Abstract
Background
The aim of this study was to determine the effects of laparoscopic surgery within an ERAS program on outcomes and immunological function in patients with a carcinoma in the right colon.
Material/Methods
Patient data were acquired from a prospectively maintained database, and 176 patients diagnosed with right colon carcinoma with surgery were selected from the database. These patients were divided into a laparoscopic group (Lap group, n=86) and an open operation group (Open group, n=90). All patients received treatment according to a standardized ERAS protocol. We collected data on CRP levels, CD4+/CD8+ ratios, and Treg values in peripheral blood, baseline and surgical characteristics, postoperative complications, and postoperative ileus (POI).
Results
Circulating CD4+/CD8+ ratios and Treg values were decreased and CRP levels were increased in both groups after the operation. However, the values in the Lap group patients recovered much more quickly than those of patients in the Open group (P<0.05). Patients undergoing laparoscopic surgery had significantly less preoperative bleeding (P<0.01), reduced ratio of overall POI (mainly early ileus), and shorter postoperative hospital stay (P=0.03). Multivariate logistic regression analysis showed that POD1 Treg value was an independent predicator for postoperative ileus in patients with right colon carcinoma resection.
Conclusions
In patients with a carcinoma in the right colon, laparoscopic surgery within an ERAS protocol leads to better immunity preservation after surgery, and POD1 Treg value may be an independent predicator for postoperative ileus, which could, at least in part, explain the shorter hospital stay after surgery.
MeSH Keywords: Colorectal Surgery; General Surgery; T-Lymphocytes, Regulatory
Background
In recent years, Enhanced Recovery after Surgery (ERAS) programs have emerged as a new standard of care for patients with elective colorectal surgery [1,2]. These programs consist of many perioperative methods proven to reduce the psychological and physiological stress of elective colorectal surgery, resulting in improved and shortened recovery after the operation [3,4]. Compared with open surgery, Laparoscopic colorectal surgery has been shown to have equal oncologic effects [5–7]. In recent years, laparoscopic has become established worldwide as a useful method in colorectal surgery as a result of better data on favorable short-term results of laparoscopic surgery [5,8]. Laparoscopic colorectal surgery reduces trauma and the manipulation of tissues, and produces less stimulation to the intestinal tract, blood vessels, and nervous system compared with open surgery [7,9]. Furthermore, many studies have demonstrated that laparoscopic colon surgery not only reduces surgical trauma, but also produces a minimal postoperative systemic immune response [10]. In parallel with this development, the use of an ERAS program in open colorectal surgery has been demonstrated to ameliorate recovery after surgery [4].
Multimodal interventions have been used in ERAS programs to improve postoperative recovery and shares the same objective as laparoscopic surgery purposed to reduce operative stress, and recent randomized trials show that laparoscopic surgery has better outcomes in an ERAS environment [1,11–13]. Furthermore, it was previously reported that regulatory T cells (Treg) and CD4/CD8 ratio play an important role in the outcomes of gastrointestinal carcinoma surgery [14,15]. However, the effect of laparoscopy within the ERAS program on postoperative immune function is uncertain. Therefore, the purpose of the present study was to determine the effect of laparoscopic surgery within an ERAS program on immunological function and clinical outcomes. To reduce selection bias, only patients with a carcinoma in the right colon were included in this study.
Material and Methods
This study was undertaken at the Department of General Surgery of Taizhou People’s Hospital, Taizhou Clinical Medical College of Nanjing Medical University (China). Patients with right colon carcinoma were identified from the prospectively maintained database and the present study was approved by the Ethics Committee of the Taizhou People’s Hospital, Taizhou Clinical Medical College of Nanjing Medical University (China).
In routine clinical practice at our hospital, patients with right colon carcinoma are generally recommended to receive laparoscopic surgery, although some patients may refuse laparoscopy because of personal choice or economic cost. Between May 2013 and Dec 2017, 176 patients diagnosed with right colon carcinoma with surgery were selected from the prospectively maintained database. The inclusion criteria were: no preoperative radiotherapy or chemotherapy, no disease of the immune system, no history of operation on abdominal and distant metastases, normal bowel habits and normal evacuation from stomach, American Society of Anesthesiology (ASA) score degree I/II/III, and self-care function prior to hospitalization. The exclusion criteria were: emergency operation, conversion from laparoscopic operation to laparotomy, association with other organ resection, need for a stoma, inability to place an epidural catheter, and inability to infuse drugs.
All patients received treatment according to a standardized ERAS protocol [16], which mainly included preoperative education, no routine mechanical bowel preparation, clear fluids should be allowed up to 2 h and solids up to 6 h prior to induction of anaesthesia, no routine use of nasogastric tubes, analgesia with non-steroidal anti-inflammatory drugs and reduce opioid analgesics, avoidance of perioperative fluid overload, early feeding, early removal of catheters, and early mobilization. These patients were divided into the laparoscopic operation group (n=86) and the open operation group (n=90). Laparoscopic right colon resection was performed using the same operative set-up and standardized technique, as described by Hildebrandt and Franco [17].
Immunological tests
Between 5: 30 and 7 a.m. on the day before surgery and after surgery (day 1, 3, and 5), samples with EDTA from venous blood were collected serially in our hospital.
Immunoturbidimetric method (Olympus, Tokyo, Japan) was used to detected C-reactive protein (CRP) levels. CD4, CD8, and CD4+CD25+Foxp3+Treg were also measured, as previous described [18]. Briefly, heparinized blood (50 μL) samples were incubated at 4°C for 30 min with each monoclonal antibody (10 μL) specific for a surface antigen (CD4, CD8, CD25, Foxp3) or isotype-matched monoclonal antibody, conjugated to different fluorescent dyes. Red blood cells were depleted from peripheral blood samples with 1 mL of FACS lysing solution (BD Biosciences) for 10 min at room temperature. We started the intracellular staining procedure after all samples were washed with PBS 2 times. Then, 0.25 mL solution of Cytofix/Cytoperm (BD Biosciences) were used to resuspend the cells, which were allowed to sit for a further 20 min to keep cells permeabilized to favor penetration of anti-Foxp3 in the subsequent incubation. Samples were then washed twice with BD Perm/Wash (BD Biosciences). To maintain the samples, we used 50 μL of BD Perm/Wash solution, which contained 10 μL of FITC-conjugated isotype-matched irrelevant antibody or FITC-conjugated anti-Foxp3 at 4°C for 30 min, and then samples were resuspended in 250 μL of PBS after being washed twice with BD Perm/Wash solution. We used a FACS Calibur instrument (BD Biosciences) to perform flow cytometry, and at least 104 events were collected for each analysis.
Clinical materials collection and evaluation
We collected data on baseline and surgical characteristics, postoperative complications, and postoperative ileus (POI). The duration of hospitalization after surgery was defined as time from the day of surgery to the day of hospital discharge, plus any hospital readmission stay within 30 days after surgery. Postoperative morbidity was divided into general and surgical complications. General complications were defined as pulmonary, cardiovascular, urinary tract, thromboembolic, and other complications. Surgical complications were defined as anastomotic leak, postoperative bleeding, wound complication, and bowel obstruction requiring reoperation. Our primary end-points were the rates of postoperative ileus, in-hospital mortality and readmission, and length of stay after surgery. Secondary outcome measures were overall complications and 30-day morbidity. Nausea, vomiting, food intake, nasogastric tube presence or reinsertion within 5 days, defecation, and gastric retention were registered daily in the database.
In this study, a more extensive definition of ileus was used to describe symptoms of POI in addition to return of defecation. Early, late, and prolonged POI were defined as previous described [19]. Early ileus was defined as presence of 1 or more of the following symptoms: more than 1 episode of vomiting or nausea, or reinsertion of the nasogastric tube in the first 5 days; the use of a nasogastric tube for more than 4 days; and no recover of euphagia after 5 days or no first-time normal defecation within 7 days. Vomiting or nausea during the first 5 days that necessitated reinsertion of the nasogastric tube or that influenced defecation and normal intake were defined as late ileus. If early ileus lasted more than 5 days, it was then redefined as prolonged ileus.
Statistical analysis
All data are expressed as means ±SD. Repeated measures analysis of variance (ANOVA) on ranks, followed by the Student-Newman-Keuls test, were used to evaluate statistical differences. The Mann-Whitney rank sum test was used to assess statistical differences between different groups. Risk factors for postoperative ileus were evaluated using multivariate logistic regression analysis. A p value <0.05 was considered as statistical significance.
Results
Altogether, 176 patients underwent a right-sided hemi-colectomy. Of these 176 patients, 86 underwent a laparoscopic operation (Lap group) and 90 underwent a conventional open operation (Open group) for a colonic carcinoma localized in the hepatic flexure, ascending colon, or cecum (Table 1). There were no significant differences between the 2 groups with respect to sex, age, body mass index (BMI), ASA score, current smoker status, comorbidity, or tumor stage (P>0.05) (Table 1).
Table 1.
Basic characteristics of 176 patients having laparoscopic and open right hemicolectomy and preoperative investigation.
| Lap group (n=86) | Open group (n=90) | P value | |
|---|---|---|---|
| Age (years) | 63.5±12.8 | 64.2±11.9 | 0.71 |
| Male/Female | 52/34 | 56/34 | 0.81 |
| BMI | 22.3±3.5 | 21.9±3.7 | 0.46 |
| ASA score | 0.83 | ||
| I | 33 | 32 | |
| II | 41 | 47 | |
| III | 12 | 11 | |
| Current smoker (n) | 14 | 17 | 0.65 |
| Comorbidity (n) | 0.95 | ||
| Angina Pectoris | 3 | 4 | |
| Heart failure | 3 | 2 | |
| Pulmonary dysfunction | 5 | 3 | |
| Insulin treatment | 5 | 4 | |
| Cortisone treatment | 2 | 2 | |
| Radiology/colonoscopy (n) | 0.87 | ||
| Caecal tumour | 42 | 46 | |
| Ascending colon tumour | 26 | 28 | |
| Right flexure tumour | 18 | 16 | |
| TNM stage | 0.82 | ||
| I | 8 | 10 | |
| II | 45 | 43 | |
| III | 33 | 37 |
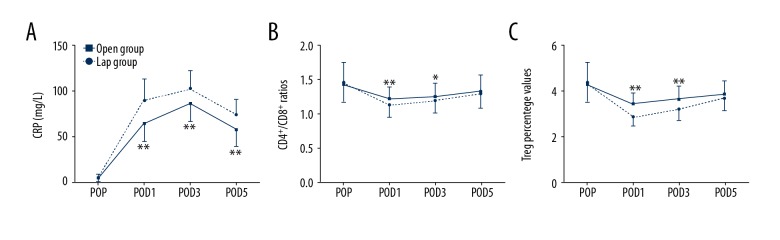
The postoperative levels of CRP were significantly higher in patients in booth groups compared to pre-operatively. The increase in the Open group was significantly higher than in the Lap group (P<0.05), and the levels of CRP on postoperative days 1, 3, and 5 in the Lap group were significantly lower than in the Open group (P<0.05, Figure 1A). Decreased circulating CD4+/CD8+ T cells ratios were found in both groups after surgery. However, the ratios in the Lap group were restored much more quickly in POD1 and POD3 compared with the Open group (P<0.05, Figure 1B). Treg plays an important role in maintaining the homeostasis of immunity. Interestingly, decreased values of Treg percentage were also found in both groups after surgery.
Figure 1.
(A–C) Evolution of peripheral blood CRP, CD4+/CD8+ ratios, and Treg percentage values in the 2 groups. Data are mean ±SD. POP – pre-operation; POD – postoperative day; Open group – open operation group; Lap group – laparoscopic group. * P<0.05, ** P<0.01 Lap group vs. open operation group.
In addition, decreased values of Treg percentage were found on POD1, and the values were gradually restored on POD3 and POD5 in the 2 groups. However, expression of Treg in the Lap group was consistently higher than in the Open group on POD1, POD3, and POD5 (P<0.05; Figure 1C).
Patients undergoing laparoscopic surgery had significantly less preoperative bleeding (91±103 ml vs. 192±215 ml, P<0.01, Table 2). Length of postoperative hospital stay and overall POI (mainly early ileus) were reduced in the Lap group compared with the Open group (P=0.03, Table 2). However, the duration of surgery was longer in the Lap group compared with the Open group (159±35 vs. 138±28, P<0.01, Table 2). There was no significant difference in time to first defecation, overall complications, readmission, and 30-day mortality between the 2 groups (P>0.05, Table 2). Further, we used multivariate logistic regression analysis to find potential predicative factors for postoperative ileus in a total of 59 patients with right colon carcinoma. We found that POD1 Treg percentage was an independent predicator for postoperative ileus in patients with right colon carcinoma resection (OR: 1.97, 95% CI: 1.17–3.32, P=0.011, Table 3).
Table 2.
Overall outcomes characteristics in Lap group and Open group
| Lap group (n=86) | Open group (n=90) | P value | |
|---|---|---|---|
| Duration of surgery (min) | 159±35 | 138±28 | <0.01 |
| Blood loss (mL) | 91±103 | 192±215 | <0.01 |
| Time to first defecation(days) | 2.6±3.1 | 2.9±3.2 | 0.53 |
| Overall POI, n | 22 | 37 | 0.03 |
| Early ileus | 8 | 20 | |
| Late ileus | 7 | 8 | |
| Prolonged ileus | 7 | 9 | |
| Post-operative hospital stay (days) | 5.5±3.7 | 6.8±4.3 | 0.03 |
| Complications overall, n | 10 | 16 | 0.25 |
| General complications | 5 | 9 | |
| Surgical complications | 5 | 7 | |
| Readmission, n | 3 | 5 | 0.51 |
| 30-day mortality, n | 1 | 0 | 0.49 |
Table 3.
Risk factors for postoperative ileus by multivariate logistic regression analysis.
| Variables (n=59) | Multivariate | P-value | |
|---|---|---|---|
| OR | 95%CI | ||
| Age | 0.94 | 0.86–1.02 | 0.54 |
| Current smoker | 1.02 | 0.94–1.19 | 0.33 |
| Comorbidity | 0.97 | 0.93–1.02 | 0.091 |
| TNM stage (I/II vs. III) | 0.98 | 0.89–1.09 | 0.19 |
| Duration of surgery | 4.15 | 0.88–10.34 | 0.084 |
| Blood loss | 1.08 | 0.96–1.22 | 0.17 |
| Time to first defecation | 1.06 | 0.67–1.77 | 0.82 |
| POD1 CRP | 0.67 | 0.17–3.74 | 0.51 |
| POD3 CRP | 0.83 | 0.23–4.15 | 0.62 |
| POD1 CD4+/CD8+ ratio | 1.34 | 0.78–2.49 | 0.28 |
| POD3 CD4+/CD8+ ratio | 1.46 | 0.59–2.48 | 0.32 |
| POD1 Treg percentage | 1.97 | 1.17–3.32 | 0.011 |
| POD3 Treg percentage | 2.51 | 0.79–8.12 | 0.13 |
Discussion
In the present study, in patients with right-side colonic carcinoma treated within an ERAS program, we found a significantly faster recovery associated with improved immunologic functions in patients who underwent laparoscopic surgery compared with those who underwent a conventional open operation.
Laparoscopy has been used in colorectal surgery for nearly 2 decades. Similar postoperative and long-term oncological results were found between laparoscopic operation and open operation, suggesting that laparoscopic surgery is a safe surgical procedure [5–7]. Furthermore, many randomized clinical trials have shown that laparoscopic colon surgery has many advantages, including improved functional bowel recovery, decreased general complications (pulmonary and cardiac complications), milder postoperative pain, and shorter hospital stay compared with traditional open surgery [20–24]. Although many large randomized multicenter studies showed that laparoscopy produced better short-term outcomes, these studies were done in a traditional care setting [20,24]. Previous studies have demonstrated that, compared with conventional open surgery without an ERAS protocol, open surgery within an ERAS program with multimodal components reduced surgical stress, resulting in less morbidity, earlier recovery, and earlier discharge after colorectal surgery [3,16]. In addition, several studies comparing open and laparoscopic operations performed within an ERAS protocol showed better results in patients undergoing laparoscopic surgery. However, some methodological limitations need to be improved, including the mixture of different surgical procedures with different outcomes, small sample-size, and absence of a control group [11,25–27]. The benefits of laparoscopic surgery within an enhanced recovery protocol were recently demonstrated by 2 multi-center randomized studies [28,29]. It is well known that abnormal postoperative immune activity can result in increased occurrence of postoperative infections and metastasis of cancer cells. Studies have reported that laparoscopic colon surgery effectively improves immune function [30]. Therefore, maintaining normal immune function is very important for patients undergoing colon surgery, especially for patients with malignant diseases that directly influence long-term oncological results. Hence, the main aim of the present study was to observe determine whether laparoscopic surgery within an ERAS protocol relieves immune suppression after surgery in patients with right-side colon carcinoma. CRP is a non-specific, acute-phase protein produced by the liver following inflammation and trauma [21]. Indeed, the serum CRP value is increased in association with stress and trauma. Thus, the degree of trauma may be reflected by the postoperative CRP level [31]. In the present study, serum CRP level increased less in patients who underwent laparoscopic surgery compared to those who underwent open surgery, suggesting that laparoscopic surgery causes less trauma and stress compared to open surgery, within an ERAS protocol. Trauma is minimal during laparoscopic surgery because the incision is significantly shorter, there is less stimulation of the intestinal tract, nerves, and blood vessels, and there is less bleeding [32,33]. In addition, surgery induces a systemic immuno-inflammatory response with increased concentrations of CRP (as a marker of inflammatory response) [34]. In present study, CRP levels were much higher immediately after the surgery and recovered more quickly in patients who underwent laparoscopic surgery in comparison to those who underwent open surgery.
CD4/CD8 ratio and regulatory T cells (Treg) were previously reported to play an important role in recovery of patients who underwent gastrointestinal carcinoma surgery [14,15]. Studies also demonstrated that increased CD4/CD8 ratio during the postoperative period were observed in patients with colorectal carcinoma receiving anti-inflammatory treatment using fish oil [35]. Zhu et al. reported that return to normal CD4/CD8 ratio may denote reduced infection as a result of recovery of the anti-infection mechanism [36]. CD4+ T cell subsets (Treg, Th1, and Th17) have remarkably opposite activities, Tregs being anti-inflammatory, and Th1 and Th17 cells being pro-inflammatory [37–39]. Effector T cells are positively correlated with Tregs, which can attenuate the activity of all these effector T cells by anti-inflammatory actions [38]. All these cells play an important role in prevention of autoimmune responses and excessive immune activation perpetrated by self-reactive T cells [37,40]. In the present study, patients who underwent laparoscopic surgery experienced an attenuated decrease and early recovery of Treg percentage values and CD4+/CD8+ ratios compared with the group who received the open surgery regimen. These results suggest better-preserved immune function in these patients, presumably as a result of a better-regulated inflammatory response. In addition, overall complications and readmission were slightly lower in patients who underwent laparoscopic surgery in this study, but the difference was not significant.
POI is a common complication in patients undergoing gastrointestinal surgery, which is accompanied by increased use of parenteral nutrition, increased patient morbidity, prolonged hospitalization, and increased costs [41,42]. An important method for reducing POI is to inhibit the inflammatory response, and in this study, overall POI (mainly early ileus) was reduced in patients who had a laparoscopic operation compared with open operation, which could at least in part explain the shorter hospital stay after surgery in patients who underwent a laparoscopic operation within an ERAS protocol. Although the mechanism underlying this effect is currently unclear, the incision in the laparoscopic operation is significantly shorter and there is less bleeding. Furthermore, Pedziwiatr et al. [43–45] demonstrated that compliance with the ERAS protocol seems to influence recovery and length of hospital stay when applied to laparoscopic colorectal cancer surgery and other abdominal surgeries, and also found an association between ERAS protocol compliance and complications. In the present study, the compliance with ERAS was similar in the 2 groups. In addition, multivariate logistic regression analysis showed that POD1 Treg percentage is an independent predicator for postoperative ileus in patients with right-side colon carcinoma resection. Laparoscopic surgery shows promise in reducing POI and hospital stay after surgery due to better postoperative immune function, especially POD1 Treg percentage, in patients within an ERAS protocol.
Conclusions
In conclusion, in patients with a carcinoma in the right colon, laparoscopic surgery within an ERAS protocol leads to better immune function preservation after surgery, and POD1 Treg value may be an independent predicator for postoperative ileus, which could, at least in part, explain the shorter hospital stays after surgery. However, this was a small-sample study, and a prospective and multi-center study with a larger number of patients is necessary to strengthen our findings of the correlation between Treg levels and postoperative recovery of bowel function.
Footnotes
Conflict of interest
None.
Source of support: This work was supported partly by funding from the National Natural Science Foundation of China (grant no. 81600434), the Jiangsu Natural Science Foundation (grant no. BK20160572 and BK20170358), the Jiangsu Provincial Medical Youth Talent (grant no. QNRC2016514), and the China Postdoctoral Science Foundation (grant no. 2018M630581)
References
- 1.Pearsall EA, Meghji Z, Pitzul KB, et al. A qualitative study to understand the barriers and enablers in implementing an enhanced recovery after surgery program. Ann Surg. 2015;261(1):92–96. doi: 10.1097/SLA.0000000000000604. [DOI] [PubMed] [Google Scholar]
- 2.Jones D, Musselman R, Pearsall E, et al. Ready to go home? Patients’ experiences of the discharge process in an enhanced recovery after surgery (ERAS) program for colorectal surgery. J Gastrointest Surg. 2017;21(11):1865–78. doi: 10.1007/s11605-017-3573-0. [DOI] [PubMed] [Google Scholar]
- 3.McLeod RS, Aarts MA, Chung F, et al. Development of an enhanced recovery after surgery guideline and implementation strategy based on the knowledge-to-action cycle. Ann Surg. 2015;262(6):1016–25. doi: 10.1097/SLA.0000000000001067. [DOI] [PubMed] [Google Scholar]
- 4.Yeung SE, Hilkewich L, Gillis C, et al. Protein intakes are associated with reduced length of stay: A comparison between enhanced recovery after surgery (ERAS) and conventional care after elective colorectal surgery. Am J Clin Nutr. 2017;106(1):44–51. doi: 10.3945/ajcn.116.148619. [DOI] [PubMed] [Google Scholar]
- 5.van Vugt JL, Reisinger KW, Derikx JP, et al. Improving the outcomes in oncological colorectal surgery. World J Gastroenterol. 2014;20(35):12445–57. doi: 10.3748/wjg.v20.i35.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di B, Li Y, Wei K, et al. Laparoscopic versus open surgery for colon cancer: A meta-analysis of 5-year follow-up outcomes. Surg Oncol. 2013;22(3):e39–43. doi: 10.1016/j.suronc.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Pedziwiatr M, Malczak P, Mizera M, et al. There is no difference in outcome between laparoscopic and open surgery for rectal cancer: A systematic review and meta-analysis on short- and long-term oncologic outcomes. Tech Coloproctol. 2017;21(8):595–604. doi: 10.1007/s10151-017-1662-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu QL, Deng YX, Yu BW, et al. Acute hypervolemic infusion can improve splanchnic perfusion in elderly patients during laparoscopic colorectal surgery. Med Sci Monit. 2018;24:614–22. doi: 10.12659/MSM.906155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang G, Jiang Z, Zhao K, et al. Immunologic response after laparoscopic colon cancer operation within an enhanced recovery program. J Gastrointest Surg. 2012;16(7):1379–88. doi: 10.1007/s11605-012-1880-z. [DOI] [PubMed] [Google Scholar]
- 10.Hildebrandt U, Kessler K, Plusczyk T, et al. Comparison of surgical stress between laparoscopic and open colonic resections. Surg Endosc. 2003;17(2):242–46. doi: 10.1007/s00464-001-9148-9. [DOI] [PubMed] [Google Scholar]
- 11.Gustafsson UO, Scott MJ, Schwenk W, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS((R))) Society recommendations. World J Surg. 2013;37(2):259–84. doi: 10.1007/s00268-012-1772-0. [DOI] [PubMed] [Google Scholar]
- 12.Gillissen F, Ament SM, Maessen JM, et al. Sustainability of an enhanced recovery after surgery program (ERAS) in colonic surgery. World J Surg. 2015;39(2):526–33. doi: 10.1007/s00268-014-2744-3. [DOI] [PubMed] [Google Scholar]
- 13.Pisarska M, Pedziwiatr M, Major P, et al. Laparoscopic gastrectomy with enhanced recovery after surgery protocol: single-center experience. Med Sci Monit. 2017;23:1421–27. doi: 10.12659/MSM.898848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang ZM, Wilmore DW, Wang XR, et al. Randomized clinical trial of intravenous soybean oil alone versus soybean oil plus fish oil emulsion after gastrointestinal cancer surgery. Br J Surg. 2010;97(6):804–9. doi: 10.1002/bjs.6999. [DOI] [PubMed] [Google Scholar]
- 15.Whiteside TL. What are regulatory T cells (Treg) regulating in cancer and why. Semin Cancer Biol. 2012;22(4):327–34. doi: 10.1016/j.semcancer.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gustafsson UO, Scott MJ, Schwenk W, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations. Clin Nutr. 2012;31(6):783–800. doi: 10.1016/j.clnu.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Hildebrandt U, Kessler K, Plusczyk T, et al. Comparison of surgical stress between laparoscopic and open colonic resections. Surg Endosc. 2003;17(2):242–46. doi: 10.1007/s00464-001-9148-9. [DOI] [PubMed] [Google Scholar]
- 18.Sellitto A, Galizia G, De Fanis U, et al. Behavior of circulating CD4+CD25+Foxp3+ regulatory T cells in colon cancer patients undergoing surgery. J Clin Immunol. 2011;31(6):1095–104. doi: 10.1007/s10875-011-9585-8. [DOI] [PubMed] [Google Scholar]
- 19.Boelens PG, Heesakkers FF, Luyer MD, et al. Reduction of postoperative ileus by early enteral nutrition in patients undergoing major rectal surgery: Prospective, randomized, controlled trial. Ann Surg. 2014;259(4):649–55. doi: 10.1097/SLA.0000000000000288. [DOI] [PubMed] [Google Scholar]
- 20.Leung KL, Kwok SP, Lam SC, et al. Laparoscopic resection of rectosigmoid carcinoma: prospective randomized trial. Lancet. 2004;363(9416):1187–92. doi: 10.1016/S0140-6736(04)15947-3. [DOI] [PubMed] [Google Scholar]
- 21.Adamina M, Steffen T, Tarantino I, et al. Meta-analysis of the predictive value of C-reactive protein for infectious complications in abdominal surgery. Br J Surg. 2015;102(6):590–98. doi: 10.1002/bjs.9756. [DOI] [PubMed] [Google Scholar]
- 22.Kim CW, Kim WR, Kim HY, et al. Learning curve for single-incision laparoscopic anterior resection for sigmoid colon cancer. J Am Coll Surg. 2015;221(2):397–403. doi: 10.1016/j.jamcollsurg.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 23.Reames BN, Sheetz KH, Waits SA, et al. Geographic variation in use of laparoscopic colectomy for colon cancer. J Clin Oncol. 2014;32(32):3667–72. doi: 10.1200/JCO.2014.57.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niitsu H, Hinoi T, Kawaguchi Y, et al. Laparoscopic surgery for colorectal cancer is safe and has survival outcomes similar to those of open surgery in elderly patients with a poor performance status: Subanalysis of a large multicenter case-control study in Japan. J Gastroenterol. 2016;51(1):43–54. doi: 10.1007/s00535-015-1083-y. [DOI] [PubMed] [Google Scholar]
- 25.Basse L, Jakobsen DH, Bardram L, et al. Functional recovery after open versus laparoscopic colonic resection: A randomized, blinded study. Ann Surg. 2005;241(3):416–23. doi: 10.1097/01.sla.0000154149.85506.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacKay G, Fearon K, McConnachie A, et al. Randomized clinical trial of the effect of postoperative intravenous fluid restriction on recovery after elective colorectal surgery. Br J Surg. 2006;93(12):1469–74. doi: 10.1002/bjs.5593. [DOI] [PubMed] [Google Scholar]
- 27.Delaney CP. Outcome of discharge within 24 to 72 hours after laparoscopic colorectal surgery. Dis Colon Rectum. 2008;51(2):181–85. doi: 10.1007/s10350-007-9126-y. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy RH, Francis A, Dutton S, et al. EnROL: A multicentre randomized trial of conventional versus laparoscopic surgery for colorectal cancer within an enhanced recovery programme. BMC Cancer. 2012;12:181. doi: 10.1186/1471-2407-12-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vlug MS, Wind J, Hollmann MW, et al. Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: A randomized clinical trial (LAFA-study) Ann Surg. 2011;254(6):868–75. doi: 10.1097/SLA.0b013e31821fd1ce. [DOI] [PubMed] [Google Scholar]
- 30.Veenhof AA, Vlug MS, van der Pas MH, et al. Surgical stress response and postoperative immune function after laparoscopy or open surgery with fast track or standard perioperative care: A randomized trial. Ann Surg. 2012;255(2):216–21. doi: 10.1097/SLA.0b013e31824336e2. [DOI] [PubMed] [Google Scholar]
- 31.Tsimogiannis KE, Tellis CC, Tselepis AD, et al. Toll-like receptors in the inflammatory response during open and laparoscopic colectomy for colorectal cancer. Surg Endosc. 2012;26(2):330–36. doi: 10.1007/s00464-011-1871-2. [DOI] [PubMed] [Google Scholar]
- 32.Reza MM, Blasco JA, Andradas E, et al. Systematic review of laparoscopic versus open surgery for colorectal cancer. Br J Surg. 2006;93(8):921–28. doi: 10.1002/bjs.5430. [DOI] [PubMed] [Google Scholar]
- 33.Abraham NS, Young JM, Solomon MJ. Meta-analysis of short-term outcomes after laparoscopic resection for colorectal cancer. Br J Surg. 2004;91(9):1111–24. doi: 10.1002/bjs.4640. [DOI] [PubMed] [Google Scholar]
- 34.Holdstock C, Lind L, Engstrom BE, et al. CRP reduction following gastric bypass surgery is most pronounced in insulin-sensitive subjects. Int J Obes (Lond) 2005;29(10):1275–80. doi: 10.1038/sj.ijo.0803000. [DOI] [PubMed] [Google Scholar]
- 35.Jie HY, Ye JL, Zhou HH, Li YX. Perioperative restricted fluid therapy preserves immunological function in patients with colorectal cancer. World J Gastroenterol. 2014;20(42):15852–59. doi: 10.3748/wjg.v20.i42.15852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu J, Paul WE. CD4 T cells: Fates, functions, and faults. Blood. 2008;112:1557–69. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barbi J, Pardoll D, Pan F. Metabolic control of the Treg/Th17 axis. Immunol Rev. 2013;252:52–77. doi: 10.1111/imr.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khazaie K, von BH. The impact of CD4+CD25+ Treg on tumor specific CD8+ T cell cytotoxicity and cancer. Semin Cancer Biol. 2006;16:124–36. doi: 10.1016/j.semcancer.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Lou J, Bai L, et al. Immune regulation of intrahepatic regulatory T cells in fibrotic livers of mice. Med Sci Monit. 2017;23:1009–16. doi: 10.12659/MSM.899725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whiteside TL. What are regulatory T cells (Treg) regulating in cancer and why. Semin Cancer Biol. 2012;22:327–34. doi: 10.1016/j.semcancer.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boelens PG, Heesakkers FF, Luyer MD, et al. Reduction of postoperative ileus by early enteral nutrition in patients undergoing major rectal surgery: Prospective, randomized, controlled trial. Ann Surg. 2014;259(4):649–55. doi: 10.1097/SLA.0000000000000288. [DOI] [PubMed] [Google Scholar]
- 42.Dai X, Ge X, Yang J, et al. Increased incidence of prolonged ileus after colectomy for inflammatory bowel diseases under ERAS protocol: A cohort analysis. J Surg Res. 2017;212:86–93. doi: 10.1016/j.jss.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 43.Pedziwiatr M, Pisarska M, Kisielewski M, et al. Is ERAS in laparoscopic surgery for colorectal cancer changing risk factors for delayed recovery. Med Oncol. 2016;33(3):25. doi: 10.1007/s12032-016-0738-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pedziwiatr M, Kisialeuski M, Wierdak M, et al. Early implementation of Enhanced Recovery After Surgery (ERAS(R)) protocol – Compliance improves outcomes: A prospective cohort study. Int J Surg. 2015;21:75–81. doi: 10.1016/j.ijsu.2015.06.087. [DOI] [PubMed] [Google Scholar]
- 45.Pedziwiatr M, Pisarska M, Kisielewski M, et al. ERAS protocol in laparoscopic surgery for colonic versus rectal carcinoma: Are there differences in short-term outcomes. Med Oncol. 2016;33(6):56. doi: 10.1007/s12032-016-0772-6. [DOI] [PMC free article] [PubMed] [Google Scholar]