Abstract
Background
The investigation of large-scale intrinsic connectivity networks in antipsychotic-naïve first-episode schizophrenia increases our understanding of system-level cerebral dysfunction in schizophrenia while enabling control of confounding effects of medication and disease progression. Reports on functional connectivity in antipsychotic-naïve patients have been mixed and the relation between network alterations, psychopathology and cognition is unclear.
Methods
A total number of 47 patients with first-episode schizophrenia who had never received antipsychotic medication and 47 healthy controls were scanned with functional magnetic resonance imaging under resting conditions. Main outcome measures were differences in functional connectivity between groups and the relationship between network alterations, psychopathology and cognition.
Results
Altered connectivity was found between right central executive network (CEN) and right ventral attention network (VAN) (patients > controls, P = .001), left CEN and left VAN (P = .002), and between posterior default mode network and auditory network (P = .006). Association between network connectivity and clinical characteristics was found as interactions between the effects of group and sustained attention (P = .005) and between group and processing speed (P = .007) on the connectivity between right CEN and right VAN.
Conclusions
Our findings suggest that the early phase of schizophrenia is characterized by increased connectivity between fronto-parietal networks suggested to be involved in the control of cognitive and sensory functions. Moreover, the present study suggests that the problem of not disengaging the VAN leads to difficulties with attention and possibly subjective awareness.
Keywords: psychopathology, cognition, functional connectivity, functional magnetic resonance imaging, resting state
Introduction
One of the most direct ways to study the functional organization of the human brain in vivo is by measuring the blood-oxygen-level-dependent (BOLD) -signal under resting conditions. This method has led to the discovery of robust patterns of correlated activity also known as intrinsic connectivity networks (ICN’s).1,2 Some of these networks have been shown to be associated with core neurocognitive domains and sensory functions like working memory and attention, (central executive network, CEN), self-monitoring (default mode network, DMN), externally directed cognition (dorsal and ventral attention networks, DAN and VAN), experience of salience (salience network, SN), and auditory processing (auditory network). Since schizophrenia is characterized by abnormalities in these functions the associated ICN’s have been incorporated into a number of models for schizophrenia. First, schizophrenia has been proposed to be associated with errors in self-monitoring as a result of decreased communication within the DMN and/or abnormal interaction between the DMN and DAN.3 Along the same lines, aberrant DMN activity has been proposed to be the result of a failure of the fronto-parietal control system (CEN, SN and VAN) to control the DMN activity.4 Second, the SN models propose that psychosis occur as a result of inappropriately attached salience.5–7 Third, other models focus on network abnormalities between regions involved in directed effort (dorsal anterior cingulate cortex [dACC], posterior cingulate cortex [PCC] and hippocampus) and auditory processing (auditory cortex).8,9 The overall strength of these models is the focus on system-level neuropathology because the diverse symptoms of schizophrenia are likely to be associated with a break down in large-scale neuronal networks.
Several studies have reported alterations in functional connectivity in medicated long-term ill patients with schizophrenia. Despite a trend towards alterations in cortico-subcortical networks, prefrontal cortex and the DMN, results so far have been inconsistent.10 This could be explained by the presence of known confounders such as medication and disease progression. Narrowing the search to studies in Antipsychotic-Naïve First-Episode (ANFE) patients does not provide a clear picture, eg, one study found higher connectivity between PCC, a central part of the DMN, and task positive regions such as dorsolateral prefrontal cortex (DLPFC)11 compared to another study reporting no alterations of DLPFC connectivity.12 Furthermore, one study found increased connectivity within the DMN and increased connectivity between DMN and fronto-parietal networks in ANFE patients,13 whereas another study reported decreased connectivity between DMN and medial prefrontal cortex (MPFC) that is a part of the DMN, and no alteration in connectivity between DMN and fronto-parietal networks.14 Because of this diversity of findings, the questions of which network alterations are present in the early course of schizophrenia and how they relate to the clinical manifestations in the antipsychotic-naïve state are largely unanswered.
In the present study, we used a large cohort of well-characterized ANFE patients to evaluate the abovementioned intrinsic large-scale network models for schizophrenia. We expected positive symptoms to be associated with DMN connectivity due to the role of the DMN in self-monitoring. Moreover, because most studies suggest a global, nonspecific relationship between network alterations and cognitive deficits,15 we expected a generalized pattern of interactions between group and different cognitive measures on network connectivity.
Methods
The study was conducted in accordance with the Declaration of Helsinki II and approved by the Danish national Committee on Biomedical Research Ethics (H-D-2008-088). All participants gave signed informed consent to their participation.
Participants
As part of a multimodal clinical study (PECANS, ClinicalTrials.gov identifier: NCT01154829) a total number of 69 ANFE patients aged 18–45 years were recruited from psychiatric centers in the capital region of Denmark from December 2008 to December 2013. Fifty patients were scanned with functional magnetic resonance imaging under resting conditions. Three patients were excluded due to excessive head motion leaving 47 patients for further analysis. The inclusion criterion was a first-episode of schizophrenia or schizoaffective disorder (ICD-10 criteria). Exclusion criteria were any prior or current treatment with antipsychotic medication. An equal number (n = 47) of healthy controls matched on age, gender, and parental socioeconomic status, with no history of psychiatric illness and no schizophrenia spectrum disorder in first-degree relatives were recruited via a specialized research website. Additional exclusion criteria for all participants were antidepressants within the last month, severe somatic illness, a history of severe head injury, current diagnosis of drug dependence (ICD-10 criteria) with the acceptance of current occasional drug use (assessed with urine test). Benzodiazepines were not allowed 12 hours prior to scans (demographic data displayed in Table 1). Data from this cohort on other modalities has been published in a number of studies.16–22
Table 1.
Demographics, Psychopathology and Cognitive Measures
| Schizophrenia Patients (n = 47) | Healthy Controls (n = 47) | |
|---|---|---|
| Age: years | 24.6 | 24.7 |
| Gender (male/female) | 29/18 | 29/18 |
| Handedness: Mean EHI-score | 72 (51) | 84 (40) |
| Education: years | 12.1 (2.6) | 14.0 (2.7) |
| Duration of untreated illness: weeks | 59 (69) | |
| Smokersa | 10/14/19/1/3 | 11/21/11/2/1 |
| PANSS totalb | 82.6 (17.4) | |
| PANSS positive | 20.3 (4.3) | |
| PANSS negative | 20.5 (7.3) | |
| PANSS general | 41.9 (9.3) | |
| GAF | 38 (16) | |
| General intelligence (estimated IQ)c | 100.8 (19.6) | 111.5 (12.2)* |
| Processing Speed (BACS Symbol coding)d | 55.8 (15.1) | 65.4 (9.5)* |
| Sustained attention (CANTAB RVP A’)e | 0.976 (0.017) | 0.987 (0.014)* |
| Shift and flexibility of attention (CANTAB IED, total errors adjusted)f | 28.1 (21.0) | 18.6 (16.5)** |
Note: PANSS, Positive And Negative Syndrome Scale; GAF, General Assessment of Functioning; IQ, intelligence quotient; RVP, Rapid Visual Information Processing; IED, Intra-Extra Dimensional set shift.
aSmoking status missing for one healthy control. Smoking categories: never tried/ tried a few times/ use regularly/ abuse/ dependency.
bPANSS data missing for 2 subjects.
c–fVarying n (b: n = 84, c: n = 92, d: n = 91, e: n = 62).
*Significant difference (P < .05); **Borderline significant difference (P = .051). Numbers in () = SD.
Clinical Assessments
To ensure a diagnosis of schizophrenia or schizoaffective disorder in patients and exclusion of psychiatric illness in healthy controls all participants underwent a Schedule of Clinical Assessment in Neuropsychiatry interview (SCAN).23 Current psychopathology was estimated with Positive and Negative Syndrome Scale (PANSS).24 Handedness was assessed with Edinburg Handedness Inventory (EHI).25 Cognitive measures were general intelligence estimated from 4 subtests from the Wechsler Adult Intelligence Scale, 3rd ed. (WAIS-III),26 processing speed assessed using the symbol coding test from Brief Assessment of Cognition in Schizophrenia (BACS),27 sustained attention assessed using the Rapid Visual Information Processing test (RVP) from Cambridge Neuropsychological Test Automated Battery (CANTAB), and executive function (attentional set shifting) was assessed using Intra-Extra Dimensional Set Shift (IED) from CANTAB (data from the clinical assessments displayed in Table 1).28
MRI Data Acquisition
Imaging was performed on a Philips Achieva 3.0T whole body MRI scanner (Philips Medical Systems, Best, The Netherlands) using an 8-channel head coil. Functional images were obtained with a T2*-weighted echo planar imaging sequence (repetition time = 2 s, echo time = 25 ms, flip angle 75°) with a matrix size of 128 × 128 × 38 and field of view = 230 × 230 × 128 mm yielding a voxel-size of 1.8 × 1.8 × 3.4 mm. Each functional run contained 300 volumes resulting in a total scan time of 10 minutes. fMRI was performed under resting conditions with instructions to stay awake with eyes closed and to not think of anything in particular. For anatomical reference T1-weighted structural images were acquired (repetition time = 10 ms, echo time = 4.6 ms, flip angle = 8° and voxel-size = 0.79 × 0.79 × 0.8).
Preprocessing of fMRI Data
In order to reduce the effect of head motion, single-subject ICA was done (Probabilistic ICA in FSL’s MELODIC vers. 4.1.9.) using MCFLIRT motion correction tool,29 and spatial smoothing with a 5 mm Gaussian kernel. For each subject, IC’s judged to be motion artifacts were discarded in a semi-automated fashion (supplementary material). After initial ICA de-noising each file was de-spiked using Artrepair.30 Before group ICA additional preprocessing steps included brain extraction, slice time correction (interleaved), high pass filtering (150 s), registration to the T1 weighted anatomical image and subsequently to the MNI-152 standard space image using FNIRT31,32 and finally resampling the 4D dataset to 4 mm isotropic voxels. Group ICA was then performed with FSL’s MELODIC vers. 4.1.9 including variance-normalization of time-courses33 and automatic dimensionality estimation, ie, MELODIC estimated the number of components automatically from the data.34,35
Statistical Analysis of Within Network Connectivity
According to the large-scale network models described above networks of interest included DMN, SN, CEN, VAN, DAN, and auditory network. Networks of interest were chosen by the criterion of maximum visual resemblance with the well-described resting state networks throughout the literature. To enable group comparisons the set of spatial maps from the group-average analysis was used to generate subject-specific versions of the spatial maps, and associated time series, using a dual regression approach.35 In order to reduce the effect of motion further, 24 motion parameters (6 rigid body estimates + their 6 derivatives + squares of the 12 parameters) were added as temporal regressors in the second regression. Finally, networks of interest were tested for voxel-wise group differences using FSL’s permutation-testing tool (Randomise) including age, gender and motion summary measures (absolute and frame-wise displacement) as nuisance regressors. For the remainder of this work we use the term “network” synonymously with the term “independent component.” Statistical analysis was made with threshold-free cluster enhancement, a significance threshold of P < .05 and FWE-correction as implemented in the Randomise tool.
Statistical Analysis of Between Network Connectivity
In order to obtain pair-wise correlations between the networks of interest we calculated a correlation matrix based on the IC time-series for each subject. After Fisher transformation, we then tested for (1) group differences, (2) correlation to PANSS scores within the patient group, and (3) interaction between the effects of group and cognitive measures on between-network connectivity with multiple linear regression including age, gender and motion summary measures. Statistical inferences were made using false discovery rate (FDR) at level δ = 0.05 with significance threshold defined as P (j) ≤ δ j/m (j is an index running from 1 to m, m is the total number of tests).36
Results
Networks of Interest
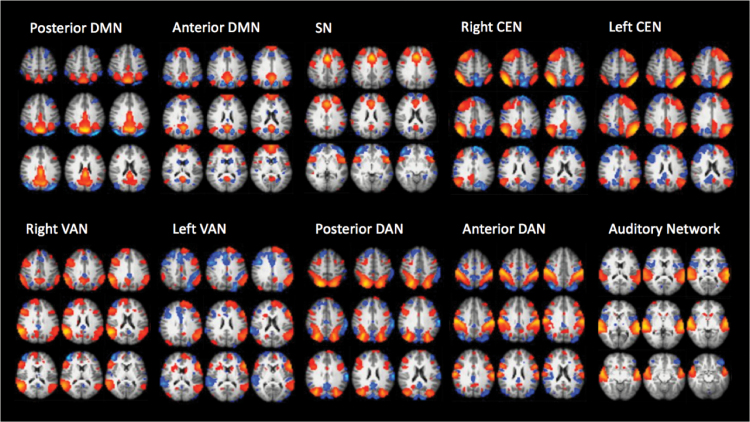
In total, group ICA resulted in 79 IC’s of which 10 components were chosen as networks of interest according to the network models (figure 1). The DMN and the DAN both appeared as anterior and posterior subnetworks whereas the CEN and VAN both were divided into a right and a left hemisphere component. Although the VAN centered on the temporo-parietal junction and fronto-lateral cortex was originally proposed as a right-lateralized network,37 we chose to include a similar network present in the left hemisphere in the model. The PCC, dACC and hippocampus, emphasized in the directed effort model, were comprised in the 2 DMN’s (PCC and hippocampus) and the SN (dACC).
Fig. 1.
Networks of interest. Default mode network (DMN), salience network (SN), central executive network (CEN), ventral attention network (VAN), dorsal attention network (DAN), and auditory network.
Functional Connectivity Within Networks
Spatial maps of networks of interest were similar between groups, and no voxel-wise group differences could be detected at corrected level.
Functional Connectivity Between Networks
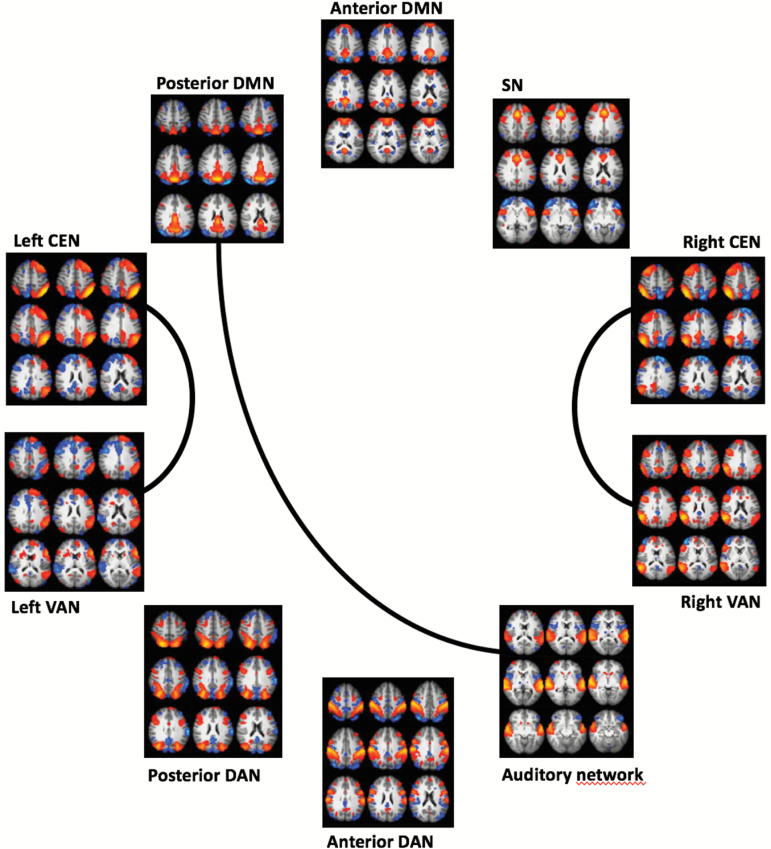
With a total number of 10 networks of interest the number of pairwise tests between networks was ((10–1) × 10)/2 = 45. This analysis revealed significant group difference in connectivity between left CEN and left VAN (P = .001, corrected), between right CEN and right VAN (P = .002, corrected), and between posterior DMN and auditory cortex (P = .006, borderline significant with correction) (figure 2). At uncorrected level group differences were seen between posterior DMN and right VAN (P = .019), posterior DMN and left VAN (P = .029), right CEN and auditory network (P = .030), and between right CEN and anterior DAN (P = .033). In all cases patients had higher connectivity compared to controls.
Fig. 2.
Group differences in pairwise correlations between networks of interest. The lines indicate stronger mean pairwise correlations in patients compared to controls, false discovery rate (FDR) corrected.
Functional Connectivity Between Networks and Clinical Characteristics
The search for associations between network connectivity and clinical symptoms was limited to the networks that showed a significant or borderline significant group difference at corrected level, ie, right and left CEN, right and left VAN, posterior DMN and auditory network.
Calculating the correlation between PANSS scores and between-network connectivity did not reveal any significant effects at corrected level. Only to indicate a trend, a positive correlation was found between positive symptoms and the connectivity between right CEN and auditory network (P = .036, uncorrected), left CEN and left VAN (P = .010, uncorrected), and between left CEN and auditory network (P = .027, uncorrected). A negative correlation was found between negative symptoms and the connectivity between left CEN and left VAN (P = .005, uncorrected), left CEN and right VAN (P = .048, uncorrected), and between left CEN and auditory network (P = .031, uncorrected). General symptoms were positively correlated with the connectivity between left CEN and left VAN (P = .017, uncorrected), left CEN and right VAN (P = .041, uncorrected), and between right VAN and posterior DMN (P = .007, uncorrected). Furthermore, general symptoms were negatively correlated with the connectivity between right CEN and left CEN (P = .032, uncorrected).
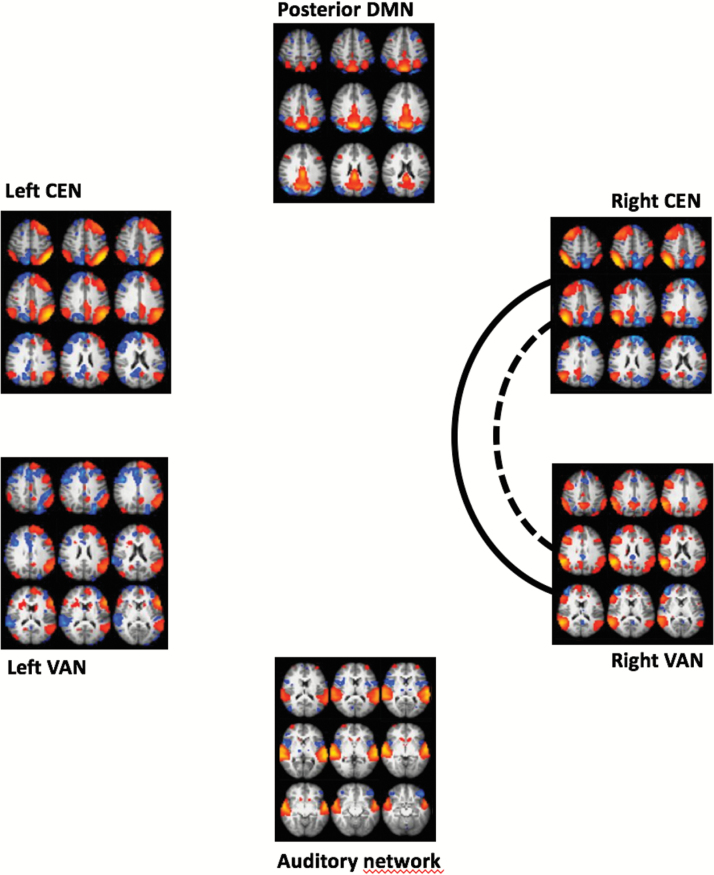
In a separate analysis, we tested for an interaction between the effects of group and cognitive measures on the between-network connectivity (figure 3). Significant interactions were revealed between the effects of group and sustained attention (RVP) on the connectivity between right CEN and right VAN (P = .006, corrected), left CEN and left VAN (P = .005, uncorrected), right VAN and posterior DMN (P = .038, uncorrected), and between posterior DMN and auditory network (P = .021, uncorrected). This analysis also revealed an interaction between the effects of group and processing speed (symbol coding) on the connectivity between right CEN and right VAN (P = .007, borderline significant at corrected level), left CEN and left VAN (P = .006, uncorrected), right CEN and auditory network (P = .045, uncorrected), and between posterior DMN and auditory network (P = .047, uncorrected). Furthermore, we found an interaction between the effects of group and general intelligence on the connectivity between right CEN and right VAN (P = .010, uncorrected), right CEN and auditory network (P = .014, uncorrected), left CEN and left VAN (P = .009, uncorrected), posterior DMN and auditory network (P = .021, uncorrected). In all these interactions, the correlation between network connectivity and cognitive measures was higher in patients compared to controls. The same analysis for an interaction between the effects of group and executive function (IED) on between network connectivity did not reveal any significant results.
Fig. 3.
Interaction between group and cognitive measures on pairwise correlations between networks of interest. The lines indicate a significant interaction between group and the specific cognitive measures on the pairwise correlation between networks, false discovery rate (FDR) corrected. Full line = sustained attention (RVP). Dotted line = processing speed (symbol coding).
Discussion
The present study of a large, well-characterized group of ANFE patients suggests that the early state of schizophrenia is associated with increased connectivity between fronto-parietal networks involved in the control of cognitive and sensory functions. Further, the results suggest that the problem of not disengaging the CEN and the VAN is associated with cognitive deficits.
Alterations in Functional Connectivity
Our finding of altered connectivity between the CEN’s and VAN’s and between posterior DMN and auditory network are in line with a few previous studies in ANFE (or partly antipsychotic-naïve) patients that have showed altered connectivity of fronto-parietal networks and DMN11,13,14,38 whereas one study found no alterations of DLPFC connectivity.12 On the other hand, these studies did not report alterations in connectivity between DMN/PCC and auditory network as revealed in the present study, although altered connectivity of PCC and auditory networks was found.13,14,38 A recent study in adolescents with auditory hallucinations also suggested altered connectivity between DMN areas and auditory cortex.39 In the present study, the SN did not show any network alterations as hypothesized by the network models. This is in line with one of the previous study in ANFE patients showing no alteration of dACC connectivity.12 Our finding of no voxel-wise alterations in any network of interest are partly in line with a previous study reporting no group differences.12 However, some previous studies have found voxel-wise alterations especially in PCC connectivity.11,40 Importantly, these inconsistencies between studies may be explained by the wide array of methods applied in functional connectivity studies.
The CEN and VAN together with the DAN and SN have been suggested to be part of an overall control-system involved broadly in the control of a range of cognitive and sensory functions.41–43 More specifically, the VAN anchored in the temporo-parietal junction (TPJ) has been suggested to be involved in the detection of behaviorally relevant stimuli and therefore act as a circuit-breaker for the DAN in order to direct attention in an adequate manner.37 Furthermore, the VAN has been shown to be involved in attentional control44,45 and in determining the intentions or mental states of other people.46,47 The CEN anchored in dorsolateral prefrontal and lateral parietal cortex has been associated with the ability to maintain and manipulate information, problem-solving in accordance with a rule and to make decisions in goal-directed behavior.5 PCC is a central part of the DMN involved in internally directed cognition,4,48 but it is also suggested to have a regulating function in terms of controlling attention49 including the shift between internal and external focus.
Interestingly, all network alterations were increased in patients compared to controls. Previous results suggest both increased and decreased connectivity in patients with schizophrenia.10,50,51 In general, it appears that reduced connectivity is the predominant finding throughout the literature regardless of methodology, disease progression and medication status.51 However, if specific networks are considered increased DMN connectivity is a predominant finding.10 Moreover, increased connectivity is a common finding in ANFE patients.14,52–57 In line with this, our results suggest a hyper-coupling between fronto-parietal control networks that make patients unable to disengage networks in a dynamic sense. If the VAN is locked in it may also explain why patients are unable to disengage the DMN during tasks.58–60
Another aspect of the present findings is that the CEN and VAN may be functionally unique to humans. One study showed that the functional connectivity of the TPJ is different in humans compared to macaques61 whereas another study suggested that certain fronto-parietal networks similar to the CEN may be found only in humans.62 If this is true, our results may point out the evolutionarily novel networks that make us vulnerable to develop the clinical characteristics seen in patients with schizophrenia.
Functional Connectivity and Clinical Characteristics
Our hypothesis of an association between positive symptoms and DMN connectivity was not verified. At the trend-level, our results may suggest that positive, negative, and general symptoms are associated with the connectivity of the left CEN and left VAN. This may indicate that a specific, left-lateralized hyper-coupling between CEN and VAN is involved broadly in psychopathology. As we expected from previous findings,15 the cognitive measures also appeared to be associated with network alterations in a generalized manner since the effects of processing speed and sustained attention showed a significant interaction with the effect of group on the connectivity between right CEN and right VAN. Because both significant interactions between network connectivity and cognitive measures revealed a higher correlation in patients compared to controls, it may indicate that connectivity between networks is more involved in cognition in patients than in healthy subjects.
The apparent unspecificity of these associations between clinical characteristics and network alterations could be a result of the presumably central role of the involved networks. If, as suggested by the present results, schizophrenia is characterized by a hyper-coupling between fronto-parietal networks involved in the control of other networks important for cognition and sensory functions, it is reasonable to expect diverse psychopathological and general cognitive deficits to arise from these control-network alterations.
It is important to note that the CEN, VAN, and DAN overlap substantially with brain regions involved in awareness. In healthy subjects the TPJ is activated by stimuli outside the current focus of attention if the stimuli are behaviorally important. On the contrary, the TPJ is deactivated when subjects are engaged in attentionally demanding tasks suggesting efficient filtering of distracting information.63 However, in a recent study investigating the distinction between attention and subjective awareness using a visual task showed that the changes in activity within the TPJ are driven by the degree of subjective awareness that co-varies with attention rather than by attention per se.64 The association between sustained attention and the connectivity between the CEN and VAN found in the present study could therefore in part reflect a problem with subjective awareness.
Methodological Considerations
An important aspect to consider is that the functions that can be ascribed to a network depend on the number of dimensions used to parcellate the resting state BOLD-signal, ie, a high number of dimensions will result in more specialized network functions rendering labels such as “the central executive network” heuristic at this stage. Moreover, differences in dimensionality estimation are likely to explain some of the inconsistencies in findings across studies, eg, within-network alterations might turn up as between-network alterations with a higher number of dimensions.
Two major concerns for studies of functional connectivity in schizophrenia are medication effects since antipsychotic medication is known to alter perfusion in grey matter,65 and motion that affects the estimates of connectivity.66–68 We have strived to deal with these effects by including only subjects who had never received antipsychotic medication and by implementing a strategy centered on single subject ICA de-noising that is currently among the most effective ways to deal with motion.69 Despite these efforts the group differences in connectivity presented here could be confounded by group differences in physiological parameters like heart rate and respiration pattern. These effects are likely to be under-sampled with a TR = 2 and represents general caveats of resting-state fMRI.
In Conclusion
As hypothesized in the large-scale network models, the present study revealed altered connectivity between CEN and VAN and between posterior DMN and auditory cortex in the early antipsychotic-naïve state of schizophrenia. As expected, these network alterations were shown to be associated with cognitive deficits in a generalized manner. Our results may indicate that psychopathology is broadly associated especially with left CEN connectivity. Because the CEN, VAN and posterior DMN, dominated by PCC/precuneus activity, have been suggested to be involved with the control of a range of cognitive and sensory functions, it is likely that a problem with disengaging these central control networks leads to the diverse clinical characteristics present in the early course of schizophrenia.
Supplementary Material
Supplementary material is available at https://academic.oup.com/schizophreniabulletin/.
Funding
This study was supported by grants from the Mental Health Services, Capital Region of Denmark and by grant R25-A2701 from the Lundbeck Foundation (Center for Clinical Intervention and Neuropsychiatric Schizophrenia Research). The funders had no influence on the design and conduct of the study, no influence on the collection, management, analysis and interpretation of the data, and no influence on the preparation, review or approval of the manuscript.
Acknowledgments
The Pan European Collaboration on Antipsychotic-Naïve First-Episode Schizophrenia (PECANS; ClinicalTrials.gov identifier: NCT01154829) cohort is a large multimodal study on first-episode patients with schizophrenia who initially had never received any antipsychotic medication. This is the first manuscript on the resting state fMRI data from the PECANS cohort although these data have been presented at conferences in a number of abstracts and posters. Data from the PECANS cohort on other modalities have been published previously in a number of papers (please see references16–22 in the reference list). The authors have no conflicts of interest.
References
- 1. Buckner RL, Krienen FM, Yeo BT. Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci. 2013;16:832–837. [DOI] [PubMed] [Google Scholar]
- 2. Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. [DOI] [PubMed] [Google Scholar]
- 3. Williamson P. Are anticorrelated networks in the brain relevant to schizophrenia?Schizophr Bull. 2007;33:994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. [DOI] [PubMed] [Google Scholar]
- 5. Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. [DOI] [PubMed] [Google Scholar]
- 6. Palaniyappan L, Liddle PF. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. J Psychiatry Neurosci. 2012;37:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Palaniyappan L, White TP, Liddle PF. The concept of salience network dysfunction in schizophrenia: from neuroimaging observations to therapeutic opportunities. Curr Top Med Chem. 2012;12:2324–2338. [DOI] [PubMed] [Google Scholar]
- 8. Northoff G, Qin P. How can the brain’s resting state activity generate hallucinations? A ‘resting state hypothesis’ of auditory verbal hallucinations. Schizophr Res. 2011;127:202–214. [DOI] [PubMed] [Google Scholar]
- 9. Williamson PC, Allman JM. A framework for interpreting functional networks in schizophrenia. Front Hum Neurosci. 2012;6:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karbasforoushan H, Woodward ND. Resting-state networks in schizophrenia. Curr Top Med Chem. 2012;12:2404–2414. [DOI] [PubMed] [Google Scholar]
- 11. He Z, Deng W, Li M et al. . Aberrant intrinsic brain activity and cognitive deficit in first-episode treatment-naive patients with schizophrenia. Psychol Med. 2013;43:769–780. [DOI] [PubMed] [Google Scholar]
- 12. Lui S, Deng W, Huang X et al. . Association of cerebral deficits with clinical symptoms in antipsychotic-naive first-episode schizophrenia: an optimized voxel-based morphometry and resting state functional connectivity study. Am J Psychiatry. 2009;166:196–205. [DOI] [PubMed] [Google Scholar]
- 13. Li M, Deng W, He Z et al. . A splitting brain: Imbalanced neural networks in schizophrenia. Psychiatry Res. 2015;232:145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lui S, Li T, Deng W et al. . Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch Gen Psychiatry. 2010;67:783–792. [DOI] [PubMed] [Google Scholar]
- 15. Sheffield JM, Barch DM. Cognition and resting-state functional connectivity in schizophrenia. Neurosci Biobehav Rev. 2016;61:108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Düring S, Glenthøj BY, Andersen GS, Oranje B. Effects of dopamine D2/D3 blockade on human sensory and sensorimotor gating in initially antipsychotic-naive, first-episode schizophrenia patients. Neuropsychopharmacology. 2014;39:3000–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ebdrup BH, Raghava JM, Nielsen MO, Rostrup E, Glenthoj B. Frontal fasciculi and psychotic symptoms in antipsychotic-naive patients with schizophrenia before and after 6 weeks of selective dopamine D2/3 receptor blockade. J Psychiatry Neurosci. 2015;41:150030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nielsen MO, Rostrup E, Wulff S et al. . Improvement of brain reward abnormalities by antipsychotic monotherapy in schizophrenia. Arch Gen Psychiatry. 2012;69: 1195–1204. [DOI] [PubMed] [Google Scholar]
- 19. Nielsen MØ, Rostrup E, Wulff S et al. . Alterations of the brain reward system in antipsychotic naïve schizophrenia patients. Biol Psychiatry. 2012;71:898–905. [DOI] [PubMed] [Google Scholar]
- 20. Nielsen MO, Rostrup E, Wulff S, Glenthoj B, Ebdrup BH. Striatal reward activity and antipsychotic-associated weight change in patients with schizophrenia undergoing initial treatment. JAMA Psychiatry. 2016;73:121–128. [DOI] [PubMed] [Google Scholar]
- 21. Nordholm D, Poulsen HE, Hjorthøj C et al. . Systemic oxidative DNA and RNA damage are not increased during early phases of psychosis: A case control study. Psychiatry Res. 2016;241:201–206. [DOI] [PubMed] [Google Scholar]
- 22. Wulff S, Pinborg LH, Svarer C et al. . Striatal D(2/3) binding potential values in drug-naïve first-episode schizophrenia patients correlate with treatment outcome. Schizophr Bull. 2015;41:1143–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wing JK, Babor T, Brugha T et al. . SCAN. Schedules for Clinical Assessment in Neuropsychiatry. Arch Gen Psychiatry. 1990;47:589–593. [DOI] [PubMed] [Google Scholar]
- 24. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 25. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. [DOI] [PubMed] [Google Scholar]
- 26. Wechsler D. WAIS-III Administration and Scoring Manual. San Antonio TX: Psychol Corp; 1997. [Google Scholar]
- 27. Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68:283–297. [DOI] [PubMed] [Google Scholar]
- 28. Sahakian BJ, Owen AM. Computerized assessment in neuropsychiatry using CANTAB: discussion paper. J R Soc Med. 1992;85:399–402. [PMC free article] [PubMed] [Google Scholar]
- 29. Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. [DOI] [PubMed] [Google Scholar]
- 30. Paul Mazaika FH, Glover GH, Reiss AL. Methods and Software for fMRI analysis for clinical subjects. Neuroimage. 2009;47:S58. [Google Scholar]
- 31. Andersson JL, Jenkinson M, Smith S.. Non-linear optimisation FMRIB Technial Report TR07JA1. 2007. https://www.fmrib.ox.ac.uk/datasets/techrep/. Accessed December 1, 2017. [Google Scholar]
- 32. Andersson JL, Jenkinson M, Smith S.. Non-linear registration, aka Spatial normalisation FMRIB Technial Report TR07JA2. 2007. https://www.fmrib.ox.ac.uk/datasets/techrep/. Accessed December 1, 2017. [Google Scholar]
- 33. Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23:137–152. [DOI] [PubMed] [Google Scholar]
- 34. Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Filippini N, MacIntosh BJ, Hough MG et al. . Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A. 2009;106:7209–7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- 37. Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. [DOI] [PubMed] [Google Scholar]
- 38. Pu W, Rolls ET, Guo S et al. . Altered functional connectivity links in neuroleptic-naïve and neuroleptic-treated patients with schizophrenia, and their relation to symptoms including volition. Neuroimage Clin. 2014;6:463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Amico F, O’Hanlon E, Kraft D et al. . Functional connectivity anomalies in adolescents with psychotic symptoms. PLoS One. 2017;12:e0169364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Anticevic A, Hu X, Xiao Y et al. . Early-course unmedicated schizophrenia patients exhibit elevated prefrontal connectivity associated with longitudinal change. J Neurosci. 2015;35:267–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cole MW, Repovš G, Anticevic A. The frontoparietal control system: a central role in mental health. Neuroscientist. 2014;20:652–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bartolomeo P. Attention disorders after right brain damage: living in halved worlds. London, New York: Springer; 2014. [Google Scholar]
- 45. Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Decety J, Lamm C. The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. Neuroscientist. 2007;13:580–593. [DOI] [PubMed] [Google Scholar]
- 47. Krall SC, Rottschy C, Oberwelland E et al. . The role of the right temporoparietal junction in attention and social interaction as revealed by ALE meta-analysis. Brain Struct Funct. 2015;220:587–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fornito A, Bullmore ET. Reconciling abnormalities of brain network structure and function in schizophrenia. Curr Opin Neurobiol. 2015;30:44–50. [DOI] [PubMed] [Google Scholar]
- 51. Pettersson-Yeo W, Allen P, Benetti S, McGuire P, Mechelli A. Dysconnectivity in schizophrenia: where are we now?Neurosci Biobehav Rev. 2011;35:1110–1124. [DOI] [PubMed] [Google Scholar]
- 52. Guo W, Liu F, Chen J et al. . Using short-range and long-range functional connectivity to identify schizophrenia with a family-based case-control design. Psychiatry Res. 2017;264:60–67. [DOI] [PubMed] [Google Scholar]
- 53. Guo W, Liu F, Chen J et al. . Hyperactivity of the default-mode network in first-episode, drug-naive schizophrenia at rest revealed by family-based case-control and traditional case-control designs. Medicine (Baltimore). 2017;96:e6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li F, Lui S, Yao L et al. . Longitudinal changes in resting-state cerebral activity in patients with first-episode schizophrenia: a 1-year follow-up functional MR Imaging Study. Radiology. 2016;279:867–875. [DOI] [PubMed] [Google Scholar]
- 55. Li T, Wang Q, Zhang J et al. . Brain-wide analysis of functional connectivity in first-episode and chronic stages of schizophrenia. Schizophr Bull. 2017;43:436–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Martino M, Magioncalda P, Yu H et al. . Abnormal resting-state connectivity in a substantia nigra-related striato-thalamo-cortical network in a large sample of first-episode drug-naive patients with schizophrenia. Schizophr Bull. 2018;44:419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang S, Zhan Y, Zhang Y et al. . Abnormal long- and short-range functional connectivity in adolescent-onset schizophrenia patients: a resting-state fMRI study. Prog Neuropsychopharmacol Biol Psychiatry. 2018;81:445–451. [DOI] [PubMed] [Google Scholar]
- 58. Hasenkamp W, James GA, Boshoven W, Duncan E. Altered engagement of attention and default networks during target detection in schizophrenia. Schizophr Res. 2011;125:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schneider FC, Royer A, Grosselin A et al. . Modulation of the default mode network is task-dependant in chronic schizophrenia patients. Schizophr Res. 2011;125:110–117. [DOI] [PubMed] [Google Scholar]
- 60. Whitfield-Gabrieli S, Thermenos HW, Milanovic S et al. . Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106:1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Patel GH, Yang D, Jamerson EC, Snyder LH, Corbetta M, Ferrera VP. Functional evolution of new and expanded attention networks in humans. Proc Natl Acad Sci U S A. 2015;112:9454–9459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mantini D, Corbetta M, Romani GL, Orban GA, Vanduffel W. Evolutionarily novel functional networks in the human brain?J Neurosci. 2013;33:3259–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shulman GL, Astafiev SV, McAvoy MP, d’Avossa G, Corbetta M. Right TPJ deactivation during visual search: functional significance and support for a filter hypothesis. Cereb Cortex. 2007;17:2625–2633. [DOI] [PubMed] [Google Scholar]
- 64. Webb TW, Igelström KM, Schurger A, Graziano MS. Cortical networks involved in visual awareness independent of visual attention. Proc Natl Acad Sci U S A. 2016;113:13923–13928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Viviani R, Graf H, Wiegers M, Abler B. Effects of amisulpride on human resting cerebral perfusion. Psychopharmacology (Berl). 2013;229:95–103. [DOI] [PubMed] [Google Scholar]
- 66. Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Satterthwaite TD, Wolf DH, Loughead J et al. . Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60:623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pruim RH, Mennes M, Buitelaar JK, Beckmann CF. Evaluation of ICA-AROMA and alternative strategies for motion artifact removal in resting state fMRI. Neuroimage. 2015;112:278–287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.