Abstract
Background
Arachis contains 80 species that carry many beneficial genes that can be utilized in the genetic improvement of peanut (Arachis hypogaea L. 2n = 4x = 40, genome AABB). Chromosome engineering is a powerful technique by which these genes can be transferred and utilized in cultivated peanut. However, their small chromosomes and insufficient cytological markers have made chromosome identification and studies relating to genome evolution quite difficult. The development of efficient cytological markers or probes is very necessary for both chromosome engineering and genome discrimination in cultivated peanut.
Results
A simple and efficient oligonucleotide multiplex probe to distinguish genomes, chromosomes, and chromosomal aberrations of peanut was developed based on eight single-stranded oligonucleotides (SSONs) derived from repetitive sequences. High-resolution karyotypes of 16 Arachis species, two interspecific F1 hybrids, and one radiation-induced M1 plant were then developed by fluorescence in situ hybridization (FISH) using oligonucleotide multiplex, 45S and 5S rDNAs, and genomic in situ hybridization (GISH) using total genomic DNA of A. duranensis (2n = 2x = 20, AA) and A. ipaënsis (2n = 2x = 20, BB) as probes. Genomes, chromosomes, and aberrations were clearly identifiable in the established karyotypes. All eight cultivars had similar karyotypes, whereas the eight wild species exhibited various chromosomal variations. In addition, a chromosome-specific SSON library was developed based on the single-copy sequence of chromosome 6A of A. duranensis. In combination with repetitive SSONs and rDNA FISH, the single-copy SSON library was applied to identify the corresponding A3 chromosome in the A. duranensis karyotype.
Conclusions
The development of repetitive and single-copy SSON probes for FISH and GISH provides useful tools for the differentiation of chromosomes and identification of structural chromosomal rearrangement. It facilitates the development of high-resolution karyotypes and detection of chromosomal variations in Arachis species. To our knowledge, the methodology presented in this study demonstrates for the first time the correlation between a sequenced chromosome region and a cytologically identified chromosome in peanut.
Electronic supplementary material
The online version of this article (10.1186/s12870-018-1468-1) contains supplementary material, which is available to authorized users.
Keywords: Arachis species, Chromosome painting, Genomic evolution, High-resolution karyotype, Oligonucleotide multiplex
Background
Cultivated peanut (Arachis hypogaea L.) is an allotetraploid species that was evolved from a cross of two wild diploid progenitors, A. duranensis and A. ipaënsis [1]. It is widely cultivated worldwide as both an oil and cash crop. In 2016, total peanut production around the world was 43,982,063 tons and average yield was 1710.49 kg ha− 1 [2]. However, peanut productivity is severely constrained by diseases and pests. The development of resistant cultivars is time-consuming and has been challenged by the existence of quantitative resistance traits and limited resistance resources in the cultivated gene pool [1].
The genus Arachis comprises 80 species [1], many of which potentially harbor beneficial genes for peanut improvement. Chromosome engineering is a useful approach by which genes from related species can be transferred and exploited. However, it is difficult to cytologically distinguish chromosomes of wild species because of their small size and the paucity of cytological markers. Therefore, many species, such as Arachis batizocoi, A. cruziana, A. krapovickasii, A. benensis, A. trinitensis, A. decora, A. palustris, and A. praecox have had incorrectly classified genomes [3, 4]. Staining with 4′, 6-diamidino-2-phenylindole (DAPI) and the use of rDNA probes are major tools that are traditionally used to differentiate peanut chromosomes [5–8]. The application of centromeric and telomeric repeat probes has significantly improved the ability to resolve Arachis karyotypes [9, 10]. Zhang et al. [11] identified seven A. duranensis bacterial artificial chromosome (BAC) clones that facilitated the differentiation of almost all chromosomes of peanut. Unfortunately, the existing markers were primarily distributed in the centromeric or telomeric regions with limited chromosomal polymorphism. In addition, the preparation of these markers is time-consuming and expensive.
SSONs, short DNA or RNA fragments, can be modified and directly hybridized with chromosomal DNA, thereby potentially replace traditional plasmid clones [12]. Oligopainting, using repetitive and single-copy sequences as probes, is a simple and efficient method that is being increasingly used in a wide variety of organisms, including humans [13], propionibacteria [14], cucumber [15], wheat [16], and maize [17]. Two types of SSON probes are currently available: repetitive and single-copy. Repetitive SSONs can be derived from simple sequence repeats (SSRs), genomic sequence repeats (GSRs), and multi-copy genetic sequences (MCGSs) [18–21]. Single-copy SSONs include single-gene and single-copy genomic oligonucleotides [15, 22]. Repetitive SSONs, generally clustered together on one or several genomes or chromosomes, are frequently used to develop high-resolution karyotypes and identify chromosomes [16, 17, 23]. Single-copy SSONs are usually chromosome-specific, and are thus used to track specific chromosomes or chromosomal fragments and identify homoeologous chromosomes in closely related species [13, 15, 24]. In addition, SSONs can be used for species identification [25], haplotype analysis of homologous chromosomes [26], and RNA analysis of single cells [27, 28].
The availability of genome sequences of peanut and its donor species A. duranensis (A-genome) and A. ipaënsis (B-genome) [29], has facilitated the development of peanut SSON probes and provided a novel method by which peanut chromosomal structure and genomic evolution could be deciphered. The aim of the present study was to investigate chromosomal structural rearrangement and genome differentiation through high-resolution chromosome painting in the genus Arachis. The specific objectives were to: 1) develop repetitive SSON probes using A. duranensis genomic sequences, SSRs, and telomeric repeats; 2) utilize multiplex probes for simple and efficient chromosome identification; 3) compare high-resolution karyotypes of eight commercial cultivars, eight wild species, two interspecific F1 hybrids, and one radiation-induced M1 plant to reveal chromosomal rearrangements and genomic evolution; and 4) construct a chromosome-specific single-copy SSON library from the sequenced chromosome 6A to identify the corresponding chromosome in the karyotype.
Results
Development of repetitive SSON probes
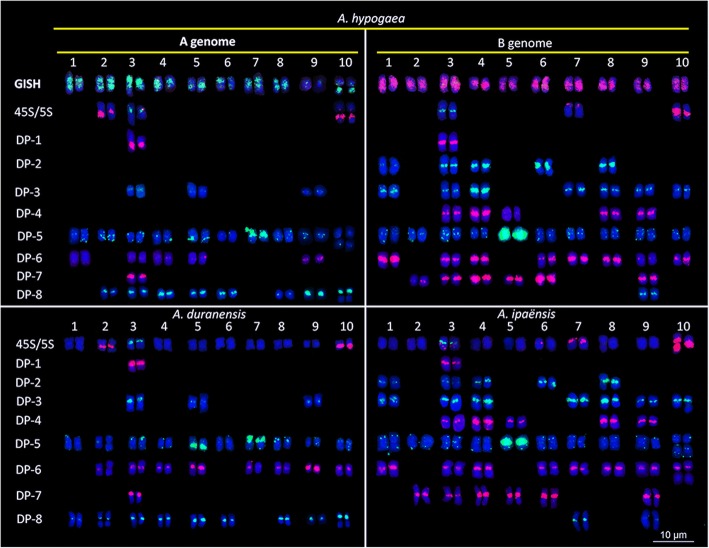
Eight repetitive SSON probes were identified and designed from different repetitive sequence sources. Probe sequences and the source of repetitive sequences are listed in Table 1. Sequential FISH/GISH was performed to position these SSONs on chromosomes of Silihong (SLH), A. duranensis (the A-genome donor), and A. ipaënsis (the B-genome donor). All repetitive SSON probes generated distinguishable, stable, and clear signals on chromosomes of SLH, A. duranensis, and A. ipaënsis (Fig. 1 and Additional file 1: Figure S1 Additional file 2: Figure S2, Additional file 3: Figure S3, Additional file 4: Figure S4). The DP-1 probe produced signals that completely overlapped with those produced by 5S rDNA plasmid clones in SLH (Fig. 1 and Additional file 1: Figure S1). Signals from DP-5 were distributed at the chromosome ends and in the centromeric, pericentromeric, and intercalary regions. They particularly formed larger clusters of strong signals in the long arm of Ahy5 and the short arm of Ahy7 of the A-genome, and in the centromeric region of Bhy5 of the B-genome (Fig. 1; Additional file 3: Figure S3). The DP-6 probe produced signals in 13 chromosomes of SLH. With the exception of one signal, which was detected on the long arm of chromosome Ahy1, all other signals were found in the centromeric regions of A- and B-genome chromosomes with various intensities. Most of the clear signals of DP-2, DP-3, DP-4, and DP-7 were observed only in the centromeric regions of B-genome chromosomes (Fig. 1). However, one exception was observed for DP-7, which revealed a clear signal in chromosome A3 near the 5S rDNA region, whereas an absence of, or sporadically weak signals were detected on the other A-genome chromosomes. In contrast to the four former SSONs, DP-8 only produced signals on eight A-genome chromosomes and on one B-genome chromosome in SLH (Fig. 1 and Additional file 4: Figure S4).
Table 1.
Single-stranded oligonucleotide (SSON) probes developed in this study
| SSON | Sequence and 5′- end modification | Origin |
|---|---|---|
| DP-1 | TAMRA-5′-TGCGATCATACCAGCACTAATGCACCGGATCCCGTCAGAACTCTGAAGTTAAGCGTG − 3′ | 5S rDNA (GenBank: M10470.1) |
| DP-2 | FAM-5′-ACTACTACTACTACTACTACTACTACTACT-3′ | (ACT)10 |
| DP-3 | FAM-5′- CATTAAATCAGTTATAGTTTGTTTGATGGTA-3′ | Genomic DNA |
| DP-3 | TAMRA −5′- CATTAAATCAGTTATAGTTTGTTTGATGGTA-3′ | Genomic DNA |
| DP-4 | TAMRA-5′-ATTATTATTATTATTATTATTATTATTATT-3′ | (ATT)10 |
| DP-5 | FAM-5′-TTTAGGGTTTAGGGTTTAGGGTTTAGGGTTTAGGGTTTAGGGTTTAGGGTTTAGGG-3′ | Telomere repeats |
| DP-6 | TAMRA-5′-AAAAAATCGGAGGAGCCTGCCGAAGATGAGG-3′ | Genomic DNA |
| DP-7 | FAM −5′-AAAAAAAAAAAAAAAAAAAAAAAAAAAAAAA-3′ | Genomic DNA |
| DP-7 | TAMRA-5′-AAAAAAAAAAAAAAAAAAAAAAAAAAAAAAA-3′ | Genomic DNA |
| DP-8 | FAM −5′-TGAAAACTTTTTATTTTTAAATTTTGAAACT-3′ | Genomic DNA |
| DP-8 | TAMRA-5′-TGAAAACTTTTTATTTTTAAATTTTGAAACT-3′ | Genomic DNA |
Fig. 1.
FISH mapping of DP-1 (red), DP-2 (green), DP-3 (green), DP-4 (red), DP-5 (green), DP-6 (red), DP-7 (red), and DP-8 (green) by sequential FISH using 45S rDNA (red), 5S rDNA (green), and A. duranensis (green) and A. ipaënsis (red) total genomic DNA as probes in Arachis hypogea cv. SLH, A. duranensis, and A. ipaënsis. Blue color represents chromosomes counterstained with DAPI
The eight SSON probes produced almost identical signals between the Bi- and Bhy-genome chromosomes of the wild donor A. ipaënsis and SLH. The only difference was that DP-8 produced a signal only on chromosome Bhy9 of SLH, but produced an additional signal on Bi7 in A. ipaënsis. Most signals generated by these eight probes were also similar between the A-genome chromosomes of A. duranensis and SLH, with only few exceptions. For instance, the signal of DP-5 at the interstitial position on the long arm of Ahy5 was weaker than that on Adu5. The DP-6 probe produced signals at centromeric positions on the chromosomes of both species (A3, A4, A5, and A9), and other signals in SLH only (Ahy1), or A. duranensis only (Adu2, Adu7, Adu8, and Adu10), or produced no signal at all (Adu1).
High-resolution karyotyping of peanut based on oligopainting using a repetitive SSON multiplex probe
A multiplex #1 repetitive SSON probe was developed according to the signal patterns of seven repetitive SSON probes that included 6-carboxyfluorescein (FAM) modified DP-2, 5, and 7, and 6-carboxytetramethylrhodamine (TAMRA) modified DP-1, 3, 4, and 6 (Table 2). Sequential FISH/GISH using Multiplex #1, total genomic DNA of A. duranensis and A. ipaënsis, and 45S and 5S rDNA probes facilitated the development of a high-resolution karyotype for SLH (Additional file 5: Figure S5). Significant differences in Ahy and Bhy chromosomes were visible in this karyotype. Among Bhy chromosomes, Bhy5 displayed distinct green centromeric signals, whereas Bhy8 and Bhy10 exhibited strong red centromeric signals. Both red and green signals were detected in the centromeric regions of all remaining chromosomes, with the exception of Bhy2, which displayed no signals. Strong signals of both colors were detected on Bhy3, Bhy4, Bhy6, and Bhy9, whereas weaker signals of both colors were detected on Bhy1, and only strong red signal was detected on Bhy7. Signals of 45S or 5S rDNA were detected on Bhy3, Bhy7, and Bhy10. Among the Ahy chromosomes, Ahy7 and Ahy5, quite unlike the other Ahy chromosomes, showed strong interstitial telomeric signals, on the short arm of Ahy7 and on the long arm of Ahy5. Signals associated with both 45S and 5S rDNA loci were detected on Ahy2, Ahy3, and Ahy10. Furthermore, Ahy6 displayed no signals, Ahy4 and Ahy8 displayed large green centromeric signals, and small red signals were detected on the long arm of Ahy1 (Fig. 2, Additional file 5: Figure S5 and Additional file 6: Figure S6).
Table 2.
Single-stranded oligonucleotide (SSON) multiplexes developed in this study
| Multiplex | SSON with 5′- end modified | Concentration (ng/μL) |
|---|---|---|
| #1 | FAM - DP-2 | 100 |
| FAM - DP-5 | 100 | |
| FAM - DP-7 | 26.67 | |
| TAMRA - DP-1 | 33.33 | |
| TAMRA - DP-3 | 100.00 | |
| TAMRA - DP-4 | 100.00 | |
| TAMRA - DP-6 | 100.00 | |
| #2 | FAM - DP-2 | 100 |
| FAM - DP-5 | 100 | |
| FAM - DP-7 | 26.67 | |
| TAMRA - DP-8 | 26.67 |
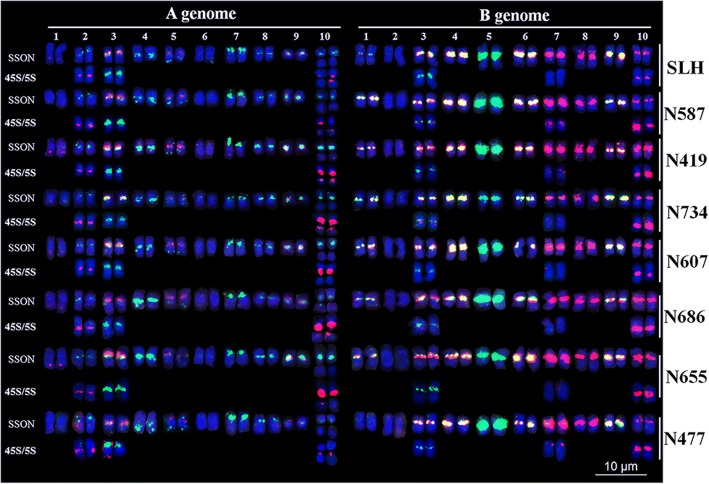
Fig. 2.
Karyotypes of eight peanut varieties. Lines labeled as SSON and 45S/5S refer to signals from Multiplex #1 and rDNAs, respectively. Blue color represents chromosomes counterstained with DAPI. In SSON, red represents signals of DP-1, DP-3, DP-4, and DP-6; and green represents signals of DP-2, DP-5, and DP-7. In 45S/5S, red represents signals of 45S rDNA and green represents signals of 5S rDNA
To validate the potential of Multiplex #1, seven other cultivars (N686, N607, N655, N734, N477, N587, and N419) were analyzed. In general, almost all chromosomes of these cultivars were similar to those of SLH, and the karyotypes of all cultivars remained conservative, with the exception of chromosomes Ahy10 and Bhy1, both of which showed minor variations in the centromeric and 45S rDNA regions of different cultivars (Fig. 2).
Chromosomal structural rearrangements revealed in wild Arachis species
To assay genomic differentiation and chromosomal rearrangements, the chromosomes of eight wild species were discriminated by sequential FISH/GISH using Multiplex #1 (Additional file 6: Figure S6 and Additional file 7: Figure S7). High-resolution karyotypes (Fig. 3) and idiograms of these species (Additional file 8: Figure S8) were developed and compared. Among these species, the genome of A. duranensis and A. ipaënsis showed a high similarity to the respective A and B genomes of SLH; however, a greater number of green signals were observed on the long arm of Adu5 than on Ahy5 (Fig. 3 and Additional file 8: Figure S8).
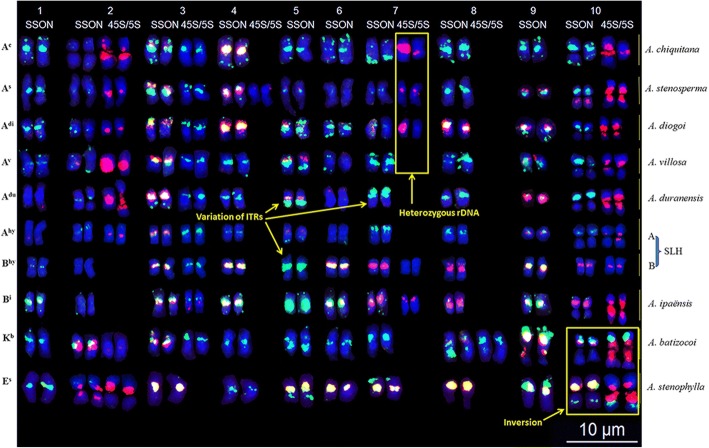
Fig. 3.
Karyotypes of nine Arachis species. Columns labeled as SSON and 45S/5S refer to signals from Multiplex #1 and rDNAs, respectively. Probe color same as Fig. 2
Significant differences were observed on chromosomes 1, 4, 5, 6, 7, 8, 9, and 10 of six A-genome species (SLH, A. duranensis, A. chiquitana, A. stenosperma, A. diogoi, and A. villosa). Signals were absent from chromosomes Ahy1, Adu1, As6, Av6, and Adu6, whereas green signals were present on the other chromosomes. Chromosomes Ahy4, Adu4, Av4, Ahy8, Adu8, Av8, As8, and Ac8 exhibited green signals only, whereas Ac4, As4, Adi4, and Adi8 displayed both red and green signals. With the exception of the much larger chromosome, Ac9, which exhibited only green signals, chromosomes A9 among the other species were similar in size and displayed both green and red signals (Fig. 3 and Additional file 8: Figure S8). Green signals were absent from the long arm of chromosomes As5 and Ac5, and red signals were absent from the centromere of Ac5. On chromosomes A7, green signals were detected at the ends of the short arms of Ahy7 and Adu7, the end of the long arm of Ac7, and in the centromeric region of Av7. Red signals were observed on chromosome As10 at the centromere. Based on these signal patterns, the genome of A. duranensis was evidently most similar to the Ahy chromosomes of SLH, followed by A. villosa, A. stenosperm, A. diogoi, and A. chiquitana (Fig. 3 and Additional file 8: Figure S8).
Karyotypes of A. batizocoi and A. stenophylla accessions were significantly different from the A and B genomes. In both species, green signals covered almost the entire short arm of chromosome 9, and 45S and 5S rDNA loci were detected on chromosome 10. The chromosomes of A. batizocoi and A. stenophylla were quite different from each other; A. batizocoi displayed 5S rDNA signals on Kb3 and Kb8, whereas A. stenophylla did not; ES5, ES6, and ES7 exhibited both red and green signals in their centromeric regions, whereas only green signals were observed in these regions on Kb5, Kb6, and Kb7. Furthermore, the distribution of 45S and 5S rDNA loci showed opposite trends between Kb10 and ES10, suggesting an inversion in one of the two species (Fig. 3 and Additional file 8: Figure S8).
Comparative analysis of all karyotypes revealed three types of structural rearrangements in the wild species studied. First, heterogeneous rDNA loci were found in different homologous chromosome pairs. For example, 45S rDNA signals differed between the homologous chromosomes 7 in species of the A-genome. In particular, the intensity of the 45S rDNA signals were identical in As7 and differed in Ac7. The 45S rDNA signal was detected only on the Adi7 chromosome; however, these signals were lacking on Adu7 (Fig. 3 and Additional file 8: Figure S8). Second, interstitial telomeric signal positions and amplifications were variable. Signals of different intensities and sizes were found at the chromosome ends (Adu7 and Es9), in the middle of chromosome arms (Adu5), or near centromeres (Bhy5) (Fig. 3 and Additional file 8: Figure S8). Third, chromosome morphology was variable among different species. For example, chromosomes Ac9 and As9 were small and metacentric; whereas Es9 was submetacentric and Es8 was subtelocentric (Additional file 8: Figure S8).
Chromosomal characterization of interspecific F1 hybrids and an irradiation-induced M1 plant
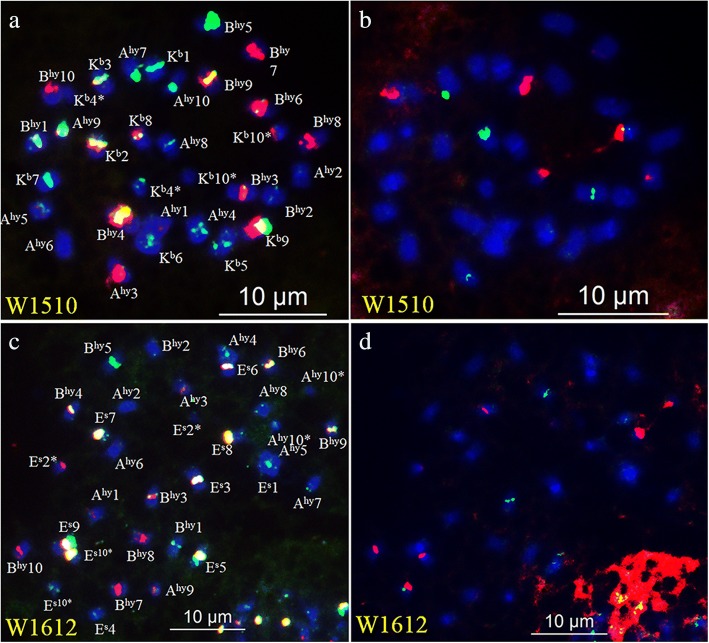
To further validate the potential of Multiplex #1, two interspecific F1 hybrids were examined by sequential FISH/GISH. Two genome sets of the hybrid w1510 were identical to Ahy and Bhy of SLH, and the third genome set was the same as Kb of A. batizocoi (Fig. 4 and Additional file 9: Figure S9). For w1612, two genome sets were identical to Ahy and Bhy of N734, and the third was similar to ES of A. stenophylla (Fig. 4 and Additional file 9: Figure S9). These results indicate that both w1510 and w1612 were true F1 hybrids.
Fig. 4.
Sequential FISH of two hybrids F1 w1510 and w1612 using Multiplex #1 (a and c), and 5S and 45S rDNA (b and d) plasmid clones as probes. a–b: w1510 derived from SLH (A. hypogaea) and A. batizocoi; c–d: w1612 derived from N734 (A. hypogaea) and A. stenophylla. Probe color as Fig. 2
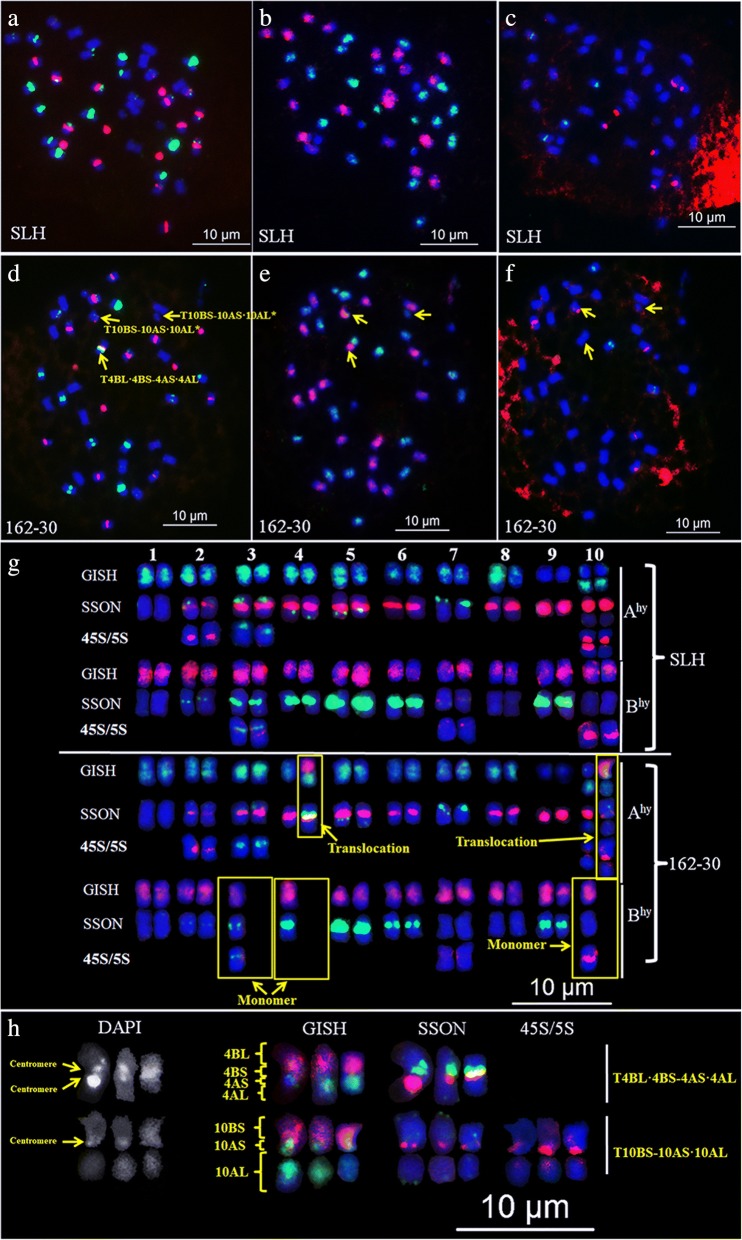
Multiplex #2, which included TAMRA-modified DP-8, and FAM-modified DP-2, DP-5, and DP-7, was used for oligopainting with sequential GISH/FISH to characterize a radiation-induced M1 plant (162–30). By applying this probe, we were able to identify two chromosomal translocations and one monosomic chromosome (Fig. 5a ~ 5f). One translocation between Ahy4 and Bhy4 that contained two centromeric DAPI bands and SSON centromeric bands of both colors, formed a potential dicentric chromosome T4BL·4BS-4AS·4AL. The other translocation, T10BS-10AS·10AL, involved a large satellite. The monosomic chromosome was identified in the Bhy3 chromosome because of its unique signal patterns (Fig. 5g and h).
Fig. 5.
Chromosome aberrations detected in radiation-induced M1 plant 162–30 of peanut cultivar SLH after sequential FISH/GISH. a–f Results of sequential FISH/GISH in SLH (a–c) and M1 plant 162–30 (d–f) using Multiplex #2 (a and d), A. duranensis genomic DNA (b and e; green), A. ipaënsis genomic DNA (b and e; red), 5S rDNA (c and f; green), and 45S rDNA (c and f; red). g Karyotypes of SLH and 162–30. h Translocated chromosomes in 162–30. Multiplex #2 contains four SSONs, including TAMRA-DP-8, FAM-DP-2, FAM-DP-5, and FAM-DP-7
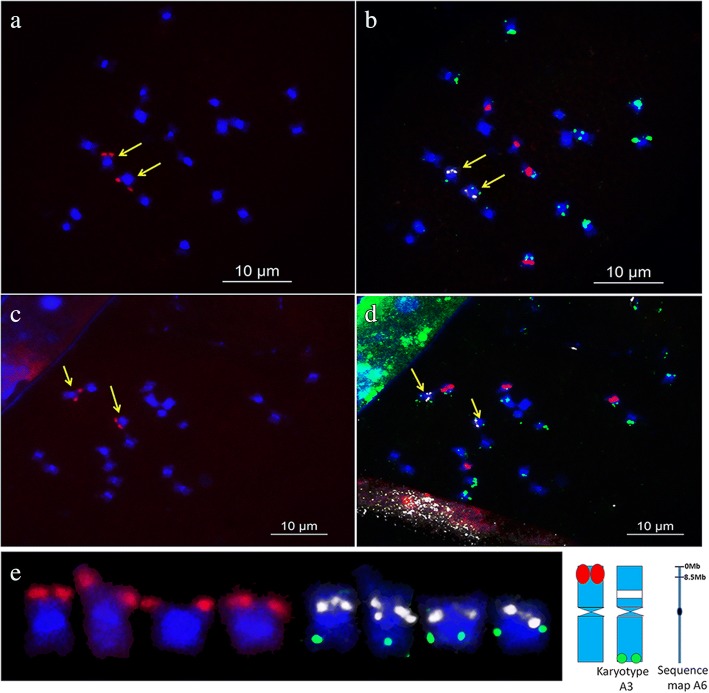
Oligopainting using the single-copy sequence library of chromosome 6A as probes, decorated the cytologically designated chromosome A3 of A. duranensis
To correlate a sequenced chromosome with a cytologically identified chromosome, a single-copy SSON library was developed from a ~ 8.5 Mb distal region of 6A and used to paint metaphase chromosomes of A. duranensis. A pair of clear signals was reputably detected on one pair of chromosomes of A. duranensis (Additional file 10: Figure S10). Sequential FISH using 45S, 5S rDNA, and DP-5 probes (Additional file 11: Figure S11) revealed that the target signals were present in cytologically designated chromosome A3, which displayed a typical distribution of 5S rDNA on its short arm (Fig. 6).
Fig. 6.
Oligopainting using single-copy oligonucleotide library 6A-1 combined with sequential FISH using DP-5, and plasmid clones 45S rDNA and 5S rDNA as probes in A. duranensis. Blue color represents chromosomes counterstained with DAPI; a and c shows oligopainting using single-copy SSON library 6A-1 (green); b and d, sequential FISH using repetitive SSON DP-5 (green), 45S rDNA (red), and 5S rDNA (white inverted from the original green); c From left to right: chromosome with signals of library 6A-1 (green); karyotype of A3, probe signals are same as (b) and (d); idiograms of the chromosome displaying signals from library 6A-1 and the karyotype of A3; and sequence map of A6 showing 0–8.5 Mb regions corresponding to library 6A-1. Yellow arrows in panels (a–d) indicate chromosomes with signals from library 6A-1
Discussion
Oligopainting using repetitive and single-copy sequences as probes provides a simple and efficient method for chromosome identification in peanut
Chromosome identification has previously been carried out in peanut using primarily FISH with 45S and 5S rDNA probes and DAPI staining [6–8]. Zhang et al. [11] recently developed seven BAC clones in Arachis as powerful new markers for chromosome identification. In the present study, eight repetitive SSON probes and one single-copy SSON library were successfully developed, and the repetitive SSON probes were combined into two powerful multiplexes (Multiplex #1 and Multiplex #2) for efficient chromosome discrimination. The two multiplexes effectively differentiated the chromosomes and genomes of eight peanut cultivars, eight wild species, two interspecific hybrids, and one radiation-induced M1 plant, and revealed structural rearrangements in these species and accessions. Repetitive SSON probes for the identification of peanut chromosomes can be easily designed and modified, thus greatly reducing the required time and expense. The repetitive SSON probes can be used advantageously in nondenaturing FISH, thereby further simplifying the FISH procedure [16]. Our findings demonstrated that repetitive SSON probes could be useful and provide powerful tools for peanut chromosome engineering. However, the current SSONs did not cover all chromosome regions and involved only limited types of repetitive sequences. A further study should utilize various sources of repetitive sequences to identify specific chromosomes or regions.
The International Peanut Sequencing Project has sequenced the reference genomes of A. duranensis and A. ipaënsis [29]. However, no direct correspondence has been established between the peanut linkage map and cytologically identified chromosomes. Based on the genome sequence of chromosome A6 of A. duranensis, we successfully developed a chromosome-specific single-copy SSON library, which could be used to paint the target chromosomes. By using both the repetitive SSONs and single-copy sequences as probes for oligopainting, we identified the target chromosomes and successfully correlated a sequenced A6 segment to the actual chromosome. This opens a path to further correlation of all sequenced chromosomes with cytologically identified chromosomes. The development of more single-copy SSON libraries for each chromosome of peanut is underway at our institute.
New clues for genomic evolution revealed by painting of Arachis species
In the cultivated peanut and its wild ancestors, chromosome painting with the eight probes generated fewer and weaker signals on A-genome chromosomes than on those of the B-genome. For example, three SSON probes, DP-2, − 4, and − 7, derived from SSRs (ACT)10, (ATT)10, and (A)31, respectively, rarely produced signals on A-genome chromosomes, but yielded strong signals on chromosomes of the B-genome, which showed more SSRs variations in A. ipaënsis than in A. duranensis. These results are consistent with the fact that although mono-, di-, and tri-nucleotides are the most abundant motifs in A. duranensis and A. ipaënsis, a greater number of SSRs are present in A. ipaënsis than in A. duranensis [30]. In addition, A. ipaënsis contains more transposable elements than A. duranensis does [29]. This suggests that the B-genome has more repeats, and may explain why the size of B-genome is larger than that of the A-genome (1.56 Gb vs 1.25 Gb) [29]. Other repetitive SSONs also produced weaker and fewer signals on A chromosomes, in comparison to B chromosomes. For example, signals between Bi and Bhy genomes, with the exception of DP-8 on chromosome 7, were almost identical in terms of position and intensity; however, fewer signals were present on four chromosomes of the Ahy genome relative to Adu. Moreover, some signals on the Ahy genome, such as Ahy5, varied according to their quantity and position (Fig. 1), suggesting that during evolution, large chromosomal rearrangements occurred in the A-genome chromosomes rather than in the B-genome chromosomes. These results correspond well with the findings of Bertioli et al. [29] and Zhang et al. [11].
Although the eight tested cultivars represented three different peanut taxa (varieties. Fastigiata, hypogea, and vulgaris), their karyotypes were similar to each other, even though a signal variation was found between chromosomes Ahy10 and Bhy1. This observation provides evidence that these cultivated peanut varieties arose from a single allotetraploid species [31].
Our results indicated that the genome of A. duranensis was most similar to the A-genome of the cultivated peanut, followed by the genomes of A. villosa, A. stenosperma, A. diogoi, and A. chiquitana, supporting the contention that A. duranensis was the donor of the A-genome [7]. Similarly, the identical karyotypes observed between A. ipaënsis and the B-genome of cultivated peanut supports the notion of A. ipaënsis as the B-genome donor [3, 8, 29, 32].
Chromosome identification by oligopainting likewise facilitates the classification of species. A. chiquitana was originally grouped with P genome species [33]. However, because it carried a small “A chromosome” and showed similar 45S rDNA, 5S rDNA, and DAPI banding patterns to those of A-genome species, it was regrouped with species of the A-genome [7]. In the present study, the karyotype of A. chiquitana was considerably different from those of other species. For example, the morphology and repetitive SSON banding pattern of Ac9 were very distinct from those of A9 chromosomes of other species with the A-genome. The genome of A. chiquitana thus does not seem to be associated with the species group bearing the A-genome. Genomes ES and Kb are more closely related to each other than they are to other genomes, even though they belong to different sections of the genus Arachis.
Comparative karyotyping by SSON DP-5 revealed that variable internal telomeric repeats (ITRs) exist in chromosomes. They might have descended from telomere-mediated chromosomal rearrangements in these repeats during genomic evolution in peanut. Heterogeneous ITRs have also been observed in tomato, and some ITRs were evidently extremely amplified [34]. Chromosome 10 of A. stenophylla and A. batizocoi both contained an obvious inversion in the region containing 45S and 5S rDNA loci, providing further evidence for chromosomal structural rearrangements.
From both our previous report [10] using the PCR-amplified telomeric repeats as probes, and the present results using multiple oligonucleotide probes, the discovery of heterogeneous distributions of rDNAs, chromosome inversions, duplications, and morphological variations in different peanut genomes has indicated that Arachis might have undergone significant chromosomal rearrangements during genome evolution. Further oligopainting using a greater number of repetitive and single-copy SSON probes should allow us to gain more information on these changes.
Conclusions
Repetitive and single-copy SSONs and multiplex repetitive SSON probes developed in the present study can be useful tools for simple and efficient chromosome identification and genomic differentiation, and facilitate chromosome engineering in peanut. Oligopainting using repetitive and single-copy sequences as probes showed that modern peanut cultivars have almost identical karyotypes, indicating low genetic diversity. In contrast, wild species exhibited an abundance of chromosomal rearrangements and variations, suggesting their genomic potential for peanut improvement. With the availability of refined reference genome sequences of peanut, additional repetitive and single-copy SSON probes can be developed and used to correlate genetic and cytological maps. Further chromosomal rearrangements, including translocations, inversions, and duplications, should be identifiable using these types of probes. The repetitive and single-copy SSON probes and methodology described in the present study provide useful tools for further application and cytogenetic study in peanut.
Methods
Plant materials
Eight accessions of cultivated peanut (A. hypogaea) (2n = 2x = 40) and eight diploid Arachis species (A. chiquitana, A. stenosperma, A. diogoi, A. villosa, A. duranensis, A. ipaënsis, A. batizocoi, and A. stenophylla) were used in this study. Five cultivars (SLH, Kainong30, Linguidachezi, Yuhua15, Liuchengzhenzhudou) were from Henan Academy of Agricultural Sciences. The other three cultivars and eight diploid species were introduced from National Germplasm Resources Laboratory of USA (Beltsville, MD). Accession number, chromosome number, genomic constitution, origin and identification of each plant material are listed in Table 3. In addition, two interspecific F1 hybrids (w1510 from a cross between the SLH variety and A. batizocoi, and w1612 from a cross between the N734 variety and A. stenophylla), and one irradiation-induced SLH M1 plant (designated as 162–30) generated by pollen irradiation at a dosage of 16 Gy using a 60Co source (Isotope Institute Co. Ltd., Henan Academy of Sciences) were also used as plant materials.
Table 3.
Plant materials used in this study
| Accession number | PI number or variety name | Identification | Origin | Section (genome) | Species (type) | References | |
|---|---|---|---|---|---|---|---|
| Collector | No. | ||||||
| SLH | Silihong | Zhang XY | China | Arachis (AhyAhyBhyBhy) | A. hypogaea (var. fastigiata) | ||
| N686 | PI 158854 | Holbrook | GP-134 | China | Arachis (AhyAhyBhyBhy) | A. hypogaea (var. hypogaea) | [42] |
| N607 | Kainong30 | Zhang XY | China | Arachis (AhyAhyBhyBhy) | A. hypogaea (var. hypogaea) | ||
| N655 | Linguidachezi | Zhang XY | China | Arachis (AhyAhyBhyBhy) | A. hypogaea (var. hypogaea) | ||
| N734 | Yuhua15 | Zhang XY | China | Arachis (AhyAhyBhyBhy) | A. hypogaea (var. hypogaea) | ||
| N477 | PI 240560 | Holbrook | GP-14 | South Africa | Arachis (AhyAhyBhyBhy) | A. hypogaea(var. hypogaea) | [42] |
| N587 | PI 290560 | Holbrook | GP-162 | India | Arachis (AhyAhyBhyBhy) | A. hypogaea (var. fastigiata) | [42] |
| N419 | Liuchengzhenzhudou | Zhang XY | China | Arachis (AhyAhyBhyBhy) | A. hypogaea (var. vulgaris) | ||
| Zw51 | PI 338280 | Hammons | 410 | Brazil | Arachis (AsAs) | A. stenosperma Krapov. & W. C. Greg. | [1, 7, 43] |
| Zw52 | PI 298639 | Krapovickas | 9484 | Bolivia | Arachis (KbKb) | A. batizocoi Krapov. & W. C. Greg. | [3, 43] |
| Zw53 | PI 468322 | Krapovickas | 30,076 | Bolivia | Arachis (BiBi) | A. ipaensis Krapov. & W. C. Greg. | [3, 43] |
| Zw55 | PI 219823 | Krapovickas | 7988 | Argentina | Arachis (AduAdu) | A. duranensis Krapov. & W. C. Greg. | [7, 43] |
| Zw56 | PI 298636 | Tweedi | 22,585 | Argentina | Arachis (AvAv) | A. villosa Benth. | [1, 7, 43] |
| Zw57 | PI 276235 | Simpson and Charles | 10,602 | Paraguay | Arachis (AdiAdi) | A. diogoi Hoehne | [1, 7, 43] |
| Zw59 | PI 476006 | Krapovickas | 36,027 | Bolivia | Arachis (AcAc) | A. chiquitana Krapov. et al. | [7, 43] |
| Zw61 | PI 468178 | Gregory | 30,136 | Brazil | Erectoides (EsEs) | A. stenophylla Krapov. & W. C. Greg. | [1, 43] |
Chromosome preparation
Seeds were germinated for 7 d on moist filter paper at 25 °C. Healthy lateral root tips were excised and pretreated with 2 mM 8-hydroxyquinoline for 3 h at 25 °C, fixed in 3:1 absolute ethanol: glacial acetic acid for 5 h at 25 °C, and stored at − 20 °C. Root meristem sections of 0.3–0.5 mm were excised, squashed in 45% glacial acetic acid, and frozen at − 80 °C for 12 h. The chromosome spreads were dehydrated in 100% ethanol and air-dried after removal of the cover slips.
Repetitive SSONs and single-copy SSON design and labeling
Eight repetitive SSON probes, named DP-1 to DP-8, were selected from different sources of repetitive sequences. The DP-2 and DP-4 probes were screened from 10 tri-nucleotide SSRs, including (AAC)10, (AAG)10, (AGG)10, (ACT)10, (CAT)10, (CAC)10, (ACG)10, (CAG)10, (AAT)10, and (GCC)10, and four di-nucleotide SSRs, namely (AT)15, (GC)15, (AG)15, and (AC)15, as described by Cuadrado et al. [18] and Du et al. [16] (Table 1). The design of the DP-5 probe was based on the repeat sequence (TTTAGGG)n, as described by Cuadrado et al. [12] and Du et al. [10]. The DP-1 probe was designed by Blasting the wheat 5S rDNA sequence against genomic sequences of A. duranensis (Table 1). Whereas DP-3, DP-6, DP-7, and DP-8 were designed following the method of Du et al. [16] using the 4 Gb genomic sequence of A. duranensis (unpublished data). The A. duranensis genomic sequence k-mers were counted using the Jellyfish tool (Version 1.1.11) [35] with the following parameters: k-mer = 31 nt and copy number > 1000. The repeat sequences were analyzed by the CD-HIT Suite and DNAMAN software, and those sequences having more than 80% identity with other repeats were discarded. From the remaining 18,052 oligonucleotides, repetitive SSONs with the highest copy number were selected. This selection resulted in 150 repetitive SSONs that were synthesized and labeled by random labeling, and analyzed on SLH chromosomes. Four repetitive SSON probes, DP-3, DP-6, DP-7, and DP-8, were then preferably selected for use in the present study because of their stronger signals. All eight repetitive SSONs were modified at the 5′-ends with TAMRA or FAM by the Invitrogen Company (Shanghai, China) and are listed in Table 1.
Single-copy SSONs were developed according to the method described by Han et al. [15], in which the genomic sequences of chromosome 6A of A. duranensis in PeanutBase [36] were downloaded and analyzed. Using the distal ~ 8.5 Mb region of chromosome 6A as a target region, all repetitive sequences were masked with RepeatMasker [37]. The remaining sequences were divided into single-copy SSONs of 42–48 nt in a stepwise size of 5 nt. Single-copy SSONs containing more than 6 nt of homopolymers were discarded. Each single-copy SSON was aligned to the genomic reference sequence of A. duranensis using the BLAT tool [38] and screened for homologs with > 75% similarity in the genome. Melting point ™ and hairpin Tm values of each single-copy SSON were calculated using the Primer3 software [39]. Single-copy SSONs with dTm > 10 °C (dTm = Tm − hairpin Tm) were retained for probe construction. As a result, 20,000 selected single-copy SSONs were synthesized by MYcroarray (Ann Arbor, MI, USA). The resulting library was amplified and labeled with digoxigenin-11-dUTP according to the MYcroarry_MYtags labeling protocol [40].
FISH and sequential FISH analysis
Total genomic DNA was extracted from fresh young leaves of A. duranensis and A. ipaënsis [41]. Clones of 5S and 45S rDNA of wheat (Triticum aestivum L.) were provided by Dr. Bikram S Gill, of Kansas State University, USA. The 5S rDNA clone and total genomic DNA of A. ipaënsis were labeled with biotin-16-dUTP (Roche) by nick translation and detected with fluorescein anti-biotin (Roche), whereas 45S rDNA and total genomic DNA of A. ipaënsis were labeled with digoxigenin-11-dUTP (Roche) and detected with anti-digoxigenin-rhodamine (Roche).
The FISH procedure followed the method described by Zhu et al. [17]. Briefly, the hybridization solution contained 7.5 μL formamide (Sigma), 1.5 μL 20× saline-sodium citrate (SSC) buffer, 1.0 μL of each probe, 2.0 μL of 50% dextran sulfate (Sigma), and ddH2O added to bring the total volume to 15 μL. The hybridization solution was denatured at 105 °C for 13 min, and the spread chromosomes were denatured in 70% formamide at 75 °C for 70 s, and then hybridized overnight at 37 °C. After hybridization, the slides were washed three times in 2× SSC at room temperature, and mounted with VECTASHIELD Mounting Medium, following which DAPI staining was performed.
Sequential FISH/GISH procedures were performed to map the signals of oligonucleotide probes. Briefly, slides were denatured after FISH using repetitive and single-copy SSON probes, and then washed to remove all signals, and subsequently dried. The GISH procedure was performed using total genomic DNA of A. duranensis and A. ipaënsis as probes to identify A- and B-genome chromosomes, respectively. A second FISH procedure using 45S rDNA and 5S rDNA probes was conducted on the same slides to develop karyotypes, as described by Du et al. [10].
Fluorescence microscopy and imaging
Slides were examined using a Leica DM6000 fluorescence microscope (Leica). Separate images from each filter set were captured using a cooled CCD camera (Leica). Images were optimized for contrast and brightness using Adobe Photoshop. To distinguish the signals produced in each chromosome by different probes after sequential FISH, the original green signal of 5S rDNA was converted to pseudo-color white. For karyotyping and chromosome diversity analysis, 3–5 cells of each accession, including 6–10 samples of each chromosome, were observed. Most karyotypes were developed from a single cell, unless they were derived from overlapping chromosomes. Chromosomes of each species were primarily ordered based on morphology, size, and unique patterns, as reported by Du et al. [10].
Additional files
Figure S1. FISH mapping of DP-2 (b, g, and l; green) and DP-1 (c, h, and m; red) by sequential FISH using 45S rDNA (e, g and j; red), 5S rDNA (e, g and j, green), and A. duranensis (a, green) and A. ipaënsis (a, red) total genomic DNA as probes in SLH (a–e), A. duranensis (f–j), and A. ipaënsis (k–o). (TIF 2370 kb)
Figure S2. FISH mapping of DP-3 (b, g, and l; green) and DP-4 (c, h, and m; red) by sequential FISH using 45S rDNA (e, g, and j; red), 5S rDNA (e, g, and j, green) and A. duranensis (a, green) and A. ipaënsis (a, red) total genomic DNA as probes in SLH (a–e), A. duranensis (f–j), and A. ipaënsis (k–o). (TIF 2188 kb)
Figure S3. FISH mapping of DP-5 (b, g, and l; green) and DP-6 (c, h, and m; red) by sequential FISH using 45S rDNA (e, g, and j; red), 5S rDNA (e, g, and j, green) and A. duranensis (a, green) and A. ipaënsis (a, red) total genomic DNA as probes in SLH (a–e), A. duranensis (f–j), and A. ipaënsis (k–o). (TIF 2587 kb)
Figure S4. FISH mapping of DP-8 (b, g, and l; green) and DP-7 (c, h, and m; red) by sequential FISH using 45S rDNA (e, g, and j; red), 5S rDNA (e, g, and j, green) and A. duranensis (a, green) and A. ipaënsis (a, red) total genomic DNA as probes in SLH (a–e), A. duranensis (f–j), and A. ipaënsis (k–o). (TIF 2283 kb)
Figure S5. Sequential FISH/GISH using Multiplex #1 (left column, SSON), A. duranensis genomic DNA, A. ipaënsis genomic DNA (middle column, GISH), 45S rDNA, and 5S rDNA (right column, 45S/5S) as probes in eight peanut varieties. (TIF 2593 kb)
Figure S6. Dual-color FISH using Multiplex #1 in SLH (a ~ d), A. duranensis (e ~ h), and A. ipaënsis (i ~ l). (TIF 1882 kb)
Figure S7. Sequential FISH using multiplex #1 (left column, SSON), 45S rDNA, and 5S rDNA (right column, 45S/5S) as probes in eight Arachis species. (TIF 3606 kb)
Figure S8. Idiogram karyotypes of SLH and eight Arachis species. sm, submetacentric; st, subtelocentric; Bar, 3 μm. (TIF 666 kb)
Figure S9. Karyotypes of two hybrid F1-derived cultivated peanut and diploid wild species using repetitive Multiplex #1 (SSON), 45S and 5S rDNA (45S/5S) plasmid clones as probes. (TIF 1258 kb)
Figure S10. FISH using oligonucleotide library 6A-1 in A. duranensis. (TIF 1066 kb)
Figure S11. Sequential FISH using DP-5 (a and e, green), 45S rDNA (b and f, green), and 5S rDNA (c and g, green) in A. duranensis, and its merged figures (d and h). (TIF 1141 kb)
Acknowledgments
We thank Ms. Kassandra Semrau (MYcroarray, Ann Arbor, Michigan, USA) and Dr. Minfeng Xue (Institute for Plant Protection and Soil Science, Hubei Academy of Agricultural Sciences, Wuhan, China) for designing the chromosome-specific oligonucleotide library and genomic repeat sequence-based oligonucleotide probes for this study. We thank Prof. Robert McIntosh (University of Sydney, Australia) and Prof. Guohao He (Center for Plant Biotechnology, Tuskegee University, USA) for their kind reviews, suggestions, and editing, and Barbara Goodson, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Funding
This research was supported by the China Agriculture Research System (CARS-13); the Henan Provincial Agriculture Research System, China (S2012–05); and the Major Technology Research and Development Program of Henan Province, China (161100111000).
Availability of data and materials
All the data pertaining to the present study have been included in table and/or figure form in the manuscript and authors are pleased to share analyzed/raw data and plant materials upon reasonable request. Other datasets supporting the conclusions of this article are included within the article and its additional files.
Abbreviations
- BAC
Bacterial artificial chromosome
- DAPI
4′, 6-diamidino-2-phenylindole
- FAM
6-carboxyfluorescein
- FISH
Fluorescence in situ hybridization
- GISH
Genomic in situ hybridization
- GSRs
Genomic sequence repeats
- ITRs
Interstitial telomeric repeats. SSC: saline-sodium citrate
- MCGSs
Multi-copy genetic sequences
- SLH
Silihong
- SSONs
Single-stranded oligonucleotides;
- SSRs
Simple sequence repeats
- TAMRA
6-carboxytetramethylrhodamine
Authors’ contributions
XZ, ZQ, BH, WD, FT, and LZ designed the experiments; PD, LL, YH, HL, LF, ZS, LQ, CC, JX, XD, SH, and ZZ performed the probes design, FISH and analyzed the data; PD, ZQ, XZ, and YH wrote the manuscript. All authors have read and approved the manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zengjun Qi, Phone: +86-25-84399029, Email: zjqi@njau.edu.cn.
Xinyou Zhang, Phone: +86-371-65729560, Email: haasz@126.com.
References
- 1.Stalker HT. Utilizing wild species for peanut improvement. Crop Sci. 2017;57:1102–1120. doi: 10.2135/cropsci2016.09.0824. [DOI] [Google Scholar]
- 2.Food and Agriculture Organization of the United Nations. http://www.fao.org/faostat/en/#data/QC. Accessed 20 Jan 2018.
- 3.Robledo G, Seijo G. Species relationships among the wild B genome of Arachis species (section Arachis) based on FISH mapping of rDNA loci and heterochromatin detection, a new proposal for genome arrangement. Theor Appl Genet. 2010;121:1033–1046. doi: 10.1007/s00122-010-1369-7. [DOI] [PubMed] [Google Scholar]
- 4.Silvestri MC, Ortiz AM, Lavia GI. rDNA loci and heterochromatin positions support a distinct genome type for ‘x = 9 species’ of section Arachis (Arachis, Leguminosae) Plant Syst Evol. 2015;301:555–562. doi: 10.1007/s00606-014-1092-y. [DOI] [Google Scholar]
- 5.Raina SN, Mukai Y. Genomic in situ hybridization in Arachis (Fabaceae) identifies the diploid wild progenitors of cultivated (A. hypogaea) and related wild (A. monticola) peanut species. Plant Syst Evol. 1999;214:251–262. doi: 10.1007/BF00985743. [DOI] [Google Scholar]
- 6.Seijo JG, Lavia GI, Fernández A, Krapovickas A, Ducasse D, Mmoscone EA. Physical mapping of the 5S and 18S–25S rRNA genes by FISH as evidence that Archis duranensis and A. ipaënsis are the wild diploid progenitors of A. hypogaea (Leguminosae) Am J Bot. 2004;91:1294–1303. doi: 10.3732/ajb.91.9.1294. [DOI] [PubMed] [Google Scholar]
- 7.Robledo G, Lavia GI, Seijo JG. Species relations among wild Arachis species with the Agenome as revealed by FISH mapping of rDNA loci and heterochromatin detection. Theor Appl Genet. 2009;118:1295–1307. doi: 10.1007/s00122-009-0981-x. [DOI] [PubMed] [Google Scholar]
- 8.Nielen S, Fonseca FC, Bertioli SL, Guimarães P, Seijo G, Town C, et al. FIDEL—a retrovirus-like retrotransposon and its distinct evolutionary histories in the A- and B-genome components of cultivated peanut. Chromosom Res. 2010;18:227–246. doi: 10.1007/s10577-009-9109-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L, Xu C, Yu W. Cloning and characterization of chromosomal markers from a Cot-1 library of peanut (Arachis hypogaea L.) Cytogenet Genome Res. 2012;137:1–41. doi: 10.1159/000339455. [DOI] [PubMed] [Google Scholar]
- 10.Du P, Li LN, Zhang ZX, Liu H, Qin L, Huang BY, et al. Chromosome painting of telomeric repeats reveals new evidence for genome evolution in peanut. J Integr Agr. 2016;15:2488–2496. doi: 10.1016/S2095-3119(16)61423-5. [DOI] [Google Scholar]
- 11.Zhang LN, Yang XY, Tian L, Chen L, Yu WC. Identification of peanut (Arachis hypogaea) chromosomes using a fluorescence in situ hybridization system reveals multiple hybridization events during tetraploid peanut formation. New Phytol. 2016;211:1424–1439. doi: 10.1111/nph.13999. [DOI] [PubMed] [Google Scholar]
- 12.Cuadrado A, Golczyk H, Jouve N. A novel, simple and rapid nondenaturing FISH (ND-FISH) technique for the detection of plant telomeres. Potential use and possible target structures detected. Chromosom Res. 2009;17:755–762. doi: 10.1007/s10577-009-9060-z. [DOI] [PubMed] [Google Scholar]
- 13.Beliveau BJ, Joyce EF, Apostolopoulos N, Yilmaz F, Fonseka CY, McCole RB, et al. Versatile design and synthesis platform for visualizing genomes with oligopaint FISH probes. Proc Nail Acad Sci USA. 2012;109:21301–21306. doi: 10.1073/pnas.1213818110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mikš-Krajnik M, Babuchowski A. 16S rRNA-targeted oligonucleotide probes for direct detection of Propionibacterium freudenreichii in presence of Lactococcus lactis with multicolour fluorescence in situ hybridization. Lett Appl Microbiol. 2014;59:320–327. doi: 10.1111/lam.12278. [DOI] [PubMed] [Google Scholar]
- 15.Han YH, Zhang T, Thammapichai P, Weng YQ, Jiang JM. Chromosome-specific painting in Cucumis species using bulked oligonucleotides. Genetics. 2015;200:771–779. doi: 10.1534/genetics.115.177642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du P, Zhuang LF, Wang YZ, Yuan L, Wang Q, Wang DR, et al. Development of oligonucleotides and multiplex probes for quick and accurate identification of wheat and Thinopyrum bessarabicum chromosomes. Genome. 2017;60:93–103. doi: 10.1139/gen-2016-0095. [DOI] [PubMed] [Google Scholar]
- 17.Zhu MQ, Du P, Zhuang LF, Chu CG, Zhao H, Qi ZJ. A simple and efficient non-denaturing FISH method for maize chromosome differentiation using single-strand oligonucleotide probes. Genome. 2017;60:657–664. doi: 10.1139/gen-2016-0167. [DOI] [PubMed] [Google Scholar]
- 18.Cuadrado A, Jouve N. Chromosomal detection of simple sequence repeats (SSRs) using nondenaturing FISH (ND-FISH) Chromosoma. 2010;119:495–503. doi: 10.1007/s00412-010-0273-x. [DOI] [PubMed] [Google Scholar]
- 19.Wang YZ. Development and characterization of small segment translocations of Thinopyrum bessarabicum and cytological mapping of interest genes. Nanjing: Master’s thesis of Nanjing Agricultural University; 2013. [Google Scholar]
- 20.Tang ZX, Yang ZJ, Fu SL. Oligonucleotides replacing the roles of repetitive sequences pAs1, pSc119.2, pTa-535, pTa71, CCS1, and pAWRC.1 for FISH analysis. J Appl Genet. 2014;55:313–318. doi: 10.1007/s13353-014-0215-z. [DOI] [PubMed] [Google Scholar]
- 21.Fu SL, Chen L, Wang YY, Li M, Yang Z. Oligonucleotide probes for ND-FISH analysis to identify rye and wheat chromosomes. Sci Rep. 2015;5:10552. doi: 10.1038/srep10552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beliveau BJ, Joyce EF, Apostolopoulosa N, Yilmaza F, Fonseka CY, McCole RB, et al. Oligopaints: highly efficient, bioinformatically designed probes for fluorescence in situ hybridization. Epigenet. Chromatin. 2013;6:5. [Google Scholar]
- 23.Danilova TV, Friebe B, Gill BS. Single-copy gene fluorescence in situ hybridization and genome analysis: Acc-2 loci mark evolutionary chromosomal rearrangements in wheat. Chromosoma. 2012;121:597–611. doi: 10.1007/s00412-012-0384-7. [DOI] [PubMed] [Google Scholar]
- 24.Li KP, Wu YX, Zhao H, Wang Y, Lu XM, Wang JM, et al. Cytogenetic relationships among Citrullus species in comparison with some genera of the tribe Benincaseae (Cucurbitaceae) as inferred from rDNA distribution patterns. BMC Evol Biol. 2016;16:85. doi: 10.1186/s12862-016-0656-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schimak MP, Kleiner M, Wetzel S, Liebeke M, Dubilier N, Fuchs BM. MiL-FISH: multilabeled oligonucleotides for fluorescence in situ hybridization improve visualization of bacterial cells. Appl Environ Micro. 2016;82:162–170. doi: 10.1128/AEM.02776-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beliveau BJ, Boettiger AN, Avendaño MS, Jungmann R, McCole RB, Joyce EF, et al. Single-molecule super-resolution imaging of chromosomes and in situ haplotype visualization using oligopaint FISH probes. Nat Commun. 2015;6:7147. doi: 10.1038/ncomms8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen KH, Boettiger AN, Moffitt JR, Wang SY, Zhuang XW. Spatially resolved, highly multiplexed RNA profiling in single cells. Science. 2015;348(6233):aaa6090. doi: 10.1126/science.aaa6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moffitta JR, Hao JJ, Wang GP, Chen KH, Babcock HP, Zhuang XW. High-throughput single-cell gene-expression profiling with multiplexed error-robust fluorescence in situ hybridization. Proc Nail Acad Sci USA. 2016;113:11046–11051. doi: 10.1073/pnas.1612826113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bertioli DJ, Cannon SB, Froenicke L, Huang GD, Farmer AD, Cannon EK, et al. The genome sequences of Arachis duranensis and Arachis ipaensis, the diploid ancestors of cultivated peanut. Nat Genet. 2016;48:438–446. doi: 10.1038/ng.3517. [DOI] [PubMed] [Google Scholar]
- 30.Zhao CZ, Qiu JJ, Agarwal G, Wang JS, Ren XZ, Xia H, et al. Genome-wide discovery of microsatellite markers from diploid progenitor species, Arachis duranensis and A. ipaënsis, and their application in cultivated peanut (A. hypogaea) Front Plant Sci. 2017;8:1209–1220. doi: 10.3389/fpls.2017.01209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seijo G, Lavia GI, Fernández A, Krapovickas A, Ducasse DA, Bertioli DJ, et al. Genomic relationships between the cultivated peanut (Arachis hypogaea, Leguminosae) and its close relatives revealed by double GISH. Am J Bot. 2007;94:1963–1971. doi: 10.3732/ajb.94.12.1963. [DOI] [PubMed] [Google Scholar]
- 32.Koppolu R, Upadhyaya HD, Dwivedi SL, Hoisington DA, Varshney RK. Genetic relationships among seven sections of genus Arachis studied by using SSR markers. BMC Plant Biol. 2010;10:15–27. doi: 10.1186/1471-2229-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mallikarjuna N. Production of hybrids between Arachis hypogaea and A. chiquitana (section Procumbentes) Peanut Sci. 2005;32:148–152. doi: 10.3146/0095-3679(2005)32[148:POHBAH]2.0.CO;2. [DOI] [Google Scholar]
- 34.He L, Liu J, Torres GA, Zhang HQ, Jiang JM, Xie CH. Interstitial telomeric repeats are enriched in the centromeres of chromosomes in Solanum species. Chromosom Res. 2013;21:5–13. doi: 10.1007/s10577-012-9332-x. [DOI] [PubMed] [Google Scholar]
- 35.Marcais G, Kingsford C. Lock-free approach for efficient parallel counting of occurrences of k-mers. Bioinformatics. 2011;27:764–770. doi: 10.1093/bioinformatics/btr011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.PeanutBase. https://peanutbase.org/gbrowse_aradu1.0. Accessed 22 Nov 2017.
- 37.RepeatMasker. http://www.repeatmasker.org. Accessed 28 Nov 2017.
- 38.Kent WJ. BLAT - the BLAST-like alignment tool. Genome Res. 2002;12:656–664. doi: 10.1101/gr.229202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, et al. Primer3 - new capabilities and interfaces. Nucleic Acids Res. 2012;40:e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murgha YE, Rouillard JM, Gulari E. Methods for the preparation of large quantities of complex single-stranded oligonucleotide libraries. PLoS One. 2014;9:e94752. doi: 10.1371/journal.pone.0094752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang CT, Huang Y, Yang XD, Jiang Y, Zhang JC, Chen DX, et al. Isolation of DNA from peanut: comparison between modified CTAB and high salt, low pH methods. J Peanut Sci. 2002;31:20–23. [Google Scholar]
- 42.Chen CY, Barkley NL, Wang ML, Holbrook CC, Dang PM. Registration of purified accessions for the U.S. Peanut mini-Core germplasm collection. J Pl Registr. 2014;8(1):77. doi: 10.3198/jpr2013.01.0003crg. [DOI] [Google Scholar]
- 43.Krapovickas A, Gregory W. Taxonomia del genero Arachis (Leguminosae) Bonplandia. 1994;8:1–187. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. FISH mapping of DP-2 (b, g, and l; green) and DP-1 (c, h, and m; red) by sequential FISH using 45S rDNA (e, g and j; red), 5S rDNA (e, g and j, green), and A. duranensis (a, green) and A. ipaënsis (a, red) total genomic DNA as probes in SLH (a–e), A. duranensis (f–j), and A. ipaënsis (k–o). (TIF 2370 kb)
Figure S2. FISH mapping of DP-3 (b, g, and l; green) and DP-4 (c, h, and m; red) by sequential FISH using 45S rDNA (e, g, and j; red), 5S rDNA (e, g, and j, green) and A. duranensis (a, green) and A. ipaënsis (a, red) total genomic DNA as probes in SLH (a–e), A. duranensis (f–j), and A. ipaënsis (k–o). (TIF 2188 kb)
Figure S3. FISH mapping of DP-5 (b, g, and l; green) and DP-6 (c, h, and m; red) by sequential FISH using 45S rDNA (e, g, and j; red), 5S rDNA (e, g, and j, green) and A. duranensis (a, green) and A. ipaënsis (a, red) total genomic DNA as probes in SLH (a–e), A. duranensis (f–j), and A. ipaënsis (k–o). (TIF 2587 kb)
Figure S4. FISH mapping of DP-8 (b, g, and l; green) and DP-7 (c, h, and m; red) by sequential FISH using 45S rDNA (e, g, and j; red), 5S rDNA (e, g, and j, green) and A. duranensis (a, green) and A. ipaënsis (a, red) total genomic DNA as probes in SLH (a–e), A. duranensis (f–j), and A. ipaënsis (k–o). (TIF 2283 kb)
Figure S5. Sequential FISH/GISH using Multiplex #1 (left column, SSON), A. duranensis genomic DNA, A. ipaënsis genomic DNA (middle column, GISH), 45S rDNA, and 5S rDNA (right column, 45S/5S) as probes in eight peanut varieties. (TIF 2593 kb)
Figure S6. Dual-color FISH using Multiplex #1 in SLH (a ~ d), A. duranensis (e ~ h), and A. ipaënsis (i ~ l). (TIF 1882 kb)
Figure S7. Sequential FISH using multiplex #1 (left column, SSON), 45S rDNA, and 5S rDNA (right column, 45S/5S) as probes in eight Arachis species. (TIF 3606 kb)
Figure S8. Idiogram karyotypes of SLH and eight Arachis species. sm, submetacentric; st, subtelocentric; Bar, 3 μm. (TIF 666 kb)
Figure S9. Karyotypes of two hybrid F1-derived cultivated peanut and diploid wild species using repetitive Multiplex #1 (SSON), 45S and 5S rDNA (45S/5S) plasmid clones as probes. (TIF 1258 kb)
Figure S10. FISH using oligonucleotide library 6A-1 in A. duranensis. (TIF 1066 kb)
Figure S11. Sequential FISH using DP-5 (a and e, green), 45S rDNA (b and f, green), and 5S rDNA (c and g, green) in A. duranensis, and its merged figures (d and h). (TIF 1141 kb)
Data Availability Statement
All the data pertaining to the present study have been included in table and/or figure form in the manuscript and authors are pleased to share analyzed/raw data and plant materials upon reasonable request. Other datasets supporting the conclusions of this article are included within the article and its additional files.