Abstract
Objective
High levels of mean platelet volume (MPV) had been found in depression subjects. We sought to examine the relationship between MPV and poststroke depression (PSD).
Methods
One hundred and eighty‐five patients with acute ischemic stroke were enrolled in our study. Peripheral venous blood samples were drawn at admission and MPV levels were measured by the automated hematology analyzer. Patients with a HAMD‐17 score >7 were diagnosed as having PSD.
Results
We found that 60 patients (32.4%) developed PSD, the MPV levels in PSD patients were significantly higher (9.3 ± 1.8 fl) compared to non‐PSD patients (8.5 ± 1.6 fl). High MPV levels (≥9.1 fl) were independently correlated with PSD (OR 2.762, 95% CI 1.138–6.702, p = 0.025).
Conclusions
Patients with higher levels of MPV at admission were correlated with the development of PSD at 1 month after stroke and might be a predictor of its presence.
Keywords: acute ischemic stroke, depression, mean platelet volume, risk factor
1. INTRODUCTION
Poststroke depression (PSD) is one of the most common neuropsychiatric sequelae following by stroke (Robinson, 2003). According to a meta‐analysis, approximately 31% of patients suffered from depression within five years after onset of acute stroke (Hackett & Pickles, 2014), which might negatively affect functional outcome as well as quality of life (Guajardo et al., 2015; Parikh et al., 1990). Thus, early detection of PSD is of great significance. However, the underlying mechanisms of PSD remain unclear.
Stroke is accompanied by changed platelet function, such as enhancement of platelet aggregation and activity (Grau et al., 1998; Tohgi, Suzuki, Tamura, & Kimura, 1991). As a marker of platelet function, Mean platelet volume (MPV) involves in platelet reactivity, including aggregation and release of platelet factor 4, thromboxane A2, as well as β‐thromboglobulin (Sharp et al., 1995). As a meta‐analysis comprising 24 trials demonstrated, MPV is a positively associated with incidence of myocardial infarction as well as restenosis after coronary angioplasty (Chu et al., 2010). Meanwhile, high MPV levels had been found in patients with aortic arterial stiffness, peripheral artery disease (Balta et al., 2014; Berger, Eraso, Xie, Sha, & Mohler, 2010). Previous studies reported that high MPV levels were correlated with increased risk of ischemic stroke as well as unfavorable prognosis among survivors of stroke (Bath, Algert, Chapman, & Neal, 2004; Muscari et al., 2009).
Besides, high MPV levels were also observed among patients who got depression or panic disorder (Buriachkovskaia et al., 2006; Kokacya et al., 2015). It has been established that MPV was associated with higher platelet reactivity (Bath & Butterworth, 1996; van der Loo & Martin, 1999; Yetkin, 2008), which is involved in the pathophysiological mechanisms of depression (Musselman et al., 1996; Nemeroff & Musselman, 2000).
Given the relationship between depression and MPV, it would be interesting to investigate whether MPV levels were correlated with the development of depression among patients after ischemic stroke. To date, however, no study has been conducted to examine the relationship between MPV and PSD. Hence, whether MPV levels correlate with PSD at 1 month after ischemic stroke was explored.
2. MATERIALS AND METHODS
2.1. Study population
Patients with acute ischemic stroke in the Stroke Unit of the First Affiliated Hospital of Wenzhou Medical University between October 2013 and May 2015 were admitted to our study. Patients were recruited if they: (a) were 18–80 years old; (b) were diagnosed with acute ischemic stroke occurring within one week and were confirmed by computed tomography (CT) or magnetic resonance imaging (MRI) reports; (c) were competent to consent to take part in the research. The exclusion criteria included: (a) significant acute or severe illness such as infection, heart failure and tumor; (b) patients with diseases which might affect MPV levels, such as bone marrow diseases and hypersplenism; (c) previous diagnosis of depression or other mental disorder or who had recently accepted antidepressants or antipsychotics; (d) severe aphasia or dysarthria; (e) a previous history of neurological illness including Alzheimer's disease and Parkinson's disease; (f) those who failed to have MPV levels measured at admission.
This study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Medical Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University. All patients had signed an informed consent.
2.2. Data collection
The demographic as well as clinical characteristics of our research were obtained through standardized questionnaires interviewed by trained neurological physicians who were blind to the patients’ laboratory results at admission. Stroke etiology was classified based on TOAST criteria (Adams, 1993). The National Institutes of Health Stroke Scale (NIHSS) was used to evaluate the stroke severity on admission. The Barthel Index (BI) as well as modified Rankin Scale (mRS) were applied for assessment of functional outcome at one month after acute ischemic stroke. Besides, The Mini‐Mental State Examination (MMSE) was used for evaluation of cognition function at one month after acute ischemic stroke.
2.3. Definition of PSD
Depressive symptoms were evaluated by trained psychiatrists who were blind to the patients’ laboratory results using the 17‐item Hamilton Depression Scale (HAMD‐17) (Zimmerman, Martinez, Young, Chelminski, & Dalrymple, 2013). Subjects with a score >7 (Park et al., 2017; Zimmerman, et al., 2013) at one month after acute ischemic stroke were considered PSD according to DSM IV.
2.4. Laboratory tests
The peripheral venous blood samples were drawn on admission and collected in a calcium ethylenediaminetetra‐acetic acid tube. Complete blood count was analyzed by the automated hematology analyzer (Sysmex Company, XE‐2100, Japan) within 1 hr after sample collection. Platelet count and MPV levels were recorded. MPV levels were further divided into tertiles (≤7.7 fl, 7.8–9.0 fl, ≥9.1 fl).
2.5. Statistical analysis
The results were expressed as percentages for categorical variables, and continuous variables according to their normal distribution were indicated as median (interquartile range, IQR) or mean standard deviation (SD). Proportions were compared employing the Chi‐squared test, and continuous variables were compared by the analysis of variance (ANOVA), Mann–Whitney test, as well as Student t test between PSD and non‐PSD groups, as appropriate. The effect of MPV on PSD was evaluated by binary logistic regression analysis in which factors with p < 0.05 in the univariate analysis between groups were included. In addition, variables such as hyperlipidemia, current drinking and lesion location were also included in the logistic regression analysis. Results were indicated as adjusted odds ratio (OR) (95% confidence interval, CI). Statistical analyses were used in IBM SPSS Statistics 19.0. All p values were two‐tailed and the significance level was set at 0.05.
3. RESULTS
3.1. Characteristics of the study population
A total of 292 acute stroke patients were screened, and 229 patients (78.4%) met the entry criteria while 63 patients (21.6%) were excluded from the study at baseline. By the time of 1‐month follow‐up, 185 patients were finally enrolled in our study (Figure 1). Compared to patients included in the study, patients excluded from the study had a higher proportion of hypertension (p < 0.05). No significant differences were found in terms of other baseline characteristics, such as age (p = 0.139), proportion of female gender (p = 0.248), years of education (p = 0.462), coronary heart disease (p = 0.670), hyperlipidemia (p = 0.681), history of stroke (p = 0.407), current smoking (p = 0.322), current drinking (p = 0.362), lesion location (p = 0.383), and stroke etiology (p = 0.438).
Figure 1.
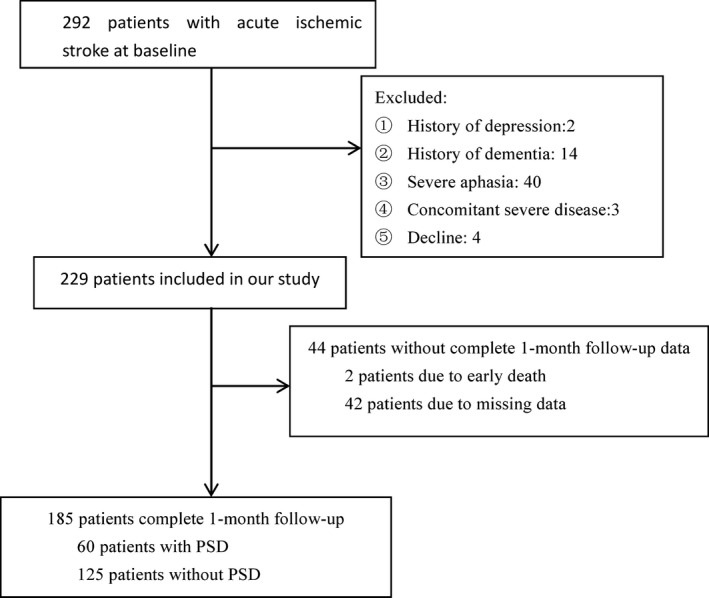
Study recruitment profile
In the study population, 60 (32.4%) were diagnosed as having PSD. Meanwhile, the average MPV levels for all patients were 8.7 ± 1.7 fl; the levels of MPV in PSD group were significantly higher than that in non‐PSD group (9.3 ± 1.8 fl vs. 8.5 ± 1.6 fl; F = 9.64, p = 0.002). Furthermore, a more severe stroke, worse functional outcome as well as worse cognitive function were observed in PSD patients (all p < 0.05). Moreover, the proportion of female gender was higher in PSD than non‐PSD (p < 0.05). No association was found between two groups in terms of platelet count, vascular risk factors, stroke etiology, and lesion location (Table 1).
Table 1.
Demographic and clinical characteristics of the samples under study
| Baseline characteristics | Non‐PSD (n = 125) | PSD (n = 60) | p value |
|---|---|---|---|
| Demographic characteristics | |||
| Female, n (%) | 36 (28.8) | 29 (48.3) | 0.009 |
| Age (years), mean ± SD | 63 ± 19 | 62 ± 10 | 0.535 |
| Educational years, median (IQR) | 5 (0–8) | 3 (0–6) | 0.227 |
| BMI (kg/m2), mean ± SD | 24.31 ± 3.52 | 25.38 ± 3.63 | 0.202 |
| Marital Status, married, n (%) | 125 (100.0) | 59 (98.3) | 0.324 |
| Vascular risk factors | |||
| Hypertension, n (%) | 91 (72.8) | 45 (75.0) | 0.751 |
| Diabetes mellitus, n (%) | 34 (27.2) | 15 (25.0) | 0.751 |
| Coronary heart disease, n (%) | 11 (8.8) | 3 (5.0) | 0.554 |
| Hyperlipidemia, n (%) | 12 (9.6) | 11 (18.3) | 0.092 |
| History of stroke, n (%) | 13 (10.4) | 5 (8.3) | 0.657 |
| Current smoking, n (%) | 46 (36.8) | 16 (26.7) | 0.172 |
| Current drinking, n (%) | 49 (39.2) | 16 (26.7) | 0.095 |
| Lesion location, n (%) | |||
| Left hemisphere | 43 (34.4) | 15 (25.0) | 0.062 |
| Right hemisphere | 35 (28.0) | 24 (40.0) | |
| Brainstem | 21(16.8) | 13 (21.7) | |
| Cerebellum | 10 (8.0) | 0 (0.0) | |
| Other | 16 (12.8) | 8 (13.3) | |
| TOAST classification, n (%) | |||
| LA | 114 (91.2) | 52 (86.7) | 0.235 |
| CE | 7 (5.6) | 2 (3.3) | |
| SA | 2 (1.6) | 3 (5.0) | |
| SOE | 0 (0.0) | 1 (1.7) | |
| SUE | 2 (1.6) | 2 (3.3) | |
| Neuropsychological function | |||
| NIHSS score, median (IQR) | 3 (1–4) | 4 (2–6) | <0.001 |
| MMSE score, median (IQR) | 24 (22–28) | 23 (20–27) | 0.017 |
| mRS (IQR) | 1 (1–2) | 2 (1–3) | 0.001 |
| BI (IQR) | 97 (100–100) | 88 (80–100) | <0.001 |
| Platelet count, mean ± SD | 219 ± 60 | 216 ± 59 | 0.821 |
| MPV(fl), mean ± SD | 8.5 ± 1.6 | 9.3 ± 1.8 | 0.002 |
BMI: body mass index; CE: cardio embolism; LA: large‐artery atherosclerosis; NIHSS: National Institutes of Health Stroke Scale; mRS: modified Rankin Scale; MMSE: mini mental state examination; BI: modified Barthel Index; MPV: mean platelet volume; IQR: interquartile range; SD: standard deviation; PSD: Poststroke depression; SA: small‐artery occlusion Lacunar; SOE: stroke of other determined etiology; SUE: stroke of other undetermined etiology.
3.2. The association between MPV and PSD
As demonstrated in Table 2, there were significant differences between PSD and non‐PSD in MPV tertiles of patients (p = 0.005). Moreover, in the lowest tertile (≤7.7 fl), PSD group had a lower proportion of patients than that in non‐PSD group (p = 0.002), while in the highest tertile (≥9.1 fl), PSD group had a higher proportion of patients than that in non‐PSD group (p = 0.010).
Table 2.
MPV tertiles of patients
| PSD patients (n = 60) | Non‐PSD patient (n = 125) | p value | |
|---|---|---|---|
| MPV, n (% of total population) | 0.005 | ||
| Tertile 1 (≤7.7 fl) | 13 (21.7%) | 51 (40.8%) | 0.002 |
| Tertile 2 (7.8–9.0 fl) | 18 (30.0%) | 42 (33.6%) | 0.624 |
| Tertile 3 (≥9.1 fl) | 29 (48.3%) | 32 (25.6%) | 0.010 |
MPV: mean platelet volume; PSD: poststroke depression.
As shown in Table 3, in the binary logistic regression analysis, tertile 2 was taken as reference and PSD presence was taken as a dependent variable, the high tertile of MPV levels (≥9.1 fl) was independently correlated with PSD (OR 2.762, 95% CI 1.138–6.702, p = 0.025). Moreover, the NIHSS score on admission as well as BI score at 1 month were significantly correlated with PSD (OR 1.237, 95% CI 1.027–1.490, p = 0.025, and OR 0.938, 95% CI 0.887–0.993, p = 0.027). Furthermore, gender and hyperlipidemia were significantly correlated with PSD (OR 2.457, 95% CI 1.058–5.704, p = 0.036, and OR 3.604, 95% CI 1.252–10.379, p = 0.018).
Table 3.
Multivariate logistic model of the clinical determinants of poststroke depression
| Variables | OR (95%CI) | p value |
|---|---|---|
| MPV | 0.009 | |
| Tertile 1 | 0.481 | |
| Tertile 3 | 2.762 (1.138–6.702) | 0.025 |
| Female | 2.457 (1.058–5.704) | 0.036 |
| Hyperlipidemia | 3.604 (1.252–10.379) | 0.018 |
| Current drinking | 0.746 | |
| Lesion location | 0.214 | |
| NIHSS | 1.237 (1.027–1.490) | 0.025 |
| BI | 0.938 (0.887–0.993) | 0.027 |
| mRS | 0.603 | |
| MMSE score | 0.277 |
MPV: mean platelet volume; OR: odds ratio; CI: confidence interval; NIHSS: National Institutes of Health Stroke Scale; mRS: modified Rankin Scale; MMSE: mini mental state examination; BI: modified Barthel Index.
4. DISCUSSION
To our best knowledge, this was the first report to examine the association between MPV levels and the presence of PSD. Our results indicated that high MPV levels were independently correlated with PSD.
In this study, 32.4% of patients developed PSD at 1 month after stroke, the prevalence is similar to previous researches (Allan et al., 2013; Hackett & Pickles, 2014; Hackett, Yapa, Parag, & Anderson, 2005). We found that female gender was significantly correlated with PSD, which was consistent with earlier findings (Guiraud et al., 2016; Mazure, Weinberger, Pittman, Sibon, & Swendsen, 2014; Zhang, Zhao, Fang, Wang, & Zhou, 2017). Our results also showed that physical disability and stroke severity were correlated with PSD, which were in line with previous researches (Eriksen, Gay, & Lerdal, 2016; Ko et al., 2018; Kutlubaev & Hackett, 2014; Sarfo et al., 2017). In addition, we found that hyperlipidemia was associated with PSD. This finding was in accord with previous studies (Armstrong et al., 2017; Lim et al., 2017). However, there was no significant association between PSD and other clinical characteristics, such as lesion location (Guiraud, et al., 2016; Hama et al., 2007; Metoki et al., 2016), although among some of previous studies the results about lesion location remained controversial.
Our results are in line with another study that demonstrated increased MPV levels were associated with depression in a large population (Canan et al., 2012). Likewise, elevated MPV levels were also found in patients with fibromyalgia syndrome as well as bipolar disorder (Vural et al., 2015; Yildirim, Solmaz, Akgol, & Ersoy, 2016). Furthermore, previous studies showed that MPV levels reduced significantly after antidepressant treatment (Ataoglu & Canan, 2009; Demircan et al., 2016).
The relationship between MPV and depression remains not completely understood. However, several mechanisms may explain the depression in patients with higher MPV levels. The regulation of the platelet activity might explain the association between MPV and depression. As a routinely available measure of platelet size at admission, MPV can be considered as a biological indicator of platelet activity (Chu, et al., 2010), which was correlated with depression (Musselman, et al., 1996; Shimbo et al., 2002). This accorded well with one study based on a large population, which reported that elevated MPV levels were associated with major depression (Canan, et al., 2012). Meanwhile, it has been shown that escitalopram effect of inhibiting reuptake of serotonin occurs in neurons as well as platelets, reducing platelet reactivity (Atar et al., 2006). Besides, as mentioned earlier, patients with depression exhibited normalization of platelet activation and significant reduction in MPV levels after escitalopram treatment (Ataoglu & Canan, 2009), indicating that MPV may play a vital role in pathophysiological process of depression. Thus, considering its role in ischemic stroke as well as depression, MPV may participate in the development of PSD.
Our study still has some limitations. First, patients with serious conditions or aphasia were excluded from our study, which may reduce the real PSD prevalence. Second, only MPV levels in the baseline period was measured, which might make us fail to reveal the dynamic change in MPV levels during the development of PSD. Finally, the application of our conclusion may be limited in those minor stroke subjects, for most of the patients with a higher NIHSS score have been excluded.
5. CONCLUSION
In conclusion, despite these limitations, the findings of this study remained important and demonstrated that high levels of MPV on admission were significantly associated with the development of PSD. High MPV levels could be considered as an independent prognostic marker of PSD. These findings suggest a potential role of MPV in the pathophysiology of PSD and indicate that patients with acute ischemic stroke should be monitored for high MPV levels. In future, multicenter and randomized controlled trials are critical to determine the causal relationship between MPV levels and PSD.
CONFLICT OF INTEREST
None declared.
ACKNOWLEDGMENTS
This study was supported by Wenzhou Municipal Sci‐Tech Bureau Program (Y20160002) as well as National Key Technology Research and Development Program of the Ministry of Science and Technology of China (grant number: 2015BAI13B01). We are greatly indebted to the staff and to the patients for their contributions during this study.
Qiu H, Liu Y, He H, et al. The association between mean platelet volume levels and poststroke depression. Brain Behav. 2018;8:e01114 10.1002/brb3.1114
REFERENCES
- Adams, H. P. Jr , Bendixen, B. H. , Kappelle, L. J. , Biller, J. , Love, B. B. , Gordon, D. L. , & Marsh, E. E. 3rd (1993). Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke, 24(1), 35–41. [DOI] [PubMed] [Google Scholar]
- Allan, L. M. , Rowan, E. N. , Thomas, A. J. , Polvikoski, T. M. , O'Brien, J. T. , & Kalaria, R. N. (2013). Long‐term incidence of depression and predictors of depressive symptoms in older stroke survivors. British Journal of Psychiatry, 203(6), 453–460. 10.1192/bjp.bp.113.128355 [DOI] [PubMed] [Google Scholar]
- Armstrong, N. M. , Meoni, L. A. , Carlson, M. C. , Xue, Q. L. , Bandeen‐Roche, K. , Gallo, J. J. , & Gross, A. L. (2017). Cardiovascular risk factors and risk of incident depression throughout adulthood among men: The Johns Hopkins Precursors Study. Journal of Affective Disorders, 214, 60–66. 10.1016/j.jad.2017.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataoglu, A. , & Canan, F. (2009). Mean platelet volume in patients with major depression: Effect of escitalopram treatment. Journal of Clinical Psychopharmacology, 29(4), 368–371. 10.1097/JCP.0b013e3181abdfd7 [DOI] [PubMed] [Google Scholar]
- Atar, D. , Malinin, A. , Takserman, A. , Pokov, A. , van Zyl, L. , Tanguay, J. F. , … Serebruany, V. (2006). Escitalopram, but not its major metabolites, exhibits antiplatelet activity in humans. Journal of Clinical Psychopharmacology, 26(2), 172–177. 10.1097/01.jcp.0000204047.76286.6e [DOI] [PubMed] [Google Scholar]
- Balta, I. , Balta, S. , Koryurek, O. M. , Demirkol, S. , Celik, T. , Akbay, G. , … … M. (2014). Mean platelet volume is associated with aortic arterial stiffness in patients with Behcet's disease without significant cardiovascular involvement. Journal of the European Academy of Dermatology and Venereology, 28(10), 1388–1393. [DOI] [PubMed] [Google Scholar]
- Bath, P. , Algert, C. , Chapman, N. , & Neal, B. (2004). Association of mean platelet volume with risk of stroke among 3134 individuals with history of cerebrovascular disease. Stroke, 35(3), 622–626. 10.1161/01.STR.0000116105.26237.EC [DOI] [PubMed] [Google Scholar]
- Bath, P. M. , & Butterworth, R. J. (1996). Platelet size: Measurement, physiology and vascular disease. Blood Coagulation & Fibrinolysis, 7(2), 157–161. 10.1097/00001721-199603000-00011 [DOI] [PubMed] [Google Scholar]
- Berger, J. S. , Eraso, L. H. , Xie, D. , Sha, D. , & Mohler, E. R. 3rd (2010). Mean platelet volume and prevalence of peripheral artery disease, the National Health and Nutrition Examination Survey, 1999–2004. Atherosclerosis, 213(2), 586–591. 10.1016/j.atherosclerosis.2010.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buriachkovskaia, L. , Poliakova, E. , Zorin, A. , Uchitel', I. , Dovlatova, N. , Masenko, V. , … … E. I. (2006). Platelet activation and inflammation markers in patients with coronary heart disease and depression. Terapevticheskii Arkhiv, 78(10), 9–14. [PubMed] [Google Scholar]
- Canan, F. , Dikici, S. , Kutlucan, A. , Celbek, G. , Coskun, H. , Gungor, A. , … Kocaman, G. (2012). Association of mean Platelet volume with DSM‐IV major depression in a large community‐based population: The MELEN study. Journal of Psychiatric Research, 46(3), 298–302. 10.1016/j.jpsychires.2011.11.016 [DOI] [PubMed] [Google Scholar]
- Chu, S. G. , Becker, R. C. , Berger, P. B. , Bhatt, D. L. , Eikelboom, J. W. , Konkle, B. , … Berger, J. S. (2010). Mean platelet volume as a predictor of cardiovascular risk: A systematic review and meta‐analysis. Journal of Thrombosis and Haemostasis, 8(1), 148–156. 10.1111/j.1538-7836.2009.03584.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demircan, F. , Gozel, N. , Kilinc, F. , Yilmaz, M. , Donder, E. , & Atmaca, M. (2016). The effect of selective serotonin reuptake inhibitor (SSRI) treatment on mean platelet volume in major depressive disorder (MDD) patients. Acta Medica Mediterranea, 32(1), 93–96. [Google Scholar]
- Eriksen, S. , Gay, C. L. , & Lerdal, A. (2016). Acute phase factors associated with the course of depression during the first 18 months after first‐ever stroke. Disability and Rehabilitation, 38(1), 30–35. 10.3109/09638288.2015.1009181 [DOI] [PubMed] [Google Scholar]
- Grau, A. J. , Ruf, A. , Vogt, A. , Lichy, C. , Buggle, F. , Patscheke, H. , &… W. (1998). Increased fraction of circulating activated platelets in acute and previous cerebrovascular ischemia. Thrombosis and Haemostasis, 80(2), 298–301. [PubMed] [Google Scholar]
- Guajardo, V. D. , Terroni, L. , Sobreiro Mde, F. , Zerbini, M. I. , Tinone, G. , Scaff, M. , … Fráguas, R. (2015). The influence of depressive symptoms on quality of life after stroke: A prospective study. Journal of Stroke and Cerebrovascular Diseases: the Official Journal of National Stroke Association, 24(1), 201–209. 10.1016/j.jstrokecerebrovasdis.2014.08.020 [DOI] [PubMed] [Google Scholar]
- Guiraud, V. , Gallarda, T. , Calvet, D. , Turc, G. , Oppenheim, C. , Rouillon, F. , & Mas, J.‐L. (2016). Depression predictors within six months of ischemic stroke: The DEPRESS Study. International Journal of Stroke, 11(5), 519–525. 10.1177/1747493016632257 [DOI] [PubMed] [Google Scholar]
- Hackett, M. L. , & Pickles, K. (2014). Part I: Frequency of depression after stroke: An updated systematic review and meta‐analysis of observational studies. International Journal of Stroke, 9(8), 1017–1025. 10.1111/ijs.12357 [DOI] [PubMed] [Google Scholar]
- Hackett, M. L. , Yapa, C. , Parag, V. , & Anderson, C. S. (2005). Frequency of depression after stroke: A systematic review of observational studies. Stroke, 36(6), 1330–1340. 10.1161/01.STR.0000165928.19135.35 [DOI] [PubMed] [Google Scholar]
- Hama, S. , Yamashita, H. , Shigenobu, M. , Watanabe, A. , Kurisu, K. , Yamawaki, S. , & Kitaoka, T. (2007). Post‐stroke affective or apathetic depression and lesion location: Left frontal lobe and bilateral basal ganglia. European Archives of Psychiatry and Clinical Neuroscience, 257(3), 149–152. 10.1007/s00406-006-0698-7 [DOI] [PubMed] [Google Scholar]
- Ko, M. , Choi‐Kwon, S. , Jun, S. E. , Kim, J. H. , Cho, K. H. , Nah, H. W. , … Kim, J. S. (2018). Poststroke Emotional Disturbances and a Tryptophan Hydroxylase 2 Gene Polymorphism. Brain and Behavior, 8(2), e00892 10.1002/brb3.892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokacya, M. H. , Copoglu, U. S. , Kivrak, Y. , Ari, M. , Sahpolat, M. , & Ulutas, K. T. (2015). Increased mean platelet volume in patients with panic disorder. Neuropsychiatric Disease and Treatment, 11, 2629–2633. 10.2147/NDT.S94147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlubaev, M. A. , & Hackett, M. L. (2014). Part II: Predictors of depression after stroke and impact of depression on stroke outcome: An updated systematic review of observational studies. International Journal of Stroke, 9(8), 1026–1036. 10.1111/ijs.12356 [DOI] [PubMed] [Google Scholar]
- Lim, S. W. , Eric Nyam, T. T. , Ho, C. H. , Shiue, Y. L. , Wang, J. J. , Chio, C. C. , & Kuo, J.‐R. (2017). Increased risk of anxiety or depression after traumatic spinal cord injury in patients with preexisting hyperlipidemia: A population‐based study. World Neurosurgery, 106, 402–408. 10.1016/j.wneu.2017.06.182 [DOI] [PubMed] [Google Scholar]
- Mazure, C. M. , Weinberger, A. H. , Pittman, B. , Sibon, I. , & Swendsen, J. (2014). Gender and stress in predicting depressive symptoms following stroke. Cerebrovascular Disease, 38(4), 240–246. 10.1159/000365838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metoki, N. , Sugawara, N. , Hagii, J. , Saito, S. , Shiroto, H. , Tomita, T. , … Yasui‐Furukori, N. (2016). Relationship between the lesion location of acute ischemic stroke and early depressive symptoms in Japanese patients. Annals of General Psychiatry, 15, 12 10.1186/s12991-016-0099-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscari, A. , Puddu, G. M. , Cenni, A. , Silvestri, M. G. , Giuzio, R. , Rosati, M. , … Zoli, M. (2009). Mean platelet volume (MPV) increase during acute non‐lacunar ischemic strokes. Thrombosis Research, 123(4), 587–591. 10.1016/j.thromres.2008.03.025 [DOI] [PubMed] [Google Scholar]
- Musselman, D. L. , Tomer, A. , Manatunga, A. K. , Knight, B. T. , Porter, M. R. , Kasey, S. , … … C. B. (1996). Exaggerated platelet reactivity in major depression. American Journal of Psychiatry, 153(10), 1313–1317. [DOI] [PubMed] [Google Scholar]
- Nemeroff, C. B. , & Musselman, D. L. (2000). Are platelets the link between depression and ischemic heart disease? American Heart Journal, 140(4 Suppl), 57–62. 10.1067/mhj.2000.109978 [DOI] [PubMed] [Google Scholar]
- Parikh, R. M. , Robinson, R. G. , Lipsey, J. R. , Starkstein, S. E. , Fedoroff, J. P. , & Price, T. R. (1990). The impact of poststroke depression on recovery in activities of daily living over a 2‐year follow‐up. Archives of Neurology, 47(7), 785–789. 10.1001/archneur.1990.00530070083014 [DOI] [PubMed] [Google Scholar]
- Park, S. C. , Kim, J. M. , Jun, T. Y. , Lee, M. S. , Kim, J. B. , Yim, H. W. , & Park, Y. C. (2017). How many different symptom combinations fulfil the diagnostic criteria for major depressive disorder? Results from the CRESCEND study. Nordic Journal of Psychiatry, 71(3), 217–222. 10.1080/08039488.2016.1265584 [DOI] [PubMed] [Google Scholar]
- Robinson, R. G. (2003). Poststroke depression: Prevalence, diagnosis, treatment, and disease progression. Biological Psychiatry, 54(3), 376–387. 10.1016/S0006-3223(03)00423-2 [DOI] [PubMed] [Google Scholar]
- Sarfo, F. S. , Jenkins, C. , Singh, A. , Owolabi, M. , Ojagbemi, A. , Adusei, N. , … Ovbiagele, B. (2017). Post‐stroke depression in Ghana: Characteristics and correlates. Journal of the Neurological Sciences, 379, 261–265. 10.1016/j.jns.2017.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp, D. S. , Benowitz, N. L. , Bath, P. M. , Martin, J. F. , Beswick, A. D. , & Elwood, P. C. (1995). Cigarette smoking sensitizes and desensitizes impedance‐measured ADP‐induced platelet aggregation in whole blood. Thrombosis and Haemostasis, 74(2), 730–735. 10.1055/s-0038-1649805 [DOI] [PubMed] [Google Scholar]
- Shimbo, D. , Child, J. , Davidson, K. , Geer, E. , Osende, J. I. , Reddy, S. , … Badimon, J. J. (2002). Exaggerated serotonin‐mediated platelet reactivity as a possible link in depression and acute coronary syndromes. American Journal of Cardiology, 89(3), 331–333. 10.1016/S0002-9149(01)02236-6 [DOI] [PubMed] [Google Scholar]
- Tohgi, H. , Suzuki, H. , Tamura, K. , & Kimura, B. (1991). Platelet volume, aggregation, and adenosine triphosphate release in cerebral thrombosis. Stroke, 22(1), 17–21. 10.1161/01.STR.22.1.17 [DOI] [PubMed] [Google Scholar]
- van der Loo, B. , & Martin, J. F. (1999). A role for changes in platelet production in the cause of acute coronary syndromes. Arteriosclerosis, Thrombosis, and Vascular Biology, 19(3), 672–679. 10.1161/01.ATV.19.3.672 [DOI] [PubMed] [Google Scholar]
- Vural, M. , Aydin, F. Y. , Bozan, B. , Kasim, A. B. , Ustun, I. , & Bes, C. (2015). Association of cognitive function with serum uric acid and mean platelet volume levels between fibromyalgia syndrome and healthy subjects. Annals of the Rheumatic Diseases, 74, 1215–1216. [Google Scholar]
- Yetkin, E. (2008). Mean platelet volume not so far from being a routine diagnostic and prognostic measurement. Thrombosis and Haemostasis, 100(1), 3–4. 10.1160/TH08-05-0336 [DOI] [PubMed] [Google Scholar]
- Yildirim, T. , Solmaz, D. , Akgol, G. , & Ersoy, Y. (2016). Relationship between mean platelet volume and vitamin D deficiency in fibromyalgia. Biomedical Research‐India, 27(4), 1265–1270. [Google Scholar]
- Zhang, Y. , Zhao, H. , Fang, Y. , Wang, S. , & Zhou, H. (2017). The association between lesion location, sex and poststroke depression: Meta‐analysis. Brain Behav, 7(10), e00788 10.1002/brb3.788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman, M. , Martinez, J. , Young, D. , Chelminski, I. , & Dalrymple, K. (2013). Severity classification on the Hamilton Depression Rating Scale. Journal of Affective Disorders, 150(2), 384–388. 10.1016/j.jad.2013.04.028 [DOI] [PubMed] [Google Scholar]


