Abstract
Effort-based decision-making paradigms are increasingly utilized to gain insight into the nature of motivation deficits. Research has shown associations between effort-based decision making and experiential negative symptoms; however, the associations are not consistent. The current study had two primary goals. First, we aimed to replicate previous findings of a deficit in effort-based decision making among individuals with schizophrenia on a test of cognitive effort. Second, in a large sample combined from the current and a previous study, we sought to examine the association between negative symptoms and effort by including the related construct of defeatist beliefs. The results replicated previous findings of impaired cognitive effort-based decision making in schizophrenia. Defeatist beliefs significantly moderated the association between negative symptoms and effort-based decision making such that there was a strong association between high negative symptoms and deficits in effort-based decision making, but only among participants with high levels of defeatist beliefs. Thus, our findings suggest the relationship between negative symptoms and effort performance may be understood by taking into account the role of defeatist beliefs, and finding that might explain discrepancies in previous studies.
Keywords: motivation, avolition, deck choice effort task
Introduction
Schizophrenia is associated with deficits in initiating and persisting in a wide range of goal-directed activities in the social, vocational, and independent living realms.1 These difficulties are considered to be related to disturbances in motivation; however, our current understanding of motivational impairments in schizophrenia is quite limited. One way to approach the study of motivation in schizophrenia is with effort-based decision-making paradigms, and these have received considerable attention in recent years (for reviews see refs. 2–4). Effort-based decision-making tasks, based on animal models of motivation, measure the amount of effort one is willing to exert for different levels of reward. Preclinical studies demonstrate a general “law of least effort” in which animals choose to exert the least amount of effort necessary to obtain a given level of reward; when reward levels increase their willingness to expend effort generally increases.5 For schizophrenia research, these tasks were designed to provide an objective, performance-based measure of motivation that is unencumbered by the pitfalls of self-report and subjective scales.6 While there is evidence of an association between performance on effort tasks and clinically rated negative symptoms, the associations are sometime modest and are not yet fully understood.
Negative symptoms can be divided into motivational negative symptoms (ie, experiential symptoms, such as avolition, anhedonia, or asociality) and diminished expression (ie, expressive symptoms, such as blunted affect and alogia). Experiential negative symptoms, more so than expressive, are robust predictors of poor functional outcome.7,8 They are theoretically linked to the effort and reward valuation processes that contribute to performance on effort-based decision making. Effort-based decision-making tasks were intended to be performance-based analogs of clinically rated negative symptom scales and, indeed, several studies find some support for this association.9–13 However, others failed to find significant associations or found them at a trend-level14–17 and one study paradoxically found the opposite pattern—higher negative symptoms correlated with more effort for higher reward amounts and probabilities.18 Most of the effort tasks used in schizophrenia research measure physical effort (eg, button pressing and grip strength). Fewer studies have been done to examine cognitive effort tasks that require mental computations or increasing cognitive load (studies that do look at cognitive effort10,13,16).
A construct closely related to motivation and engagement in goal-oriented behavior is dysfunctional attitudes, also called defeatist beliefs. Defeatist beliefs refer to negative appraisals about one’s self and one’s ability to perform goal-directed tasks (eg, “Why bother, I’ll just fail again”).19 In cognitive-behavioral models of negative symptoms, defeatist beliefs are closely related to poor motivation.20 They are endorsed at higher rates among individuals with schizophrenia than the general population and are associated with impaired real-world functioning.7,21–24 Defeatist beliefs mediate the relationship between cognition and negative symptoms, as well as the relationship between cognition and impaired functional outcome.19,22,23,25,26 Some studies have reported that interventions targeting defeatist beliefs reduce negative symptoms and improve functional outcome,27,28 while others found no association between change in defeatist beliefs and amotivation or functioning.29
According to the widely accepted cognitive-behavioral model of negative symptoms, defeatist beliefs directly contribute to poor motivation in schizophrenia.22 In Grant and Beck’s theory, cognitive impairment and both negative symptoms and functioning are linked through defeatist beliefs. Individuals with schizophrenia have higher levels of defeatist beliefs than controls and defeatist beliefs are more prevalent in those with higher negative symptoms. Defeatist beliefs have been shown to mediate the association between cognitive deficits and negative symptoms and poor functioning: without defeatist beliefs, poor outcomes would be less associated with poor cognitive ability. Indeed, an empirically tested causal model showed a direct relationship from impaired ability (including cognition) to defeatist beliefs to motivational negative symptoms to poor daily functioning.7 This model is consistent with Beck and Grant’s theory that low expectations for success, based on past experiences, may contribute to social and instrumental withdrawal and diminished motivation. Defeatist beliefs may play a prominent role in affecting reward-related and effort-based behavior and may help explain the association between negative symptoms and performance on effort tasks. We are positing a moderation role for defeatist beliefs based on the idea that endorsement of defeatist beliefs may enhance the effect of experiential negative symptoms such that increasing the level of defeatist beliefs may increase the effect of experiential negative symptoms on effort-based decision making.
The current study had two goals. The first goal was to replicate the findings of our original study in which individuals with schizophrenia displayed decreased willingness to exert effort for rewards, compared to controls, on the Deck Choice Effort Task.30 For this goal, we collected data from a sample of participants with schizophrenia and demographically matched healthy controls. The second goal of the current study was to extend our previous findings by combining our replication sample with the original sample and examining the combined group to better understand the relationships among performance-based effort, negative symptoms, and defeatist beliefs. In this larger sample, we evaluated bivariate associations between these constructs, as well as interactions among them. We wanted to test the possible moderating role of any key component of motivation, namely defeatist beliefs.
Methods
Participants
The replication sample included 33 individuals with schizophrenia and 30 demographically matched healthy controls; the combined replication and original sample includes 126 individuals with schizophrenia and 69 controls. Selection criteria for participants with schizophrenia included: (1) Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV-TR) diagnosis of schizophrenia determined with the Structured Clinical Interview for DSM-IV (SCID-I/P),31 (2) age 18–60 years, (3) no clinically significant neurological disease, (4) no history of serious head injury, (5) no evidence of substance dependence in the past 6 months and no evidence of substance abuse in past month, (6) no history of mental retardation or developmental disability, and (7) clinically stable (ie, no inpatient hospitalizations for 3 months prior to enrollment, no changes in antipsychotic medication type in the 4 weeks prior to enrollment). Neurocognition and clinical symptom assessments were conducted by interviewers trained according to established procedures that included a library of videotaped interviews developed by the Treatment Unit of the Department of Veterans Affairs (VA) VISN 22 Mental Illness Research, Education, and Clinical Center (MIRECC). Symptom raters were trained to a minimum ICC of 0.80.
Selection criteria for both the original and replication healthy control samples included (1) no psychiatric history involving schizophrenia spectrum disorder (including avoidant, paranoid, schizotypal, or schizoid personality disorders), or other psychotic or recurrent axis I mood disorder, according to the SCID-I and SCID-II, (2) no family history of a psychotic disorder among first-degree relatives based on participant report, (3) no history of substance or alcohol dependence and no current substance use, (4) age 18–60 years, (5) no clinically significant neurological disease, and (6) no history of serious head injury. For all study participants, written informed consent was obtained prior to participation after providing a complete description of the study in accordance with approval from the Institutional Review Boards at the VA Greater Los Angeles Healthcare System and UCLA.
Procedures
Deck Choice Effort Task
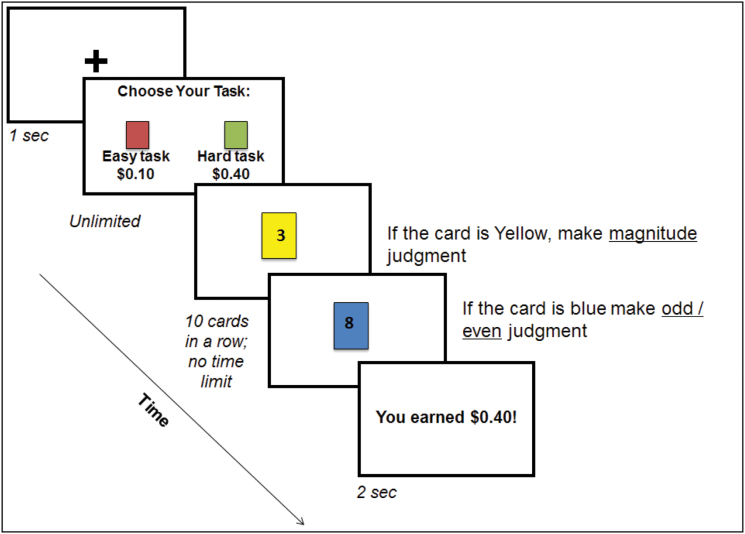
This task is based on a paradigm used in healthy samples to measure willingness to exert cognitive effort for different levels of monetary rewards.32,33 The task includes 36 forced-choice trials in which participants make a series of choices from one of two “decks” of cards (figure 1). The hard deck is composed of cards that alternate between two colors (each color requires a different mental activity) and participants alternate on each trial between making judgments about whether the numbers are odd/even or if they are greater than/less than 5. The easy deck includes cards that are all the same color (requiring a single mental activity, such as saying whether the numbers are odd or even). Participants learn which judgment is associated with which color during practice rounds, which are repeated until over 70% accuracy is achieved. The easy deck always earns $0.10 reward, while the hard deck includes equal number of trials worth $0.10, $0.20, and $0.40. There are 10 cards in each deck and 12 choices of decks in each of the three high-demand reward conditions. Based on our initial psychometric and external validation studies of effort-based decision making tasks,13,30 the primary dependent variable used in correlational and hierarchical modeling is the difference score between percent of “hard” choices at the highest reward condition and the percent of “hard” choices at the lowest reward condition. Higher scores indicate greater willingness to exert effort for large versus small rewards. Difference scores present a problem for “inflexible responders” who selected either 100% hard tasks across all reward levels or 100% easy tasks across all reward levels. With difference scores, participants who select either all hard or all easy would both be assigned a value of “0” (ie, no difference between the highest and the lowest reward levels). Because these two subgroups of participants reflect qualitatively different response profiles and willingness to exert effort for rewards, it is a problem to give these very different subtypes the same value. For this reason, we removed from analyses participants who made only hard selections across all reward levels (that is, they had no room to demonstrate increases in effort allocation). Of the 126 individuals with schizophrenia included in the combined sample, 118 completed the deck choice task. Thirteen selected all hard trials across all three reward levels and were excluded from the combined sample analyses. Thus, the sample for these analyses includes 105 individuals with schizophrenia. We examined task performance using reaction time and accuracy metrics. Following the practice rounds, in which feedback was provided, there were easy and hard nonfeedback trials without reward contingencies. These were used to compute mean reaction time and accuracy for each type of trial. We also analyzed mean accuracy across all hard decks (ie, high effort) selected in the reward section of the task.
Fig. 1.
Deck choice effort task.
Symptoms
The psychiatric symptoms of participants with schizophrenia were evaluated using the Positive and Negative Syndrome Scale (PANSS)34 and the Clinical Assessment Interview for Negative Symptoms (CAINS).35 The PANSS is a 30-item structured interview that yields symptom factor scores on five dimensions; we used the positive, negative, and depression symptom factor scores to characterize the sample.36 The CAINS is a 13-item instrument that yields two subscales (0–4), which measure the two primary negative symptom factors: Motivation and Pleasure (MAP) which measures experiential negative symptoms and Expression.
Cognition
The MATRICS Consensus Cognitive Battery (MCCB)37 was used to assess cognitive functioning. The MCCB includes 10 tests to measure seven domains of cognition: speed of processing, attention/vigilance, working memory, verbal memory, visual memory, reasoning and problem solving, and social cognition. Standardized T-scores were computed for each of the seven domains, correcting for age and gender. The composite score was based on the average T-score from each of the domains and served as the primary dependent measure in this study.
Defeatist Beliefs
Defeatist Performance Attitude Scale (DPAS)38 is a 15-item subscale of the Dysfunctional Attitude Scale. It assesses an individual’s tendency to overgeneralize from past failures to expected future failures (eg, “If I fail partly, it is as bad as being a complete failure,” “People will probably think less of me if I make a mistake,” “If I ask a question it makes me look inferior”). Scoring for these items is on a 7-point scale (1–7) with higher scores indicating greater severity of defeatist attitudes (range for DPAS total = 15–105).
Statistical Analysis
First, two-tailed t tests and chi-squared analyses were used to examine demographic and clinical characteristics across groups for the replication and combined samples. Second, for the replication sample, we examined main effects of reward and group, as well as interactions between reward level and group using repeated measures ANOVA with reward level as a within-subject factor. We also computed effect sizes of these effects to compare to the original study. The remaining analyses were conducted with the combined sample. Pearson’s correlations were used to examine associations between performance on the effort task and negative symptoms, defeatist beliefs, cognition, and depression. Hierarchical multiple linear regression was used to examine whether there was an interaction between experiential negative symptoms and defeatist performance beliefs on effort-based decision making. We used the SPSS macro PROCESS (model template number 1 by Hayes)39 to formally test a moderating effect using the bootstrapping method. To avoid potentially problematic high multicollinearity with the interaction term, the variables were centered and an interaction between MAP and DPAS was created.40
Results
Participants
Table 1 provides the demographic characteristics, symptoms, cognition, and task performance of the replication sample and the larger combined sample (the original sample is described in detail in ref. 30). For both samples, the schizophrenia and control groups did not differ in age, parental education, sex, or ethnicity. As expected, participants with schizophrenia had significantly lower personal education than controls. The replication sample did not differ from the original sample in any clinical characteristic (demographics or symptoms; Supplemental table 1). We compared the 13 participants that were excluded because of inflexible responding (selected all hard trials across all three reward levels) with the remaining schizophrenia sample and found no differences in demographics, cognition, or clinical symptoms. We also compared the two samples in terms of task performance: reaction time and accuracy. There were no differences in either the patient groups or control groups included in the replication and combined samples in terms of task performance.
Table 1.
Descriptive Characteristics of the Replication and Combined Samples
| Replication | Combined | |||||
|---|---|---|---|---|---|---|
| Schizophrenia (n = 33) | Controls (n = 30) | Group Comparisons | Schizophrenia (n = 126) | Controls (n = 69) | Group Comparisons | |
| Sex (% male) | 61% | 53% | x 2 = 0.3, P = .56 | 67% | 57% | x 2 = 2.3, P = .13 |
| Age (SD) | 46.2 (10.2) | 47.9 (7.8) | t = −0.7, P = .48 | 48.2 (11.2) | 47.3 (10.1) | t = 0.6, P = .55 |
| Education (SD) | 13.1 (2.0) | 14.6 (1.7) | t = −3.2, P = .002 | 13.1 (1.9) | 14.5 (1.8) | t = −5.0, P < .001 |
| Parental education (SD) | 13.4 (3.1) | 14.1 (2.4) | t = −0.9, P = .35 | 13.2 (3.3) | 13.3 (3.4) | t = 0.5, P = .60 |
| Race (%) | x 2 = 5.5, P = .36 | X 2 = 5.2, P = .39 | ||||
| Black | 30% | 27% | 37% | 30% | ||
| White | 61% | 50% | 55% | 55% | ||
| Asian | 1% | 1% | 3% | 3% | ||
| Mixed/Other | 1% | 2% | 5% | 12% | ||
| Ethnicity (% Hispanic/Latino) | 24% | 23% | x 2 = 0.01, P = .93 | 17% | 22% | X 2 = 0.5, P = .47 |
| MCCB Composite (SD) | 37.7 (11.3) | 45 (11.8) | t = −2.4, P = .02 | 33.3 (11.9) | 46.2 (9.8) | t = −7.5, P < .001 |
| Symptoms (SD) | ||||||
| PANSS positive (1–7) | 2.2 (1.0) | 2.3 (0.9) | ||||
| PANSS negative (1–7) | 2.0 (0.9) | 2.2 (1.0) | ||||
| PANSS depression (1–7) | 1.8 (0.8) | 1.8 (0.7) | ||||
| CAINS MAP (0–4) | 1.7 (0.9) | 1.7 (0.8) | ||||
| CAINS expressive (0–4) | 1.1 (0.9) | 1.2 (1.0) | ||||
| Antipsychotic medication | ||||||
| Typical (%) | 6% | 10% | ||||
| Atypical (%) | 88% | 84% | ||||
| Both (%) | 3% | <1% | ||||
| None (%) | 3% | <1% | ||||
| CPZ equivalent | 548.2 (363) | 744.1 (831.0) | ||||
| Deck choice effort task performance | ||||||
| Easy trial reaction time | 972 (296) | 866 (212) | t = 1.4, P = .17 | 998.6 (261.4) | 872.4 (172.2) | t = 3.2, P < .01 |
| Hard trial reaction time | 1291 (355) | 1241 (221) | t = 0.6, P = .58 | 1373.3 (336.7) | 1294.2 (248.6) | t = 1.5, P = .13 |
| Easy trial accuracy | .84 (.2) | .93 (.1) | t = −1.8, P = .08 | .85 (.2) | .94 (.1) | t = −3.1, P < .01 |
| Hard trial accuracy | .73 (.1) | .84 (.2) | t = −2.7, P = .01 | .73 (.2) | .85 (.1) | t = −4.7, P < .01 |
| Total hard accuracy | .54 (.4) | .69 (.4) | t = −1.3, P = .19 | .46 (.4) | .75 (.3) | t = −4.7, P < .01 |
Note: SD, Standard deviation; MCCB, MATRICS Consensus Cognitive Battery; PANSS, Positive and Negative Symptom Scale; CAINS, Clinical Assessment Interview for Negative Symptoms; MAP, Motivation and Pleasure; CPZ, Chlorpromazine.
Schizophrenia—Control Group Differences (Replication Sample)
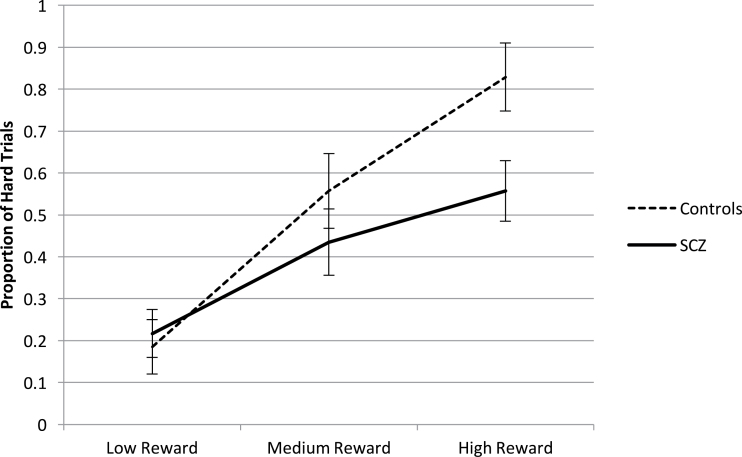
There was a significant main effect of reward (F (2, 96) = 42.3, P < .001), with increased willingness to exert effort at the higher reward levels for both participants with schizophrenia and controls. The main effect of group was nonsignificant (F (1,48) = 2.0, P = .16). Importantly, we found a significant group by reward interaction (F (2, 96) = 4.0, P = .02, partial eta squared = .08), such that participants with schizophrenia and controls were roughly equivalent in proportion of hard trial choices at the lowest level of reward, but controls were more willing to select hard trials as reward values increased compared with participants with schizophrenia (figure 2). Hence, the previous results for group differences in response to reward were replicated.
Fig. 2.
Schizophrenia-control group differences on willingness to exert effort for rewards on the deck choice effort task.
Correlations with Clinical and Cognitive Variables (Combined Sample)
Correlations between variables of interest are presented in table 2. There were no significant correlations between the Deck Choice Effort Task difference score and the CAINS subscales (MAP and Expressive), DPAS, or depression. There was a significant correlation between cognition and effort (r = .33, P < .01). This finding is consistent with that from our original sample in which cognitive functioning was significantly associated with effort expenditure on the Deck Choice Effort Task. We conducted correlational analyses between the Deck Choice Effort Task difference score and CPZ equivalents and found no correlation.
Table 2.
Correlations Between Deck Choice Effort Task Difference Score Between Highest and Lowest Reward Levels and Clinical Variables of Interest
| 1. | 2. | 3. | 4. | |
|---|---|---|---|---|
| 1.Deck Choice Effort Task difference score | – | |||
| 2.CAINS MAP | −.15 | – | ||
| 3.DPAS | −.04 | .31** | – | |
| 4.MCCB Composite | .32** | −.20* | −.10 | – |
| 5.PANSS Depression | −.02 | .06 | .35** | .10 |
Interaction Between Negative Symptoms and Defeatist Beliefs (Combined Sample)
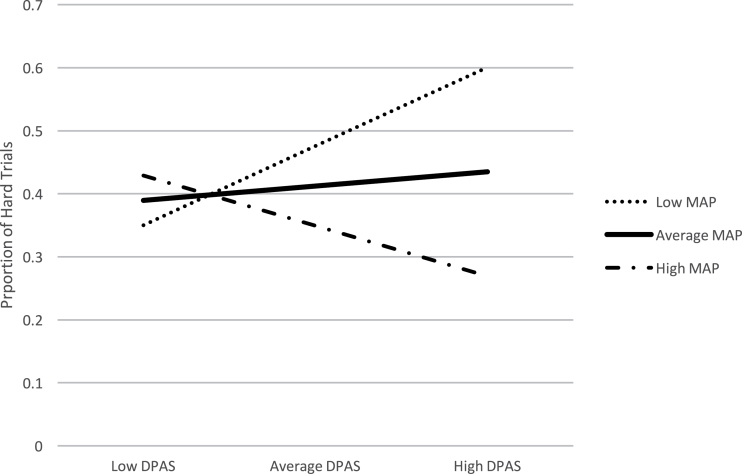
For this analysis, the difference score between hard-effort choices at the high and low reward levels was the dependent variable. Because we were primarily interested in the part of effort performance related to motivation per se and not cognitive ability, we conducted the hierarchical linear regression models with and without cognition included as a covariate. Results with cognition are included here and without are included in Supplementary material. In the first step of the hierarchical multiple regression analysis, three variables were included: MAP, DPAS, and cognition. These variables accounted for a significant amount of variance in effort expenditure R2 = .11, F (3, 102) = 4.0, P =.01 (table 3). Next, the interaction term between DPAS and MAP was added to the model, which accounted for a significant proportion of the additional variance in effort expenditure, Change R2 = .06, Change F (1, 98) = 7.25, P < .01, b = −.01, t (98) = −3.24, P = .002. Examination of the interaction plot showed that, as experiential negative symptoms and defeatist performance beliefs both increased, willingness to exert effort decreased (see green line in figure 3). Results are presented with and without cognition included in the model (see Supplementary material for model specifics). The contribution of MAP and DPAS and their interaction term were equivalent with and without cognition. Cognition did significantly contribute to performance on the Deck Choice Effort Task.
Table 3.
Standardized Regression Weights for Predictors of Deck Choice Effort Task Performance, with and without Cognition in the Model
| Standardized Beta Weights | Standardized Beta Weights | |
|---|---|---|
| Step 1 | ||
| CAINS MAP | −.10 | −.16 |
| DPAS | .05 | .01 |
| MCCB Cognition | .30** | |
| Step 2 | ||
| CAINS MAP | −.13 | −.18 |
| DPAS | .09 | .06 |
| MCCB Cognition | .29** | |
| MAP × DPAS | −.25** | −.26** |
Note: Abbreviations are explained in the first footnote to table 1.
Fig. 3.
Interaction between experiential negative symptoms and defeatist beliefs, controlling for neurocognition. DPAS, Defeatist Performance Attitudes Scale; MAP, Motivation and Pleasure scale
We followed up this analysis with subgroup correlations to further understand the relationship between the defeatist beliefs, negative symptoms, and effort deficits within the schizophrenia group. We divided subjects into three groups based on their DPAS scores using a tertiary split (low DPAS range: 15–46; medium DPAS range: 46–56; high DPAS range: 56–98). The group with the lowest defeatist beliefs had a nonsignificant positive correlation between MAP and willingness to exert effort to earn rewards (n = 41; r = .09, P = .57). The middle group had a non-significant correlation in the negative direction (n = 31; r = −.23, P = .21). The high defeatist beliefs group had a significant, negative correlation (n = 33; r = −.37; P = .03). When we compared these correlations using Fisher’s r to z transformation, there were no significant differences at the .05 alpha level. Hence, the predicted association between performance-based effort and experiential negative symptoms is seen and is most clearly for those with high defeatist beliefs, although our sample size may limit significant differences between the groups.
Discussion
In the current study, we replicated the finding of a significant interaction between group and reward level in an independent replication sample on the Deck Choice Effort Task. Analysis of effect sizes support the consistency of these results: both studies showed small to medium effect sizes (original sample Cohen’s d = .37; replication sample Cohen’s d = .49). That is, individuals with schizophrenia did not increase the amount of effort they were willing to exert with increasing rewards to the same extent as controls. When we examined bivariate correlations in the combined sample, we did not find significant associations between effort-based decision making and either negative symptoms or defeatist beliefs. However, we found a significant interaction between experiential negative symptoms and defeatist beliefs. Specifically, defeatist beliefs moderated the relationship between experiential negative symptoms and willingness to exert effort. Those with high levels of defeatist beliefs and high experiential negative symptoms were less inclined to work harder to earn higher amounts of reward. This finding provides additional evidence of the importance of considering defeatist beliefs to understand motivation and outcome in schizophrenia.7,41,42
We found evidence of defeatist beliefs as moderators in the relationship between experiential negative symptoms and effort-based decision making. Previous studies have found evidence for defeatist beliefs as mediators in the association between negative symptoms and outcome22 or as proximal to negative symptoms in a sequence originating with cognitive or perceptual deficits (eg, refs 7,43). We examined defeatist beliefs as a moderator because several studies have shown the association between negative symptoms and effort, but the lack of consistent large correlations indicated other variables may be at play. A recent review highlighted the fact that although there is evidence for a relationship between defeatist beliefs, negative symptoms, and functional outcome, it is not known when in a developmental sequence defeatist beliefs might come online or what accounts for the variability in defeatist belief severity within a sample of people with negative symptoms.44 Our current findings do not preclude defeatist beliefs as mediators and do not directly speak to the developmental sequence of factors. The moderation influence in this study indicates that under certain conditions (ie, high levels of defeatist performance beliefs), experiential negative symptoms are significantly related to low levels of willingness to exert effort on a cognitive effort-based decision-making task. We do know that those with the most severe negative symptoms have significantly higher levels of defeatist beliefs,43 and it appears that the confluence of these factors is useful in predicting low levels of willingness to exert cognitive effort. Not surprisingly, cognition also significantly contributed to performance on the Deck Choice Effort Task. The task is designed to elicit exertion of cognitive effort and performance has been shown to correlate with cognitive ability in a previous study.13 Importantly, including cognition as a predictor in the regression model did not reduce the predictive contribution of the negative symptom and defeatist belief interaction; thus, irrespective of cognition, the motivational, and psychological factors predict willingness to exert effort on the task.
Although individuals with schizophrenia are often aggregated together in clinical research, the clinical heterogeneity of the disorder calls for examination of clinically meaningful subgroups or dimensions. In the current study, bivariate associations were not apparent, but the multimeasure approach illuminated meaningful patterns. Motivation is a multifaceted construct that includes not only the ability to experience and seek pleasure but also value estimation and reward prediction, effort valuation, and decision-making processes.45 Assessing various dimensions of motivation, and using multimodal techniques, may be an important avenue to elucidate meaningful interactions between the various components. One example of such multimodal analysis is demonstrated in a recent study that examined defeatist beliefs in relation to effort exertion as measured by pupil dilation and found participants with high defeatist beliefs, as opposed to those with mild levels of defeatist beliefs, did not show an increase in the physiological index of effort from easy to hard tasks (ie, digit span tasks).46 This finding suggests that those with high defeatist beliefs were less willing to exert effort on this task and had poorer performance. Other neuroscientific techniques including EEG and fMRI could tap into certain processing components of motivation and complement the information gathered from self-report and performance-based measures.
Clinical research further supports the notion of various determinants of motivation. Randomized clinical trials targeting negative symptoms and functional outcome show that the interaction between motivation and defeatist beliefs is relevant for meaningful clinical gains.27,28,47 For example, Granholm et al. found that defeatist beliefs were related to response to a cognitive-behavioral social skills training intervention such that reduction in defeatist beliefs was associated with more improvement in functioning following treatment, and those with more severe defeatist beliefs at baseline had more benefit from treatment than participants with less severe defeatist beliefs.28 Those individuals with high defeatist beliefs may be the most vulnerable to poor functional outcomes and could be identified as appropriate for cognitive and behavioral therapies that target defeatist cognitive schemas (this has been done in several recent studies; see refs 42,47–49).
Effort-based decision-making tasks are still in the early stages of development. They are likely important tools to assess effort-based decisional processes that may contribute to negative symptoms, but it is not yet entirely clear how they fit into a complex construct like motivation. Furthermore, psychometric properties and test-retest reliability (reproducibility) of findings are important to describe for each developed task. We have partially answered our question regarding why there is not a stronger association between experiential negative symptoms and performance on effort-based decision making—defeatist beliefs appear to play an important role, but there may be other contributing factors as well. In the current paper, we can only comment on the types of motivation we examined and we were limited in the range of constructs and assessments we included. In effort-based decision making, we included a measure of cognitive but not physical effort. In dysfunctional attitudes, we considered a measure of defeatist performance beliefs but did not include other potentially important aspects of motivation, such as asocial attitudes, social motivation, or social preference.50 In negative symptoms, we focused our attention on experiential negative symptoms as they theorized to be are the most closely related to reward-seeking behavior.
Supplementary Material
Supplementary material is available at Schizophrenia Bulletin online.
Funding
This project was funded by a VA MERIT Award to WPH (5101CX000593).
Supplementary Material
Acknowledgments
The authors have declared there are no conflicts of interest in relation to the subject of this study.
References
- 1. Blanchard JJ, Kring AM, Horan WP, Gur R. Toward the next generation of negative symptom assessments: the collaboration to advance negative symptom assessment in schizophrenia. Schizophr Bull. 2011;37:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fervaha G, Graff-Guerrero A, Zakzanis KK, Foussias G, Agid O, Remington G. Incentive motivation deficits in schizophrenia reflect effort computation impairments during cost-benefit decision-making. J Psychiatr Res. 2013;47:1590–1596. [DOI] [PubMed] [Google Scholar]
- 3. Gold JM, Waltz JA, Frank MJ. Effort cost computation in schizophrenia: a commentary on the recent literature. Biol Psychiatry. 2015;78:747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Green MF, Horan WP, Barch DM, Gold JM. Effort-based decision making: a novel approach for assessing motivation in schizophrenia. Schizophr Bull. 2015;41:1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Solomon RL. The influence of work on behavior. Psychol Bull. 1948;45:1–40. [DOI] [PubMed] [Google Scholar]
- 6. Moran EK, Culbreth AJ, Barch DM. Ecological momentary assessment of negative symptoms in schizophrenia: relationships to effort-based decision making and reinforcement learning. J Abnorm Psychol. 2017;126:96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Green MF, Hellemann G, Horan WP, Lee J, Wynn JK. From perception to functional outcome in schizophrenia: modeling the role of ability and motivation. Arch Gen Psychiatry. 2012;69:1216–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rassovsky Y, Horan WP, Lee J, Sergi MJ, Green MF. Pathways between early visual processing and functional outcome in schizophrenia. Psychol Med. 2011;41:487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barch DM, Treadway MT, Schoen N. Effort, anhedonia, and function in schizophrenia: reduced effort allocation predicts amotivation and functional impairment. J Abnorm Psychol. 2014;123:387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Culbreth A, Westbrook A, Barch D. Negative symptoms are associated with an increased subjective cost of cognitive effort. J Abnorm Psychol. 2016;125:528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gold JM, Strauss GP, Waltz JA, Robinson BM, Brown JK, Frank MJ. Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biol Psychiatry. 2013;74:130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hartmann MN, Hager OM, Reimann AV et al. . Apathy but not diminished expression in schizophrenia is associated with discounting of monetary rewards by physical effort. Schizophr Bull. 2015;41:503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Horan WP, Reddy LF, Barch DM et al. . Effort-based decision-making paradigms for clinical trials in schizophrenia: part 2—external validity and correlates. Schizophr Bull. 2015;41:1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Strauss GP, Whearty KM, Morra LF, Sullivan SK, Ossenfort KL, Frost KH. Avolition in schizophrenia is associated with reduced willingness to expend effort for reward on a progressive ratio task. Schizophr Res. 2016;170:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Docx L, de la Asuncion J, Sabbe B et al. . Effort discounting and its association with negative symptoms in schizophrenia. Cogn Neuropsychiatry. 2015;20:172–185. [DOI] [PubMed] [Google Scholar]
- 16. Gold JM, Kool W, Botvinick MM, Hubzin L, August S, Waltz JA. Cognitive effort avoidance and detection in people with schizophrenia. Cogn Affect Behav Neurosci. 2015;15:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Treadway MT, Peterman JS, Zald DH, Park S. Impaired effort allocation in patients with schizophrenia. Schizophr Res. 2015;161:382–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McCarthy JM, Treadway MT, Bennett ME, Blanchard JJ. Inefficient effort allocation and negative symptoms in individuals with schizophrenia. Schizophr Res. 2016;170:278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Quinlan T, Roesch S, Granholm E. The role of dysfunctional attitudes in models of negative symptoms and functioning in schizophrenia. Schizophr Res. 2014;157:182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kirkpatrick B, Fenton WS, Carpenter WT Jr, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32:214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Couture SM, Blanchard JJ, Bennett ME. Negative expectancy appraisals and defeatist performance beliefs and negative symptoms of schizophrenia. Psychiatry Res. 2011;189:43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grant PM, Beck AT. Defeatist beliefs as a mediator of cognitive impairment, negative symptoms, and functioning in schizophrenia. Schizophr Bull. 2009;35:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Horan WP, Rassovsky Y, Kern RS, Lee J, Wynn JK, Green MF. Further support for the role of dysfunctional attitudes in models of real-world functioning in schizophrenia. J Psychiatr Res. 2010;44:499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rector NA. Dysfunctional attitudes and symptom expression in schizophrenia: Differential associations with paranoid delusions and negative symptoms. J Cogn Psychother. 2004;18:163–173. [Google Scholar]
- 25. Luther L, Salyers MP, Firmin RL, Marggraf MP, Davis B, Minor KS. Additional support for the cognitive model of schizophrenia: evidence of elevated defeatist beliefs in schizotypy. Compr Psychiatry. 2016;68:40–47. [DOI] [PubMed] [Google Scholar]
- 26. Rector NA, Beck AT, Stolar N. The negative symptoms of schizophrenia: a cognitive perspective. Can J Psychiatry. 2005;50:247–257. [DOI] [PubMed] [Google Scholar]
- 27. Granholm E, Holden J, Link PC, McQuaid JR. Randomized clinical trial of cognitive behavioral social skills training for schizophrenia: improvement in functioning and experiential negative symptoms. J Consult Clin Psychol. 2014;82:1173–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Granholm E, Holden J, Link PC, McQuaid JR, Jeste DV. Randomized controlled trial of cognitive behavioral social skills training for older consumers with schizophrenia: defeatist performance attitudes and functional outcome. Am J Geriatr Psychiatry. 2013;21:251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pillny M, Lincoln TM. Predictors of improved functioning in patients with psychosis: The role of amotivation and defeatist performance beliefs. Psychiatry Res. 2016;244:117–122. [DOI] [PubMed] [Google Scholar]
- 30. Reddy LF, Horan WP, Barch DM et al. . Effort-based decision-making paradigms for clinical trials in schizophrenia: Part 1—Psychometric characteristics of 5 paradigms. Schizophr Bull. 2015;41:1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. First MB, Spitzer R, Robert G, Gibbon M, Gibbon W, Janet BW.. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York: Biometics Research Department, New York State Psychiatric Institute; 2002. [Google Scholar]
- 32. Kool W, McGuire JT, Rosen ZB, Botvinick MM. Decision making and the avoidance of cognitive demand. J Exp Psychol Gen. 2010;139:665–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McGuire JT, Botvinick MM. Prefrontal cortex, cognitive control, and the registration of decision costs. Proc Natl Acad Sci U S A. 2010;107:7922–7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kay SR, Opler LA, Lindenmayer JP. The Positive and Negative Syndrome Scale (PANSS): Rationale and standardization. Br J Psychiatry 1989;155(Suppl. 7):59–65. [PubMed] [Google Scholar]
- 35. Horan WP, Kring AM, Gur RE, Reise SP, Blanchard JJ. Development and psychometric validation of the Clinical Assessment Interview for Negative Symptoms (CAINS). Schizophr Res. 2011;132:140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. J Clin Psychiatry. 1997;58:538–546. [DOI] [PubMed] [Google Scholar]
- 37. Nuechterlein KH, Green MF.. MATRICS Consensus Cogntive Battery. Los Angeles, CA: MATRICS Assessment, Inc; 2006. [Google Scholar]
- 38. Weissman AR, Beck AT.. Development and validation of the Dysfunctional Attitudes Scale: a preliminary investigation. In: Presented at the 62nd Annual Meeting of the American Educational Research Association, March 27–31, 1978. Toronto, Ontario, Canada. [Google Scholar]
- 39. Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36:717–731. [DOI] [PubMed] [Google Scholar]
- 40. Aiken LS, West SG.. Multiple Regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- 41. Kiwanuka JN, Strauss GP, McMahon RP, Gold JM. Psychological predictors of functional outcome in people with schizophrenia. Schizophr Res. 2014;157:299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Staring AB, Ter Huurne MA, van der Gaag M. Cognitive Behavioral Therapy for negative symptoms (CBT-n) in psychotic disorders: a pilot study. J Behav Ther Exp Psychiatry. 2013;44:300–306. [DOI] [PubMed] [Google Scholar]
- 43. Beck AT, Grant PM, Huh GA, Perivoliotis D, Chang NA. Dysfunctional attitudes and expectancies in deficit syndrome schizophrenia. Schizophr Bull. 2013;39:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Campellone TR, Sanchez AH, Kring AM. defeatist performance beliefs, negative symptoms, and functional outcome in schizophrenia: a meta-analytic review. Schizophr Bull. 2016;42:1343–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Marder SR, Galderisi S. The current conceptualization of negative symptoms in schizophrenia. World Psychiatry. 2017;16:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Granholm E, Ruiz I, Gallegos-Rodriguez Y, Holden J, Link PC. Pupillary responses as a biomarker of diminished effort associated with defeatist attitudes and negative symptoms in schizophrenia. Biol Psychiatry. 2016;80:581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Grant PM, Huh GA, Perivoliotis D, Stolar NM, Beck AT. Randomized trial to evaluate the efficacy of cognitive therapy for low-functioning patients with schizophrenia. Arch Gen Psychiatry. 2012;69:121–127. [DOI] [PubMed] [Google Scholar]
- 48. Morrison AP, Turkington D, Pyle M et al. . Cognitive therapy for people with schizophrenia spectrum disorders not taking antipsychotic drugs: a single-blind randomised controlled trial. Lancet. 2014;383:1395–1403. [DOI] [PubMed] [Google Scholar]
- 49. Velligan DI, Roberts D, Mintz J et al. . A randomized pilot study of MOtiVation and Enhancement (MOVE) Training for negative symptoms in schizophrenia. Schizophr Res. 2015;165:175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee J, Green MF. Social preference and glutamatergic dysfunction: underappreciated prerequisites for social dysfunction in schizophrenia. Trends Neurosci. 2016;39:587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.