Abstract
Serotonin (5HT) is a constituent of the so-called “inflammatory soup” that sensitizes nociceptors during inflammation. Nevertheless, receptors and signaling mechanisms that mediate an excitation of dorsal root ganglion (DRG) neurons by 5HT remained controversial. Therefore, capsaicin-sensitive nociceptive neurons dissociated from rat DRGs were used to investigate effects of 5HT on membrane excitability and currents through ligand- as well as voltage-gated ion channels. In 58% of the neurons tested, 5HT increased action potential firing, an effect that was abolished by the 5HT2 receptor antagonist ritanserin, but not by the 5HT3 antagonist tropisetron. Unlike other algogenic mediators, such as PGE2 and bradykinin, 5HT did not affect currents through TTX-resistant Na+ channels or Kv7 K+ channels. In all neurons investigated, 5HT potentiated capsaicin-evoked currents through TRPV1 channels, an effect that was attenuated by antagonists at 5HT2A (4 F 4 PP), 5HT2B (SB 204741), as well as 5HT2C (RS 102221) receptors. 5HT triggered slowly arising inward Cl− currents in 53% of the neurons. This effect was antagonized by the 5HT2C receptor blocker only, and the current was prevented by an inhibitor of Ca2+-activated chloride channels (CaCC). The 5HT-induced increase in action potential firing was also abolished by this CaCC blocker and by the TRPV1 inhibitor capsazepine. Amongst the subtype selective 5HT2 antagonists, only RS 102221 (5HT2C-selectively) counteracted the rise in action potential firing elicited by 5HT. These results show that 5HT excites DRG neurons mainly via 5HT2C receptors which concomitantly mediate a sensitization of TRPV1 channels and an opening of CaCCs.
Keywords: Dorsal root ganglion neurons, 5HT2 receptors, TRPV1 channels, Ca2+-activated chloride channels, TTX-resistant Na+ channels, Kv7 K+ channels
1. Introduction
Nociceptive dorsal root ganglion (DRG) neurons are excited by noxious stimuli such as heat, cold, excessive pressure, or chemical irritation, but not by nonhazardous stimuli such as warming or light touch which, however, suffice to trigger firing in non-nociceptive DRG neurons (Julius and Basbaum, 2001). Under conditions of tissue damage and inflammation, nociceptors get sensitized towards such non-noxious stimuli by a plethora of signaling molecules that are released from surrounding cells. These mediators are collectively characterized as “inflammatory soup” (Basbaum et al., 2009) and contain protons, kinins, prostanoids, nucleotides, histamine, and serotonin/5HT (Dray, 1995).
The role of serotonin in pain sensation is particularly complex, as this monoamine is known to exert pro- as well as antinociceptive effects in the central as well as peripheral nervous system. Even at the level of DRG neurons, serotonin has been found to either boost or mitigate nociceptive signaling (Sommer, 2004). This diversification of serotonin actions in DRG neurons is related to the large number of different 5HT receptors that are available for the monoamine to elicit its effects. This receptor family comprises seven subclasses, 5HT1 to 5HT7, and each of these groups can harbor one (5HT4, -6, -7) to five (5HT1) different proteins (Hoyer et al., 2002). With the exception of 5HT3, which belongs to the cys-loop family of ligand-gated ion channels, all these receptors display a 7 transmembrane segment topology and signal via heterotrimeric G proteins. Members of the 5HT1 group couple to Gi/o, 5HT2 to Gq/11, and the remaining ones mainly to Gs (Hannon and Hoyer, 2008). In fact, 5HT1A, 5HTIB, 5HT1D, 5HT1E, 5HT1F, 5HT2A, 5HT2B, 5HT2C, 5HT3A, 5HT3B, 5HT4, 5HT5A, 5HT5B, 5HT6, and 5HT7 receptors have been reported to be expressed in DRGs (Nicholson et al., 2003; Pierce et al., 1996).
Incipiently, 5HT released from platelets and mast cells during tissue damage and inflammation was believed to excite nociceptors via 5HT3 receptors (Julius and Basbaum, 2001), but then it turned out that this ionotropic receptor was only relevant in a minor subset of DRG neurons and only for delayed nociceptive responses (Zeitz et al., 2002). Therefore, 5HT must be expected to act on DRG neurons rather via G protein-coupled receptors (GPCRs) as do bradykinin (Liu et al., 2010), prostaglandin E2 (England et al., 1996) and nucleotides (Yousuf et al., 2011). The excitatory actions of such algogenic mediators on DRG neurons in vitro parallel their proalgetic effects in vivo (Liu et al., 2010). Vice versa, a decrease in the excitability of DRG neurons due to ion channel modulation in vitro is correlated with analgesia in vivo (Du et al., 2014). Excitation of sensory neurons by the aforementioned inflammatory mediators via GPCRs involves changes in the gating of various ion channels: activation of bradykinin B2 receptors facilitates the opening of TRPV1 channels and TMEM16A Ca2+-activated Cl− channels (CaCCs) and simultaneously inhibits Kv7 K+ channels (Brown and Passmore, 2010; Liu et al., 2010); prostaglandin E2 enhances currents through tetrodotoxin (TTX) resistant Na+ channels via EP2 as well as EP4 receptors (Matsumoto et al., 2005) and currents through TRPV1 channels via EP1 receptors (Moriyama et al., 2005); nucleotides promote the gating of TRPV1 channels and impede the gating of Kv7 K+ channels, both via P2Y1 as well as P2Y2 receptors (Yousuf et al., 2011). In comparison, in DRG neurons 5HT has been proposed to potentiate TRPV1 channels via 5HT2, 5HT4, and/or 5HT7 receptors (Ohta et al., 2006; Sugiuar et al., 2004) and TTX resistant Na+ channels via 5HT4 receptors (Cardenas et al., 1997). Although 5HT is also known to increase the excitability of DRG neurons, receptor subtypes and mechanisms involved therein have not been elucidated (Cardenas et al., 2001). In particular, it remained unclear whether ion channels other than TRPV1 and TTX resistant Na+ channels might be relevant for the control of neuronal excitability in DRGs by 5HT and which of the three distinct 5HT2 receptor subtypes were involved. These issues are addressed below, and the results reveal a previously unrecognized role of 5HT2C receptors linked to Ca2+-activated Cl− channels.
2. Materials and methods
2.1. Materials
5-Hydroxytryptamine (serotonin, 5HT), gamma-aminobutyric acid (GABA), capsaicin, ritanserin, tropisetron hydrochloride along with bulk chemicals were obtained from Sigma-Aldrich (Vienna, Austria). Bradykinin, 6-(1,1-Dimethylethyl)-2-[(2-furanylcarbonyl) amino]-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxylic acid (CaCC Inh. - A01), SB 204741 (N-(1-Methyl-1H-5-indolyl)-N′ -(3-methyl-5-isothiazolyl)urea), thapsigargin, and XE991 (10,10-bis(4-pyridinylmethyl)-9(10H)-anthracenone) were purchased from Tocris (Bristol, UK). 4-(4-fluorobenzoyl)-1-(4phenylbutyl) piperidine (4 F 4 PP) oxalate was obtained from Santa Cruz Biotechnology, Inc. (Heidelberg, Germany), capsazepine from Sanova Pharma Ges. m. b. H. (Vienna, Austria), 8-[5-(2,4-dimethoxy-5-(4-trifluoromethylphenylsulphonamido) phenyl-5-oxopentyl]-1,3,8-triazaspiro[4,5]decane-2,4-dione (RS102221) from Szabo-Scandic (Vienna, Austria), and tetrodotoxin (TTX) from Latoxan (Valence, France).
2.2. Primary cultures of DRG neurons
Our methods of DRG culture preparation have been described in detail before (Klinger et al., 2012). Rats were sacrificed by decapitation after short CO2 asphyxia in accordance with the ARRIVE guidelines and the Austrian animal protection law (see http://www.ris.bka.gv.at/Dokumente/BgblAuth/BGBLA_2012_I_114/BGBLA_2012_I_114.pdf) and the Austrian animal experiment by-laws (see http://www.ris.bka.gv.at/Dokumente/BgblAuth/BGBLA_2012_II_522/BGBLA_2012_II_522.pdf) which implement European (DIRECTIVE 2010/63/EU; see http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:en:PDF) in Austrian law. Collected ganglia from all levels were incubated in collagenase (1.5 mg ml−1, Sigma-Aldrich) and dispase (3.0 mg ml−1, Boehringer Mannheim, Vienna, Austria) for 30 min followed by 0.25% trypsin (Worthington, Lakewood, NJ, USA) for 15 min at 37 °C. After trituration, cells were resuspended in Dulbecco's Modified Eagle Medium containing 4.5 g l−1 glucose, 10 mg l−1 insulin, 25 000 IU l−1 penicillin, 25 mg l−1 streptomycin (all Sigma-Aldrich), 50 μg l−1 nerve growth factor (Biomedica, Vienna, Austria), and 5% heat-inactivated fetal calf serum (Biochrom, Berlin, Germany) and seeded onto 35 mm culture dishes coated with poly d-lysine (Sigma-Aldrich). Cultures were kept at 37 °C in a humidified 5% CO2 atmosphere. On days 1 and 3 after preparation, the medium was exchanged entirely.
2.3. Electrophysiology
Electrophysiological experiments were carried out as described recently (Klinger et al., 2015) using small diameter DRG neurons. Unless stated otherwise, each of the neurons included in the analysis showed capsaicin-induced currents (Icaps) as an indication of nociceptive functions. Recordings were performed at room temperature (20 - 24 °C) using an Axopatch 200B amplifier, the pCLAMP 10.4 hard- and software and digitized with a Digidata 1440 (Molecular Devices, Sunnyvale, California). All current clamp recordings as well as voltage-clamp recordings of currents through ligand-gated ion channels (P2X, 5HT3), TRPV1 and Kv7 voltage-gated potassium channels were conducted in the perforated patch-clamp mode, whereas currents through TTX-resistant voltage-gated sodium channels were recorded in conventional whole-cell mode. Traces of voltage-gated sodium currents were low-pass filtered at 10 kHz and digitized at 50 kHz. All other recordings were low-pass filtered at 2 kHz and digitized at 10 kHz. Data were analyzed offline using the Clampfit 10.4 software (Molecular Devices). Patch-pipettes were pulled from borosilicate glass capillaries (GB150-8P, Science Products) with a Sutter P-97 puller (Sutter Instruments, Novato, CA, USA) using a trough filament (FT330B, Science Products, Hofheim, Germany). For perforated-patch recordings, the pipette resistances were between 1 and 3.5 MΩ. Pipettes were front-filled with internal solution and then backfilled with the same solution containing 500 μg ml−1 amphotericin B. When the series resistance had dropped below 20 MΩ after 20–30 min, recordings were started. For conventional whole-cell recordings, tip resistances were between 2.5 and 3.5 MΩ. After achieving a GΩ seal, cell membranes were ruptured by applying negative pressure to reach series resistances of 3–7 MΩ. Cells were allowed to equilibrate for 2 min before the start of recordings.
The internal solution for recordings of membrane excitability and currents through TRPV1 channels, 5HT3 receptors, P2X receptors as well as Kv7 channels contained (mM): K2SO4 (75), KCl (55), MgCl2 x 6H2O (8.0), and HEPES (10) and was adjusted to pH 7.4 with KOH. In recordings of currents through calcium-activated chloride channels, a pipette solution previously optimized for this purpose (Salzer et al., 2014) was used instead and consisted of (mM): KCl (140), CaCl2 x 2H2O (1.0), MgCl2 (0.7), EGTA (10), HEPES (10), and KOH (29.4), thereby achieving pH 7.4. In experiments focusing on the reversal potential of Cl− currents, the intracellular solution had to contain K+ channel blockers and therefore comprised (mM) CsCl (140), TEA-Cl (20), CaCl2 x 2H2O (1.0), EGTA (5), Glucose (10), HEPES (10), and CsOH (15), thereby achieving pH 7.4. For recordings of TTX-resistant voltage-gated sodium currents, the internal solution was composed as follows (mM): Cs aspartate (145), NaCl (10), HEPES (10), EGTA (1.0), MgCl2 (2.0) Na-ATP (1.0), Na-GTP (0.3) adjusted to pH 7.4 with CsOH. The bath solution for all perforated-patch recordings contained (mM): NaCl (140), Glucose (20), HEPES (10), CaCl2 x 2H2O (2.5), MgCl2 (2.0), KOH (3.0), and NaOH (2.0) to achieve pH 7.4. In whole-cell recordings of Na+ currents, the composition was the following (mM): TEA-Cl (125), NaCl (15), Glucose (11), HEPES (5.0), CsCl (3.0), MgCl2 (1.0), and TTX (0.0005), adjusted to pH 7.4 with TEA-OH. The pipette solution containing KCl did not result in a liquid junction potential. The pipette solution containing K2SO4 resulted in a junction potential of −7.4 mV, the combination of solutions for recordings of voltage-gated sodium currents led to junction potentials of −20 mV, both of which were corrected for during experimentation. Stock solutions of 4 F 4 PP, BAPTA-AM, CaCC-Inh. A01, capsazepine, GF 109203X, RS 102221, SB 204741, ritanserin, thapsigargin, tropisetron, and U73122 were prepared in DMSO, stock solutions of capsaicin were prepared in ethanol and were then diluted in external solutions. In control recordings, DMSO was used as solvent in appropriate concentrations. Drugs were either applied with a DAD-12 drug application device (ALA Scientific Instruments Inc., Westbury, NY) or an OctaFlow perfusion system (ALA Scientific Instruments Inc.), which both permit complete exchange of solutions surrounding the patched cell within 100 ms.
To test the effect of 5HT on membrane excitability, small diameter neurons were injected with 5 evenly increasing current steps (0.1–0.3 nA). Changes in excitability were evaluated by the number of evoked action potentials in presence of 5HT as compared to those in its absence. Blockers of ion channels or receptor antagonists were present for 60 s before application of 5HT.
Currents through 5HT3, P2X and TRPV1 channels were recorded at a steady holding voltage of −70 mV. ATP and 5HT were applied for 2 s, respectively. For recordings of currents through TRPV1 channels (Icaps), capsaicin (0.3 μM) was applied for 15 s once every 2 min 5HT was present for 30 s before application of capsaicin. Peak current amplitudes were analyzed. Currents through Kv7 channels were evoked by clamping cells to −30 mV followed by a 1 s step to -55 mV once every 15 s to deactivate Kv7 channels. The deactivation amplitude between 20 ms after the start and 20 ms before the end of the hyperpolarizing voltage step was evaluated as the amplitude of currents through Kv7 channels (Klinger et al., 2012). For recordings of currents through calcium-activated chloride channels, cells were clamped to −65 mV (which approximately equals the resting membrane potentials; see 3.1), and 5HT was applied twice for 120 s with a 6 min washout period between the applications (Salzer et al., 2014). Antagonists of GPCRs and calcium-activated chloride channels were present for 60 s prior to and throughout the application of 5HT and during the 5 min washout phase after the application. An average of mean current levels during 10 s periods before the application of 5HT and 10 s periods at the end of the 5 min washout phase were taken as baseline and compared to the mean current levels during the last 10 s period of the 5HT application. Voltage-activated Na+ currents were activated routinely by 15 ms voltage steps from −90 mV to −10 mV once every 5 s. For current voltage relations of Na+ channels, DRG neurons were depolarized for 10 ms from a holding potential of −90 mV to voltages between −60 mV and +20 mV. In between the voltage steps, cells were clamped to −80 mV and a P/6 protocol was used for leak subtraction. Voltage-gated sodium channels were exclusively examined on freshly dissociated neurons; all other recordings were performed on days 1–5 after dissociation.
2.4. Statistics
All data are represented as arithmetic means ± SEM; n values give the number of single cells measured. The statistical tests used are indicated in the text and the figure legends, respectively.
3. Results
3.1. Serotonin increases the excitability of DRG neurons via 5HT2 receptors
To analyze effects of 5HT on the excitability of DRG neurons, current clamp experiments were performed, and currents of evenly increasing amplitudes were injected in order to elicit action potential firing. Via this procedure, excitability enhancing effects of bradykinin (Liu et al., 2010), prostaglandin E2 (England et al., 1996), and nucleotides (Yousuf et al., 2011) have been demonstrated for DRG neurons. In the perforated patch configuration, the resting membrane potential of DRG neurons amounted to −65.2 ± 0.5 mV (n = 131), and injection of a sequence of 5 depolarizing currents with amplitudes increasing from 0.1 to 0.3 nA led to the firing of 5.4 ± 0.4 action potentials (n = 131). In an initial set of 64 neurons, 37 displayed increased excitability in the presence of 10 μM 5HT: the number of action potentials fired in the absence of 5HT was 6.3 ± 0.7, and this value rose to 25.8 ± 3.4 in the presence of the monoamine (see Fig. 1a for a representative recording). In these 37 neurons, 5HT also caused a depolarization of the membrane potential by 3.7 ± 0.6 mV. In the remaining 27 neurons, the number of action potentials fired in the absence (3.5 ± 0.4) and presence of 5HT (4.4 ± 0.4) was comparable and the membrane potential remained apparently unaffected.
Fig. 1. Increase in membrane excitability of rat DRG neurons caused by 5HT.
Action potentials were recorded in perforated current clamp mode (a and b) and were triggered by injecting five increasing current steps (shown in a, left panel). Application of 5HT, tropisetron and ritanserin was started 60 s before current injection. In perforated voltage clamp mode (c and d), currents through 5HT3 and P2X receptors were induced by 2 s applications of the indicated concentrations of 5HT and ATP, respectively, both at a voltage of −70 mV. a. Representative voltage traces recorded before (control) or during application of 10 μM 5HT, either alone or in the presence of 30 nM tropisetron (5HT + tropisetron) or 1 μM ritanserin (5HT + ritanserin). b. Total number of action potentials triggered by the 5 increasing current injections in presence of 5HT either alone or together with ritanserin and tropisetron, respectively (n = 5). * indicates significant difference vs. 5HT at p < 0.05 (Kruskal-Wallis test, Dunn's multiple comparison). c. Representative traces of currents evoked by application of the indicated concentrations of either 5HT or ATP. d. Peak amplitudes of currents evoked by application of the indicated concentrations of 5HT and ATP, respectively (n = 9). **, *** indicate significant differences vs. ATP at p < 0.01 and p < 0.001, respectively (Kruskal-Wallis test, Dunn's multiple comparison).
To reveal which receptors might be involved in the excitatory actions described above, 5HT was applied again in the presence of 30 nM tropisetron to 7 of the responsive neurons; this concentration is sufficient to abolish the effects of 10 μM serotonin at neuronal 5HT3 receptors (Dorostkar and Boehm, 2007). However, the effects of 5HT were not altered by this 5HT3 antagonist (Fig. 1a and b). Therefore, 1 μM of the 5HT2 receptor antagonist ritanserin (Knight et al., 2004) was applied after removal of tropisetron and turned out to abrogate the actions of the agonist (Fig. 1a and b). Thus, the 5HT-dependent increase in the excitability of DRG neurons did not involve 5HT3 receptors, but was rather mediated by 5HT2 receptors.
To reveal whether DRG neurons do express functionally significant levels of 5HT3 receptors, voltage clamp experiments were performed to search for 5HT-induced currents. However, at concentrations up to 100 μM the monoamine failed to induce significant inward currents at negative membrane potentials (Fig. 1c and d). For comparison, in the very same neurons 10 μM ATP caused inward currents with amplitudes of several hundred pA (Fig. 1c and d) as described before (Yousuf et al., 2011). Hence, from the data obtained in current as well as voltage clamp experiments one may conclude that 5HT3 receptors cannot be of major importance.
3.2. Serotonin sensitizes TRPV1 channels via three 5HT2 receptor subtypes
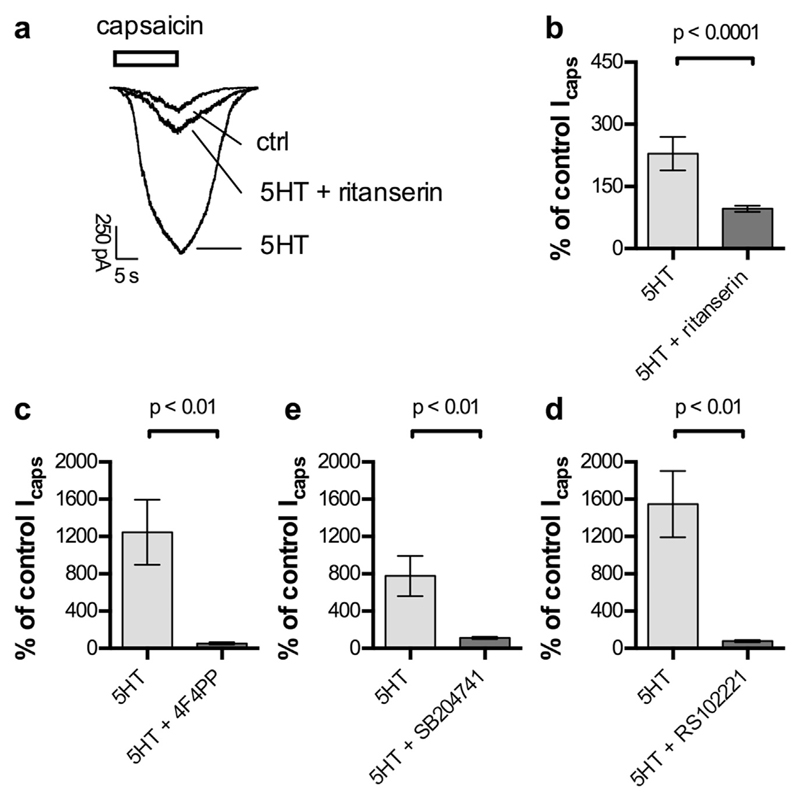
In DRG neurons dissected from either rats (Ohta et al., 2006) or mice (Sugiuar et al., 2004), 5HT has been found to sensitize TRPV1 channels. This mechanism is also known to contribute to the raised excitability of DRG neurons in the presence of bradykinin, prostaglandins, and nucleotides, as outlined in the introduction. To reveal whether such an action could also contribute to the 5HT2 receptor-mediated increase in DRG excitability described above, currents through TRPV1 channels were elicited by 0.3 μM capsaicin in the absence and presence of 10 μM 5HT. The monoamine potentiated capsaicin-induced currents in all neurons (n = 28) investigated, but the effect varied considerably between single cells (Fig. 2b to d). Nevertheless, in all subsets of neurons (Fig. 2b to d), the 5HT dependent increase in current amplitudes was statistically significant (p < 0.05, Friedman test followed by Dunn's post-hoc comparison). As observed with the 5HT-induced increase in excitability, the effect of 5HT on TRPV1 currents was significantly attenuated by 1 μM ritanserin (Fig. 2a and b). Moreover, in the presence of 1 μM ritanserin, 10 μM 5HT failed to significantly augment capsaicin-induced currents (n = 12; p > 0.05, Friedman test followed by Dunn's post-hoc comparison).
Fig. 2. Potentiation of currents through TRPV1 channels by 5HT.
Currents through TRPV1 channels, recorded in perforated voltage clamp mode at −70 mV, were evoked by application of capsaicin (0.3 μM) for 15 s in the absence and presence of 5HT2 receptor ligands. a. Representative traces of currents evoked by capsaicin before (ctrl) and during the presence of either 10 μM 5HT (5HT) or 10 μM 5HT plus 1 μM ritanserin (5HT + ritanserin). b. Potentiation of capsaicin-evoked currents (Icaps) by 10 μM 5HT applied either alone (5HT) or together with 1 μM ritanserin (5HT + ritanserin; n = 12). c. Potentiation of capsaicin-induced currents (Icaps) by 10 μM 5HT applied either alone (5HT) or together with 100 nM 4F4PP (5HT + 4F4PP; n = 5). d. Potentiation of capsaicin-evoked currents (Icaps) by 10 μM 5HT applied either alone (5HT) or in combination with 200 nM SB204741 (5HT + SB204741; n = 6). e. Potentiation of capsaicin-induced currents (Icaps) by 10 μM 5HT applied either alone (5HT) or together with 30 nM RS102221 (5HT + RS102221; n = 5). p < 0.01 and p < 0.0001 indicate levels of significance determined by a Mann-Whitney test for differences between the two bars being compared.
As evidence for the expression of each of the three 5HT2 receptor subtypes (5HT2A, 5HT2B, 5HT2C) in DRGs has been presented (see Introduction), the subtype-selective antagonists RS 102221, 4 F 4 PP (Acuña-Castillo et al., 2002), and SB 204741 (Forbes et al., 1995) were used to reveal which of these subtypes contribute to the potentiation of TRPV1 channels by 5HT. Each of these agents attenuated the potentiation of capsaicin-evoked currents by 10 μM 5HT (Fig. 2 c to d), and in the presence of 100 nM 4 F 4 PP, 200 nM SB 204741, and 30 nM RS 102221, respectively, this 5HT concentration failed to cause significant changes in TRPV1 current amplitudes (n = 5 to 6 in each case; p > 0.05, Friedman test followed by Dunn's post-hoc comparison). Thus, all three 5HT2 receptor subtypes can contribute to the potentiation of TRPV1 channels by 5HT.
3.3. Serotonin does not affect Kv7 K+ or TTX-resistant Na + channels
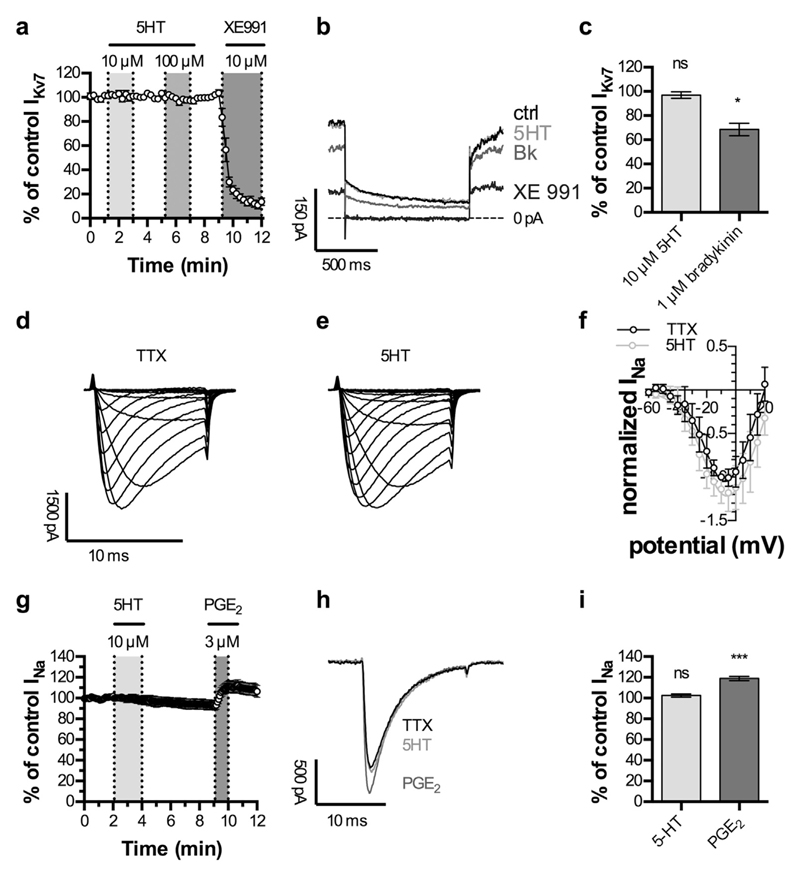
Algogenic mediators other than 5HT, such as bradykinin and nucleotides, are known to excite DRG neurons through an inhibition of voltage-gated Kv7 K+ channels (see Introduction). However, currents carried by Kv7 channels, also known as M currents (IM; Brown and Passmore, 2010) were not altered in the presence of up to 100 μM 5HT, but reduced in amplitude by >90% when 10 μM XE991, a specific M current blocker, was applied (Fig. 3a and b). In contrast to 5HT, bradykinin, at a concentration of 1 μM, reduced M current amplitudes by 30% (Fig. 3c).
Fig. 3. Lack of effect of 5HT on currents through Kv7 K+ and TTX-resistant Na+ channels.
Currents through Kv7 channels (IKv7) were recorded in perforated voltage clamp mode. Cells were clamped to −30 mV and hyperpolarized to −55 mV for 1 s, once every 15 s. Currents through voltage-gated Na+ channels (INa) were recorded in whole-cell voltage clamp mode in the presence of 0.5 μM tetrodotoxin (TTX). a. Time course of IKv7 in capsaicin positive DRG neurons. 10 μM and 100 μM 5HT were applied as indicated by grey shaded areas, respectively. XE 991 (10 μM) was applied to confirm the recording of IKv7 (n = 9). b. Representative traces of IKv7 under control conditions (ctrl), during the application of 10 μM 5HT (5HT), 1 μM bradykinin (Bk) or 10 μM XE991 (XE991). c. Effects of 10 μM 5HT (5HT) and 1 μM bradykinin (Bk) on IKv7 (n = 6). * indicates significant difference vs. amplitudes of control IKv7 (Friedman test, Dunn's post-hoc comparison). d. Na+ currents in a freshly dissociated, capsaicin sensitive DRG neuron evoked by 10 ms depolarizations from −90 mV to potentials between −60 mV and +20 mV in the presence of 0.5 μM TTX. e. Na+ currents in the very same neuron evoked again by 10 ms depolarizations from −90 mV to potentials between −60 mV and +20 mV, but this time in the presence of 0.5 μM TTX plus 10 μM 5HT. f. Current voltage relations for currents evoked as shown in d and e in 5 different neurons. For each neuron, all peak current amplitudes were normalized to the peak amplitude obtained at −10 mV (maximum) in the presence of TTX only. Shown are mean values of normalized current amplitudes (normalized INa). g. Time course of INa evoked by 15 ms depolarizations from −90 mV to −10 mV, applied once every 5 s. 10 μM 5HT and 3 μM prostaglandin E2 (PGE2) were applied as indicated by the grey shaded areas (n = 7). h. Representative traces of voltage-gated Na+ currents before (TTX) and during application of 10 μM 5HT (5HT) or 3 μM prostaglandin E2 (PGE2). i. Effects of 10 μM 5HT (5HT) and 3 μM prostaglandin E2 (PGE2) on voltage-gated Na+ currents (n = 7). *** indicates a significant difference vs. amplitudes of control INa (Friedman test, Dunn's post-hoc comparison).
Another type of voltage-gated ion channel that is known to be modulated by inflammatory mediators such as prostaglandins (see Introduction) is TTX-resistant Na+ channels. To learn whether the gating of such channels might be sensitive towards 5HT, current voltage curves were obtained in the presence of either of 0.5 μM TTX alone or TTX plus 10 μM 5HT, but no significant differences were to be seen (Fig. 3d to f). In subsequent experiments, Na+ currents were elicited by depolarizations to −10 mV, and either 10 μM 5HT or 3 μM PGE2 were added to 0.5 μM TTX. Again, current amplitudes remained unaltered in the presence of 5HT, but were enhanced by PGE2 (Fig. 3g to i).
3.4. Serotonin leads to the gating of Ca2+ activated Cl− channels
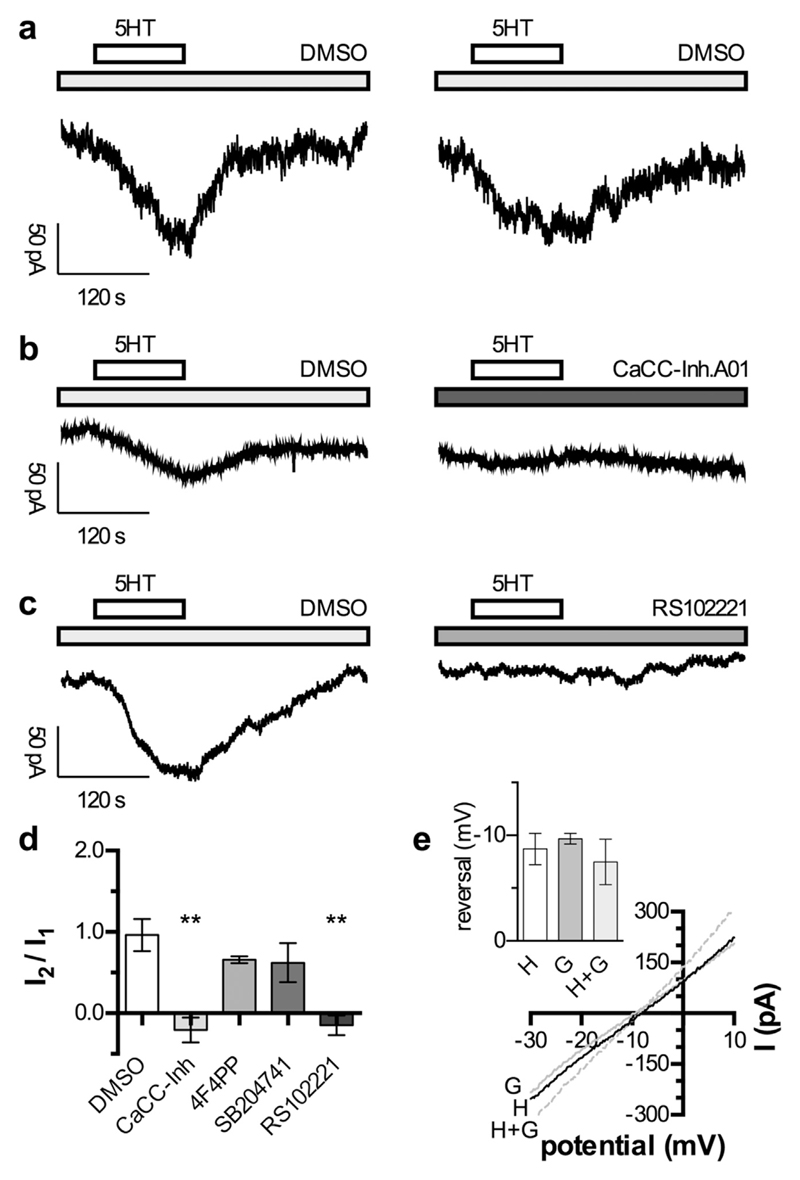
In addition to Kv7 K+ and TTX-resistant Na+ channels, CaCCs have been reported to be involved in the excitation of DRG neurons via GPCRs (see Introduction). At a holding potential close to the resting membrane potential (−65 mV), the application of 10 μM 5HT led to an inward current that developed with a considerable delay (Fig. 4a). Such inward currents were observed in 25 out of 47 cells tested. On average, the beginning of the downward deflection of the current trace was found at 20.5 ± 2.2 s after the start of 5HT application, and the maximum current amplitude was reached after 99.5 ± 3.8 s (n = 25). To verify that this slow inward current was mediated by CaCCs, the specific blocker CaCC Inh. - A01 (Namkung et al., 2011) was used. In the presence of solvent (DMSO), the inward current was evoked by two consecutive applications of 5HT, and the ratio of the two current amplitudes (I2/I1) was close to one (Fig. 4a and d) since the two current amplitudes were not significantly different from each other (n = 5; p > 0.05, Mann-Whitney test). When the second 5HT application was performed in the presence of 3 μM CaCC-Inh. A01, no obvious inward current was to be seen; the amplitudes of the second currents were significantly smaller (n = 5; p < 0.05, Mann-Whitney test), and the I2/I1 ratio dropped below zero (Fig. 4b and d). This proves that the slowly developing 5HT-induced inward current was mediated by CaCCs.
Fig. 4. Activation of currents through CaCCs channels by 5HT.
Cells were recorded in perforated voltage clamp mode at a holding potential of −65 mV (a to d). 5HT was applied twice for 120 s (indicated by the bar) separated by 300 s washout and 60 s wash-in of the respective modulator. a. Original traces of currents induced by two subsequent applications of 10 μM 5HT in presence of 0.03% DMSO. b. Original traces of currents induced by two subsequent applications of 10 μM 5HT in the presence of 0.03% DMSO and 3 μM CaCC-Inh. A01 (as indicated by the grey bar), respectively. c. Original traces of currents induced by two subsequent applications of 10 μM 5HT in the presence of 0.03% DMSO and 30 nM RS102221 (as indicated by the grey bar), respectively. d. Relation of the currents induced by two subsequent applications of 10 μM 5HT (I2/I1) in the presence of 0.03% DMSO (n = 5), 3 μM CaCC-Inh. A01 (n = 5), 100 nM 4F4PP (n = 5), 200 nM SB204741 (n = 5), and 30 nM RS102221 (n = 5), respectively. ** indicate significant differences vs. the I2/I1 ratio obtained in DMSO at p < 0.01, respectively (Kruskal-Wallis test, followed by Dunn's multiple comparison). e. Currents were evoked by the application of 10 μM 5HT (H), 10 μM GABA (G), or 10 μM 5HT plus 10 μM GABA (H + G). During peak current responses, 50 ms ramp depolarizations from −30 mV to +10 mV were applied in order to determine reversal potentials, and the resulting current traces are shown. In the inset, the values of reversal potentials obtained with 10 μM 5HT (H), 10 μM GABA (G), or 10 μM 5HT plus 10 μM GABA (H + G) in 5 different neurons are indicated. There were no significant differences between these three values (p > 0.05; Kruskal-Wallis test, followed by Dunn's multiple comparison).
To reveal whether this 5HT effect was mediated by 5HT2 receptors, and if so, by which of the three subtypes, the subtype-selective 5HT2 receptor antagonists were employed again at the concentrations mentioned above. These antagonists were tested in the same manner as described for the CaCC inhibitor. Of these three subtype-selective blockers, only RS 102221 significantly depressed the second current as compared to the preceding one (n = 5; p < 0.05, Mann-Whitney test) and reduced the I2/I1 ratio to a value below zero (Fig. 4b and d). Thus, 5HT activates CaCCs in DRG neurons via 5HT2C receptors.
To further prove that these currents were carried by Cl− ions, the reversal potential of the currents induced by 5HT was compared to that of GABA-evoked currents. In both cases, the reversal values lay between −5 and −10 mV and were not different from each other (Fig. 4e). Considering that these values were obtained in perforated patch recordings, they are in reasonable agreement with the calculated value of −2 mV. To reveal whether 10 μM 5HT might affect the Cl− equilibrium, GABA-evoked currents were also determined in the presence of the monoamine, but the resulting reversal potential was not different from that obtained in its absence (Fig. 4e). Thus, 5HT did evoke Cl− currents, but did not cause significant alterations in the Cl− equilibrium.
3.5. The excitation of DRG neurons by serotonin involves 5HT2C receptors as well as TRPV1 and Ca2+ activated Cl− channels
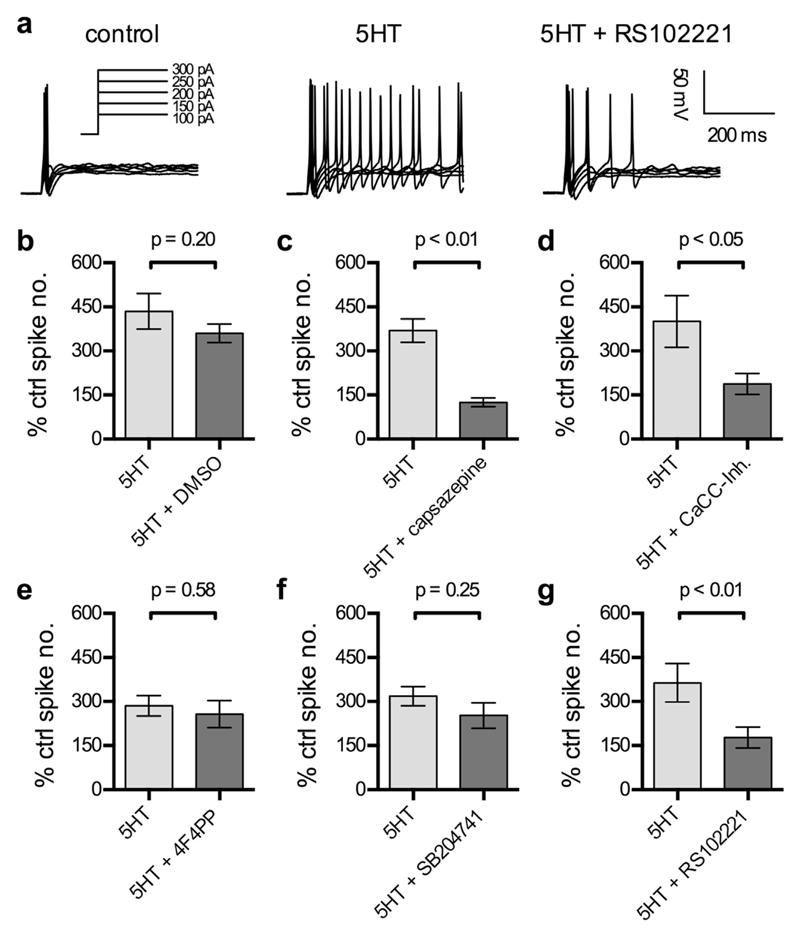
Knowing that 5HT acted on TRPV1 as well as Ca2+ activated Cl− channels, the contribution of these two ion channels to the 5HT-induced increase in excitability was explored. In the presence of DMSO used as solvent, two consecutive applications of 10 μM 5HT raised the number of action potentials fired in response to current injections to a comparable extent (about 400% of control; Fig. 5b); with both 5HT challenges, the increase in the number of action potentials was significant (n = 9; p < 0.05, Friedman test followed by Dunn's post-hoc comparison). In the presence of either 10 μM capsazepine, a TRPV1 antagonist (Dickenson and Dray, 1991), or 3 μM CaCC-Inh. A01, the relative increase in firing rate caused by 5HT was significantly diminished (Fig. 5c and d). Moreover, both channel blockers prevented 5HT from inducing a significant rise in the number of action potentials seen in response to current injections (n = 9 for capsazepine and 8 for CaCC-Inh. A01; p > 0.05, Friedman test followed by Dunn's post-hoc comparison). Hence, both channels contributed to the excitation of DRG neurons by 5HT.
Fig. 5. Roles of ion channels and 5HT2 receptor subtypes in the excitation of DRG neurons by 5HT.
Cells were recorded in perforated current-clamp mode. Action potentials were evoked by five increasing current injections (shown in a, left panel). The application of 10 μM 5HT was started 45 s before the start of recordings. DMSO 0.03% or antagonists were present from 60 s onwards prior to the recording. a. Representative traces recorded before (ctrl) or during the application of 10 μM 5HT, either alone (5HT) or in the presence of 30 nM RS102221 (5HT + RS102221). b-g Increases in the number of action potentials evoked by 5 increasing current injections caused by 10 μM 5HT applied either alone (5HT) or in the presence of 0.03% DMSO (b, 5HT + DMSO, n = 9), 10 μM capsazepine (c, 5HT + capsazepine, n = 8), 3 μM CaCC-Inh. A01 (d, 5HT + CaCC-Inh, n = 7), 100 nM 4 F 4 PP (e, 5HT + 4F4PP, n = 8), 200 nM SB204741 (f, 5HT + SB204741, n = 9), and 30 nM RS102221 (g, 5HT + RS102221, n = 9), respectively. p < 0.05, p < 0.01 indicate levels of significance determined by a Mann-Whitney test for differences between the two bars being compared.
To unveil which of the three 5HT2 receptor subtypes may contribute to this excitatory action of 5HT, the subtype-selective antagonists were assayed in the same way as the two channel blockers. Only 30 nM RS 102221, but not 4 F 4 PP or SB 204741, was able to significantly reduce the increase in firing rate caused by 5HT (Fig. 5e to g), and in the presence of the former 10 μM 5HT failed to significantly affect the number of action potentials triggered by the current injections (n = 9; p > 0.05, Friedman test followed by Dunn's post-hoc comparison); with the two other 5HT2 receptor antagonists, 5HT did raise the number of action potentials significantly (n = 8 for 4 F 4 PP and 9 for SB 204741; p < 0.05 in both cases, Friedman test followed by Dunn's post-hoc comparison). Thus, the excitation of DRG neurons by 5HT involved 5HT2C, but no other 5HT2 receptor subtype.
4. Discussion
Although it is well established that 5HT does excite peripheral nociceptors to cause pain, the underlying mechanisms have remained controversial. Most of the available evidence attributed a central role to the sensitization of TRPV1 channels, but several different 5HT receptor subtypes and associated signaling cascades were suggested to be involved (Loyd et al., 2013). In support of this general concept, the present results reveal 5HT (i) to potentiate capsaicin-evoked currents in DRG neurons and (ii) to enhance action potential firing in response to current injections. Such increases as well as decreases in the excitability of DRG neurons in vitro parallel pro- and analgetic effects in vivo, respectively (Liu et al., 2010; Du et al., 2014). Here, the causal relation between the sensitization of TRPV1 channels and the increased excitability of DRG neurons is also established, as the TRPV1 blocker capsazepine prevented the escalation of action potential firing by 5HT. Hence, the essential role of TRPV1 sensitization in the excitation of DRG neurons was documented by these findings, but the receptor subtype involved remained to be determined.
The 5HT-induced rise in neuronal excitability was blocked by the general 5HT2 receptor antagonist ritanserin, but not by the 5HT3 receptor antagonist tropisetrone, thereby excluding a key role of ionotropic 5HT receptors. This confirms that 5HT3 receptors control excitability in only a minor subgroup of DRG neurons (Zeitz et al., 2002) members of which must have been either underrepresented or even absent from the group of nociceptive neurons investigated here. Ritanserin also abolished the potentiating effect of 5HT on TRPV1 currents. This latter 5HT effect was also prevented by the three subtype-specific 5HT2 antagonists 4 F 4 PP, SB 204741, and RS 102221 which display about 100-fold selectivity for 5HT2A, 5HT2B, and 5HT2C, respectively (Acuña-Castillo et al., 2002; Forbes et al., 1995). Each of these three subtypes have been found to be expressed in DRGs (Nicholson et al., 2003; Pierce et al., 1996). Therefore, one can conclude that all three 5HT2 receptor subtypes can mediate the sensitization of TRPV1 channels by 5HT.
Nevertheless, only the 5HT2C-selective antagonist interfered with the 5HT-induced rise in neuronal excitability. From this apparent discrepancy one can conclude the following: (i) the chosen concentrations of the three antagonists (4 F 4 PP, SB 204741, and RS 102221) acted in a selective manner, as not all 5HT-dependent responses were affected by each of these three agents. (ii) Additional, 5HT2C-specific signaling mechanisms must contribute to the 5HT-dependent excitation of DRG neurons. (iii) Even though the 5HT-induced increase in action potential firing was prevented by the TRPV1 blocker capsazepine, a sensitization of TRPV1 channels alone does not appear to be sufficient for the 5HT-dependent rise in neuronal excitability. With respect to additional signaling mechanisms that might contribute to the rise in excitability in the presence of 5HT, the monoamine was tested for effects on currents through TTX-resistant Na+ channels and Kv7 K+ channels. Neither of these two types of channels was affected by 5HT, but they were enhanced and inhibited by PGE2 and bradykinin, respectively. With respect to the Na+ channels, a similar pattern of modulation, potentiation by PGE2, but no effect of 5HT, has been reported for mouse DRG neurons before (Rush and Waxman, 2004). In a subpopulation of rat DRG neurons, an enhancement of Na+ currents by 5HT has been described before (Cardenas et al., 1997), but it is not known whether and to what extent that population might be present under the culture conditions chosen for the present study. With respect to Kv7 channels, an inhibition via 5HT2 receptors has only been described in hypothalamic (Roepke et al., 2012), but not in DRG neurons.
Another type of ion channel that has recently gained attention with respect to pain sensation is TMEM16a, also known as anoctamin1, a CaCC (Oh and Jung, 2016). Here, 5HT induced slowly arising inward directed Cl− currents that were abolished in the presence of CaCC-Inh. A01, an agent that is known to block all subtypes of CaCCs (Liu et al., 2014). Amongst the three subtype-specific 5HT2 antagonists 4 F 4 PP, SB 204741, and RS 102221, only the latter prevented the gating of CaCCs by 5HT, thereby indicating that this effect was mediated by 5HT2C receptors. Previously, activation of B2 bradykinin receptors has been shown to induce currents through CaCCs in DRG neurons (Liu et al., 2010). Analogous results have been obtained with these three antagonists with respect to the increase in action potential firing in the presence of 5HT. Moreover, this 5HT-dependent excitation of DRG neurons was also prevented when the Cl− channels were blocked by CaCC-Inh. A01. Together, these data reveal the 5HT2C receptor mediated activation of CaCCs as decisive step in the excitatory action of 5HT on DRG neurons.
The key role of CaCCs in the excitation of DGR neurons by 5HT is also evidenced by the number of neurons that display specific responses towards 5HT. In 100% of the neurons tested, currents through TRPV1 channels were enhanced by 5HT. However, in just 53% of the neurons did 5HT induce currents through CaCCs, and in only 58% of the neurons the monoamine was able to enhance action potential firing. This further supports the conclusion that the increase in excitability triggered by 5HT was rather related to the gating of CaCCs than to the sensitization of TRPV1 channels.
In light of the above reasoning, one hast to pose the question as to why blockade of TRPV1 channels by capsazepine was also sufficient to prevent excitation of DRG neurons by 5HT. TRPV1 and TMEM16a channels have been found to directly interact with each other, and blockers of TMEM16a were reported to reduce the excitatory actions of capsaicin on DRG neurons. The underlying process is an activation of the CaCC by Ca2+ influx via TRPV1 channels which due to high intracellular Cl− concentrations in DRG neurons leads to depolarization and to a resultant increase in capsaicin-evoked action potential firing (Deba and Bessac, 2015; Takayama et al., 2015). This mechanism can also contribute to the interpretation of the data shown above. Activation of each of the three 5HT2 receptor subtypes expressed in DRG neurons, 5HT2A, 5HT2B, and 5HT2C, did cause sensitization of TRPV1 channels, but only activation of 5HT2C receptors led to a concomitant gating of CaCCs. Obviously, the simultaneous sensitization of TRPV1 and opening of CaCCs is key to the excitation of DRG neurons by 5HT, and this is only provided by activation of 5HT2C receptors.
A similar pattern of signaling events in DRG neurons triggered by an inflammatory mediator has been described for bradykinin (Liu et al., 2010). The prominent role of CaCCs in the sensitization of DRG neurons by algogenic mediators is further highlighted by the finding that the excitatory action of an inflammatory soup containing bradykinin, prostaglandin E2, histamine, and 5HT on action potential firing was largely attenuated or even abolished in neurons that lacked TMEM16a (Lee et al., 2014). Moreover, inflammation-dependent thermal hyperalgesia and mechanical allodynia were both found to be reduced in animals that did not express TMEM16a in DRG neurons (Lee et al., 2014). Thus, activation of CaCCs appears to be a mechanism that is shared by several constituents of the inflammatory soup, and it will be interesting to learn whether aside of 5HT and bradykinin other algogenic agents, such as prostaglandin E2, histamine, and/or nucleotides, can also act on CaCCs.
DRG neurons, at least during development, express 5HT transporters (Singh et al., 2009). Hence, 5HT might also act via these transporters. Activation of 5HT transporters heterologously expressed in muscle cells leads to depolarization and ensuing gating of voltage activated Ca2+ channels (Ruchala et al., 2014) which can then result in increases in intracellular Ca2+. Whether such a mechanism can also occur in DRG neurons is currently unknown. Moreover, the fact that the 5HT induced increase in neuronal excitability was abolished by highly divergent types of selective blocker (RS 102221, capsazepine, CaCC-Inh. A01) renders a contribution of 5HT transporters rather unlikely.
In summary, the present results show that 5HT excites DRG neurons mainly via 5HT2C receptors, the activation of which simultaneously leads to sensitization of TRPV1 channels and to opening of CaCCs.
Acknowledgements
The perfect technical assistance of G. Gaupmann is acknowledged gratefully.
Funding
This work was supported by the Austrian Science Fund (FWF; W1205). I.S. is a member of CCHD, a PhD program supported by FWF and Medical University of Vienna.
Abbreviations
- 4F 4 PP
4-(4-fluorobenzoyl)-1-(4phenylbutyl) piperidine
- CaCC
Ca2+-activated Cl− channel
- DRG
dorsal root ganglion
- EGTA
ethylene glycol-bis(2-aminoethylether)-N,N,N′,N'-tetraacetic acid
- GPCR
G-protein coupled receptor
- HEPES
4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid, N-(2-Hydroxyethyl) piperazine-N′ -(2-ethanesulfonic acid)
- Icaps
capsaicin-evoked current through TRPV1 channels
- IM
M current through KV7 channels
- PGE2
prostaglandin E2
- RS102221
8-[5-(2,4-dimethoxy-5-(4-trifluoromethylphenylsulphonamido)phenyl-5-oxopentyl]-1,3,8-triazaspiro[4,5]decane-2,4-dione
- SB 204741
N-(1-Methyl-1H-5-indolyl)-N′ -(3-methyl-5-isothiazolyl)urea
- TTX
tetrodotoxin
- XE991
10,10-bis(4-pyridinylmethyl)-9(10H)-anthracenone
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Acuña-Castillo C, Villalobos C, Moya PR, Sáez P, Cassels BK, Huidobro-Toro JP. Differences in potency and efficacy of a series of phenylisopropylamine/phenylethylamine pairs at 5-HT(2A) and 5-HT(2C) receptors. Br J Pharmacol. 2002;136:510–519. doi: 10.1038/sj.bjp.0704747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Passmore GM. Some new insights into the molecular mechanisms of pain perception. J Clin Invest. 2010;120:1380–1383. doi: 10.1172/JCI42143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas CG, Del Mar LP, Cooper BY, Scroggs RS. 5HT4 receptors couple positively to tetrodotoxin-insensitive sodium channels in a subpopulation of capsaicin-sensitive rat sensory neurons. J Neurosci. 1997;17:7181–7189. doi: 10.1523/JNEUROSCI.17-19-07181.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas LM, Cardenas CG, Scroggs RS. 5HT increases excitability of nociceptor-like rat dorsal root ganglion neurons via cAMP-coupled TTX-resistant Na(+) channels. J Neurophysiol. 2001;86:241–248. doi: 10.1152/jn.2001.86.1.241. [DOI] [PubMed] [Google Scholar]
- Deba F, Bessac BF. Anoctamin-1 Cl(-) channels in nociception: activation by an N-aroylaminothiazole and capsaicin and inhibition by T16A[inh]-A01. Mol Pain. 2015;11:55. doi: 10.1186/s12990-015-0061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson AH, Dray A. Selective antagonism of capsaicin by capsazepine: evidence for a spinal receptor site in capsaicin-induced antinociception. Br J Pharmacol. 1991;104:1045–1049. doi: 10.1111/j.1476-5381.1991.tb12547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorostkar MM, Boehm S. Opposite effects of presynaptic 5-HT3 receptor activation on spontaneous and action potential-evoked GABA release at hippocampal synapses. J Neurochem. 2007;100:395–405. doi: 10.1111/j.1471-4159.2006.04218.x. [DOI] [PubMed] [Google Scholar]
- Dray A. Inflammatory mediators of pain. Br J Anaesth. 1995;75:125–131. doi: 10.1093/bja/75.2.125. [DOI] [PubMed] [Google Scholar]
- Du X, Hao H, Gigout S, Huang D, Yang Y, Li L, Wang C, Sundt D, Jaffe DB, Zhang H, Gamper N. Control of somatic membrane potential in nociceptive neurons and its implications for peripheral nociceptive transmission. Pain. 2014;155:2306–2322. doi: 10.1016/j.pain.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England S, Bevan S, Docherty RJ. PGE2 modulates the tetrodotoxin-resistant sodium current in neonatal rat dorsal root ganglion neurones via the cyclic AMP-protein kinase A cascade. J Physiol. 1996;495(Pt 2):429–440. doi: 10.1113/jphysiol.1996.sp021604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes IT, Jones GE, Murphy OE, Holland V, Baxter GS. N-(1-methyl-5-indolyl)-N’-(3-methyl-5-isothiazolyl)urea: a novel, high-affinity 5-HT2B receptor antagonist. J Med Chem. 1995;38:855–857. doi: 10.1021/jm00006a001. [DOI] [PubMed] [Google Scholar]
- Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/S0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Klinger F, Bajric M, Salzer I, Dorostkar MM, Khan D, Pollak DD, Kubista H, Boehm S, Koenig X. δ Subunit-containing GABAA receptors are preferred targets for the centrally acting analgesic flupirtine. Br J Pharmacol. 2015;172:4946–4958. doi: 10.1111/bph.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger F, Geier P, Dorostkar MM, Chandaka GK, Yousuf A, Salzer I, Kubista H, Boehm S. Concomitant facilitation of GABAA receptors and KV7 channels by the non-opioid analgesic flupirtine. Br J Pharmacol. 2012;166:1631–1642. doi: 10.1111/j.1476-5381.2011.01821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight AR, Misra A, Quirk K, Benwell K, Revell D, Kennett G, Bickerdike M. Pharmacological characterisation of the agonist radioligand binding site of 5-HT(2A), 5-HT(2B) and 5-HT(2C) receptors. Naunyn Schmiedeb Arch Pharmacol. 2004;370:114–123. doi: 10.1007/s00210-004-0951-4. [DOI] [PubMed] [Google Scholar]
- Lee B, Cho H, Jung J, Yang YD, Yang D-J, Oh U. Anoctamin 1 contributes to inflammatory and nerve-injury induced hypersensitivity. Mol Pain. 2014;10:5. doi: 10.1186/1744-8069-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Linley JE, Du X, Zhang X, Ooi L, Zhang H, Gamper N. The acute nociceptive signals induced by bradykinin in rat sensory neurons are mediated by inhibition of M-type K+ channels and activation of Ca2+-activated Cl-channels. J Clin Invest. 2010;120:1240–1252. doi: 10.1172/JCI41084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang H, Huang D, Qi J, Xu J, Gao H, Du X, Gamper N, Zhang H. Characterization of the effects of Cl– channel modulators on TMEM16A and bestrophin-1 Ca2+ activated Cl– channels. Pflügers Arch Eur J Physiol. 2014;467:1417–1430. doi: 10.1007/s00424-014-1572-5. [DOI] [PubMed] [Google Scholar]
- Loyd DR, Henry MA, Hargreaves KM. Serotonergic neuromodulation of peripheral nociceptors. Semin Cell Dev Biol. 2013;24:51–57. doi: 10.1016/j.semcdb.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S, Ikeda M, Yoshida S, Tanimoto T, Takeda M, Nasu M. Prostaglandin E2-induced modification of tetrodotoxin-resistant Na+ currents involves activation of both EP2 and EP4 receptors in neonatal rat nodose ganglion neurones. Br J Pharmacol. 2005;145:503–513. doi: 10.1038/sj.bjp.0706212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama T, Higashi T, Togashi K, Iida T, Segi E, Sugimoto Y, Tominaga T, Narumiya S, Tominaga M. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol Pain. 2005;1:3. doi: 10.1186/1744-8069-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namkung W, Phuan P-W, Verkman AS. TMEM16A inhibitors reveal TMEM16A as a minor component of calcium-activated chloride channel conductance in airway and intestinal epithelial cells. J Biol Chem. 2011;286:2365–2374. doi: 10.1074/jbc.M110.175109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson R, Small J, Dixon AK, Spanswick D, Lee K. Serotonin receptor mRNA expression in rat dorsal root ganglion neurons. Neurosci Lett. 2003;337:119–122. doi: 10.1016/S03. [DOI] [PubMed] [Google Scholar]
- Oh U, Jung J. Cellular functions of TMEM16/anoctamin. Pflügers Arch Eur J Physiol. 2016;468:443–453. doi: 10.1007/s00424-016-1790-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T, Ikemi Y, Murakami M, Imagawa T, Otsuguro K, Ito S. Potentiation of transient receptor potential V1 functions by the activation of metabotropic 5-HT receptors in rat primary sensory neurons. J Physiol Oxf U K. 2006;576:809–822. doi: 10.1113/jphysiol.2006.112250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce PA, Xie GX, Levine JD, Peroutka SJ. 5-Hydroxytryptamine receptor subtype messenger RNAs in rat peripheral sensory and sympathetic ganglia: a polymerase chain reaction study. Neurosci. 1996;70:553–559. doi: 10.1016/0306-4522(95)00329-0. [DOI] [PubMed] [Google Scholar]
- Roepke TA, Smith AW, Rønnekleiv OK, Kelly MJ. Serotonin 5-HT2C receptor-mediated inhibition of the M-current in hypothalamic POMC neurons. Am J Physiol Endocrinol Metab. 2012;302:E1399–E1406. doi: 10.1152/ajpendo.00565.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchala I, Cabra V, Solis E, Glennon RA, De Felice LJ, Eltit JM. Electrical coupling between the human serotonin transporter and voltage-gated Ca(2+) channels. Cell Calcium. 2014;56:25–33. doi: 10.1016/j.ceca.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AM, Waxman SG. PGE2 increases the tetrodotoxin-resistant Nav1.9 sodium current in mouse DRG neurons via G-proteins. Brain Res. 2004;1023:264–271. doi: 10.1016/j.brainres.2004.07.042. [DOI] [PubMed] [Google Scholar]
- Salzer I, Gafar H, Gindl V, Mahlknecht P, Drobny H, Boehm S. Excitation of rat sympathetic neurons via M1 muscarinic receptors independently of K v7 channels. Pflugers Arch. 2014;466:2289–2303. doi: 10.1007/s00424-014-1487-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RP, Cheng Y-H, Nelson P, Zhou FC. Retentive multipotency of adult dorsal root ganglia stem cells. Cell Transpl. 2009;18:55–68. doi: 10.3727/096368909788237177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer C. Serotonin in pain and analgesia: actions in the periphery. Mol Neurobiol. 2004;30:117–125. doi: 10.1385/MN:30:2:117. [DOI] [PubMed] [Google Scholar]
- Sugiuar T, Bielefeldt K, Gebhart GF. TRPV1 function in mouse colon sensory neurons is enhanced by metabotropic 5-hydroxytryptamine receptor activation. J Neurosci. 2004;24:9521–9530. doi: 10.1523/JNEUROSCI.2639-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama Y, Uta D, Furue H, Tominaga M. Pain-enhancing mechanism through interaction between TRPV1 and anoctamin 1 in sensory neurons. Proc Natl Acad Sci U S A. 2015:1–6. doi: 10.1073/pnas.1421507112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousuf A, Klinger F, Schicker K, Boehm S. Nucleotides control the excitability of sensory neurons via two P2Y receptors and a bifurcated signaling cascade. Pain. 2011;152:1899–1908. doi: 10.1016/j.pain.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitz KP, Guy N, Malmberg AB, Dirajlal S, Martin WJ, Sun L, Bonhaus DW, Stucky CL, Julius D, Basbaum AI. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci. 2002;22:1010–1019. doi: 10.1523/JNEUROSCI.22-03-01010.2002. doi:22/3/1010 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]