Abstract
We recently reported that resting hippocampal, basal ganglia and midbrain perfusion is elevated in people at ultra high risk (UHR) for psychosis. The present study sought to replicate our previous finding in an independent UHR cohort, and examined the relationship between resting perfusion in these regions, psychosis and depression symptoms, and traumatic experiences in childhood. Pseudo-Continuous Arterial Spin Labelling (p-CASL) imaging was used to measure resting cerebral blood flow (rCBF) in 77 UHR for psychosis individuals and 25 healthy volunteers in a case-control design. UHR participants were recruited from clinical early detection services at 3 sites in the South of England. Symptoms levels were assessed using the Comprehensive Assessment of At Risk Mental States (CAARMS), the Hamilton Depression Scale (HAM-D), and childhood trauma was assessed retrospectively using the Childhood Trauma Questionnaire (CTQ). Right hippocampal and basal ganglia rCBF were significantly increased in UHR subjects compared to controls, partially replicating our previous finding in an independent cohort. In UHR participants, positive symptoms were positively correlated with rCBF in the right pallidum. CTQ scores were positively correlated with rCBF values in the bilateral hippocampus and negatively associated with rCBF in the left prefrontal cortex. Elevated resting hippocampal and basal ganglia activity appears to be a consistent finding in individuals at high risk for psychosis, consistent with data from preclinical models of the disorder. The association with childhood trauma suggests that its influence on the risk of psychosis may be mediated through an effect on hippocampal function.
Keywords: schizophrenia, ultra high-risk, cerebral blood flow, childhood trauma
Introduction
Alterations in hippocampal anatomy and function are among the most robust biological findings in schizophrenia,1,2 and have also been reported in people at ultra high risk (UHR) of developing psychosis.3–7 These observations are consistent with preclinical models, which posit a key role for the hippocampus in the development of psychosis. Such models also suggest that resting hippocampal activity is increased prior to illness onset and linked to elevated activity in regions involved in dopamine signaling in the striatum and midbrain.8 Resting cerebral activity in these regions can be assessed in vivo by measuring resting cerebral blood flow (rCBF), which is closely correlated with the level of local neural function due to neuro-vascular coupling,9,10 and can be measured using a Magnetic Resonance Imaging technique called pseudo-Continuous Arterial Spin Labelling (p-CASL). In a previous study using this approach, we found that subjects at UHR for psychosis exhibited increased rCBF in the bilateral hippocampus/subiculum, basal ganglia and midbrain, relative to controls.11 These data, along with independent findings using a different method for measuring cerebral perfusion,5 provided the first evidence that the increased resting activity evident in preclinical models of psychosis12 was also evident in humans at high risk for psychosis.
However, initial findings in psychosis research have not always been replicated, and recently this has become a particular issue for neuroimaging studies because of concerns about image analysis methods.13,14 The present study sought to address this issue by aiming to replicate our previous finding of elevated hippocampal, basal ganglia and midbrain rCBF in UHR individuals. We repeated the study using the same neuroimaging methods in a second, and completely independent sample of UHR subjects and healthy controls. We tested the hypothesis that the UHR group would again show elevated hippocampal, basal ganglia and midbrain rCBF relative to the controls. We then tested if elevated rCBF in these regions was associated with psychotic symptoms. Because depressive symptoms are also prevalent in about 40% of UHR subjects,15 and major depressive disorder is associated with alterations in hippocampal volume and function,16,17 we also tested if elevated rCBF in the hippocampus was specific to psychosis, or was also associated with depressive symptoms scores.
We then sought to examine the relationship between rCBF in hippocampal, basal ganglia and midbrain regions and childhood trauma in UHR subjects. Childhood adversity is an important risk factor for psychosis,18–21 and for other psychiatric disorders.22 Exposure to environmental risk factors for psychosis may be especially influential during developmentally sensitive periods such as childhood.23 However, the mechanisms through which environmental factors such as trauma in childhood alter brain development and increase risk for psychosis in adulthood remains unclear. One approach that can be used to address this issue is to examine the relationship between neuroimaging findings in adults and a measure of the extent to which they experienced trauma in childhood. A recent Positron Emission Tomography (PET) study employing this approach found that adversity in childhood was linked to elevated striatal dopamine function in adulthood.24 However, while volumetric25,26 and functional neuroimaging studies27 in adults with a history of childhood trauma report alterations in hippocampal and other regions, no studies have examined the relationship between rCBF and childhood trauma in an UHR cohort. Experimental studies in rodents have shown that peri-pubertal stress28 can lead to alterations in striatal and cortical development and function.29,30 Based on these rodent studies and findings in human subjects, we predict that in UHR subjects, childhood trauma will be associated with increased rCBF in hippocampal, basal ganglia and midbrain regions.
METHODS
Participants and Assessment
The study had National Research Ethics Service (NRES) approval and all participants gave written informed consent to participate. One hundred two participants (25 healthy controls [CTRL] and 77 participants at UHR of psychosis) participated in the study. UHR subjects were recruited through clinical early detection services at 3 sites: OASIS (Outreach and Support in South London),31 part of the South London and Maudsley NHS Trust; the West London Early Intervention Service, part of the West London Mental Health NHS trust; and CAMEO, part of the Cambridge and Peterborough NHS trust. All of the neuroimaging data were acquired at the Centre for Neuroimaging Sciences, King’s College London. Diagnosis of the UHR state was made according to PACE criteria, using information acquired from the Comprehensive Assessment of At Risk Mental States (CAARMS32). Briefly, this required that participants had 1 or more of the following: (1) attenuated psychotic symptoms (APS); (2) brief limited intermittent psychotic symptoms (BLIP: a history of 1 or more episodes of frank psychotic symptoms that resolved spontaneously within 1 week in the past year); or (3) a recent decline in function, together with either the presence of schizotypal personality disorder or a family history of psychosis in a first degree relative. All UHR participants met criteria for APS, 5 additionally met criteria for a BLIP and 2 for a recent decline in function/family history. Social and occupational functioning was measured using the GAF.33
Eight of the UHR participants were being treated with low doses of antipsychotic medications (Quetiapine n = 4, Olanzapine n = 2, Risperidone n = 2) and 19 with antidepressant medications (Mirtazapine = 3, Citalopram = 2, Sertraline = 9, Fluoxetine = 3, Amitriptyline = 1, Venlafaxine = 1). Healthy controls were recruited from the local community. Control participants with a history of psychiatric disorders or who were receiving prescription medications were excluded. None of the control subjects had a history of neurological illness, or met DSM-IV criteria for drug or alcohol dependence. All participants (in both groups) had an estimated pre-morbid IQ in the normal range (ie, 80–110), as assessed using the National Adult Reading Scale (NART).34 Depression was assessed using the Hamilton Depression Scale (HAM-D).35 Hamilton Anxiety (HAM-A)35 scores were also obtained for use as a covariate in statistical models (see below). Subjects were asked to provide information on tobacco (number of cigarettes per day) and cannabis use (0 = no use, 1 = experimental use, 2= occasional use, 3 = moderate use, 4 = heavy use). Subjects who met DSM-IV criteria for a substance use disorder were excluded. Childhood trauma was assessed using the Childhood Trauma Questionnaire (CTQ).36 This widely used instrument provides a retrospective measure of physical, emotional and sexual abuse that occurred before the age of 17 years. CTQ data were available in 38 UHR participants but not in CTRL.
p-CASL Protocol and Image Preprocessing
Arterial spin labeling allows the quantification of rCBF measures in units of ml/100g of tissue/per second. To optimize the sensitivity to regional tissue perfusion and neural activity, p-CASL images were acquired after a long (1.5 s) post-labeling delay, to ensure that the data reflected perfusion at the level of capillary micro-circulation, which is most closely associated with neural function.9 p-CASL acquisition parameters and p-CASL image pre-processing procedures are detailed in the supplementary material and elsewhere.11
Statistical Analysis
Analyses of demographic and global rCBF data were performed in SPSS version 22 using appropriate parametric and nonparametric tests. Statistical analyses of regional rCBF data were performed using Statistical Parametric Mapping Version 8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8). We tested for significant group effects in rCBF quantities in CTRL and UHR using a region of interest (ROI) approach. ROIs were specified using coordinates from our previous rCBF study in a completely independent sample of UHR and CTRL subject (based on the contrast UHR > CTRLS11) (MNI coordinate system). ROIs were specified in the bilateral hippocampus/subiculum region (right ROI x, y, z = 20, −28, −8 and left ROI x, y, z = −22, −28, −8), the bilateral basal ganglia (right pallidum/putamen ROI x, y, z = 22, −12, −4, and the left pallidum/putamen ROI x, y, z = −18, −8, −4), and the left midbrain (ROI x, y, z = −10, −32, −18). Spheres (6mm) were then constructed to form a mask containing all ROIs. Statistical inferences were made at P < .05 with Family Wise Error (FEW) correction for multiple comparisons at the voxel-level after applying small volume correction (SVC). Regional (ROI) group effects were tested using independent t-tests in SPM-8 including nuisance covariates (see below). Mean global rCBF was extracted from each individual subject to assess global effects and an independent t-test was performed in SPSS. rCBF values ([ml/100g/min] × 10) were extracted from peak activations for use in the plots shown in figures 1 and 2 (for illustrative purposes and to check for outliers). As antipsychotic (AP) medication is known to affect rCBF,37 additional analyses were conducted after UHR subjects receiving AP medication (n = 8) had been excluded. To ensure group tests were conducted in the same way as our previous study11 the following covariates were included in statistical models: age, gender, global rCBF, anxiety (HAM-A scores) and cigarettes per day.
Fig. 1.
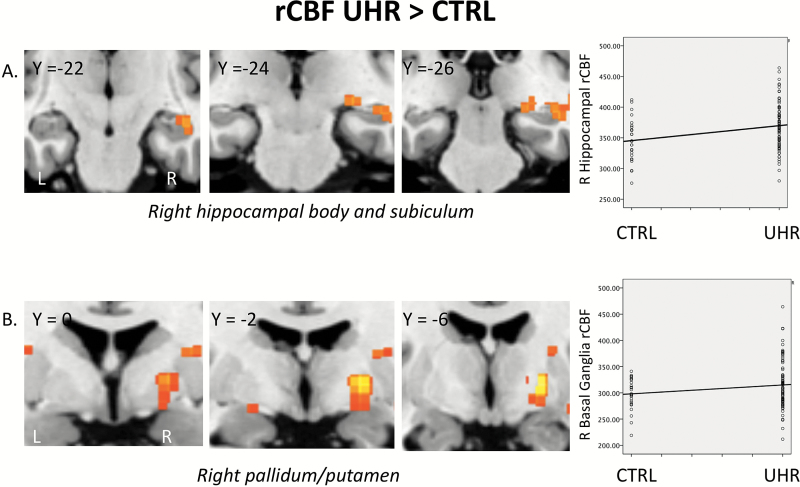
(A) Coronal sections through the medial temporal lobe showing elevated resting cerebral blood flow (rCBF) in UHR relative to CTRL subjects (PFWE = .021) and scatter plot showing rCBF levels in each case. (B) Coronal sections through basal ganglia regions showing elevated rCBF in UHR relative to CTRL subjects (PFWE = .03) and scatter plot showing rCBF levels in each case. rCBF levels are quantified in (ml/100g/sec) × 10.
To establish the effect of symptoms and childhood trauma scores on regional rCBF values, we used CAARMS positive symptom, HAM-D and CTQ scores (available in 38 UHR subjects) as regressors in separate statistical models. Cigarettes per day and cannabis use were included as covariates of no interest in the regression model as both have been reported to affect rCBF.38,39 Statistical inferences were made at P < .05 with FWE correction for multiple comparisons at the voxel-level after applying SVC. For completeness an exploratory whole brain analysis was also conducted to assess wider effects of symptoms and childhood trauma on rCBF. Significant results are reported at a FWE cluster level (P < .05) using a cluster detection threshold of P < .00114,40 to reduce likelihood of false positive results.
RESULTS
Demographic, Clinical and Medication Data
These data are summarized in table 1. CTRL and UHR participants did not differ significantly in terms of age, gender, handedness, premorbid IQ or cigarettes smoked per day. However UHR participants were less educated and used more cannabis, and as would be expected, UHR participants had higher levels of anxiety and depression. All of the UHR participants met the Attenuated Psychotic Symptoms criteria for inclusion in the study. A minority also met criteria for BLIPS (n = 5) or the schizoptypy/familial risk criterion (n = 2). The mean CTQ score for UHR participants was 56 meaning that as a group these UHR participants reported moderate to severe levels of childhood trauma.36
Table 1.
Participant Characteristics for UHR and CTRL Groups
| UHR Mean (n = 77) | UHR SD | CTRL Mean (n = 25) | CTRL SD | Statistics | P | |
|---|---|---|---|---|---|---|
| Age (y) | 22.6 | 3.64 | 23.9 | 2.85 | t = 1.77 | .09 |
| NART IQ (estimated) | 102.15 | 14.89 | 102.83 | 13.33 | t = .20 | .84 |
| Years of Education | 14.59 | 2.22 | 15.84 | 3.56 | t = 2.13 | .04 |
| Cigarettes per day | 6.28 | 8.18 | 3.72 | 5.50 | t = −1.46 | .14 |
| Cannabis use (Median)a | 2 | — | 1 | — | Z = −1.91 | .05 |
| GAF | 59.8 | 9.23 | 92.68 | 5.02 | t = 15.24 | <.001 |
| Symptoms | 58.61 | 11.70 | 92.40 | 5.11 | t = 14.98 | <.001 |
| Disability | 61.66 | 12.43 | 92.60 | 4.97 | t = 14.93 | <.001 |
| CAARMS Total | 42.17 | 21.96 | — | — | ||
| CAARMS Pos | 10.08 | 4.32 | — | — | ||
| CAARMS Neg | 4.97 | 4.11 | — | — | ||
| HAM-A | 18.34 | 9.54 | 3.04 | 3.83 | t = −7.79 | <.001 |
| HAM-D | 16.88 | 10.35 | 1.33 | 2.93 | t = − 6.73 | <.001 |
| CTQb | 56.00 | 8.10 | — | — | ||
| N | % | N | % | Statistics | P | |
| Past or present MDD/anxiety disorder |
24 | 31 | ||||
| Antipsychotic medication | 8 | 10.3% | — | — | ||
| Antidepressant medication | 19 | 24.6% | ||||
| Gender (male) | 44 | 57 | 13 | 52 | 0.66 | .72 |
| Handedness (right) | 63 | 81 | 23 | 92 | 5.09 | .08 |
Note: NART, National Adult Reading Test; GAF, Global Assessment of Function; CAARMS, Comprehensive Assessment of At Risk Mental State; HAM-A, Hamilton Anxiety scale; HAM-D, Hamilton Depression Scale; MDD, Major Depressive disorder.
Data missing in 5 cases.
Data available in 38 UHR.
Global rCBF
Mean global rCBF (grey and white mater) did not differ significantly between the 2 groups (35.6 [SD = 8.1] vs 36.1 [SD = 6.61] ml/100g/min, respectively) (t(101)= 0.63, P = .94).
Regions of Interest
Hippocampal/Subiculum rCBF.
Relative to the CTRL group, UHR participants showed increased rCBF in the right hippocampal ROI (hippocampal body extending to the subiculum/parahippocampal gyrus [x, y, z = 24, −24, −6; Z = 2.99; KE = 42; PFWE = .021; cohen’s d = .62]) (figure 1A). The group effect in the left hippocampal ROI (x, y, z = −30, −32, −4; Z = 2.14; KE = 15; PFWE = .15; cohen’s d = .40) was nonsignificant. There were no hippocampal regions in which the UHR group showed reduced rCBF relative to the CTRL group. When the 8 UHR using antipsychotic medication were removed from the model the result in the right hippocampal ROI remained significant (x, y, z = 24, −24 −6; Z = 3.00; KE = 38; PFWE = .024; cohen’s d = .63).
Basal Ganglia rCBF.
Relative to the CTRL group, UHR participants showed increased rCBF in the right basal ganglia ROI (in the pallidum/putamen [x, y, z = 22, −8, −2; Z = 2.85; KE = 16; PFWE = .03; cohen’s d = .65]) (figure 1B). The group effect in the left basal ganglia ROI (x, y, z = −22, −12, −6; Z = 1.69; KE = 4; PFWE = .25; cohen’s d = .30) was nonsignificant. There were no basal ganglia regions in which the UHR group showed reduced rCBF relative to the CTRL group. When the 8 UHR using antipsychotic medication were removed from the model the result in the right basal ganglia ROI remained significant (x, y, z = 22, −8 −2; Z = 2.98; KE = 38; PFWE = .021; cohen’s d = .68).
Midbrain ROI rCBF.
There were no suprathreshold group effects within the midbrain ROI.
rCBF Associations With Symptoms and Childhood Trauma
CAARMS Positive Symptoms.
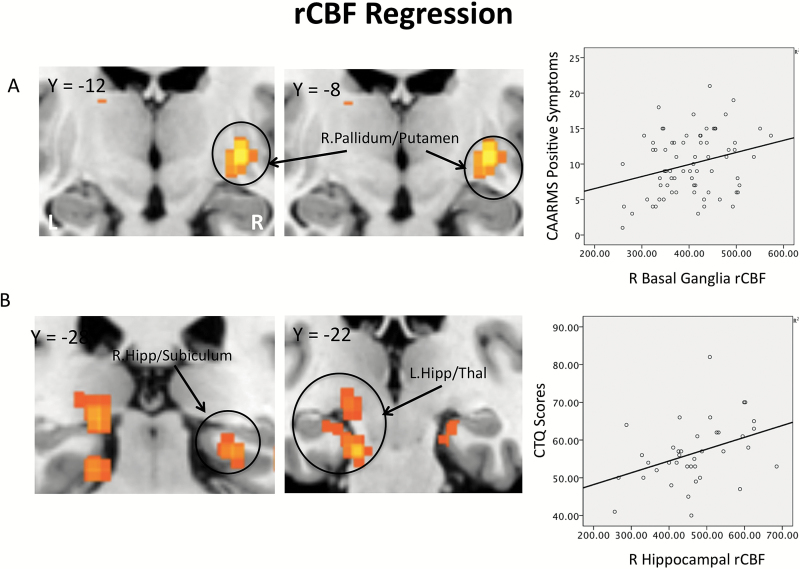
There was no association between CAARMS positive symptom scores and rCBF in the bilateral hippocampal or midbrain ROIs. There was a significant positive correlation between CAARMS positive scores in the right basal ganglia ROI (globus pallidus/putamen (x, y, z = 28, −12, −4; Z = 3.32: KE = 29: PFWE = .008) (figures 2A and D). Exploratory whole brain analysis was nonsignificant.
Fig. 2.
(A) Coronal sections and scatter plot, basal ganglia regions where resting cerebral blood flow (rCBF) is significantly correlated with CAARMS positive symptom scores (PFWE = .008). (B) Coronal section and scatter plot, medial temporal lobe regions where rCBF is positively correlated with CTQ scores (PFWE = .024 [left] and .031 [right]).
Depressive Symptoms (HAM-D).
There were no significant associations between HAM-D scores and rCBF in any ROI. Exploratory whole brain analysis was also nonsignificant.
Childhood Trauma and rCBF.
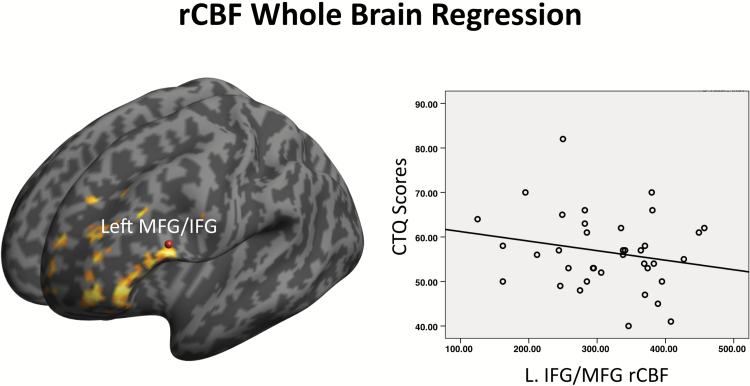
ROI analysis revealed a positive association between CTQ scores and rCBF in right hippocampus/subiculum (right: x, y, z = 24, −30, −12; Z = 3.82; KE = 59; PFWE = .034) and the left parahippocampal gyrus extending to the thalamus (left: x, y, z = −18, −28, −4; Z = 3.00; KE = 60; PFWE = .021) (figures 2B and D). The association between CTQ scores and rCBF in basal ganglia and midbrain ROIs was nonsignificant. Whole brain analysis revealed that CTQ scores were negatively associated with rCBF in a large cluster spanning the left inferior frontal gyrus (x, y, z = −58, 18, 22; Z = 4.42; KE = 308; PFWE < .01) and superior/medial prefrontal cortex (x, y, z = −4, 6, 70; Z = 4.33; KE = 209; PFWE < .001; figure 3).
Fig. 3.
Render and scatter plot, left prefrontal regions where rCBF is negatively correlated with CTQ scores (whole brain analysis) (PFWE < .001).
Discussion
The first aim of the present study was to replicate our previous finding of elevated hippocampal, basal ganglia, and midbrain rCBF11 in a larger, independent cohort of UHR individuals. We were unable to replicate our previous finding of elevated rCBF in the midbrain. Furthermore, elevated hippocampal and basal ganglia rCBF were not seen bilaterally, but were instead restricted to the right hemisphere. It is unclear why elevated midbrain and left hippocampal/basal ganglia rCBF were not observed in this second cohort. Both cohorts presented with similar levels of UHR symptoms although the current group of UHR subjects were better matched to their control group in terms of IQ and cigarette smoking. However, elevated rCBF in the right hippocampus/subiculum and basal ganglia does appear to be a robust finding in UHR subjects (effect sizes in these regions were similar to those seen in our previous study, ie, in the small to medium range). This finding remained significant after excluding the minority of UHR participants taking antipsychotic medication, and was not attributable to a difference in global rCBF levels, which were not significantly different between groups. Elevated hippocampal rCBF is also consistent with evidence from studies using other MRI techniques reporting that UHR subjects show increased resting hippocampal perfusion,5,41 reductions in hippocampal grey matter volume3 and activation during cognitive tasks.6,7 Findings are also in line with data from preclinical models of psychosis that indicate that hippocampal neuronal activity is increased, leading to altered activity in striatal/basal ganglia regions involved in dopamine regulation.8 Consistent with our previous study however, elevated hippocampal rCBF was not associated with levels of attenuated positive symptoms. Neither, in this second cohort, were hippocampal rCBF levels associated with depressive symptoms. Interestingly, rCBF levels in the right pallidum were associated with attenuated positive symptoms. The pallidum is part of the basal ganglia and a network of subcortical regions involved in the regulation of striatal dopamine function,8 which, has been shown to be aberrant in UHR subjects.42,43
We also aimed to investigate the relationship between rCBF levels and childhood trauma in UHR subjects. We found that CTQ scores in our UHR subjects were in the moderate to severe range,36 consistent with previous reports of increased levels of childhood trauma in UHR cohorts,19,20,24 and the well-established link between childhood adversity and psychotic disorders in adulthood.18 Within our UHR sample, CTQ scores were positively correlated with rCBF levels in the bilateral hippocampus extending to the thalamus and parahippocampal gyrus (left ROI). Whole brain analysis showed that CTQ scores were also negatively associated with rCBF in the left inferior and superior frontal gyrus. Previous neuroimaging studies in UHR subjects have reported alterations in rCBF,5,11 activation6,7,44 and volume3,4 in hippocampal and prefrontal regions. However, surprisingly few studies have examined the relationship between neuroimaging measures in UHR subjects and a history of childhood trauma. The only previous study of this kind in subjects at UHR for psychosis reported that childhood adversity was linked to increased striatal dopamine synthesis capacity in adulthood, although this effect was evident across both UHR subjects and controls.24 In patients with psychosis, one study described an association between childhood trauma and reduced prefrontal volume,45 but another failed to find an association between childhood trauma and hippocampal volume.46 However, the sample sizes in the studies to date have been relatively small; investigations involving larger samples are needed, particularly given the heterogeneity of the UHR category.47
A recent meta analysis of volumetric imaging studies across psychiatric diagnoses found a robust relationship between a history of childhood trauma and reduced hippocampal and dorsolateral prefrontal volumes in adulthood.25 It is possible that alterations in volume and function in hippocampal and prefrontal regions, due to childhood trauma, underlie vulnerability to a range of psychiatric disorders. Indeed, within UHR cohorts there are high levels of comorbidity, particularly with depression.15 A previous perfusion study reported altered prefrontal and hippocampal rCBF in patients with depression.48 However, in the present study, we did not observe an association between rCBF levels and depressive symptoms.
Interestingly, the results of the present study show that elevated hippocampal rCBF, while associated with childhood trauma, was not directly related to levels of attenuated psychotic symptoms. It seems reasonable to speculate that elevated hippocampal rCBF in UHR subjects may be associated with a general psychiatric vulnerability. Accordingly, it is well established that the majority of UHR subjects do not go on to develop a psychotic disorder49 and a significant proportion have additional clinical needs.50
Mechanistically, interactions between the prefrontal cortex, hippocampus (and amygdala) are thought to be critical for normal emotional and stress regulation,51 and these regions have well-established roles in cognitive and mnemonic processing, which are known to be impaired across a range of psychiatric diagnoses. Hippocampal and prefrontal regions seem to be particularly susceptible to effects of environmental stressors, particularly in early life.25 Adverse environmental experiences can lead to stress sensitisation and increased stress responsivity, which is thought to reflect disruption of hippocampal-prefrontal interactions.52
Limitations
Although our sample was a good size, UHR and CTRL participants were not matched for education levels, cannabis use or anxiety levels. While this is not uncommon in case control studies comparing psychosis or psychosis risk populations to healthy controls, we accounted for these group differences by including these factors in our analyses. Because CTQ data were not available from our healthy control participants, we could not assess whether the relationship between childhood trauma and hippocampal rCBF that we identified is specific to UHR subjects. The relationship between childhood trauma and rCBF in healthy populations has not been examined before, but a recent meta-analysis found that childhood adversity was associated with reduced hippocampal volume in nonclinical and general population samples.26 Further, CTQ scores were not available for all of the subjects in the UHR sample, and this may have limited our power to detect significant associations between childhood trauma and rCBF in other brain regions. Some participants were unwilling to complete a questionnaire on this sensitive topic, while others were unable to provide accurate or complete information, thus reducing the number of participants in which CTQ data were available. It is also worth noting that a recent study reported that young adults that retrospectively recalled having been being maltreated (ie, using the CTQ) had a particularly elevated risk for psychopathology. However, when prospective informant-reports from caregivers and clinicians are used instead, the relationship between childhood trauma and later psychiatric problems appears to be less robust.53 Nevertheless, the number of subjects in whom these data were available was comparable to that in previous studies of this type.24,25 Although most of our UHR subjects were medication-naïve, a minority (8 of 77) had been treated with low doses of antipsychotic drugs which could have altered both the severity of psychotic symptoms and rCBF.37 However, the main findings remained significant after exclusion of these subjects. UHR subjects typically go on to have diverse clinical outcomes, with some developing psychotic or other Axis-I disorders, others having persistent attenuated symptoms, and some improving such that they no longer meet the inclusion criteria for the UHR state.54 The UHR sample we studied remains to be followed up, at which point it will be possible to examine the relationship between baseline rCBF and these different outcomes.
Conclusions
Elevated resting activity in the right hippocampus and pallidum appears to be a consistent finding in people at UHR for psychosis. Increased rCBF in the hippocampus may be related to the severity of traumatic experiences in childhood.
Supplementary Material
Supplementary material is available at https://academic.oup.com/schizophreniabulletin/.
Funding
This work was supported by a Wellcome Trust Programme Grant (grant number 091667, 2011).
Acknowledgments
The authors wish to thank the study volunteers for their participation, and we gratefully thank members of the OASIS, CAMEO, and Warwick & Coventry clinical teams. O.D.H. has received unrestricted investigator-led charitable funding from or spoken at meetings organized by Astra-Zeneca, Bristol-Myers Squibb, Jenssen, Hoffman la Roche, Leyden-Delta and Eli Lilly. R.M. has received honoraria from Jenssen, Lilly, Astra-Zeneca and Roche. A.A.G. receives consulting fees from Johnson & Johnson, Lundbeck, Pfizer, GSK, Merck, Takeda, Dainippon Sumitomo, Otsuka, Lilly, Roche, Asubio, and Abbott; and receives research funding from Lundbeck, Lilly, Autofony, Alkermes and Johnson & Johnson. The other authors declare no competing financial interests.
References
- 1. Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162:2233–2245. [DOI] [PubMed] [Google Scholar]
- 2. Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010;167:1178–1193. [DOI] [PubMed] [Google Scholar]
- 3. Mechelli A, Riecher-Rössler A, Meisenzahl EM et al. . Neuroanatomical abnormalities that predate the onset of psychosis: a multicenter study. Arch Gen Psychiatry. 2011;68:489–495. [DOI] [PubMed] [Google Scholar]
- 4. Pantelis C, Velakoulis D, McGorry PD et al. . Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. [DOI] [PubMed] [Google Scholar]
- 5. Schobel SA, Chaudhury NH, Khan UA et al. . Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78:81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Allen P, Seal ML, Valli I et al. . Altered prefrontal and hippocampal function during verbal encoding and recognition in people with prodromal symptoms of psychosis. Schizophr Bull. 2011;37:746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Allen P, Luigjes J, Howes OD et al. . Transition to psychosis associated with prefrontal and subcortical dysfunction in ultra high-risk individuals. Schizophr Bull. 2012;38:1268–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lodge DJ, Grace AA. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol Sci. 2011;32:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hirano Y, Stefanovic B, Silva AC. Spatiotemporal evolution of the functional magnetic resonance imaging response to ultrashort stimuli. J Neurosci. 2011;31:1440–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2011;468:232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Allen P, Chaddock CA, Egerton A et al. . Resting hyperperfusion of the hippocampus, midbrain, and basal ganglia in people at high risk for psychosis. Am J Psychiatry. 2016;173:392–399. [DOI] [PubMed] [Google Scholar]
- 12. Lodge DJ, Grace AA. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol Sci. 2011;32:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Poldrack RA, Baker CI, Durnez J et al. . Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat Rev Neurosci. 2017;18:115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eklund A, Nichols TE, Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 2016;113:7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fusar-Poli P, Nelson B, Valmaggia L, Yung AR, McGuire PK. Comorbid depressive and anxiety disorders in 509 individuals with an at-risk mental state: impact on psychopathology and transition to psychosis. Schizophr Bull. 2014;40:120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Malykhin NV, Coupland NJ. Hippocampal neuroplasticity in major depressive disorder. Neuroscience. 2015;309:200–213. [DOI] [PubMed] [Google Scholar]
- 17. MacQueen G, Frodl T. The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research?Mol Psychiatry. 2011;16:252–264. [DOI] [PubMed] [Google Scholar]
- 18. Varese F, Smeets F, Drukker M et al. . Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophr Bull. 2012;38:661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Binbay T, Drukker M, Elbi H et al. . Testing the psychosis continuum: differential impact of genetic and nongenetic risk factors and comorbid psychopathology across the entire spectrum of psychosis. Schizophr Bull. 2012;38:992–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fusar-Poli P, Tantardini M, De Simone S et al. . Deconstructing vulnerability for psychosis: meta-analysis of environmental risk factors for psychosis in subjects at ultra high-risk. Eur Psychiatry. 2017;40:65–75. [DOI] [PubMed] [Google Scholar]
- 21. Kraan TC, Ising HK, Fokkema M et al. . The effect of childhood adversity on 4-year outcome in individuals at ultra high risk for psychosis in the Dutch Early Detection Intervention Evaluation (EDIE-NL) Trial. Psychiatry Res. 2017;247:55–62. [DOI] [PubMed] [Google Scholar]
- 22. Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence?Nat Rev Neurosci. 2008;9:947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468:203–212. [DOI] [PubMed] [Google Scholar]
- 24. Egerton A, Valmaggia LR, Howes OD et al. . Adversity in childhood linked to elevated striatal dopamine function in adulthood. Schizophr Res. 2016;176:171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Paquola C, Bennett MR, Lagopoulos J. Understanding heterogeneity in grey matter research of adults with childhood maltreatment-A meta-analysis and review. Neurosci Biobehav Rev. 2016;69:299–312. [DOI] [PubMed] [Google Scholar]
- 26. Calem M, Bromis K, McGuire P, Morgan C, Kempton MJ. Meta-analysis of associations between childhood adversity and hippocampus and amygdala volume in non-clinical and general population samples. Neuroimage Clin. 2016;14:471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Teicher MH, Samson JA, Anderson CM, Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci. 2016;17:652–666. [DOI] [PubMed] [Google Scholar]
- 28. Gomes FV, Grace AA. Prefrontal cortex dysfunction increases susceptibility to schizophrenia-like changes induced by adolescent stress exposure. Schizophr Bull. 2017. ;43:486–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Molet J, Maras PM, Avishai-Eliner S, Baram TZ. Naturalistic rodent models of chronic early-life stress. Dev Psychobiol. 2014;56:1675–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nikiforuk A, Popik P. Ketamine prevents stress-induced cognitive inflexibility in rats. Psychoneuroendocrinology. 2014;40:119–122. [DOI] [PubMed] [Google Scholar]
- 31. Fusar-Poli P, Byrne M, Badger S, Valmaggia LR, McGuire PK. Outreach and support in south London (OASIS), 2001–2011: ten years of early diagnosis and treatment for young individuals at high clinical risk for psychosis. European Psychiatry: The Journal of the Association of European Psychiatrists 2013;28:315–326. [DOI] [PubMed] [Google Scholar]
- 32. Yung AR, Phillips LJ, McGorry PD et al. . Prediction of psychosis. A step towards indicated prevention of schizophrenia. Br J Psychiatry Suppl. 1998;172:14–20. [PubMed] [Google Scholar]
- 33. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders.4th ed (DSM-IV) ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 34. Nelson HE. National Adult Reading Scale (NART). 2nd ed. National Foundation for Educational Research-Nelson, Windsor, UK; 1991. [Google Scholar]
- 35. HAMILTON M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. [DOI] [PubMed] [Google Scholar]
- 36. Bernstein DP, Stein JA, Newcomb MD et al. . Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27:169–190. [DOI] [PubMed] [Google Scholar]
- 37. Handley R, Zelaya FO, Reinders AA et al. . Acute effects of single-dose aripiprazole and haloperidol on resting cerebral blood flow (rCBF) in the human brain. Hum Brain Mapp. 2013;34:272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zubieta JK, Heitzeg MM, Xu Y et al. . Regional cerebral blood flow responses to smoking in tobacco smokers after overnight abstinence. Am J Psychiatry. 2005;162:567–577. [DOI] [PubMed] [Google Scholar]
- 39. Mathew RJ, Wilson WH, Turkington TG et al. . Time course of tetrahydrocannabinol-induced changes in regional cerebral blood flow measured with positron emission tomography. Psychiatry Res. 2002;116:173–185. [DOI] [PubMed] [Google Scholar]
- 40. Woo CW, Krishnan A, Wager TD. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage. 2014;91:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schobel SA, Lewandowski NM, Corcoran CM et al. . Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry. 2009;66:938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Howes OD, Montgomery AJ, Asselin MC et al. . Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20. [DOI] [PubMed] [Google Scholar]
- 43. Egerton A, Chaddock CA, Winton-Brown TT et al. . Presynaptic striatal dopamine dysfunction in people at ultra-high risk for psychosis: findings in a second cohort. Biol Psychiatry. 2012;74:106–112. [DOI] [PubMed] [Google Scholar]
- 44. Benetti S, Mechelli A, Picchioni M, Broome M, Williams S, McGuire P. Functional integration between the posterior hippocampus and prefrontal cortex is impaired in both first episode schizophrenia and the at risk mental state. Brain. 2009;132:2426–2436. [DOI] [PubMed] [Google Scholar]
- 45. Sheffield JM, Williams LE, Woodward ND, Heckers S. Reduced gray matter volume in psychotic disorder patients with a history of childhood sexual abuse. Schizophr Res. 2013;143:185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aas M, Navari S, Gibbs A et al. . Is there a link between childhood trauma, cognition, and amygdala and hippocampus volume in first-episode psychosis?Schizophr Res. 2012;137:73–79. [DOI] [PubMed] [Google Scholar]
- 47. Fusar-Poli P, Cappucciati M, Borgwardt S et al. . Heterogeneity of psychosis risk within individuals at clinical high risk: a meta-analytical stratification. JAMA Psychiatry. 2016;73:113–120. [DOI] [PubMed] [Google Scholar]
- 48. Lui S, Parkes LM, Huang X et al. . Depressive disorders: focally altered cerebral perfusion measured with arterial spin-labeling MR imaging. Radiology. 2009;251:476–484. [DOI] [PubMed] [Google Scholar]
- 49. Fusar-Poli P, Bonoldi I, Yung AR et al. . Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69:220–229. [DOI] [PubMed] [Google Scholar]
- 50. Fusar-Poli P, Nelson B, Valmaggia L, Yung AR, McGuire PK. Comorbid depressive and anxiety disorders in 509 individuals with an at-risk mental state: impact on psychopathology and transition to psychosis. Schizophr Bull. 2014;40:120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Godsil BP, Kiss JP, Spedding M, Jay TM. The hippocampal-prefrontal pathway: the weak link in psychiatric disorders?Eur Neuropsychopharmacol. 2013;23:1165–1181. [DOI] [PubMed] [Google Scholar]
- 52. Gomes FV, Rincón-Cortés M, Grace AA. Adolescence as a period of vulnerability and intervention in schizophrenia: insights from the MAM model. Neurosci Biobehav Rev. 2016;70:260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Newbury JB, Arseneault L, Moffitt TE et al. . Measuring childhood maltreatment to predict early-adult psychopathology: comparison of prospective informant-reports and retrospective self-reports. J Psychiatr Res. 2017;96:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fusar-Poli P, Bonoldi I, Yung AR et al. . Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69:220–229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.