Abstract
Objective
Diet is increasingly recognized as a potentially modifiable factor influencing the onset and outcomes of psychiatric disorders. Whereas, previous research has shown long-term schizophrenia is associated with various nutritional deficiencies, this meta-analysis aimed to determine the prevalence and extent of nutritional deficits in first-episode psychosis (FEP).
Method
A search of electronic databases conducted in July 2017 identified 28 eligible studies, examining blood levels of 6 vitamins and 10 minerals across 2612 individuals: 1221 individuals with FEP and 1391 control subjects. Meta-analyses compared nutrient levels in FEP to nonpsychiatric controls. Clinical correlates of nutritional status in patient samples were systematically reviewed.
Results
Significantly lower blood levels of folate (N = 6, n = 827, g = −0.624, 95% confidence interval [CI] = −1.176 to −0.072, P = .027) and vitamin D (N = 7, n = 906, g = −1.055, 95% CI = −1.99 to −0.119, P = .027) were found in FEP compared to healthy controls. Synthesis of clinical correlates found both folate and vitamin D held significant inverse relationships with psychiatric symptoms in FEP. There was also limited evidence for serum level reductions of vitamin C (N = 2, n = 96, g = −2.207, 95% CI = −3.71 to −0.71, P = .004). No differences were found for other vitamins or minerals.
Conclusions
Deficits in vitamin D and folate previously observed in long-term schizophrenia appear to exist from illness onset, and are associated with worse symptomology. Further research must examine the direction and nature of these relationships (ie, mediator, moderator, or marker) with clinical status in FEP. Future trials assessing efficacy of nutrient supplementation in FEP samples should consider targeting and stratifying for baseline deficiency.
Keywords: folic acid, cholecalciferol, nutrients, dietary, trace elements, nutrition, antioxidants
Introduction
Nutritional deficiencies, resulting from insufficient intake or absorption of nutrients critical to human health, are now a recognized risk factor for psychiatric disorders.1,2 For instance, excessive intake of nutritionally devoid foods is predictive of poor mental health,3 whereas a healthy diet reduces risk.4 Although previous research has focused on common mental disorders,1,5 recent attention has been drawn to the food intake of people with schizophrenia—who may have the worst diet, poorest metabolic health, and greatest premature mortality across all severe mental illnesses.6–8
Blood levels of certain nutrients are also significantly reduced in psychiatric disorders. Folate (B9) and B12 are often deficient in schizophrenia,9,10 and be associated with symptom severity.11 Furthermore, B-vitamin supplementation can significantly reduce symptoms of schizophrenia12 and reverse some neurological deficits associated with the disorder.13 This is perhaps due to the neuroprotective properties of these nutrients,14 or the ability of B vitamins to lower homocysteine levels, which adversely affect mental health.14,15 Antioxidant vitamins C and E are also reduced in long-term schizophrenia, potentially contributing to the elevated oxidative stress observed in this population.16
Additionally, vitamin D is implicated in schizophrenia onset, with a body of research showing developmental deficiencies in vitamin D3 increase later risk.17–20 Furthermore, vitamin D deficiencies persist over long-term illness and may be associated with worsened physical and mental health outcomes.21,22 Studies have also indicated that certain dietary minerals, such as zinc and selenium, are lowered in people with schizophrenia;23–25 as has been observed in other conditions such as depression.26,27 The direction of these nutrient/pathology associations however need to be clarified by prospective research.
Previous meta-analyses examining individual nutrient levels in individuals with long-term schizophrenia have shown clear deficits in B vitamins (folate28 and B1229), antioxidant vitamins (C and E),16 and vitamin D.30 However, which nutritional deficiencies are present at the first episode of psychosis (FEP), independent of antipsychotic treatment, has yet to be determined. This is a particularly pertinent issue, given that diet quality appears reduced from psychosis onset,31,32 and inflammation and oxidative stress are highest at this point of illness16,33; both of which may be also linked to poor nutrition. Additionally, the FEP phase has been identified as a “critical period” as firstly it is the stage where the process of neuroprogression appears most active,34 and secondly for reducing physical health inequalities,35 as the initiation of antipsychotic treatment is associated with rapid weight gain and metabolic dysfunction.36 Therefore, determining which nutritional deficits are present from the onset of antipsychotic treatment, and identifying which nutritional deficiencies are associated with physical and mental health outcomes in FEP, will identify key targets for dietary/nutrient interventions to improve nutritional status and attenuate neuroprogression and metabolic risk. Indeed, randomized controlled trials (RCTs) in long-term schizophrenia have already indicated that vitamin supplementation has greatest efficacy among individuals with shorter illness duration.12
In the current study, we used meta-analytic techniques to quantify the presence and severity of nutritional deficits in FEP, across every class of vitamin and/or dietary mineral examined in this population to date. Along with determining where deficits may exist, we also sought to systematically review the clinical correlates of nutritional status, to identify which vitamins and minerals are related to physical and mental health outcomes in FEP.
Methods
This meta-analysis followed the PRISMA statement37 to ensure comprehensive and transparent reporting (supplement 1).
Search Strategy
An electronic database search Cochrane Central Register of Controlled Trials, Health Technology Assessment Database, AMED, HMIC, MEDLINE, PsycINFO, and EMBASE was conducted on July 7, 2017. A keyword search algorithm (supplement 2) was developed to identify all studies assessing blood levels of any vitamins and/or minerals in FEP. The reference lists of retrieved articles and Google Scholar were also searched to identify any additional relevant articles.
Screening and Selection Process
Only peer-reviewed research articles available in English were included. Eligible samples were those in which >75% of individuals were specified as “first-episode psychosis.” Studies in which the term “first-episode psychosis” was not explicitly used were only eligible if the entire patient sample was specified as either (1) currently receiving treatment from “early intervention in psychosis” services, or (2) within the first 3 years of receiving antipsychotic treatment for schizophrenia or other nonaffective psychotic disorders (as this period is broadly accepted and used as an appropriate timeframe for “early intervention” programs for psychosis).38–40 This criterion was applied to capture all relevant studies of patients in relatively early stages of illness and antipsychotic treatment, while excluding samples likely representative of those with more established illness. Eligible studies were those which either: (1) compared blood levels (including whole blood, plasma, serum, erythrocyte, leukocyte, or other blood components) of any vitamin and/or dietary mineral (as defined by the British Nutrition Foundation, 201641) in FEP to a non-FEP control sample, or (2) reported on clinical correlates (ie, any metabolic, psychiatric, or neurocognitive parameters assessed using a clinically validated measure) of vitamin/mineral levels in FEP samples. For inclusion in the systematic review, no restriction was placed on the nature of control sample used. However, meta-analyses used only the data from studies which compared FEP to “healthy” control samples (ie, individuals with no psychiatric diagnosis).
Data Extraction
Articles were screened for eligibility by 2 independent reviewers (J.F. and R.C.). Disagreements were resolved through discussion until consensus was reached. Where further information or study data were required, the corresponding authors of the respective articles were contacted twice over the period of 1 month to request this. This was attempted for one paper but data were not retrieved.42 A systematic tool was used to extract the following data from each study:
Study characteristics: study design, country, sample size (n).
FEP sample: age, % male, inpatient/outpatient status, duration of untreated psychosis, medication type, and duration of antipsychotic treatment.
Control sample: age, % male, clinical/healthy population, any sociodemographic characteristics matched to FEP sample for.
Study findings: blood levels of vitamin/minerals in FEP and controls, outcomes of all reported statistical comparisons between groups, relationships with any physical/psychiatric/neurocognitive assessment measures.
Statistical Analyses
Meta-analyses were performed in Comprehensive Meta-Analysis 2.0.43 A random effects model was applied to all analyses to account for methodological heterogeneity between eligible studies,44 in terms of differences in both blood sampling procedures and assay measures used across studies. Individual analyses were performed for each vitamin and mineral examined in FEP patients, using pooled comparisons with blood levels observed in nonpsychiatric control samples. Where studies used multiple measures of an individual vitamin or mineral, the more complex measure which best reflects bioavailable levels or long-standing nutrient was preferentially used. The overall difference between FEP and control groups for each vitamin/mineral was computed as Hedge’s G from the raw (ie, unadjusted) mean levels reported in each study.
Variance between studies was assessed using Cochran’s Q and I2 values, both of which estimate the degree of variance resulting from between-study heterogeneity, rather than chance. For all statistically significant findings, Egger’s regression test was applied to quantify the risk of publication bias. Additionally, a “Fail-Safe N”45 was calculated to determine the number of unpublished null studies which would invalidate the findings, and Duval and Tweedie’s trim-and-fill analysis was applied. We also performed post hoc sensitivity analyses to assess if comparable differences were still observed when excluding outlier studies with very high effect sizes.
Finally, for all individual study findings which could not be combined in meta-analyses, we produced a systematic narrative synthesis reporting on: (1) differences between FEP samples and other “non healthy” (ie, clinical/psychiatric) populations, and (2) all clinical correlates of vitamin/mineral levels in FEP.
Results
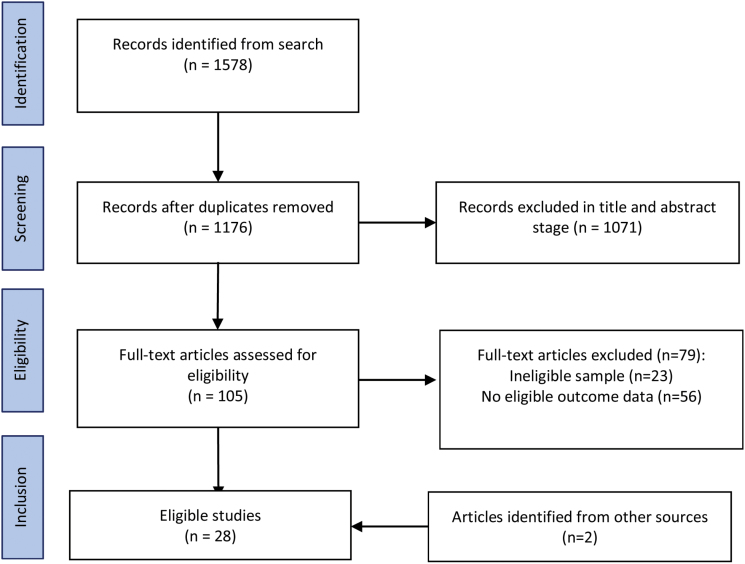
The initial database search was performed on July 7, 2017. The search returned 1578 results, reduced to 1176 after duplicates were removed. A further 1071 articles were excluded after and abstract screening. Full text versions were retrieved for 105 articles, of which were 26 eligible for inclusion. A further 2 eligible articles were identified from an additional search of Google Scholar. Full details of the search results and reasons for exclusion and are summarized in figure 1.
Fig. 1.
PRISMA search diagram.
A total of 28 articles, reporting data from 24 unique samples of 2612 participants (1221 FEP, 1391 controls) were included. These assessed differences in blood levels of 6 vitamins (A, B12, C, D, E, and folate) and 10 minerals (zinc, magnesium, sodium, potassium, calcium, copper, chromium, iron, manganese, and selenium). Four studies were conducted in India, 3 in Pakistan and China, 2 in United States of America, United Kingdom, Turkey, Spain, and Nigeria, and individual studies in Poland, Romania, Norway, and Singapore. Full details and findings of all included studies are shown in table 1a–d.
Table 1.
Study Details
| Study (Country) | Population Studied + Treatment Information | FEP Sample Age (Yrs) % Male | Ctrl Sample Age (Yrs) % Male | DUP (Yrs) | Study Design and Comparator | Vitamin/Minerals Measured | Differences Found | Clinical Correlates |
|---|---|---|---|---|---|---|---|---|
| a. B vitamin studies | ||||||||
| Ayesa-Arriola et al.90 (Spain) | In + outpatients | N = 139 | N = 99 | 1.13 | Cross-sectional with healthy controls | Vitamin B12 (serum) | Serum folate and vitamin B12 did not differ between groups | None examined |
| Medication-naïve | 32.9 yrs | 27.0 yrs | Folate (serum) | |||||
| FEP classification | 54% male | 59% male | ||||||
| Ipcioglu et al46 (Turkey) | Inpatients | N = 14 | N = 34 | N = 30 | Cross-sectional with healthy and psychiatric controls | Vitamin B12 (serum) | Serum folate and vitamin B12 did not differ between groups | None examined |
| Medication n/s | 21.8 yrs | 30.6 yrs | 33 yrs | Folate (serum) | ||||
| FEP classification | 50% male | 50% male [HC] | 53% male (Depression) | |||||
| Kale et al.52 (India) | Outpatients | N = 31 | N = 48 | 0.52 | Cross-sectional with healthy controls | Vitamin B12 (plasma) | Significantly lower plasma folate (P = .02) and RBC (P = .01), and lower B12 plasma (P = .09) | Vitamin B12 showed trend level negative correlation with positive symptoms on PANSS, but no significant correlations for plasma folate and PANSS scores |
| Medication-naïve | 33 yrs | 34 yrs | Folate (plasma + RBC) | |||||
| FEP classification | 56% male | 55% male | ||||||
| Misiak et al.51 (Poland) | 67% SGA | N = 135 | N = 146 | 1.16 yrs | Cross-sectional compared with healthy controls | Vitamin B12 (serum) | Significantly lower serum folate in FEP group (P < .001) but no difference in B12 | Inverse correlation between PANSS negative scores and serum B12, but no relationship found for folate or other clinical correlates |
| 18% Medication-naïve | 27.2 yrs56% male | 27.6 yrs46% male | Folate (serum) | |||||
| 15% FGA | ||||||||
| FEP classification | ||||||||
| Misiak et al.50 (Poland) | Subsample of above | N = 56 | N = 53 | Not specified | Cross-sectional compared with healthy controls | Vitamin B12 (plasma) | Significantly lower plasma folate in FEP group (P < .001) but no difference in B12 | Plasma B12 correlated inversely with severity of negative symptoms. Higher folate levels were associated with lower general symptom scores. |
| 27.2 yrs | 25.7 yrs | Folate (plasma) | ||||||
| 56% male | 49% male | |||||||
| Misiak et al.54 (Poland) | Subsample of above | N = 39 | N/A | Not specified | 12 week observational study of SGAs | Vitamin B12 (serum) | N/A | 12 weeks of treatment with olanzapine, but not risperidone, was associated with significant reductions in folate and B12 |
| 26.0 yrs | Folate (serum) | |||||||
| 60% male | ||||||||
| Misiak et al.91 (Poland) | Subsample of above | N = 83 | N/A | Not specified | Cross-sectional | Vitamin B12 (serum)Folate (serum) | N/A | Folate or B12 plasma were not associated with childhood trauma |
| 25.1 yrs (m) | ||||||||
| 28.8 yrs (f) | ||||||||
| 57% male | ||||||||
| Song et al.48 (China) | Inpatient | N = 46 | N = 30 | 0.65 yrs | Cross-sectional compared with healthy controls | Folate (serum) | Serum folate did not differ between groups | Inverse relationship between serum levels of folate and PANSS total and PANSS negative symptom scores |
| Medication-naïve | 22.5 yrs | 24.3 yrs | ||||||
| FEP classification | 61% male | 57% male | ||||||
| Xuimei et al.49 (China) | Inpatient | N = 60 | N = 60 | Not specified | Cross-sectional compared with healthy controls | Folate (serum) | Serum folate significantly lower in the FEP group (P < .01). | Negative correlation between serum levels of folate and negative symptoms (PANSS) but not positive symptoms, general psychopathology or cognitive function scores |
| Medication-naïve | 22 yrs | 23 yrs | ||||||
| FEP classification | 57% male | 53% male | ||||||
| b. Vitamin D studies | ||||||||
| Crews et al.92 (UK) | Inpatient | N = 69 | N = 69 | Cross-sectional with healthy controls | Vitamin D (serum) | Serum level vitamin D significantly lower in FEP group than controls (P < .001) and higher level of deficiencies (OR = 2.99, P = .008) | No correlation between vitamin D and time as an inpatient. No difference according to antipsychotic or anticonvulsant use but vitamin D levels higher in SSRI users than nonusers (P = .04). | |
| 86% antipsychotic (not specified) | 31 yrs39% male | 31 yrs39% male | ||||||
| 25% SSRIs | ||||||||
| 6% anticonvulsant FEP | ||||||||
| Graham et al.57 (USA) | Outpatient | N = 20 | N = 20 | <5 yrs | Cross-sectional with healthy controls | Vitamin D (serum) | No significant difference in vitamin D serum levels | More severe negative and total symptoms (PANSS) associated with lower vitamin D levels (P = .03). Trend level association found for positive symptoms. No relationship with depressive symptoms (CDRS). Lower vitamin D associated with more severe cognitive deficits including verbal fluency scores in patient group. |
| Medicated <16 weeks FEP | 23 yrs60% male | 25 yrs60% male | ||||||
| Malik et al.54 (Pakistan) | Inpatients | N = 100 | N = 100 | Not specified | Cross-sectional with general population | Vitamin D (serum) | Vitamin D levels significantly lower in people with newly diagnosed schizophrenia compared with controls | None reported |
| Unmedicated “newly diagnosed” | Age and gender not reported | Age and gender not reported | ||||||
| Nerhus et al.55 | In + outpatient | N = 71 | N = 142 | 2.8 yrs | Cross-sectional with healthy controls and established SZ | Vitamin D (serum) | No significant differences in vitamin D with healthy controls | Lower levels of vitamin D related to higher levels of depressive symptoms in FEP. No significant relationship between vitamin D and positive or negative symptoms (PANSS). |
| 83% medicated | 27 yrs | 28 yrs | ||||||
| 77% AP; 3% lithium; 6% antiepileptic; 24% AD; 6% hypnotic | 65% male | 65% male[HC] | ||||||
| FEP classification | ||||||||
| N = 71 | No significant differences in vitamin D of people experiencing a FEP and those with established SZ | |||||||
| 28 yrs | ||||||||
| 65% male | ||||||||
| [Established SZ] | ||||||||
| Salavert et al.56 (Spain) | Medication-naïve FEP | N = 45 | N = 22 | Not specified | Cross-sectional with healthy controls | Vitamin D (serum) | Significantly reduced vitamin D levels in FEP sample compared to control condition | Patients who later received diagnosis of schizophrenia had trend-level lower vitamin D than those who were diagnosed with other psychoses (P = .06) |
| 33.7 yrs | 36.1 yrs | |||||||
| 60% male | 27.3% male | |||||||
| Yee et al.58 (Singapore) | <4 weeks AP FEP classification | N = 3129 yrs48% male | N = 3129 yrs45% male | 1.8 yrs | Cross-sectional with healthy controls | Vitamin D (serum and bioavailable levels) | No significant difference in serum levels of vitamin D between groups, but controls had significantly higher levels of serum bioavailable vitamin D (P = .05) | Significant negative association between vitamin D and negative symptoms (PANSS) even after controlling for gender, ethnicity, and DUP |
| Calcium (serum) | ||||||||
| Zhu et al.93 (China) | Outpatients | N = 93 | N = 93 | <5 yrs | Cross-sectional with family members | Vitamin D (plasma) | Plasma vitamin D significantly lower in FEP than healthy family members (P < .001). Mean vitamin D levels 40% lower in patients. People in lower quartiles of vitamin D levels had significantly increased proportions of SZ. | None reported |
| <16 weeks AP | 30 yrs | 43 yrs | ||||||
| FEP classification | 41% male | 52% male | ||||||
| c. Antioxidant vitamin studies | ||||||||
| Dadheech et al.59 (India) | <40 years old“Newly diagnosed” | N = 3072% male | N = 40 | Not specified | Cross-sectional with healthy controls and older SZ | Vitamin E (plasma) | Patient sample had significantly lower vitamin E and C. Older patients had lower vitamin E than younger patients. | Not reported |
| 73% male [HC] | Vitamin C (plasma + leukocyte) | |||||||
| N = 28 | ||||||||
| >40 years old | ||||||||
| 72% male [older SZ] | ||||||||
| Dakhale et al.62 (India) | Inpatients | N = 40 | Not specified | RCT of vitamin C | Vitamin C (plasma) | N/A | Negative symptoms (BPRS) were significantly reduced after 8 weeks of treatment with 500 mg/day of vitamin C compared with placebo (P < .01). Significant and negative correlation was found between plasma ascorbic acid levels and BPRS score (r = −0.38, P < .05). | |
| Unmedicated | 38.5 yrs | |||||||
| Sarandol et al.94 (Turkey) | Medication naïve | N = 26 | N = 25 | Not specified | Longitudinal single arm trial + cross-sectional with healthy controls | Vitamin E (plasma) | No significant difference in plasma levels of vitamin E | None reported |
| FEP classification | 26 yrs | 24 yrs | ||||||
| 39% male | 40% male | |||||||
| Scottish Schizophrenia Research Group61 (UK) | Inpatients | N = 30 | N = 30 | Not specified | Cross-sectional with general population | Vitamin E (serum) | Serum levels of vitamin E significantly lower in FEP group, but no difference for vitamin A. 77% of FEP and 70% controls had ratio of vitamin E to cholesterol <5 (level necessary to protect against heart disease). | Vitamin levels not related to psychiatric symptoms (PANSS, CGI). |
| Medication-naïveFEP classification | 28 yrs (m); 33 yrs (f) | 30 yrs70% male | Vitamin A (serum) | |||||
| 70% male | ||||||||
| Surapaneni60 (India) | “Newly diagnosed” | N = 48 | N = 48 | Not specified | Cross-sectional with healthy controls | Vitamin E (plasma) | Significantly lower vitamin E and vitamin C in patient sample | None reported |
| 63% male | 63% male | Vitamin C (plasma) | ||||||
| d. Dietary mineral studies | ||||||||
| Akinladel et al.63 (Nigeria) | <3 years illness | N = 19 | N = 30 [HC] | Cross-sectional compared with healthy controls and long term SZ | Sodium, potassium (serum) | Significantly lower potassium and sodium in FEP than controls. No difference between FEP and long- term SZ. | None reported | |
| Medication naïveFEP classification | N = 41 [Established SZ] | |||||||
| Arinola and Idonije 95 (Nigeria) + Arinola et al.65 (Nigeria) | InpatientMedication-naive | N = 1524 yrs73% male | N = 20 | 0.15 yrs | Cross-sectional compared with healthy controls and medicated SZ | Zinc, iron, manganese, chromium, selenium, magnesium, copper (all plasma) | Iron, selenium, and chromium were all significantly higher in unmedicated FEP patients compared with healthy controls (P < .01). No significant difference was found for other minerals. | Zinc was significantly lower and manganese and chromium were significantly higher in unmedicated than medicated SZ |
| 28 yrs60% male[HC] | ||||||||
| N = 20 | ||||||||
| 27 yrs | ||||||||
| 65% male [Established SZ] | ||||||||
| Gunduz-Bruce et al.96 (USA) | <6 months medication | N = 16 25.7 yrs75% maleN = 75Age and gender not reported | N = 28 28.3 yrs36% maleN = 25Age and gender not reported | Not specified | Cross-sectional compared with healthy controls | Sodium (plasma) | Plasma sodium levels significantly higher in patient group (P = .05) | None reported |
| FEP classification | ||||||||
| Jamil et al.98 (Pakistan) | Inpatient | Not specified | Cross-sectional compared with healthy controls. | Calcium (serum) | Higher levels of serum calcium (P = .04) and sodium (P = .01), and lower levels of potassium (P < .01) and magnesium (P = .06) were observed in newly diagnosed patients compared with controls | None reported | ||
| Unmedicated“Newly diagnosed” | Sodium (serum)Potassium (serum)Magnesium (serum) | |||||||
| Nawaz et al.97 (Pakistan) | Outpatients | N = 12 | N = 19 | Not specified | Cross-sectional compared with siblings and established SZ | Chromium, zinc, copper, magnesium, iron, manganese, selenium (all plasma) | No significant difference in any trace metals in the newly diagnosed group compared with their siblings | None reported |
| Unmedicated“Newly diagnosed” | Age and gender not reported | Age and gender not reported[Sibling] | ||||||
| N = 22 | No significant difference between newly diagnosed and established SZ | |||||||
| Age and gender not reported [Established SZ] | ||||||||
| Nechifor et al.66 (Romania) | Inpatient | N = 56 | N = 20 | Not specified | Longitudinal; 3 week trial of haloperidol v risperidone compared with controls | Magnesium (plasma + erythrocyte) calcium, zinc, copper | Significantly lower erythrocyte magnesium and zinc levels in FEP group (P < .01) but no difference in plasma magnesium, calcium or copper | Both haloperidol and risperidone treatments were associated with increased levels of erythrocyte |
| 71% SGA29% FGA“Newly treated” | 38 yrs (median)43% male | Not reported | ||||||
| Magnesium and plasma zinc after 3 weeks | ||||||||
Note: AP, antipsychotic; DEP, depression; DUP, duration untreated psychosis; FEP, first-episode psychosis; FGA, first generation antipsychotics; N/A, not applicable; PANSS, Positive and Negative Syndrome Scale; RBC, red blood count; RCT, randomized controlled trial; SANS, Scale for the assessment of negative symptoms; SAPS, Scale for the assessment of positive symptoms; SGA, second generation antipsychotics; SSRI, selective serotonin reuptake inhibitors; SZ, schizophrenia; yrs, years.
B Vitamins in FEP
Nine studies, with 6 independent samples, reported on B vitamin levels across 872 participants: 425 with FEP, all of whom were medication-naïve (except for one study where this was no specified; table 1), and 447 controls. Two B vitamins were examined: folate (B9) and cobalamin (B12).
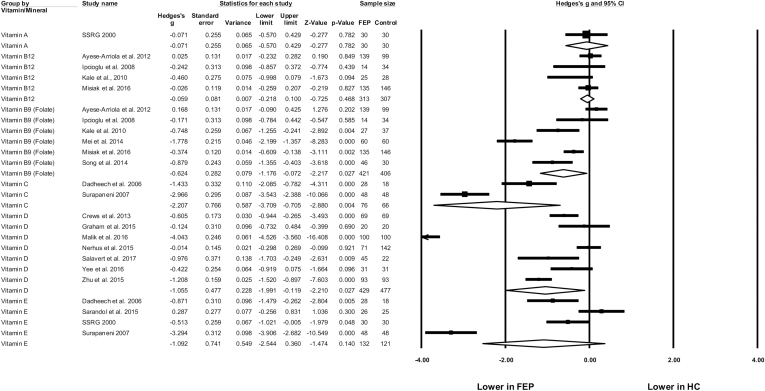
Random effects meta-analyses found significantly lower blood levels of folate in FEP compared with healthy controls (figure 2), with a moderately large effect size (N = 6, n = 827, g = −0.624, 95% CI = −1.176 to −0.072, P = .027). There was significant heterogeneity (Q = 66.0, P < .01, I2 = 92.4%), and a range of blood measures applied across studies, showing significant differences between FEP and controls in plasma, serum, and red blood cell levels (table 1). There was no evidence of publication bias (Eggers regression, P = .16) and the trim-and-fill analysis did not identify any outlier studies. Furthermore, the fail-safe N was 71, indicating 71 additional null studies would be required to make the observed difference nonsignificant.
Fig. 2.
Meta-analysis of blood levels of vitamins in first-episode psychosis (FEP) and healthy controls (HC). Box size represents study weighting. Diamond represents overall effect size and 95% confidence intervals.
Four studies (n = 620) examined blood levels of vitamin B12, finding no significant difference between FEP and healthy controls (g = −0.059, 95% CI = −0.22 to 0.10, P = .468, Q = 2.96, I2 = 0%). A single study compared serum folate and vitamin B12 levels in FEP to people with depression,46 finding no significant differences between the 2 patient groups. However, no firm conclusions could this be drawn due to the low number of FEP patients examined (n = 14).
Table 1a shows all clinical correlates of B vitamin levels in FEP. Five studies examined relationships between folate levels and psychiatric symptoms (measured using the Positive and Negative Syndrome Scale; “PANSS”47).48–52 Only one found a significant correlation with PANSS total scores.48 However, 3 found significant correlations between serum folate and PANSS subscales; with lower folate levels predicting more severe negative48,49 or general53 symptoms. No associations were found for positive symptoms or cognition.
Three studies examined clinical correlates of vitamin B12 in FEP. One found trend-level correlation with reduced positive symptoms,52 and 2 found significant inverse correlation between B12 and negative symptom scores.51,53 One further study examining the impact of antipsychotic medications found significant reductions in serum levels of both folate and B12 after 12 weeks of treatment with olanzapine, but no reduction from risperidone in FEP.50 Finally, one study found that neither folate or B12 levels were related to childhood trauma.53
Vitamin D in FEP
Seven studies examined blood levels of vitamin D using plasma and serum measures across 906 participants; 429 with FEP, 477 controls (table 1b). Meta-analyses comparing FEP to healthy control samples (all matched for age and ethnicity) showed that people with FEP had reduced vitamin D levels; with a large, significant difference between the 2 groups (g = −1.055, 95% CI = −1.99 to −0.119, P = .027) (figure 2). There was significant heterogeneity but no evidence of publication bias (Q = 216.6, P < .01, I2 = 97.2%, Egger’s regression P = .27, Fail Safe N = 265). A sensitivity analysis excluding the single study with large effect size (g = −4.04) which did not use established FEP criterion (classifying patients as “newly diagnosed”)54 found the difference was still significant among 6 remaining studies (g = −0.554, 95% CI = −1.00 to −0.113, P = .014). A study comparing vitamin D in FEP and multi-episode schizophrenia found no significant differences between groups.55 However, one study found that the FEP patients later diagnosed with schizophrenia had trend-level lower vitamin D than FEP patients who were later diagnosed with other psychoses (P = .06).56
Three studies examined correlations between vitamin D and psychiatric symptoms; all finding some link between low vitamin D and worse mental health (table 1b). Graham et al57 found serum levels of vitamin D were negatively associated with PANSS totals and negative subscale scores, overall neurocognitive functioning, and specific tests of verbal fluency. There was also a trend-level association (P = .054) with PANSS positive symptoms. Yee et al58 found low vitamin D levels were only associated with greater PANSS negative symptoms, whereas Nerhus et al55 only observed a significant relationship with depressive symptoms (PANSS factor scale).
Antioxidant Vitamins in FEP
Five studies assessed blood levels of antioxidant vitamins (A, C, and E) in FEP (table 1c). As shown in figure 2, significant differences were only observed for vitamin C (g = −2.207, 95% CI = −3.71 to −0.71, P = .004) from 2 studies observing large deficits of vitamin C in FEP samples.59,60 However, there was a small sample (n = 96), substantial heterogeneity (Q = 11.9, P < .01, I2 = 91.6%), and studies did not report the specifics of FEP classification (samples only described as “newly diagnosed for schizophrenia”). Differences in vitamin E were nonsignificant (N = 4, n = 253, g = −1.09, 95% CI = −2.54 to 0.36, P = .14). The sole study examining vitamin A levels in FEP (n = 30) found no difference from healthy controls (n = 30).61
Regarding clinical correlates of antioxidant vitamins, neither A or E levels correlated with psychiatric symptoms (measured with PANSS or Clinical Global Impression scales).61 Nonetheless, Dakhale et al62 found that higher plasma vitamin C in newly diagnosed schizophrenia patients receiving vitamin C supplementation (n = 40) were associated with greater symptomatic improvement over 8 weeks (assessed with the Brief Psychiatric Rating Scale) (r = −.38, P < .05).
Dietary Minerals in FEP
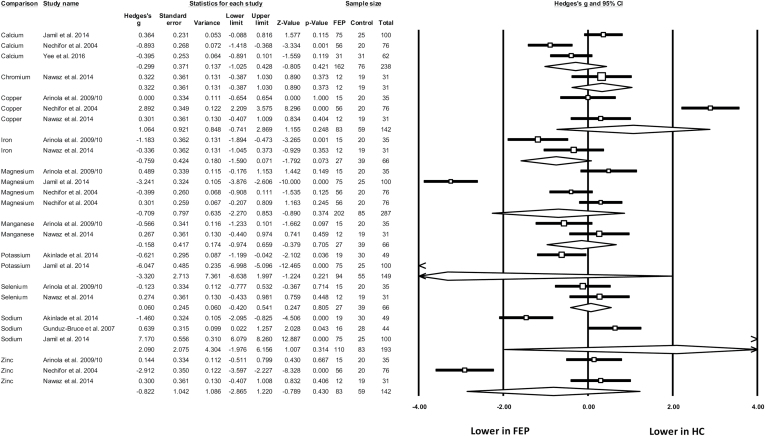
Ten dietary minerals were assessed across 8 studies (table 1d) with 480 participants (224 FEP, 173 healthy controls, 83 older/longer-term schizophrenia). Only one study used the term “first-episode psychosis,”58 with all others describing their samples as “medication-naïve” or “newly treated/diagnosed schizophrenia patients.” There were 3 studies examining blood levels of calcium, copper, magnesium, sodium, and zinc; 2 studies for iron, manganese, potassium, and selenium; and 1 study for chromium. Meta-analyses found no significant differences between FEP samples and healthy controls for any dietary mineral (figure 3).
Fig. 3.
Meta-analysis of blood levels of dietary minerals in first-episode psychosis (FEP) and healthy controls (HC). Box size represents study weighting. Diamond represents overall effect size and 95% confidence intervals.
When comparing patient groups, 2 studies found no difference in mineral levels between newly diagnosed patients and those with established schizophrenia.63,64 However, Arinola et al65 found that zinc, manganese, and chromium were all significantly lower in newly diagnosed, medication-naïve patients (n = 15) compared to medicated schizophrenia patients (n = 20). Furthermore, in a longitudinal study, Nechifor et al66 found that both magnesium and zinc were raised significantly following antipsychotic treatment (with either haloperidol or risperidone). No studies examined correlations between mineral levels and symptomology in FEP.
Discussion
This is the first study to examine serum nutrient status in FEP, and the first to show that compared to nonpsychiatric controls, reduced nutritional status exists independently, and in some cases, before antipsychotic treatment. To date, meta-analyses have only examined nutritional status in people with long-term schizophrenia,28–30 and each of these have focused on single nutrients in isolation.28–30 In this study, we included all vitamins and minerals examined in FEP to date, to identify which particular nutrient levels may act as specific biomarkers and/or therapeutic targets for this population. Our systematic search found 28 studies with 24 independent samples comparing blood levels of 6 vitamins and 10 dietary minerals in 1221 people with FEP to 1391 controls. Random effects meta-analysis found significant reductions in folate, vitamin D, and vitamin C among people with FEP compared to nonpsychiatric controls, with no significant differences for other vitamins or minerals.
The strongest evidence was found for vitamin D deficits; with pooled data from 7 independent studies showing a large, significant reduction across 429 individuals with FEP compared to 477 nonpsychiatric controls (g = −1.1, P = .027), all matched for age and ethnicity. Given the prevalence of vitamin D deficiencies in long-term schizophrenia,30 and the compelling evidence for low vitamin D status during brain development being linked to schizophrenia onset,67 it is perhaps unsurprising that FEP is associated with lower vitamin D. However, the extent of the deficit in FEP samples compared to the general population observed in this meta-analysis is troubling, especially considering that vitamin D levels are often low even among healthy adults,68 with 20%–40% of young adults in UK population showing insufficiencies.69 Furthermore, within the FEP samples, lower vitamin D levels were found to be associated with more severe symptomology.55,57,58
Currently, there is an absence of RCTs examining the efficacy of vitamin D supplementation as an adjunctive treatment in FEP—although previous studies have shown vitamin D supplements used during childhood can reduce the risk of developing schizophrenia.20 The efficacy of vitamin D supplementation in young people with FEP is currently being examined in a placebo-controlled RCT, the “D-Fend” study (“Vitamin D First Episode Neuroprotection Design”) (ISRCTN12424842). This will provide the first insights into potential benefits for this population. Vitamin D is an essential nutrient for both metabolic and neurological health.70,71 Thus, supplementing at the first-episode phase may attenuate the metabolic and neurological abnormalities which arise during the early stages of treatment. Indeed, preliminary research has already shown that vitamin D levels hold positive correlations with metabolic health,21 along with brain structure and functioning in people with psychosis.57,72
We also found that FEP samples had significantly lower serum folate than nonpsychiatric controls; as has previously been observed for long-term schizophrenia.28 Folate deficiencies, even in the general population, are among the most widespread of all nutritional deficiencies globally, and adversely affect intellectual development and mortality risk.73 Folate also plays an important role in maintaining neuronal integrity74 and lowering levels of homocysteine—which has been linked to aetiology of schizophrenia.15 Indeed, 2 studies also found higher serum folate correlated with reduced negative symptoms in FEP,48,49 as has been previously observed in long-term schizophrenia.11,13
Interestingly, the one study which measured dietary folate intake found that reduced folate levels in FEP could not be accounted for by differences in diet.52 Instead, genotypic differences in folate absorption efficiency may be responsible for deficiencies observed in schizophrenia.15,52,75 In long-term schizophrenia, this hypothesis is supported by strong experimental evidence showing that symptomatic benefits from standard folic acid supplements are moderated by genotypic differences in absorption,75,76 whereas administration of “L-methlyfolate” (the most bioactive folate type; readily absorbed regardless of genotype) significantly reduces negative symptoms across entire schizophrenia samples.13 Symptomatic benefits of L-methylfolate have also been shown in multiple RCTs in people with depression.77,75 Although there are no RCTs of L-methylfolate to date in individuals with FEP, research in long-term schizophrenia samples suggests that patients with shorter illness durations are more responsive to B vitamin treatments.12 Given the very low side-effect profile of L-methylfolate (with RCTs in other populations administering over 100 times the recommended daily requirement without any negative effects),78 this could potentially be trialed as an adjunctive treatment in FEP, or even as a prevention strategy for individuals identified as at-risk of psychosis. Additionally, B12 (another homocysteine-reducing B vitamin) may also be as useful adjunctive treatment, as although no pooled differences were observed between FEP and controls for this nutrient, studies consistently indicated that higher B12 was related to better mental health in FEP.51–53
Although only examined in 2 studies, vitamin C was also significantly reduced in FEP. This is concordant with data suggesting low fruit and vegetable intake in this population.79 Additionally, a single RCT in patients undergoing first antipsychotic treatment has shown 500 mg of vitamin C per day significantly reduces psychiatric symptoms.62 Furthermore, increases in plasma vitamin C were correlated with symptomatic improvements following the trial. Meta-analyses of other antioxidant vitamins A and E found no evidence for deficits in FEP.
However, a limitation of this meta-analysis is that despite the large number of total studies, certain individual nutrients were only examined in a small number of FEP samples—reducing statistical power to detect significant differences. Therefore, although the review provides evidence that folate and vitamin D are reduced in FEP compared to healthy populations, the nonsignificance of differences for other vitamins does not necessarily rule these out as deficient. Indeed, further adequately powered studies could ultimately find a spectrum of deficiencies impacting on the physical and mental health of people with FEP. Additionally, the lack of significant differences found between FEP samples and other psychiatric populations, such as depression46 and long-term schizophrenia,55,63,64 could be due to limited sample sizes in respective studies. Thus, further research is required to determine if any nutritional deficiencies are particularly pronounced in FEP, or if these deficiencies are prevalent across all phases/types of severe mental illness.
Another limitation is that the causative and mechanistic links between nutritional deficiencies and mental health outcomes has yet to be established.80 Further longitudinal and interventional research in individuals identified at “ultra-high risk” for psychosis would provide valuable insights into both the predictive value of nutritional deficiencies in the onset of psychosis, along with potentially determining if nutritional supplementation can confer any benefit for reducing psychosis risk. A note of caution is warranted, as in disorders such as cardiovascular disorders and cancer, where nutrient deficiencies have also been noted, the research has been underwhelming in supporting supplementation. For instance, while vitamin D insufficiency is widespread across noncommunicable medical conditions, RCTs of vitamin D supplementation in cardiovascular disorders, cancer, and osteoporosis have generally failed to robustly support causative hypotheses.80–82 It should also be considered that although nutritional deficiencies could feasibly exacerbate psychiatric symptoms, it is also possible that therapeutic benefits of supplementation may not be reliant upon vitamin/mineral deficiencies. For instance, beneficial effects of other nutrient-based adjunctive treatments (such as NAC83 and Taurine84) are not due to restoring specific nutritional deficits, but instead attributed to these amino acids targeting pathological neurological processes.83,84 Therefore, even in the absence of clear deficiencies, certain nutrients may confer positive effects in FEP through the neurochemical properties of these compounds, regardless of deficiency status.
Given the marked reductions in vitamin D and folate observed in FEP compared to nonpsychiatric samples, future research should also examine what proportion of individuals with FEP reach clinical thresholds for nutritional deficiencies in comparison to established reference values, which in turn could have implications for routine screening of nutritional deficiencies in FEP. Additionally, much of the emphasis for dietary intervention in schizophrenia so far lies with reducing over-consumption of obesogenic foods.7,85 Thus, this review also holds implications for dietary interventions to further emphasize the importance of consuming a good quality diet containing adequate nutrients. Indeed, large-scale epidemiological studies show that low levels of both vitamin D and folate are linked to cardiovascular mortality.86,87 Further efforts toward resolving these deficiencies holds promise to reduce the physical health inequalities observed in schizophrenia. Additionally, multi-ingredient “nutraceuticals,” which combine various beneficial nutrients to target specific nutritional deficits and neurological processes implicated in psychiatric disorders,88,89 may also provide safe and effective adjunctive treatments for FEP. These data also support stratification of individuals based on nutrient intake/status as criteria for nutraceutical interventions and in trial design. Given the clear lack of experimental evidence in this area,12 there is a now a clear need for clinical trials to evaluate the use of whole diet interventions as well as nutrient-based interventions for improving the typically poor recovery rates observed among people with FEP.
Supplementary Material
Supplementary data are available at Schizophrenia Bulletin online.
Funding
J.F. is supported by a Blackmores Institute Fellowship and an MRC Doctoral Training Grant P117413F07. J.S. is supported by an NHMRC Research Fellowship (APP1125000) and has received either presentation honoraria, travel support, clinical trial grants, book royalties, or independent consultancy payments from Integria Healthcare & MediHerb, Pfizer, Scius Health, Key Pharmaceuticals, Taki Mai, Bioceuticals & Blackmores, Soho-Flordis, Healthworld, HealthEd, HealthMasters, Elsevier, Chaminade University, International Society for Affective Disorders, Complementary Medicines Australia, Terry White Chemists, ANS, Society for Medicinal Plant and Natural Product Research, UBiome, Omega-3 Centre, the National Health and Medical Research Council, CR Roper Fellowship. M.B. is supported by a NHMRC Senior Principal Research Fellowship (GNT1059660) and has received Grant/Research Support from the NIH, Cooperative Research Centre, Simons Autism Foundation, Cancer Council of Victoria, Stanley Medical Research Foundation, MBF, NHMRC, Beyond Blue, Rotary Health, Geelong Medical Research Foundation, Bristol Myers Squibb, Eli Lilly, Glaxo SmithKline, Meat and Livestock Board, Organon, Novartis, Mayne Pharma, Servier, Woolworths, Avant, and the Harry Windsor Foundation, has been a speaker for Astra Zeneca, Bristol Myers Squibb, Eli Lilly, Glaxo SmithKline, Janssen Cilag, Lundbeck, Merck, Pfizer, Sanofi Synthelabo, Servier, Solvay, and Wyeth, and served as a consultant to Allergan, Astra Zeneca, Bioadvantex, Bionomics, Collaborative Medicinal Development, Eli Lilly, Grunbiotics, Glaxo SmithKline, Janssen Cilag, LivaNova, Lundbeck, Merck, Mylan, Otsuka, Pfizer, and Servier. R.C. is funded by an Economic and Social Research Council grant (ESJ5000991). S.T. is funded by the South Eastern Sydney Local Health District. B.S. is part funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Supplementary Material
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Sarris J, Logan AC, Akbaraly TN et al. ; International Society for Nutritional Psychiatry Research. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry. 2015;2:271–274. [DOI] [PubMed] [Google Scholar]
- 2. Sarris J, Logan AC, Akbaraly TN et al. . International Society for Nutritional Psychiatry Research consensus position statement: nutritional medicine in modern psychiatry. World Psychiatry. 2015;14:370–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akbaraly TN, Brunner EJ, Ferrie JE, Marmot MG, Kivimaki M, Singh-Manoux A. Dietary pattern and depressive symptoms in middle age. Br J Psychiatry. 2009;195:408–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jacka FN, Pasco JA, Mykletun A et al. . Association of Western and traditional diets with depression and anxiety in women. Am J Psychiatry. 2010;167:305–311. [DOI] [PubMed] [Google Scholar]
- 5. O’Neil A, Quirk SE, Housden S et al. . Relationship between diet and mental health in children and adolescents: a systematic review. Am J Public Health. 2014;104:e31–e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heald A, Sein K, Anderson S et al. . Diet, exercise and the metabolic syndrome in schizophrenia: A cross-sectional study. Schizophr Res. 2015;169:494–495. [DOI] [PubMed] [Google Scholar]
- 7. Dipasquale S, Pariante CM, Dazzan P, Aguglia E, McGuire P, Mondelli V. The dietary pattern of patients with schizophrenia: a systematic review. J Psychiatr Res. 2013;47:197–207. [DOI] [PubMed] [Google Scholar]
- 8. Laursen TM. Life expectancy among persons with schizophrenia or bipolar affective disorder. Schizophr Res. 2011;131:101–104. [DOI] [PubMed] [Google Scholar]
- 9. García-Miss Mdel R, Pérez-Mutul J, López-Canul B et al. . Folate, homocysteine, interleukin-6, and tumor necrosis factor alfa levels, but not the methylenetetrahydrofolate reductase C677T polymorphism, are risk factors for schizophrenia. J Psychiatr Res. 2010;44:441–446. [DOI] [PubMed] [Google Scholar]
- 10. Bouaziz N, Ayedi I, Sidhom O et al. . Plasma homocysteine in schizophrenia: determinants and clinical correlations in Tunisian patients free from antipsychotics. Psychiatry Res. 2010;179:24–29. [DOI] [PubMed] [Google Scholar]
- 11. Goff DC, Bottiglieri T, Arning E et al. . Folate, homocysteine, and negative symptoms in schizophrenia. Am J Psychiatry. 2004;161:1705–1708. [DOI] [PubMed] [Google Scholar]
- 12. Firth J, Stubbs B, Sarris J et al. . The effects of vitamin and mineral supplementation on symptoms of schizophrenia: a systematic review and meta-analysis. Psychol Med. 2017;47:1515–1527. [DOI] [PubMed] [Google Scholar]
- 13. Roffman J, Petruzzi L, Tanner A et al. . Biochemical, physiological and clinical effects of l-methylfolate in schizophrenia: a randomized controlled trial. Mol Psychiatry. 2017. doi: 10.1038/mp.2017.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mattson MP, Haberman F. Folate and homocysteine metabolism: therapeutic targets in cardiovascular and neurodegenerative disorders. Curr Med Chem. 2003;10:1923–1929. [DOI] [PubMed] [Google Scholar]
- 15. Muntjewerff JW, Kahn RS, Blom HJ, den Heijer M. Homocysteine, methylenetetrahydrofolate reductase and risk of schizophrenia: a meta-analysis. Mol Psychiatry. 2006;11:143–149. [DOI] [PubMed] [Google Scholar]
- 16. Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74:400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McGrath J. Is it time to trial vitamin D supplements for the prevention of schizophrenia?Acta Psychiatr Scand. 2010;121:321–324. [DOI] [PubMed] [Google Scholar]
- 18. McGrath J, Eyles D, Burne T. Environmental risk factors for schizophrenia: does developmental vitamin D deficiency play a role?Aust N Z J Psychiatry. 2010;44:A4. [Google Scholar]
- 19. McGrath J, Eyles D, Mowry B, Yolken R, Buka S. Low maternal vitamin D as a risk factor for schizophrenia: a pilot study using banked sera. Schizophr Res. 2003;63:73–78. [DOI] [PubMed] [Google Scholar]
- 20. McGrath J, Saari K, Hakko H et al. . Vitamin D supplementation during the first year of life and risk of schizophrenia: a Finnish birth cohort study. Schizophr Res. 2004;67:237–245. [DOI] [PubMed] [Google Scholar]
- 21. Lally J, Gardner-Sood P, Firdosi M et al. . Clinical correlates of vitamin D deficiency in established psychosis. BMC Psychiatry. 2016;16:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Adamson J, Lally J, Gaughran F, Krivoy A, Allen L, Stubbs B. Correlates of vitamin D in psychotic disorders: a comprehensive systematic review. Psychiatry Res. 2017;249:78–85. [DOI] [PubMed] [Google Scholar]
- 23. Cai L, Chen T, Yang J et al. . Serum trace element differences between Schizophrenia patients and controls in the Han Chinese population. Sci Rep. 2015;5 doi:10.1038/srep15013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sharma SK, Sharma A, Sood S, Gupta I. Study of serum selenium levels in schizophrenic patients. Indian Medical Gazette. 2014;148:403–407. [Google Scholar]
- 25. Liu T, Lu QB, Yan L et al. . Comparative study on serum levels of 10 trace elements in schizophrenia. PLoS One. 2015;10:e0133622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Swardfager W, Herrmann N, Mazereeuw G, Goldberger K, Harimoto T, Lanctôt KL. Zinc in depression: a meta-analysis. Biol Psychiatry. 2013;74:872–878. [DOI] [PubMed] [Google Scholar]
- 27. Pasco JA, Jacka FN, Williams LJ et al. . Dietary selenium and major depression: a nested case–control study. Complement Ther Med. 2012;20:119–123. [DOI] [PubMed] [Google Scholar]
- 28. Wang D, Zhai JX, Liu DW. Serum folate levels in schizophrenia: a meta-analysis. Psychiatry Res. 2016;235:83–89. [DOI] [PubMed] [Google Scholar]
- 29. Cao B, Wang DF, Xu MY et al. . Vitamin B12 and the risk of schizophrenia: a meta-analysis. Schizophr Res. 2016;172:216–217. [DOI] [PubMed] [Google Scholar]
- 30. Valipour G, Saneei P, Esmaillzadeh A. Serum vitamin D levels in relation to schizophrenia: a systematic review and meta-analysis of observational studies. J Clin Endocrinol Metab. 2014;99:3863–3872. [DOI] [PubMed] [Google Scholar]
- 31. Williamson K, Kilner K, Clibbens N. A comparison of the nutrient intake of a community-dwelling first-episode psychosis cohort, aged 19–64 years, with data from the UK population. J Nutr Sci. 2015;4:e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Teasdale SB, Ward PB, Rosenbaum S et al. . A nutrition intervention is effective in improving dietary components linked to cardiometabolic risk in youth with first-episode psychosis. Br J Nutr. 2016;115:1987–1993. [DOI] [PubMed] [Google Scholar]
- 33. Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Andreasen NC, Nopoulos P, Magnotta V, Pierson R, Ziebell S, Ho BC. Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biol Psychiatry. 2011;70:672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Phutane VH, Tek C, Chwastiak L et al. . Cardiovascular risk in a first-episode psychosis sample: a ‘critical period’ for prevention?Schizophr Res. 2011;127:257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tek C, Kucukgoncu S, Guloksuz S, Woods SW, Srihari VH, Annamalai A. Antipsychotic-induced weight gain in first-episode psychosis patients: a meta-analysis of differential effects of antipsychotic medications. Early Interv Psychiatry. 2016;10:193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269, W64. [DOI] [PubMed] [Google Scholar]
- 38. Marshall M, Lockwood A, Lewis S, Fiander M. Essential elements of an early intervention service for psychosis: the opinions of expert clinicians. BMC Psychiatry. 2004;4:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Addington J, Addington D. Early intervention for psychosis: the Calgary early psychosis treatment and prevention program. Can Psychiatr Assoc Bull. 2001;33:11–16. [Google Scholar]
- 40. Craig TK, Garety P, Power P et al. . The Lambeth Early Onset (LEO) team: randomised controlled trial of the effectiveness of specialised care for early psychosis. BMJ. 2004;329:1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Foundation BN. Nutrition Requirements 2016. https://wwwnutritionorguk/attachments/article/234/Nutrition Requirements_Revised Oct 2016pdf.
- 42. Kirkpatrick B, Miller BJ, Garcia-Rizo C, Fernandez-Egea E, Bernardo M. Is abnormal glucose tolerance in antipsychotic-naive patients with nonaffective psychosis confounded by poor health habits?Schizophr Bull. 2012;38:280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive meta-analysis 2.0. Biostatistics. 2005. [Google Scholar]
- 44. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 45. Orwin RG. A fail-safe N for effect size in meta-analysis. J Educ Stat. 1983;8:157–159. [Google Scholar]
- 46. Ipcioglu OM, Ozcan O, Gultepe M, Ates A, Basoglu C, Cakir E. Reduced urinary excretion of homocysteine could be the reason of elevated plasma homocysteine in patients with psychiatric illnesses. Clin Biochem. 2008;41:831–835. [DOI] [PubMed] [Google Scholar]
- 47. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 48. Song X, Fan X, Li X et al. . Serum levels of BDNF, folate and homocysteine: in relation to hippocampal volume and psychopathology in drug naïve, first episode schizophrenia. Schizophr Res. 2014;159:51–55. [DOI] [PubMed] [Google Scholar]
- 49. Xuimei C, Yue Z, Wei Z et al. . Serum folic acid and homocysteine levels in patients with first-episode schizophrenia and their relationship with cognitive function. Chin J Med Sci. 2014;94:990–993. [Google Scholar]
- 50. Misiak B, Frydecka D, Laczmanski L, Lezak R, Kiejna A. Effects of second-generation antipsychotics on selected markers of one-carbon metabolism and metabolic syndrome components in first-episode schizophrenia patients. Eur J Clin Pharmacol. 2014;70:1433–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Misiak B, Laczmanski L, Sloka K et al. . Methylenetetrahydrofolate reductase (MTHFR) gene polymorphisms and antipsychotic-induced metabolic disturbances in first-episode schizophrenia patients. Eur Psychiatry. 2016;33:S104. [Google Scholar]
- 52. Kale A, Naphade N, Sapkale S et al. . Reduced folic acid, vitamin B12 and docosahexaenoic acid and increased homocysteine and cortisol in never-medicated schizophrenia patients: Implications for altered one-carbon metabolism. Psychiatry Res. 2010;175:47–53. [DOI] [PubMed] [Google Scholar]
- 53. Misiak B, Frydecka D, Slezak R, Piotrowski P, Kiejna A. Elevated homocysteine level in first-episode schizophrenia patients—the relevance of family history of schizophrenia and lifetime diagnosis of cannabis abuse. Metab Brain Dis. 2014;29:661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Malik A, Saleem S, Ashraf MAB, Qazi MH. 1, 25-dihydroxyvitamin D3, a potential role player in the development of thyroid disorders in schizophrenics. Pak J Med Sci. 2016;32:1370–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nerhus M, Berg AO, Dahl SR et al. . Vitamin D status in psychotic disorder patients and healthy controls—the influence of ethnic background. Psychiatry Res. 2015;230:616–621. [DOI] [PubMed] [Google Scholar]
- 56. Salavert J, Grados D, Ramiro N et al. . Association between vitamin D status and schizophrenia: a first psychotic episode study. J Nerv Ment Dis. 2017;205:409–412. [DOI] [PubMed] [Google Scholar]
- 57. Graham KA, Keefe RS, Lieberman JA, Calikoglu AS, Lansing KM, Perkins DO. Relationship of low vitamin D status with positive, negative and cognitive symptom domains in people with first-episode schizophrenia. Early Interv Psychiatry. 2015;9:397–405. [DOI] [PubMed] [Google Scholar]
- 58. Yee JY, See YM, Abdul Rashid NA, Neelamekam S, Lee J. Association between serum levels of bioavailable vitamin D and negative symptoms in first-episode psychosis. Psychiatry Res. 2016;243:390–394. [DOI] [PubMed] [Google Scholar]
- 59. Dadheech G, Mishra S, Gautam S, Sharma P. Oxidative stress, α-tocopherol, ascorbic acid and reduced glutathione status in schizophrenics. Indian J Clin Biochem. 2006;21:34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Surapaneni K. Status of lipid peroxidation, glutathione, ascorbic acid, vitamin E and antioxidant enzymes in schizophrenic patients. J Clin Diagn Res. 2007;1:39–44. [PubMed] [Google Scholar]
- 61. Scottish Schizophrenia Research Group. Smoking habits and plasma lipid peroxide and vitamin E levels in never-treated first-episode patients with schizophrenia. Br J Psychiatry 2000;176:290–293. [DOI] [PubMed] [Google Scholar]
- 62. Dakhale GN, Khanzode SD, Khanzode SS, Saoji A. Supplementation of vitamin C with atypical antipsychotics reduces oxidative stress and improves the outcome of schizophrenia. Psychopharmacology (Berl). 2005;182:494–498. [DOI] [PubMed] [Google Scholar]
- 63. Akinladel KS, Fagbomedo FO, Rahamon SK, Makanjuola VA, Ampitan AB. Serum copeptin and its diagnostic performance in schizophrenia. Afr J Med Med Sci. 2014;43:259–264. [PubMed] [Google Scholar]
- 64. Nawaz R, Siddiqui S. Association of single nucleotide polymorphisms in catechol-omethyltransferase and serine-threonine protein kinase genes in the Pakistani schizophrenic population: a study with special emphasis on cannabis and smokeless tobacco. CNS Neurol Disord Drug Targets. 2015;14:1086–1095. [DOI] [PubMed] [Google Scholar]
- 65. Arinola G, Idonije B, Akinlade K, Ihenyen O. Essential trace metals and heavy metals in newly diagnosed schizophrenic patients and those on anti-psychotic medication. J Res Med Sci. 2010;15:1–5. [PMC free article] [PubMed] [Google Scholar]
- 66. Nechifor M, Vaideanu C, Palamaru I, Borza C, Mindreci I. The influence of some antipsychotics on erythrocyte magnesium and plasma magnesium, calcium, copper and zinc in patients with paranoid schizophrenia. J Am Coll Nutr. 2004;23:549S–551S. [DOI] [PubMed] [Google Scholar]
- 67. McGrath JJ, Burne TH, Féron F, Mackay-Sim A, Eyles DW. Developmental vitamin D deficiency and risk of schizophrenia: a 10-year update. Schizophr Bull. 2010;36:1073–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hyppönen E, Power C. Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr. 2007;85:860–868. [DOI] [PubMed] [Google Scholar]
- 69. Prentice A. Vitamin D deficiency: a global perspective. Nutr Rev. 2008;66(10 suppl 2):153–164. [DOI] [PubMed] [Google Scholar]
- 70. Balion C, Griffith LE, Strifler L et al. . Vitamin D, cognition, and dementia: a systematic review and meta-analysis. Neurology. 2012;79:1397–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jorde R, Grimnes G. Vitamin D and metabolic health with special reference to the effect of vitamin D on serum lipids. Prog Lipid Res. 2011;50:303–312. [DOI] [PubMed] [Google Scholar]
- 72. Shivakumar V, Kalmady SV, Amaresha AC et al. . Serum vitamin D and hippocampal gray matter volume in schizophrenia. Psychiatry Res. 2015;233:175–179. [DOI] [PubMed] [Google Scholar]
- 73. Bailey RL, West KP Jr, Black RE. The epidemiology of global micronutrient deficiencies. Ann Nutr Metab. 2015;66(suppl 2):22–33. [DOI] [PubMed] [Google Scholar]
- 74. Krebs MO, Bellon A, Mainguy G, Jay TM, Frieling H. One-carbon metabolism and schizophrenia: current challenges and future directions. Trends Mol Med. 2009;15:562–570. [DOI] [PubMed] [Google Scholar]
- 75. Hill M, Shannahan K, Jasinski S et al. . Folate supplementation in schizophrenia: a possible role for MTHFR genotype. Schizophr Res. 2011;127:41–45. [DOI] [PubMed] [Google Scholar]
- 76. Roffman JL, Lamberti JS, Achtyes E et al. . Randomized multicenter investigation of folate plus vitamin B12 supplementation in schizophrenia. JAMA Psychiatry. 2013;70:481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Papakostas GI, Shelton RC, Zajecka JM et al. . L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. Am J Psychiatry. 2012;169:1267–1274. [DOI] [PubMed] [Google Scholar]
- 78. Passen M, Cucinotta D, Abate G et al. . Oral 5′-methyltetrahydrofolic acid in senile organic mental disorders with depression: results of a double-blind multicenter study. Aging Clin Exp Res. 1993;5:63–71. [DOI] [PubMed] [Google Scholar]
- 79. McCreadie R, Elizabeth M, Blacklock C et al. . Dietary intake of schizophrenic patients in Nithsdale, Scotland: case–control study. BMJ. 1998;317:784–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. McGrath JJ. Vitamin D and mental health—the scrutiny of science delivers a sober message. Acta Psychiatr Scand. 2017;135:183–184. [DOI] [PubMed] [Google Scholar]
- 81. Keum N, Giovannucci E. Vitamin D supplements and cancer incidence and mortality: a meta-analysis. Br J Cancer. 2014;111:976–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Thadhani R, Appelbaum E, Pritchett Y et al. . Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA. 2012;307:674–684. [DOI] [PubMed] [Google Scholar]
- 83. Berk M, Copolov D, Dean O et al. . N-acetyl cysteine as a glutathione precursor for schizophrenia–a double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 2008;64:361–368. [DOI] [PubMed] [Google Scholar]
- 84. O’Donnell CP, Allott KA, Murphy BP et al. . Adjunctive taurine in first-episode psychosis: a phase 2, double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2016;77:e1610–e1617. [DOI] [PubMed] [Google Scholar]
- 85. Teasdale SB, Ward PB, Rosenbaum S, Samaras K, Stubbs B. Solving a weighty problem: systematic review and meta-analysis of nutrition interventions in severe mental illness. Br J Psychiatry. 2016. doi:10.1192/bjp.bp.115.177139 [DOI] [PubMed] [Google Scholar]
- 86. Loria CM, Ingram DD, Feldman JJ, Wright JD, Madans JH. Serum folate and cardiovascular disease mortality among US men and women. Arch Intern Med. 2000;160:3258–3262. [DOI] [PubMed] [Google Scholar]
- 87. Schöttker B, Jorde R, Peasey A et al. . Vitamin D and mortality: meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. BMJ. 2014;348:g3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sarris J, Stough C, Bousman C et al. . An adjunctive antidepressant nutraceutical combination in treating major depression: study protocol, and clinical considerations. Adv Integr Med. 2015;7:49–55. [Google Scholar]
- 89. Dean OM, Turner A, Malhi GS et al. . Design and rationale of a 16-week adjunctive randomized placebo-controlled trial of mitochondrial agents for the treatment of bipolar depression. Rev Bras Psiquiatr. 2015;37:3–12. [DOI] [PubMed] [Google Scholar]
- 90. Ayesa-Arriola R, Pérez-Iglesias R, Rodríguez-Sánchez JM et al. . Homocysteine and cognition in first-episode psychosis patients. European Archives of Psychiatry and Clinical Neuroscience. 2012;262:557–564. [DOI] [PubMed] [Google Scholar]
- 91. Misiak B, Kiejna A, Frydecka D. The history of childhood trauma is associated with lipid disturbances and blood pressure in adult first-episode schizophrenia patients. General Hospital Psychiatry. 2015;37:365–367. [DOI] [PubMed] [Google Scholar]
- 92. Crews M, Lally J, Gardner-Sood P et al. . Vitamin D deficiency in first episode psychosis: a case–control study. Schizophrenia research. 2013;150:533–537. [DOI] [PubMed] [Google Scholar]
- 93. Zhu DM, Liu Y, Zhang AG et al. . High levels of vitamin D in relation to reduced risk of schizophrenia with elevated C-reactive protein. Psychiatry Research. 2015;228:565–570. [DOI] [PubMed] [Google Scholar]
- 94. Sarandol A, Sarandol E, Acikgoz HE, Eker SS, Akkaya C, Dirican M. First‐episode psychosis is associated with oxidative stress: effects of short‐term antipsychotic treatment. Psychiatry and Clinical Neurosciences. 2015;69:699–707. [DOI] [PubMed] [Google Scholar]
- 95. Arinola OG, Idonije OB. Status of plasma nitric oxide and non-enzymatic antioxidants before and after antipsychotic treatment in Nigerian patients with schizophrenia. Journal of Research in Medical Sciences: the Official Journal of Isfahan University of Medical Sciences. 2009;14:37–42. [PMC free article] [PubMed] [Google Scholar]
- 96. Gunduz-Bruce H, Narr KL, Gueorguieva R et al. . CSF sub-compartments in relation to plasma osmolality in healthy controls and in patients with first episode schizophrenia. Psychiatry Research: Neuroimaging. 2007;155:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Nawaz R, Zahir E, Siddiqui S, Usmani A, Shad KF. The role of trace metals and environmental factors in the onset and progression of schizophrenia in Pakistani population. World Journal of Neuroscience. 2014;4:450–460. [Google Scholar]
- 98. Jamil MHQ, Saleem S, Malik A, Shuja N, Manan A. Inter-relationship of circulating biochemical markers of oxidative stress and thyroid hormones in newly diagnosed schizophrenics: perspective study from local population of Punjab pakistan. Medical Forum. 2014;25:19–22. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.