Abstract
Self-generated speech produces a smaller N1 amplitude in the auditory-evoked potential than externally generated speech; this phenomenon is known as N1-suppression. Schizophrenia patients show less N1-suppression than healthy controls. This failure to self-suppress may underlie patients’ characteristic tendency to misattribute self-generated thoughts and actions to external sources. While the cause of N1-suppression deficits to speech in schizophrenia remains unclear, structural damage to the arcuate fasciculus is a candidate, due to its ostensible role in transmitting the efference copy of the motor plan to speak. Fifty-one patients with early illness schizophrenia (ESZ), 40 individuals at clinical high-risk for psychosis (CHR), and 59 healthy control (HC) participants underwent an electroencephalogram while they spoke and then listened to a recording of their speech. N1-suppression to the spoken sounds was calculated. Participants also underwent a diffusion-tensor imaging (DTI) scan, from which the arcuate fasciculus and pyramidal tract were extracted with deterministic tractography. ESZ patients exhibited significantly less N1-suppression to self-generated speech than HC participants, with CHR participants exhibiting intermediate levels. ESZ patients also exhibited structural abnormalities in the arcuate fasciculus—specifically, reduced fractional anisotropy and increased radial diffusivity—relative to both HC and CHR. There were no between-group differences in the structural integrity of the pyramidal tract. Finally, level of N1-suppression was linearly related to the structural integrity of the arcuate fasciculus, but not the pyramidal tract, across groups. These results suggest that the self-suppression deficits to willed speech consistently observed in schizophrenia patients may be caused, at least in part, by structural damage to the arcuate fasciculus.
Keywords: sensory attenuation, corollary discharge, efference copy, N1, auditory-evoked potential, diffusion-tensor imaging (DTI)
Introduction
In healthy people, self-generated speech evokes a smaller amplitude of the N1-component of the auditory evoked potential, compared to when the same speech is prerecorded and played back to participants while they are listening passively.1–3 Evidence from the animal literature suggests that this electrophysiological suppression may be caused by an efference copy of the motor signal used to predict the sensory consequences of self-generated vocalizations, thereby tagging the sensations as coming from the self, and suppressing the resultant activity in the primary auditory cortex.4
There is substantial evidence that schizophrenia patients show subnormal levels of electrophysiological suppression to self-generated speech.5–7 These deficits in self-suppression (also known as sensory attenuation) are significant as they provide a prima facie account for some of the most distinctive and characteristic symptoms of schizophrenia. An example are the passivity experiences, in which patients seem to misattribute self-generated actions and thoughts to external sources.8,9 There is also evidence to indicate that individuals at clinical high-risk (CHR) of developing psychosis show levels of electrophysiological self-suppression (ESS) that are intermediate between schizophrenia patients and healthy controls, suggesting that ESS deficits could potentially reflect an underlying vulnerability for, and precede the onset of, schizophrenia or psychotic disorders more generally.10
While the underlying causes of ESS abnormalities in schizophrenia are unclear, one possibility, suggested previously,11 is that they are caused by damage to the white matter fiber bundle along which the efference copy initiated by self-generated speech travels. While the fasciculus in question has not been definitively established, the arcuate fasciculus is a likely possibility as it provides a direct physical connection between the primary sites of speech production (ie, the ventral premotor cortex/Broca’s area) and the primary auditory cortex,12,13 consistent with forward models of motor control.14 Consistent with this hypothesis, schizophrenia patients have been found to exhibit structural damage in the arcuate fasciculus relative to healthy controls, as assessed with diffusion-tensor imaging (DTI).15–20
If N1-suppression to willed speech is associated with the structural integrity of the arcuate fasciculus (ie, the likely route of the efference copy), then variation in arcuate integrity should account for significant variance in N1-suppression, across clinical groups. The integrity of the arcuate fasciculus may covary with the integrity of other white matter tracts, including the pyramidal tract that transmits speech motor commands to muscle groups involved in vocalization, due to factors that generally influence white matter microstructure across the entire brain. However, if the association between arcuate fasciculus integrity and N1-suppression is specifically due to the efference-copy associated with willed speech, arcuate integrity should uniquely account for variance in N1-suppression, over and above the amount accounted for by the pyramidal tract.21,22
The present study aimed to investigate the unique association between N1-suppression to self-generated speech and the structural integrity of the arcuate fasciculus, controlling for the structural integrity of the pyramidal tract, across 3 clinical populations: namely, individuals with early illness schizophrenia (ESZ), individuals at CHR of developing psychosis, and healthy controls (HC). All participants underwent a DTI scan, and their arcuate fasciculus and pyramidal tract were identified with deterministic tractography. The structural integrity of these 2 fasciculi were quantified with 2 established diffusion metrics: fractional anisotropy (FA), which is a general measure of fasciculus integrity,23 and radial diffusivity (RD), which is a more specific measure of myelin integrity.24 Participants also underwent our previously established “Talk–Play” protocol, which has been used to assess the electrophysiological correlates of willed speech,25 and their level of N1-suppression to self-generated speech was calculated. Finally, multiple linear regression was used to investigate whether (a) the integrity of the participants’ arcuate fasciculus significantly predicted their level of N1-suppression in a similar manner across groups, and (b) whether the integrity of the arcuate fasciculus was a unique predictor of N1-suppression, when controlling for the integrity of the pyramidal tract.
Methods
Participants
Three groups of participants were recruited for the study: ESZ, CHR, and HC. The ESZ participants were diagnosed with DSM-IV schizophrenia based on the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (SCID).26 All interviews were conducted by a trained psychiatrist, clinical psychologist, or research assistant. The ESZ participants were all within 2.5 years of their first hospitalization for psychosis and/or their first exposure to antipsychotic medication. The CHR participants were assessed by trained clinicians administering the Structured Interview of Psychosis-Risk Syndromes (SIPS)27,28 and met criteria for at least 1 of the 3 psychosis-risk sub-syndromes defined by the Criteria of Psychosis-Risk Syndromes (COPS),27,28 namely (1) attenuated positive symptoms (n = 37), (2) brief intermittent psychotic states (n = 0), and (3) genetic risk with deterioration in social/occupational functioning (n = 5) (Note: the sub-syndromes were not mutually exclusive). ESZ and CHR participants were either self-referred, or referred by community clinicians to an early psychosis clinic and research program at the University of California, San Francisco (UCSF). HC participants were recruited by advertisements and word-of- mouth. In total, 51 ESZ, 40 CHR, and 59 HC individuals participated in the study. The N1-suppression data from a subset (85/150) of these participants have been previously reported10; the DTI data have not been reported previously. The demographic and clinical details of the study participants are summarized in table 1. The average time between electrophysiological recording and DTI scanning was 33.39 days (SD = 67.49, range = 0–399).
Table 1.
Demographic and Clinical Information for the Study Participants
| Early Illness Schizophrenia | Clinical High-Risk | Healthy Controls | Between-Group Comparison (P-value) | |
|---|---|---|---|---|
| Number of participants | 51 | 40 | 59 | |
| Age (years) | 21.2 (3.5) [14–30] | 20.3 (4.0) [12–28] | 21.4 (5.9) [12–31] | .46 |
| Gender | 19F, 32M | 15F, 25M | 26F, 33M | .72 |
| Average parental SES | 33.3 (14.0) | 34.7 (16.1) | 30.4 (15.2) | .34 |
| Handedness | 45R, 2L, 4A | 32R, 4L, 4A | 56R, 3L | |
| Antipsychotic medication class | 2U, 48A, 1T, 1A + T | 33U, 7A | 59U | |
| SANS global attention | 2.30 (1.31) | |||
| SANS alogia | 1.30 (1.46) | |||
| SANS avolition | 2.14 (1.46) | |||
| SANS affective flattening | 1.94 (1.45) | |||
| SAPS hallucinations | 1.42 (1.67) | |||
| SAPS delusions | 1.70 (1.42) | |||
| SAPS thought disorder | 0.70 (1.17) | |||
| SAPS bizarre behaviour | 0.30 (0.71) | |||
| SOPS unusual thought content | 3.28 (1.58) | |||
| SOPS suspiciousness | 2.00 (1.71) | |||
| SOPS grandiosity | 1.31 (1.51) | |||
| SOPS hallucinations | 2.24 (1.50) | |||
| SOPS disorganization | 1.48 (1.64) | |||
| SOPS negative symptom total | 10.93 (7.09) | |||
| SOPS general symptom total | 8.10 (4.90) |
Note: Mean, standard deviation, and range are presented where appropriate. Handedness: R, right handed; L, left-handed; A, ambidextrous. Antipsychotic medication class: U, unmedicated; A, atypical antipsychotic; T, typical antipsychotic. SAPS, Scale for the Assessment of Positive Symptoms; SANS, Scale for the Assessment of Negative Symptoms; SOPS, Scale of Prodromal Symptoms; Average parental socioeconomic status (SES) based on Hollingshead.29
Exclusion criteria for all groups included a history of alcohol or substance dependence within the past year (excepting nicotine dependence), a history of a significant medical or neurological illness, a history of head injury resulting in loss of consciousness, and an age-at-scan of ≥32 years. Additional exclusion criteria for the HC participants were a present or past diagnosis of an Axis I disorder (based on the SCID—non-patient edition30), or self-report of a first-degree relative with a psychotic disorder. The study was approved by the UCSF Institutional Review Board, and all participants provided written informed consent, or in the case of minors, written parent/guardian informed consent and participant assent, to participate.
Symptom severity ratings were obtained from ESZ patients by trained raters administering the Scale of Positive Symptoms (SAPS31) and the Scale of Negative Symptoms (SANS32) during semi-structured interviews. Symptom ratings for CHR participants were obtained using the Scale of Psychosis-Risk Symptoms (SOPS), imbedded in the SIPS.28 Parental socioeconomic status29 and handedness33 were also assessed in all participants.
Materials and Procedure
DTI Acquisition and Preprocessing.
Diffusion-weighted images were collected with an echo-planar imaging sequence on a 3-T Siemens Tim Trio system. The parameters for diffusion-weighted imaging were TR = 9000 ms, TE = 84 ms, 35 gradient directions (b = 800 s/mm2), matrix = 128 mm × 128 mm, slices = 72; voxel size = 2 mm3. Diffusion-tensor images were constructed from the diffusion-weighted images on the basis of least-squares estimation. Intra-scan misalignments due to head movements and eddy currents were removed through affine registration of the diffusion-weighted images to the baseline image for each individual participant (Functional MRI of the Brain Software Library; FSL). All images were masked in order to remove non-brain areas and background noise by manually editing a label map, which was initialized using the OTSU module in the Slicer-3 software package (www.slicer.org).
Diffusion-Tensor Tractography.
The Slicer-3 software package (www.slicer.org) was used to extract the 2 fasciculi-of-interest namely the arcuate fasciculus and the pyramidal tract. Both fasciculi were extracted from the left hemisphere using deterministic (streamline) tractography. The left hemisphere was chosen due to the fact that the arcuate fasciculus is often poorly defined in the right hemisphere.34
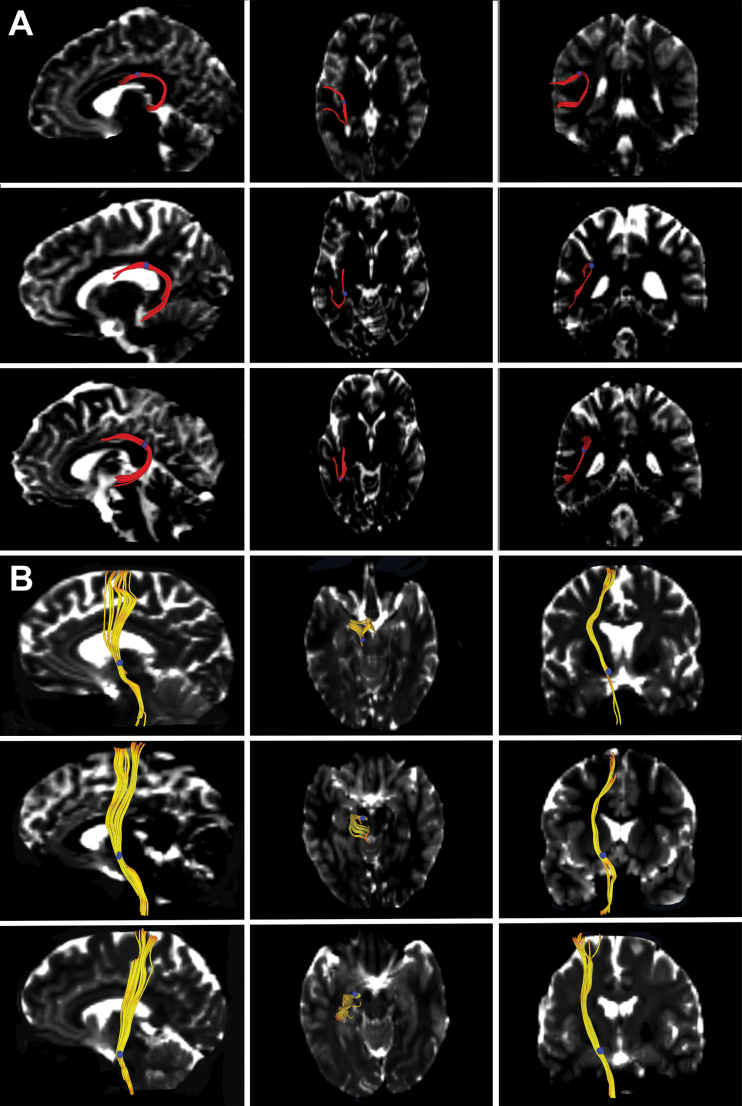
The 2 fasciculi of interest were extracted using a manual, fiducial-based approach, based on our previously published protocol15,17—see supplementary material for more details. Examples of the left arcuate fasciculus and left pyramidal tract, extracted from 3 representative participants, are provided in figure 1. Following tractography, a binary label-map was generated for each of the 2 fasciculi by labeling the voxels through which any of the streamlines passed.
Fig. 1.
Extracting the arcuate fasciculus and pyramidal tract with diffusion-tensor tractography. The arcuate fasciculus (A; fibers in red) and pyramidal tract (B; fibers in yellow) are each shown extracted from 3 representative participants. The left column shows the saggital view, the middle column the axial view, and the right column the coronal view. The fiducials used for real-time tractography are shown as blue circles. The extracted fibers are shown overlaid on a b0 image from each participant.
Electrophysiology: Talk–Play Paradigm.
Participants completed the Talk–Play paradigm, summarized below, which has been described in detail previously.25 In the Talk condition, participants were trained to pronounce short (<300 ms), sharp vocalizations of the phoneme “ah” repeatedly in a self-paced manner, approximately every 1–2 s for 187 s in total. The speech was recorded using a microphone connected to the stimulus presentation computer, and transmitted back to subjects, in real time, through Etymotic ER3-A ear-inserts. In the Play condition, the recording from the Talk condition was played back to participants while they listened passively. The Talk–Play paradigm was run on Presentation software (www.neurobs.com/presentation).
ERP Acquisition and Preprocessing.
The procedure for ERP acquisition and preprocessing has been described in detail previously.10 Electroencephalographic data were acquired from 64 channels using a BioSemi ActiveTwo system (www.biosemi.com), referenced to CMS/DRL. Additional electrodes were placed on the outer canthi of both eyes and above and below the right eye to measure electro-oculogram (EOG) data reflecting horizontal (HEOG) and vertical (VEOG) eye movements, respectively, as well as blinks. EEG data were continuously digitalized at 1024 Hz and referenced offline to the average earlobe electrodes. A 1 Hz high-pass filter was then applied using EEGLAB (http://sccn.ucsd.edu/eeglab/). Data were next subjected to Fully Automated Statistical Thresholding for EEG artifact Rejection (FASTER), using a freely distributed toolbox,35 as described in our prior studies36,37—see supplementary material for more detail. Epochs were time locked to the onset of each “ah,” and baseline corrected to the 100 ms preceding vocalization. N1 amplitude was quantified separately for the Talk and Listen conditions, for each participant, as the most negative local minimum potential between 50 and 175 ms following the onset of the stimulus.
Statistical Analysis
DTI Component.
Two diffusion metrics were used to quantify the structural integrity of the 2 fasciculi of interest: FA and RD. FA was calculated according to the formula described by Basser,38 while RD was calculated as (λ2+ λ3)/2.38 Mean arcuate FA and RD were calculated by averaging the respective FA and RD values across the voxels constituting each participant’s arcuate label map. Mean pyramidal FA and RD were calculated similarly. Four separate univariate ANOVAs were used to compare arcuate FA, arcuate RD, pyramidal FA, and pyramidal RD between the 3 participant groups. In the case of a significant group effect, pairwise post hoc Tukey–Kraemer tests were conducted to determine which groups differed.
Electrophysiology Component.
Univariate ANOVA was used to compare N1-suppression between the 3 participant groups. N1-suppression was calculated as the difference in N1 amplitude between the Talk and Play conditions at electrode Cz. Electrode Cz was chosen as it is the site at which the amplitude of the N1 component is often maximal, which was verified in the current dataset. A significant group effect was followed up with pairwise post hoc Tukey–Kraemer tests to determine which groups differed.
Predicting N1-Suppression From White Matter Integrity.
Multiple linear regression was employed for this analysis. Participants’ level of N1-suppression at electrode Cz was entered as the dependent variable. The predictor variables comprised 2 group indicator variables representing Group (HC, CHR, ESZ), and the 4 white matter integrity variables arcuate FA, arcuate RD, pyramidal FA, and pyramidal RD. Before testing the “common slope” effects of the white matter variables (each controlling for all the others), 8 group × white matter integrity interaction terms (2 indicator variables × 4 white matter predictors) were added to the model to test for significant slope differences between the groups for any of the white matter integrity predictors. Significant slope differences were followed up by characterizing and testing the white matter integrity variable’s associations with N1 suppression separately in each group. If the interaction terms did not account for a significant increment in R2, slope differences were ruled out and the interaction terms were dropped from the model, allowing the common slopes across groups to be tested for significance for each white matter integrity measure while controlling for the remaining 3 white matter measures.
Results
ERP Component
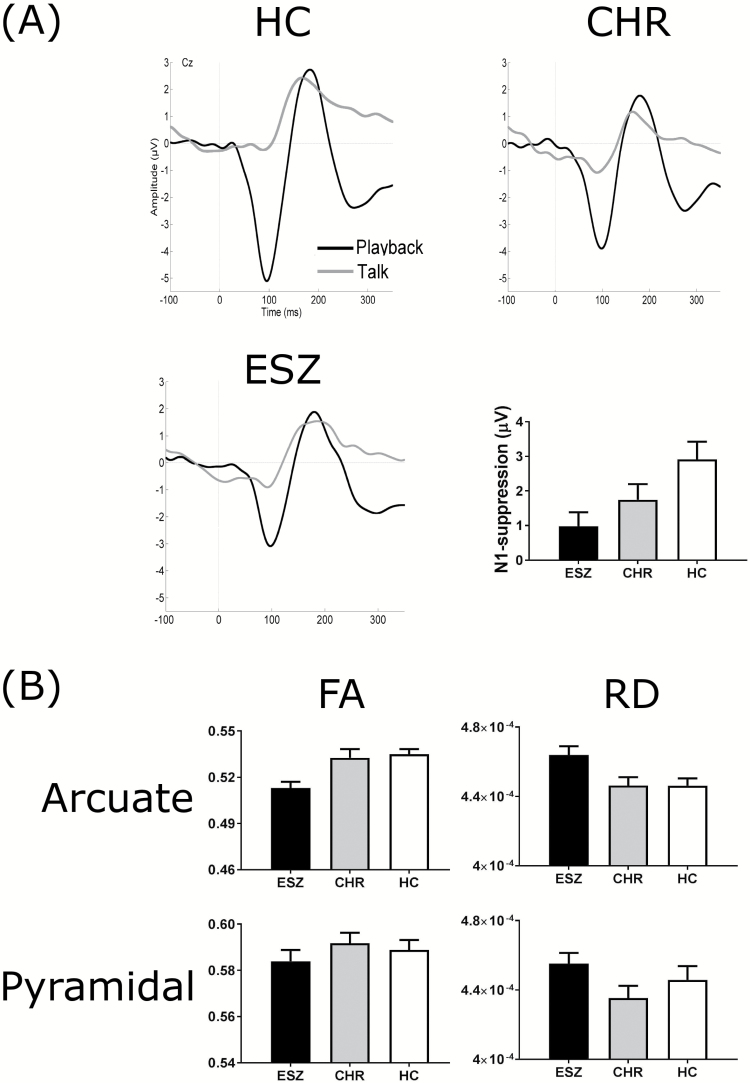
The auditory-evoked potential waveforms for the Talk–Play data and the means and standard errors for N1-suppression across the 3 participant groups are presented in figure 2A. Univariate ANOVA of the N1-suppression data revealed a significant main effect of group [F(2, 149) = 4.639, P = .011]. Follow-up post hoc tests (Tukey–Kraemer) revealed that the ESZ group exhibited significantly lower N1-suppression than the HC group [t(108) = 3.01, P = .008]. There was no significant difference between the ESZ and CHR groups [t(89) = 1.08, P = .526], nor between the CHR and HC groups [t(97) = 1.68, P = .215]. However, a linear contrast analysis revealed a highly significant linear trend across the group means [F(1, 149) = 9.089, P = .003], indicating that the level of N1-suppression increased in a linear fashion from ESZ to CHR to HC.
Fig. 2.
(A) Auditory-evoked potential waveforms for the Talk–Play data for the 3 participant groups. The grand average waveforms for the Talk condition and Playback condition are shown for the healthy control (HC, n = 59), clinical high-risk (CHR; n = 40) and early illness schizophrenia (ESZ; n = 51) participants. The recording electrode was Cz. Bar graphs illustrating the between-group differences in mean N1-suppression are shown at the bottom right of A. N1-suppression was calculated as the N1-amplitude in the Playback condition subtracted from the N1-amplitude in the Talk condition. Error bars represent the standard error of the mean. (B) Between-group comparisons of the structural integrity of the arcuate fasciculus and pyramidal tract. The data for the arcuate fasciculus are presented on the top row and the pyramidal tract on the bottom row. Fractional anisotropy (FA) is presented in the left column, while radial diffusivity (RD) is presented in the right column. The ESZ participants are shown as black bars, the CHR participants as gray bars, and the HC participants as white bars. Error bars represent the standard error of the mean.
DTI Component
Arcuate Fasciculus.
The means and standard errors of the FA and RD data for the arcuate fasciculus across the 3 groups are presented in figure 2B.
Fractional Anisotropy
Univariate ANOVA of the FA data revealed a significant main effect of group [F(2, 149) = 8.527, P < .001]. Follow-up post hoc tests (Tukey–Kraemer) revealed that the ESZ group had significantly lower FA compared to both the HC group [t(108) = 3.88, P < .001] and the CHR group [t(89) = 3.13, P = .006]. There was no significant difference in FA between the CHR and the HC groups [t(97) = 0.40, P = .917].
Radial Diffusivity
Univariate ANOVA of the RD data revealed a significant main effect of group [F(2, 149) = 4.820, P = .009]. Follow-up post hoc tests (Tukey–Kraemer) revealed that the ESZ group had significantly higher RD compared to the HC group [t(108) = 2.80, P = .016] and the CHR group [t(89) = 2.53, P = .033]. There was no significant difference in RD between the CHR and the HC groups [t(97) = 0.01, P = .999].
Pyramidal Tract.
The means and standard errors of the FA and RD data for the pyramidal tract across the 3 participant groups are presented in figure 2B.
Fractional Anisotropy
Univariate ANOVA of the FA data revealed a nonsignificant main effect of group [F(2, 149) = 0.666, P = .515]. As the main effect of group was nonsignificant, follow-up post hoc tests were not performed.
Radial Diffusivity
Univariate ANOVA of the RD data revealed a nonsignificant main effect of group [F(2, 149) = 1.663, P = .193]. As the main effect of group was nonsignificant, follow-up post hoc tests were not performed.
Predicting N1-Suppression From White Matter Integrity
To investigate whether N1-suppression could be predicted based on the structural integrity of the arcuate fasciculus and pyramidal tract, a multiple linear regression was performed. N1-suppression at electrode Cz was the dependent variable. Structural integrity was quantified on the basis of the diffusion metrics of FA and RD.
In the full model, arcuate FA and RD, pyramidal FA and RD, and 2 indicator variables coding for group were entered in the first step. The 8 interaction terms (ie, Indicator1*arcuate FA, Indicator1*arcuate RD, Indicator1*pyramidal FA, Indicator1*pyramidal RD, and repeated forIndicator2) were entered in the second step. Adding the 8 interaction terms did not significantly increase R2 [R2-change = 0.010, F(8, 134) = 0.187, P = .992], and none of the 8 interaction terms had significant beta values (all Ps > .5). Thus, there was no significant evidence of slope differences between the groups. Therefore, the interaction terms were dropped from the model and the common slopes for the white matter integrity measures were tested in the reduced model.
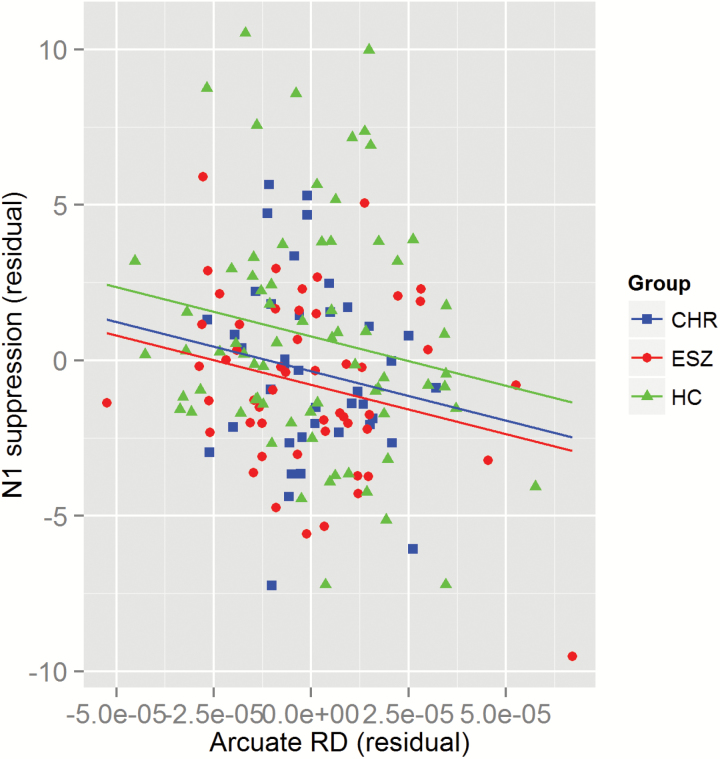
In the reduced model, only arcuate RD showed a statistically significant association with N1-suppression (t = −2.627, P = .010, partial r = −.215), controlling for all other white matter integrity variables (figure 3). Of note, in this reduced model, the ESZ vs HC mean difference in N1-suppression remained significant (P = .007), and the CHR vs HC mean difference was at trend level (P = .068) after controlling for the 4 white matter integrity covariates. The parameter estimates for all of the predictor variables and the tests of their significance are listed for the reduced model in table 2.
Fig. 3.
Scatterplot of N1-suppression vs arcuate radial diffusivity residuals from the regression model. Healthy controls (HC) are plotted as green triangles, clinical high-risk (CHR) as blue squares and early illness schizophrenia (ESZ) as red circles. The common slope lines-of-best-fit are plotted separately for the 3 clinical groups. The common slope was significantly different from zero (P = .01).
Table 2.
Model Estimates for the Linear Regression Analysis
| Model | Effect | β Estimate | SE | t | P-value |
|---|---|---|---|---|---|
| Reduced | Intercept | 33.98 | 15.57 | 2.183 | .031 |
| Group (CHR vs HC indicator) | −1.248 | 0.6776 | −1.842 | .068 | |
| Group (ESZ vs HC indicator) | −1.795 | 0.6609 | −2.716 | .007 | |
| Pyramidal FA | −2.175 | 12.22 | −0.178 | .859 | |
| Pyramidal RD | −3596 | 7934 | −0.453 | .651 | |
| Arcuate FA | −23.29 | 14.51 | −1.606 | .111 | |
| Arcuate RD | −35240 | 13410 | −2.627 | .010 |
The predictor variables were fractional anisotropy (FA) and radial diffusivity (RD) in the arcuate fasciculus and pyramidal tract. The dependent variable was N1-suppression at electrode Cz.
Discussion
To summarize our results, firstly, with regards to N1-suppression: as predicted, the ESZ group exhibited significantly lower levels of N1-suppression in response to self-generated speech, relative to the HC group, with the CHR group exhibiting intermediate levels of N1-suppression. Secondly, with regards to the structural integrity of the arcuate fasciculus: as predicted, the ESZ group exhibited significantly lower levels of FA and significantly higher levels of RD in the arcuate, relative to the HC group. However, unpredicted was the fact that there were no observable differences between the HC and CHR groups with respect to either FA or RD in the arcuate. Thirdly, with regards to the hypothesized relationship between N1-suppression and arcuate integrity: arcuate RD (but not arcuate FA) was found to account for a small but significant (3.7%) amount of unique variance in N1-suppression, in a similar manner across groups. This was over-and-above the nonsignificant variance accounted for by arcuate FA, and the pyramidal tract integrity measures (FA and RD). In light of the fact that both N1-suppression deficits to speech5,6 and diffusion abnormalities in the arcuate fasciculus15–19 have been found to be a consistent feature of schizophrenia (ie, reported both in the present study, and previously), the observed linear association between arcuate RD and N1-suppression suggests that these abnormalities may be causally related. On the other hand, the regression model results indicate that the significant N1-suppression abnormalities in ESZ patients, and the trend level N1-suppression abnormalities in CHR individuals cannot be fully accounted for by compromised integrity of the arcuate fasciculus, since group differences persisted even after accounting for the influence of arcuate integrity.
The observed differences in N1-suppression between ESZ and HC are consistent with previous studies that have identified ESS deficits to speech in schizophrenia (see reviews in refs.39,40) and other psychotic disorders.6 The current results, and our overlapping previous report,10 suggest that ESS deficits to self-generated speech are present early in the illness course, and may even precede the onset of psychosis. These results suggest that ESS deficits to self-generated speech may represent a potential vulnerability or endophenotypic marker of psychosis, which may complement other electrophysiological biomarkers that have previously been identified.41–44 Previously, we found intermediate ESS deficits to self-generated speech in the unaffected first-degree relatives of patients with schizophrenia, schizoaffective disorder, or bipolar psychosis,6 similar to what we observed in CHR individuals in the current study. Furthermore, a recent study by Oestreich et al,45 compared N1-suppression in the Talk–Play task in undergraduate students who scored high and low on a commonly used psychometric scale of schizotypy,46 and found that the high-schizotypy participants showed significantly reduced levels of N1-suppression relative to low-schizotypy participants. Taken together, these findings suggest that subnormal levels of N1-suppression to self-generated vocalizations may be not limited to patients with schizophrenia but may also be present, at least in attenuated form, in individuals exhibiting the psychosis-risk syndrome, patients across the broader schizophrenia-bipolar psychosis spectrum and their first-degree relatives, and individuals with high levels of schizotypic traits. In addition to providing empirical evidence for the hypothesized “continuum of psychosis,”47 these findings raise the possibility that N1-suppression deficits to self-generated vocalizations may represent an electrophysiological biomarker for psychosis-proneness, and could potentially show utility in predicting future transition to psychosis in clinical high-risk individuals. Future research investigating this possibility is warranted.
The present study is one of the few studies to directly investigate the association between neuroimaging measures of brain structure and electrophysiological measures of brain function.48–50 In regards to brain structure, the ESZ patients were found to exhibit significant abnormalities in the arcuate fasciculus (as quantified by significantly decreased FA and increased RD) relative to both HC and CHR. While several previous DTI studies have identified arcuate abnormalities in schizophrenia patients relative to matched healthy controls,15–20 few previous studies have compared arcuate integrity between schizophrenia patients and CHR individuals (see Karlsgodt et al51 for a review of the evidence for DTI abnormalities in CHR individuals). While most (though not all52) previous studies have reported arcuate abnormalities in schizophrenia patients relative to both HC and CHR participants, arcuate abnormalities have typically not been observed in CHR53–56 relative to HC. Taken together, these results suggest that while there is strong evidence for the existence of structural abnormalities in the arcuate fasciculus in patients with schizophrenia—both chronic and early-illness—it is less clear whether arcuate abnormalities are present in individuals at clinical or genetic high-risk for psychosis.
The arcuate fasciculus is a plausible route for an efference copy initiated by willed speech, as it provides direct physical connection between the primary sites of speech production and auditory perception.13 Previously, a source localization analysis of scalp EEG localized pre-speech activity to the inferior frontal gyrus (Broca’s area), and found it to be associated with post-speech N1-suppression in the auditory cortex, consistent with this hypothesis.57 The present study found that the integrity of the arcuate fasciculus accounted for a small but significant amount of variance (3.7%) in participants’ levels of N1-suppression, across groups, over and above the nonsignificant amount of variance accounted for by the integrity of the pyramidal tract. RD of the arcuate was the strongest DTI predictor in the model (β = −35240) indicating that participants with the highest levels of RD had the lowest levels of N1-suppression. RD has traditionally been considered an index of myelin integrity, as increased levels of RD have been associated with demyelination/dysmyelination in both animal24,58 and human59 studies (though other studies have cautioned that the relationship between RD and myelin is complicated60,61). The ultrastructural specificity of RD stands in contrast to FA, which is known to be influenced by numerous different parameters, including axonal integrity, fiber packing density, axonal caliber, etc.—as well as myelination.23,61 Furthermore, the reduced levels of FA typically observed in schizophrenia patients have been found to be driven largely by increases in RD, which is suggestive of myelin damage.62
In contrast to the arcuate results, the present study failed to identify any group differences in the integrity of the pyramidal tract. While abnormalities in the pyramidal tract have previously been reported in patients with adolescent-onset schizophrenia,63 the null results of the present study are consistent with a large meta-analyses that did not identify FA reductions in this tract in schizophrenia patients.64 The pyramidal tract is known to be involved in regulating movements of the mouth and tongue during the production of willed speech,22,65 among other motor actions. This null result is informative as it suggests that while abnormalities are present in the likely route of the efference copy associated with self-generated speech (ie, the arcuate fasciculus), they are not present in the ultimate route of the motor efference per se (ie, the pyramidal tract). In the terminology of the comparator model,66 this result is consistent with the idea that the pathway that transmits the efference copy, or copy of the motor plan, is disrupted in schizophrenia while the pathway that transmits the efference, or motor plan itself, is preserved.
In conclusion, N1-suppression to willed vocalizations was found to be significantly reduced in ESZ patients relative to HC participants, with CHR individuals showing intermediate levels of N1-suppression. This result provides support for the idea that schizophrenia is associated with a failure to suppress the sensory consequences of self-generated actions, leading to their misattribution to external sources. The results also indicated that the structural integrity of the arcuate fasciculus was a better predictor of N1-suppression to self-generated speech level than the structural integrity of the pyramidal tract. This result is consistent with the idea that self-suppression abnormalities to willed speech—which have consistently been observed in patients with schizophrenia—are caused by structural damage to the arcuate fasciculus, given its ostensible role in conveying the efference copy associated with willed speech to the auditory cortex.
Supplementary Material
Supplementary data are available at Schizophrenia Bulletin online.
Funding
This work was supported by a Career Development Fellowship from the National Health and Medical Research Council of Australia (APP1090507) and Discovery Projects from the Australian Research Council (DP140104394; DP170103094) to T.J.W. This work was also supported by National Institute of Mental Health (K02MH067967 to J.M.F.; R01MH076989 to D.H.M.) and the Department of Veterans Affairs (J.M.F.).
Supplementary Material
References
- 1. Ford JM, Gray M, Faustman WO, Roach BJ, Mathalon DH. Dissecting corollary discharge dysfunction in schizophrenia. Psychophysiology. 2007;44:522–529. [DOI] [PubMed] [Google Scholar]
- 2. Ford JM, Mathalon DH, Heinks T, Kalba S, Faustman WO, Roth WT. Neurophysiological evidence of corollary discharge dysfunction in schizophrenia. Am J Psychiatry. 2001;158:2069–2071. [DOI] [PubMed] [Google Scholar]
- 3. Houde JF, Nagarajan SS, Sekihara K, Merzenich MM. Modulation of the auditory cortex during speech: an MEG study. J Cogn Neurosci. 2002;14:1125–1138. [DOI] [PubMed] [Google Scholar]
- 4. Eliades SJ, Wang X. Neural substrates of vocalization feedback monitoring in primate auditory cortex. Nature. 2008;453:1102–1106. [DOI] [PubMed] [Google Scholar]
- 5. Ford JM, Mathalon DH, Kalba S, Whitfield S, Faustman WO, Roth WT. Cortical responsiveness during talking and listening in schizophrenia: an event-related brain potential study. Biol Psychiatry. 2001;50:540–549. [DOI] [PubMed] [Google Scholar]
- 6. Ford JM, Mathalon DH, Roach BJ et al. Neurophysiological evidence of corollary discharge function during vocalization in psychotic patients and their nonpsychotic first-degree relatives. Schizophr Bull. 2013;39:1272–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heinks-Maldonado TH, Mathalon DH, Houde JF, Gray M, Faustman WO, Ford JM. Relationship of imprecise corollary discharge in schizophrenia to auditory hallucinations. Arch Gen Psychiatry. 2007;64:286–296. [DOI] [PubMed] [Google Scholar]
- 8. Feinberg I. Efference copy and corollary discharge: implications for thinking and its disorders. Schizophr Bull. 1978;4:636–640. [DOI] [PubMed] [Google Scholar]
- 9. Frith CD. The Cognitive Neuropsychology of Schizophrenia. Hove, UK: Lawrence Erlbaum Associates; 1992. [Google Scholar]
- 10. Perez VB, Ford JM, Roach BJ et al. Auditory cortex responsiveness during talking and listening: early illness schizophrenia and patients at clinical high-risk for psychosis. Schizophr Bull. 2012;38:1216–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Whitford TJ, Ford JM, Mathalon DH, Kubicki M, Shenton ME. Schizophrenia, myelination, and delayed corollary discharges: a hypothesis. Schizophr Bull. 2012;38:486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guenther FH, Hickok G. Role of the auditory system in speech production. Handb Clin Neurol. 2015;129:161–175. [DOI] [PubMed] [Google Scholar]
- 13. Pynn LK, DeSouza JF. The function of efference copy signals: implications for symptoms of schizophrenia. Vision Res. 2013;76:124–133. [DOI] [PubMed] [Google Scholar]
- 14. Wolpert DM, Miall RC. Forward models for physiological motor control. Neural Netw. 1996;9:1265–1279. [DOI] [PubMed] [Google Scholar]
- 15. Whitford TJ, Mathalon DH, Shenton ME et al. Electrophysiological and diffusion tensor imaging evidence of delayed corollary discharges in patients with schizophrenia. Psychol Med. 2011;41:959–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Geoffroy PA, Houenou J, Duhamel A et al. The arcuate fasciculus in auditory-verbal hallucinations: a meta-analysis of diffusion-tensor-imaging studies. Schizophr Res. 2014;159:234–237. [DOI] [PubMed] [Google Scholar]
- 17. McCarthy-Jones S, Oestreich LK, Australian Schizophrenia Research Bank , Whitford TJ. Reduced integrity of the left arcuate fasciculus is specifically associated with auditory verbal hallucinations in schizophrenia. Schizophr Res. 2015. [DOI] [PubMed] [Google Scholar]
- 18. Abdul-Rahman MF, Qiu A, Woon PS, Kuswanto C, Collinson SL, Sim K. Arcuate fasciculus abnormalities and their relationship with psychotic symptoms in schizophrenia. PLoS One. 2012;7:e29315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Weijer AD, Mandl RC, Diederen KM et al. Microstructural alterations of the arcuate fasciculus in schizophrenia patients with frequent auditory verbal hallucinations. Schizophr Res. 2011;130:68–77. [DOI] [PubMed] [Google Scholar]
- 20. Seitz J, Zuo JX, Lyall AE et al. Tractography analysis of 5 white matter bundles and their clinical and cognitive correlates in early-course schizophrenia. Schizophr Bull. 2016;42:762–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Duffau H. The anatomo-functional connectivity of language revisited. New insights provided by electrostimulation and tractography. Neuropsychologia. 2008;46:927–934. [DOI] [PubMed] [Google Scholar]
- 22. Love RJ, Webb WG.. Neurology for the speech-language pathologist. 2nd ed. London, UK: Elsevier Health Services; 1992. [Google Scholar]
- 23. Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51:527–539. [DOI] [PubMed] [Google Scholar]
- 24. Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. [DOI] [PubMed] [Google Scholar]
- 25. Ford JM, Roach BJ, Mathalon DH. Assessing corollary discharge in humans using noninvasive neurophysiological methods. Nat Protoc. 2010;5:1160–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. First M, Spitzer R, Gibbon M, Williams J.. Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID-I), Clinician Version. Washington, DC: American Psychiatric Publishing; 1997. [Google Scholar]
- 27. Miller TJ, McGlashan TH, Rosen JL et al. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. 2002;159:863–865. [DOI] [PubMed] [Google Scholar]
- 28. McGlashan T, Walsh BC, Woods S.. The Psychosis-Risk Syndrome: Handbook for Diagnosis and Follow-Up. 1st ed. New York, NY: Oxford University Press; 2010. [Google Scholar]
- 29. Hollingshead A. Two Factor of Index of Social Position. New Haven: Yale Station; 1965. [Google Scholar]
- 30. First MB, Spitzer RL, Gibbon M, Williams BJ.. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP). New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 31. Andreasen NC. Scale for the Assessment of Positive Symptoms. Iowa City: Iowa University Press; 1984. [Google Scholar]
- 32. Andreasen NC. Scale for the Assessment of Negative Symptoms. Iowa City: Iowa University Press; 1984. [Google Scholar]
- 33. Crovitz HF, Zener K. A group-test for assessing hand- and eye-dominance. Am J Psychol. 1962;75:271–276. [PubMed] [Google Scholar]
- 34. Parker GJ, Luzzi S, Alexander DC, Wheeler-Kingshott CA, Ciccarelli O, Lambon Ralph MA. Lateralization of ventral and dorsal auditory-language pathways in the human brain. Neuroimage. 2005;24:656–666. [DOI] [PubMed] [Google Scholar]
- 35. Nolan H, Whelan R, Reilly RB. FASTER: fully automated statistical thresholding for EEG artifact rejection. J Neurosci Methods. 2010;192:152–162. [DOI] [PubMed] [Google Scholar]
- 36. Ford JM, Palzes VA, Roach BJ, Mathalon DH. Did I do that? Abnormal predictive processes in schizophrenia when button pressing to deliver a tone. Schizophr Bull. 2014;40:804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kort NS, Ford JM, Roach BJ et al. Role of N-methyl-D-aspartate receptors in action-based predictive coding deficits in schizophrenia. Biol Psychiatry. 2017;81:514–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8:333–344. [DOI] [PubMed] [Google Scholar]
- 39. Ford JM, Mathalon DH. Corollary discharge dysfunction in schizophrenia: can it explain auditory hallucinations?Int J Psychophysiol. 2005;58:179–189. [DOI] [PubMed] [Google Scholar]
- 40. Mathalon DH, Ford JM. Corollary discharge dysfunction in schizophrenia: evidence for an elemental deficit. Clin EEG Neurosci. 2008;39:82–86. [DOI] [PubMed] [Google Scholar]
- 41. Cadenhead KS, Light GA, Shafer KM, Braff DL. P50 suppression in individuals at risk for schizophrenia: the convergence of clinical, familial, and vulnerability marker risk assessment. Biol Psychiatry. 2005;57:1504–1509. [DOI] [PubMed] [Google Scholar]
- 42. Light GA, Swerdlow NR, Rissling AJ et al. Characterization of neurophysiologic and neurocognitive biomarkers for use in genomic and clinical outcome studies of schizophrenia. PLoS One. 2012;7:e39434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Javitt DC, Spencer KM, Thaker GK, Winterer G, Hajós M. Neurophysiological biomarkers for drug development in schizophrenia. Nat Rev Drug Discov. 2008;7:68–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Luck SJ, Mathalon DH, O’Donnell BF et al. A roadmap for the development and validation of event-related potential biomarkers in schizophrenia research. Biol Psychiatry. 2011;70:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Oestreich LK, Mifsud NG, Ford JM, Roach BJ, Mathalon DH, Whitford TJ. Subnormal sensory attenuation to self-generated speech in schizotypy: electrophysiological evidence for a ‘continuum of psychosis’. Int J Psychophysiol. 2015;97:131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Raine A. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr Bull. 1991;17:555–564. [DOI] [PubMed] [Google Scholar]
- 47. van Os J, Hanssen M, Bijl RV, Ravelli A. Strauss (1969) revisited: a psychosis continuum in the general population?Schizophr Res. 2000;45:11–20. [DOI] [PubMed] [Google Scholar]
- 48. Fusar-Poli P, Crossley N, Woolley J et al. White matter alterations related to P300 abnormalities in individuals at high risk for psychosis: an MRI-EEG study. J Psychiatry Neurosci. 2011;36:239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Whitford TJ, Kubicki M, Ghorashi S et al. Predicting inter-hemispheric transfer time from the diffusion properties of the corpus callosum in healthy individuals and schizophrenia patients: a combined ERP and DTI study. Neuroimage. 2011;54:2318–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64:521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Karlsgodt KH, Jacobson SC, Seal M, Fusar-Poli P. The relationship of developmental changes in white matter to the onset of psychosis. Curr Pharm Des. 2012;18:422–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Peters BD, de Haan L, Dekker N et al. White matter fibertracking in first-episode schizophrenia, schizoaffective patients and subjects at ultra-high risk of psychosis. Neuropsychobiology. 2008;58:19–28. [DOI] [PubMed] [Google Scholar]
- 53. Muñoz Maniega S, Lymer GK, Bastin ME et al. A diffusion tensor MRI study of white matter integrity in subjects at high genetic risk of schizophrenia. Schizophr Res. 2008;106:132–139. [DOI] [PubMed] [Google Scholar]
- 54. de Weijer AD, Neggers SF, Diederen KM et al. Aberrations in the arcuate fasciculus are associated with auditory verbal hallucinations in psychotic and in non-psychotic individuals. Hum Brain Mapp. 2013;34:626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Voineskos AN, Lerch JP, Felsky D et al. The ZNF804A gene: characterization of a novel neural risk mechanism for the major psychoses. Neuropsychopharmacology. 2011;36:1871–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kubicki M, Shenton ME, Maciejewski PK et al. Decreased axial diffusivity within language connections: A possible biomarker of schizophrenia risk. Schizophrenia Res. 2013;148:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang J, Mathalon DH, Roach BJ et al. Action planning and predictive coding when speaking. Neuroimage. 2014;91:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. [DOI] [PubMed] [Google Scholar]
- 59. Klawiter EC, Schmidt RE, Trinkaus K et al. Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. Neuroimage. 2011;55:1454–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chang EH, Argyelan M, Aggarwal M et al. The role of myelination in measures of white matter integrity: combination of diffusion tensor imaging and two-photon microscopy of CLARITY intact brains. Neuroimage. 2017;147:253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jones DK, Knösche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage. 2013;73:239–254. [DOI] [PubMed] [Google Scholar]
- 62. Seal ML, Yücel M, Fornito A et al. Abnormal white matter microstructure in schizophrenia: a voxelwise analysis of axial and radial diffusivity. Schizophr Res. 2008;101:106–110. [DOI] [PubMed] [Google Scholar]
- 63. Douaud G, Smith S, Jenkinson M et al. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130:2375–2386. [DOI] [PubMed] [Google Scholar]
- 64. Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res. 2009;108:3–10. [DOI] [PubMed] [Google Scholar]
- 65. Urban PP, Hopf HC, Fleischer S, Zorowka PG, Müller-Forell W. Impaired cortico-bulbar tract function in dysarthria due to hemispheric stroke. Functional testing using transcranial magnetic stimulation. Brain. 1997;120(pt 6):1077–1084. [DOI] [PubMed] [Google Scholar]
- 66. Frith C. Explaining delusions of control: the comparator model 20 years on. Conscious Cogn. 2012;21:52–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.