Abstract
Background
Traditional diagnostic methods for tuberculosis (TB) cannot be reliably applied to tuberculous pleurisy. Therefore, this prospective, randomized, controlled trial was performed to compare the diagnostic sensitivity and safety of ultrasound-guided cutting-needle pleural biopsy versus thoracoscopic pleural biopsy in patients suspected of tuberculous pleurisy following inconclusive thoracentesis.
Material/Methods
A total of 196 adult patients with acid-fast bacillus (AFB)-negative exudative pleural effusions clinically suspected of tuberculous pleurisy were recruited. Enrollees were randomized into 2 cohorts: ultrasound-guided cutting-needle pleural biopsy (n=96) or thoracoscopic pleural biopsy (n=96). The overall diagnostic yields, diagnostic sensitivities for tuberculous pleurisy, and post-procedural complications for both cohorts were statistically compared.
Results
Ultrasound-guided pleural biopsy displayed an overall diagnostic yield of 83%, while thorascopic pleural biopsy displayed a similar overall diagnostic yield of 86% (χ2=1.88, df=1, p=0.17). There were 127 patients conclusively diagnosed with tuberculous pleurisy, resulting in a tuberculous pleurisy prevalence of 65% in this patient population (66% in the ultrasound cohort vs. 63% in the thoracoscopy cohort; p>0.05). Ultrasound-guided pleural biopsy displayed a sensitivity of 82% in detecting tuberculous pleurisy, while thorascopic pleural biopsy displayed a similar sensitivity of 90% (χ2=1.05, df=1, p=0.30). The sensitivities of these 2 modalities did not significantly differ based on the degree of pleural thickening (p>0.05). Post-procedural complications were minor.
Conclusions
Ultrasound-guided and thoracoscopic pleural biopsy both display strong (>80%) but statistically similar overall diagnostic yields for diagnosing pleural effusions following inconclusive thoracentesis. Both modalities also display strong (>80%) but statistically similar sensitivities in detecting tuberculous pleurisy.
MeSH Keywords: Biopsy; Pleural Diseases; Sound; Thoracoscopy; Tuberculosis, Avian
Background
Tuberculosis (TB) is an airborne disease produced by the inhaled pathogen Mycobacterium tuberculosis that typically affects lung tissue, resulting in severe cough, fever, and chest pains [1]. In 2016, the World Health Organization (WHO) estimated there were 10.4 million new TB cases and 1.7 million TB-related deaths [2]. Notably, the vast majority (95%) of these reported deaths occur in emerging low- and middle-income countries, with the preponderance of cases appearing in India, Indonesia, China, Philippines, and Pakistan, in descending order [2]. More than 50 million patient lives have been saved through TB diagnosis and therapy since 2000, but improving the diagnosis and treatment of TB remains a pressing global health issue [2].
Tuberculous pleurisy – characterized by subpleural caseous focal rupture followed by delayed hypersensitivity reactions to M. tuberculosis antigens – is the second most common extrapulmonary manifestation of TB [3,4]. Tuberculous pleurisy has been positively associated with pleural fibrosis, calcification, and residual thickening, which can result in serious ventilatory limitation [5,6]. Unfortunately, the traditional diagnostic methods for TB (such as the acid-fast bacillus (AFB) smear, tubercle bacillus culture, and molecular tests) cannot be reliably applied to tuberculous pleurisy; only 10–35% of true-positive cultures and 20–81% of true-positive molecular tests actually reveal mycobacteria in thoracentesis-derived pleural fluid [7]. Therefore, more sensitive diagnostic modalities for tuberculous pleurisy are still needed.
To this end, image-guided pleural biopsies (i.e., ultrasound-guided or computer tomography (CT)-guided) have become popular modalities for diagnosing pleural effusions following inconclusive thoracentesis [8]. In particular, ultrasound-guided pleural biopsy shows certain practical advantages over CT-guided pleural biopsy, as ultrasound-guided procedures can be carried out in real-time at the bedside or on an outpatient basis, and do not involve costly CT facilities, complex CT positioning, or radiation exposure [9]. Although the diagnostic sensitivity of ultrasound-guided pleural biopsy in malignant pleural effusions has been verified [10,11], its diagnostic sensitivity in tuberculous pleurisy has not been reported.
Thoracoscopic pleural biopsy, although more invasive than ultrasound-guided pleural biopsy, has also been shown to be a sensitive and safe modality in diagnosing pleural effusions following inconclusive thoracentesis [12]. However, there is a lack of published evidence regarding the sensitivity and safety of ultrasound-guided pleural biopsy vis-à-vis thoracoscopic pleural biopsy in diagnosing tuberculous pleurisy. Therefore, this prospective, randomized, controlled trial was performed to compare the diagnostic sensitivity and safety of ultrasound-guided cutting-needle pleural biopsy vs. thoracoscopic pleural biopsy in patients suspected of having tuberculous pleurisy following inconclusive thoracentesis. In addition, as pleural thickening has been shown to affect diagnostic efficacy [13], we also analyzed any potential correlations between pleural thickening and diagnostic sensitivities for these 2 modalities.
Material and Methods
Ethics statement
All enrollees were informed of the procedural risk before examination and provided signed, written informed consent prior to inclusion. This trial was approved by the Ethics Committee of the Bengbu Medical College (approval no. 2012-037) and was registered at the Chinese Clinical Trial Registry as ChiCTR-DCD-15007167 (www.chictr.org.cn).
Patient selection
This trial sequentially screened all candidates admitted to the Department of Respiratory Medicine at the First Affiliated Hospital of Bengbu Medical College between June 2012 and June 2015 who were clinically suspected of having tuberculous pleurisy. All candidates underwent standard chest radiography and one-time thoracentesis. Clinical suspicion of tuberculous pleurisy was based on reasonable suspicion of tuberculous pleurisy from patient history, clinical presentation, chest radiography, and thoracentesis findings after ruling out malignancy and other pleural diseases [14]. Specifically, reasonable suspicion of tuberculous pleurisy was defined as the combination of: (i) evidence of exudative pleural effusions by chest radiography and (ii) presence of AFB-negative pleural effusions containing lymphocytes without malignant cells via thoracentesis. Adenosine deaminase levels were assessed in the pleural fluid. However, more advanced molecular tests (e.g., GeneXpert MTB/RIF) were not available.
The inclusion criterion for this trial was reasonable suspicion of tuberculous pleurisy as defined above. Exclusion criteria for this trial were: (1) age less than 18 or over 85 years; (2) pre-existing diagnosis of tuberculous pleurisy; (3) presence of AFB-positive pleural effusion(s) (indicating tuberculous pleurisy); (4) pleural adenosine deaminase values exceeding 70 U/l (indicating tuberculous pleurisy); (5) currently taking anti-TB therapy; (6) the presence of suspected pulmonary malignant mass by radiological imaging; (7) invasive procedures contraindicated due to cardiopulmonary dysfunction and/or coagulopathy; or (8) pregnancy.
Considering that the published sensitivities for thoracoscopic and ultrasound-guided pleural biopsy in diagnosing pleural effusions are 94% and 87%, respectively [15,16], the minimal sample size per arm for the current trial was pre-estimated to be 89 patients using an α-value of 0.05 and a maximal 7% marginal error of estimate [17]. The number of patients in each arm was increased by 10% to 98 cases per arm to accommodate potential withdrawals. On this basis, a total of 196 patients (111 males and 85 females, mean age: 57.8±17.7 years) were finally selected for inclusion in this trial. Most enrollees presented with symptoms of fever, cough, night sweats, and/or chest pains, lasting between 1 and 6 weeks. Fifty-five enrollees had pre-existing diabetes, 8 enrollees had pre-existing coronary heart disease, and 4 enrollees had pre-existing hypertension.
Trial design
The enrollees were blindly randomized into 2 cohorts using a random number table: the ultrasound group or the thoracoscopy group. Due to administrative and technical limitations, neither patients nor providers were blinded to the procedure undertaken. In the ultrasound group, patients received an initial ultrasound-guided cutting-needle pleural biopsy, and those who were not diagnosed after the first attempt underwent a second ultrasound-guided pleural biopsy. Those who were still indeterminate after the second attempt then received a thoracoscopic pleural biopsy. In the thoracoscopy group, patients received an initial thoracoscopic pleural biopsy. Patients with pleural adhesions who were unable to undergo thoracoscopy after artificial pneumothorax then underwent an ultrasound-guided pleural biopsy.
Pre-procedural preparation
Before the procedure, all patients underwent routine blood screening (e.g., CBC, coagulation function, hepatitis B and C, HIV, and syphilis), ECG, liver and kidney function tests, and arterial blood gas analysis. All patients received a pre-procedural posterior-anterior (PA) chest X-ray as well as a pre-procedural B-mode ultrasound on the affected side to explore the degree of pleural effusion, thickness, and adhesion. Pleural thickness was measured and recorded for later analysis.
In the thoracoscopy group, the pleural effusion on the affected side was slowly drained using central venous catheterization (100 ml/h) 48 h before the procedure, resulting in a total drainage of 800–1000 ml. Then, thoracoscopy patients were injected with 600–800 ml of filtered oxygen 24 h before the procedure. After the procedure, PA chest X-rays were obtained to rule out artificial pneumothorax.
Instrumentation
Thoracoscopy was carried out using the following standard instruments: a flexible electronic medical thoracoscope (LTF-240, Olympus, Japan), a disposable trocar for thoracic puncture (Olympus, Japan), pleural biopsy forceps (FB-55CR-1, Olympus, Japan), a surgical kit for closed thoracic drainage, a closed thoracic drainage catheter, a closed drainage bottle, a nasal catheter for oxygen delivery, and an ECG monitor.
Ultrasound-guided cutting-needle biopsy was performed using a GE Voluson E8 color Doppler ultrasonic diagnostic apparatus equipped with a 3.5-MHz convex array probe (General Electric, Schenectady, NY, USA), a Bard biopsy instrument (MG15–22, Bard Biopsy Systems, Tempe, AZ, USA), and a 18G disposable biopsy needle (Bard-Magnum, Bard Biopsy Systems, Tempe, AZ, USA).
Thoracoscopy procedure
All thoracoscopies were performed in respiratory endoscopy rooms by 2 licensed operators per procedure (XZ, PJ, XH, and WL) (Supplementary Figure 1A). This procedure is routinely performed in our respiratory intervention center and is typically completed in approximately 30 minutes at a cost of €300.
The patients were placed in a lateral decubitus position and received oxygen inhalation through a nasal catheter. Intra-procedural electrocardiography (ECG), blood pressure, and pulse oximetry were monitored. Patients received an intravenous injection of midazolam and fentanyl and routine disinfection 10 minutes prior to the initial incision. After inducing an artificial pneumothorax, the surgical incision was localized according to the pre-procedural chest X-ray at the level of the fifth to seventh intercostal space on the mid- to anterior axillary line. The incision site was then locally anesthetized using 10–20 ml of 2% lidocaine. A 15-mm incision was made along the upper edge of the rib, and the subcutaneous tissue was bluntly dissected to expose the parietal pleura. The trocar was placed, and the thoracoscope was inserted to successively explore the lung apex, the posterior costal pleura, the anterior costal pleura, the lateral pleura, the diaphragmatic pleura, the visceral pleura, and the pleura mediastinalis.
After locating the parietal pleural lesions, multiple biopsies were performed under direct visualization. Four to 6 pieces of tissue were collected and immediately transferred into formalin for histopathological examination. The trocar was then retracted, a 24F thoracic drainage catheter was placed, and the closed drainage bottle was connected. Postoperative patients were managed according to routine thoracoscopic nursing care.
Ultrasound-guided biopsy procedure
All ultrasound procedures were performed at the bedside by 2 licensed operators per procedure (YC, HG, and XQ), either in a respiratory endoscopy room or a patient’s suite (Supplementary Figure 1). This procedure is routinely performed in our respiratory intervention center and is typically completed in approximately 30 minutes at a cost of €120.
Patients were positioned in a sitting or semi-recumbent position. The chest walls were thoroughly examined via ultrasound using a superior-to-inferior approach on the anterior, lateral, and posterior regions of the chest wall. After locating diffuse or localized pleural thickening, the pleural thickness, color Doppler blood flow, and spectral parameters were recorded. The patients were shifted to a semi-Fowler or lateral decubitus position and subjected to routine disinfection. Multi-point local infiltration anesthesia was performed subcutaneously with 2% lidocaine to ensure sufficient pleural anesthesia. The biopsy needle was perpendicularly punctured into the lesion site under ultrasound guidance using Bard biopsy needles. When the tip was within range of the pleural lesion, firing and tissue sampling were performed. Following sampling, the needle was quickly retracted, and the needle tract was compressed. Four to 6 pieces of tissue were collected and immediately transferred into formalin for histopathological examination. The biopsy procedure was then repeated at a later date.
Histopathological diagnosis
All pleural samples were blindly and independently assessed by 2 licensed pathologists. The location, size, and gross morphology of the samples were recorded and then subjected to routine histopathological examination. In addition, the samples were subjected to the Ziehl-Neelsen staining test for AFB. Diagnosis of tuberculous pleurisy was confirmed by the presence of tuberculous granulomas (with or without caseation) and/or a positive AFB test after excluding other granulomatous diseases [18]. The 2 pathologists reached the same diagnosis in the vast majority of cases. A third licensed pathologist was consulted for a final decision in cases of disagreement. Patients diagnosed with tuberculous pleurisy received standard anti-TB therapy and were followed-up for 6 months.
Statistical analysis
Statistical analyses were performed using SPSS 13.0 software. The data are expressed as means ± standard deviations. Significant differences between the 2 cohorts were analyzed using the t test with a p-value significance threshold of 0.05. Diagnostic sensitivities were compared using the chi-squared (χ2) test with an α-level of 0.05.
Results
Comparative analysis of cohorts
This study assessed a total of 205 patients for eligibility, of which 196 patients were included (Figure 1). The CONSORT flow diagram detailing the enrollment and randomized allocation of the 196 enrollees (n=98 per arm) is provided in Figure 2. The ultrasound group consisted of 51 males (52%) and 47 females (48%) with a mean age ±SD of 58.3±18.5 years. The thoracoscopy group consisted of 61 males (62%) and 37 females (38%) with a mean age ±SD of 56.9±15.4 years. There were no significant differences in sex (χ2=0.98, df=1, p=0.33) or age (t=0.376, df=1, p=0.71) distributions between the 2 cohorts.
Figure 1.
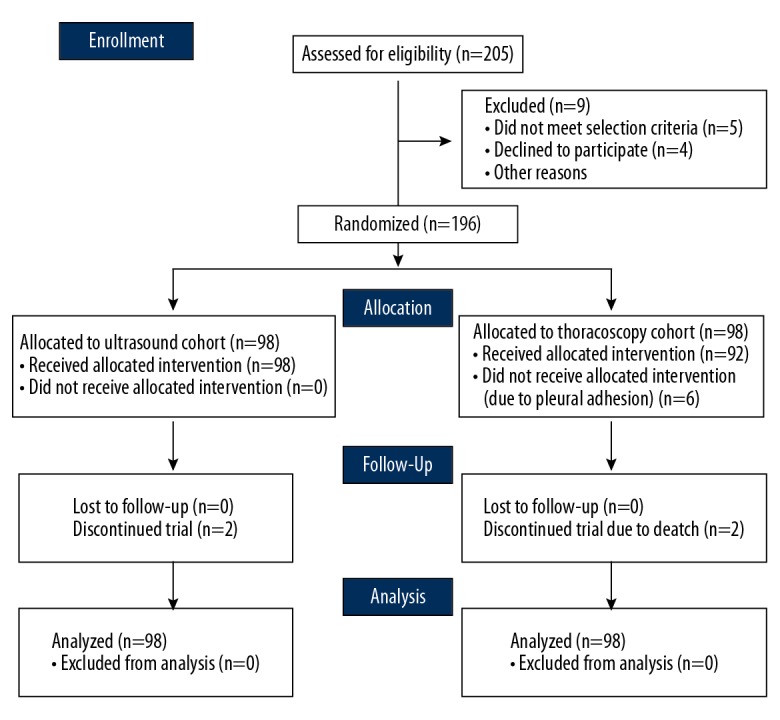
CONSORT 2010 flow diagram.
Figure 2.
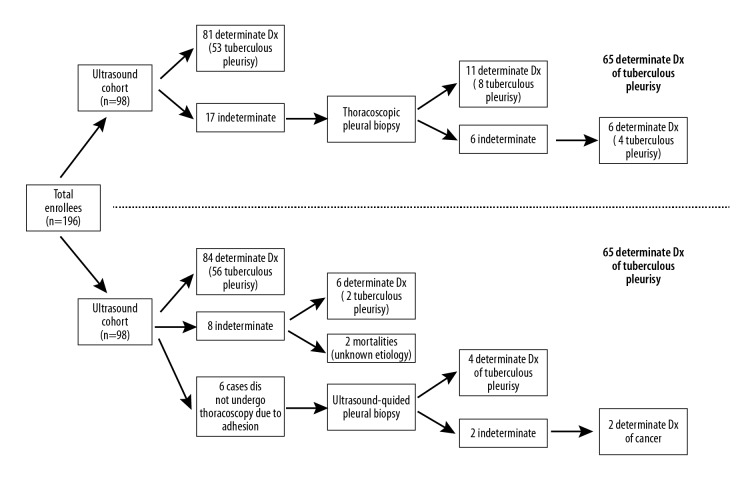
Diagnostic flowchart. The flowchart diagrams the diagnostic progress of the enrollees throughout the trial. In the ultrasound cohort (top panel), there were 53 positive test results among 65 patients with tuberculous pleurisy. In the thoracoscopy cohort (bottom panel), there were 56 positive test results among 62 patients with tuberculous pleurisy.
Ultrasound-guided pleural biopsy findings
The diagnostic flowchart for the ultrasound cohort is provided in Figure 2, and the final diagnostic findings for the ultrasound cohort are summarized in Table 1. A total of 81 patients (81/98, 83%) received a determinate diagnosis from the 2 initial rounds of ultrasound-guided pleural biopsy; 51 of these patients were conclusively diagnosed with tuberculous pleurisy after the first biopsy and 53 patients (with 2 additional cases) were conclusively diagnosed by the second biopsy. This left 17 indeterminate cases. Eleven out of these 17 indeterminate cases were then conclusively diagnosed using thoracoscopy, of which 8 cases were tuberculous pleurisy. This left 6 remaining indeterminate cases, 4 of which received anti-TB treatment due to abnormally elevated adenosine deaminase levels within the pleural effusion. Anti-TB therapy was effective in all 4 cases, as no recurrence of pleural effusion was found during the 6-month follow-up. Due to the efficacy of anti-TB therapy, these 4 cases were post-therapeutically diagnosed as tuberculous pleurisy. The remaining 2 indeterminate cases displayed worsening pleural effusions, and these 2 patients were eventually diagnosed with pleural mesothelioma by a second thoracoscopic examination. We observed complete cessation of all TB signs and symptoms in all tuberculous pleurisy patients after 6 months of anti-TB therapy.
Table 1.
Final diagnostic findings for the 2 patient cohorts.
| Diagnosis | Ultrasound group, n (%) | Thoracoscopy group, n (%) | Total, n (%) |
|---|---|---|---|
| Tuberculous pleurisy | 65 (66%) | 62 (63%) | 127 (65%) |
| Metastatic malignancy | 18 (18%) | 22 (22%) | 40 (20%) |
| Emphysema | 4 (4%) | 6 (6%) | 10 (5%) |
| Non-specific inflammation | 7 (7%) | 2 (2%) | 9 (5%) |
| Lymphoma | 2 (2%) | 0 (0%) | 2 (1%) |
| Mesothelioma | 2 (2%) | 4 (4%) | 6 (3%) |
| Indeterminate | 0 (0%) | 2 (2%) | 2 (1%) |
| Totals | 98 (100%) | 98 (100%) | 196 (100%) |
Thoracoscopic pleural biopsy findings
The diagnostic flowchart for the thoracoscopy cohort is provided in Figure 2, and the final diagnostic findings for the thoracoscopy cohort are summarized in Table 1. A total of 84 patients (84/98, 86%) received a determinate diagnosis by the initial thoracoscopic pleural biopsy, of which 56 patients were diagnosed with tuberculous pleurisy. Of the remaining 14 cases, 8 were indeterminate cases and 6 were unable to undergo thoracoscopy due to pronounced pleural adhesions.
Of the 8 indeterminate cases, 2 cases received anti-inflammatory therapy for 2 weeks during follow-up. The symptoms were not alleviated, and the pleural effusions increased in magnitude. Thereafter, both patients were started on anti-TB therapy, and the symptoms ameliorated thereafter. A chest CT performed after 2 months of anti-TB therapy revealed complete absorption of the pleural effusions. These 2 patients were post-therapeutically diagnosed with tuberculous pleurisy and experienced no recurrence of pleural effusion during the 6-month follow-up period. Of the 8 indeterminate cases, 4 cases displayed a gradually increase in pleural effusions, and malignant cells were detected during repeated hydrothorax exfoliative cytological examination. Two of these cases were determined to be breast carcinoma, while the other 2 cases were diagnosed as lung carcinoma. The remaining 2 indeterminate cases died of non-procedural causes during the follow-up period; the etiology remains unknown since post-mortem testing was refused.
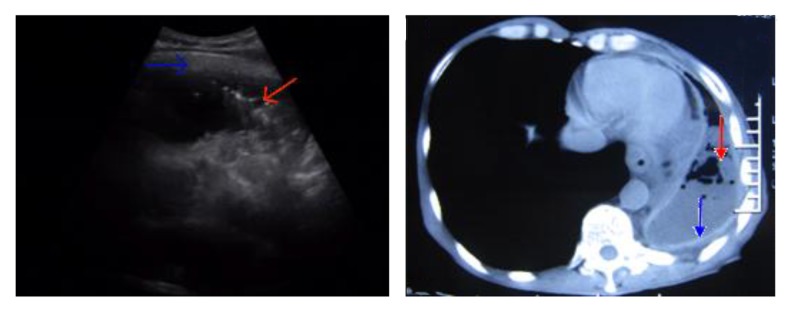
Six cases failed thoracoscopy due to pleural adhesions and underwent ultrasound-guided pleural biopsy (Figure 3); 4 of these 6 cases were diagnosed as tuberculous pleurisy. The remaining 2 cases refused the repeat ultrasound-guided pleural biopsy and manifested increased pleural effusions during follow-up. Both cases revealed the presence of malignant cells via hydrothorax exfoliative cytological examination. We observed complete cessation of all TB signs and symptoms in all tuberculous pleurisy patients after 6 months of anti-TB therapy.
Figure 3.
Ultrasound-guided pleural biopsies with pronounced pleural adhesions. Ultrasound-guided pleural biopsies were performed in 6 cases with pronounced pleural adhesions (indicated by the blue arrow) that contraindicated thoracoscopy. Areas of pleural thickening (indicated by the red arrow) were selected for biopsy.
Gross thoracoscopic observations in tuberculous pleurisy cases
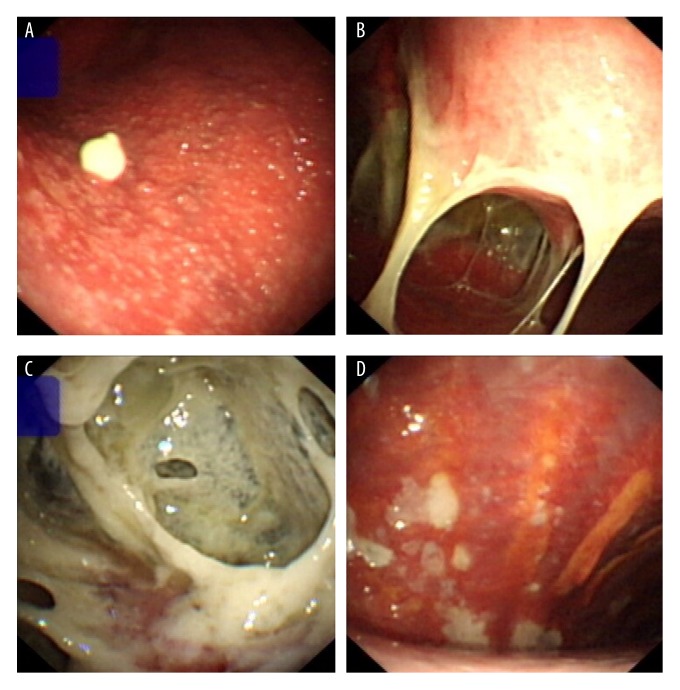
Tuberculous pleural effusions presented as different morphologies under thoracoscopy (Figure 4). The most common morphologies were: (1) parietal pleura congestion with extensive, uniformly-distributed miliary nodules on the pleural surface, appearing gray or yellow (42/62, 68%; Figure 4A), (2) white fibrous band-like adhesions in the pleural cavity, with newly-generated adhesions appearing white and transparent and older adhesions appearing dark with visible vascularization (22/62, 35%; Figure 4B), (3) extensive intrathoracic capsular fibrin deposition, with more advanced cases showing significant fibrin deposition in the parietal and visceral pleura, leading to an unrecognizable thoracic structure (13/62, 21%; Figure 4C), and (4) parietal pleural congestion with white scarring in the pleural space (9/62, 15%; Figure 4D).
Figure 4.
Thoracoscopic views of tuberculous pleurisy. Representative photographs of tuberculous pleurisy captured during thoracoscopy. (A) Severe congestion of the parietal pleura, visible extensive miliary nodules, and granulomatous neoplasms. (B) White fibrous band-like adhesions in the pleural cavity. (C) Extensive intrathoracic capsular fibrin deposition coupled with disappearance of normal intrathoracic structures. (D) Parietal pleural congestion with white scars.
Histopathological findings in tuberculous pleurisy cases
The key histopathological characteristics of tuberculous granulomas included infused epithelial cell nodules with caseous necrotic cores and deposition of fibrous connective tissue (Figure 5). Epithelial cells, lymphocytes, and Langhans multinucleated giant cells were also visible around the granulomatous lesion (Figure 5).
Figure 5.
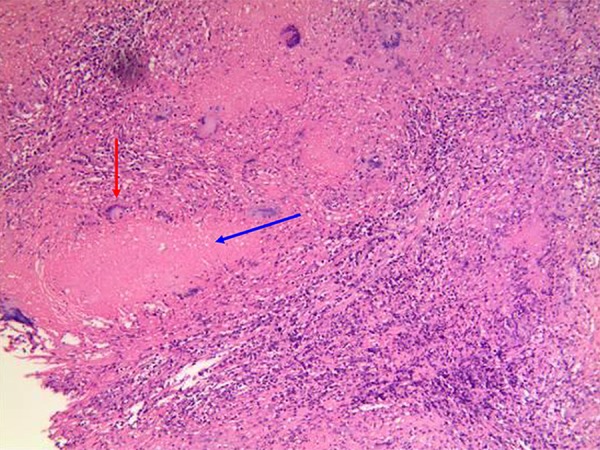
Histopathological diagnosis of tuberculous pleurisy. Representative microscopic imaging of tuberculous granulomas present in tuberculous pleurisy cases. Typical changes include the presence of infused epithelial cell nodules with a caseous necrotic core (indicated by the blue arrow), fibrous connective tissue, epithelial cells, and lymphocytes in the peripheral area. Langhans multinucleated giant cells are visible around the lesion (indicated by the red arrow).
Diagnostic analyses
Diagnostic yield is defined as the proportion of patients achieving a conclusive positive diagnosis within a particular patient population (i.e., the likelihood that the diagnostic modality provides the desired information) [19]. In the ultrasound cohort, there were 68 positive test results after the first biopsy among 98 cases, resulting in an initial diagnostic yield of 69% for single ultrasound-guided pleural biopsy. Then, there were 81 positive test results after the second biopsy (Figure 2), resulting in a final diagnostic yield of 83% for repeat ultrasound-guided pleural biopsy. In the thoracoscopy cohort, there were 84 positive test results among 98 cases (Figure 2), resulting in a diagnostic yield of 86% for thorascopic pleural biopsy. The diagnostic yields of these 2 modalities were not significantly different (χ2=1.88, df=1, p=0.17).
Disease prevalence is defined as the proportion of a particular patient population who have the disease in question (i.e., true positives and false negatives divided by the total sample size) [20]. In the patient population under study, there were 127 patients with tuberculous pleurisy in a total cohort of 196 patients (Figure 2), resulting in a tuberculous pleurisy prevalence of 65% in patients clinically suspected of tuberculous pleurisy following inconclusive thoracentesis. The tuberculous pleurisy prevalence rates were not significantly different between the 2 cohorts (66% in the ultrasound cohort vs. 63% in the thoracoscopy cohort; p>0.05).
Diagnostic sensitivity is defined as the proportion of positive test results among patients with the disease in question [21]. In the ultrasound cohort, there were 51 positive test results after the first biopsy among 65 tuberculous pleurisy cases, resulting in an initial sensitivity of 78% for single ultrasound-guided pleural biopsy in detecting tuberculous pleurisy. Then, there were 53 positive test results after the second biopsy (Figure 2), resulting in a final sensitivity of 82% for repeat ultrasound-guided pleural biopsy in detecting tuberculous pleurisy. In the thoracoscopy cohort, there were 56 positive test results among 62 patients with tuberculous pleurisy (Figure 2), resulting in a sensitivity of 90% for thorascopic pleural biopsy in detecting tuberculous pleurisy. The diagnostic sensitivities of these 2 modalities in detecting tuberculous pleurisy were not significantly different (χ2=1.05, df=1, p=0.30).
Notably, the sensitivity of AFB smear testing in the ultrasound cohort was 3% (2/65), while the sensitivity of AFB smear testing in the thoracoscopy cohort was 6% (4/62). There was no significant difference between these 2 cohorts (χ2=0.38, df=1, p=0.54).
No significant correlation between pleural thickness and sensitivity
The correlations between pleural thickness and diagnostic sensitivity were evaluated for both diagnostic modalities (Table 2). In the ultrasound group, the sensitivity was 94% in cases of pleural thickness ≥1 cm and 66% in cases of pleural thickness <1 cm. The difference in diagnostic sensitivity was not statistically significant but showed a trend toward lower sensitivity in cases of pleural thickness <1 cm (p=0.06). In the thoracoscopy group, the diagnostic sensitivity was 94% in cases of pleural thickness ≥1 cm and 86% in cases of pleural thickness <1 cm. The difference in diagnostic sensitivity was not statistically significant (p=0.58).
Table 2.
Correlation analysis for pleural thickening and sensitivity.
| Pleural thickness | Ultrasound group sensitivity | P-value* | Thoracoscopy group sensitivity | P-value* |
|---|---|---|---|---|
| ≥1 cm | 94% (34/36) | 0.06 | 94% (32/34) | 0.57 |
| <1 cm | 66% (19/29) | 86% (24/28) |
Two-sided Fisher’s exact test comparing ≥1 cm cases versus <1 cm cases.
Safety findings
Post-procedural complications arising from the 2 diagnostic methods are detailed in Table 3. The thoracoscopy cohort displayed overall greater percentages of post-procedural complications, most commonly subcutaneous emphysema (20/98, 20%) and chest pain (18/98, 18%). These complications were amenable to symptomatic treatment or resolved spontaneously. In the ultrasound cohort, 4 cases (4/98, 4%) resulted in pneumothorax. Two of these cases required closed thoracic drainage, leading to complete recovery within 3 days.
Table 3.
Post-procedural complications.
| Complication | Ultrasound groupn (%) | Thoracoscopy group, n (%) | Total, n (%) |
|---|---|---|---|
| Chest pain | 6 (6%) | 18 (18%) | 24 (12%) |
| Fever | 10 (10%) | 6 (6%) | 16 (8%) |
| Subcutaneous emphysema | 0 (0%) | 20 (20%) | 20 (10%) |
| Pneumothorax | 4 (4%) | 0 (0%) | 4 (2%) |
| Hemoptysis | 8 (8%) | 4 (4%) | 12 (6%) |
| Wound empyema/infection | 0 (0%) | 2 (2%) | 2 (1%) |
Discussion
In China and other high TB-burden countries, TB is the most common cause of lymphocyte-dominant pleural effusion [16]. Since thoracentesis yields very few M. tuberculosis bacilli, AFB smears display very poor sensitivities in detecting tuberculous pleurisy [22]. For instance, despite an actual tuberculous pleurisy prevalence of 65% in this study’s population, the sensitivity of AFB smear testing ranged from only 3% to 6%. These findings are consistent with previously reported data [23].
This situation demands the development and use of other more sensitive diagnostic methods in detecting tuberculous pleurisy. Unfortunately, TB culture facilities with high-quality microscopy services and more advanced molecular diagnostics for TB are not commonly available in lower-income countries like China [24]. As a result, in countries like China, pleural biopsy has become an important modality for diagnosing pleural effusions following inconclusive thoracentesis, especially in differentiating TB from malignancy [25,26]. Therefore, our prospective, randomized, controlled trial is the first to compare the sensitivity and safety of ultrasound-guided versus thoracoscopic pleural biopsy in diagnosing tuberculous pleurisy. We demonstrate that ultrasound-guided and thoracoscopic pleural biopsy display strong (>80%) but statistically similar overall diagnostic yields for diagnosing pleural effusions following inconclusive thoracentesis. We also demonstrate that ultrasound-guided and thoracoscopic pleural biopsy display strong (>80%) but statistically similar sensitivities in detecting tuberculous pleurisy.
As initially described by Abrams and Cope, percutaneous pleural biopsy is a relatively low-cost procedure for diagnosing pleural effusions [27,28]. However, the practitioner operates blindly during a traditional closed-blind pleural biopsy, resulting in lower diagnostic yields and inferior sensitivity rates compared to image-guided methods [29,30]. Ultrasound-guided pleural biopsy can overcome these limitations by providing a real-time imaging capability [9]. In the present trial, we found an initial sensitivity of 78% after the first ultrasound-guided pleural biopsy and a final sensitivity of 82% after the second ultrasound-guided pleural biopsy. Therefore, ultrasound-guided pleural biopsy shows a strong sensitivity in detecting tuberculous pleurisy, and repeat ultrasound-guided pleural biopsy may provide some marginal benefit. Notably, 8 of the 12 tuberculous pleurisy patients in this 17-case cohort following repeat ultrasound-guided biopsy were conclusively diagnosed with tuberculous pleurisy by thoracoscopic pleural biopsy. These numbers yield a 67% sensitivity for thoracoscopic pleural biopsy following inconclusive repeat ultrasound-guided biopsy. Therefore, patients suspected of tuberculous pleurisy may benefit from thoracoscopic pleural biopsy following inconclusive repeat ultrasound-guided biopsy. Larger trials are needed to investigate this possibility.
In contrast to ultrasound, thoracoscopy enables the direct visualization of suspicious pleural lesions [31]. Current semi-rigid thoracoscopy involves a 5-cm flexible distal end and a 22-cm rigid operating lever, producing superior diagnostic yields over rigid thoracoscopy when combined with special pleural biopsy forceps [32]. In this trial, we found a sensitivity of 90% for thoracoscopic pleural biopsy in detecting tuberculous pleurisy. Of note, thoracoscopy was not feasible in 6 cases due to pleural adhesions. Four of the 6 tuberculous pleurisy cases in this 6-patient cohort were conclusively diagnosed with tuberculous pleurisy by ultrasound-guided pleural biopsy. These numbers yield a 67% sensitivity for ultrasound-guided pleural biopsy in patients with pleural adhesions contraindicating thoracoscopy. Therefore, patients suspected of tuberculous pleurisy may benefit from ultrasound-guided pleural biopsy where thoracoscopy is contraindicated due to pleural adhesions. Larger trials are needed to investigate this possibility.
One important sequelae of tuberculous pleurisy is pleural thickening, a phenomenon which has been reported in up to half of tuberculous pleurisy patients[6]. Our correlation analysis, shows that pleural thickness did not significantly affect the sensitivity of either ultrasound-guided or thoracoscopic pleural biopsies, which is consistent with the findings Metintas et al. [15]. That being said, the ultrasound group showed a non-significant trend (p=0.06) toward lower sensitivity in cases of pleural thickness under 1 cm. Thus, larger trials are still needed to determine the effects of pleural thickness upon the diagnostic efficacy of ultrasound-guided pleural biopsy.
With respect to safety considerations, no serious post-procedural complications were found in the 2 cohorts. The frequency of pneumothorax was higher in the ultrasound cohort, while the frequencies of subcutaneous emphysema and chest pain were significantly higher in the thoracoscopy cohort. The latter finding may be attributable to the use of a 32F trocar for thoracic drainage, as there were no chest pain complaints following replacement with a smaller 24F trocar. We speculate that the use of the smaller-diameter trocar reduces irritation to the surrounding intercostal nerves. Our findings are consistent with previous reports demonstrating the safety of thoracoscopy and ultrasound-guided pleural biopsy [27,28,33].
There are several limitations to the present study. First, although adequately powered, this trial was conducted with a single patient population recruited from 1 hospital in China, which may limit the generalizability of the findings. Second, we did not analyze the relationship between adenosine deaminase levels as a marker of TB severity [34] or the diagnostic sensitivity of the 2 modalities. Third, neither patients nor providers were blinded to the procedure undertaken, which may have adversely affected conclusions of the trial.
Conclusions
In conclusion, ultrasound-guided and thoracoscopic pleural biopsy both display strong (>80%) but statistically similar overall diagnostic yields for diagnosing pleural effusions following inconclusive thoracentesis. Both modalities also display strong (>80%) but statistically similar sensitivities in detecting tuberculous pleurisy. That being said, ultrasound-guided pleural biopsy may be preferable to thoracoscopy on account of ultrasound’s simplicity, lower invasiveness, and lower cost. Ultrasound-guided pleural biopsy also shows promise in patients with pleural adhesions contraindicating thoracoscopy.
Supplementary Figure
Representtaive photographs of the pleural biopsy procedures. (A) Thoracoscopic pleural biopsy and (B) ultrasound-guided pleural biopsy.
Footnotes
Conflicts of Interest
None.
Source of support: This work was funded by the National Natural Science Foundation of Tibet (XZ2017ZR-ZY033), the Key Laboratory Performance Assessment Subsidy Program of Anhui Province (1206c0805025), the National Natural Science Foundation of Anhui Province (1408085MH144), the Key Foundation of Anhui Educational Committee (KJ2016A487), and Shannan City in Tibet Science and Technology Project (SNKJYFJF2017-3)
References
- 1.Fogel N. Tuberculosis: A disease without boundaries. Tuberculosis. 2015;95(5):527–31. doi: 10.1016/j.tube.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Tuberculosis, Fact Sheet N°104. last updated October [Internet] [Google Scholar]
- 3.Christopher DJ, Dinakaran S, Gupta R, et al. Thoracoscopic pleural biopsy improves yield of Xpert MTB/RIF for diagnosis of pleural tuberculosis. Respirology. 2018;23(7):714–17. doi: 10.1111/resp.13275. [DOI] [PubMed] [Google Scholar]
- 4.Sehgal IS, Dhooria S, Aggarwal AN, et al. Diagnostic performance of Xpert MTB/RIF in tuberculous pleural effusion: Systematic review and meta-analysis. J Clin Microbiol. 2016;54(4):1133–36. doi: 10.1128/JCM.03205-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie S, Lu L, Li M, et al. The efficacy and safety of adjunctive corticosteroids in the treatment of tuberculous pleurisy: A systematic review and meta-analysis. Oncotarget. 2017;8(47):83315–22. doi: 10.18632/oncotarget.18160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakrabarti B, Davies P. Pleural tuberculosis. Monaldi Arch Chest Dis. 2006;65(1):26–33. doi: 10.4081/monaldi.2006.582. [DOI] [PubMed] [Google Scholar]
- 7.Vorster MJ, Allwood BW, Diacon AH, Koegelenberg CF. Tuberculous pleural effusions: Advances and controversies. J Thorac Dis. 2015;7(6):981–91. doi: 10.3978/j.issn.2072-1439.2015.02.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metintas M, Yildirim H, Kaya T, et al. CT scan-guided Abrams’ needle pleural biopsy versus ultrasound-assisted cutting needle pleural biopsy for diagnosis in patients with pleural effusion: a randomized, controlled trial. Respiration. 2016;91(2):156–63. doi: 10.1159/000443483. [DOI] [PubMed] [Google Scholar]
- 9.Kuwal A, Dutt N, Chauhan N. Image-guided pleural biopsy: Issue of the expertise and availability of the resources. Respiration. 2016;92(1):56. doi: 10.1159/000446443. [DOI] [PubMed] [Google Scholar]
- 10.Qureshi NR, Rahman NM, Gleeson FV. Thoracic ultrasound in the diagnosis of malignant pleural effusion. Thorax. 2009;64(2):139–43. doi: 10.1136/thx.2008.100545. [DOI] [PubMed] [Google Scholar]
- 11.Sivakumar P, Jayaram D, Rao D, et al. Ultrasound-guided abrams pleural biopsy vs. CT-guided Tru-Cut pleural biopsy in malignant pleural disease, a 3-year follow-up study. Lung. 2016;194(6):911–16. doi: 10.1007/s00408-016-9933-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Y-B, Xu L-L, Wang X-J, et al. Diagnostic value of medical thoracoscopy in malignant pleural effusion. BMC Pulm Med. 2017;17(1):109. doi: 10.1186/s12890-017-0451-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lau KK, Ng CS. Principles and Practice of Cardiothoracic Surgery. InTech; 2013. Malignant pleural mesothelioma and the role of non-operative therapies. [Google Scholar]
- 14.Ruan S-Y, Chuang Y-C, Wang J-Y, et al. Revisiting tuberculous pleurisy: Pleural fluid characteristics and diagnostic yield of mycobacterial culture in an endemic area. Thorax. 2012;67(9):822–27. doi: 10.1136/thoraxjnl-2011-201363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metintas M, Ak G, Dundar E, et al. Medical thoracoscopy vs. CT scan-guided Abrams pleural needle biopsy for diagnosis of patients with pleural effusions: A randomized, controlled trial. Chest. 2010;137(6):1362–68. doi: 10.1378/chest.09-0884. [DOI] [PubMed] [Google Scholar]
- 16.Diacon AH, Theron J, Schubert P, et al. Ultrasound-assisted transthoracic biopsy: Fine-needle aspiration or cutting-needle biopsy? Eur Respir J. 2007;29(2):357–62. doi: 10.1183/09031936.00077706. [DOI] [PubMed] [Google Scholar]
- 17.Hajian-Tilaki K. Sample size estimation in diagnostic test studies of biomedical informatics. J Biom Inform. 2014;48:193–204. doi: 10.1016/j.jbi.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Gopi A, Madhavan SM, Sharma SK, Sahn SA. Diagnosis and treatment of tuberculous pleural effusion in 2006. Chest. 2007;131(3):880–89. doi: 10.1378/chest.06-2063. [DOI] [PubMed] [Google Scholar]
- 19.Jernlås B, Nyberger H, Ek, et al. Diagnostic yield and efficacy of endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal lymphadenopathy. Clin Respir J. 2012;6(2):88–95. doi: 10.1111/j.1752-699X.2011.00251.x. [DOI] [PubMed] [Google Scholar]
- 20.Brenner H, Gefeller O. Variation of sensitivity, specificity, likelihood ratios and predictive values with disease prevalence. Stat Med. 1997;16(9):981–91. doi: 10.1002/(sici)1097-0258(19970515)16:9<981::aid-sim510>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 21.Glas AS, Lijmer JG, Prins MH, et al. The diagnostic odds ratio: A single indicator of test performance. J Clin Epidemiol. 2003;56(11):1129–35. doi: 10.1016/s0895-4356(03)00177-x. [DOI] [PubMed] [Google Scholar]
- 22.Chakravorty S, Sen MK, Tyagi JSL. Diagnosis of extrapulmonary tuberculosis by smear, culture, and PCR using universal sample processing technology. J Clin Microbiol. 2005;43(9):4357–62. doi: 10.1128/JCM.43.9.4357-4362.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Botana-Rial M, Leiro-Fernandez V, Represas-Represas C, et al. Thoracic ultrasound-assisted selection for pleural biopsy with Abrams needle. Respir Care. 2013;58(11):1949–54. doi: 10.4187/respcare.02378. [DOI] [PubMed] [Google Scholar]
- 24.Siddiqi K, Lambert M-L, Walley J. Clinical diagnosis of smear-negative pulmonary tuberculosis in low-income countries: The current evidence. Lancet Infect Dis. 2003;3(5):288–96. doi: 10.1016/s1473-3099(03)00609-1. [DOI] [PubMed] [Google Scholar]
- 25.Hallifax RJ, Corcoran JP, Ahmed A, et al. Physician-based ultrasound-guided biopsy for diagnosing pleural disease. Chest. 2014;146(4):1001–6. doi: 10.1378/chest.14-0299. [DOI] [PubMed] [Google Scholar]
- 26.Blanc FX, Atassi K, Bignon J, Housset B. Diagnostic value of medical thoracoscopy in pleural disease: A 6-year retrospective study. Chest. 2002;121(5):1677–83. doi: 10.1378/chest.121.5.1677. [DOI] [PubMed] [Google Scholar]
- 27.James P, Gupta R, Christopher DJ, Balamugesh T. Evaluation of the diagnostic yield and safety of closed pleural biopsy in the diagnosis of pleural effusion. Indian J Tuberc. 2010;57(1):19–24. [PubMed] [Google Scholar]
- 28.Bhattacharya S, Bairagya TD, Das A, et al. Closed pleural biopsy is still useful in the evaluation of malignant pleural effusion. J Lab Physicians. 2012;4(1):35–38. doi: 10.4103/0974-2727.98669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rezk NASA, Aly NYA, El-Hadidy TA, Dashti K. CT-guided biopsy versus conventional Abram’s needle biopsy in malignant pleural effusion. Egyptian Journal of Chest Diseases and Tuberculosis. 2015;64(2):405–9. [Google Scholar]
- 30.Koegelenberg CFN, Bolliger CT, Theron J, et al. Direct comparison of the diagnostic yield of ultrasound-assisted Abrams and Tru-Cut needle biopsies for pleural tuberculosis. Thorax. 2010;65(10):857–62. doi: 10.1136/thx.2009.125146. [DOI] [PubMed] [Google Scholar]
- 31.Maturu VN, Dhooria S, Bal A, et al. Role of medical thoracoscopy and closed-blind pleural biopsy in undiagnosed exudative pleural effusions: A single-center experience of 348 patients. J Bronchology Interv Pulmonol. 2015;22(2):121–29. doi: 10.1097/LBR.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 32.Noppen M. The utility of thoracoscopy in the diagnosis and management of pleural disease. Semin Respir Crit Care Med. 2010;31(6):751–59. doi: 10.1055/s-0030-1269835. [DOI] [PubMed] [Google Scholar]
- 33.Agarwal R, Aggarwal AN, Gupta D. Diagnostic accuracy and safety of semirigid thoracoscopy in exudative pleural effusions: A meta-analysis. Chest. 2013;144(6):1857–67. doi: 10.1378/chest.13-1187. [DOI] [PubMed] [Google Scholar]
- 34.Saini V, Lokhande B, Jaswal S, et al. Role of serum adenosine deaminase in pulmonary tuberculosis. Indian J Tuberc. 2018;65(1):30–34. doi: 10.1016/j.ijtb.2017.08.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representtaive photographs of the pleural biopsy procedures. (A) Thoracoscopic pleural biopsy and (B) ultrasound-guided pleural biopsy.