Abstract
A growing body of evidence suggests that mechanical signals emanating from the cell's microenvironment are fundamental regulators of cell behaviour. Moreover, at the macroscopic scale, the influence of forces, such as the ones generated by blood flow and muscle contraction, gravity, as well as overall tissue rigidity (for example inside of a tumor lump) are central to our understanding of physiology and disease pathogenesis. And yet, how mechanical cues are sensed and transduced at the molecular level to regulate gene expression has long remained enigmatic. The identification of the transcription factors YAP and TAZ as mechanotransducers started to fill this gap. YAP and TAZ read a broad range of mechanical cues, from shear stress to cell shape and extracellular matrix rigidity, and translate them into cell-specific transcriptional programmes. YAP and TAZ mechanotransduction is critical for driving stem cell behaviour and regeneration, and sheds new light on the mechanisms by which aberrant cell mechanics is instrumental for the onset of multiple diseases, such as atherosclerosis, fibrosis, pulmonary hypertension, inflammation, muscular dystrophy and cancer.
A myriad of mechanical forces operate in a living body, including heart pumping, fluid shear stress, pressure and tensional forces in the skeletal system. In truth, physical forces affect every cell of our organs for the simple reason that our tissues have complex architectures, which are the product of an equilibrium of forces: internal pulling forces, dictated by the tension and organization of the cytoskeleton, counterbalancing external forces, such as topology and rigidity of the surrounding extracellular matrix (ECM) and other cells. As such, mechanical forces are informational systems by which cells perceive their position, shape and perturbations in their environment, inducing them to react by building, growing and healing tissues until a proper mechanical equilibrium is attained. Disturbance of these homeostatic mechanisms, caused by abnormal mechanical signals from the environment or cell-generated ones, is associated to a vast number of inborn or acquired diseases1–8.
The profound influence of mechanical and physical constraints and forces on cell behaviour had long been recognized: for more than a century, before the advent of reductionist approaches in the late '60, cell and developmental biology were primarily explained in terms of the material properties and mechanical interactions between cells and tissues9. However, lack of mechanistic, molecular understanding of these events sidestepped such view of living systems, placing instead emphasis on signal transduction and genetics. The renaissance of modern mechanobiology started by seminal discoveries on the mechanisms of mechanoperception at the level of cell–cell and cell–ECM adhesion sites7,10–13, on the role of cytoskeletal proteins in these events, and by key demonstrations that cell mechanics and cell shape control cell proliferation, death and stem cell differentiation. That said, how mechanical forces, from the macroscopic to the microscopic scale, regulate cell fate by controlling gene expression, remained a major black box in biology. We will discuss in the Review how the identification of YAP and TAZ as nuclear transducers of cell mechanics starts to fill this gap, linking the physicality of cells and tissues to potent transcriptional responses.
YAP and TAZ are transcriptional co-regulators, which bind primarily to enhancer elements using TEAD factors as DNA binding platforms14–17, an interaction originally described and functionally validated in Drosophila melanogaster18, 19. YAP and TAZ are at the nexus of multiple cellular inputs. They are best understood in the context of the Hippo signalling cascade, where upstream kinases, MST1/2, trigger phosphorylation and activation of LATS1/2 kinases, in turn phosphorylating and inactivating YAP and TAZ by inducing their cytoplasmic retention and degradation. The reader can refer to excellent reviews for details on the Hippo and other pathways impacting on YAP and TAZ activity20–23. That said, a wealth of recent reports indicates that an overarching determinant of YAP and TAZ regulation is cellular mechanotransduction, operating primarily in a LATS-independent manner24–35. Remarkably, YAP and TAZ read very diverse biomechanical signals and transduce them into biological effects in a manner that is specific for each type of cell and mechanical-stress. As reviewed here, this includes cues emanating from ECM rigidity and topology, geometries of individual cells and whole tissues, stretching epithelial monolayers in 2D and 3D, tensional forces generated by asymmetric cell division in early embryos, as well as diverse types of macroscopic forces, including stress generated by blood flow, muscle contraction and cyclic stretching of vascular smooth muscle cells. This ability to respond to the different mechanical inputs highlights the central role of YAP and TAZ as universal mechanotransducers and mechanoeffectors. Moreover, these roles of YAP and TAZ offer new means of interpreting and understanding classic aspects of tissue physiology and pathology in molecular terms. In this light, the discovery of YAP and TAZ as mechanotransducers is finally revealing the contribution of aberrant cell mechanics to the onset of multiple diseases, including atherosclerosis, fibrosis, cardiac hypertrophy, muscular dystrophy and cancer.
YAP and TAZ mechanotransduction
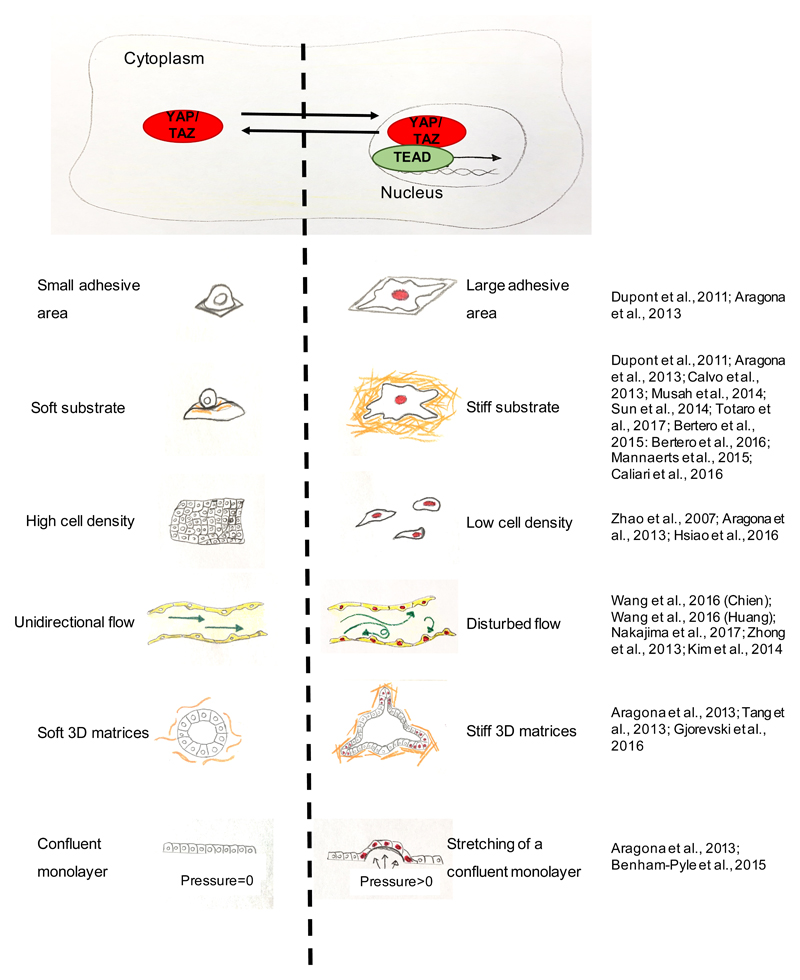
A primary layer of YAP and TAZ regulation occurs at the level of their subcellular distribution as YAP and TAZ activation entails their accumulation in the nucleus. This is controlled by cell shape, rigidity and topology of the ECM substrate24,27,36–39, and by shear stress40–42 (Figure 1). For example, YAP and TAZ are localized in the cytoplasm in cells experiencing low levels of mechanical signalling, such as in rounded cells attached to a soft ECM (typically below 1.5 kPa for epithelial cells) or to a small adhesive area (i.e., 300 μm2); YAP and TAZ are instead nuclear in cells perceiving high mechanical signalling, such as cells cultured on rigid substrates (typically at >5/10 kPa) or experiencing deformation and cytoskeletal tension (e.g., cells stretching over an area > 3000 μm2), while remaining evenly distributed at more intermediate levels of mechanical signals24,27,43 (Figure 1).
Figure 1. Schematic representations of mechanical stimuli influencing YAP and TAZ subcellular localization and activity.
A) When YAP and TAZ are mechanically activated (red) they translocate to the nucleus, where they interact with TEAD factors to regulate gene expression. B) schematics illustrating how different matrix, geometry, and physical conditions influence YAP and TAZ localization and activity: left panels show conditions, when YAP and TAZ are inhibited and localized to the cytoplasm, whereas right panels show conditions that promote YAP and TAZ nuclear localization (indicated by red colouring of cell nuclei). For references see: part a:24,27; part b:24,27,36,43,72,76,105,106,108,109; part c:24,75,91; part d:40–42,83,84; part e:24,45,80; part f:24,44.
Moreover, YAP and TAZ are mechanically regulated by various regimens of cell stretching, such as deformations of epithelial monolayers, or by the rigidity and deformability of the ECM supporting cellular outgrowths in 3D, such as organoids24,27,44,45 (Figure 1 and see Box1).
Box 1. YAP and TAZ-mechanotransduction drives organoid biology.
Organoids are 3D cultures of primary cells, whose cellular composition and spatial organization closely recapitulate those of the tissue from which they derive (reviewed in134). YAP and TAZ are essential for the maintenance and self-renewal of organoids derived from distinct tissues65; regulation of organoids by cell mechanics is also instrumental for their "self-organizing" and "self-renewing" properties, that is, to preserve their stem cells and, at the same time, allow differentiation. Accordingly, YAP and TAZ are genetically required for the maintenance of intestinal stem cell function in organoids derived from adult intestinal crypts 45,64,135 and YAP protein was reported to be active in the growing (i.e., "crypt-like") compartment of the organoids, while it is confined to the cytoplasm of their more differentiated compartment45. Typically, organoids are grown in Matrigel, a natural basement membrane substrate that is chemically and physically heterogeneous, making impossible to dissect the relative contribution of distinct inputs on organoid biology. Intriguingly, when intestinal organoids are embedded in fully defined synthetic hydrogels, their growth is inhibited in soft substrates, but dramatically fostered by more rigid matrices, a condition accompanied by, and requiring, nuclear accumulation of YAP and TAZ45 (Figure 1). As cells accumulate and the organoid expands, increased cell crowding progressively limits YAP and TAZ activity (Figure 1), promoting differentiation. As such, to support long-term organoid expansion, colonies must be transferred into a cleavable, and thus mechanically dynamic, artificial matrix that allows budding of “crypt-like” finger-shaped protrusions at whose tips stem cells are preserved by nuclear active YAP and TAZ45.
As another example, in human airway organoids YAP and TAZ are nuclear in basal (progenitor) cells and absent in differentiated cells. Similarly to intestinal organoids, mechano-activation of YAP and TAZ by raising tension in F-actin cytoskeleton is essential for branching morphogenesis of these lung organoids in vitro136.
In all these contexts, YAP and TAZ are not just reading out cell and tissue mechanics but, crucially, also mediating the biological effects associated to mechanical signalling in each biological system, as detailed below (Figures 2 and 3). Importantly, none of this could have been discovered without the development of new technologies and biomaterials that allowed studying mechanical signals independently from other variables first in vitro and then translating this knowledge in vivo10,11,24,45,46.
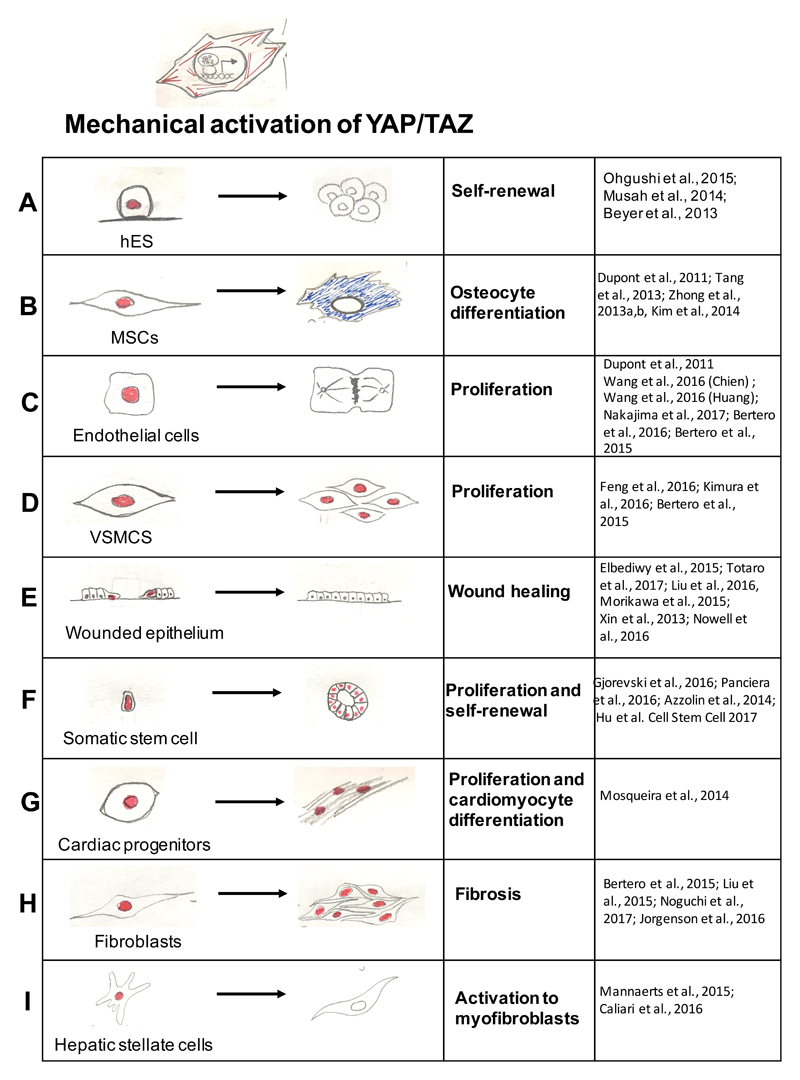
Figure 2. List of biological responses caused by nuclear accumulation and activation of YAP and TAZ by high mechanical signalling.
When YAP and TAZ are activated by raised mechanical inputs (see Figure 1, right panels), this causes a host of YAP and TAZ-dependent biological effects (indicated in boxes) specific for each cell type. Red-labelled nuclei indicate the nuclear activation of YAP and TAZ. For references see: part a:71–73; part b:27,80,82–84; part c:27,40–42,105,106; part d:102,103,105; part e:43,93,94,99,100,116 ; part f:28,45,64,65 ; part g:98; part h:105,110–112; part i:108,109. Legend: hES, human embryonic stem cells; MSC, mesenchymal stem cell; VSMCs, vascular smooth muscle cells.
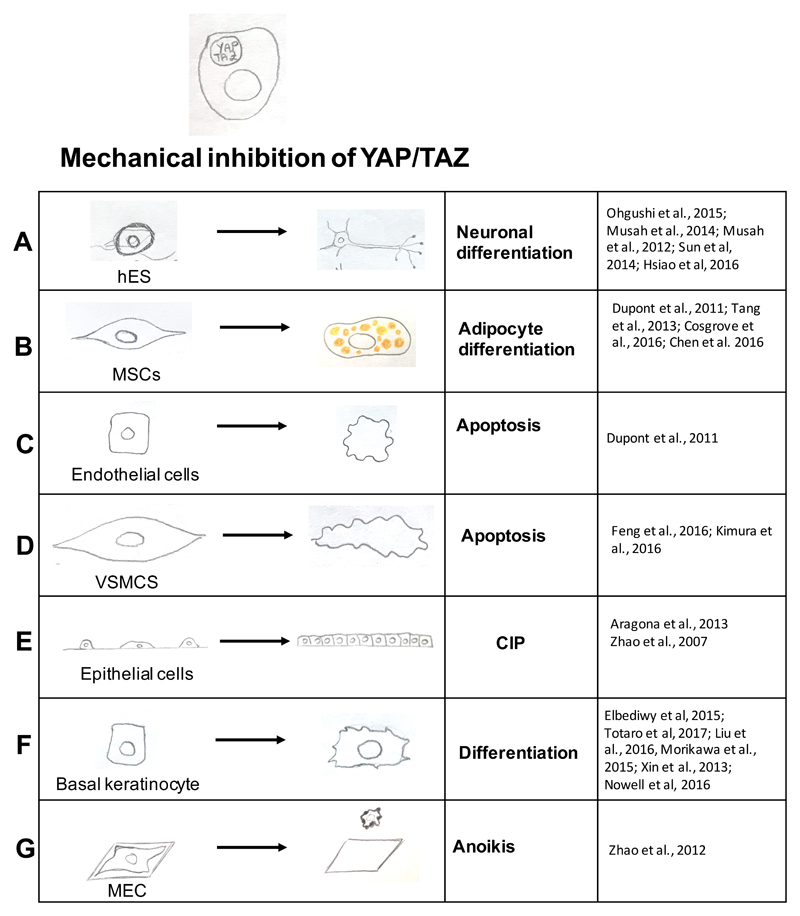
Figure 3. List of biological responses caused by cytoplasmic YAP and TAZ retention and inhibition due to low mechanical stimuli.
Low mechanical forces (see Figure 1, left panels) blunt YAP and TAZ activity and this results in a panel of YAP and TAZ-dependent biological effects in diverse cell types. For references see: part a:71,72,74–76; part b:27,80,81,85 ; part c:27 ; part d:102,103 ; part e:24,91 ; part f:43,93,94,99,100,116; part g:47. Legend: CIP, contact inhibition of proliferation; hES, human embryonic stem cells; MSC, mesenchymal stem cell; VSMCs, vascular smooth muscle cells; MEC, mammary epithelial cells.
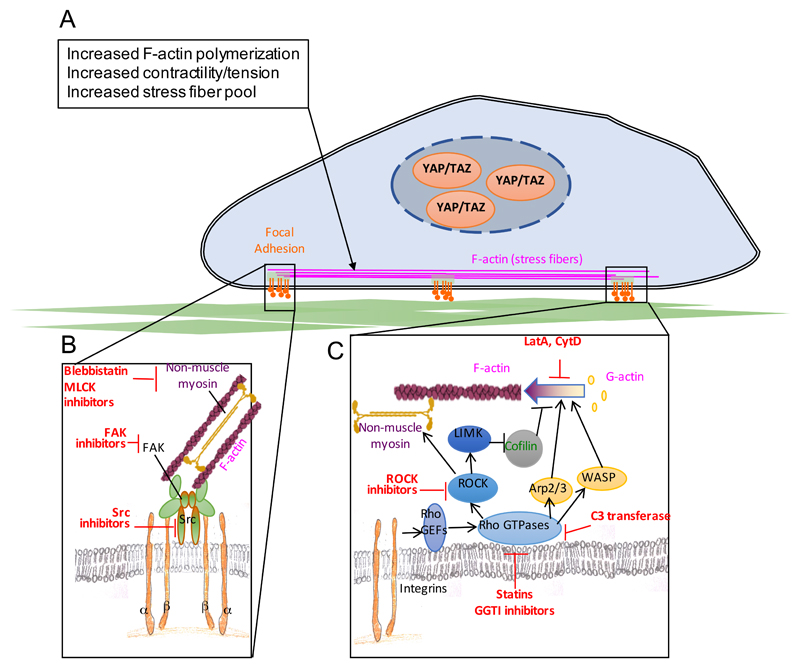
YAP and TAZ mechanotransduction requires integrity of the actin cytoskeleton and YAP and TAZ activity is experimentally abolished by inhibitors of filamentous actin (F-actin)22,27,32,34,47 (Figure 4). That said, YAP and TAZ regulation by mechanotransduction is unrelated to the ratio of free monomeric actin (G-actin) vs. F-actin. For example, rounded cells contain more total F-actin than spread cells48, and yet, in rounded cells YAP and TAZ are inhibited. Rather, YAP and TAZ mechanotransduction relies on a specific organization of the F-actin cytoskeleton, as revealed by the potent inhibitory effects of F-actin capping proteins, such as CAPZ (capping actin protein of muscle Z-line), ADF (actin depolymerizing factor) and CFL (Cofilin), on YAP and TAZ activity24,37,49, and similar regulations by other F-actin regulatory proteins, such as AMOT (angiomotin)50,51, Spectrins52,53 and ZO-2 (zonula occludens-2)54–58 (Figure 4). In addition, actin contractility and increased tension of actin cytoskeleton have been linked to YAP and TAZ activation24,27. However, the exact mechanisms of how actin cytoskeleton impacts YAP and TAZ signalling in the context of mechanotransduction are not known.
Figure 4. Molecular players involved in YAP and TAZ mechanotransduction.
(a) Cell–extracellular matrix (ECM) adhesion complexes undergo force-dependent conformational changes to trigger increase in actin polymerization and consequent increase in stress fibres contractility, resulting in YAP and TAZ activation. (b) a stiffer matrix causes integrin clustering which results in the activation of focal-adhesion associated kinases such as focal adhesion kinase (FAK) and Src, which in turn favour stress fibre growth, stability and contractility, thereby activating YAP and TAZ28,33,62,63,114,115. Src has also been shown to phosphorylate YAP and this was linked to YAP activation downstream of Src. However, whether such phosphorylation events by Src are indeed causal for effective YAP (and TAZ) activation remains to be determined. (c) signalling from focal adhesions activates Rho GTPases which can directly activate actin regulatory proteins such as actin-related protein 2/3 complex (Arp2/3) and Wiskott-Aldrich syndrome protein (WASP) or can favour filamentous -actin (F-actin) polymerization through the activation of Rho-associated protein kinase (ROCK); active ROCK promotes acto-myosin contractility and activates LIM domain protein kinase (LIMK), which in turn inhibits the F-actin severing protein Cofilin. In parts a and b boxes with inhibitory arrows list relevant chemical inhibitors of key players in the YAP and TAZ mechanotransduction pathway24,27,32,34,47. Legend: MLCK: myosin light chain kinase; GEFs: guanine nucleotide exchange factors; LatA: LatrunculinA; CytD: Cytochalasin D; GGTI: geranyl-geranyl transferase I; C3: clostridium botulinum C3 exoenzyme.
Once mechanical forces are detected at the cell surface, primarily at integrin–ECM and cell–cell adhesion sites, the information they carry must be propagated and preserved within the cytoskeleton before force dissipation59–61. This is mediated by mechanosensory systems that include integrins and adherens junctions, adaptor proteins such as vinculin and talins, as well the focal adhesion kinase (FAK) and Src-family of kinases that connect the extracellular mechanical world to the F-actin cytoskeleton62,63. Notably, these mechanosensory proteins have been involved in YAP and TAZ regulation28,33 (Figure 4).
Finally, cells are not passive force receivers but dynamically respond to external forces with a proportional cell-generated force. This occurs through assembly of an acto-myosin contractile network and cytoskeletal remodelling1. Consistently, in some cell types, YAP activation by a stiff ECM can be blunted by myosin light chain kinase (MLCK), non-muscle myosin type II or Rho-associated protein kinase (ROCK) inhibitors27. Moreover, integrin activation restructures the cytoskeleton by activating Rho-GTPases. Consistently, Rho signalling is essential for YAP and TAZ activity, and this has been experimentally exploited in a variety of systems, either genetically, or by Rho inhibitors24,27,32,34,47 (Figure 4; see also below).
Stem cell mechanobiology
In adult tissues, nuclear YAP and TAZ are typically found in locations enriched for somatic stem cells or progenitors, such as at the bottom of intestinal crypts, in cells of the basal layer of the skin, in basal and luminal mammary gland progenitors25,57,64,65. YAP and TAZ are remarkably essential for tissue repair in vivo66, or for the growth of organ-specific stem cells ex-vivo as organoids (Box 1). In contrast, they appear dispensable for normal homeostasis of most adult epithelial organs66. These seemingly at odd observations may be reconciled if one considers that, under homeostatic conditions, adult organs may keep their cell mechanics below the threshold required to activate YAP and TAZ-transcriptional effects. Recent results on mutant mice lacking cofilin/ADF in skin and liver, where extensive cytoskeletal remodeling and F-actin accumulation is accompanied by induction of massive organ overgrowth occurring in just few days (phenocopying the effects of YAP and TAZ activation)67 are consistent with the view that the level of mechanical tension and cytoskeletal organization of normal epithelial tissues are insufficient to sustain YAP and TAZ responses.
YAP and TAZ mechanobiology in embryonic stem cells
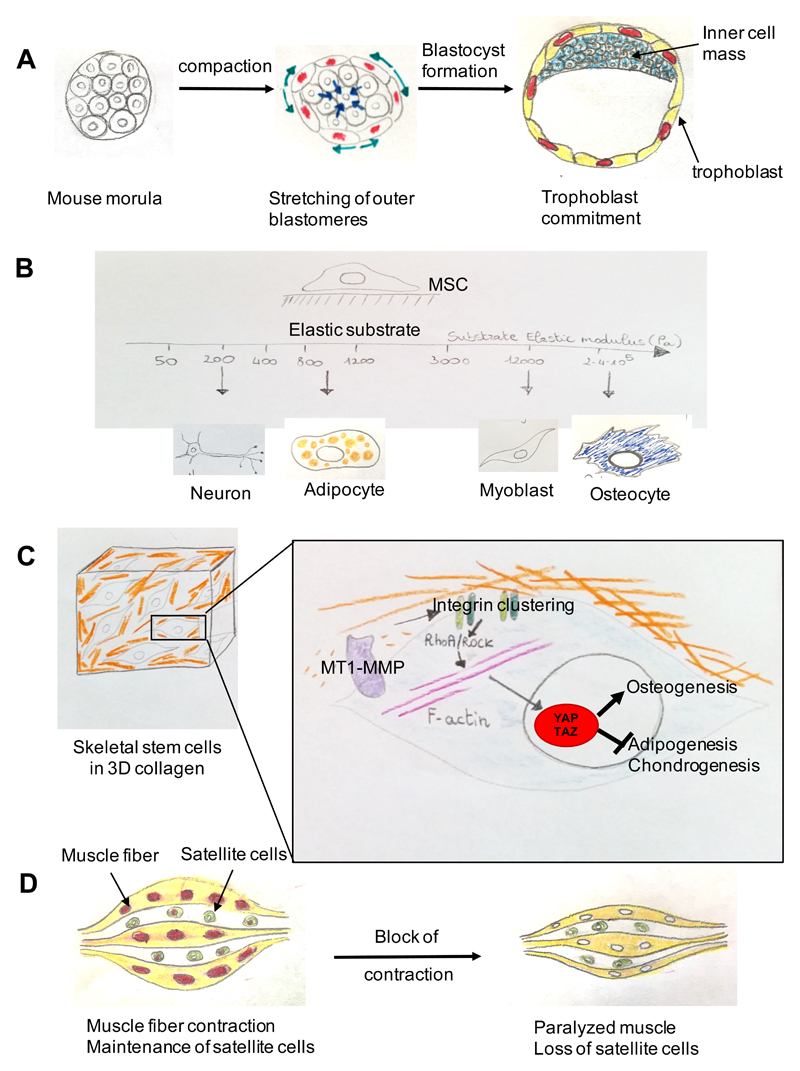
YAP and TAZ and their regulation by cell mechanics control the first cell fate decision of in the mammalian embryo, namely, the specification into trophoblast, or into inner cell mass cells (ICM). YAP and TAZ are required for the definition of the trophoblast fate, and experimental overactivation of YAP and TAZ in ICM cells is sufficient for them to acquire trophoblast markers68. In line, in a normal blastocyst YAP and TAZ are nuclear in trophoblast and cytoplasmic in ICM cells, respectively. The Hippo kinases LATS1/2 are relevant to blunt YAP and TAZ activity at these stages; yet, it remains unclear whether Hippo signalling itself is primarily involved in patterning YAP and TAZ in the blastocyst, or whether it provides an inhibitory signal setting a threshold above which the trophoblast fate is induced by other patterning signalling. Strikingly, recent work highlighted cell contractility and cell shape as signals regulating YAP and TAZ in the early mouse embryo. Contraction of ICM cells forces more polarized outer cells to stretch over the external surface of the embryo, fixing their fate as trophoblast cells. Inhibition of such pulling forces by exposing mouse embryos to Blebbistatin (an inhibitor of myosin II and microfilament contractility), or ROCK inhibitors, abolishes nuclear localization of YAP in outer cells and their trophoblast commitment69,70 (Figure 5a).
Figure 5. YAP and TAZ mechanotransduction in stem cell biology.
(a) Trophoblast commitment in the early mouse embryo involves a phase of stretching of the outer cells caused by compaction (inward pointing arrows) of the embryo. This stretching causes YAP and TAZ nuclear translocation in the outer cells and the acquisition of a trophoblast fate. Cells that do not activate YAP and TAZ become the cells of the embryo proper (the inner cell mass)69,70. (b) In vivo cells experience a range of different ECM elasticities in different tissues. By recapitulating these different stiffnesses in vitro, it was found that mesenchymal stem cells (MSCs) differentiate optimally into neurons, adipocytes, skeletal muscle cells or osteoblasts at specific elasticities that match the physiological ECM stiffness of their corresponding natural niche. (c) Control of skeletal stem cell lineage commitment mediated by ECM remodelling by membrane type 1-matrix metalloproteinase 1 (MT1-MMP). By affecting the ECM and thereby the cell shape of mesenchymal stem cells in a collagen-based 3D environment, the MT1-MMP promotes integrin clustering and the concomitant activation of the integrin–RhoA pathway, thereby triggering YAP and TAZ activation, which switches on a programme favouring osteogenic differentiation over alternative adipogenic and chondrogenic cell fates80. (d) YAP- and TAZ-dependent maintenance of satellite cells (muscle progenitors) by muscle contraction. In the chick embryo contraction of post-mitotic muscle fibres triggers YAP-dependent, expression of the Delta-like ligand JAG2, that activates NOTCH in muscle progenitor cells and prevents their differentiation. Blocking embryonic muscle contraction (muscle paralysis) inhibits YAP and transcription of JAG2 in myofibres, which causes a shift towards satellite cell differentiation. Enhanced differentiation eventually depletes the pool of muscle progenitor cells and interferes with regenerative potential of the tissue87. Similarly, expression of Delta-like ligands, such as DLL1 and JAG2, are also downstream of YAP and TAZ in epidermal stem cells43.
YAP and TAZ are required to foster survival and prevent differentiation of human embryonic stem cells (hESCs) allowing their long-term propagation71–73. YAP and TAZ are nuclear localized in hESCs cultured under standard conditions on stiff substrates, but relocalize to the cytoplasm when cells are forced to adopt a rounded shape by low mechanical signals. Coherently with YAP and TAZ inhibition, hESCs differentiate in these mechanically inhibited conditions71,72,74,75,76 (Figures 2 and 3). Based on these reports, it is tempting to speculate that differentiation of pluripotent ESCs might be directed toward specific cell fates by gradients of mechanical signals quantitatively tuning the strength and duration of YAP and TAZ activity, in a manner conceptually similar to the effects of gradients of soluble growth factors, or morphogens, that are thought to pattern the early embryo.
YAP and TAZ mechanotransduction guides mesenchymal stem cell differentiation
Mesenchymal stem cells (MSCs) are paradigm model systems in the mechanotransduction field, and it has been shown that differentiation of MSCs along distinct cell fates is actually dictated by the physical features of the cell microenvironment, and not by soluble signals77. This can be experimentally controlled by allowing cells to stretch on stiff substrates or, conversely, by inducing cell rounding either by confining cells to small adhesive areas, or culturing them on soft substrates (Figure 1).
Human MSCs (hMSCs) adopt an osteogenic fate when allowed to stretch (Figure 2), whereas they turn into adipocytes at low mechanical stresses (Figure 3). Treatments with inhibitors of actomyosin contractile units block osteogenesis and favor adipogenesis, indicating that mechanically-driven cell fate determination relies on the contractility of the actomyosin cytoskeleton. In line, overexpression of constitutive active ROCK forces osteogenic differentiation in cells plated on small fibronectin islands77. In another study it has been demonstrated that hMSCs differentiation could be regulated by the elasticity of the underlying substrate11. By matching the elastic modulus of distinct tissues, it was shown that MSCs adopt neurogenic, myogenic, or osteogenic fates at increasing substrate rigidities (figure 5b). This occurs independently from soluble cues, but depending on the degree of hMSC spreading and cytoskeletal contractility.
Despite these elegant bioassays, the nuclear effectors of MSC mechanosensitivity remained elusive until the identification of YAP and TAZ27. Previous work had shown that TAZ was required for osteogenic differentiation of MSCs78. Now it is known that YAP and TAZ transcriptional activity couples substrate compliance to hMSC differentiation, as YAP and TAZ depletion impairs osteogenic differentiation on stiff substrates, skewing MSCs towards adipogenic differentiation; conversely, overexpression of an hyperactive form of YAP is sufficient to redirect hMSCs towards an osteogenic fate on soft substrates27. Importantly, YAP and TAZ are genetically required for proper osteogenic differentiation, as Yap+/-;Taz+/- MSCs display impaired osteogenesis despite being cultured under permissive conditions79.
YAP and TAZ act as key mechanotransducers also in specialized MSCs, such as in skeletal progenitors (or skeletal stem cells, SSCs), when these are embedded within 3D collagen gels80. ECM remodeling is well known to occur during morphogenesis and wound healing; the discovery of YAP and TAZ allows linking such remodeling to nuclear responses. The matrix metalloproteinase MT1-MMP (Mmp14) is key to remodel collagen fibres in the 3D environment of SSCs, in turn promoting cytoskeletal tension through a RhoA-ROCK pathway and acquisition of a spread cell-shape, essential for YAP and TAZ activation. Indeed, Mmp14-/- SSCs display impaired osteogenic differentiation and enhanced adipogenic differentiation, both in vitro and in vivo in a manner that is genetically dependent on YAP and TAZ dosage80 (Figure 5c).
Mechanical regulation of hMSC fate is not only dependent on the physical properties of the underlying substrates, but also modulated by the physical contacts with other cells. During development, MSCs engage in cell-–cell and cell–ECM contacts simultaneously, raising questions on how cells integrate distinct mechanical inputs. This has been addressed in elegant in vitro experiments, which indicated that the engagement of N-cadherin (involved in cell–cell interactions) reduced the contractile state and thereby nuclear YAP and TAZ localization in MSCs, resulting in altered interpretation of ECM rigidity81.
MSCs can be mechanically regulated also by the shear stress generated in tissues by extravascular liquid flow. Culturing MSCs on microfluidic chips that mimic interstitial shear stress increases YAP and TAZ activity in a Rho–ROCK dependent manner, to enhance osteogenic and fibrochondrogenic differentiation82–84. Moreover, TAZ-dependent mechanotransduction has been proposed as the mechanism responsible for the microgravity-induced bone loss observed in space flights; culturing MSCs under microgravity indeed resulted in blunted TAZ nuclear accumulation and reduced osteogenesis85.
Integrin–FAK signalling to YAP and TAZ in tooth progenitors
The mouse incisors constantly grow, in part through constant deposition of enamel by epithelial cells generated from a pool of progenitor cells. This pool needs to expand before differentiation, and high nuclear YAP and TAZ activity is instrumental for this process. YAP and TAZ activation in this context requires signalling from the surrounding ECM transduced by integrin α3 and FAK, and is LATS-independent28.
YAP, muscle stem cells and muscle hypertrophy
Muscles grow in response to physical exercise and degenerate when muscle contraction is impaired. This is regulated in YAP-dependent manner. In mouse models, mechanical overload sustains YAP activity. This overload in turn can induce hypertrophy and is required to protect from atrophy caused by denervation. In skeletal muscle cells cultured in vitro, high YAP activity promotes proliferation of muscle stem cells, whereas YAP inactivation by soft substrates fosters myogenic differentiation86. The underlying mechanisms are only partially understood: in the chick embryo contraction of post-mitotic muscle fibres led to nuclear YAP, in turn leading to activation of NOTCH signalling in adjacent muscle progenitor cells, which prevented their differentiation87 (Figure 5d). Moreover, muscle size is known to be controlled by TGFβ–Myostatin–Smad signalling and by the mTOR–Akt cascade, which, in other contexts, have been shown to crosstalk with YAP and TAZ activity28,88–90. This raises the intriguing possibility that in the skeletal muscle YAP and TAZ may relay mechanical signals to other transcription factors or metabolic pathways, as such harmonizing complex cell behaviours to the cell's mechanical state.
Contact inhibition of proliferation
A classic "social-like" behaviour of epithelial cells is contact inhibition of proliferation (CIP), whereby the tissue stops growing after reaching a critical cell density. CIP is transiently relieved at a wound's margin after tissue damage, and more permanently overridden in tumours66. CIP can be molecularly explained by inhibition of YAP and TAZ91, simply because crowding confines cells to small areas, putting them into a low contractility and YAP and TAZ repressive cytoskeletal states24 (Figure 3). To a substantial extent, this occurs in LATS-independent manner24. Consistently with CIP being a mechanically regulated biological response, CIP is overcome either by ECM stiffening92, loss of actin capping and severing proteins, or cell stretching24,44 — processes that activate YAP and TAZ transcription (Figure 1). More generally, CIP provides a proof-of-principle that cells can read patterns of mechanical forces associated to specific 2D topologies and 3D architectures of living tissues, turning them into a pattern of YAP and TAZ activity that can then guide specific cell behaviours.
Mechanoregulation of regeneration
The healing of damaged tissues entails several steps, including changes in the stiffness of surrounding ECM and increased cellular mechanoresponsiveness in the cells bordering the wound. This may lead to dedifferentiation, transdifferentiation and acquisition of migratory and/or progenitor-like properties. A causal relationship between changes in the tissue's mechanical properties and YAP and TAZ activation during regeneration has been proposed in several tissues (Figure 2). One example is YAP-driven lung regeneration caused by surgical removal of a part of lung tissue (pneumonectomy)93. Pneumonectomy induces expansion of the remaining lung epithelia by activating latent stem cell properties of alveolar type II (AT2) cells, which eventually proliferate and differentiate into alveolar type I (AT1) cells, the main cell type of the alveolar epithelium. Pneumonectomy induces stretching and actin polymerization in AT2 cells through a mechanism that depends on the Rho-GTPase CDC42. In turn, cell stretching promotes YAP nuclear localization and transcriptional activities (Figure 2), which are required for the expansion of AT2 cells and replenishment of AT1 cells. Accordingly, genetic deletion of CDC42 or YAP impairs regeneration after pneumonectomy93.
Skin injury represents another model for YAP and TAZ-dependent regeneration. The epidermis is a paradigm mechanosensitive tissue: upon stretching - such as during growth-associated post-natal development or pregnancy - it adapts to body expansion by increasing its own size through an elevated proliferation of the basal cell layer, containing epidermal progenitors. Wound repair occurs through activation of basal keratinocytes at the wound's margin, which migrate toward the injured area and proliferate to mediate wound re-epithelialization. YAP and TAZ are activated in basal keratinocytes close to the wound (Figure 2), and are required for proper wound closure94. How YAP and TAZ are activated in the proximity of the wound margins remains only partially understood, but at least in vitro, "wounding" a cell monolayer results in a localized loss of cell polarity, loss of contact inhibition (see above), and cell stretching (Figure 2), whereas in vivo, increased rigidity of the underlying dermis occurs — all conditions associated to mechanical activation of YAP and TAZ. Intriguingly, loss of integrin-β1 results in impaired wound closure similarly to YAP and TAZ knockout95.
Indeed, mechanical signals, such as cell spreading and ECM rigidity can activate YAP and TAZ in basal keratinocytes (Figure 2), and this is required for preserving epidermal stemness (Figure 3), which occurs through the suppression of the Notch pathway, a potent inducer of epidermal differentiation and stratification43. Consistently, unscheduled activation of actomyosin-mediated cellular tension in the skin of transgenic mice by expressing ROCK2 induces epidermal hyperplasia in a manner that is attenuated by inhibition of FAK, LIM domain Kinase 1 (LIMK) or myosin ATPase96.
Mechanically-regulated YAP and TAZ activity may also have a role in cardiac regeneration. Myocardial infarction is accompanied by a prominent remodelling and stiffening of the myocardial ECM97 and YAP and TAZ are expressed and localized in the nucleus of murine cardiac cells at the infarction border zone98. YAP and TAZ have key functions in adult cardiac progenitor mechano-sensing responses, being instrumental for cardiomyocyte proliferation at specific rigidities98. YAP activation indeed increases cardiomyocyte proliferation, reduces scar size, and improves cardiac regeneration in a myocardial infarction model99,100 (Figure 2). Thus, activation of YAP through biomaterial design may offer new means of promoting cardiac repair after injury.
Mechanotransduction in diseases
Aberrant mechanical signalling has been associated with the pathogenesis of multiple diseases, and this can be caused by changes in the physical and structural features of the cell microenvironment or by defects in how cells perceive mechanical inputs1–8. Strikingly, most of these defects eventually impact on cell behaviour through YAP and TAZ deregulation.
Atherosclerosis and cardiovascular diseases
YAP and TAZ are key factors in the pathophysiology of the cardiovascular system, whose development and homeostasis are intimately connected to mechanical forces associated with blood flow, and for which defects in mechanical parameters are often the primary initiators of disease3. Endothelial cells are a convenient model in mechanobiology. These cells experience vascular shear stress, and blood pressure, and their behaviour is under the control of cell mechanics: by using small and large microprinted adhesive areas, it has been shown that individual endothelial cells could be induced to proliferate or die depending on cell shape, that is, by the degree of cytoskeletal distortion and reorganization allowed by the geometry of their environment10.
YAP and TAZ knockdown impairs proliferation of endothelial cells allowed to stretch over big microprinted islands, whereas YAP overexpression inhibits apoptosis and rescues proliferation of endothelial cells seeded on small islands27 (Figures 2 and 3). This prompted further studies showing that YAP and TAZ mechanotransduction mediates the pathologic effects of disturbed blood flow on atherogenesis3. YAP and TAZ are inhibited in endothelial cells cultured under static fluid flow (which is atheroprotective), whereas they localize to the nucleus when cultured under disturbed (oscillatory) flow conditions (which mimic atherogenic mechanical stress)40,41 (Figure 1). In these in vitro conditions, nuclear YAP and TAZ foster endothelial cell proliferation and expression of adhesive factors for monocytes, correlating with key pathogenic events in atherosclerosis, such as endothelial thickening, and recruitment of monocytes, which eventually turn into plaque-forming foam cells.
YAP is also genetically required for this atherogenic response of endothelial cells in vivo41, thereby offering an unexpected therapeutic opportunity for atherosclerosis. Main anti-atherosclerotic drugs are statins which are cholesterol-lowering compounds and are commonly used as first-line therapy for patients with cardiovascular diseases; however, the cholesterol biosynthetic pathway that is targeted by statins also generates the geranylgeranyl lipid intermediates that anchor Rho GTPases to the plasma membrane and are essential for cytoskeletal tension and remodelling in the YAP and TAZ mechanotransduction pathway32 (Figure 4). Consequently, anti-inflammatory and anti-plaque effects of statins are now being re-interpreted as being mediated, at least in part, by their capacity to inhibit YAP and TAZ40. More broadly, this suggests that YAP and TAZ mechanotransduction could be specifically targeted for improved atherosclerosis treatment.
Recent work on Yap1-null zebrafish embryos showed vessel regression in mutant larvae, with vessel stenosis and retraction between 4.5 and 6.5 days post fertilization. This phenotype can be associated with the requirement of YAP and TAZ activation by shear stress and acto-myosin contractility in endothelial cells for vasculogenesis at the onset of blood circulation42.
Apart from endothelial cells, mechanically-regulated YAP and TAZ activity plays a part in vascular smooth muscle cells (VSMCs) for vessel development and homeostasis. Yap knockout in smooth muscle cells in mice results in embryonic lethality possibly due to abnormal aortic development101. YAP and TAZ also drive proliferation and migration of VSMCs (Figure 2) in the context of vessel restenonsis after coronary angioplasty. These responses can be blunted by inhibition of Rho, ROCK or F-actin remodelling, which interfere with YAP and TAZ activation and result in VSMC apoptosis102,103 (Figures 3 and 4).
Pulmonary hypertension is a deadly vascular disease, characterized by increases in proliferation of endothelial cells and VSMCs, and by abundant deposition and stiffening of the vascular ECM104. It has been recently proposed that YAP and TAZ activation downstream of ECM stiffening serves as molecular driver of pulmonary hypertension105,106. Indeed, a stiff ECM activates YAP and TAZ in vivo and in vitro, triggering proliferation of adventitial fibroblasts, endothelial cells and VSMCs; in turn, YAP and TAZ increase ECM deposition and remodelling via collagen synthesis, lysil oxidase (LOX) production, and secretion of pro-fibrotic factors105, all in all fuelling a YAP and TAZ mechanoactive feedback. Of note, YAP and TAZ mechanotransduction may contribute to well-established metabolic dysfunctions associated with pulmonary hypertension, including increased glycolysis at the expenses of mitochondrial oxidative phosphorylation because YAP and TAZ directly control the transcription of key metabolic enzymes responsible for glycolysis and glutaminolysis105,106. Importantly, reduction of ECM stiffening with LOX inhibitors blunted nuclear YAP and TAZ and ameliorated the end-stage manifestations of pulmonary hypertension in murine models106. It is tempting to propose that targeting mechano-activated YAP and TAZ and/or their downstream effectors in pulmonary hypertension could be a valuable tool to treat patients affected by this devastating disease.
Tissue fibrosis
Fibrotic diseases, which include liver cirrhosis, pulmonary, kidney and cardiac fibrosis, and systemic sclerosis, account for at least one-third of deaths worldwide. The cardinal traits shared by these diseases is the abnormal remodelling and excessive deposition of ECM by activated fibroblasts, also known as myofibroblasts, over a prolonged period of time; this triggers tissue stiffening, cellular dysfunction and eventually organ failure. There is thus great interest in developing means to oppose or even reverse fibrosis. Although the cellular players involved are well understood, the signalling pathways underlying fibrosis are less clear107. Importantly, YAP and TAZ-mechanotransduction is emerging as a key player in these processes.
In liver fibrosis, YAP is activated in hepatic stellate cells in response to liver damage in vivo and to ECM stiffening ex-vivo108,109. Activation of hepatic stellates by tissue damage triggers YAP nuclear translocation, and increase in YAP total levels and activity. This also results in the increase in collagen deposition and smooth muscle actin expression, which are associated with the generation of myofibroblasts (Figure 2). Interestingly, in the fibrotic areas of livers from patients suffering from hepatitis C, myofibroblasts displayed strong YAP nuclear staining and hepatic stellate cell activation could be reversed by pharmacologic inhibition of YAP108.
YAP and TAZ are also induced in the lungs of patients suffering from idiopathic pulmonary fibrosis, and YAP and TAZ sustain a pro-fibrotic transcriptional programme in human lung fibroblasts both in vitro and in vivo110,111 (Figure 2), which includes increased collagen and fibronectin deposition and secretion of pro-fibrotic cytokines. Consistently, Taz-heterozygous mice were resistant to bleomycin-induced lung fibrosis112. Of note YAP and TAZ-dependent collagen deposition is itself fostered by ECM stiffness, indicating the presence of mechanically self-reinforcing positive loops, similarly to the feedback regulation described for pulmonary hypertension.
A fascinating open issue is whether targeting YAP and TAZ mechanotransduction might have therapeutic effects, or even reverse tissue fibrosis.
YAP and TAZ as mediators of inflammatory responses
Inflammation is the response to injury, which entails recruitment of leukocytes and plasma proteins, activation of a fibrogenic response, and ECM remodelling. Acute inflammation is a transient response, integral to the regenerative response of normal tissues that stops after the healing process is completed. Importantly, YAP mediates epithelial tissue regeneration after inflammatory damage66,113; in these contexts, YAP activation is Hippo/LATS-independent but dependent on SRC kinases33. SRC activity is a potent inducer of cytoskeletal remodelling downstream of FAK and integrin signalling114,115 raising the enticing possibility that inflammation may activate YAP and TAZ also by inducing increased mechano-responsiveness in epithelial cells.
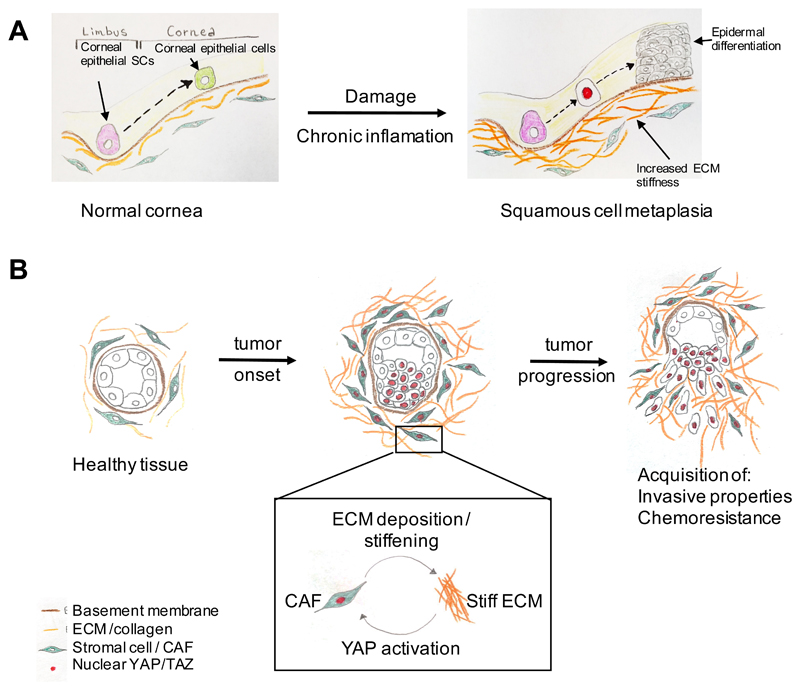
The potential links between inflammation and YAP and TAZ-mechanotransduction are particularly appealing in the context of chronic inflammatory diseases, which, differently from acute inflammation, represent persistent conditions, characterized by constant tissue damage and repair that progressively erode tissue integrity and function. The causes of chronic inflammation are poorly understood, but the persistence of a disturbed tissue architecture that never heals may causally contribute to such maladaptive responses. Although still awaiting further validation, a link between chronic inflammation, ECM stiffening and altered YAP and TAZ signalling has recently been demonstrated. In a mouse model of corneal squamous cell metaplasia, corneal wounding induces chronic inflammation and excessive deposition of ECM by the corneal stroma and ensuing YAP and TAZ nuclear localization. In this mechanically aberrant environment, epithelial stem and progenitor cells are then driven to transdifferentiate into keratinizing, epidermal-like cells, resulting in metaplasia and ocular surface blindness116 (Figure 6a).
Figure 6. Deregulation of YAP and TAZ mechanotransduction in disease.
Aberrant mechanotransduction can lead to abnormal YAP and TAZ activation (red nuclei) in a variety of pathological conditions. These include: (a) the change of fate of corneal epithelial cells into epidermal cells resulting in squamous cell metaplasia116; this is induced by tissue damage and chronic inflammation, which results in extracellular matrix (ECM) stiffening (b) cancer development36,66,118; this involves the activation of YAP in cancer associated fibroblasts (CAFs) by ECM stiffening, which in turn promotes the activity of CAFs and leads to collagen deposition and further stiffening of the ECM. As a result, a mechanically-activated positive feedback loop operates that exacerbates the pathological outcome (see box)36. Stiff ECM also promotes YAP and TAZ-mediated mechanotransduction in the cells of the developing tumour, which promotes their metastatic dissemination and acquisition of chemoresistance. Intriguingly, a similar mechanical feedback mechanism is likely to occur also in the case of pulmonary hypertension105,106 and fibrosis110,111.
Muscular dystrophy
Defects in YAP-mediated mechanosensing have been reported in patients carrying mutations in lamin-type A (LMNA) associated with congenital muscular dystrophy. These cells present several defects in the organization of the cytoskeleton, causing aberrant YAP and TAZ mechanotransduction: YAP is cytoplasmic in healthy control myoblasts when these are cultured on a soft ECM, but remains nuclear in soft hydrogel cultures of LMNA mutant cells. Paradoxically, YAP activity is blunted in these mutant cells when they are challenged by cycles of stretch and relaxation117.
Instructive roles of YAP and TAZ in cancer development
YAP and TAZ are pervasively activated in human cancers, where they are required to instruct malignant properties, including unrestrained proliferation, cell survival, chemoresistance and metastasis66,118. These effects may be explained, at least in part, by the ability of active YAP and TAZ to convert benign neoplastic cells into cancer stem cells66,119. It should be noted, however, that in spite of their widespread activation in tumours, activating mutations in YAP or TAZ have not been reported in human cancers; moreover, Hippo pathway mutations are rare in human tumors and not yet detected in carcinomas. Mechanical inputs from the aberrant tumor microenvironment are thus prime candidates to induce YAP and TAZ over-activity in cancer cells. These inputs include aberrant tissue organization, accumulation of stromal cells, inflammation, increased compression forces and interstitial pressure, metalloprotease-mediated ECM remodelling by cancer activated fibroblasts (CAFs) and overall ECM stiffening1,120,121 (Figure 6b).
YAP and TAZ regulation by the cytoskeleton might represent an interesting target for the design of anti-cancer therapeutics. Of note, recent studies suggest that mechanical activation of YAP and TAZ in cancer cells is also relevant for acquisition of chemoresistance (122,123,124; see also ref66 for review). However, given the toxicity of anti-cytoskeletal treatments, therapeutic options are currently limited to inhibitors of Rho and its upstream inducers, such as Src, FAK and integrins. As in the case of atherosclerosis, drugs that target geranylgeranyl synthesis, thereby blocking Rho GTPase recruitment to the membrane, such as statins, bisphosphonates and geranylgeranyl transferase-1 inhibitors, display YAP and TAZ inhibiting abilities in several cancer cell types32. Statins and bisphosphonates also reduce the growth of tumour xenografts32,35,125, although their effects appeared stronger in vitro than in vivo. Some epidemiological studies showed that patients under statin therapies display reduced risk to develop cancer126, although there are conflicting data on this topic127 highlighting the need of further clinical studies on the potential of these drugs for cancer treatment and/or prevention.
In several tumours, cancer-associated fibroblasts (CAFs) are responsible for increasing ECM stiffness, and for changing the cellular composition of tumour niches by secreting chemokines, as such promoting tumor invasion and angiogenesis. These properties rely on activation of YAP in CAFs, and occur downstream of ECM stiffening36. In turn, increased YAP activity in CAFs promotes contractility and restructuring of the ECM, locking fibroblast in a vicious mechanically-activated positive feedback loop that self-sustains YAP and TAZ activation in both CAFs and tumour cells (Figure 6b).
Perspectives and key questions
In this review, we highlighted the role of YAP and TAZ as a molecular entry point to understand how mechanical forces impact cell behaviour and tissue pathophysiology from the micrometer to the macroscopic scales. Despite rapid progress, however, this field is still in its infancy and several outstanding questions remain unanswered.
First, the mechanisms by which cell mechanics regulate YAP and TAZ are elusive. F-actin integrity and organization is now broadly accepted as a dominant input to regulate YAP and TAZ. But how does it occur exactly? Many hypothetical scenarios can be proposed; for example YAP and TAZ may be normally kept inactive by inhibitory proteins, and F-actin itself or F-actin associated proteins may oppose this inhibitory association. Also unclear is where YAP and TAZ regulation by mechanotransduction occurs. Several lines of evidence indicate that nuclear localization of YAP and TAZ may not automatically translate into transcriptional activation. Actomyosin-generated cytoskeletal tension regulates nuclear shape and, intriguingly, force transfer from the cytoplasm to the nucleus through the linker of nucleoskeleton and cytoskeleton (LINC) protein complex is essential for activation of the YAP and TAZ pathway128. Moreover, the nuclear lamina has been recently shown to be mechanosensitive and indeed affecting YAP and TAZ activity129. This raises an interesting question of whether nuclear mechanics are critical for YAP and TAZ activity. Unfortunately, the nuclear lamina and cytoskeleton are not preserved in a typical cell lysate, hampering our ability to capture perhaps the most relevant YAP and TAZ interacting partners by Mass Spectrometry.
The exact connections between Hippo signalling and mechanotransduction of YAP and TAZ are also elusive. Redundant lines of evidence from various model systems indicate that YAP and TAZ mechanotransduction occurs independently from Hippo kinases24–35, although these two pillars of YAP and TAZ regulation must obviously intercept to act coherently. There is currently no evidence that Hippo/LATS activity is patterned in contexts in which YAP and TAZ activity is instead regulated in an exquisitely cell- and temporal-specific manner66. Rho and F-actin cytoskeleton are overarching determinants for the effect of the Hippo kinases on YAP and TAZ, and the integrity of the F-actin cytoskeleton is essential for YAP inhibition by LATS. At the same time, it is also clear that LATS activity itself is under cytoskeletal control47,130 and that, LATS can also affect the cytoskeleton23. Addressing these issues is imperative to attain an integrated molecular overview of YAP and TAZ regulation by cell mechanics. This should allow to shed light on one of the outstanding problems in cell biology, that is, understanding the nature of self-organizing properties of stem cells and tissues, whereby a sequence of mechanical and architectural checkpoints must be achieved to allow unperturbed development, tissue repair and homeostasis.
The role of YAP and TAZ and their regulation by the multitude of physical tissue deformations occurring during gastrulation and morphogenesis of distinct organs remains, to date, a largely unexplored area of investigation. Embryonic over-activation of YAP and TAZ has been connected to organ overgrowth at least in some tissues (liver, hear and stomach); intriguingly, this is a reversible process, as the organ shrinks back to its "correct" size after normalization of YAP and TAZ levels. Thus, each cell of an organ must perceive the presence of all other cells in order to stop its growth when the organ has reached its final size. Diffusion of morphogen gradients is poorly suited to carry out these effects. Instead, mechanical forces transmitted through the interconnected network of cytoskeletal and ECM proteins may ideally serve as long-range messengers of this global control informing individual cell with nanometer accuracy. This remains an enticing speculation, and in its favour growth and shaping of the fly wing primordium is YAP dependent and controlled by global patterns of mechanical stress that originate from its stretched tissue border131. Already in 1917 D'Arcy Thompson published his visionary book "On Growth and Form"132 where he proposed that "diagrams of forces" underlie tissue patterning and that force-oriented changes in the 3D shape of tissues may dictate the evolutionary aspects of morphological diversities between related species. The integration of YAP and TAZ biology with evo-devo approaches, along with material science and mathematical modelling may offer fresh experimental perspectives to these visionary ideas. Crucially, addressing these issues would require the implementation of new tools allowing, on the one hand, the ablation of YAP and TAZ in individual organs during development, and, on the other, genetic manipulation of cell mechanics in vivo.
One of the most remarkable properties of mechanically-activated YAP and TAZ, at least in vitro, is their ability to trigger conversion of differentiated cells into their corresponding tissue-specific stem cells61. Is this exploited in vivo? Are YAP/TAZ specifically induced when the homeostatic, repressive tissue architecture is lost after damage and inflammation? Experimental overexpression of YAP also awakens regenerative potentials that are normally not exploited by mature tissues. Heart regeneration is a point in case, as forced YAP activation rescues cardiomyocyte proliferation and fosters organ recovery after myocardial infarction in mice99,100. Is lack of regeneration linked to the installment of a mechanical environment that is not permissive for proper YAP/TAZ activation? Can biomaterials be exploited as therapeutic tools to foster such latent healing powers?
Finally, what are the evolutionary roots of the YAP and TAZ involvement in mechanotransduction? The widespread relevance of a unique set of transcription factors, in multiple tissues and cell-types, downstream of a broad array of pathophysiological mechanical stresses, is both stunning and surprising. Of note, Drosophila YAP (Yki) is also under control of F-actin organization37,49. All this likely reflects a conserved function of YAP and TAZ in extracting information from mechanical signals. Intriguingly, YAP appearance predates multicellularity, being first detected in the genome of the closest unicellular relatives of the animals133. It would be interesting to test whether also in these unicellular systems YAP activity is connected to sensing substrate mechanics.
Key points.
-
1)
Mechanical signals are fundamental regulators of cell behaviour, but how mechanical cues are sensed and transduced at the molecular level to regulate gene expression has long remained enigmatic.
-
2)
YAP and TAZ have been identified as conserved mechanotransducers, reading a very diverse set of mechanical cues, from shear stress to cell shape and extracellular matrix rigidity, and translate them into cell-specific transcriptional programmes.
-
3)
YAP and TAZ mechanotransduction offers new interpretational keys and experimental opportunities tools to re-define classic aspects of tissue physiology and pathology in molecular terms.
-
4)
YAP and TAZ as mechanotransducers provides insight into how aberrant cell mechanics drive the onset of multiple diseases, including atherosclerosis, fibrosis, cardiac hypertrophy, muscular dystrophy and cancer.
Glossary.
Shear stress: It is the force vector component coplanar with a material cross-section; a fluid moving along a solid boundary will generate a shear stress on that boundary.
TEAD factors: TEA domain family members (also known as TEF) are a family of DNA-binding transcription factors that cannot induce target gene transcription on their own, but need to associate with specific co-factors, such as YAP and TAZ.
Mechanotransduction: mechanisms by which cells convert mechanical signals conveyed by the microenvironment into biochemical signals to adapt their behavior to the environment.
Capping proteins: Proteins (e.g. CapZ but also ADF, Cofilin that are also F-actin severing proteins) that bind to the growing (barbed) end of F-actin filaments, blocking the binding of other actin subunits to the growing filament.
Vinculin: Focal adhesion protein that links the cytosolic domain of integrins to the F-actin cytoskeleton.
Talins: Focal adhesion proteins that link integrin to the F-actin cytoskeleton either directly or indirectly (through binding to vinculin or α-actinin).
Focal Adhesion Kinase: FAK (also known as PTK2) is a cytosolic tyrosine kinase associated with focal adhesions and required for integrin-dependent cell motility, cell spreading and cell survival.
Src-family of kinases: A family of tyrosine kinases (composed of nine different members: Src, Yes, Fyn, Fgr, Lck, Hck, Blk, Lyn, Frk) that can target cytosolic, nuclear or membrane proteins, having a broad impact on the phosphotyrosine proteome and on diverse cell behaviours.
Rho-associated protein kinase (ROCK): Serine-threonine kinase best understood as downstream effector of the small GTPase RhoA. ROCK favors formation of stress fibres and contractility of the acto-myosin cytoskeleton by phosphorylating LIMK and Myosin light chain kinase.
Intestinal crypts: simple tubular invaginations at the base of intestinal villi (also known as crypts of Lieberkühn). Intestinal crypts are lined by different cell types: goblet, enteroendocrine, tuft, paneth cells and intestinal stem cells.
Trophoblast: Outer layer of a blastocyst, composed of polarized cells from which part of the placenta originates.
Inner cell mass (ICM): mass of cells lying on one side of the blastocyst cavity from which all the adult tissues eventually derive
Blastocyst: Structure formed during the development of mammalian embryos following morula stage, before implantation; composed by an external layer (trophoblast) surrounding an internal cavity (blastocoele) and the ICM.
Elastic modulus: Measure of the resistance of an object to elastic deformation.
Enamel: One of the four different tissues that compose the tooth; it is the outermost highly mineralized tissue formed by epithelial cells known as ameloblasts.
LIM domain kinase (LIMK1 and LIMK2): actin-bound kinases that phosphorylate and inactivate actin binding and severing proteins ADF/Cofilin, thereby fostering the stabilization of F-actin filaments. These kinases are activated by Rho GTPases.
Foam cells: Lipid-filled M2 macrophages that form when monocytes are recruited to an atherogenic area of the arterial wall; these are derived from monocytes that engulf lipoprotein particles and turn into foam cells that contribute to the formation of the atherosclerotic plaque.
Vessel stenosis: Abnormal narrowing of a blood vessel (might be referred to as coarctation in the case of the carotid artery).
Coronary angioplasty: Surgical endovascular procedure performed to widen the narrowed (stenotic) coronary arteries of the heart.
Adventitial fibroblasts: fibroblasts that are part of the area between the external elastic lamina and the outermost edge of a blood vessel (adventitia).
Lysil oxidase: enzyme that promotes collagen and elastin crosslinking.
Hepatic stellate cells: Pericytes found in the perisinusoidal space of the liver; these are the major cell type involved in fibrosis in response to liver damage.
Metaplasia: Reversible transition of a differentiated cell type to another differentiated cell type, that can be caused in a tissue by an abnormal stimulus; once the stimulus ceases, the tissue returns to its normal differentiation condition. Metaplasia is often considered an early phase of the tumorigenic process.
Myoblasts: embryonic progenitor cells that can differentiate into muscle cells. Myoblasts that do not differentiate into muscle cells in the skeletal muscle fibre can turn into satellite cells (somatic muscle stem cells).
Linker of nucleoskeleton and cytoskeleton (LINC) complex: Protein complex associated to the nuclear membrane composed of SUN-domain and KASH-domain proteins. It serves as linker between nuclear lamins and chromatin with cytosolic actin microfilaments, microtubule filaments and intermediate filaments.
Evo-devo (evolutionary developmental biology): A field in biological studies that compares the developmental processes of different organisms to trace their evolutionary relationships and to infer the evolution of developmental processes.
Acknowledgments
We thank all members of SP lab for discussion. This work is supported by AIRC Special Program Molecular Clinical Oncology “5 per mille”, by an AIRC PI-Grant to S.P, and by Epigenetics Flagship project CNR-MIUR grants to S.P. This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No 670126-DENOVOSTEM).
Author biographies
Tito Panciera is a post-doctoral fellow at the University of Padua Medical School (DMM). He contributed to the discovery of cell mechanics as YAP and TAZ regulator in epithelial sheets and recently reported the ability of YAP and TAZ to endow cell plasticity and induce stem cell traits in differentiated cells.
Luca Azzolin is a post-doctoral fellow at the University of Padua Medical School (DMM). He contributed to the discovery of YAP and TAZ as transducers of the Wnt pathway, as essential for organoid growth and self-renewal and for the in vivo pivotal roles of YAP and TAZ for cell transformation, cancer emergence and inflammation by pioneering combined YAP and TAZ conditional mouse models.
Michelangelo Cordenonsi is Professor of Embryology and Histology at University of Padua Medical School (DMM). He discovered the interplay between p53 family members and TGFb signalling in development, cancer and metastasis. He then moved to study the Hippo pathway, revealing the role of cell polarity as structural determinant of YAP and TAZ activation and first reported the ability of TAZ expression to confer stemness properties to otherwise benign tumor cells.
Stefano Piccolo is Professor of Molecular Biology at University of Padua Medical School. He contributed to the molecular mechanisms shared by embryonic patterning, cancer and metastasis. He contributed to the fields of TGFβ, Wnt and Hippo signalling. He discovered YAP and TAZ mechanotransduction.
Footnotes
Author contributions
All authors contributed equally to all aspects of the article (researching data for article, substantial contribution to discussion of content, writing, review/editing of manuscript before submission).
The authors declare no competing financial interests.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Subject categories
Biological sciences / Cell biology / Cell adhesion / Mechanotransduction [URI /631/80/79/2066]
Biological sciences / Cell biology / Cell adhesion / Extracellular matrix [URI /631/80/79/750]
Biological sciences / Cell biology / Cell migration / Integrin signalling [URI /631/80/84/2342]
Biological sciences / Cell biology / Cytoskeleton / Actin [URI /631/80/128/1276]
Biological sciences / Cell biology / Mechanisms of disease [URI /631/80/304]
eTOC
YAP and TAZ transcription factors have recently emerged as conserved transducers of mechanical inputs from the environment into cellular responses, including proliferation, migration and cell fate decisions. Instructive roles of YAP and TAZ mechanotransduction have now been documented in many physiological and pathological contexts, providing novel insights into cellular mechano-responses and their consequences.
References
- 1.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nature reviews Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nat Rev Mol Cell Biol. 2009;10:63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mammoto A, Ingber DE. Cytoskeletal control of growth and cell fate switching. Curr Opin Cell Biol. 2009;21:864–870. doi: 10.1016/j.ceb.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Provenzano PP, Keely PJ. Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling. Journal of cell science. 2011;124:1195–1205. doi: 10.1242/jcs.067009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 8.Wozniak MA, Chen CS. Mechanotransduction in development: a growing role for contractility. Nat Rev Mol Cell Biol. 2009;10:34–43. doi: 10.1038/nrm2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keller R. Developmental biology. Physical biology returns to morphogenesis. Science. 2012;338:201–203. doi: 10.1126/science.1230718. [DOI] [PubMed] [Google Scholar]
- 10.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [A classic in the field, elegantly showing that cellular mechanotransduction can affect a classic binary cell decision, such as cell death and proliferation. Later on, Dupont et al., 2011 showed that this control is indeed a YAP and TAZ-dependent event.] [DOI] [PubMed] [Google Scholar]
- 11.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [Another classic in the field, remarkably demonstrating that ECM ridigity instructs cellular differentiation. Dupont et al., 2011 then demonstrated that this is caused by YAP and TAZ-regulation.] [DOI] [PubMed] [Google Scholar]
- 12.Folkman J, Moscona A. Role of cell shape in growth control. Nature. 1978;273:345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- 13.Watt FM, Jordan PW, O'Neill CH. Cell shape controls terminal differentiation of human epidermal keratinocytes. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:5576–5580. doi: 10.1073/pnas.85.15.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zanconato F, et al. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nature cell biology. 2015;17:1218–1227. doi: 10.1038/ncb3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galli GG, et al. YAP Drives Growth by Controlling Transcriptional Pause Release from Dynamic Enhancers. Mol Cell. 2015;60:328–337. doi: 10.1016/j.molcel.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stein C, et al. YAP1 Exerts Its Transcriptional Control via TEAD-Mediated Activation of Enhancers. PLoS Genet. 2015;11:e1005465. doi: 10.1371/journal.pgen.1005465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vassilev A, Kaneko KJ, Shu H, Zhao Y, DePamphilis ML. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes & development. 2001;15:1229–1241. doi: 10.1101/gad.888601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Wu S, Liu Y, Zheng Y, Dong J, Pan D. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Developmental cell. 2008;14:388–398. doi: 10.1016/j.devcel.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Pan D. The hippo signaling pathway in development and cancer. Developmental cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu FX, Zhao B, Guan KL. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell. 2015;163:811–828. doi: 10.1016/j.cell.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiological reviews. 2014;94:1287–1312. doi: 10.1152/physrev.00005.2014. [DOI] [PubMed] [Google Scholar]
- 23.Gaspar P, Tapon N. Sensing the local environment: actin architecture and Hippo signalling. Curr Opin Cell Biol. 2014;31:74–83. doi: 10.1016/j.ceb.2014.09.003. [A well balanced review outlining the role of F-actin organization as central for YAP and TAZ biology, the evolutionary conservation of this connection and the potential crosstalks and mutual regulations between YAP and TAZ regulation by mechanotransduction and Hippo signalling.] [DOI] [PubMed] [Google Scholar]
- 24.Aragona M, et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154:1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 25.Barry ER, et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature. 2013;493:106–110. doi: 10.1038/nature11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das A, Fischer RS, Pan D, Waterman CM. YAP Nuclear Localization in the Absence of Cell-Cell Contact Is Mediated by a Filamentous Actin-dependent, Myosin II- and Phospho-YAP-independent Pathway during Extracellular Matrix Mechanosensing. J Biol Chem. 2016;291:6096–6110. doi: 10.1074/jbc.M115.708313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dupont S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [This is the original paper reporting YAP/TAZ as readers and effectors of mechanical signalling.] [DOI] [PubMed] [Google Scholar]
- 28.Hu JK, et al. An FAK-YAP-mTOR Signaling Axis Regulates Stem Cell-Based Tissue Renewal in Mice. Cell stem cell. 2017 doi: 10.1016/j.stem.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rashidian J, et al. Ski regulates Hippo and TAZ signaling to suppress breast cancer progression. Sci Signal. 2015;8:ra14. doi: 10.1126/scisignal.2005735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reginensi A, et al. Yap- and Cdc42-dependent nephrogenesis and morphogenesis during mouse kidney development. PLoS Genet. 2013;9:e1003380. doi: 10.1371/journal.pgen.1003380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ren F, Zhang L, Jiang J. Hippo signaling regulates Yorkie nuclear localization and activity through 14-3-3 dependent and independent mechanisms. Developmental biology. 2010;337:303–312. doi: 10.1016/j.ydbio.2009.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sorrentino G, et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nature cell biology. 2014;16:357–366. doi: 10.1038/ncb2936. [DOI] [PubMed] [Google Scholar]
- 33.Taniguchi K, et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519:57–62. doi: 10.1038/nature14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wada K, Itoga K, Okano T, Yonemura S, Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development. 2011;138:3907–3914. doi: 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, et al. Interplay of mevalonate and Hippo pathways regulates RHAMM transcription via YAP to modulate breast cancer cell motility. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E89–98. doi: 10.1073/pnas.1319190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calvo F, et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nature cell biology. 2013;15:637–646. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandez BG, et al. Actin-Capping Protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila. Development. 2011;138:2337–2346. doi: 10.1242/dev.063545. [DOI] [PubMed] [Google Scholar]
- 38.Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol. 2012;13:591–600. doi: 10.1038/nrm3416. [DOI] [PubMed] [Google Scholar]
- 39.Schroeder MC, Halder G. Regulation of the Hippo pathway by cell architecture and mechanical signals. Semin Cell Dev Biol. 2012;23:803–811. doi: 10.1016/j.semcdb.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 40.Wang KC, et al. Flow-dependent YAP/TAZ activities regulate endothelial phenotypes and atherosclerosis. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:11525–11530. doi: 10.1073/pnas.1613121113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L, et al. Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature. 2016 doi: 10.1038/nature20602. [DOI] [PubMed] [Google Scholar]
- 42.Nakajima H, et al. Flow-Dependent Endothelial YAP Regulation Contributes to Vessel Maintenance. Developmental cell. 2017;40:523–536 e526. doi: 10.1016/j.devcel.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 43.Totaro A, et al. YAP/TAZ link cell mechanics to Notch signalling to control epidermal stem cell fate. Nature communications. 2017;8 doi: 10.1038/ncomms15206. 15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benham-Pyle BW, Pruitt BL, Nelson WJ. Cell adhesion. Mechanical strain induces E-cadherin-dependent Yap1 and beta-catenin activation to drive cell cycle entry. Science. 2015;348:1024–1027. doi: 10.1126/science.aaa4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gjorevski N, et al. Designer matrices for intestinal stem cell and organoid culture. Nature. 2016;539:560–564. doi: 10.1038/nature20168. [DOI] [PubMed] [Google Scholar]
- 46.Eyckmans J, Boudou T, Yu X, Chen CS. A hitchhiker's guide to mechanobiology. Developmental cell. 2011;21:35–47. doi: 10.1016/j.devcel.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao B, et al. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes & development. 2012;26:54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Connelly JT, et al. Actin and serum response factor transduce physical cues from the microenvironment to regulate epidermal stem cell fate decisions. Nature cell biology. 2010;12:711–718. doi: 10.1038/ncb2074. [DOI] [PubMed] [Google Scholar]
- 49.Sansores-Garcia L, et al. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. The EMBO journal. 2011;30:2325–2335. doi: 10.1038/emboj.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mana-Capelli S, Paramasivam M, Dutta S, McCollum D. Angiomotins link F-actin architecture to Hippo pathway signaling. Molecular biology of the cell. 2014;25:1676–1685. doi: 10.1091/mbc.E13-11-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moleirinho S, et al. Regulation of localization and function of the transcriptional co-activator YAP by angiomotin. eLife. 2017;6 doi: 10.7554/eLife.23966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deng H, et al. Spectrin regulates Hippo signaling by modulating cortical actomyosin activity. eLife. 2015;4:e06567. doi: 10.7554/eLife.06567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fletcher GC, et al. The Spectrin cytoskeleton regulates the Hippo signalling pathway. The EMBO journal. 2015;34:940–954. doi: 10.15252/embj.201489642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oka T, et al. Functional complexes between YAP2 and ZO-2 are PDZ domain-dependent, and regulate YAP2 nuclear localization and signalling. Biochem J. 2010;432:461–472. doi: 10.1042/BJ20100870. [DOI] [PubMed] [Google Scholar]
- 55.Pan CQ, Sudol M, Sheetz M, Low BC. Modularity and functional plasticity of scaffold proteins as p(l)acemakers in cell signaling. Cell Signal. 2012;24:2143–2165. doi: 10.1016/j.cellsig.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 56.Remue E, et al. TAZ interacts with zonula occludens-1 and -2 proteins in a PDZ-1 dependent manner. FEBS Lett. 2010;584:4175–4180. doi: 10.1016/j.febslet.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 57.Schlegelmilch K, et al. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Silvis MR, et al. alpha-catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1. Sci Signal. 2011;4:ra33. doi: 10.1126/scisignal.2001823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 2014;15:802–812. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iskratsch T, Wolfenson H, Sheetz MP. Appreciating force and shape-the rise of mechanotransduction in cell biology. Nat Rev Mol Cell Biol. 2014;15:825–833. doi: 10.1038/nrm3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Low BC, et al. YAP/TAZ as mechanosensors and mechanotransducers in regulating organ size and tumor growth. FEBS Lett. 2014;588:2663–2670. doi: 10.1016/j.febslet.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 62.Galbraith CG, Yamada KM, Sheetz MP. The relationship between force and focal complex development. J Cell Biol. 2002;159:695–705. doi: 10.1083/jcb.200204153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tzima E, del Pozo MA, Shattil SJ, Chien S, Schwartz MA. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. The EMBO journal. 2001;20:4639–4647. doi: 10.1093/emboj/20.17.4639. [A key contribution on the role of Rho, downstream of integrin, in the response to a classic mechanical signal. Dupont et al., 2011 then connected Rho-GTPase signaling and their biological effects in cell mechanics to YAP/TAZ regulation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Azzolin L, et al. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 65.Panciera T, et al. Induction of Expandable Tissue-Specific Stem/Progenitor Cells through Transient Expression of YAP/TAZ. Cell stem cell. 2016;19:725–737. doi: 10.1016/j.stem.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the Roots of Cancer. Cancer cell. 2016;29:783–803. doi: 10.1016/j.ccell.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kanellos G, et al. ADF and Cofilin1 Control Actin Stress Fibers, Nuclear Integrity, and Cell Survival. Cell reports. 2015;13:1949–1964. doi: 10.1016/j.celrep.2015.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nishioka N, et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Developmental cell. 2009;16:398–410. doi: 10.1016/j.devcel.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 69.Kono K, Tamashiro DA, Alarcon VB. Inhibition of RHO-ROCK signaling enhances ICM and suppresses TE characteristics through activation of Hippo signaling in the mouse blastocyst. Developmental biology. 2014;394:142–155. doi: 10.1016/j.ydbio.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maitre JL, et al. Asymmetric division of contractile domains couples cell positioning and fate specification. Nature. 2016;536:344–348. doi: 10.1038/nature18958. [A recent report on the role of cell mechanics and YAP and TAZ regulation as determinants of the first cell fate decision in mammalian embryos.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ohgushi M, Minaguchi M, Sasai Y. Rho-Signaling-Directed YAP/TAZ Activity Underlies the Long-Term Survival and Expansion of Human Embryonic Stem Cells. Cell stem cell. 2015;17:448–461. doi: 10.1016/j.stem.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 72.Musah S, et al. Substratum-induced differentiation of human pluripotent stem cells reveals the coactivator YAP is a potent regulator of neuronal specification. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:13805–13810. doi: 10.1073/pnas.1415330111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beyer TA, et al. Switch enhancers interpret TGF-beta and Hippo signaling to control cell fate in human embryonic stem cells. Cell reports. 2013;5:1611–1624. doi: 10.1016/j.celrep.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 74.Musah S, et al. Glycosaminoglycan-binding hydrogels enable mechanical control of human pluripotent stem cell self-renewal. ACS nano. 2012;6:10168–10177. doi: 10.1021/nn3039148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hsiao C, et al. Human pluripotent stem cell culture density modulates YAP signaling. Biotechnology journal. 2016;11:662–675. doi: 10.1002/biot.201500374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun Y, et al. Hippo/YAP-mediated rigidity-dependent motor neuron differentiation of human pluripotent stem cells. Nature materials. 2014;13:599–604. doi: 10.1038/nmat3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Developmental cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [Another seminal paper introducing the role of Rho signalling and cell shape as dictators of the behaviour of mesenchymal stem cells.] [DOI] [PubMed] [Google Scholar]
- 78.Hong JH, et al. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science. 2005;309:1074–1078. doi: 10.1126/science.1110955. [DOI] [PubMed] [Google Scholar]
- 79.Tang Y, Feinberg T, Keller ET, Li XY, Weiss SJ. Snail/Slug binding interactions with YAP/TAZ control skeletal stem cell self-renewal and differentiation. Nature cell biology. 2016;18:917–929. doi: 10.1038/ncb3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tang Y, et al. MT1-MMP-dependent control of skeletal stem cell commitment via a beta1-integrin/YAP/TAZ signaling axis. Developmental cell. 2013;25:402–416. doi: 10.1016/j.devcel.2013.04.011. [A key demonstration that cells must remodel their microenvironment through metalloproteases in order to exploit new mechanical niches to foster YAP and TAZ activity in progenitor cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cosgrove BD, et al. N-cadherin adhesive interactions modulate matrix mechanosensing and fate commitment of mesenchymal stem cells. Nature materials. 2016;15:1297–1306. doi: 10.1038/nmat4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhong W, et al. Mesenchymal stem cell and chondrocyte fates in a multishear microdevice are regulated by Yes-associated protein. Stem Cells Dev. 2013;22:2083–2093. doi: 10.1089/scd.2012.0685. [DOI] [PubMed] [Google Scholar]
- 83.Zhong W, Zhang W, Wang S, Qin J. Regulation of fibrochondrogenesis of mesenchymal stem cells in an integrated microfluidic platform embedded with biomimetic nanofibrous scaffolds. PloS one. 2013;8:e61283. doi: 10.1371/journal.pone.0061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim KM, et al. Shear stress induced by an interstitial level of slow flow increases the osteogenic differentiation of mesenchymal stem cells through TAZ activation. PloS one. 2014;9:e92427. doi: 10.1371/journal.pone.0092427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen Z, Luo Q, Lin C, Kuang D, Song G. Simulated microgravity inhibits osteogenic differentiation of mesenchymal stem cells via depolymerizing F-actin to impede TAZ nuclear translocation. Scientific reports. 2016;6 doi: 10.1038/srep30322. 30322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Judson RN, et al. The Hippo pathway member Yap plays a key role in influencing fate decisions in muscle satellite cells. Journal of cell science. 2012;125:6009–6019. doi: 10.1242/jcs.109546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Esteves de Lima J, Bonnin MA, Birchmeier C, Duprez D. Muscle contraction is required to maintain the pool of muscle progenitors via YAP and NOTCH during fetal myogenesis. eLife. 2016;5 doi: 10.7554/eLife.15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Varelas X, Wrana JL. Coordinating developmental signaling: novel roles for the Hippo pathway. Trends Cell Biol. 2012;22:88–96. doi: 10.1016/j.tcb.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 89.Strassburger K, Tiebe M, Pinna F, Breuhahn K, Teleman AA. Insulin/IGF signaling drives cell proliferation in part via Yorkie/YAP. Developmental biology. 2012;367:187–196. doi: 10.1016/j.ydbio.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 90.Tumaneng K, et al. YAP mediates crosstalk between the Hippo and PI(3)K-TOR pathways by suppressing PTEN via miR-29. Nature cell biology. 2012;14:1322–1329. doi: 10.1038/ncb2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhao B, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes & development. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [A classic paper on the role of YAP and TAZ as determinant of CIP. Later on, Aragona et al., 2013 showed that CIP regulate cell proliferation primarily by F-actin organization and mechanical signals, such as stretching vs. cell compaction.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim JH, Asthagiri AR. Matrix stiffening sensitizes epithelial cells to EGF and enables the loss of contact inhibition of proliferation. Journal of cell science. 2011;124:1280–1287. doi: 10.1242/jcs.078394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu Z, et al. MAPK-Mediated YAP Activation Controls Mechanical-Tension-Induced Pulmonary Alveolar Regeneration. Cell reports. 2016;16:1810–1819. doi: 10.1016/j.celrep.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 94.Elbediwy A, et al. Integrin signalling regulates YAP and TAZ to control skin homeostasis. Development. 2016;143:1674–1687. doi: 10.1242/dev.133728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grose R, et al. A crucial role of beta 1 integrins for keratinocyte migration in vitro and during cutaneous wound repair. Development. 2002;129:2303–2315. doi: 10.1242/dev.129.9.2303. [DOI] [PubMed] [Google Scholar]
- 96.Samuel MS, et al. Actomyosin-mediated cellular tension drives increased tissue stiffness and beta-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer cell. 2011;19:776–791. doi: 10.1016/j.ccr.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiological reviews. 2007;87:1285–1342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 98.Mosqueira D, et al. Hippo pathway effectors control cardiac progenitor cell fate by acting as dynamic sensors of substrate mechanics and nanostructure. ACS nano. 2014;8:2033–2047. doi: 10.1021/nn4058984. [DOI] [PubMed] [Google Scholar]
- 99.Morikawa Y, et al. Actin cytoskeletal remodeling with protrusion formation is essential for heart regeneration in Hippo-deficient mice. Sci Signal. 2015;8:ra41. doi: 10.1126/scisignal.2005781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xin M, et al. Hippo pathway effector Yap promotes cardiac regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:13839–13844. doi: 10.1073/pnas.1313192110. [Refs 99 and 100 reveal the remarkable capacity of YAP activation to trigger cardiac regeneration in mammals, a property lost more than 300 millions years ago, and requiring actin cytoskeletal remodelling.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang Y, et al. Deletion of yes-associated protein (YAP) specifically in cardiac and vascular smooth muscle cells reveals a crucial role for YAP in mouse cardiovascular development. Circ Res. 2014;114:957–965. doi: 10.1161/CIRCRESAHA.114.303411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Feng X, et al. Thromboxane A2 Activates YAP/TAZ Protein to Induce Vascular Smooth Muscle Cell Proliferation and Migration. J Biol Chem. 2016;291:18947–18958. doi: 10.1074/jbc.M116.739722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kimura TE, et al. The Hippo pathway mediates inhibition of vascular smooth muscle cell proliferation by cAMP. J Mol Cell Cardiol. 2016;90:1–10. doi: 10.1016/j.yjmcc.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lammers S, et al. Mechanics and function of the pulmonary vasculature: implications for pulmonary vascular disease and right ventricular function. Compr Physiol. 2012;2:295–319. doi: 10.1002/cphy.c100070. [DOI] [PubMed] [Google Scholar]
- 105.Bertero T, et al. Matrix Remodeling Promotes Pulmonary Hypertension through Feedback Mechanoactivation of the YAP/TAZ-miR-130/301 Circuit. Cell reports. 2015;13:1016–1032. doi: 10.1016/j.celrep.2015.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bertero T, et al. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J Clin Invest. 2016;126:3313–3335. doi: 10.1172/JCI86387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rockey DC, Bell PD, Hill JA. Fibrosis--a common pathway to organ injury and failure. N Engl J Med. 2015;372:1138–1149. doi: 10.1056/NEJMra1300575. [DOI] [PubMed] [Google Scholar]
- 108.Mannaerts I, et al. The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. Journal of hepatology. 2015;63:679–688. doi: 10.1016/j.jhep.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 109.Caliari SR, et al. Stiffening hydrogels for investigating the dynamics of hepatic stellate cell mechanotransduction during myofibroblast activation. Scientific reports. 2016;6 doi: 10.1038/srep21387. 21387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu F, et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. American journal of physiology. Lung cellular and molecular physiology. 2015;308:L344–357. doi: 10.1152/ajplung.00300.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jorgenson AJ, et al. TAZ activation drives fibroblast spheroid growth, expression of profibrotic paracrine signals, and context-dependent ECM gene expression. American journal of physiology. Cell physiology. 2017;312:C277–C285. doi: 10.1152/ajpcell.00205.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Noguchi S, et al. TAZ contributes to pulmonary fibrosis by activating profibrotic functions of lung fibroblasts. Scientific reports. 2017;7 doi: 10.1038/srep42595. 42595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cai J, et al. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes & development. 2010;24:2383–2388. doi: 10.1101/gad.1978810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sorrentino G, et al. Glucocorticoid receptor signalling activates YAP in breast cancer. Nature communications. 2017;8 doi: 10.1038/ncomms14073. 14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huveneers S, Danen EH. Adhesion signaling - crosstalk between integrins, Src and Rho. Journal of cell science. 2009;122:1059–1069. doi: 10.1242/jcs.039446. [DOI] [PubMed] [Google Scholar]
- 116.Nowell CS, et al. Chronic inflammation imposes aberrant cell fate in regenerating epithelia through mechanotransduction. Nature cell biology. 2016;18:168–180. doi: 10.1038/ncb3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bertrand AT, et al. Cellular microenvironments reveal defective mechanosensing responses and elevated YAP signaling in LMNA-mutated muscle precursors. Journal of cell science. 2014;127:2873–2884. doi: 10.1242/jcs.144907. [DOI] [PubMed] [Google Scholar]
- 118.Zanconato F, Battilana G, Cordenonsi M, Piccolo S. YAP/TAZ as therapeutic targets in cancer. Curr Opin Pharmacol. 2016;29:26–33. doi: 10.1016/j.coph.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cordenonsi M, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147:759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 120.Erler JT, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 121.Levental KR, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [A seminal contribution on the role of cell mechanics as driver of malignancy. Aragona et al., 2013 then showed that stiffening the 3D environment of mammary tumor cells indeed fostered YAP/TAZ activation and malignant properties.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lin CH, et al. Microenvironment rigidity modulates responses to the HER2 receptor tyrosine kinase inhibitor lapatinib via YAP and TAZ transcription factors. Molecular biology of the cell. 2015;26:3946–3953. doi: 10.1091/mbc.E15-07-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hirata E, et al. Intravital imaging reveals how BRAF inhibition generates drug-tolerant microenvironments with high integrin beta1/FAK signaling. Cancer cell. 2015;27:574–588. doi: 10.1016/j.ccell.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim MH, et al. Actin remodeling confers BRAF inhibitor resistance to melanoma cells through YAP/TAZ activation. The EMBO journal. 2016;35:462–478. doi: 10.15252/embj.201592081. [A very original paper on the role of YAP and TAZ mechanotransduction as inducer of chemoresistance.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li Z, et al. The Hippo transducer TAZ promotes epithelial to mesenchymal transition and cancer stem cell maintenance in oral cancer. Molecular oncology. 2015;9:1091–1105. doi: 10.1016/j.molonc.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Karp I, Behlouli H, Lelorier J, Pilote L. Statins and cancer risk. The American journal of medicine. 2008;121:302–309. doi: 10.1016/j.amjmed.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 127.Goldstein MR, Mascitelli L, Pezzetta F. Do statins prevent or promote cancer? Curr Oncol. 2008;15:76–77. doi: 10.3747/co.v15i2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Driscoll TP, Cosgrove BD, Heo SJ, Shurden ZE, Mauck RL. Cytoskeletal to Nuclear Strain Transfer Regulates YAP Signaling in Mesenchymal Stem Cells. Biophys J. 2015;108:2783–2793. doi: 10.1016/j.bpj.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Swift J, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341 doi: 10.1126/science.1240104. 1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yu FX, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Legoff L, Rouault H, Lecuit T. A global pattern of mechanical stress polarizes cell divisions and cell shape in the growing Drosophila wing disc. Development. 2013;140:4051–4059. doi: 10.1242/dev.090878. [DOI] [PubMed] [Google Scholar]
- 132.Thompson DAW. On growth and form. 1st edn. University Press; 1917. [Google Scholar]
- 133.Sebe-Pedros A, Zheng Y, Ruiz-Trillo I, Pan D. Premetazoan origin of the hippo signaling pathway. Cell reports. 2012;1:13–20. doi: 10.1016/j.celrep.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bartfeld S, Clevers H. Stem cell-derived organoids and their application for medical research and patient treatment. Journal of molecular medicine. 2017 doi: 10.1007/s00109-017-1531-7. [DOI] [PubMed] [Google Scholar]
- 135.Gregorieff A, Liu Y, Inanlou MR, Khomchuk Y, Wrana JL. Yap-dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer. Nature. 2015;526:715–718. doi: 10.1038/nature15382. [DOI] [PubMed] [Google Scholar]
- 136.Tan Q, Choi KM, Sicard D, Tschumperlin DJ. Human airway organoid engineering as a step toward lung regeneration and disease modeling. Biomaterials. 2017;113:118–132. doi: 10.1016/j.biomaterials.2016.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]