4 hours after the last dose of morphine treatment on day 7. Immediately after naloxone injection, to monitor the morphine withdrawal symptoms manifestations (jumping, rearing, teeth chattering), each animal was placed in a transparent cylindrical container for half an hour [20].
Method of co-administration use of PFE and morphine on morphine dependency and withdrawal syndrome
Rats in groups 3, 4, 5, and 9 received PFE at doses of 100 mg/kg, 200 mg/kg, and 300 mg/kg and normal saline respectively, by NG tube concurrently with morphine twice daily for 6 days; To examine the challenge, 4 hours prior to naloxone injection, animals in groups 3, 4, and 5 received the last dose on day 7, and group 9 received saline with morphine. In order to investigate the effect of PFE on the weakening of morphine dependence, behavioral symptoms of withdrawal in animals of groups 3, 4, 5, and 9 were compared.
Method of co-administration use of luteolin and morphine on morphine dependency and withdrawal syndrome
Rats in groups 6, 7, 8, and 9 received luteolin at 30 mg/kg, 60 mg/kg, and 90 mg/kg, and saline respectively, by NG tube concurrently with morphine twice daily for 6 days; To examine the challenge, 4 hours prior to naloxone injection, animals in groups 6, 7, and 8 received the last dose on day 7, and group 9 received saline with morphine. In order to investigate the effect of luteolin on the weakening of morphine dependence, behavioral symptoms of withdrawal in animals of groups 6, 7, 8, and 9 were compared.
Method of co-administration use of clonidine hydrochloride and morphine on morphine dependency and withdrawal syndrome
In group 2, rats received clonidine hydrochloride at (0.2 mg/ kg i.p.) twice daily for 6 days with morphine; and to examine the challenge, 4 hours prior to naloxone injection, animals in group 2 received the last dose on day 7.
Statistical analysis
The animal study results were presented as mean ± standard error of the mean (SEM). One-way analysis of variance (ANOVA) followed by Tukey post hoc test was used for data analysis. P-value <0.05 was considered as statistically significant.
Results
Extraction and identification
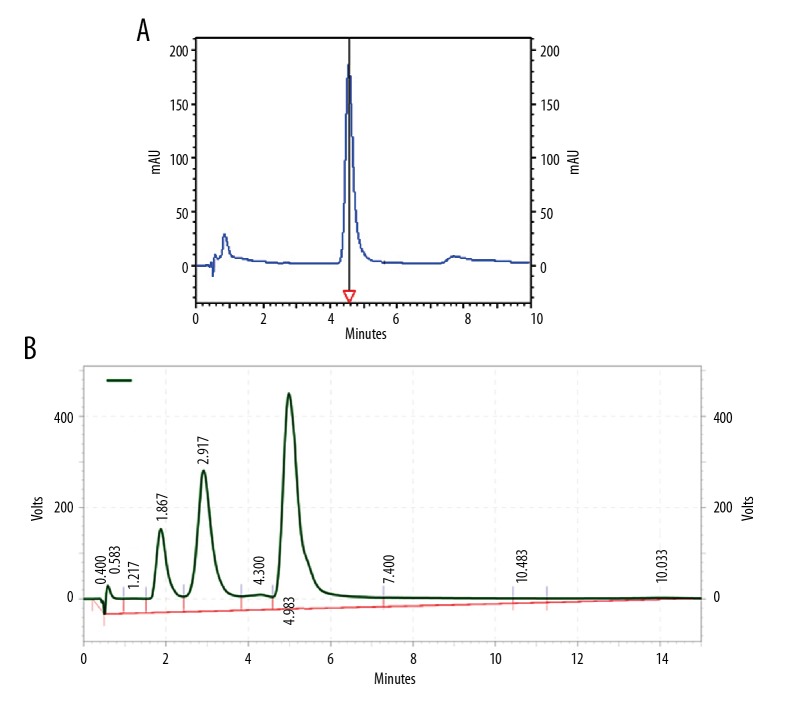
The luteolin HPLC chromatogram was obtained as a standard broad peak with a retention time at 4.898 min at a wavelength of 348 nm. In the same conditions, the standard chromatogram of the methanolic PFE was similar to that of standard luteolin in 4.983 min (Figure 1A, 1B). Upon application of the developed method, well-separated peaks were obtained for luteolin in PFE as shown, compared to its respective standards as in Figure 1. The quantitative analysis revealed that luteolin was found to be predominant in the methanol fraction (2.64 mg/g of luteolin) of PFE. The assay method was validated, and the calibration curve was linear (R2=0.9999, y=178399x–4122.01).
Figure 1.
High-performance liquid chromatography (HPLC) of (A) luteolin standard and (B) PFE; HPLC conditions were the same for both luteolin and PFE. PFE – Prosopis farcta extract.
Determination of mortality
Mortality and LD50 values of PFE and luteolin were 540 and 150 mg/kg within 72 hours after administration (i.p.). The mortality rate increased with an increase in the dose concentration of PFE and luteolin. There was no mortality reported in animals administered orally with of 100, 200, or 300 mg/kg body weight of PFE and 30, 60, or 90 mg/kg body weight of luteolin.
Morphine-dependent rats
Administration of 200 and 300 mg/kg of PFE (P<0.05 vs. control and P<0.001 vs. control, respectively) and all doses of luteolin (30, 60, and 90 mg/kg) decreased significantly the number of jumps in morphine-dependent rats (P<0.001 vs. control) precipitated by administration of naloxone (5 mg/kg, i.p.) 2 hours after the last dose of morphine. The effect of PFE was dose-dependent. As expected, clonidine also reduced the number of jumps in animals (Figure 2).
Figure 2.
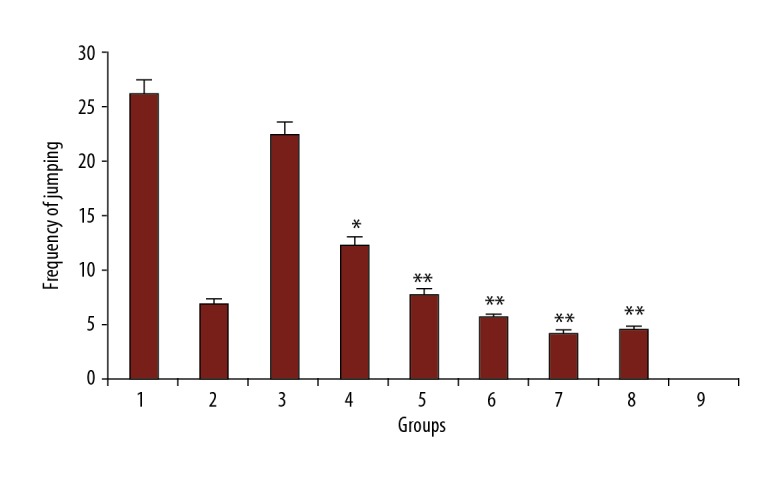
The frequency of jumping in rats. Group 1; frequency of jumping in morphine-dependent rats (control). Group 2; frequency of jumping in morphine-dependent rats with co-administration of clonidine hydrochloride (0.2 mg/kg i.p.) (positive control). Group 3, 4, and 5; frequency of jumping in morphine-dependent rats with co-administration of 100 mg/kg, 200 mg/kg, and 300 mg/kg PFE, respectively. Group 6, 7, and 8; frequency of jumping in morphine-dependent rats with co-administration of 30 mg/kg, 60 mg/ kg, and 90 mg/kg luteolin, respectively. Group 9; frequency of jumping in morphine nondependent rats (saline). PFE – Prosopis farcta extract.
Effect of the PFE and luteolin on the naloxone-induced rearing in morphine-dependent rats
Different doses of PFE (200 and 300 mg/kg) and luteolin (30, 60, and 90 mg/kg) decreased the rearing in morphine-dependent rats precipitated by administration of naloxone (5 mg/kg, i.p.) 2 hours after the last dose of morphine (P<0.001 vs. control). The effect of PFE and luteolin was dose-dependent. As expected, clonidine also reduced the number of the incidence of rearing in the study animals (Figure 3).
Figure 3.
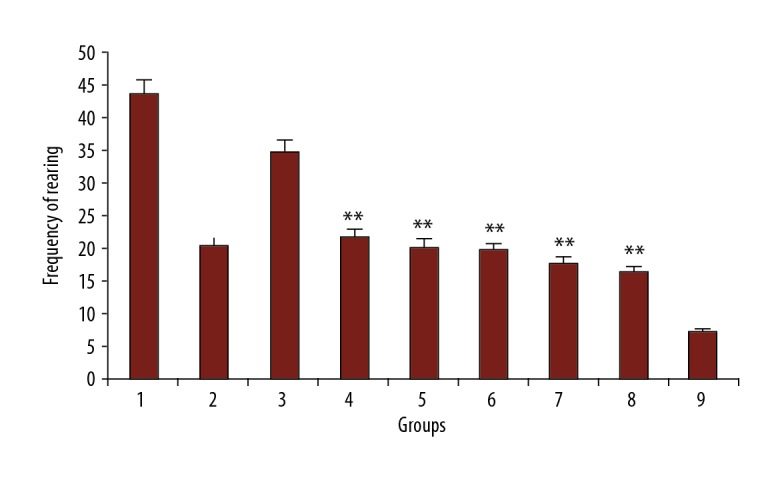
The frequency of rearing in rats. Group 1; frequency of jumping in morphine-dependent rats (control). Group 2; frequency of rearing in morphine-dependent rats with co-administration of clonidine hydrochloride (0.2 mg/kg i.p.) (positive control). Group 3, 4, and 5; frequency of rearing in morphine-dependent rats with co-administration of 100 mg/kg, 200 mg/kg and 300 mg/kg PFE, respectively. Group 6, 7, and 8; frequency of rearing in morphine-dependent rats with co-administration of 30 mg/kg, 60 mg/kg, and 90 mg/kg luteolin, respectively. Group 9; frequency of jumping in morphine nondependent rats (saline). PFE – Prosopis farcta extract.
Effect of the PFE and luteolin on the naloxone-induced teeth chattering in morphine-dependent rats
Different doses of PFE (200 and 300 mg/kg) and luteolin (30, 60, and 90 mg/kg) decreased the teeth chattering in morphine-dependent rats precipitated by administration of naloxone (5 mg/kg, i.p.) 2 hours after the last dose of morphine (P<0.001 vs. control). The effect of PFE and luteolin was dose-dependent. Also, clonidine reduced the number of the teeth chattering in animals (Figure 4).
Figure 4.
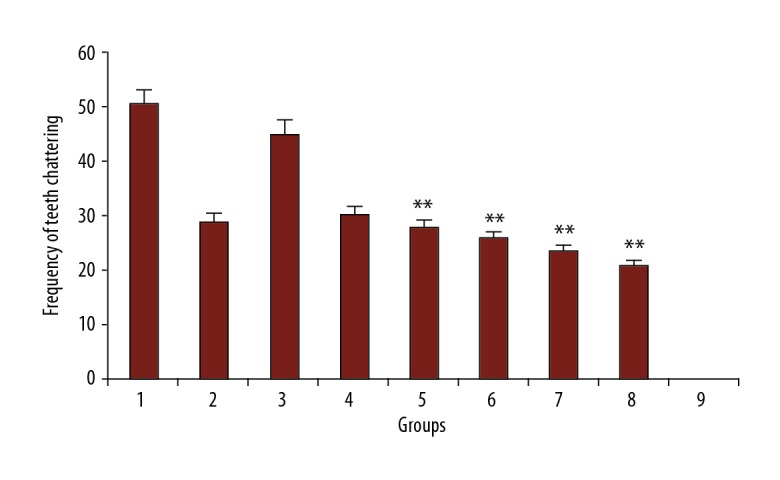
The frequency of teeth chattering in rats. Group 1; frequency of jumping in morphine-dependent rats (positive control). Group 2; frequency of teeth chattering in morphine-dependent rats with co-administration of clonidine hydrochloride (0.2 mg/kg i.p.). Group 3, 4, and 5; frequency of teeth chattering in morphine-dependent rats with co-administration of 100 mg/kg, 200 mg/kg, and 300 mg/ kg PFE, respectively. Group 6, 7, and 8; frequency of teeth chattering in morphine-dependent rats with co-administration of 30 mg/kg, 60 mg/ kg, and 90 mg/kg luteolin, respectively. Group 9; frequency of teeth chattering in morphine nondependent rats (saline). PFE – Prosopis farcta extract.
Discussion
In the present study, it was found that co-administration of PFE with morphine reduced the severity of the signs of morphine withdrawal syndrome induced by naloxone. These findings showed that the PFE was able to reduce the symptoms of morphine withdrawal syndrome. Our results demonstrated that the co-administration of either PFE or clonidine hydrochloride with morphine prior to withdrawal syndrome reduced the expected symptoms of naloxone in morphine-dependent rats. Also, our results indicated that increasing the dose of PFE significantly decreased the frequency of the signs of withdrawal syndrome including jumping, teeth chattering, and rearing following a naloxone trial. It has been reported that some substances such as magnesium, agmatine sulfate, yohimbine, midazolam and betacarbolines, harman and harmine, and 7-nitroindazole reduce the symptoms of withdrawal in the rat [21]. Also, it has been demonstrated that clonidine and lofexidine are more effective than placebo for the management of withdrawal from heroin or methadone [22]. The effect of medicinal plants to improve symptoms of morphine dependence has been studied. It has been reported that some medicinal plants, such as Mitragyna speciosa and Matricaria chamomilla, have been shown to relieve morphine withdrawal symptoms in rats [23,24]. However, no study has been published in scientific journals that investigated the effects of PFE on morphine withdrawal syndrome in rats. It has been reported that some species (i.e., P. spicigera) exhibited significant antidepressant-like effect and skeletal muscle relaxant activity by using the forced swimming and tail suspension tests [25]. Another species of this genus, such as the P. juliflora, has been shown to stimulate the immune system [26] and function as an acetylcholinesterase inhibitor and butyrylcholinesterase inhibitor with Ca2+ channel blocking activity [27]. It has been shown that the methanol stem bark extract of P. africana does possess significant analgesic and anti-inflammatory activities in laboratory animals and this supports the ethnomedical use of the plant in painful and inflammatory conditions [28]. However, more scientific research about this genus is necessary to promote its use as a source of natural medicines, analgesic compounds, and remedies for improvements in withdrawal syndrome. A number of phenolic compounds with strong antioxidant activity have been identified in PFEs such as vicenin-2, apigenin C-glycoside, isoorientin, vitexin, luteolin, and caffeic acid [11]. In this study, the luteolin HPLC chromatogram was obtained as a standard broad peak with a retention time at 4.898 min at a wavelength of 348 nm. Similar to our results, it has previously been reported that one of the active ingredients of PFE is luteolin [11]. The quantitative analysis revealed that luteolin was found to be predominant in methanol fraction (2.64 mg/g of luteolin) of P. farcta. In this study, it was observed that the concomitant use of luteolin and morphine before the naloxone challenge reduced the expression of withdrawal syndrome in morphine-dependent animals. It was demonstrated that the chronic co-administration of either luteolin or clonidine with morphine before the induction of withdrawal syndrome blocked naloxone-precipitated morphine withdrawal syndrome in morphine-dependent animals. Our results indicated that increasing the dose of luteolin significantly decreased the frequency of the signs of withdrawal syndrome including teeth chattering, jumping, and rearing following naloxone challenge. Luteolin, a flavonoid which is abundant in many herbs has been reported to possess neurotrophic properties via promoting neuronal survival and neuritis outgrowth [29]. It has also been reported that luteolin treatment increased protein kinase A (PKA) activity and cyclic AMP response element-binding protein (CREB) phosphorylation. Taken together, these results suggest that luteolin might upregulate small non-coding RNA micro RNA-132 (miR-132), an important regulator of neurotrophic actions by activating cAMP/PKA/CREB signaling pathways in PC12 cells [30]. In addition, it has been reported that the flavonoids such as luteolin, quercetin, naringenin, and chrysin promotes reduced iNOS (inducible nitric oxide synthase, an enzyme for synthesis of NO) and NO production at the same time, inhibiting neuronal death in microglia [31]. Some also have been confirmed to reduce inflammatory cytokines, thus highlighting their potential in reducing morphine tolerance via lowering neuroinflammation [32]. Furthermore, it has been demonstrated that pretreatment with luteolin suppressed seizure induction, duration, and severity following PTZ (interval on pentylenetetrazol) injection, reversed cognitive impairment, reduced neuronal and oxidative stress damage, and increased phosphoactivation of PKA and CREB as well as brain-derived neurotrophic factor (BDNF) expression [33]. In this study, taken together, these results demonstrate that the ameliorative effects of luteolin against naloxone challenge in rats may be modulated by α2 receptor or PKA pathway, and indicating that luteolin might be developed as a chemopreventive supplementary agent to ameliorate withdrawal syndrome. The inhibitory effect of the P. farcta total extract and luteolin fraction on the opioid withdrawal syndrome is comparable to clonidine. However, luteolin alleviates the morphine withdrawal syndrome markedly more than the total PFE.
LD50 of the total PFE and luteolin was 540 mg/kg and 150 mg/kg, respectively. It has been demonstrated that substances having LD50 values in the range of 50–500 mg/kg and 500–5000 mg/kg were considered moderately and slightly toxic, respectively [34]. The 90% of the total PFE and luteolin administered orally were moderately and slightly toxic, respectively in the rats. Moreover, the LD50 of the total PFE was 73% higher than that of luteolin, showing less toxicity of the total PFE compared to luteolin.
Conclusions
This study showed that clonidine compared with PFE at a dose of 100 mg/kg was effective in reducing symptoms of morphine withdrawal, but at a dose of 200 mg/kg and 300 mg/kg, no significant difference was found. However, luteolin at a dose of 30, 60, and 90 mg/kg had no significant difference compared to clonidine in attenuating the morphine withdrawal syndrome. LD50 of the extract of P. farcta and luteolin were 540 mg/kg and 150 mg/kg, respectively. Our findings suggest that PFE and luteolin can reduce the symptoms of withdrawal of narcotic drugs, and its effect in reducing these symptoms is equivalent to clonidine and may have potential for human therapies. Thus, these data suggest that perhaps PFE and luteolin might inhibit morphine abstinence by a clonidine-like effect.
Footnotes
Conflict of interest
None.
Source of support: This study was supported by Ilam University of Medical Sciences (Grant No. 87-04-33-8073)
References
- 1.Klein JW. Pharmacotherapy for substance use disorders. Med Clin North Am. 2016;100(4):891–910. doi: 10.1016/j.mcna.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Soyka M, Mutschler J. Treatment-refractory substance use disorder: Focus on alcohol, opioids, and cocaine. Prog Neuropsychopharmacol Biol Psychiatry. 2016;70:148–61. doi: 10.1016/j.pnpbp.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Reed K, Day E, Keen J, Strang J. Pharmacological treatments for drug misuse and dependence. Expert Opin Pharmacother. 2016;16(3):325–33. doi: 10.1517/14656566.2015.983472. [DOI] [PubMed] [Google Scholar]
- 4.Tzschentke TM. Where do we stand in the field of anti-abuse drug discovery? Expert Opin Drug Discov. 2015;9(11):1255–58. doi: 10.1517/17460441.2014.948415. [DOI] [PubMed] [Google Scholar]
- 5.Ward J, Rosenbaum C, Hernon C, et al. Herbal medicines for the management of opioid addiction: Safe and effective alternatives to conventional pharmacotherapy? CNS Drugs. 2011;25(12):999–1007. doi: 10.2165/11596830-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Tabatabai SM, Dashti S, Doosti F, Hosseinzadeh H. Phytotherapy of opioid dependence and withdrawal syndrome: A review. Phytother Res. 2014;28(6):811–30. doi: 10.1002/ptr.5073. [DOI] [PubMed] [Google Scholar]
- 7.Ebrahimie M, Bahmani M, Shirzad H, et al. A review study on the effect of Iranian herbal medicines on opioid withdrawal syndrome. J Evid Based Complementary Altern Med. 2015;20(4):302–9. doi: 10.1177/2156587215577896. [DOI] [PubMed] [Google Scholar]
- 8.Abdelmoteleb A, Valdez-Salas B, Ceceña-Duran C, et al. Silver nanoparticles from Prosopis glandulosa and their potential application as biocontrol of Acinetobacter calcoaceticus and Bacillus cereus. Chem Spec Bioavailab. 2017;29(1):1–5. [Google Scholar]
- 9.Sharifi-Rad J, Hoseini-Alfatemi SM, Sharifi-Rad M, et al. Phytochemical screening and antibacterial activity of Prosopis farcta different parts extracts against methicillin-resistant Staphylococcus aureus (MRSA) Minerva Biotec. 2014;26:287–93. [Google Scholar]
- 10.Mollashahi M, Tehranipour M, Khayyatzade J, et al. The neuroprotective effects of Prosopis farcta pod aqueous and ethanol extracts on spinal cord α-motoneurons neuronal density after sciatic nerve injury in rats. Life Sci J. 2013;10:293–97. [Google Scholar]
- 11.Harzallah-Skhiri F, BenJannet H. Flavonoids diversification in organs of two Prosopis farcta (banks & sol.) eig (Leguminosae, Mimosoideae) populations occurring in the northeast and the southeast of Tunisia. J Appl Sci Res. 2005;1(2):130–36. [Google Scholar]
- 12.López-Lázaro M. Distribution and biological activities of the flavonoid luteolin. Mini Rev Med Chem. 2009;9:31–59. doi: 10.2174/138955709787001712. [DOI] [PubMed] [Google Scholar]
- 13.Lin TY, Lu CW, Wang SJ. Luteolin protects the hippocampus against neuron impairments induced by kainic acid in rats. Neurotoxicology. 2016;55:48–57. doi: 10.1016/j.neuro.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Cheng H, Che Z. Ameliorating effect of luteolin on memory impairment in an Alzheimer’s disease model. Mol Med Rep. 2016;13:4215–20. doi: 10.3892/mmr.2016.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez A, de Tangil MS, Vega-Orellana O, et al. Phenolic constituents, antioxidant and preliminary antimycoplasmic activities of leaf skin and flowers of Aloe vera (L.)Burm. f. (syn. A. barbadensis Mill.) from the Canary Islands (Spain) Molecules. 2013;18(5):4942–54. doi: 10.3390/molecules18054942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu QZ, Yu AH, Xi YW. Development and evaluation of penciclovir-loaded solid lipid nanoparticles for topical delivery. Int J Pharm. 2009;272:191–98. doi: 10.1016/j.ijpharm.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Parmar NS, Prakash S. Screening methods in pharmacology. New Delhi: Alpha Science International Ltd.; 2006. [Google Scholar]
- 18.El Kabbaoui M, Chda A, El-Akhal J, et al. Acute and sub-chronic toxicity studies of the aqueous extract from leaves of Cistus ladaniferus L. in mice and rats. J Ethnopharmacol. 2017;209:147–56. doi: 10.1016/j.jep.2017.07.029. [DOI] [PubMed] [Google Scholar]
- 19.Romandini S, Cervo L, Samanin R. Evidence that drugs increasing 5-hydroxytryptamine transmission block jumping but not dog shakes in morphineabstinent rats: A comparison with clonidine. J Pharm Pharmacol. 1984;36:68–70. doi: 10.1111/j.2042-7158.1984.tb02995.x. [DOI] [PubMed] [Google Scholar]
- 20.Feily A, Abbasi N. The inhibitory effect of Hypericum perforatum extract on morphine withdrawal syndrome in rat and comparison with clonidine. Phytother Res. 2009;23(11):1549–52. doi: 10.1002/ptr.2807. [DOI] [PubMed] [Google Scholar]
- 21.Aricioglu-Kartal F, Uzbay IT. Inhibitory effect of agmatine on naloxone-precipitated abstinence syndrome in morphine dependent rats. Life Sci. 1997;61:1775–81. doi: 10.1016/s0024-3205(97)00801-1. [DOI] [PubMed] [Google Scholar]
- 22.Gowing L, Farrell MF, Ali R, White JM. Alpha2-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev. 2014;3:1–74. doi: 10.1002/14651858.CD002024.pub4. [DOI] [PubMed] [Google Scholar]
- 23.Cheaha D, Reakkamnuan C, Nukitram J, et al. Effects of alkaloid-rich extract from Mitragyna speciosa (Korth.) Havil. on naloxone-precipitated morphine withdrawal symptoms and local field potential in the nucleus accumbens of mice. J Ethnopharmacol. 2017;208:129–37. doi: 10.1016/j.jep.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Gomaa A, Hashem T, Mohamed M, Ashry E. Matricaria chamomilla extract inhibits both development of morphine dependence and expression of abstinence syndrome in rats. J Pharmacol Sci. 2003;92:50–55. doi: 10.1254/jphs.92.50. [DOI] [PubMed] [Google Scholar]
- 25.George M, Joseph L, Sharma A. Antidepressant and skeletal muscle relaxant effects of the aqueous extract of the Prosopis cineraria. Braz J Pharm Sci. 2012;48(3):577–81. [Google Scholar]
- 26.Ahmad A, Khursheed KA, Ahmad V. Immunomodulating effect of juliflorine on the antibody response to Listeria hemolysin. Med J Islam World Acad Sci. 1992;5:189–93. [Google Scholar]
- 27.Choudhary MI, Nawaz SA, Azim MK, et al. Juliflorine: A potent natural peripheral anionic-site-binding inhibitor of acetylcholinesterase with calcium-channel blocking potential, a leading candidate for Alzheimer’s disease therapy. Biochem Biophys Res Commun. 2005;332(4):1171–79. doi: 10.1016/j.bbrc.2005.05.068. [DOI] [PubMed] [Google Scholar]
- 28.Ayanwuyi LO, Yaro AH, Abodunde OM. Analgesic and anti-inflammatory effects of the methanol stem bark extract of Prosopis africana. Pharm Biol. 2010;48(3):296–99. doi: 10.3109/13880200903121006. [DOI] [PubMed] [Google Scholar]
- 29.Cheng HY, Hsieh MT, Tsai FS, et al. Neuroprotective effect of luteolin on amyloid β protein (25–35)-induced toxicity in cultured rat cortical neurons. Phytother Res. 2010;24(Suppl 1):S102–8. doi: 10.1002/ptr.2940. [DOI] [PubMed] [Google Scholar]
- 30.Lin LF, Chiu SP, Wu MJ, et al. Luteolin induces microRNA-132 expression and modulates neurite outgrowth in PC12 cells. PLoS One. 2012;7:293. doi: 10.1371/journal.pone.0043304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spencer JPE, Vafeiadou K, Williams RJ, et al. Neuroinflammation: Modulation by flavonoids and mechanisms of action. Mol Aspects Med. 2012;33:83–97. doi: 10.1016/j.mam.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 32.Abd Aziz CB, Ismail CAN, Che Hussin CM, et al. The antianalgesic effects of A. dorsata honey in male Sprague-Dawley rats: A preliminary study. J Tradit Complement Med. 2014;4:298–302. doi: 10.4103/2225-4110.139115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhen JL, Chang YN, Qu ZZ, et al. Luteolin rescues pentylenetetrazole-induced cognitive impairment in epileptic rats by reducing oxidative stress and activating PKA/CREB/BDNF signaling. Epilepsy Behav. 2016;57:177–84. doi: 10.1016/j.yebeh.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Ruiz P, Begluitti G, Tincher T, et al. Prediction of acute mammalian toxicity using QSAR methods: A case study of sulfur mustard and its breakdown products. Molecules. 2012;17(8):8982–9001. doi: 10.3390/molecules17088982. [DOI] [PMC free article] [PubMed] [Google Scholar]