Abstract
The present double-blind crossover study examines the effects of cerebellar transcranial direct current stimulation (tDCS) in controls and in an analogue population to psychosis: individuals reporting elevated symptoms of nonclinical psychosis (NCP). A total of 18 controls and 24 NCP individuals were randomized into conditions consisting of 25 minutes of anodal (active) or sham cerebellar tDCS. Following this, both groups completed a pursuit rotor task designed to measure procedural learning performance. Participants then returned 1-week later and received the corresponding condition (either active or sham) and repeated the pursuit rotor task. Results indicate that in the sham condition, control participants showed significantly greater rates of motor learning when compared with the NCP group. In the active condition, the NCP group exhibited significant improvements in the rate of motor learning and performed at a level that was comparable to controls; these data support the link between cerebellar dysfunction and motor learning. Taken together, tDCS may be a promising treatment mechanism for patient populations and a useful experimental approach in elucidating our understanding of psychosis.
Keywords: tDCS, transcranial direct current stimulation, procedural learning, motor learning, psychosis, schizophrenia, nonclinical psychosis, cerebellum
Introduction
The cerebellum is a brain region involved in both motor and cognitive function. The cerebellum has been implicated in procedural learning processes, which is the development of skill through routine practice. Accumulating evidence suggests that procedural learning deficits are present along the psychosis continuum.1–5 Influential theories such as cognitive dysmetria suggest that the cerebellum helps to coordinate and time movement with thought and deficits in cerebellar function may impact several cognitive domains; it is unclear what types of interventions may help to enhance cerebellar function.6 Transcranial direct current stimulation (tDCS) is a noninvasive brain stimulation technique that has shown promise in improving symptomatology and cognition in patients with schizophrenia.7–15 Much of the current work has focused on applying tDCS to cortical regions such as the dorsal lateral prefrontal cortex (DLPFC); however, no study to date has applied cerebellar tDCS to this group for improving procedural learning. The current study is a double-blind randomized investigation, and anodal (active) cerebellar tDCS is administered to an analogue population to psychosis, consisting of participants endorsing high levels of nonclinical psychosis (NCP; ie, non-help-seeking participants reporting infrequent symptoms along the lower end of the psychosis continuum).16 Findings may contribute to our understandings of tDCS as a possible treatment modality and psychosis spectrum disorders more broadly.
In contrast to declarative learning, which is the rote learning of factual information and is inclusive of episodic memory content, procedural learning occurs with routine practice and becomes more automatic the more the procedure is engaged (eg, riding a bike).17 Procedural learning involves both intact motor abilities and cognition, and deficits in these processes have been related to frontal-striatal dysfunction.18,19 The real world implications of impairments in procedural learning are vast in that they can contribute to difficulties in completing everyday routine tasks, and ultimately may impact social and occupational functioning. Cross-sectional studies suggest that procedural learning is slower in samples of NCP individuals and those at clinical high-risk for psychosis.1,4,5 Furthermore, procedural learning may be impacted by poor sleep habits and is related to physical abnormalities suggestive of impacted brain development prior to the onset of psychosis.1,4,5
There is evidence to suggest that there may be an etiological role of the cerebellum in the psychosis continuum. There is work highlighting links between cerebellar deficits and impairments in both symptoms and cognition in at risk groups1,20,21 as well as individuals experiencing psychosis.6,22,23 Many studies involving cerebellar damage and lesions have provided foundations for our understanding of the cerebellum and the pathogenesis of psychosis.24–27 For example, Bielawski and Bondurant28 conducted a case report about a 56-year-old man that had a bilateral cerebellar stroke. As a result, this man experienced persistent persecutory delusions, hallucinations, cognitive deficits, and low affect suggesting that the cerebellum may be linked with psychosis symptoms and there may be impairments in relevant cerebrocerebllar pathways. There is also evidence in the earlier stages of the psychosis continuum. For example, Jukuri and colleagues29 investigated functional resting state magnetic resonance imaging (MRI) patterns in individuals at familial high risk for psychosis and found that this group had increased activity in the anterior lobe of the right cerebellum compared to controls and associated this with increased vulnerability to psychosis. There have also been studies identifying cerebellar deficits in individuals at clinical high risk for developing psychosis such as deficits in procedural learning linked to cerebellar volume.1,21,30 Taken together, cerebellar deficits are exhibited in several stages along the psychosis spectrum and studying cerebellar function along the psychosis continuum may be an informative pathway to understanding the developments of psychotic disorders such as schizophrenia.
Although processes within the prefrontal cortex may drive much of cognition, the cerebellum has also been suggested to be involved in cognitive processes such as procedural learning.31 The cerebellum modulates a number of cognitive processes and motor functions such as coordination and the timing of movement and thought.6,22 Procedural learning relies heavily on cerebellar function as exemplified by studies investigating cerebellar lesions and damage.24,25 Furthermore, smaller volume of motor and cognitive areas of the cerebellum is related to poorer rate of learning on the pursuit rotor task in a clinical high-risk population.1 Given the literature linking the cerebellum with procedural learning, the cerebellum may be a useful target for stimulation.
To date, there has been increasing interest in treatments such as tDCS for enhancing cerebellar function.10,32–34 For example, in a study conducted by Demirtas-Tatlidede and colleagues,35 individuals diagnosed with schizophrenia underwent 8 sessions of intermittent theta burst stimulation (TBS) to the cerebellar vermis (involved in emotion and affect), which is a relatively new refractory transcranial magnetic stimulation (rTMS) technique that modulates neuronal activity for a shorter period of time but longer effects may be observed compared to traditional TMS approaches. Findings from this study suggest improvements in negative and depressive symptoms a well as working memory.35 It is perhaps surprising, however, there have been no studies along the psychosis continuum (ie, in schizophrenia, other spectrum disorders, or continuum populations) that have examined the impacts of cerebellar tDCS on procedural learning. This technique has several advantages in that it is easy to administer, affordable, has minimal side effects, and overall is noninvasive. This procedure works by releasing a weak electrical current to the brain surface, which facilitates cortical excitability.36 There have been some studies investigating the impact of tDCS on both behavioral symptoms and cognitive function among schizophrenia populations.7–15 However, while these studies provide a useful foundation, this work is still limited in that relatively small sample sizes are used, and some of these studies do not have sham conditions to rule out placebo effects and they lack control groups.
There are promising results for tDCS studies that indicate reductions in positive symptoms such as auditory hallucinations.8,12,37 For example, in a highly influential study, Brunelin and colleagues38 were the first group to investigate tDCS and auditory hallucinations among a sample of schizophrenia patients and with a control group and found that cathodal stimulation over the left temporo-parietal cortex and anodal stimulation over the left dorsolateral prefrontal cortex resulted in reductions in auditory verbal hallucinations and negative symptoms. Similarly, there is research investigating tDCS and cognition, and findings point to improvements in functions such as working memory and social cognition in psychosis.10,13–15,39 For example, Rassovsky and colleagues13 employed stimulation over the DLPFC and observed improvements on facial emotion identification following anodal stimulation.
In the current study, we implemented a double-blind randomized investigation to examine whether cerebellar tDCS can improve the rate of procedural learning performance in NCP. Studying this group is important because this population can serve as a safe analogue for informing psychosis broadly; this group tends to share similar vulnerabilities with other groups on the psychosis continuum, including deficits in procedural learning.4,5,40,41 Furthermore, this population tends to be free of confounds such as antipsychotic medications and substance abuse that can convolute research designs and findings.42,43 Control and NCP individuals were randomized into anodal (active) cerebellar tDCS or sham conditions and following 25 minutes of active/sham stimulation, both groups completed a pursuit rotor task. Given the literature suggesting impairments in procedural learning in schizophrenia,2,3,44 and NCP populations,5 we predicted that (1) following the sham condition, the NCP group would exhibit a slower rate of learning compared to controls and (2) following the active stimulation, the performance of the NCP group would normalize similar to the control group performance.
Materials and Method
Participants
A total of 42 young adults (18 controls and 24 NCP), aged 18–22 (mean = 19.05, SD = 0.99) were recruited at Northwestern University’s Adolescent Development and Preventive Treatment (ADAPT) research program. All participants were in the Northwestern University Psychology recruitment pool. The research pool (n = 841) was administered the Community Assessment of Psychic Experiences45 (CAPE) positive symptom dimension, a questionnaire focusing on symptoms of NCP. The option to participate in the study was made available to those scoring in the top and bottom 15th percentile on the CAPE (control mean = 3.89, SD = 3.77; NCP mean = 21.50, SD = 8.12). A within subjects design was used for the tDCS portion of the study. There were 2 randomized sessions: one with active cerebellar tDCS and one with sham. The University Institutional Review Board approved the protocol and informed consent. All individuals received informed consent and were consented into the study prior to participation. See table 1 for demographic characteristics of the sample. See supplementary material for additional details.
Table 1.
Demographics
| Controls | NCP | Total | Statistic | P | |
|---|---|---|---|---|---|
| Age | |||||
| Mean (SD) | 18.78 (0.55) | 19.25 (1.19) | 19.05 (0.99) | t(40) = −1.72 | .10 |
| Gender | |||||
| Male | 8 | 10 | 18 | χ2(1) = .032 | .86 |
| Female | 10 | 14 | 24 | ||
| Total | 18 | 24 | 42 | ||
| Parent education (y) | |||||
| Mean (SD) | 16.81 (1.67) | 15.58 (2.31) | 16.11 (2.13) | t(40) = 1.90 | .07 |
| CAPE total | |||||
| Mean (SD) | 3.89 (3.77) | 21.50 (8.12) | 13.95 (10.99) | t(40) = −9.36 | ≤.001 |
| Beck Depression Inventory | |||||
| Mean (SD) | 4.53 (3.07) | 5.30 (4.55) | 5.00 (4.00) | t(36) = −0.58 | .57 |
| Beck Anxiety Inventory | |||||
| Mean (SD) | 7.94 (7.38) | 8.08 (6.71) | 8.02 (6.92) | t(40) = −0.06 | .95 |
Note: NCP, nonclinical psychosis; CAPE, Community Assessment of Psychic Experiences. NCP symptom scores reflect the total sum from the positive symptom dimension of the CAPE. 45 Parent education is the average of mother and father education. Beck Depression Inventory and Beck Anxiety Inventory reflect sum scores of all items.
Clinical Symptoms
As noted, the CAPE45 inventory was used to measure the frequency of NCP symptoms on a 4-item likert scale including “Never,” “Sometimes,” “Often,” and “Nearly Always.” The positive symptom section of the CAPE contains 20 items and is one of the most widely used, reliable, and well-validated instruments for examining NCP.46,47 Trained raters (graduate students and professional research assistants) administered the SCID48 B module after reaching reliability (κ > .80). The Beck Depression Inventory-II (BDI-II)50 and the Beck Anxiety Inventory,51 both 21-item self-report questionnaires, were used to assess depressive or anxious symptoms, respectively. See supplementary material for more information.
Cerebellar Transcranial Direct Current Stimulation
Stimulation was delivered using a battery-driven NeuroConn DC-stimulator Plus (NeuroConn GmbH) with 5 × 7 cm conductive-rubber electrodes placed on the scalp, over the cerebellum (1–2 cm below the inion and on the midline of the scalp) and the cathode was placed over the right arm deltoid muscle. This is a reliable montage used in past research.33,36 Stimulation was administered for 25 minutes (ramp-up/ramp-down: 5 s) with a current intensity of 2 mA (maximum current density: 0.057 mA/cm2, total charge of 0.0512 C/cm2). In the sham condition, all settings were identical to the active conditions except the stimulation duration (ramp-up: 5 s; stimulation: 30 s; ramp-down: 5 s).52 See supplementary material for more information.
Stimulation Design
Participants were randomly assigned to receive active cerebellar tDCS and sham in a counterbalanced order on separate laboratory visits, 1-week apart. We used Neuroconn’s built-in study-mode software, which allows for double-blind stimulation by entering a 5-digit code linked to either active or sham stimulation. Study-mode software comes with a list of 5-digit codes for active or sham stimulation. The 5-digit codes for active and sham stimulation were assigned a code of 0 and 1 respectively. A random list of 0s and 1s was created using the randomization tools at random.org. A blinded code list was used during experiment sessions and a separate investigator who did not interact with participants controlled the blinded code list. All participants received both active and sham stimulation.
Procedural Learning
After 25 minutes of cerebellar tDCS (active or sham), participants completed a pursuit rotor task in order to examine motor and cognitive function. We used a computerized version of the pursuit rotor task53,54 taken from the PEBL task battery freely available online (http://pebl.sourceforge.net/battery.html), where participants were instructed to follow a moving target around a circular track with a mouse held in their right hand. Subjects were given 4 blocks with 3 trials each, and blocks were separated by 15 minutes. Procedural learning was computed by calculating the mean of the 3 trials during each block, to yield a single index of performance for each block. The study focused on percentage of time on target at each block, and changes across the 4-block period (45 min), as an index of procedural learning. Rate of learning, also described as the change in learning across the 4 blocks, was calculated by subtracting the average percent time on target from the 3 trials in block 1 from the percent time on target from the 3 trials in block 4. See supplementary material for more information.
Statistical Approach
SPSS Statistics 23 was used to conduct behavioral analyses. Demographic characteristics were evaluated using independent t tests and Chi-square tests. We first investigated the effects of group (control vs NCP) and brain stimulation (active vs sham) on pursuit rotor performance across the 4 blocks in a 2 × 2 × 4 mixed model analysis of variance (ANOVA). Post-hoc tests were employed to further understand interactions within each group and condition. In addition, analyses were conducted to examine the effects of group (control vs NCP) on rate of learning in each brain stimulation condition (rate of learning in sham vs rate of learning in active) in a 2 × 2 mixed model ANOVA and post hoc tests were conducted to compare performance across conditions in each individual group.
Results
Demographics
There were no significant differences in demographic characteristics between the controls and NCP groups including age, t(40) = 1.72 P = .10, gender, χ2(1) = .032, P = .86 or parental education, t(40) = 1.90, P = .07. There were also no significant group differences in reported depressive symptoms, t(36) = −0.58, P = .57 and anxious symptoms t(40) = −0.06, P = .95. As expected, the NCP group reported significantly more NCP symptoms, t(40) = −9.36, P ≤ .001, d = 2.78 when compared with controls (see table 1 for means and SDs). A total of 6 participants reported occasional drug use (4 individuals reported cannabis use and 2 individuals did not indicate which drug type). There were no discontinuations of tDCS. During the debriefing session, a total of 50% of participants reported experiencing tingling and 19% reported feeling an itching sensation during stimulation. In our examination of blinding, blinding was effective for both sham and active conditions and following each visit, both groups guessed the correct condition at rates below chance.
Transcranial Direct Current Stimulation and Procedural Learning Performance
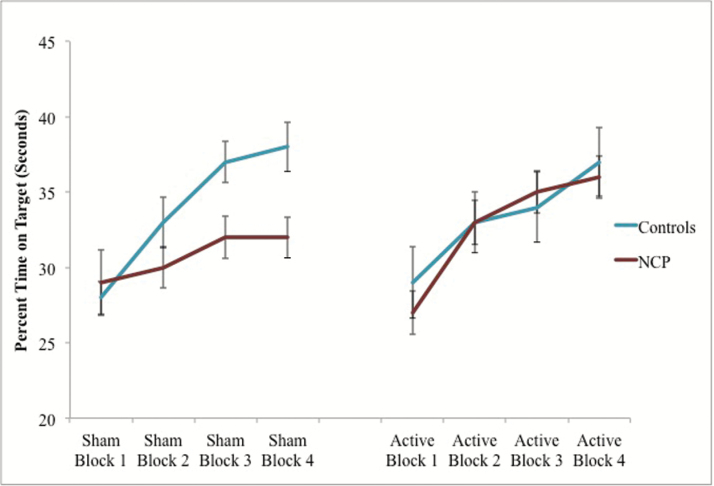
There was a significant 3-way interaction, F (3, 234) = 4.31, P = .006, partial ƞ2 = .05, indicating there was a significant interaction between condition (sham vs active), group (control vs NCP), and pursuit rotor performance (blocks 1–4). To unpack this interaction, we examined the relationship between tDCS condition on pursuit rotor performance separately for controls and NCP individuals. In the control group, there was no block by stimulation interaction, F (3,99) = 1.48, P = .225, ƞ2 = .04. This suggests that the control group performed similarly regardless of whether they received active or sham stimulation. For the NCP group, there was a significant interaction, F (3,135) = 3.91, P = .010, partial ƞ2 = .08 indicating that the active stimulation did impact performance on the pursuit rotor task. Within the NCP group, descriptively speaking, the rate of learning was higher in the active stimulation condition than in the sham condition (figure 1).
Fig. 1.
Sham compared to active condition and procedural learning in control and nonclinical psychosis (NCP) groups. Note: Scores plotted represent the average percent time on target in seconds for each pursuit rotor block. Error bars represent SE.
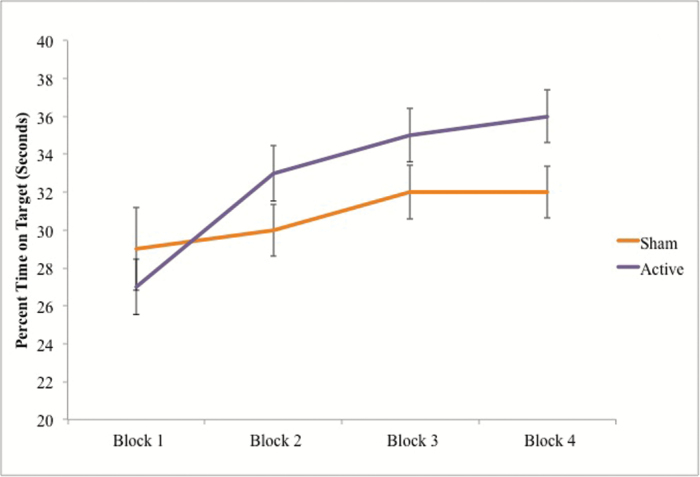
To further understand differences between the groups and tDCS conditions, the interaction between block and group was investigated within the sham and active stimulation conditions separately. In the sham condition, there was a significant interaction between time and group, F (3, 114) = 3.43, P = .019, ƞ2 = .08. These data indicate the control group performed better on the pursuit rotor task than the NCP group after the sham tDCS condition. After receiving active stimulation, the NCP group performed closer to the level of the control group on the pursuit rotor task as evidenced by the lack of a significant interaction between time and group within the stimulation condition, F (3,120) = 1.51, P = .215, ƞ2 = .04 (figure 2).
Fig. 2.
Sham compared to active condition and procedural learning in the nonclinical psychosis (NCP) group. Note: Scores plotted represent the average percent time on target in seconds for each pursuit rotor block within the NCP group. Error bars represent SE.
In further analyses, we were interested in looking at the interactions between group and rate of learning, an index of procedural learning in each condition. We observed a significant interaction between group (control vs NCP), and condition (rate of learning in sham vs rate of learning in active), F (1,39) = 4.53, P = .04, ƞ2 = .10. To unpack this interaction, we examined differences in the rate of learning between sham and active conditions within each group. We found that there were no significant differences between conditions in rate of learning within the control group, t(17) = 0.92, P = .37. However, in the NCP group, there were significant differences between the sham and active condition in rate of learning in that there was significantly greater rate of learning in the active condition compared to the sham condition, t(22) = −2.11, P = .046, driving the interaction.
Discussion
The present study is the first to evaluate the efficacy of cerebellar tDCS in a psychosis spectrum population. Consistent with our predictions, the NCP group exhibited a slower rate of procedural learning when compared with controls. This is relevant as motor learning is a foundational cognitive function underlying a host of integral activities, and related deficits may contribute to significant disability in patients with psychosis. Given this issue, it is highly noteworthy that present findings indicate that procedural learning deficits may be remediated by cerebellar tDCS. Taken together, findings suggest that this method may be an important tool for further improving our understanding of psychosis as well as for designing targeted treatments. However, replication in patient groups and experiments including multiple sessions of active cerebellar tDCS will be necessary before we make definitive conclusions about the efficacy of cerebellar stimulation for procedural learning in psychosis.
In the sham condition (ie, akin to placebo, where functionally meaningful active stimulation is not applied to the cerebellum), NCP individuals exhibited deficits in time on target across each of the 4 blocks, as well as a slower rate procedural learning. These findings are consistent with the broader literature suggesting that motor learning deficits are present in psychosis risk groups1,4 and that cerebellar deficits may be present even in the lowest levels of the psychosis vulnerability spectrum.5,49
Cerebellar tDCS, as reflected in the current findings, may have important clinical implications given the improvements observed in procedural learning performance after receiving active stimulation. Specifically, we found that in the condition in which there was no prolonged stimulation, the NCP group performed significantly worse on the pursuit rotor task compared to controls. However, after active stimulation, participants performed at a similar level compared to controls. These findings suggest that tDCS may have benefits for psychosis populations. These data are positive findings and lay the groundwork for future work in this domain. While the results of this study are encouraging, it is important to consider that more broadly, tDCS as a treatment modality still remains controversial. Much of the work to date has stemmed from case reports and a few randomized control trials. Overall, tDCS seems to be improving cognition as identified by some groups10,13,14,39; however, not all studies have shown these results. For example, Vercammen and colleagues47 in a sample of 21 patients with schizophrenia, implemented both anodal stimulation and sham conditions in a randomized order. This group found improvements in probabilistic association learning after tDCS only in a subgroup of schizophrenia patients. Further, another study utilized daily stimulation and placed the anode over the prefrontal cortex and the cathode over the temporoparietal junction in 24 patients with schizophrenia and compared this bimodal approach to sham stimulation.32 In this study, there were no improvements in hallucinations or negative symptoms. These data are critical because they show that while tDCS is innovative and offers an important perspective, there still is work that is needed in order to understand aspects of this intervention such as electrode montages (ie, where to place the electrodes), sham conditions, number of sessions (ie, daily, weekly) and dosage.
Findings that anodal cerebellar tDCS did not impact the control group are important to consider. This result is inconsistent with findings from a study conducted by Ferrucci and colleagues,33 which observed improvements in a serial reaction time task (SRTT) in 21 healthy volunteers following cerebellar tDCS. The differences between studies may be attributed to procedural learning task. Specifically, it is possible that the pursuit rotor task (used presently) may have a lower performance ceiling (ie, in this case, the controls became so proficient with keeping the task on target, that there was no further room for improvement). In contrast, the SRTT may be more challenging for controls, allowing for greater room to change. Both in the sham and active stimulation conditions, the control group performed about the same, and it could be that there was not enough room to improve (ie, performance at 35%–40% of time on target may be the ceiling of what normative samples can reach at this speed, with this amount of practice); this is supported by performance of controls in distinct samples,4,5 while the NCP group performed significantly worse in the sham condition and had more room to change following active stimulation. Furthermore, it is also possible that the control group may have not improved on the pursuit rotor task because, along with experiencing ceiling effects, did not respond to cerebellar tDCS because there is no cerebellar dysfunction present. Future work is needed to compare clinical populations with control groups in order to better understand these relationships. Additionally, future studies should incorporate other procedural learning paradigms.
Abnormalities in the cerebello-thalamo-cortical circuit (CTCC) have been implicated in psychosis.6,22,55 Disruptions in this circuitry may produce cognitive dysmetria, which is the disconnect between the timing of movement and thought and may lead to “poor mental coordination.”55 Deficits in this circuitry may be observed even prior to the onset of psychosis. For example, Bernard and colleagues56 found that abnormalities in this network may be predictive of positive symptoms in individuals at ultra-high risk for developing psychosis.54 Dysfunctions in this network could contribute to a broader array of types of symptoms including cognitive deficits and even the etiology of psychosis.55 Enhancing cerebellar function by using tDCS may also be improving the dynamic communication between the CTCC. Remediating cerebellar function may contribute to feedback and feed-forward processes.57,58 Feedback control processes compare sensory errors with predicted movement in order to adjust the movement. Feed-forward control processes updates internal models so that the timing of the movement is appropriate and efficient. Similarly, the cerebellum consists of inhibitory purkinje cells and excitatory granule cells that may be relevant and implicated when implementing cerebellar tDCS.6,55 Taken together, while there are important theories that may be relevant to the mechanism of change through cerebellar tDCS, studies could benefit from continuing to tease apart the neurobehavioral impacts this technique may have.
There are several strengths and limitations to the current study. First, the NCP population is a readily accessible and convenient sample to examine proof of concept. Second, we recruited a total of 24 NCP individuals to the study, which is consistent with other tDCS studies in schizophrenia populations.8,10,30,39 Third, our study design offers the ability to limit potential biases in our data collection (eg, double-blind and randomized). Fourth, we used a sham condition, which is a critical aspect of the current study design given that many studies in the tDCS and schizophrenia literature have not used control conditions, which without, adds the complication of results being driven by possible placebo effects.37 However, the findings of this study could be strengthened with the use of a larger sample of participants at different stages of illness with varying types of symptom expression and other cognitive measures to understand clinical and cognitive characteristics of samples that receive tDCS. Medication use and genetic risk is also important to investigate in terms of tDCS and procedural learning performance. Also, in the present study, our sample sizes were unequal due to participants not attending sessions and future work should try to recruit more equal samples. We did not assess whether participants were naïve to tDCS and it will be important to implement questions to assess for this component in the future.
Future studies should incorporate additional tasks to assess procedural learning and investigate the impacts of various numbers of pursuit rotor trials as some work have implemented shorter intervals59 and the amount of time in between for rest. Additionally, in the future, it will be important to investigate the impacts of multiple sessions of tDCS, duration of lasting effects, placements (in the present study, we placed the cathode on the right arm, and participants completed the pursuit rotor task with their right hand after, which may be a confounding variable), sizes of electrodes, parameters, and the amount of stimulation as the current literature is still understanding what is considered gold standard and most effective. Finally, there may be utility in applying tDCS to other patient groups (ie, attenuated positive symptom or genetic risk inclusion) and using other cerebellar tasks. In conclusion, the present study introduced a novel treatment and experimental approach by using tDCS to improve procedural learning. Not only does the current study provide implications for future clinical intervention, but it also highlights that studying NCP individuals can be useful in understanding prevention of and intervention for psychotic disorders.
Supplementary Material
Supplementary material is available at Schizophrenia Bulletin online.
Funding
This work was supported by National Institutes of Health grants R01 MH094650, R21/R33 MH103231, R21 MH110374 to V.A.M., and T32 NS047987, and in part by Brain and Behavior Research Foundation (NARSAD) Independent Investigator Award to V.A.M.
Acknowledgments
V.A.M. is a consultant to Takeda Pharmaceuticals. No other authors have any disclosures.
References
- 1. Dean DJ, Bernard JA, Orr JM, et al. . Cerebellar morphology and procedural learning impairment in neuroleptic-naive youth at ultrahigh risk of psychosis. Clin Psychol Sci. 2014;2:152–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eysenck H, Frith C.. Reminiscence, Motivation, and Personality. New York, NY: Plenum Press; 1977. [Google Scholar]
- 3. Huston PE, Shakow D. Learning capacity in schizophrenia; with special reference to the concept of deterioration. Am J Psychiatry. 1949;105:881–888. [DOI] [PubMed] [Google Scholar]
- 4. Lunsford-avery JR, Dean DJ, Mittal VA. Self-reported sleep disturbances associated with procedural learning impairment in adolescents at ultra-high risk for psychosis. Schizophr Res. 2017;190:160–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mittal VA, Dean DJ, Pelletier A. Dermatoglyphic asymmetries and fronto-striatal dysfunction in young adults reporting non-clinical psychosis. Acta Psychiatr Scand. 2012;126:290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biol Psychiatry. 2008;64:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bose A, Sowmya S, Shenoy S, et al. . Clinical utility of attentional salience in treatment of auditory verbal hallucinations in schizophrenia using transcranial direct current stimulation (tDCS). Schizophr Res. 2015; 164:1–5. [DOI] [PubMed] [Google Scholar]
- 8. Brunelin J, Bernard C. Transcranial direct current stimulation for the treatment of refractory symptoms of schizophrenia. Curr Pharm Des. 2015;21:3373–3383. [DOI] [PubMed] [Google Scholar]
- 9. Hasan A, Strube W, Palm U, Wobrock T. Repetitive noninvasive brain stimulation to modulate cognitive functions in schizophrenia: a systematic review of primary and secondary outcomes. Schizophr Bull. 2016;42(suppl 1):S95–S109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoy KE, Arnold SL, Emonson MR, Daskalakis ZJ, Fitzgerald PB. An investigation into the effects of tDCS dose on cognitive performance over time in patients with schizophrenia. Schizophr Res. 2014;155:96–100. [DOI] [PubMed] [Google Scholar]
- 11. Mondino M, Jardri R, Suaud-Chagny MF, Saoud M, Poulet E, Brunelin J. Effects of fronto-temporal transcranial direct current stimulation on auditory verbal hallucinations and resting-state functional connectivity of the left temporo-parietal junction in patients with schizophrenia. Schizophr Bull. 2016;42:318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Palm U, Keeser D, Hasan A, et al. . Prefrontal transcranial direct current stimulation for treatment of schizophrenia with predominant negative symptoms: a double-blind, sham-controlled proof-of-concept study. Schizophr Bull. 2016;42:1253–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rassovsky Y, Dunn W, Wynn J, et al. . The effect of transcranial direct current stimulation on social cognition in schizophrenia: a preliminary study. Schizophr Res. 2015;165:171–174. [DOI] [PubMed] [Google Scholar]
- 14. Smith RC, Boules S, Mattiuz S, et al. . Effects of transcranial direct current stimulation (tDCS) on cognition, symptoms, and smoking in schizophrenia: a randomized controlled study. Schizophr Res. 2015;168:260–266. [DOI] [PubMed] [Google Scholar]
- 15. Vercammen A, Rushby JA, Loo C, Short B, Weickert CS, Weickert TW. Transcranial direct current stimulation influences probabilistic association learning in schizophrenia. Schizophr Res. 2011;131:198–205. [DOI] [PubMed] [Google Scholar]
- 16. Kelleher I,Cannon M. Psychotic like experiences in the general population: characterizing a high risk group for psychosis. Psychol Med. 2011;41:1–6. [DOI] [PubMed] [Google Scholar]
- 17. Censor N, Sagi D, Cohen LG. Common mechanisms of human perceptual and motor learning. Nat Rev Neurosci. 2012;13:658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Granholm E, Bartzokis G, Asarnow RF, Marder SR. Preliminary associations between motor procedural learning, basal ganglia T2 relaxation times, and tardive dyskinesia in schizophrenia. Psychiatry Res. 1993;50:33–44. [DOI] [PubMed] [Google Scholar]
- 19. Sarazin M, Deweer B, Merkl A, Von Poser N, Pillon B, Dubois B. Procedural learning and striatofrontal dysfunction in Parkinson’s disease. Mov Disord. 2002;17:265–273. [DOI] [PubMed] [Google Scholar]
- 20. Bernard JA, Mittal VA. Cerebellar-motor dysfunction in schizophrenia and psychosis-risk: the importance of regional cerebellar analysis approaches. Front Psychiatry. 2014;5:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mittal VA, Dean DJ, Bernard JA, et al. . Neurological soft signs predict abnormal cerebellar-thalamic tract development and negative symptoms in adolescents at high risk for psychosis: a longitudinal perspective. Schizophr Bull. 2014;40:1204–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Picard H, Amado I, Mouchet-Mages S, Olié JP, Krebs MO. The role of the cerebellum in schizophrenia: an update of clinical, cognitive, and functional evidences. Schizophr Bull. 2008;34:155–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bernard JA, Mittal VA. Dysfunctional activation of the cerebellum in schizophrenia: a functional neuroimaging meta-analysis. Clin Psychol Sci. 2015;3:545–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Flament D, Ellermann JM, Kim SG, Ugurbil K, Ebner TJ. Functional magnetic resonance imaging of cerebellar activation during the learning of a visuomotor dissociation task. Hum Brain Mapp. 1996;4:210–226. [DOI] [PubMed] [Google Scholar]
- 25. Jenkins IH, Brooks DJ, Nixon PD, Frackowiak RS, Passingham RE. Motor sequence learning: a study with positron emission tomography. J Neurosci. 1994;14:3775–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Molinari M, Leggio MG, Solida A, et al. . Cerebellum and procedural learning: evidence from focal cerebellar lesions. Brain. 1997;120(Pt 10):1753–1762. [DOI] [PubMed] [Google Scholar]
- 27. Pascual-Leone A, Grafman J, Clark K, et al. . Procedural learning in Parkinson’s disease and cerebellar degeneration. Ann Neurol. 1993;34:594–602. [DOI] [PubMed] [Google Scholar]
- 28. Bielawski M, Bondurant H. Psychosis following a stroke to the cerebellum and midbrain: a case report. Cerebellum Ataxias. 2015;2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jukuri T, Kiviniemi V, Nikkinen J, et al. . Cerebellar activity in young people with familial risk for psychosis–The Oulu Brain and Mind Study. Schizophr Res. 2015;169:46–53. [DOI] [PubMed] [Google Scholar]
- 30. Bernard JA, Dean DJ, Kent JS, et al. . Cerebellar networks in individuals at ultra high-risk of psychosis: impact on postural sway and symptom severity. Hum Brain Mapp. 2014;35:4064–4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grimaldi G, Argyropoulos GP, Bastian A, et al. . Cerebellar Transcranial Direct Current Stimulation (ctDCS): a novel approach to understanding cerebellar function in health and disease. Neuroscientist. 2016;22:83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boehringer A, Macher K, Dukart J, Villringer A, Pleger B. Cerebellar transcranial direct current stimulation modulates verbal working memory. Brain Stimul. 2013;6:649–653. [DOI] [PubMed] [Google Scholar]
- 33. Ferrucci R, Brunoni AR, Parazzini M, et al. . Modulating human procedural learning by cerebellar transcranial direct current stimulation. Cerebellum. 2013;12:485–492. [DOI] [PubMed] [Google Scholar]
- 34. Zhou J, Hao Y, Wang Y, et al. . Transcranial direct current stimulation reduces the cost of performing a cognitive task on gait and postural control. Eur J Neurosci. 2014;39:1343–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Demirtas-Tatlidede A, Freitas C, Cromer JR, et al. . Safety and proof of principle study of cerebellar vermal theta burst stimulation in refractory schizophrenia. Schizophr Res. 2010;124:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ferrucci R, Cortese F, Priori A. Cerebellar tDCS: how to do it. Cerebellum. 2015;14:27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Koops S, van den Brink H, Sommer IE. Transcranial direct current stimulation as a treatment for auditory hallucinations. Front Psychol. 2015;6:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brunelin J, Mondino M, Gassab L, et al. . Examining transcranial direct-current stimulation (tDCS) as a treatment for hallucinations in schizophrenia. Am J Psychiatry. 2012;169:719–724. [DOI] [PubMed] [Google Scholar]
- 39. Göder R, Baier PC, Beith B, et al. . Effects of transcranial direct current stimulation during sleep on memory performance in patients with schizophrenia. Schizophr Res. 2013;144:153–154. [DOI] [PubMed] [Google Scholar]
- 40. Pelletier AL, Dean DJ, Lunsford-Avery JR, et al. . Emotion recognition and social/role dysfunction in non-clinical psychosis. Schizophr Res. 2013;143:70–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zammit S, Kounali D, Cannon M, et al. . Psychotic experiences and psychotic disorders at age 18 in relation to psychotic experiences at age 12 in a longitudinal population-based cohort study. Am J Psychiatry. 2013;170:742–750. [DOI] [PubMed] [Google Scholar]
- 42. Verdoux H, van Os J. Psychotic symptoms in non-clinical populations and the continuum of psychosis. Schizophr Res. 2002;54:59–65. [DOI] [PubMed] [Google Scholar]
- 43. Kelleher I, Harley M, Murtagh A, Cannon M. Are screening instruments valid for psychotic-like experiences? A validation study of screening questions for psychotic-like experiences using in-depth clinical interview. Schizophr Bull. 2011;37:362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schérer H, Stip E, Paquet F, Bédard MA. Mild procedural learning disturbances in neuroleptic-naive patients with schizophrenia. J Neuropsychiatry Clin Neurosci. 2003;15:58–63. [DOI] [PubMed] [Google Scholar]
- 45. Stefanis NC, Hanssen M, Smirnis NK, et al. . Evidence that three dimensions of psychosis have a distribution in the general population. Psychol Med. 2002;32:347–358. [DOI] [PubMed] [Google Scholar]
- 46. Barkus E, Stirling J, Hopkins R, McKie S, Lewis S. Cognitive and neural processes in non-clinical auditory hallucinations. Br J Psychiatry. 2007; 191: 76–81. [DOI] [PubMed] [Google Scholar]
- 47. Vellante M, Larøi F, Cella M, Raballo A, Petretto DR, Preti A. Hallucination-like experiences in the nonclinical population. J Nerv Ment Dis. 2012;200:310–315. [DOI] [PubMed] [Google Scholar]
- 48. First MB, Spitzer RL, Gibbon M, Williams JB.. Structured Clinical Interview for the DSM-IV Axis I Disorders (SCID-I) Patient Edition, January 1995 Final: SCID-I/P Version 2.0). New York: NY: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 49. Mittal VA, Smolen A, Dean DJ, Pelletier AL, Lunsford-Avery J, Smith A. BDNF Val66Met and spontaneous dyskinesias in non-clinical psychosis. Schizophr Res. 2012;140:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–597. [DOI] [PubMed] [Google Scholar]
- 51. Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. [DOI] [PubMed] [Google Scholar]
- 52. Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117:845–850. [DOI] [PubMed] [Google Scholar]
- 53. Mueller ST, Piper BJ. The psychology experiment building language (PEBL) and PEBL test battery. J Neurosci Methods. 2014;222:250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mueller ST. The Psychology Experiment Building Language, Version 0.13 2012. http://pebl.sourceforge.net. Accessed May 01, 2016.
- 55. Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry?Schizophr Bull. 1998;24:203–218. [DOI] [PubMed] [Google Scholar]
- 56. Bernard JA, Orr JM, Mittal VA. Cerebllo-thalamo-cortical networks predict positive symptom progression in individuals at ultra-high risk for psychosis. Neuroimage Clin. 2017;14:622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Marr D. A theory of cerebellar cortex. J Physiol. 1969;202:437–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Seidler RD, Noll DC, Thiers G. Feedforward and feedback processes in motor control. Neuroimage. 2004;22:1775–1783. [DOI] [PubMed] [Google Scholar]
- 59. Noguchi T, Demura S, Aoki H. Superiority of the dominant and nondominant hands in static strength and controlled force exertion. Percept Mot Skills. 2009;109:339–346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.