Abstract
GRP78 (an Mr 78 kDa calcium dependent glucose binding protein) is located in ER lumen. It functions as ER chaperone and translocates proteins for glycosylation at the asparagine residue present in the sequon Asn-X-Ser/Thr. Paraffin sections from N-glycosylation inhibitor tunicamycin treated ER–/PR–/HER2+ (double negative) breast tumor in athymic nude mice exhibited reduced N-glycan but increased GRP78 expression. We have evaluated the effect of tunicamycin on cellular localization of GRP78 in metastatic human breast cancer cells MDA-MB-231 (ER–/PR–/HER2–). Tunicamycin inhibited cell proliferation in a time and dose-dependent manner. Nonmetastatic estrogen receptor positive (ER+) MCF-7 breast cancer cells were also equally effective. GRP78 expression (protein and mRNA) was higher in tunicamycin (1.0 μg/mL) treated MCF-7 and MDA-MB-231 cells. GRP78 is an ER stress marker, so we have followed its intracellular localization using immunofluorescence microscopy after subjecting the cancer cells to various stress conditions. Unfixed cells stained with either FITC-conjugated Concanavalin A (Con A) or Texas-red conjugated wheat germ agglutinin (WGA) exhibited surface expression of N-glycans but not GRP78. GRP78 became detectable only after a brief exposure of cells to ice-cold methanol. Western blotting did not detect GRP78 in conditioned media of cancer cells whereas it did for MMP-1. The conclusion, GRP78 is expressed neither on the outer-leaflet of the (ER–/PR–/HER2–) human breast cancer cells nor it is secreted into the culture media during tunicamycin-induced ER stress. Our study therefore suggests strongly that anti-tumorigenic action of tunicamycin can be modeled to develop next generation cancer therapy, i.e., glycotherapy for treating breast and other sold tumors.
Keywords: asparagine-linked glycoprotein, breast cancer, ER stress, GRP78, tunicamycin
Introduction
The complex functions of endoplasmic reticulum (ER) are significantly influenced by a multitude of factors present in the cellular microenvironment (Alberts et al. 2008). For example, the availability of oxygen (i.e., the hypoxia) or glucose (i.e., the hypoglycemia), hyperthermia, acidosis, calcium levels, the redox milieu, energy levels, etc. influence and perturb the functioning of the ER resulting in the development of ER stress and impacting the protein folding in the ER lumen. Protein folding is a complex process and depends on the dynamic interaction of chaperone proteins, foldases, and glycosylating enzymes, as well as appropriate calcium levels and an oxidizing environment. Impairment of the protein folding due to ER stress results in the accumulation of unfolded or misfolded proteins. The consequence of which is the activation of a specific cellular process called unfolded protein response (upr; Malhotra and Kaufman 2007; Ron and Walter 2007). Activation of upr thus represents the defining criterion of ER stress, although oftentimes the terms upr and ER stress are used interchangeably (Schönthal 2012).
ER lumen is rich in calcium-dependent molecular chaperone, such as GRP78 (also called BiP: immunoglobulin heavy chain-binding protein) (Haas and Wabl 1983; Munro and Pelham 1986). GRP78 is an Mr 78 kDa glucose-regulated protein which carries an N-terminal signal sequence and a C-terminal ER localization KDEL sequence reminiscent of an ER resident protein. It also has an ATP binding site, a hydrophobic domain and a Ca2+ binding site. A large number of studies have now shown that a great variety of cellular and microenvironmental disturbances, as well as many pharmacological interventions, could lead to increased GRP78 expression, along with aggravated ER stress (Lee et al. 1981; Lee 2001; Healy et al. 2009; Zhang et al. 2010; Luo and Lee 2013). For example, under hypoglycemia (i.e., low glucose supply) N-linked protein glycosylation (Kornfeld and Kornfeld 1985; Helenius and Aebi 2004) is impaired (Roth et al. 2010; Csala et al. 2012) and GRP78 expression stands out resulting in ER stress induced upr signaling. Thus, GRP78 becomes a master initiator of early ER stress/upr signaling.
We have observed earlier (Martínez et al. 1999) that tunicamycin (a glucosamine-containing pyrimidine nucleoside and a competitive inhibitor of N-acetylglucosaminyl 1-phosphate transferase) (Duksin and Mahoney 1982; Elbein 1987) a protein N-glycosylation inhibitor reduces angiogenesis (in vitro and in vivo) as well as the progression of ER–/PR–/HER2+ (double-negative) and ER–/PR–/HER2– (triple-negative) breast tumors in athymic nude mice. There was no behavioral nor skeletal tissue toxicity. In addition, proliferation, chemoinvasion as well as the chemotactic activity of the cells were all impaired in cells treated with tunicamycin. The molecular mechanism supported induction of ER stress in tumor microvasculature as well as in ER–/PR–/HER2+ breast tumors (Banerjee et al. 2011). Immunofluorescence microscopy of formalin-fixed paraffin-embedded tissue sections from tunicamycin treated breast tumor exhibited increased level of GRP78 expression in regressing tumors and in their microvasculature. This is in sharp contrast with the current dogma claiming that GRP78 is located on the surface of the tumor cell (Arap et al. 2004; Misra et al. 2005; Misra et al. 2006; Shani et al. 2008; Liu et al. 2007; Zhang et al. 2010) and its increased surface expression in cancer tissue/cells and/or secretion contributes to drug resistance and is anti-tumorigenic (Quiñones et al. 2008; Lee 2014). These conclusions are erroneous and based on the experimental protocols that used either formalin-fixed tumor tissue, or after diluting the secondary antibody in a buffer containing Triton X-100 (Lee 2005; Yao et al. 2015).
Formalin-fixed paraffin-embedded tumor tissue sections have inherent membrane properties, and consequently cannot determine the precise cell surface location of GRP78. Therefore, to evaluate the cellular localization of GRP78, we have used immunofluorescence microscopy as a tool and nonfixed triple negative human breast cancer cells MDA-MB-231 as a model. Tunicamycin inhibited tumor cell growth. Western blotting as well as qPCR supported upregulated expression of both GRP78 protein and the mRNA in tunicamycin treated cells. Immunofluorescence microscopy detected no surface expression of GRP78 in these breast cancer cells irrespective of their culture conditions, e.g., in low-serum (i.e., 0% serum) media, or treating them with tunicamycin (1 μg/mL), etc. There was no GRP78 present either in the conditioned media. GRP78, however, becomes detectable only after brief exposing of cells to ice-cold methanol. Hence, we concluded that GRP78 is located intracellularly and not on the cell surface or it is secreted in the conditioned media.
Results
Tunicamycin induces ER stress in ER–/PR–/HER2+ breast tumor
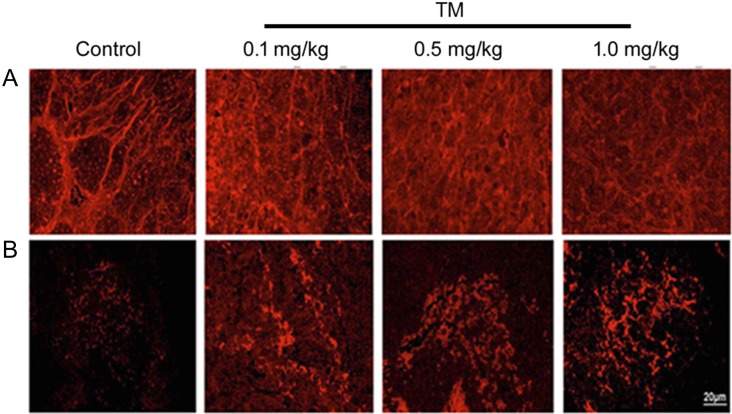
Breast tumors developed orthotopically in athymic nude mice [Balb/c (nu/nu)] with ER–/PR–/HER2+ (double negative) human breast cancer cells MDA-MB-435 was susceptible to tunicamycin treatment. Tumors regressed ~55% in 3 weeks when tunicamycin (1 mg/kg) was given by intravenous injection (i.v.) once a week. Theses tumors expressed reduced WGA-specific N-glycans in paraffin sections (Figure 1A) as opposed to the untreated control. Furthermore, the GRP78 expression was minimal in untreated tumors but increased significantly as the tumors regressed (Figure 1B). To evaluate, if the ER stress in tumor was a direct effect of tunicamycin, or was an indirect effect on tumor microenvironment, such as nutritional deprivation due to a reduced blood flow in the tumor because of reduced number of blood vessels, GRP78 was analyzed in cancer cells.
Fig. 1.
Expression of cell surface glycan and induction of ER stress in ER–/PR–/HER2+ breast tumor. Orthotopic double negative (ER–/PR–/HER2+) breast tumor was developed in athymic nude mice with MDA-MB-435 human breast cancer cells. Formalin-fixed and paraffin-embedded sections were processed as before [Quinoñes et al. 2008; Banerjee et al. 2011]. (A) WGA staining for N-glycan in control and tunicamycin (TM) (0–1.0 mg/kg)-treated breast tumor tissue sections. Images were captured under a fluorescence microscope. (B) Immunocytochemistry of GRP78 in paraffin section of breast tumor tissue. The sections were stained with anti-GRP78 antibody followed by rhodamine-conjugated secondary antibody. Histology scale: 20 μm.
Tunicamycin induces ER stress and inhibits the proliferation of ER–/PR–/HER2– and ER+ human breast cancer cells
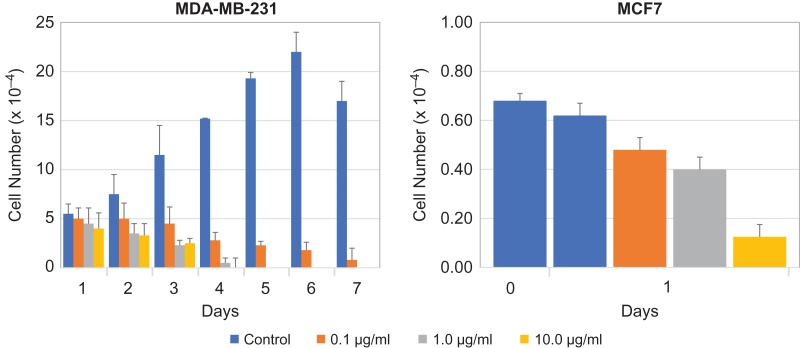
Paraffin-embedded tumor tissue slices are not ideal for surface localization studies of the ER stressor marker GRP78. Therefore, we used triple negative (ER–/PR–/HER2–) human breast cancer cells MDA-MB-231. These cells proliferate normally but the rate of proliferation retarded significantly following tunicamycin treatment (Figure 2). Thus, supporting unequivocally that tunicamycin inhibited the proliferation of breast tumor cells in a dose and time-dependent manner. To examine if tunicamycin is similarly effective to other type of breast cancer cells, we used estrogen receptor positive human breast cancer cells MCF-7. There was ~33% reduction in cell proliferation in 24 h at tunicamycin concentration of 1.0 μg/mL (Figure 2). The inhibition however was between 80% and 90% at 10 μg/mL of tunicamycin.
Fig. 2.
Tunicamycin inhibits proliferation of ER–/PR–/HER2– (triple negative; MDA-MB-231) and estrogen receptor positive (MCF-7) breast cancer cells. Triple negative human breast cancer cells (MDA-MB-231) were seeded (2 × 104 cells/well) in 24-well plates, synchronized in serum-free media for 24 h and treated with tunicamycin (0, 0.1, 1.0 and 10.0 μg/mL) in a 2% serum-containing media for 7 days. The cells were counted after every 24 h in a haemocytometer. Estrogen receptor positive human breast cancer cells (MCF-7) were seeded (2 × 104 cells/well) in 24-well plates, synchronized in serum-free media for 48 h and treated with tunicamycin (0, 0.1, 1.0 and 10.0 μg/mL) in a 2% serum-containing media for 24 h. The cells were counted in a haemocytometer. The error bars represent the standard deviations (mean ± SD) for n = 3.
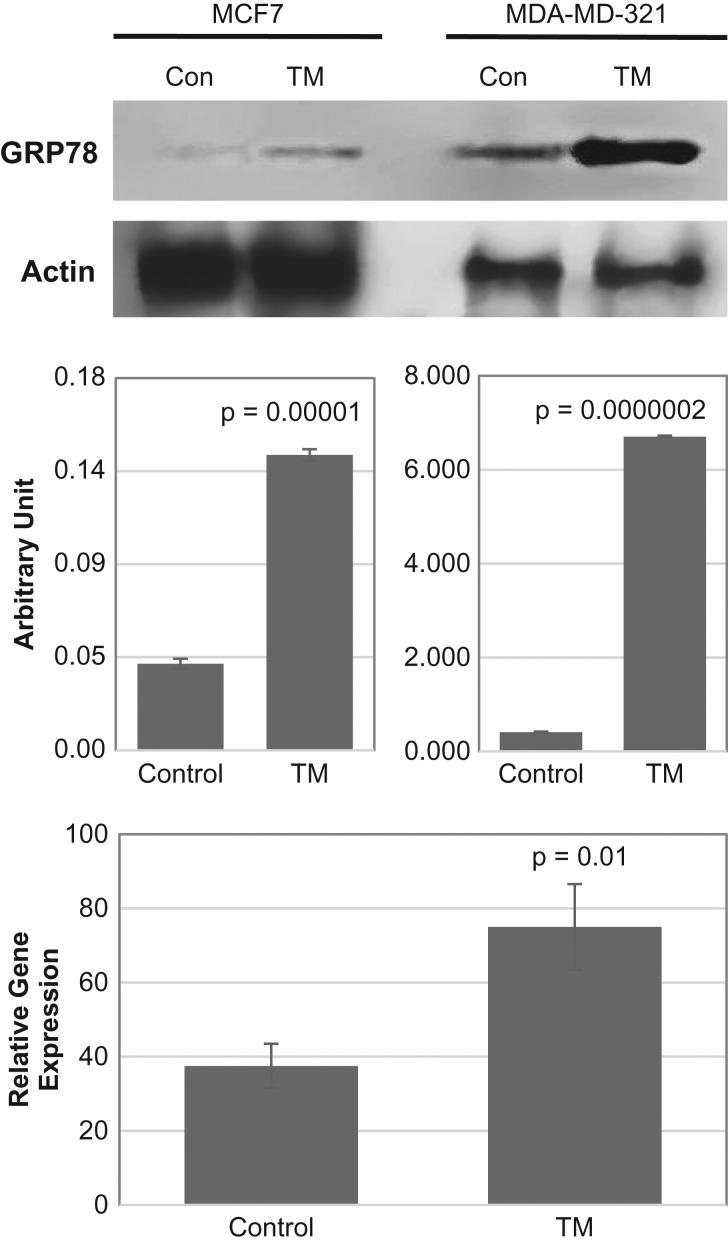
To quantify the expressed ER stress marker GRP78 in tunicamycin treated MCF7 and MDA-MB-231 breast cancer cells, GRP78 protein was analyzed by western blotting and the mRNA by qPCR. The results (Figure 3) explained that both GRP78 protein and mRNA expression increased quantitatively in tunicamycin treated cells. Thus, confirming that tunicamycin indeed induces ER stress in MCF-7 and MDA-MB-231 human breast cancer cells.
Fig. 3.
GRP78 Protein and mRNA expression in ER+ (estrogen receptor positive) and ER–/PR–/HER2– (triple negative; MDA-MB-231) human breast cancer cells. Triple negative MDA-MB-231 and ER+ MCF-7 breast cancer cells were cultured, synchronized and treated with tunicamycin (1 μg/mL) for 48 h. Top: GRP78 protein expression was examined by immunoblotting (30 μg of total protein) from control (Con) and Tunicamycin (TM) treated cells. The blots were developed with anti-GRP78 antibody (1:1000; v/v) and anti-actin antibody (1:5000; v/v). Middle: The histogram representing the quantification of GRP78 expression by Image J software, respectively. The results are an average from three blots done independently. Bottom: Quantification of GRP78 mRNA expression in triple negative breast cancer cells before and after tunicamycin treatment. The error bars represent the standard error (mean ± SE) for n = 3, and the P-values are from Student t-test.
Localization of GRP78 in ER–/PR–/HER2– human breast cancer cells by immunofluorescence microscopy
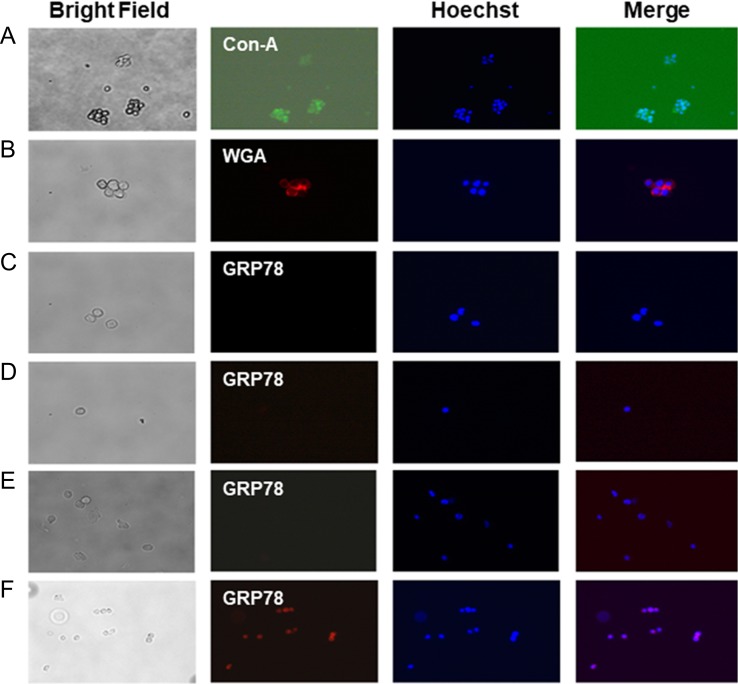
Above observations supported that upregulated expression of GRP78 during ER stress could initiate the upr signaling, and the process is truly intracellular. This argues against the claims made earlier that GRP78 expression is increased on the tumor cell surface during ER stress while treating with anti-tumor agents/drugs and promotes tumor growth not inhibition. Unfortunately, these claims were based on using either formalin-fixed tissues or cells and/or diluting the secondary antibody in Triton X-100. Formalin-fixation makes the breast tumor tissue section lose their plasma membrane integrity. To evaluate, the localization of GRP78 in MDA-MB-231 human breast cancer cells under ER stress, we used an experimental design where plasma membrane integrity was preserved. We have used here unfixed cells and monitored the surface expression of N-glycans using FITC-conjugated Con A (specificity = α-d-mannose, α-d-glucose, branched mannose) and/or Texas Red-conjugated WGA [specificity = GlcNAc-β-(1,4)-GlcNAc)1–4 > β-GlcNAc-NeuAc]. The results in Figure 4A and B (first and second columns from the left) detected the expression of Con A and WGA-positive N-glycans on tumor cell surface. A similar protocol also used anti-GRP78 antibody and Rhodamine-conjugated secondary antibody to detect GRP78. Figure 4C–E analyzed the expression of GRP78 in tumor cells cultured for 24 h in EMEM containing 10% fetal bovine serum (Figure 4C), or for 24 h in serum-free EMEM (Figure 4D), or cultured with tunicamycin (1.0 μg/mL) for 24 h in EMEM with 2% fetal bovine serum (Figure 4E). In all cases, the cells were stained with anti-GRP78 antibody followed by Rhodamine-conjugated secondary antibody and examined under a fluorescence microscope (Axioskop 2, Carl Zeiss, Germany). No fluorescence for GRP78 was detected under any of these three conditions (Figure 4C–E) suggesting the absence of GRP78 on the outer surface of the triple negative human breast cancer cells. The GRP78 (i.e., the red fluorescence) however was detected only when the cells were briefly exposed to methanol (ice-cold) prior to incubating with anti-GRP78 antibody and the corresponding secondary antibody (Figure 4F). This explained conclusively that GRP78 expression in tumor cells is intracellular and not on the cell surface.
Fig. 4.
Localization of GRP78 in ER–/PR–/HER2– (triple negative; MBA-MB-231) breast cancer cells. Cells were cultured overnight, removed by nonenzymatic cell dissociation solution. The surface N-glycans were stained with (A) FITC-conjugated Con-A (green, 20×), and (B) Texas-red conjugated WGA (red; 20×) in a 50 mM Tris buffer, pH 7.0 containing 0.15 M NaCl and 4 mM CaCl2 but no fixative. The images were collected by a fluorescence microscope (Axioskop 2, Carl Zeiss, Germany) with AxioCam MRc5 camera and Axion Vision Rel 4.6 software. (C–E) Detection of GRP78 (red, 40×); (C) cells were cultured for 24 h in EMEM with 10% serum and then stained with anti-GRP78 antibody; (D) cells were cultured for 24 h in EMEM without serum (i.e., serum-free; 0% FBS) and then stained with anti-GRP78 antibody; (E) cells were treated with tunicamycin (1 μg/mL) for 24 h in EMEM containing 2% serum and the stained with anti-GRP78 antibody; (F) cells were treated with tunicamycin (1 μg/mL) as in (E) and then permeabilized by exposing to ice-cold methanol for 15 s and stained with anti-GRP78 antibody.
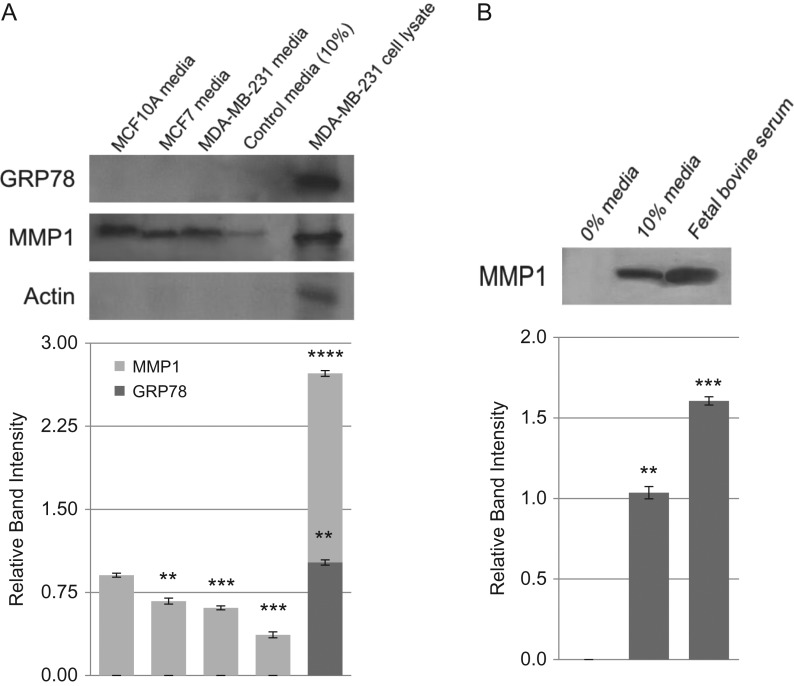
Secretion of GRP78 and MMP-1 in conditioned media of breast cancer cells
To evaluate if breast cancer cells secret GRP78 we have analyzed the conditioned media from MDA-MB-231 and MCF-7 human breast cancer cells as well as that from control breast cells MCF-10A (Figure 5). GRP78 was not detected in the conditioned media from the cell types studied here (Figure 5A). There was no GRP78 either in the cell culture media (i.e., EMEM with 10% serum). MDA-MB-231 total cell lysate however detected GRP78 and served as a positive control. MMP-1 (Figure 5A), on the other hand, is secreted and found in conditioned media from tumor cells as well as from nontumor cells. In fact, the secretion of MMP-1 was very high in MCF-10A cells compared to MCF-7 and MDA-MB-231 cancer cells. MDA-MB-231 cell lysate also detected a large intracellular level of MMP-1. To evaluate if the presence of MMP-1 in control cell culture media was contributed by serum, a western blot for MMP-1 was developed with the culture media (EMEM with 10% serum) as well as with serum alone. The results (Figure 5B) confirmed that fetal bovine serum does contain MMP-1.
Fig. 5.
Secretion of GRP78 and MMP-1 in conditioned media of breast cancer cells. Breast cancer cells MCF-7 and MDA-MB-231 as well as the control MCF-10A were all cultured overnight in EMEM containing 10% fetal bovine serum. The conditioned media were collected, dialyzed overnight at 4°C in 50 mM Tris-HCl, pH 7.0, and concentrated approximately 1000-fold over Aquacide II™. Equal amount of protein from each sample was analyzed by western blotting. (A) GRP78; lane 1 = media from MCF-10A cells, lane 2 = media from MCF-7 cells, lane 3 = media from MDA-MB-231 cells, lane 4 = EMEM with 10% serum, lane 5 = MDA-MB-231 whole cell lysate; (B) MMP-1; lane 1 = EMEM with 0% fetal bovine serum, lane 2 = EMEM with 10% fetal bovine serum, lane 3 = fetal bovine serum from stock. The error bars represent the standard error (Mean ± SE) for n = 3. P-values are from the Student t-test (** = P < 0.005; *** = P > 0.00005; **** = P > 0.000005).
Discussion
Anti-angiogenic/anti-tumorigenic action of tunicamycin is like a dual-action therapeutic which treats breast cancer of diverse backgrounds by inducing ER stress-mediated upr signaling mediated apoptotic death. Such breast cancer therapy is not only rare but has never been described before. The master regulator is GRP78 and its intracellular expression. Unfortunately, earlier claims on poor prognosis and aggressive behavior of melanoma (Papalas et al. 2010), gastric carcinoma (Zhang et al. 2006; Zheng et al. 2008, 2010), hepatocellular carcinoma (Su et al. 2010), and head and neck cancer (Chiu et al. 2008) were based on a positive relationship between increased GRP78 expression and aggressive tumor behavior (Zhang and Zhang 2010). The conclusion was faulty and based perhaps on inadequate biochemical evidence and inaccurate experimental design. For example, there is (i) no biochemical study identifies the ER resident protein GRP78 and the GRP78 expressed on the cell surface are identical; (ii) immunocytochemical detection of GRP78 in tumor specimens uses paraffin-fixed sections and those analyzed tumor cells use secondary antibody diluted/suspended in a buffer containing Triton X-100 (Lee 2005; Yao et al. 2015); (iii) no evidence for a protease that cleaves the GRP78 from the ER membrane, etc.
A common method used in these studies was siRNA knockdown of GRP78 to demonstrate the functional importance of this protein in tumor cell behavior. The investigators however suggested that such approach is problematic since it caused a significant reduction in the major ER pool of GRP78 as well as its surface expression (Misra et al. 2002). Consequently, it will invariably hamper any cellular behavior requiring protein synthesis. This plus the dysregulation of ER-based upr-signaling that resulted from GRP78 knockdown (Pyrko et al. 2007) have made it impossible to reasonably distinguish the effects of decreased cell-membrane GRP78 from decreased intracellular protein. In other scenarios: (i) U251 glioma and BXPC3 pancreatic adenocarcinoma cell lines resistant to epidermal growth factor receptor (EGFR)-targeted therapies markedly reduce RTK (receptor tyrosine kinase) signaling through Akt but become radiosensitive upon treating with ER stressor and protein N-glycosylation inhibitor tunicamycin (Contessa et al. 2008); and (ii) tunicamycin reverses the multiple drug resistance (MDR) phenotype. When added in vitro to drug-resistant NIH-3T3-MDR and KB-8-5-11 cells, they developed an increased sensitivity to doxorubicin, epirubicin, vincristine and colchicine. Similarly, the sensitivity of NIH-3T3-MDR cells to cisplatin also enhanced by tunicamycin. In addition, the presence of tunicamycin, drug-sensitive NIH-3T3-parental cells exhibited greater susceptibility to the toxic effects of doxorubicin, epirubicin, vincristine (marginally significant), and colchicine, but not to cisplatin (Hiss et al. 1996). The evidence is overwhelming to support that GRP78 is neither expressed on the tumor cell surface nor secreted in the culture media from cells where ER stress is induced by tunicamycin. Therefore, we conclude that tunicamycin can be modeled to develop next generation cancer therapy, i.e., glycotherapy for treating breast and other solid tumors.
Materials and methods
Materials
Minimal essential media with Earl’s salt (EMEM), glutamine, antibiotic mix (penicillin–streptomycin–fungizone), trypsin-EDTA and TRIzol were from Invitrogen (Carlsbad, CA). Fetal bovine serum was purchased from HyClone Laboratories (Logan, UT). Dimethyl sulfoxide, nystatin, cell dissociation solution, tunicamycin and methylthiazolyl diphenyl tetrazolium bromide (MTT) were obtained from Sigma-Aldrich (St. Louis, MO). Hoechst 33,342 was from Molecular Probe (Eugene, OR). Mouse monoclonal antibody for actin was from BD-Bioscience (San Diego, CA). Rabbit polyclonal antibody for GRP78 was from Santa Cruz Biotechnology (Santa Cruz, CA). WGA (Texas-Red conjugated) and Concanavalin A (FITC-conjugated) were from EY Laboratories (San Mateo, CA). HRP-conjugated goat anti-rabbit IgG, HRP-conjugated rabbit anti-mouse IgG, HRP-conjugated streptavidin, and ECL chemiluminescence detection kit were from GE Healthcare (Piscataway, NJ). Biotinylated protein molecular weight markers and all electrophoresis reagents were obtained from Bio-Rad Laboratories (Hercules, CA). Aquacide II™ and anti-MMP-1 mouse monoclonal antibody were purchased from Calbiochem (La Jolla, CA). Cell culture plastic wares were from Sarstedt (Newton, NC). All other chemicals and solvents used were of highest purity available.
Cell lines and animals
Human breast carcinoma cells MDA-MB-231, MDA-MB-435 and MCF-7 as well as MCF-10A were from the American Type Culture Collection (ATCC, Manassas, VA). Athymic Balb/c female (nu/nu) mice were from Charles River Laboratories (Wilmington, MA). The cell stocks were maintained in culture as reported earlier (Banerjee et al. 2011).
Culturing of human breast cancer cells and cell proliferation assay
To measure proliferation, MDA-MB-231 and MCF-7 breast cancer cells were plated at a density of 2.5 × 104 cells/mL/well in 24-well plates in EMEM containing 10% fetal bovine serum (Banerjee et al. 2011). Following synchronization in serum-free EMEM for 24 h, the cells were cultured for 7 days in the presence or absence of tunicamycin (0.0, 0.1, 1.0 and 10.0 μg/mL) in EMEM containing 2% fetal bovine serum. The cells were removed every 24 h and counted. For cell viability assay, 1.6 × 104 cells/well were plated in triplicate in 96-well plates. After synchronization in serum-free EMEM for 24 h, the cells were cultured in the presence or absence of tunicamycin (0.0, 1.0, 2.0 and 5.0 μg/mL) in EMEM containing 2% fetal bovine serum. Plates were removed every 24 h and the cell number was counted in a hemocytometer (Banerjee et al. 1985).
Development of breast tumor in athymic nude mice
4 × 106 MDA-MB-435 human breast cancer cells (ER–/PR–/HER2+) (Robinson et al. 2000; Shibuya 2001) were mixed with 0.3 mL of Matrigel™ and injected into mammary fat pads of 6-week-old athymic Balb/c (nu/nu) female mice. One week later, mice bearing tumors were divided into two groups (10 mice per group). Mice in the treatment group received tunicamycin intravenously (i.v.) at 0.1 mg/kg, 0.5 mg/kg and 1.0 mg/kg per week while the mice in the control group received i.v. injection of vehicle only. Tumor growth was monitored by caliper measurements of length and width for 23 days and, the tumor volume was calculated. At the end, the mice were sacrificed; the excised tumor was formalin (10%) fixed and paraffin embedded. 5-μm thick tumor tissue sections were analyzed histologically and immunohistochemically (Axioskop 2, Carl Zeiss, Germany). All animal studies were approved by the Institutional Animal Care and Use Committee (IACUC).
Immunofluorescence microscopy of tumor tissue sections
De-paraffinized tumor tissue sections were treated with 0.3% hydrogen peroxide (10 min) at room temperature, washed with PBS, pH 7.4 (3 × 3 min each), and blocked with solutions A and B for 30 min and 10 min, respectively (Histomouse™ Max kit; Zymed Laboratories Inc.). The sections were incubated at room temperature with anti-GRP78 antibody in a moist chamber for 3 h. After rinsing with PBS, pH 7.4 (3 × 2 min each), the sections were incubated with 100 μL of Rhodamine-conjugated goat anti-rabbit IgG for 10 min at room temperature. The sections were rinsed three times with PBS, pH 7.4, mounted and analyzed under a fluorescence microscope (Axioskop 2, Carl Zeiss, Germany).
Detection of GRP78 in breast cancer cells by immunofluorescence microscopy
Breast cancer cells were cultured in 35 mm dishes for 24 h in EMEM with 10% fetal bovine serum (heat-inactivated) at 37°C in 95% air and 5% CO2. The cells were removed after 24 h by cell dissociation medium (nonenzymatic), blocked with 3% BSA in PBS, pH 7.4 for 30 min. After rinsing with PBS, pH 7.4, cells were incubated with anti-GRP78 antibody (1:30 dilution; v/v) in blocking solution for 2 h at room temperature. Cells after washing with PBS, pH 7.4 (three times; 10 min each) treated with Rhodamine-conjugated goat anti-rabbit IgG for 1 h at room temperature. To stain nuclei, the cells were incubated with Hoechst 33,342 for 10 min at room temperature. After washing in PBS, pH 7.4 (three times; 5 min each) the cells were mounted and the images were collected under a fluorescence microscope (Axioskop 2, Carl Zeiss, Germany). To induce ER stress, cells were incubated either in serum-free media for 24 h and/or treated with tunicamycin (1 μg/mL) in EMEM with 2% fetal bovine serum for 24 h before processing for GRP78 immunofluorescence microscopy. To permeabilize, the cells were exposed to ice-cold methanol for 15 s.
SDS-PAGE and western blotting
SDS-PAGE and western blot analyses were performed in cancer cell lysates as described before (Banerjee et al. 2011) with 10% gel concentration. To analyze if GRP78 is secreted, the conditioned media were collected, dialyzed overnight at 4°C in 50 mM Tris-HCl, pH 7.0, and concentrated over Aquacide II™. Equal amount of protein from each sample was subjected to western blotting.
Quantitative real-time PCR (qPCR)
(a) Isolation of RNA: total RNA was isolated in TRIzol, treated with DNase, and quantified in a NanoDrop Bioanalyzer. The integrity of RNA was verified by agarose gel electrophoresis followed by ethidium bromide staining. (b) qPCR: total RNA (2 μg) was used to generate cDNA using the iScript™ cDNA synthesis kit. qPCR was performed in an iCycler (Bio-Rad) using iQ™ SYBR Green Supermix for the double-stranded DNA. After optimizing the PCR condition, reactions with SYBR Green PCR master mix, forward/reverse primers (10 μM), and cDNA (100 ng) were performed. PCR parameters used were 95°C for 3 min (required for iTaq™ DNA polymerase activation), 40 cycles at 95°C for 10 s, and 1 min at 50°C (annealing). The PCR products were confirmed by agarose gel electrophoresis. The quantification of the target gene expression relative to the total DNA was carried out by melting curve analysis and calculated by ∆Ct = Cthouse keeping – Cttarget gene. The changes in target gene expression in tunicamycin-treated cells compared to the control are reported as ∆∆Ct = ∆Ctcontrol – ∆Cttunicamycin where the Cttarget and CtGAPDH are the fractional cycle number at which fluorescence generated by reporter dye exceeded the fixed level above baseline for target and housekeeping genes. The relative expression of the target gene in the control and tunicamycin-treated samples was calculated as 2∆∆Ct. Each sample was run three times. The PCR primer pairs used were: human GRP78 (forward): 5′GTTACAATCAAGGTCTAT3′; (reverse): 5′CATTCACATCTATCTCAA3′; human GAPDH (forward): 5’TTGCCATCAATGACCCCTTCA3′; (reverse): 5′CGCCCCACTTGATTTTGGA3′.
Acknowledgements
We thank Aurymar Sanchez and Uldaliz Trujillo for their technical help.
Abbreviations
- Con A
concanavalin A
- ER
endoplasmic reticulum
- GRP
glucose-regulated protein
- UPR
unfolded protein response
- WGA
wheat germ agglutinin
Funding
The authors gratefully acknowledge the support from the Office of the Dean, University of Puerto Rico School of Medicine, the infrastructural support grant from the National Institues of Health NIH-NIMHD G12MD007600 as well the grants from Susan G. Komen for the Cure BCTR0600582 (D.K.B.), the National Science Foundation NSF- EPS-1002410 (D.K.B.) and the grant from the National Institutes of Health NIH-NIMHD 2G12MD007583 (K.B.).
Conflict of interest statement
The authors do not have any conflict of interest
References
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Peter Walter P. 2008. Molecular Biology of the Cell, 5th ed New York: Garland Science. [Google Scholar]
- Arap MA, Lahdenranta J, Mintz PJ, Hajitou A, Sarkis AS, Arap W, Pasqualini R. 2004. Cell surface expression of the stress response chaperone GRP78 enables tumor targeting by circulating ligands. Cancer Cell. 6:275–284. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Lang JY, Hung MC, Sengupta K, Banerjee SK, Baksi K, Banerjee DK. 2011. Unfolded protein response is required in nu/nu mice microvasculature for treating breast tumor with tunicamycin. J Biol Chem. 286:29127–29138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee DK, Ornberg RL, Youdim MB, Heldman E, Pollard HB. 1985. Endothelial cells from Bovine adrenal medulla develop capillary-like growth patterns in culture. Proc Natl Acad Sci USA. 82:4702–4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CC, Lin CY, Lee LY, Chen YJ, Kuo TF, Chang JT, Liao CT, Wang HM, Yen TC, Shen CR et al. 2008. Glucose-regulated protein 78 regulates multiple malignant phenotypes in head and neck cancer and may serve as a molecular target of therapeutic intervention. Mol Cancer Ther. 7:2788–2797. [DOI] [PubMed] [Google Scholar]
- Contessa JN, Bhojani MS, Freeze HH, Rehemtulla A, Lawrence TS. 2008. Inhibition of N-linked glycosylation disrupts receptor tyrosine kinase signaling in tumor cells. Cancer Res. 68:3803–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csala M, Kereszturi E, Mandl J, Banhegyi G. 2012. The endoplasmic reticulum as the extracellular space inside the cell: role in protein folding and glycosylation. Antioxid Redox Signal. 16:1100–1108. [DOI] [PubMed] [Google Scholar]
- Duksin D, Mahoney W. 1982. Relationship of structure and biological activity of the naturalhomologues of tunicamycin. J Biol Chem. 257:3105–3109. [PubMed] [Google Scholar]
- Elbein AD. 1987. Inhibitors of the biosynthesis and processing of N-linked oligosaccharide chains. Annu Rev Biochem. 56:497–534. [DOI] [PubMed] [Google Scholar]
- Haas IG, Wabl M. 1983. Immunoglobulin heavy chain binding protein. Nature. 306:387–389. [DOI] [PubMed] [Google Scholar]
- Healy SJM, Gorman AM, Mousavi-Shafaei P, Gupta S, Samali A. 2009. Targeting the endoplasmic reticulum-stress response as an anticancer strategy. Eur J Pharmacol. 625:234–246. [DOI] [PubMed] [Google Scholar]
- Helenius A, Aebi M. 2004. Roles of N-linked glycans in the endoplasmic reticulum. Annu Rev Biochem. 73:1019–1049. [DOI] [PubMed] [Google Scholar]
- Hiss D, Gabriels G, Jacobs P, Folb P. 1996. Tunicamycin potentiates drug cytotoxicity and Vincristine retention in multidrug resistant cell lines. Eur J Cancer. 32:2164–2172. [DOI] [PubMed] [Google Scholar]
- Kornfeld R, Kornfeld S. 1985. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 54:631–664. [DOI] [PubMed] [Google Scholar]
- Lee AS, Delegeane A, Scharff D. 1981. Highly conserved glucose-regulated protein in hamster and chicken cells: preliminary characterization of its cDNA clone. Proc Natl Acad Sci USA. 8:4922–4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AS. 2001. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci. 26:504–510. [DOI] [PubMed] [Google Scholar]
- Lee AS. 2005. The ER chaperone and signaling regulator GRP78/Bip as a monitor of endoplasmic reticulum stress. Methods. 35:373–381. [DOI] [PubMed] [Google Scholar]
- Lee AS. 2014. Glucose-regulated proteins in cancer: molecular mechanisms and therapeutic potential. Nat Rev. 14:263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Steiniger SCJ, Kim Y, Kaufmann GF, Felding-Habermann B, Janda KD. 2007. Mechanistic studies of a peptidic GRP78 ligand for cancer cell-specific drug delivery. Mol Pharm. 4:435–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo B, Lee AS. 2013. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene. 32:805–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. 2007. The endoplasmic reticulum and the unfolded protein response. Sem Cell and Dev Biol. 18:716–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez JA, Torres-Negrón I, Amigó LA, Banerjee DK. 1999. Expression of Glc3Man9GlcNAc2-PP-Dol is a prerequisite for capillary endothelial cell proliferation. Cell Mol Biol. 45:137–152. [PubMed] [Google Scholar]
- Misra UK, Gonzalez-Gronow M, Gawdi G, Hart JP, Johnson CE, Pizzo SV. 2002. The role of Grp 78 in alpha 2-macroglobulin-induced signal transduction. Evidence from RNA interference that the low density lipoprotein receptor-related protein is associated with, but not necessary for, GRP 78-mediated signal transduction. J Biol Chem. 277:42082–42087. [DOI] [PubMed] [Google Scholar]
- Misra UK, Gonzalez-Gronow M, Gawdi G, Pizzo SV. 2005. The role of MTJ-1 in cell surface translocation of GRP-78, a receptor for alpha 2- macroglobulin-dependent signaling. J Immunol. 174:2092–2097. [DOI] [PubMed] [Google Scholar]
- Misra UK, Deedwania R, Pizzo SV. 2006. Activation and cross-talk between Akt, NF-κB, and unfolded protein response signaling in 1-LN prostate cancer cells consequent to ligation of cell surface-associated GRP78. J Biol Chem. 281:13694–13707. [DOI] [PubMed] [Google Scholar]
- Munro S, Pelham HRB. 1986. An hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 46:291–300. [DOI] [PubMed] [Google Scholar]
- Papalas JA, Vollmer RT, Gonzalez-Gronow M, Pizzo SV, Burchette J, Youens KE, Johnson KB, Selim MA. 2010. Patterns of GRP78 and MTJ1 expression in primary cutaneous malignant melanoma. Mod Pathol. 23:134–143. [DOI] [PubMed] [Google Scholar]
- Pyrko P, Kardosh A, Liu YT, Soriano N, Xiong W, Chow RH, Uddin J, Petasis NA, Mircheff AK, Farley RA et al. 2007. Calcium-activated endoplasmic reticulum stress as a major component of tumor cell death induced by 2,5-dimethyl-celecoxib, a non-coxib analogue of celecoxib. Mol Cancer Ther. 6:1262–12675. [DOI] [PubMed] [Google Scholar]
- Quiñones QJ, de Ridder GG, Pizzo SV. 2008. GRP78: a chaperone with diverse roles beyond the endoplasmic reticulum. Histol Histopathol. 23:1409–1416. [DOI] [PubMed] [Google Scholar]
- Robinson DR, Wu YM, Lin SF. 2000. The protein tyrosine kinase family of the human. Oncogene. 20:5548–5557. [DOI] [PubMed] [Google Scholar]
- Ron D, Walter P. 2007. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 8:519–529. [DOI] [PubMed] [Google Scholar]
- Roth J, Zuber C, Park S, Jang I, Lee Y, Kysela KG, Le Fourn V, Santimaria R, Guhl B, Cho JW. 2010. Protein N-glycosylation, protein folding, and protein quality control. Mol Cells. 30:497–506. [DOI] [PubMed] [Google Scholar]
- Schönthal AH. 2012. Endoplasmic reticulum stress: its role in disease and novel prospects for therapy. Scientifica (Cairo). 2012:857516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shani G, Fischer WH, Justice NJ, Kelber JA, Vale W, Gray PC. 2008. GRP78 and cripto form a complex at the cell surface and collaborate to inhibit transforming growth factor β signaling and enhance cell growth. Mol Cell Biol. 28:666–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M. 2001. Structure and function of VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct Funct. 26:25–35. [DOI] [PubMed] [Google Scholar]
- Su R, Li Z, Li H, Song H, Bao C, Wei J, Chen L. 2010. Grp78 promotes the invasion of hepatocellular carcinoma. BMC Cancer. 10:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Liu H, Zhang X, Zhang L, Wang C, Sun S. 2015. Cell surface GRP78 accelerated breast cancer cell proliferation and migration by activating STAT3. PLoS ONE. 10:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang J, Jiang Y, Jia Z, Li Q, Gong W, Wang L, Wei D, Yao J, Fang S, Xie K. 2006. Association of elevated GRP78 expression with increased lymph node metastasis and poor prognosis in patients with gastric cancer. Clin Exp Metastasis. 23:401–410. [DOI] [PubMed] [Google Scholar]
- Zhang LH, Zhang X. 2010. Roles of GRP78 in physiology and cancer. J Cell Biochem. 110:1299–1305. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu R, Ni M, Gill P, Lee AS. 2010. Cell surface relocalization of the endoplasmic reticulum chaperone and unfolded protein response regulator GRP78/BiP. J Biol Chem. 285:15065–15075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng HC, Takahashi H, Li XH, Hara T, Masuda S, Guan YF, Takano Y. 2008. Overexpression of GRP78 and GRP94 are markers for aggressive behavior and poor prognosis in gastric carcinomas. Hum Pathol. 39:1042–1049. [DOI] [PubMed] [Google Scholar]
- Zheng HC, Zheng YS, Xia P, Xu XY, Xing YN, Takahashi H, Guan YF, Takano Y. 2010. The pathobiological behaviors and prognosis associated with Japanese gastric adenocarcinomas of pure WHO histological subtypes. Histol Histopathol. 25:445–452. [DOI] [PubMed] [Google Scholar]