Abstract
Background
Bile acids (BAs) are signaling molecules that participate in maintaining glucose homeostasis. Acute enteral infusion of BAs potently reduces the glycemic response to glucose, associated with an increase of incretin hormones. However, the effect of long-term supplementation of BAs on glucose metabolism has not been fully investigated.
Material/Methods
Thirty diabetic rats were assigned to a control group (n=10), a low TCA group (L-TCA group, n=10), and a high TCA group (H-TCA group, n=10). Rats in the control group were fed a regular high-fat diet (HFD), while rats in the L-TCA group and H-TCA group were fed a TCA (taurocholic acid)-mixed HFD with the concentrations of 0.05% and 0.3%, respectively, to control the intake of HFD and TCA. Energy intake, body weight, serum insulin, glucose tolerance, insulin sensitivity, GLP-1, and total serum BAs were measured at week 2 and week 12.
Results
At week 2 there were no significant differences in body weight, daily energy intake, glucose tolerance, serum insulin, insulin sensitivity, GLP-1, or fasting total serum BAs between the 3 groups. At week 12, fasting blood glucose and intragastric glucose tolerance were better in the H-TCA group, with significantly greater insulin and GLP-1 secretion and better insulin sensitivity; no significant differences in body weight, energy intake, or total fasting serum BAs were observed.
Conclusions
Long-term supplementation with small doses of TCA was demonstrated to improve glucose metabolism in a diabetic rat model and may be a potential target for diabetes control.
MeSH Keywords: Diabetes Mellitus, Type 2; Rats; Taurocholic Acid
Background
Bile acids have been recognized as signaling molecules that take part in multiple physiological metabolism-regulation pathways [1,2]. It has been reported that BAs have the capacity of maintaining glucose homeostasis, mostly via G protein-coupled bile acid receptor (TGR5) [3] and Farnesoid X receptor (FXR) [1,4]. Interestingly, BAs can also be importantly associated with bariatric surgery, as BAs have been found to be elevated postoperatively [5,6] and the bile diversion procedures can partially mimic the metabolism-improving benefits of bariatric procedures [7,8]. Additionally, acute small-intestinal infusion of BAs potently reduces the glycemic response to small-intestinal glucose, associated with an increase in GLP-1 and C-peptide/glucose ratio, indicating the potential for BAs-based therapy in type 2 diabetes [9]. Nevertheless, due to diverse physiological effects and toxicity of certain BA species, long-term supplementation of BAs has not been fully investigated. In the present study, we used taurocholic acid (TCA), which was found to be elevated following duodenal-jejunal bypass in our previous report [10] and observed its long-term gavage effect on metabolism in a rat model of diabetes.
Material and Methods
Rats and TCA gavage
Male Sprague-Dawley rats (8 weeks old, 250 g average weight) were purchased from Beijing (Huafukang Bioscience, Inc., China) and housed individually in cages with normal diurnal rhythm (12-h light/dark cycle), temperature 24–26°C, and humidity at 50–70%. All rats had free access to water and HFD feed (product number: H10141, Beijing Huafukang Bioscience, Inc, China; the ingredients list is shown in Table 1) to improve insulin resistance and fat accumulation for 1 month. After 12-h fasting, 35 mg/kg streptozocin solution (Sigma Aldrich, USA) was intraperitoneally injected. At 72 h after the injection, blood glucose was tested randomly with a glucometer (Roche Diagnostics, Germany). Among these rats, 30 with blood glucose ≥16.7 mmol/L were considered as diabetic and were divided into 3 groups: a control group (n=10), a high TCA group (H-TCA group, n=10), and a low TCA group (L-TCA group, n=10). Rats in the control group were fed a regular HFD, while rats in the L-TCA group and H-TCA group were fed a TCA (Sigma Aldrich, USA)-mixed HFD with concentrations of 0.05% and 0.3%, respectively. All rats were sacrificed humanely at week 12 after a 12 h-fast. The research was approved by the Ethics Committee of Qilu Hospital of Shandong University.
Table 1.
HFD ingredient list.
| Ingredients | Content (g) | Energy (kcal) |
|---|---|---|
| Casein | 195.00 | 780.00 |
| Methionine | 3.00 | 12.00 |
| Corn starch | 50.00 | 200.00 |
| Maltodextrin | 100.00 | 400.00 |
| Saccharose | 341.00 | 1364.00 |
| Cellulose | 50.00 | 0.00 |
| Corn oil | 10.00 | 90.00 |
| Anhydrous milk fat | 200.00 | 1800.00 |
| Mineral mixture | 35.00 | 0.00 |
| Calcium carbonate | 4.00 | 0.00 |
| Vitamin mixture | 10.00 | 40.00 |
| Hydrocholine tartrate | 2.00 | 0.00 |
| Cholesterin | 1.50 | 0.00 |
| Antioxidant | 0.04 | 0.00 |
| Total | 1000 | 4686.00 |
Intragastric glucose tolerance test (IGGTT)
After a 12-h fast, the rats were given an intragastric gavage with 20% glucose (1 g/kg). Then, the blood glucose was measured from the tail vein with a glucometer at t=0, 10, 30, 60, and 120 min. IGGTT was performed at baseline, week 2, and week 12.
Serum insulin and GLP-1
After intragastric gavage with 20% glucose (1 g/kg) at 0, 10, 30, 60, and 120 min, we collected blood specimens from the retrobulbar venous plexus. The blood supernatant was obtained after centrifugation and was stored at −80°C. We used Rat Insulin ELISA kits (Millipore, USA) and Multi-Species GLP-1 Total ELISA kits (Millipore, USA) to measure the serum insulin and total GLP-1, respectively.
Matsuda index
Whole-body insulin sensitivity was estimated using the Matsuda index, as previously described [11].
Fasting serum total bile acids (TBA)
We measured serum total TBA by using the 3α-enzyme cycling method (Roche Cobas 8000 system).
Statistical analyses
Data are expressed as mean ± standard error (mean ±SE). Trapezoidal integration was used to calculate areas under the curve (AUC) for IGGTT. GLP-1, IGGTT, insulin, energy intake, and body weight over time were analyzed using 2-way analysis of variance (ANOVA), treatment and time were factors, and P (tx) values were used to report results of repeated-measures ANOVA for differences over time (time), differences by treatment, and differences by reason of interaction of treatment and time (tx*time). Post hoc comparisons were performed appropriately with Bonferroni correction. GLP-1, insulin, total bile acids, and fasting blood glucose were statistically tested by 1-way ANOVA or t test with Bonferroni correction. P<0.05 was set as statistical significance. IBM SPSS version 19.0 (Chicago, USA) was used in all calculations.
Results
All rats survived the whole study.
Body weight
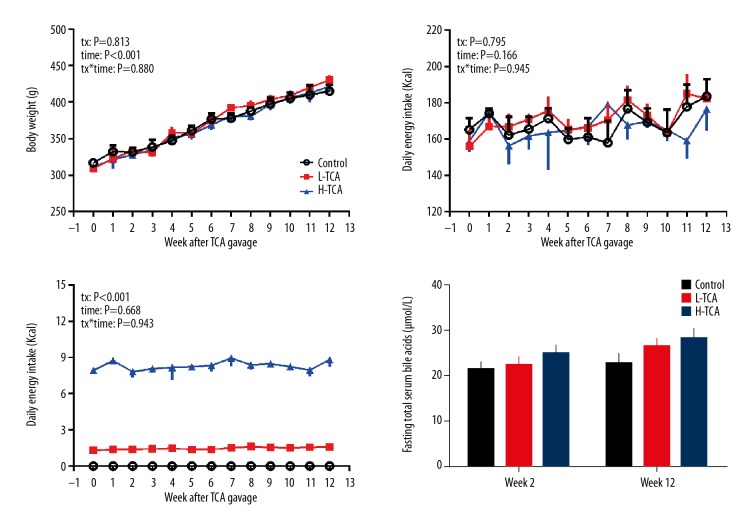
The body weights in all 3 groups increased significantly during the whole study (time: P<0.001) and peaked at week 12 (control: 415±18.7 g, L-TCA: 431±13.3 g, H-TCA: 422±25.5 g). Body weights of all rats in the 3 groups did not differ significantly at any time during the whole study (P>0.05 each) (Figure 1).
Figure 1.
Body weight, daily energy intake, daily TCA intake, and fasting total serum bile acids.
Daily energy intake and TCA intake
During the 12 weeks, daily energy intake did not change significantly (time: P=0.166) and no difference was observed between the 3 groups at any time (tx: P=0.795; tx*time: P=0.945), indicating that TCA supplementation did not affect the appetite of rats (Figure 1).
Based on daily energy intake, TCA intake was calculated. As illustrated in Figure 1, the TCA intake excursion was relatively stable, with the average intake of 1.5±0.1 mg/d and 8.3±0.1 mg/d in the L-TCA and H-TCA groups, respectively (Figure 1).
Fasting blood glucose, Intragastric glucose tolerance test (IGGTT), and AUCIGGTT
Fasting blood glucose was not significantly different among control, L-TCA, and H-TCA groups (P>0.05). At week 2, fasting blood glucose of the control group did not change significantly (16.1±0.9 mmol/L, P>0.05); fasting blood glucose of the L-TCA group tended to be decreased (15.5±1.0 vs. 13.6±0.8mmol/L, P=0.086) and fasting blood glucose of the H-TCA group was significantly decreased compared to baseline (16.7±1.0 vs. 13.8±0.7, P=0.042). At week 12, fasting blood glucose of the control group continued to increase, tending to be worse than baseline (17.4±1.1 vs. 15.9±1.2, P=0.093); interestingly, fasting blood glucose of the L-TCA group returned to 15.3±0.9 mmol/L, which was not significantly different from baseline (P>0.05); but in the H-TCA group, fasting blood glucose was decreased (12.9±0.9 mmol/L) and was significantly lower than at baseline (P=0.049) but was not different from week 2 (P>0.05).
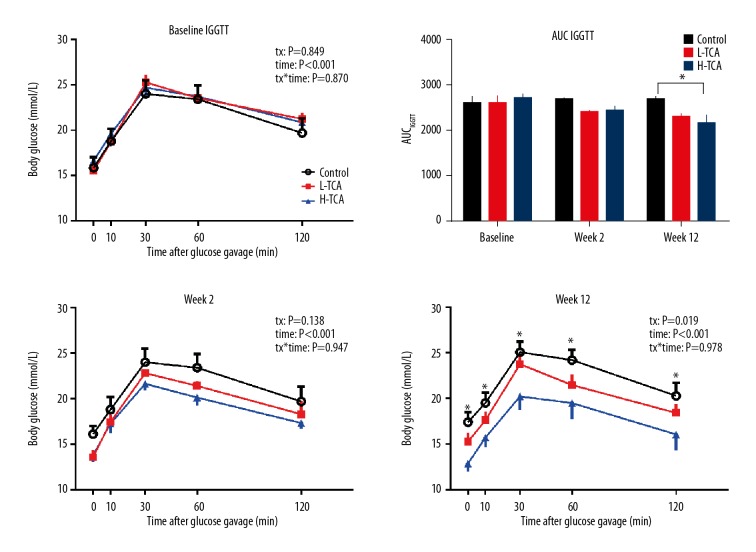
IGGTT did not show any difference between the 3 groups at baseline and week 2 (baseline: tx: P=0.849, tx*time: P=0.870; week 2: tx: P=0.138, tx*time: P=0.947). At week 12, there were significant differences among the 3 groups (tx: P=0.019), with the blood glucose concentrations of the control group being higher than those of the H-TCA group at t=0, 10, 30, 60, and 120 min after glucose gavage (P<0.05 each).
For AUCIGGTT, there were no significant differences among the 3 groups at either baseline or week 2 (P>0.05 both). At week 12, AUCIGGTT of the H-TCA group was significantly lower than in the control group (P=0.02) (Figure 2).
Figure 2.
IGGTT and AUCIGGTT.
Serum insulin and Matsuda index
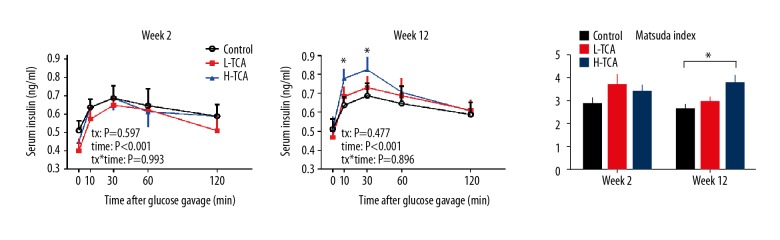
Baseline serum insulin did not significantly differ among the 3 groups at week 2 or week 12 (P>0.05 all). At week 2, in response to glucose gavage, serum insulin was increased, peaking at t=30 min, and then decreased gradually, without any significant differences among the 3 groups at any time (time: P<0.001, tx: P=0.597). At week 12, in response to glucose gavage, the pattern of serum insulin did not differ from week 2; however, the serum insulin concentrations in the H-TCA group were significantly higher than that of the control group at t=10 min and 30 min (P<0.05 both). No other differences were observed among the 3 groups (P>0.05).
The Matsuda index of the 3 groups did not differ at week 2. At week 12, the Matsuda index of the H-TCA group was significantly higher than that of the control group (3.8±0.4 vs. 2.6±0.2, P=0.024), indicating higher insulin sensitivity in the H-TCA group (Figure 3).
Figure 3.
Serum insulin at weeks 2 and 12, and Matsuda index.
Serum GLP-1
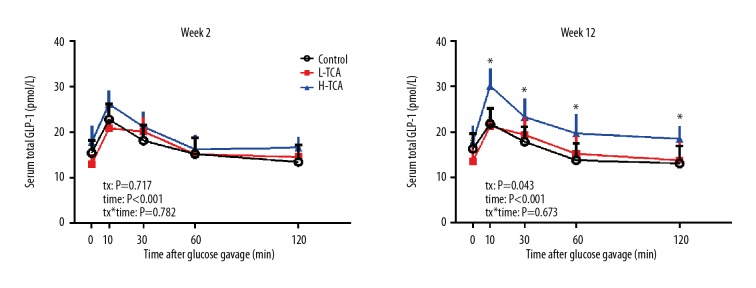
Fasting serum GLP-1 did not differ among the three groups at either week 2 or week 12 (P>0.05 both). At week 2, in response to glucose gavage, serum GLP-1 increased, peaking at t=10 min and then gradually decreased, but there was no significant difference among the 3 groups, (tx: P=0.717). At week 12, in response to glucose gavage, there was significant difference between the 3 groups (time: P<0.001; tx: P=0.043), with the concentrations of serum GLP-1 in the H-TCA group being greater than those of the control group at t=10, 30, 60, and 120 min (P<0.05 all) (Figure 4).
Figure 4.
Serum total GLP-1 at weeks 2 and 12.
Fasting serum total bile acids
Fasting serum total bile acids did not differ among the 3 groups at either week 2 or week 12 (P>0.05 all) (Figure 1, Table 2).
Table 2.
Statistical data of control group and experimental group.
| Control | L-TCA | H-TCA | P value | |
|---|---|---|---|---|
| Baseline body weight (g) | 317±15.1 | 309±24.9 | 312±13.8 | P=0.560 |
| Baseline daily energy intake (kcal) | 165±6.3 | 156±9.3 | 159±6.0 | P=0.665 |
| Baseline fasting blood glucose (mmol/L) | 15.9±1.2 | 15.5±1.0 | 16.7±1.0 | P=0.743 |
| Fasting blood glucose at week 2 (mmol/L) | 16.1±0.9 | 13.6±0.8 | 13.8±0.7 | P=0.067 |
| Fasting blood glucose at week 12 (mmol/L) | 17.4±1.1 | 15.3±0.9 | 12.9±0.9 | P=0.009 |
| Fasting serum insulin at week 2 (ng/ml) | 0.51±0.05 | 0.40±0.07 | 0.45±0.03 | P=0.352 |
| Fasting serum insulin at week 12 (ng/ml) | 0.50±0.04 | 0.47±0.02 | 0.51±0.07 | P=0.807 |
| Matsuda index at week 2 | 2.8±0.3 | 3.7±0.5 | 3.4±0.3 | P=0.262 |
| Matsuda index at week 12 | 2.6±0.2 | 2.9±0.2 | 3.8±0.4 | P=0.023 |
| Fasting serum GLP-1 at week 2 (pmol/L) | 15.4±2.8 | 13.0±2.2 | 18.0±3.6 | P=0.584 |
| Fasting serum GLP-1 at week 12 (pmol/L) | 16.3±3.4 | 13.5±3.2 | 17.8±3.6 | P=0.666 |
| Fasting serum total bile acids at week 2 (μmol/L) | 21.3±1.7 | 22.4±2.0 | 24.8±2.2 | P=0.462 |
| Fasting serum total bile acids at week 12 (μmol/L) | 22.7±2.3 | 26.6±2.0 | 28.1±4.1 | P=0.246 |
Discussion
We found that long-term dietary supplementation with a small dose of TCA improved glucose metabolism in a diabetic rat model. To the best of our knowledge, this is the first observational study of the effect of long-term TCA gavage on glucose homeostasis.
Bile acids, formerly known as “intestinal soap”, have been confirmed as signaling molecules since 1999 [1,2]. Later, bariatric/metabolic surgery was found to postoperatively elevate serum bile acids in animal models and in humans [12,13]. Therefore, it is reasonable to infer that bile acids might be an essential factor mediating the metabolic benefits of bariatric/metabolic surgery. Related studies were performed in 2 directions: bile acid re-routing studies and bile acid gavage studies. Bile acid re-routing studies mimic the anatomical or physiological alterations of bile acids made by bariatric surgery, and confirmed that bile acid manipulation was capable of improving glucose metabolism [7,8]. In our previous report, we performed a bile diversion procedure and concluded that bile acid diversion was an important mechanism, albeit not the only one, in restoring glucose and lipid homeostasis in diabetes [14]. Bile acid re-routing per se did not initially change bile acid production, but led to bile acid redistribution, diverting bile acids to more metabolism-friendly regions, such as the distal small intestine [15].
In contrast, bile acid gavage studies concentrate on bile acid supplementation, based on the metabolism-regulating effects of certain bile acid species. In 2013, Kohli et al. used ursodeoxy cholic acid (UDCA) and tauro ursodeoxy cholic acid (TUDCA) gavage in diet-induced obese rats for 3 weeks, and found that UDCA/TUDCA gavage reduced hepatic steatosis and ER stress (a mechanism for insulin resistance in T2DM) and a concomitant increase in serum TUDCA, without any influence on body weight [7]. In 2017, Mathavan et al. developed a TCA-capsule, which delayed the release of TCA; however, supplementation with a single dose (1.2 mg/300 g) to T1DM rats did not exert hypoglycemic effects [16]. In the present study, consistent with Kohli’s findings, we did not find any difference in body weight between control, L-TCA, and H-TCA groups. Nevertheless, unlike Mathavan’s report, the H-TCA group showed significantly improved glucose intolerance. The explanation for this discrepancy might be multifactorial: first, the animal model we used was induced by a small dose of STZ after HFD gavage, which was closer to a T2DM rat model than a T1DM rat model; second, the dose of TCA in H-TCA group was 8.3±0.1mg/d, which was greater than 1.2 mg/300g; third, the TCA supplementation manner in the present study was physiological gavage, not a capsule, which is technically difficult to prepare and may exert additional stress reaction when feeding.
As this was an observational study, we did not pay much attention to mechanisms. Based on limited data, we found augmented insulin secretion in response to glucose gavage, together with increased insulin sensitivity (evaluated by Matsuda index) at week 12 in the H-TCA group. These findings might be explained by incretin hormones, glucose-dependent insulinotropic polypeptide (GIP), and glucagon-like peptide-1 (GLP-1), which account for up to 70% of postprandial insulin secretion after oral glucose in a glucose-dependent manner in healthy subjects [17]. In T2DM, the insulinotropic effect of GIP is markedly impaired, while GLP-1 still has the capability to enhance insulin secretion [18,19]. As the enteroendocrine L cells, which secrete GLP-1, are widely distributed along the gut [20], it is not surprising to observe an enhanced postprandial GLP-1 response with enteral TCA supplementation (8.3 mg). One previous study reported that the acute metabolism-beneficial effect of TCA was preserved, albeit attenuated, in GLP-1R−/− mice, indicating that other mechanisms might also be involved [21].
This study has several limitations. First, as the conclusions were drawn from an animal model, they may not be generalizable to humans. Second, the underlying mechanisms were not deeply investigated, which warrants further research.
Conclusions
Long-term supplementation with small doses of taurocholic acid was demonstrated to improve glucose metabolism in a diabetic rat model and may be a potential target for diabetes control.
Footnotes
Source of support: National Natural Science Foundation of China (No. 81700708/H0712)
References
- 1.Parks DJ, Blanchard SG, Bledsoe RK, et al. Bile acids: Natural ligands for an orphan nuclear receptor. Science. 1999;284(5418):1365–68. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 2.Wang H, Chen J, Hollister K, et al. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3(5):543–53. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 3.Maruyama T, Miyamoto Y, Nakamura T, et al. Identification of membrane-type receptor for bile acids (M-BAR) Biochem Biophys Res Commun. 2002;298(5):714–19. doi: 10.1016/s0006-291x(02)02550-0. [DOI] [PubMed] [Google Scholar]
- 4.Makishima M, Okamoto AY, Repa JJ, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284(5418):1362–65. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 5.Jahansouz C, Xu H, Hertzel AV, et al. Bile acids increase independently from hypocaloric restriction after bariatric surgery. Ann Surg. 2016;264(6):1022–28. doi: 10.1097/SLA.0000000000001552. [DOI] [PubMed] [Google Scholar]
- 6.Albaugh VL, Flynn CR, Cai S, et al. Early increases in bile acids post Roux-en-Y gastric bypass are driven by insulin-sensitizing, secondary bile acids. J Clin Endocrinol Metab. 2015;100(9):E1225–33. doi: 10.1210/jc.2015-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohli R, Setchell KD, Kirby M, et al. A surgical model in male obese rats uncovers protective effects of bile acids post-bariatric surgery. Endocrinology. 2013;154(7):2341–51. doi: 10.1210/en.2012-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flynn CR, Albaugh VL, Cai S, et al. Bile diversion to the distal small intestine has comparable metabolic benefits to bariatric surgery. Nat Commun. 2015;6:7715. doi: 10.1038/ncomms8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu T, Bound MJ, Standfield SD, et al. Effects of taurocholic acid on glycemic, glucagon-like peptide-1, and insulin responses to small intestinal glucose infusion in healthy humans. J Clin Endocrinol Metab. 2013;98(4):E718–22. doi: 10.1210/jc.2012-3961. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Wang Y, Zhong M, et al. Duodenal-jejunal bypass preferentially elevates serum taurine-conjugated bile acids and alters gut microbiota in a diabetic rat model. Obes Surg. 2016;26(8):1890–99. doi: 10.1007/s11695-015-2031-x. [DOI] [PubMed] [Google Scholar]
- 11.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–70. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 12.Han H, Wang L, Du H, et al. Expedited biliopancreatic juice flow to the distal gut benefits the diabetes control after duodenal-jejunal bypass. Obes Surg. 2015;25(10):1802–9. doi: 10.1007/s11695-015-1633-7. [DOI] [PubMed] [Google Scholar]
- 13.Nakatani H, Kasama K, Oshiro T, et al. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism. 2009;58(10):1400–7. doi: 10.1016/j.metabol.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Liu T, Wang Y, et al. Comparative effects of bile diversion and duodenal-jejunal bypass on glucose and lipid metabolism in male diabetic rats. Obes Surg. 2016;26(7):1565–75. doi: 10.1007/s11695-015-1925-y. [DOI] [PubMed] [Google Scholar]
- 15.Cummings BP, Strader AD, Stanhope KL, et al. Ileal interposition surgery improves glucose and lipid metabolism and delays diabetes onset in the UCD-T2DM rat. Gastroenterol. 2010;138(7):2437–46. 46e1. doi: 10.1053/j.gastro.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Islam KB, Fukiya S, Hagio M, et al. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterol. 2011;141(5):1773–81. doi: 10.1053/j.gastro.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 17.Nauck MA, Homberger E, Siegel EG, et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab. 1986;63(2):492–98. doi: 10.1210/jcem-63-2-492. [DOI] [PubMed] [Google Scholar]
- 18.Wu T, Ma J, Bound MJ, et al. Effects of sitagliptin on glycemia, incretin hormones, and antropyloroduodenal motility in response to intraduodenal glucose infusion in healthy lean and obese humans and patients with type 2 diabetes treated with or without metformin. Diabetes. 2014;63(8):2776–87. doi: 10.2337/db13-1627. [DOI] [PubMed] [Google Scholar]
- 19.Nauck MA, Heimesaat MM, Orskov C, et al. Preserved incretin activity of glucagon-like peptide 1 [7–36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91(1):301–7. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eissele R, Goke R, Willemer S, et al. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur J Clin Invest. 1992;22(4):283–91. doi: 10.1111/j.1365-2362.1992.tb01464.x. [DOI] [PubMed] [Google Scholar]
- 21.Rafferty EP, Wylie AR, Hand KH, et al. Investigating the effects of physiological bile acids on GLP-1 secretion and glucose tolerance in normal and GLP-1R(−/−) mice. Biol Chem. 2011;392(6):539–46. doi: 10.1515/BC.2011.050. [DOI] [PubMed] [Google Scholar]