Abstract
This study considered the involvement of the mesial temporal lobe (MTL) in language and verbal memory functions in healthy children and adolescents. We investigated 30 healthy, right-handed children and adolescents, aged 7 to 16, with a fMRI language paradigm and a comprehensive cognitive test battery. We found significant MTL activations during language fMRI in all participants; 63% of them had left lateralized MTL activations, 20% exhibited right MTL lateralization, and 17% showed bilateral MTL involvement during the fMRI language paradigm. Group analyses demonstrated a strong negative correlation between the lateralization of MTL activations and language functions. Specifically, children with less lateralized MTL activation showed significantly better vocabulary skills. These findings suggest that the mesial temporal lobes of both hemispheres play an important role in language functioning, even in right-handers. Our results furthermore show that bilateral mesial temporal lobe involvement is advantageous for vocabulary skills in healthy, right-handed children and adolescents.
Index Medicus: functional neuroimaging, hippocampus, parahippocampal gyrus, language, cognitive function
1. Introduction
The mesial temporal lobe (MTL) is traditionally considered critical for episodic memory functions, yet its contribution to language processing is a recent finding. Our work and others demonstrate with functional magnetic resonance imaging (fMRI) that the MTL plays a role in vocabulary and verbal fluency in healthy right- and left-handed adults (Alessio et al., 2006; Bartha et al., 2003; Bonelli et al., 2011; Tomaszewki Farias, Harrington, Broomand, & Seyal, 2005). Clinical evidence suggests that focal lesions in the left, but also the right MTL induce linguistic deficits, especially in naming and category fluency (Bartha-Doering & Trinka, 2014; Bartha, Benke, Bauer, & Trinka, 2005a; Bartha et al., 2004; Gabrieli, Cohen, & Corkin, 1988; Powell et al., 2008; Sabsevitz et al., 2003). Furthermore, it has been shown that left hippocampal pathology affects language reorganization in adults and children (Hamberger et al., 2007; Liegeois et al., 2004).
These findings underscore the role of the MTL in naming and word retrieval, though its involvement in broader language skills is not yet fully understood. The hippocampal formations have long been known for their role in relational binding and in online information processing in memorization (Eichenbaum, Yonelinas, & Ranganath, 2007). Duff and colleagues have proposed that these processes are not only important for episodic memory, but also support the language processing system in rapid access of information and integration of contextual information (Duff & Brown-Schmidt, 2012; Kurczek, Brown-Schmidt, & Duff, 2013). This hypothesis is supported by findings in patients with hippocampal amnesia that have deficits in establishing, recovering, maintaining, and the use of relational memory representations in conversation (Duff, Gupta, Hengst, Tranel, & Cohen, 2011; Duff, Hengst, Tranel, & Cohen, 2008).
The lateralization of language is of clinical and theoretical relevance. The degree of language lateralization represents a prognostic factor of language deficits after unilateral brain lesions (Jansen et al., 2006; Knecht et al., 2002). The determination of language lateralization is also important for the preoperative investigation of epilepsy patients, as the risk of postoperative language and memory deficits is related to preoperative language lateralization (Bell, Davies, Haltiner, & Walters, 2000; Bonelli et al., 2012; Sabsevitz et al., 2003). Furthermore, disorders like autism (Kleinhans, Muller, Cohen, & Courchesne, 2008; Knaus et al., 2010), specific language impairment (Badcock, Bishop, Hardiman, Barry, & Watkins, 2012; de Guibert et al., 2011), and dyslexia (Heim, Eulitz, & Elbert, 2003) have been linked to abnormal language lateralization.
For the recognition and understanding of atypical language lateralization patterns in children and adolescents with neurological diseases, knowledge about language lateralization in healthy children and its possible impact on language functioning is important. Some studies in healthy children and adolescents show a correlation between better linguistic abilities and greater left lateralization of language regions (Everts et al., 2009; Groen, Whitehouse, Badcock, & Bishop, 2012). However, not all studies find that increased lateralization is associated with better performance; rather some studies suggest that the relationship between language lateralization and language functioning in healthy children may be task and region dependent (Berl et al., 2010; Berl et al., 2014; Lidzba et al., 2011).
Accordingly, the relevance of laterality for MTL involvement in language processing is unknown. Clinical studies in adults reported linguistic deficits in both left and right temporal lobe epilepsy patients (Bartha-Doering & Trinka, 2014). However, a significant association of left hippocampal activations during a language fMRI task with naming performance has been shown in healthy participants, and in patients before and after epilepsy surgery (Bartha et al., 2005b; Bonelli et al., 2011). Moreover, better naming correlated with larger activation in the remaining left posterior hippocampus following epilepsy surgery (Bonelli et al., 2012). Few studies have been conducted with children. Recently, Sepeta et al. (2016) demonstrated MTL activation during an auditory description definition task not only in healthy adults, but also in the majority of healthy children, thus underlining the role of MTL in language early in development. Yet, the developmental finding was that activation in the MTL was more bilateral in children than in adults. Thus, there may be developmental differences in the role of left and right MTL, respectively, in neural language organization. However, these authors did not examine how language laterality in the MTL is associated with language performance.
In the present study, we investigated the association between MTL activation during an auditory description definition task and language competence in healthy children and adolescents. We hypothesized that, similar to lateralization of classical language areas, stronger lateralized activation of the MTL will be associated with better language performance in healthy children and adolescents.
2. Methods
2.1. Participants
Thirty healthy children and adolescents, aged 7 to 16 years (mean 10.27, sd 2.80), participated in the study. All participants (12 girls, 18 boys) were native, monolingual German speakers with no history of neurological disease and no clinical evidence of neurological dysfunction or developmental delay. No study participant was on medication. All children and adolescents had normal or corrected-to-normal vision and normal hearing. All participants were right-handed, Edinburgh Handedness Inventory EHI (Oldfield, 1971) ranging from +50 to +100 (mean 93.33, sd 12.95). Participants were recruited by flier distribution at the Medical University Vienna and received a 30 € voucher for a book store. The study was approved by the Ethics Committee of the Medical University Vienna in accordance with the Helsinki Declaration of 1975.
2.2. Data acquisition
2.2.1. Neuropsychological examinations
All participants underwent standardized cognitive assessment. Expressive vocabulary was investigated with the Wortschatz- und Wortfindungstest WWT (Glück, 2011). This test provides information about expressive vocabulary in different lexical categories including nouns, verbs, and adverbs/adjectives. Immediate auditory attention, short-term, and working memory was measured by digit span forward and backwards tasks of the Hamburger-Wechsler-Intelligenztest für Kinder IV (Petermann & Petermann, 2008). Verbal learning was assessed using the Verbaler Lern- und Merkfähigkeitstest (Helmstaedter, Lendt, & Lux, 2001), the German version of the Auditory Verbal Learning Test (Lezak, 1995). This test provides scores for verbal learning learning curve, verbal short-term recall after distraction, verbal long-term recall, and verbal recognition. Semantic verbal fluency was evaluated with the Regensburger Wortflüssigkeitstest (Aschenbrenner, Tucha, & Lange, 2001) which requires the participant to name, within two minutes, as many words as possible in the category of animals (Regensburger Wortflüssigkeitstest, RWT).
In order to gain information about non-linguistic cognitive functioning, a perceptual reasoning index was measured with the subtests block design, matrix reasoning, and picture completion of the Hamburg-Wechsler Intelligenztest für Kinder HAWIK IV (Petermann & Petermann, 2008), the German equivalent of the Wechsler Intelligence Scale for Children.
Raw scores of cognitive tests were transformed into age adjusted percentiles for each cognitive test. For the WWT vocabulary norms are only available from 6 to 10;11 years of age. For the ten adolescents aged 11-16, we therefore decided to transform the WWT raw scores into percentiles based on the 10;11 year old children with the risk of an overestimation of percentile rank results in the elder participants. We therefore included analyses for the WWT results without the ten adolescents aged 11-16 yielding a sample of n=20 (marked in the analyses with n=20).
2.2.2. fMRI Paradigm
During fMRI assessment, the German version of an auditory description definition task was applied. This paradigm reliably lateralizes language and elicits mesial temporal lobe activation in healthy children (Balsamo, Xu, & Gaillard, 2006; Berl et al., 2014; Gaillard et al., 2007; Sepeta et al., 2016; You et al., 2011). During this task, participants listened via headphones to a definition of an object followed by a noun. Participants were instructed to press a button each time they judged that the description did not match the noun. For instance, “A long yellow fruit is a banana.” (true response) or “Something you sit on is a spaghetti.” (not true). Seventy percent of items were correct targets, matching pairs were pseudo-randomly distributed. This paradigm requires comprehension of a phrase, vocabulary knowledge, semantic recall, and a semantic decision. During the control condition, participants listened to verbal stimuli presented in reverse speech. The participants were instructed to press a button each time he/she heard a tone following the auditory string. The baseline was designed to control for first and second order auditory processing, attention, and motor response, while engaging the broad language processing network on an individual basis (You et al., 2011). Task performance was evaluated by the overall percentage accuracy for the language task and the control task separately.
Target nouns were selected according to linguistic criteria including word frequency, word length, and word complexity and were well balanced within five semantic categories. Three age appropriate levels of difficulty were available (7–9 years old, 10–12 years old, 13-16 years old). The difficulty level was achieved by manipulating the task vocabulary based on word normative data (http://www.wortschatz-unileipzig.de).
A block design was used composed by five language task blocks alternating with five baseline blocks. Each block lasted for 40 seconds and consisted of 10 sentences presented every four seconds. Total fMRI scan time was 6 minutes 40 seconds. Prior to the MRI measurement, children were prepared for the MRI session with a video clip and a training session. The video showed the MRI setting, including the MRI noise, and followed a child from entrance to the MRI institute until the scanning procedure. In addition, the study participants were asked to practice the fMRI paradigm with 10 training-items of the language task and the control task, respectively.
2.2.3. MRI Image acquisition
All participants were scanned on a 3T Siemens TIM Trio scanner (Siemens Medical Solutions, Erlangen, Germany) equipped with a high-performance gradient system to support fast, high-resolution whole-brain echo-planar imaging. 3D structural MRI scans were performed using an isocubic magnetization-prepared rapid gradient-echo (MPRAGE, T1-weighted, TE/TR _ 4.21/2300ms, inversion time 900, with a matrix size of 240 x 256 x 160, voxel size 1 x 1 x 1.10mm, flip angle 9°) sequence. FMRI was acquired using a phase corrected blipped gradient echo, single shot echo planar imaging (EPI) sequence. Altogether, 200 EPI volumes were acquired with a square FOV of 210 mm, voxel size 2.1 x 2.1 x 4 mm, 25 percent gap and 20 slices aligned parallel to the AC-PC plane using a repetition time (TR) of 2000 msec, echo time (TE) 42 msec, and a flip angle of 90.
2.3. Data analysis
Pre-processing and statistical analyses were carried out in SPM8 (Wellcome Department of Cognitive Neurology, London, United Kingdom) using MATLAB (Version 8.3 Mathworks, Inc., Sherborn, MA, U.S.A.). EPI volumes were spatially realigned and corrected for movement. Employing the Template-O-Matic toolbox TOM (Wilke, Holland, Altaye, & Gaser, 2008), customized prior probability maps and one customized T1 template were created, matched to age and gender composition of the study group. This approach to template creation employs the general linear model and is based on pediatric imaging data from the NIH study on healthy brain development (Evans & The Brain Development Cooperative Group, 2006). Each subject’s anatomical image was segmented with the customized priors and the customized T1 template. After coregistration, the derived spatial normalization parameters were used to normalize the functional volumes. Normalized EPI volumes were smoothed using a spatial filter kernel of FWHM of 8 mm. BOLD signal increases pertaining to task-evoked responses in brain activity were modeled as a block design using a general linear model as implemented in SPM. The canonical double-gamma hemodynamic response function (HRF) was convolved with the block design. Regressors modeling residual movement related variance (translational and rotational movement) were included in the model as covariates of no-interest. An anatomical MTL mask incorporating hippocampus and parahippocampal gyrus was created using the WFU PickAtlas (Maldjian, Laurienti, Kraft, & Burdette, 2003). This region of interest (ROI) was applied to whole brain activation t-maps calculated without restrictions.
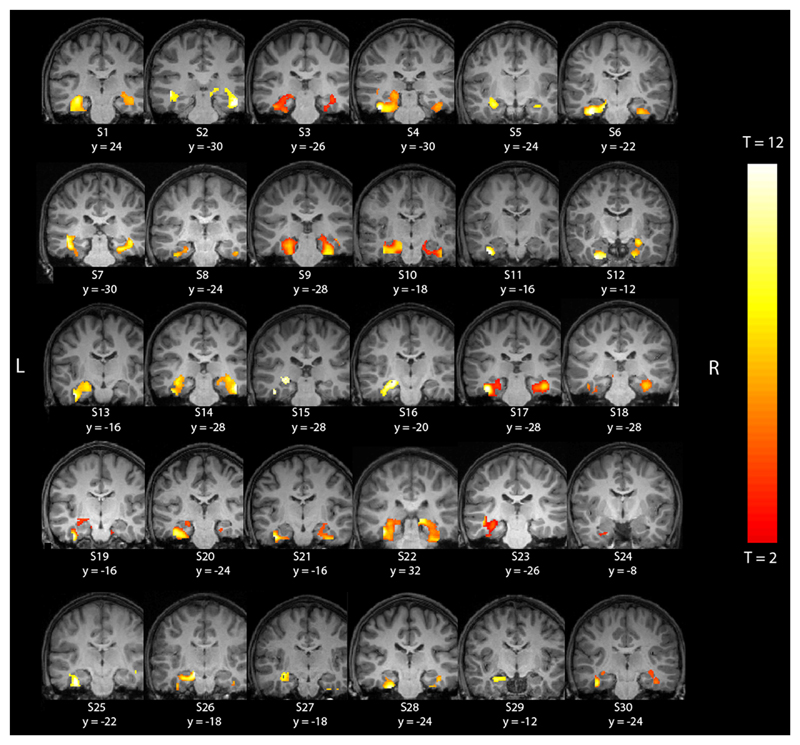
At the individual level, statistical parametrical maps were thresholded at puncorr < .001 to provide a balance between the risk of type-I versus type-II errors (Ahmad, Balsamo, Sachs, Xu, & Gaillard, 2003; Holland et al., 2007; Norrelgen, Lilja, Ingvar, Amark, & Fransson, 2015). For illustration of individual activations (Figure 2), threshold was set at T = 2. Lateralization of activations was estimated on the single-subject level by use of the LI-toolbox (Wilke & Lidzba, 2007). The MTL ROI was used as inclusive mask. In order to avoid the threshold dependency of simple lateralization indices, a bootstrapping approach was employed (Lidzba et al., 2011). The algorithm was applied to statistical maps (t-maps). With this approach, a multitude of bootstrapping resamples from the original dataset is analyzed at different thresholds, yielding a single, weighted mean laterality index (LI) which is based on the whole of the underlying dataset (Wilke & Schmithorst, 2006). This was done for each of the 30 subjects. LI was calculated according to the formula :
where “∑activation” is the sum of activated voxels and “mwf” is the mask weighting factor (Wilke & Lidzba, 2007). Based upon previous studies (Lidzba, de Haan, Wilke, Krageloh-Mann, & Staudt, 2017; Lidzba, Kupper, Kluger, & Staudt, 2017), for single subject analyses LI was categorized as left lateralized if ≥ .20, bilateral if within -.20 and +.20, or right if ≤ -.20. Furthermore, to investigate laterality regardless of the direction (left or right), we calculated the strength of laterality using the absolute value of the laterality index |LI|. This measurement avoids predetermined categorical allocations of MTL activations into “bilateral” or “lateralized”, but provides information about the absolute degree of lateralization.
Figure 2.
Individual analyses of mesial temporal lobe activation in 30 participants. Individual activation is displayed on the individual, normalized brain. Left is left hemisphere.
To examine the group effect of MTL activations, one-sample t tests were used, corrected for multiple comparisons family-wise error (FWE) with p < .05.
Statistical analyses of behavioral data were conducted using SPSS Statistics (version 22.0). Because laterality indices and behavioral data were not normally distributed, nonparametric testing was conducted whenever analyses included laterality indices and/or cognitive test results. The strength of the relationship between continuous variables (cognitive test scores, laterality indices, strength of lateralization, age at examination) was examined using Spearman’s rank correlation coefficient. Partial nonparametric correlation was calculated using the partial Spearman coefficient. Nonparametric Mann-Whitney U test was used to examine if cognitive test scores, laterality indices, or strength of lateralization differed by sex. Significance of correlations was set based on a strict Bonferroni correction factor, i.e. α = .05 / number of comparisons, which corresponds to a significance threshold of .006. Fisher’s r-to-z transformation was used to assess the significance of the difference between two correlation coefficients.
3. Results
3.1. In-scanner performance
On-site check of in-scanner performance showed that all children and adolescents were able to follow the fMRI instructions and perform the fMRI paradigm. However, task accuracy is missing for 8 participants due to technical reasons. In-scanner behavioral test results are presented in Table 1. In-scanner performances did not correlate significantly with age indicating proper matching of task demands to age. Furthermore, task performance did not differ significantly by gender.
Table 1. In-scanner performance and cognitive test results.
| Mean (SD) | age r (p) |
gender U (p) |
|
|---|---|---|---|
| in-scanner task performance | |||
| language task | 95.00 (6.20) | +.33 (.123) | 51.5 (.647) |
| control task | 95.18 (6.28) | +.26 (.248) | 53.0 (.744) |
| cognitive test results | |||
| expressive vocabulary | 70.37 (29.38) | +.19 (.326) | 81.5 (.267) |
| expressive vocabulary (n=20) | 67.30 (29.07) | -.08 (.729) | 40.0 (.503) |
| verbal span | 59.83 (21.95) | +.10 (.607) | 84.5 (.325) |
| verbal learning curve | 43.13 (32.60) | +.11 (.582) | 71.5 (.124) |
| verbal short-term memory | 50.90 (32.58) | -.02 (.911) | 93.0 (.545) |
| verbal long-term memory | 55.37 (30.82) | -.02 (.911) | 74.0 (.158) |
| verbal recognition | 37.57 (28.24) | +.50 (.005)* | 77.0 (.200) |
| verbal fluency | 34.17 (27.38) | +.19 (.321) | 80.0 (.249) |
| perceptual reasoning index | 71.06 (20.37) | +.11 (.580) | 101.5 (.787) |
Mean percentiles and standard deviations of in-scanner task performances and out-scanner cognitive test results of study participants, non-parametric correlation with age, and differences by gender. Uncorrected p-values are given, statistical significance after Bonferroni correction is indicated with *.
3.2. Cognitive test results
Cognitive test results are depicted in Table 1. As expected, the participants performed in the average range or better, though there was considerable variability. Even with age adjusted scores, verbal recognition correlated with age, with older participants performing better in verbal recognition than younger ones (r = .50, p = .005). Cognitive test results did not differ significantly by gender.
Correlation between cognitive test results revealed significant correlations between in-scanner language task and in-scanner control task (r = .67, p = .001), between all verbal memory measures (r = .69 to .88, p = .000), and between expressive vocabulary and verbal learning (r = .59, p = .001), verbal short-term memory (r = .49, p = .005), verbal long-term memory (r = .52, p = .003), and verbal recognition (r = .52, p = .004), respectively. No significant association surviving Bonferroni correction was found between perceptual reasoning and any language measure.
3.3. Direction and strength of lateralization of MTL activations
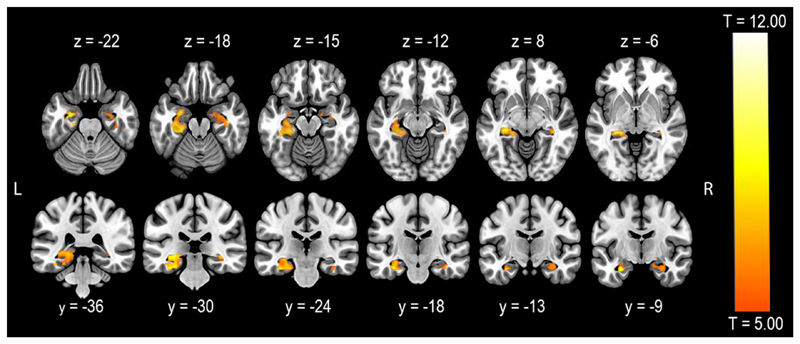
In fMRI group analysis, second level one sample t-test revealed significant MTL activation, with a larger cluster in the left hemisphere than in the right hemisphere and with a group weighted mean MTL laterality index of +.55 (Table 2, Figure 1).
Table 2. Clusters of MTL activations during the auditory description definition task.
| Cluster size | Z | mean coordinate | SD | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| left MTL formation | 1078 | 6.15 | -28 | -8 | -24 | 0.03 |
| right MTL formation | 55 | 6.08 | 36 | -32 | -4 | 0.02 |
| right MTL formation | 73 | 5.17 | 42 | -20 | -20 | 0.02 |
| right MTL formation | 8 | 4.83 | 18 | -8 | -18 | 0.05 |
Figure 1.
One sample t-test of mesial temporal lobe activation in the whole group of participants (p < .05, FWE-corr). Activation is displayed on a single brain in MNI space. Left is left-hemisphere in axial and coronal views.
In single subject analyses, all participants exhibited MTL activations at the significance threshold at puncorr < .001 (Figure 2). In the individual analyses, left LI in the MTL was found in 20 children (66.7 %), bilateral LI in four participants (13.3 %), and right LI in six participants (20.0 %). Individual MTL laterality indices ranged from -.55 to +.91 (Table 3). MTL laterality indices did not significantly differ by gender and did not correlate with age or handedness. Individual strength of laterality of MTL activations—absolute value of LI value—ranged from .02 to .91 and did not differ significantly by gender nor correlated with handedness. A modest negative correlation with age was found (r = -.40), though correlation did not survive Bonferroni correction.
Table 3. MTL Language laterality in single subject analyses.
| mean (SD) | age r (p) |
gender (p) |
handedness r (p) |
|
|---|---|---|---|---|
| laterality index in the MTL (LI) | +.30 (.43) | -.35 (.058) | .305 | +.34 (.063) |
| strength of laterality in the MTL |LI| | .47 (.24) | -.40 (.029) | .950 | +.05 (.798) |
uncorrected p-values are given
3.4. Relationship between cognitive test results and MTL laterality
Even though the correlation between age and language laterality did not survive correction for multiple comparisons, we took a conservative approach and controlled for age effects with partial Spearman correlation because of the modest strength of the correlation coefficients.
We found a significant negative correlation between expressive vocabulary and the laterality index of the MTLs (r = .61, p = .001), however, this finding was no longer significant in the analysis of the n=20 group (Table 4). Individual MTL laterality indices did not correlate significantly with in-scanner task performance, verbal memory test results, or verbal fluency. Moreover, there was no correlation between MTL laterality and non-linguistic perceptual reasoning.
Table 4. Correlation of cognitive test results with laterality indices and strength of laterality, controlled for age effects.
| Laterality index r (p) |
Strength of laterality r (p) |
|
|---|---|---|
| in-scanner task performance | ||
| language task | -.53 (.014) | -.46 (.036) |
| cognitive test results | ||
| expressive vocabulary | -.61 (.001) * | -.71 (.000) * |
| expressive vocabulary (n=20) | -.51 (.025) | -.73 (.000) * |
| verbal span | -.28 (.139) | -.34 (.074) |
| verbal learning curve | -.37 (.046) | -.47 (.011) |
| verbal short-term memory | -.36 (.054) | -.38 (.040) |
| verbal long-term memory | -.25 (.199) | -.39 (.035) |
| verbal recognition | -.34 (.071) | -.46 (.011) |
| verbal fluency | -.11 (.571) | -.21 (.287) |
| perceptual reasoning index | -.40 (.033) | -.30 (.112) |
uncorrected p-values are given, statistical significance after Bonferroni correction is indicated with *
We furthermore found a significant negative correlation between the strength of MTL laterality and expressive vocabulary (r = -.71, p = .000), and this significance of correlation remained so in the analysis of the n=20 group (r = -.73, p = .000). No further language or memory test result, nor the perceptual reasoning index, correlated significantly with the strength of laterality of the MTL activations. However, some modest correlations between the strength of laterality and cognitive test results were found which did not survive the strict correction of multiple comparisons, but all showed a negative relationship. Thus, with a rigorous approach, stronger left MTL lateralization was associated with worse expressive vocabulary, and greater lateralization of MTL activations was associated with worse expressive vocabulary, which was consistent with an overall pattern across all measures that stronger lateralized MTL activations were associated with worse performance of various verbal functions.
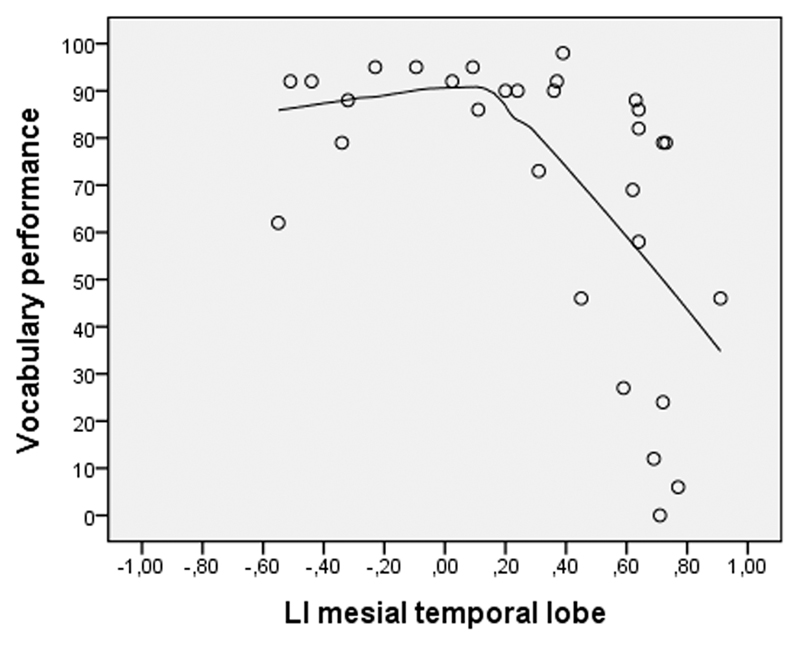
A comparison of r-values reveals that absolute laterality has a stronger relationship to expressive vocabulary (n=20) than left laterality (z = 2.02, p = 0.02). This non-linear relationship between language laterality and expressive vocabulary is illustrated in Figure 3, where the MTL laterality indices and expressive vocabulary results are fitted with LOESS locally weighted scatterplot smoothing. Figure 3 shows an inverted L shaped curve illustrating the relationship between the laterality index of MTL activation and expressive vocabulary performance.
Figure 3.
Relationship between the laterality index of MTL activations and expressive vocabulary performance, fitted with LOESS locally weighted scatterplot smoothing
3.4.1. Relationship between the strength of MTL lateralization and expressive vocabulary: variation by age
In a post-hoc analysis, we examined whether the relationship between the strength of MTL language lateralization and expressive vocabulary varied by age. For this purpose, we divided study participants into three age groups with 10 participants each. In group 1 (aged 7 to 8), nonparametric Spearman correlation analysis revealed a significant negative correlation between the strength of lateralization and expressive vocabulary (r = -.74, p = .015). Group 2 (aged 9 to 10) still showed a high correlation, though less significant (r = -.69, p = .029). Finally, group 3 (aged 11 to 16) revealed a moderate, but not significant correlation between the strength of MTL language lateralization and expressive vocabulary (r = -.48, p =. 163). Fisher r-to-z transformation shows no significant differences between the correlation coefficients of the age groups.
4. Discussion
The present study focuses on the MTL involvement in language and verbal memory functions in children and adolescents. We therefore tested 30 healthy, right-handed children and adolescents with a well-known fMRI language paradigm and a comprehensive cognitive test battery. We found significant MTL activations during the language fMRI in all participants; the majority of them had a left lateralized MTL laterality index, but 20% exhibited right MTL language lateralization, and 13% showed a bilateral MTL laterality index during the fMRI language paradigm. Greater lateralization of MTL activation to the left or right hemisphere was significantly correlated with worse expressive vocabulary abilities in healthy children and adolescents. The lack of a significant correlation between MTL laterality and non-linguistic perceptual reasoning furthermore shows that the lateralization findings were specific to language.
The findings of the present study suggest that the MTL of both hemispheres may play an important role in language functioning in healthy right-handed children. Previous clinical studies support this view. Investigating neurosurgical patients, Jacobs et al. (2016) showed that deep brain stimulation in both the entorhinal region and hippocampus caused decreased memory performance for both verbal and spatial information. It is furthermore known that unilateral mesial temporal lobe epilepsy may lead to deficits in semantic fluency and expressive vocabulary, often irrespective of the epileptogenic side, both in children and in adults (Bartha-Doering & Trinka, 2014; Bartha et al., 2005c). In a large study on episodic and semantic memory in 66 children with temporal lobe epilepsy, Smith and Lah (2011) observed high rates of impairments across tasks and did not find differences related to the laterality of temporal lobe pathology. Specifically, damage to either left or right MTL may result in verbal memory deficits. Furthermore, Skirrow et al. (2015) showed that better verbal memory following temporal lobe epilepsy surgery in children was linked to larger post-surgical residual hippocampal volumes, more robustly in left surgical participants, but also in children after right temporal lobe surgery. In addition, Bonelli et al. (2012) showed that ipsilateral recruitment involving the posterior hippocampal remnant is important for maintaining language, while reorganization to the contralateral hemisphere is less effective in adult patients following epilepsy surgery and can be associated with a decrease of proficiency in language abilities.
Our results furthermore suggest that lateralized MTL activation during a semantic language paradigm does not necessarily engender better language performance. We are not aware of previous studies that focus on the impact of MTL lateralization on language functioning. There are, however, neuropediatric studies that investigate laterality in cortical regions during semantic language tasks and the relationship to performance. While many of them reported a positive correlation between linguistic abilities and left lateralization of language in the brain across different populations (Lillywhite et al., 2009; Elkana et al., 2011; de Guibert et al., 2011; Everts et al., 2009), there are also some instances when better verbal skills are associated with less lateralized language (Lidzba et al., 2011). Yeatman et al. (2010) furthermore showed that frontal regions bilaterally become more active in response to increasing task demands in children with superior receptive language skills than in children with average language abilities. One possible explanation for discrepant results is that less language lateralization may reflect a different underlying mechanism in children with language deficits compared to healthy children, and the recruitment of contralateral homologues may represent a different strategy. In children with language deficits, less lateralization may represent a compensatory mechanism while in children with strong language skills, less lateralization may represent a superior mechanism.
An alternative explanation is that the relationship between language lateralization and language functioning may be task and region dependent. This is illustrated by studies in healthy children who showed that stronger left temporal lateralization during an auditory description definition task was associated with better object naming and right lateralized cerebellar activation was significantly correlated with better core language skills (Berl et al., 2014). In a second study they found that greater left frontal lateralization during reading fMRI was associated with better post-scan performance, and frontal activation during story listening was positively correlated with better performance on comprehension questions, whereas temporal activations during both fMRI tasks showed no correlations with post-task performance or cognitive skills (Berl et al., 2010). Thus, laterality may be advantageous or not depending on the specific region of the brain and the skill being assessed. These studies focused on specific language ROIs and do not report specific findings of MTL contributions to language abilities.
Our present study shows a strong negative linear correlation between the strength of language laterality in the MTL and vocabulary, thus the less lateralized MTL activation, the better was a child’s vocabulary. The relationship between MTL activation and linguistic performance is however best described in using a LOESS-approach of the hippocampal language laterality and linguistic performance which forms a non-linear, inverted L shaped curve. It may be hypothesized that with the inclusion of more participants with negative LI values, this curve may form a U-shape reflecting lower vocabulary performance with greater left- or right-sided strength of laterality. Such a curve has already been described by Hirnstein, Leask, Rose, and Hausmann (2010) who used visual half-field tasks of word-matching and face-decision and found a slight to moderate degree of hemispheric asymmetry to achieve best cognitive performance, while performance deteriorated towards extreme ends of lateralization. Interestingly, Boles, Barth, and Merrill (2008) demonstrated with visual half-field and dichotic listening that the asymmetry-performance relationship is not only U shaped, but also modality dependent, with a strong lateralization being associated with increased performance for auditory language tasks, but decreased performance in visual lexical processes. Thus, the relationship between language abilities and functional asymmetry may be more complex than a simple linear relationship, and for further studies in this field, a non-linear approach of data analyses should be taken into consideration.
In the present study, our hypothesis that stronger left lateralized activation of the MTL would be significantly associated with better language performance was not supported. Instead, we found that children with strongly left lateralized MTL activation had worse expressive vocabulary, whereas bilateral activation during an auditory description definition task was associated with better expressive vocabulary.
We found a trend of a negative correlation of the strength of laterality with age, i.e. younger participants revealed more lateralized MTL activations, though significance of this finding did not survive Bonferroni correction. Given that the in-scanner cognitive stimulus set varies as a function of age, this effect could be driving the age affect. However, age-related differences in functional laterality may be related to the protracted development of the hippocampus. Although some investigations found little to no relationship between hippocampal volume and age (Giedd et al., 1996; Yurgelun-Todd, Killgore, & Cintron, 2003) and conclude that the development of the hippocampal formation, except the dentate gyrus, is completed in the first years of life (Gilmore et al., 2012; Seress, 2007; Seress & Ribak, 1995), others found that total hippocampal volume increases with age (Ostby et al., 2009). When investigated in greater structural detail, studies report age-related variations in hippocampal volume along the longitudinal axis of the hippocampus (Gogtay et al., 2006; Insausti, Cebada-Sanchez, & Marcos, 2010). More recently, when measuring across different subregions along the longitudinal axis of the hippocampus, age-related differences in language and memory were found (DeMaster, Pathman, Lee, & Ghetti, 2014; Deniz Can, Richards, & Kuhl, 2013). Another study showed that the relation between bilateral hippocampal volume and expressive language increases with age (Lee et al., 2015). Parsing functional activation into subregions of the hippocampus is a technical challenge but is underway (Eldridge, Engel, Zeineh, Bookheimer, & Knowlton, 2005); however, it has not been applied in children or epilepsy populations. In addition to not acquiring the data in a way that would allow this analysis, our study population is not large enough to study this relationship. However, in a post-hoc explanatory analysis, we examined whether the relation between MTL language lateralization and expressive vocabulary varied by age group. In the youngest age-group, the negative correlation coefficient between MTL language activations and expressive vocabulary was the highest, indicating that the involvement of both MTLs is more advantageous for expressive vocabulary in younger children. However, this difference by age groups was not statistically significant. Though this post-hoc analysis is limited due to the small subgroups and the artificial subdivision in age-groups, our data indicate that age is not a factor moderating the relationship between language lateralization in the MTL and expressive vocabulary.
The present study found lateralized MTL activation to a greater degree and more consistently than the study of Sepeta et al. (2016). Though we used the same paradigm, these findings may be due to a number of differences. Differences in fMRI parameters may contribute to the different results between Sepeta et al. and the current study, given that our task acquisition had a shorter TR which allows for more datapoints to be collected during the same paradigm time. In addition to technical differences, the differences may also be related to presenting the tasks in different languages. Although English and German belong to the same language family, these languages differ in their syntactic complexity. Relative to English, German possesses a comparatively rich morphology and a complex word order (Hawkins, 1986). Cross-linguistic studies in native speakers of different languages reveal common language networks, but also considerable differences in brain areas involved in language comprehension (Bick, Goelman, & Frost, 2011; Ge et al., 2015; Szlachta, Bozic, Jelowicka, & Marslen-Wilson, 2012). Furthermore, differences in language processing speed have been found between English and German native speakers, affected by factors such as word length, syllable structure, compounding, initial frication, and degree of word order flexibility (Bates et al., 2003; Thierry, 2016). These differences in processing the English and German language, respectively, may help to explain the different findings of our study compared to the study of Sepeta and coworkers.
Moreover, the participants in our study were older than the children in the study of Sepeta and investigators. Whereas the mean age of their pediatric study sample was 9.9 years with a range of 6 to 13, the mean age of our sample was 10.3 with a range of 7 to 16. Sepeta et al. found a significant increase of language lateralization towards the left MTL with increasing age in their pediatric and adult samples combined, thus older study participants exhibited more left lateralized language activations in the MTL than younger study participants. Hence, the more left lateralized MTL activations in our study sample can be attributed to the older age of our study participants, which underlines a developmental shift in the relationship between MTL and language lateralization.
The mesial temporal lobe mask we used in our study covers the hippocampus proper as well as the parahippocampal gyrus. In the current study, the acquisition does not allow for us to reliably parse activation into smaller ROIs. Thus our findings are based on the whole MTL and conclusions specific to the role of the hippocampus proper in language localization cannot be made. However, these two regions differ with respect to their cortical and subcortical connections (Eichenbaum et al., 2007; Furtak, Wei, Agster, & Burwell, 2007). If and to which degree they also differ in their role in semantic memory and language functions, is still a matter of debate (de Haan, Mishkin, Baldeweg, & Vargha-Khadem, 2006). On the one hand, cognitive investigations of patients with developmental amnesia support the view that the hippocampus plays little to no role in semantic memory. The patients who acquired bilateral hippocampal damage during their first years of life exhibited impaired episodic memory, but revealed intact semantic knowledge and intact language functions (Bindschaedler, Peter-Favre, Maeder, Hirsbrunner, & Clarke, 2011; Gadian et al., 2000; Vargha-Khadem et al., 1997). These findings suggest that while the acquisition of context-dependent episodic memory is dependent on the hippocampus proper, perirhinal and entorhinal cortices support the formation of context-free semantic memory. While it is possible that reorganizational processes take place in early hippocampal damage, this hypothesis is further supported by two case studies of adult amnestic patients with acquired selective bilateral damage to the hippocampus proper (Holdstock et al., 2002; Verfaellie, Koseff, & Alexander, 2000).
In contrast, Squire and others emphasize that at least some patients with isolated hippocampal damage exhibit semantic memory deficits and conclude that the hippocampus itself supports semantic memory as well as episodic memory (Luo & Niki, 2002; Manns, Hopkins, & Squire, 2003; Squire, 2004; Squire & Zola, 1998). Furthermore, research with functional neuroimaging in healthy subjects has shown hippocampal activation during retrieval of various kinds of semantic knowledge, such as retrieval of elements of semantic categories (Ryan, Cox, Hayes, & Nadel, 2008), semantic decisions (Bartha et al., 2003), public events (Maguire & Mummery, 1999), and famous people (Bernard et al., 2004).
Projections from lateral temporal to MTL regions are proposed to play a key role in initial language learning (Alessio et al., 2004; Breitenstein et al., 2005; Davis, Di Betta, Macdonald, & Gaskell, 2009; Knecht et al., 2004; Liegeois et al., 2004; Richardson, Strange, Duncan, & Dolan, 2003). In fMRI studies on memory encoding and recognition of recently learned spoken words, the MTL is co-activated with the lateral temporal lobe (Gagnepain et al., 2011; Takashima, Bakker, van Hell, Janzen, & McQueen, 2014). Davis and Gaskell (2009) introduced a model for word learning which involves cortico-hippocampal connections during two stages of lexical acquisition: whereas the rapid, initial acquisition of words is supported by medial temporal and hippocampal learning, slower overnight consolidation of previously acquired information is achieved by neocortical involvement. This model is supported by findings of an association of spindle activity and overnight lexical integration as well as overnight chances in cortical responses to learned words (Davis, 2016; Tamminen, Payne, Stickgold, Wamsley, & Gaskell, 2010).
In addition, the syntactic frame of the fMRI language task was set so that the participant could confirm whether the sentence was accurate only at the end of the sentence (“a long yellow fruit is a banana”, “something you sit on is spaghetti”). Thus, the initial part of the sentence allows for multiple semantic interpretations, which are integrated and maintained incrementally in real-time to process the meaning of the sentence and decide whether it is true or not. Studies have shown that language listeners make on-line predictions about upcoming words and are capable of modulating these predictions based on the integration of new linguistic information with semantic knowledge (Altmann & Kamide, 2007; Federmeier, 2007). Duff and colleagues have hypothesized that the same processes by which the hippocampal system creates and integrates representations in the formation of new memories and maintain them on-line are also necessary for the on-line processing of language (Duff & Brown-Schmidt, 2012; Duff et al., 2011; Duff et al., 2008; Kurczek et al., 2013).
Overall, studies demonstrate that acquiring new semantic facts, making semantic decisions, and initial language learning rely on MTL structures. This is in concert with the present study which shows a significant association of expressive vocabulary performance with MTL laterality during a task that requires vocabulary knowledge.
Children with larger expressive vocabulary were also significantly better in verbal learning, short- and long-term recall, and recognition, while there was no association between verbal and nonverbal functioning. This result and the lack of a significant association between MTL laterality and nonverbal functioning supports that our findings are language-specific. Our findings highlight that the mental lexicon uses neural systems that also serve the acquisition of episodic information (Bartha et al., 2003; Breitenstein et al., 2005; Ullman, 2001).
Increased knowledge about all regions related to language functioning may not only enhance our understanding of the relationship between brain and behavior in healthy children and adolescents, but will also expand our range of targets for intervention. There are, for example, medicines that target hippocampal functioning which may have secondary positive effects on verbal functioning (Breitenstein et al., 2004; Breitenstein et al., 2015). In this respect, the present study adds important information on the role of both MTL in language functioning. Future studies may use the present findings as a basis for investigating the contribution of both MTL to verbal functioning in neuropediatric populations.
Limitations
Our study population consisted of right-handed children and adolescents. While 67 % of them showed left MTL lateralization, only 20 % revealed language activations in the MTL lateralized to the right hemisphere. Thus, this sample does not provide a balanced distribution of MTL laterality indices. Statistical comparisons revealed that the strength of laterality has a stronger relationship to expressive vocabulary than left laterality, but an inclusion of more right-handed participants with right lateralized MTL activations would have enhanced the statistical power and increased the representativeness of the study.
Furthermore, the findings of the present study can not necessarily be transferred to left-handed children and adolescents. From our data, we hypothesize that less asymmetry of MTL contributions is also favorable for language abilities in left-handed children and adolescents, and that data of left-handers would complete the inverted U shape of the relationship between laterality index and performance we found in our study. On the other hand, previous studies have shown that left-handers represent a much more heterogeneous group in both lateralization and performance, and that the relationship between functional asymmetry and performance in left-handers is much more complex than in right-handers (Somers, Shields, Boks, Kahn, & Sommer, 2015; Szaflarski et al., 2002; Szaflarski et al., 2012). In a further step, it would therefore be very interesting to investigate the relationship between functional asymmetry and performance in left-handed children to form a complete picture of the relationship between MTL language contribution and performance in childhood.
The lack of normative data for the expressive vocabulary test for children older than 10;11 years is a further limitation of the study. As the mean difficulty to name the items of the WWT decreases exponentially and phases out in a flat curve with 10 years of age (Glück, 2011), we transformed the WWT raw scores of ten elder participants into percentiles based on the 10;11 year old children. Thus, for the interpretation of the results of the whole study sample, the risk of an overestimation of percentile rank results for ten elder study participants has to be taken into account. However, in a more conservative approach, we excluded all adolescents aged 11-16 yielding a sample of 20 children and repeated the analyses. While the correlation between MTL language lateralization and expressive vocabulary did not survive Bonferroni correction in the reduced sample, the relationship between the strength of language lateralization in the MTL and expressive vocabulary remained highly significant.
Finally, using a fMRI memory task in addition to the language task in the future will be an important step in directly testing the relationship between language and memory lateralization. Thus, the question if language lateralization parallels memory lateralization in the same individual, and if bilateral hippocampal activation during a memory task is also associated with better memory and/or language functions remains an open question.
Supplementary Material
Acknowledgement
We are very grateful to Andreas Gleiss from the Section for Clinical Biometrics, Medical University Vienna, for his professional help on the statistical analyses.
Funding
This work was supported by funds of the Oesterreichische Nationalbank (Anniversary Fund, project number 15356) and by the Austrian Science Fund (FWF), Grant KLI544-B27.
References
- Ahmad Z, Balsamo LM, Sachs BC, Xu B, Gaillard WD. Auditory comprehension of language in young children: neural networks identified with fMRI. Neurology. 2003;60(10):1598–1605. doi: 10.1212/01.wnl.0000059865.32155.86. [DOI] [PubMed] [Google Scholar]
- Alessio A, Bonilha L, Rorden C, Kobayashi E, Min LL, Damasceno BP, et al. Memory and language impairments and their relationships to hippocampal and perirhinal cortex damage in patients with medial temporal lobe epilepsy. Epilepsy and Behavior. 2006;8(3):593–600. doi: 10.1016/j.yebeh.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Alessio A, Damasceno BP, Camargo CH, Kobayashi E, Guerreiro CA, Cendes F. Differences in memory performance and other clinical characteristics in patients with mesial temporal lobe epilepsy with and without hippocampal atrophy. Epilepsy and Behavior. 2004;5(1):22–27. doi: 10.1016/j.yebeh.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Altmann GTM, Kamide Y. The real-time mediation of visual attention by language and world knowledge: Linking anticipatory (and other) eye movements to linguistic processing. Journal of Memory and Language. 2007;57(4):502–518. [Google Scholar]
- Aschenbrenner S, Tucha O, Lange KW. Regensburger Wortflüssigkeitstest. Göttingen: Testzentrale; 2001. [Google Scholar]
- Badcock NA, Bishop DV, Hardiman MJ, Barry JG, Watkins KE. Co-localisation of abnormal brain structure and function in specific language impairment. Brain and Language. 2012;120(3):310–320. doi: 10.1016/j.bandl.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsamo LM, Xu B, Gaillard WD. Language lateralization and the role of the fusiform gyrus in semantic processing in young children. Neuroimage. 2006;31(3):1306–1314. doi: 10.1016/j.neuroimage.2006.01.027. [DOI] [PubMed] [Google Scholar]
- Bartha-Doering L, Trinka E. The interictal language profile in adult epilepsy. Epilepsia. 2014;55(10):1512–1525. doi: 10.1111/epi.12743. [DOI] [PubMed] [Google Scholar]
- Bartha L, Benke T, Bauer G, Trinka E. Interictal language functions in temporal lobe epilepsy. Journal of Neurology, Neurosurgery, and Psychiatry. 2005a;76(6):808–814. doi: 10.1136/jnnp.2004.045385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartha L, Brenneis C, Schocke M, Trinka E, Koylu B, Trieb T, et al. Medial temporal lobe activation during semantic language processing: fMRI findings in healthy left- and right-handers. Cognitive Brain Research. 2003;17(2):339–346. doi: 10.1016/s0926-6410(03)00135-6. [DOI] [PubMed] [Google Scholar]
- Bartha L, Marien P, Brenneis C, Trieb T, Kremser C, Ortler M, et al. Hippocampal formation involvement in a language-activation task in patients with mesial temporal lobe epilepsy. Epilepsia. 2005b;46(11):1754–1763. doi: 10.1111/j.1528-1167.2005.00292.x. [DOI] [PubMed] [Google Scholar]
- Bartha L, Marien P, Brenneis C, Trieb T, Kremser C, Ortler M, et al. Hippocampal formation involvement in a language-activation task in patients with mesial temporal lobe epilepsy. Epilepsia. 2005c;46(11):1754–1763. doi: 10.1111/j.1528-1167.2005.00292.x. [DOI] [PubMed] [Google Scholar]
- Bartha L, Trinka E, Ortler M, Donnemiller E, Felber S, Bauer G, et al. Linguistic deficits following left selective amygdalohippocampectomy: a prospective study. Epilepsy and Behavior. 2004;5(3):348–357. doi: 10.1016/j.yebeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Bates E, D'Amico S, Jacobsen T, Szekely A, Andonova E, Devescovi A, et al. Timed picture naming in seven languages. Psychonomic Bulletin and Review. 2003;10(2):344–380. doi: 10.3758/bf03196494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell BD, Davies KG, Haltiner AM, Walters GL. Intracarotid amobarbital procedure and prediction of postoperative memory in patients with left temporal lobe epilepsy and hippocampal sclerosis. Epilepsia. 2000;41(8):992–997. doi: 10.1111/j.1528-1157.2000.tb00284.x. [DOI] [PubMed] [Google Scholar]
- Berl MM, Duke ES, Mayo J, Rosenberger LR, Moore EN, VanMeter J, et al. Functional anatomy of listening and reading comprehension during development. Brain and Language. 2010;114(2):115–125. doi: 10.1016/j.bandl.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berl MM, Mayo J, Parks EN, Rosenberger LR, VanMeter J, Ratner NB, et al. Regional differences in the developmental trajectory of lateralization of the language network. Human Brain Mapping. 2014;35(1):270–284. doi: 10.1002/hbm.22179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard FA, Bullmore ET, Graham KS, Thompson SA, Hodges JR, Fletcher PC. The hippocampal region is involved in successful recognition of both remote and recent famous faces. Neuroimage. 2004;22(4):1704–1714. doi: 10.1016/j.neuroimage.2004.03.036. [DOI] [PubMed] [Google Scholar]
- Bick AS, Goelman G, Frost R. Hebrew brain vs. English brain: language modulates the way it is processed. Journal of Cognitive Neuroscience. 2011;23(9):2280–2290. doi: 10.1162/jocn.2010.21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindschaedler C, Peter-Favre C, Maeder P, Hirsbrunner T, Clarke S. Growing up with bilateral hippocampal atrophy: from childhood to teenage. Cortex. 2011;47(8):931–944. doi: 10.1016/j.cortex.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Boles DB, Barth JM, Merrill EC. Asymmetry and performance: toward a neurodevelopmental theory. Brain and Cognition. 2008;66(2):124–139. doi: 10.1016/j.bandc.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Bonelli SB, Powell R, Thompson PJ, Yogarajah M, Focke NK, Stretton J, et al. Hippocampal activation correlates with visual confrontation naming: fMRI findings in controls and patients with temporal lobe epilepsy. Epilepsy Research. 2011;95(3):246–254. doi: 10.1016/j.eplepsyres.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonelli SB, Thompson PJ, Yogarajah M, Vollmar C, Powell RH, Symms MR, et al. Imaging language networks before and after anterior temporal lobe resection: results of a longitudinal fMRI study. Epilepsia. 2012;53(4):639–650. doi: 10.1111/j.1528-1167.2012.03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitenstein C, Jansen A, Deppe M, Foerster AF, Sommer J, Wolbers T, et al. Hippocampus activity differentiates good from poor learners of a novel lexicon. Neuroimage. 2005;25(3):958–968. doi: 10.1016/j.neuroimage.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Breitenstein C, Korsukewitz C, Baumgartner A, Floel A, Zwitserlood P, Dobel C, et al. L-dopa does not add to the success of high-intensity language training in aphasia. Restor Neurol Neurosci. 2015;33(2):115–120. doi: 10.3233/RNN-140435. [DOI] [PubMed] [Google Scholar]
- Breitenstein C, Wailke S, Bushuven S, Kamping S, Zwitserlood P, Ringelstein EB, et al. D-amphetamine boosts language learning independent of its cardiovascular and motor arousing effects. Neuropsychopharmacology. 2004;29(9):1704–1714. doi: 10.1038/sj.npp.1300464. [DOI] [PubMed] [Google Scholar]
- Davis MH. The neurobiology of lexical access. In: Hickok G, Small SL, editors. Neurobiology of Language. Amsterdam and Boston: Elsevier; 2016. pp. 541–551. [Google Scholar]
- Davis MH, Di Betta AM, Macdonald MJ, Gaskell MG. Learning and consolidation of novel spoken words. Journal of Cognitive Neuroscience. 2009;21(4):803–820. doi: 10.1162/jocn.2009.21059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MH, Gaskell MG. A complementary systems account of word learning: neural and behavioural evidence. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 2009;364(1536):3773–3800. doi: 10.1098/rstb.2009.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guibert C, Maumet C, Jannin P, Ferre JC, Treguier C, Barillot C, et al. Abnormal functional lateralization and activity of language brain areas in typical specific language impairment (developmental dysphasia) Brain. 2011;134(Pt 10):3044–3058. doi: 10.1093/brain/awr141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan M, Mishkin M, Baldeweg T, Vargha-Khadem F. Human memory development and its dysfunction after early hippocampal injury. Trends in Neuroscience. 2006;29(7):374–381. doi: 10.1016/j.tins.2006.05.008. [DOI] [PubMed] [Google Scholar]
- DeMaster D, Pathman T, Lee JK, Ghetti S. Structural development of the hippocampus and episodic memory: developmental differences along the anterior/posterior axis. Cerebral Cortex. 2014;24(11):3036–3045. doi: 10.1093/cercor/bht160. [DOI] [PubMed] [Google Scholar]
- Deniz Can D, Richards T, Kuhl PK. Early gray-matter and white-matter concentration in infancy predict later language skills: a whole brain voxel-based morphometry study. Brain and Language. 2013;124(1):34–44. doi: 10.1016/j.bandl.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff MC, Brown-Schmidt S. The hippocampus and the flexible use and processing of language. Frontiers in Human Neuroscience. 2012;6:69. doi: 10.3389/fnhum.2012.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff MC, Gupta R, Hengst JA, Tranel D, Cohen NJ. The use of definite references signals declarative memory: evidence from patients with hippocampal amnesia. Psychological Sciences. 2011;22(5):666–673. doi: 10.1177/0956797611404897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff MC, Hengst JA, Tranel D, Cohen NJ. Collaborative discourse facilitates efficient communication and new learning in amnesia. Brain and Language. 2008;106(1):41–54. doi: 10.1016/j.bandl.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annuals Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge LL, Engel SA, Zeineh MM, Bookheimer SY, Knowlton BJ. A dissociation of encoding and retrieval processes in the human hippocampus. Journal of Neuroscience. 2005;25(13):3280–3286. doi: 10.1523/JNEUROSCI.3420-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkana O, Frost R, Kramer U, Ben-Bashat D, Hendler T, Schmidt D, et al. Cerebral reorganization as a function of linguistic recovery in children: An fMRI study. Cortex. 2011;47(2):202–216. doi: 10.1016/j.cortex.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Everts R, Lidzba K, Wilke M, Kiefer C, Mordasini M, Schroth G, et al. Strengthening of laterality of verbal and visuospatial functions during childhood and adolescence. Human Brain Mapping. 2009;30(2):473–483. doi: 10.1002/hbm.20523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federmeier KD. Thinking ahead: the role and roots of prediction in language comprehension. Psychophysiology. 2007;44(4):491–505. doi: 10.1111/j.1469-8986.2007.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Baker JM, Whiteside J, Eoute D, Jr, Moser D, Vesselinov R, et al. Treating visual speech perception to improve speech production in nonfluent aphasia. Stroke. 2009;40(3):853–858. doi: 10.1161/STROKEAHA.108.532499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtak SC, Wei SM, Agster KL, Burwell RD. Functional neuroanatomy of the parahippocampal region in the rat the: perirhinal and postrhinal cortices. Hippocampus. 2007;17(9):709–722. doi: 10.1002/hipo.20314. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD, Cohen NJ, Corkin S. The impaired learning of semantic knowledge following bilateral medial temporal-lobe resection. Brain and Cognition. 1988;7(2):157–177. doi: 10.1016/0278-2626(88)90027-9. [DOI] [PubMed] [Google Scholar]
- Gadian DG, Aicardi J, Watkins KE, Porter DA, Mishkin M, Vargha-Khadem F. Developmental amnesia associated with early hypoxic-ischaemic injury. Brain. 2000:499–507. doi: 10.1093/brain/123.3.499. [DOI] [PubMed] [Google Scholar]
- Gagnepain P, Henson R, Chetelat G, Desgranges B, Lebreton K, Eustache F. Is neocortical-hippocampal connectivity a better predictor of subsequent recollection than local increases in hippocampal activity? New insights on the role of priming. Journal of Cognitive Neuroscience. 2011;23(2):391–403. doi: 10.1162/jocn.2010.21454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard WD, Berl MM, Moore EN, Ritzl EK, Rosenberger LR, Weinstein SL, et al. Atypical language in lesional and nonlesional complex partial epilepsy. Neurology. 2007;69(18):1761–1771. doi: 10.1212/01.wnl.0000289650.48830.1a. [DOI] [PubMed] [Google Scholar]
- Ge J, Peng G, Lyu B, Wang Y, Zhuo Y, Niu Z, et al. Cross-language differences in the brain network subserving intelligible speech. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(10):2972–2977. doi: 10.1073/pnas.1416000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4-18 years. Journal of Comparative Neurology. 1996;366(2):223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gill SK, Leff AP. Dopaminergic therapy in aphasia. Aphasiology. 2012;28(2):155–170. doi: 10.1080/02687038.2013.802286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Shi F, Woolson SL, Knickmeyer RC, Short SJ, Lin W, et al. Longitudinal development of cortical and subcortical gray matter from birth to 2 years. Cerebral Cortex. 2012;22(11):2478–2485. doi: 10.1093/cercor/bhr327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glück CW. Wortschatz- und Wortfindungstest für 6- bis 10-Jährige. München: Elsevier; 2011. [Google Scholar]
- Gogtay N, Nugent TF, 3rd, Herman DH, Ordonez A, Greenstein D, Hayashi KM, et al. Dynamic mapping of normal human hippocampal development. Hippocampus. 2006;16(8):664–672. doi: 10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]
- Groen MA, Whitehouse AJ, Badcock NA, Bishop DV. Does cerebral lateralization develop? A study using functional transcranial Doppler ultrasound assessing lateralization for language production and visuospatial memory. Brain and Behavior. 2012;2(3):256–269. doi: 10.1002/brb3.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberger MJ, Seidel WT, Goodman RR, Williams A, Perrine K, Devinsky O, et al. Evidence for cortical reorganization of language in patients with hippocampal sclerosis. Brain. 2007;130(Pt 11):2942–2950. doi: 10.1093/brain/awm187. [DOI] [PubMed] [Google Scholar]
- Hawkins JA. A Comparative Typology of English and German: Unifying the Contrasts. Oxon, New York: Taylor & Francis; 1986. [Google Scholar]
- Heim S, Eulitz C, Elbert T. Altered hemispheric asymmetry of auditory N100m in adults with developmental dyslexia. Neuroreport. 2003;14(3):501–504. doi: 10.1097/00001756-200303030-00041. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Lendt M, Lux S. Verbaler Lern- und Merkfähigkeitstest VLMT. Göttingen: Beltz Test; 2001. [Google Scholar]
- Hirnstein M, Leask S, Rose J, Hausmann M. Disentangling the relationship between hemispheric asymmetry and cognitive performance. Brain and Cognition. 2010;73(2):119–127. doi: 10.1016/j.bandc.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Holdstock JS, Mayes AR, Roberts N, Cezayirli E, Isaac CL, O'Reilly RC, et al. Under what conditions is recognition spared relative to recall after selective hippocampal damage in humans? Hippocampus. 2002;12(3):341–351. doi: 10.1002/hipo.10011. [DOI] [PubMed] [Google Scholar]
- Holland SK, Vannest J, Mecoli M, Jacola LM, Tillema JM, Karunanayaka PR, et al. Functional MRI of language lateralization during development in children. International Journal of Audiology. 2007;46(9):533–551. doi: 10.1080/14992020701448994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Cebada-Sanchez S, Marcos P. Postnatal development of the human hippocampal formation. Advances in Anatomy, Embryology and Cell Biology. 2010;206:1–86. [PubMed] [Google Scholar]
- Jacobs J, Miller J, Lee SA, Coffey T, Watrous AJ, Sperling MR, et al. Direct Electrical Stimulation of the Human Entorhinal Region and Hippocampus Impairs Memory. Neuron. 2016;92(5):983–990. doi: 10.1016/j.neuron.2016.10.062. [DOI] [PubMed] [Google Scholar]
- Jansen A, Menke R, Sommer J, Forster AF, Bruchmann S, Hempleman J, et al. The assessment of hemispheric lateralization in functional MRI--robustness and reproducibility. Neuroimage. 2006;33(1):204–217. doi: 10.1016/j.neuroimage.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Muller RA, Cohen DN, Courchesne E. Atypical functional lateralization of language in autism spectrum disorders. Brain Research. 2008;1221:115–125. doi: 10.1016/j.brainres.2008.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus TA, Silver AM, Kennedy M, Lindgren KA, Dominick KC, Siegel J, et al. Language laterality in autism spectrum disorder and typical controls: a functional, volumetric, and diffusion tensor MRI study. Brain and Language. 2010;112(2):113–120. doi: 10.1016/j.bandl.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht S, Breitenstein C, Bushuven S, Wailke S, Kamping S, Floel A, et al. Levodopa: faster and better word learning in normal humans. Annals of Neurology. 2004;56(1):20–26. doi: 10.1002/ana.20125. [DOI] [PubMed] [Google Scholar]
- Knecht S, Floel A, Drager B, Breitenstein C, Sommer J, Henningsen H, et al. Degree of language lateralization determines susceptibility to unilateral brain lesions. Nature Neuroscience. 2002;5(7):695–699. doi: 10.1038/nn868. [DOI] [PubMed] [Google Scholar]
- Kurczek J, Brown-Schmidt S, Duff M. Hippocampal contributions to language: evidence of referential processing deficits in amnesia. Journal of Experimental Psychology: General. 2013;142(4):1346–1354. doi: 10.1037/a0034026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Nordahl CW, Amaral DG, Lee A, Solomon M, Ghetti S. Assessing hippocampal development and language in early childhood: Evidence from a new application of the Automatic Segmentation Adapter Tool. Human Brain Mapping. 2015;36(11):4483–4496. doi: 10.1002/hbm.22931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological assessment. third edition. Oxford: University Press; 1995. Vol. [Google Scholar]
- Lidzba K, de Haan B, Wilke M, Krageloh-Mann I, Staudt M. Lesion characteristics driving right-hemispheric language reorganization in congenital left-hemispheric brain damage. Brain and Language. 2017;173:1–9. doi: 10.1016/j.bandl.2017.04.006. [DOI] [PubMed] [Google Scholar]
- Lidzba K, Kupper H, Kluger G, Staudt M. The time window for successful right-hemispheric language reorganization in children. European Journal of Paediatric Neurology. 2017;21(5):715–721. doi: 10.1016/j.ejpn.2017.06.001. [DOI] [PubMed] [Google Scholar]
- Lidzba K, Schwilling E, Grodd W, Krageloh-Mann I, Wilke M. Language comprehension vs. language production: age effects on fMRI activation. Brain and Language. 2011;119(1):6–15. doi: 10.1016/j.bandl.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Liegeois F, Connelly A, Cross JH, Boyd SG, Gadian DG, Vargha-Khadem F, et al. Language reorganization in children with early-onset lesions of the left hemisphere: an fMRI study. Brain. 2004;127(Pt 6):1229–1236. doi: 10.1093/brain/awh159. [DOI] [PubMed] [Google Scholar]
- Lillywhite LM, Saling MM, Harvey AS, Abbott DF, Archer JS, Vears DF, et al. Neuropsychological and functional MRI studies provide converging evidence of anterior language dysfunction in BECTS. Epilepsia. 2009;50(10):2276–2284. doi: 10.1111/j.1528-1167.2009.02065.x. [DOI] [PubMed] [Google Scholar]
- Luo J, Niki K. Role of medial temporal lobe in extensive retrieval of task-related knowledge. Hippocampus. 2002;12(4):487–494. doi: 10.1002/hipo.10027. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Mummery CJ. Differential modulation of a common memory retrieval network revealed by positron emission tomography. Hippocampus. 1999;9(1):54–61. doi: 10.1002/(SICI)1098-1063(1999)9:1<54::AID-HIPO6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Manns JR, Hopkins RO, Squire LR. Semantic memory and the human hippocampus. Neuron. 2003;38(1):127–133. doi: 10.1016/s0896-6273(03)00146-6. [DOI] [PubMed] [Google Scholar]
- Meinzer M, Mohammadi S, Kugel H, Schiffbauer H, Floel A, Albers J, et al. Integrity of the hippocampus and surrounding white matter is correlated with language training success in aphasia. Neuroimage. 2010;53(1):283–290. doi: 10.1016/j.neuroimage.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Norrelgen F, Lilja A, Ingvar M, Amark P, Fransson P. Presurgical language lateralization assessment by fMRI and dichotic listening of pediatric patients with intractable epilepsy. Neuroimage: Clinical. 2015;7:230–239. doi: 10.1016/j.nicl.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ostby Y, Tamnes CK, Fjell AM, Westlye LT, Due-Tonnessen P, Walhovd KB. Heterogeneity in subcortical brain development: A structural magnetic resonance imaging study of brain maturation from 8 to 30 years. Journal of Neuroscience. 2009;29(38):11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petermann F, Petermann U. HAWIK-IV. Hamburger-Wechsler-Intelligenztest für Kinder IV. Bern: Hogrefe; 2008. [Google Scholar]
- Powell HW, Parker GJ, Alexander DC, Symms MR, Boulby PA, Barker GJ, et al. Imaging language pathways predicts postoperative naming deficits. Journal of Neurology, Neurosurgery, and Psychiatry. 2008;79(3):327–330. doi: 10.1136/jnnp.2007.126078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson MP, Strange BA, Duncan JS, Dolan RJ. Preserved verbal memory function in left medial temporal pathology involves reorganisation of function to right medial temporal lobe. Neuroimage. 2003;20(Suppl 1):S112–119. doi: 10.1016/j.neuroimage.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Ryan L, Cox C, Hayes SM, Nadel L. Hippocampal activation during episodic and semantic memory retrieval: comparing category production and category cued recall. Neuropsychologia. 2008;46(8):2109–2121. doi: 10.1016/j.neuropsychologia.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabsevitz DS, Swanson SJ, Hammeke TA, Spanaki MV, Possing ET, Morris GL, III, et al. Use of preoperative functional neuroimaging to predict language deficits from epilepsy surgery. Neurology. 2003;60(11):1788–1792. doi: 10.1212/01.wnl.0000068022.05644.01. [DOI] [PubMed] [Google Scholar]
- Seniow J, Litwin M, Litwin T, Lesniak M, Czlonkowska A. New approach to the rehabilitation of post-stroke focal cognitive syndrome: effect of levodopa combined with speech and language therapy on functional recovery from aphasia. Journal of the Neurological Sciences. 2009;283(1–2):214–218. doi: 10.1016/j.jns.2009.02.336. [DOI] [PubMed] [Google Scholar]
- Sepeta LN, Berl MM, Wilke M, You X, Mehta M, Xu B, et al. Age-dependent mesial temporal lobe lateralization in language fMRI. Epilepsia. 2016;57(1):122–130. doi: 10.1111/epi.13258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seress L. Comparative anatomy of the hippocampal dentate gyrus in adult and developing rodents, non-human primates and humans. Progress in Brain Research. 2007;163:23–41. doi: 10.1016/S0079-6123(07)63002-7. [DOI] [PubMed] [Google Scholar]
- Seress L, Ribak CE. Postnatal development and synaptic connections of hilar mossy cells in the hippocampal dentate gyrus of rhesus monkeys. Journal of Comparative Neurology. 1995;355(1):93–110. doi: 10.1002/cne.903550111. [DOI] [PubMed] [Google Scholar]
- Skirrow C, Cross JH, Harrison S, Cormack F, Harkness W, Coleman R, et al. Temporal lobe surgery in childhood and neuroanatomical predictors of long-term declarative memory outcome. Brain. 2015;138(Pt 1):80–93. doi: 10.1093/brain/awu313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Lah S. One declarative memory system or two? The relationship between episodic and semantic memory in children with temporal lobe epilepsy. Neuropsychology. 2011;25(5):634–644. doi: 10.1037/a0023770. [DOI] [PubMed] [Google Scholar]
- Somers M, Shields LS, Boks MP, Kahn RS, Sommer IE. Cognitive benefits of right-handedness: a meta-analysis. Neuroscience and Biobehavioral Reviews. 2015;51:48–63. doi: 10.1016/j.neubiorev.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory systems of the brain: a brief history and current perspective. Neurobiology of Learning and Memory. 2004;82(3):171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola SM. Episodic memory, semantic memory, and amnesia. Hippocampus. 1998;8(3):205–211. doi: 10.1002/(SICI)1098-1063(1998)8:3<205::AID-HIPO3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Hippocampal function in cognition. Psychopharmacology. 2004;174(1):99–110. doi: 10.1007/s00213-004-1795-9. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Binder JR, Possing ET, McKiernan KA, Ward BD, Hammeke TA. Language lateralization in left-handed and ambidextrous people: fMRI data. Neurology. 2002;59(2):238–244. doi: 10.1212/wnl.59.2.238. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Rajagopal A, Altaye M, Byars AW, Jacola L, Schmithorst VJ, et al. Left-handedness and language lateralization in children. Brain Research. 2012;1433:85–97. doi: 10.1016/j.brainres.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szlachta Z, Bozic M, Jelowicka A, Marslen-Wilson WD. Neurocognitive dimensions of lexical complexity in Polish. Brain and Language. 2012;121(3):219–225. doi: 10.1016/j.bandl.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Takashima A, Bakker I, van Hell JG, Janzen G, McQueen JM. Richness of information about novel words influences how episodic and semantic memory networks interact during lexicalization. Neuroimage. 2014;84:265–278. doi: 10.1016/j.neuroimage.2013.08.023. [DOI] [PubMed] [Google Scholar]
- Tamminen J, Payne JD, Stickgold R, Wamsley EJ, Gaskell MG. Sleep spindle activity is associated with the integration of new memories and existing knowledge. Journal of Neuroscience. 2010;30(43):14356–14360. doi: 10.1523/JNEUROSCI.3028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry G. Neurolinguistic Relativity: How Language Flexes Human Perception and Cognition. Language Learning. 2016;66(3):690–713. doi: 10.1111/lang.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaszewki Farias S, Harrington G, Broomand C, Seyal M. Differences in functional MR imaging activation patterns associated with confrontation naming and responsive naming. AJNR American Journal of Neuroradiology. 2005;26(10):2492–2499. [PMC free article] [PubMed] [Google Scholar]
- Ullman MT. A neurocognitive perspective on language: the declarative/procedural model. Nature Reviews Neuroscience. 2001;2(10):717–726. doi: 10.1038/35094573. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277(5324):376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- Verfaellie M, Koseff P, Alexander MP. Acquisition of novel semantic information in amnesia: effects of lesion location. Neuropsychologia. 2000;38(4):484–492. doi: 10.1016/s0028-3932(99)00089-5. [DOI] [PubMed] [Google Scholar]
- Wilke M, Holland SK, Altaye M, Gaser C. Template-O-Matic: a toolbox for creating customized pediatric templates. Neuroimage. 2008;41(3):903–913. doi: 10.1016/j.neuroimage.2008.02.056. [DOI] [PubMed] [Google Scholar]
- Wilke M, Lidzba K. LI-tool: a new toolbox to assess lateralization in functional MR-data. Journal of Neuroscience Methods. 2007;163(1):128–136. doi: 10.1016/j.jneumeth.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Wilke M, Schmithorst VJ. A combined bootstrap/histogram analysis approach for computing a lateralization index from neuroimaging data. Neuroimage. 2006;33(2):522–530. doi: 10.1016/j.neuroimage.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Yeatman JD, Ben-Shachar M, Glover GH, Feldman HM. Individual differences in auditory sentence comprehension in children: An exploratory event-related functional magnetic resonance imaging investigation. Brain and Language. 2010;114(2):72–79. doi: 10.1016/j.bandl.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You X, Adjouadi M, Guillen MR, Ayala M, Barreto A, Rishe N, et al. Sub-patterns of language network reorganization in pediatric localization related epilepsy: a multisite study. Human Brain Mapping. 2011;32(5):784–799. doi: 10.1002/hbm.21066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Killgore WD, Cintron CB. Cognitive correlates of medial temporal lobe development across adolescence: a magnetic resonance imaging study. Percept Mot Skills. 2003;96(1):3–17. doi: 10.2466/pms.2003.96.1.3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.