Abstract
Zirconium-89 is currently being used in numerous clinical trials involving monoclonal antibodies and positron emission tomography. This report describes a revised strategy that reduces preparation time while increasing the specific activity of clinically relevant immuno-PET agents. Additionally, it demonstrates that n-acetyl-L-cysteine acts as a superior radioprotective agent that improves long-term stability without compromising antigen affinity in vivo.
Introduction
The use of monoclonal antibodies (mAbs), which represent one of the fastest growing classes of anti-cancer therapeutics, continues to accelerate in the clinic.1–4 Given the high cost of these therapies, the need arises for companion diagnostic agents that can non-invasively assist physicians in accurately identifying patients that would experience an effective clinical response from treatment. Zirconium-89 immuno-PET, which is the use of 89Zr-radiolabelled mAbs in conjunction with positron emission tomography (PET) to non-invasively probe receptor expression on tumors, is being evaluated in this capacity. Currently, it is being assessed in numerous clinical trials to detect disease, stratify patients for appropriate treatment and monitor their response to therapy.5
Zirconium-89 chelator development remains an active area of research.1, 6–31 Currently, desferrioxamine-B (DFO) and its bifunctional chelator derivatives are used to attach 89Zr to a mAb for clinical applications, and several strategies to accomplish this have been published in the literature.10, 21, 32–34 Initially, 89Zr-immuno-PET preparation strategies were technically cumbersome involving sublimation or cation exchange.33, 35 However, Vosjan, et al. published a more practical method,34 and since then minor modifications to this method have been published by Knight and co-workers.36 Still, challenges remain for researchers attempting to incorporate 89Zr-immuno-PET into their research and clinical programs. For example, the mass of mAb and chelate-to-mAb ratio, which can directly affect the final specific activity of the radiopharmaceutical, varies between protocols.37 Additionally, while some reports describe the use of phosphate buffered saline as a reaction solvent,36 others recommend avoiding chloride containing solvents altogether.25, 34 Moreover, radioactive thin-layer chromatography (radio-TLC) and centrifugal-filter analysis are often used to determine radiochemical purity and radiopharmaceutical stability. However, depending on the pore size of the filter membrane, radiolabelled high-molecular weight aggregates may be retained with the 89Zr-immuno-PET agents leading to an overestimation of purity and yield. Radio-TLC only distinguishes unchelated 89Zr from all other radiolabelled species. Furthermore, gel electrophoresis, which is also used to establish purity and stability, is time intensive and not easily adapted for use at clinical sites. Finally, while radiopharmaceutical stability is influenced by time and environmental conditions such as temperature and storage medium, little guidance is available on preservation methods that protect radiopharmaceutical integrity during transport or long-term storage. This final point becomes increasingly important as many clinical sites rely upon centralized radiopharmaceutical production facilities, which are often not in the same geographical location, to supply them with an 89Zr-immuno-PET agent so that they may provide precision medicine strategies to patients in a cost effective manner.38 This report provides guidance on these matters through a comprehensively revised strategy that can be easily implemented by researchers in academic, industrial and clinical settings.
Experimental
Materials and methods.
Zirconium-89 (89Zr: t½ = 78.4 h, β+: 22.8 %, Eβ+max = 901 keV; EC: 77%, Eγ = 909 keV) was purchased from Sophie, Inc. (Dulles, VA). Unless noted, all other chemicals were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA), and solutions were prepared using ultrapure water (18 MΩ-cm resistivity). p-isothiocyanatobenzyl-desferrioxamine (DFO-Bz-NCS) was purchased from Marcocyclics, Inc. (Dallas, TX). The trastuzumab and cetuximab antibodies were obtained from Myoderm, Inc. (Philadelphia, PA) and used as received.
Radiochemistry reaction progress and purity were monitored using a Size Exclusion (SE) chromatography system (Waters, Milford, MA), which runs Empower3 software and is configured with a 1525 binary pump, 2707 autosampler, 2998 photodiode array detector, fraction collector, size exclusion Superdex 200 Increase 10/300 GL (Code no. 28–9909-04, GE Healthcare Life Sciences, Piscataway, NJ) column, a Carrol Ramsey 105-s radioactivity detector (Berkeley, CA), and an isocratic mobile phase consisting of phosphate buffered saline (DPBS, Lonza, Catalog no. 17–512F) with a flow rate of 0.5 mL·min-1. Radio-ITLC analysis was performed on Bioscan AR 2000 radio-TLC scanner equipped with a 10% methane:argon gas supply, a PC interface running Winscan v.3 analysis software (Eckert & Ziegler, Berlin, Germany), and Varian ITLC-SG strips (Agilent Technologies, Santa Clara, CA), with 20 mM citric acid (pH 5) as eluent. Radioactive samples were counted using either a CRC-25R radioisotope calibrator (Capintec, Inc.) or a Perkin Elmer 2480 Wizard® gamma counter (Waltham, MA) with an energy window of 500–1500 keV. PET and CT images were acquired using a Trifoil small animal PET/CT scanner (Chatworth, CA).
Preparation of [89Zr]Zr-DFO-mAbs and [89Zr]Zr-DFO-IgG without Na2CO3 solution neutralization and using n-acetyl-L-cysteine (NAC) as a radioprotectant.
DFO-mAbs (cetuximab, trastuzumab, and IgG) were prepared using a modified literature procedure,34 and the chelator to antibody ratio was determined in triplicate using a reported process,39 (details can be found in the ESI). The radiopharmaceuticals, [89Zr]Zr-DFO-cetuximab and [89Zr]Zr-DFO-trastuzumab were prepared and formulated in 0.9% saline, 20 mM histidine/240 mM sucrose or 5 mg·mL−1 2,5,-dihydroxbenzoic acid (gentisic acid; GA) in 0.25 M sodium acetate buffer according to known protocols.25, 34 For radiopharmaceuticals prepared without sodium carbonate solution neutralization, [89Zr]Zr-oxalate (1.3–1.5 mCi, 48–55 MBq in 25–30 μL, 1.0 M oxalic acid) was placed in a 1.5 mL tube and pH was adjusted to 6.8–7.2 using 0.5 M HEPES buffer (500 μL, pH 7.2). NAC (100 μL, 5 mg·mL−1 in 0.5 M sodium acetate buffer, pH 6.8–7.0) and 330 μg of DFO-mAb conjugate (in 121 μL saline) were then added; the resulting mixture was incubated at 21 °C for 15 min. followed by PD-10 column purification using NAC (0.5 mg·mL−1 in 0.25 M sodium acetate buffer, pH 6.8–7.0) as eluent.
In vitro stability of [89Zr]Zr-DFO-mAbs determination using centrifugal filtration analysis, radio-ITLC or size exclusion chromatography (SEC).
For in vitro stability studies, [89Zr]Zr-DFO-mAbs (0.25 mCi (9.3 MBq), n = 3), which were preserved in different buffer-excipient combinations were evaluated at either 4 °C or 21 °C over the course of seven days. For centrifugal filtration analysis, samples were analyzed at 0, 1, 3, 5 and 7 days. Briefly, a 4 μL aliquot was applied to a 30 kDa centrifugal filter unit (Ultracel YM-30, Merck Millipore) and diluted with 96 μL of PBS (containing 5 % DMSO (v/v)). The unit containing the activity underwent centrifugation for 7 min. The filter was washed with PBS (2 × 100 μL) followed by centrifugation (2 × 7 min.). The radioactivity associated with the filter and filtrate was measured in a gamma counter. The percent (%) intact [89Zr]Zr-DFO-mAb was calculated as previously described.25 For radio-ITLC analysis, samples were analysed at 0, 1, 3, and 7 days by using Varian ITLC-SG strips and 20 mM citric acid (pH 5.0) as the mobile phase. In this system, unchelated 89Zr formed a complex with citric acid and eluted with solvent front (Rf ∼ 1), while [89Zr]Zr-DFO-mAbs remained at the origin (Rf ∼ 0). For samples analyzed by SEC, aliquots were injected at 0, 1, 3, 5 and 7 days. Fractions (0.5 mL/tube) were collected and the activity in each fraction was measured in a gamma counter. The percent intact radiopharmaceutical was determined by subtracting the total integrated area under the product peak from the total integrated area generated for all peaks in the chromatogram and multiplying by a factor of 100%.
In vitro serum stability of [89Zr]Zr-DFO-mAbs.
In vitro serum stability was carried out by adding 50 μCi (4.2 MBq) of [89Zr]Zr-DFO-mAbs (50 μL, NaOAc containing NAC, 0.5 mg·mL−1, pH 6.8–7.0) to 500 μL human serum. The solutions (n = 3) were incubated at 37 °C, and at 1, 3, 5 and 7 days, a 4 μL aliquot was removed and applied to a 30 kDa centrifugal filter unit (Ultracel YM-30, Merck Millipore) and diluted with 96 μL of PBS (containing 5 % DMSO (v/v)). The sample containing unit underwent centrifugation for 7 min. The filter was washed with PBS (2 × 100 μL) followed by centrifugation (2 × 7 min.). The activity associated with the filter and filtrate were measured in a gamma counter. The percent (%) intact of [89Zr]Zr-DFO-mAb was calculated as described previously.25
Small animal PET/CT imaging and post-PET biodistribution studies.
PET imaging with [89Zr]Zr-DFO-cetuximab or [89Zr]Zr-DFO-IgG was performed on NOD-SCIDγ (NSG) mice bearing PDX tumors (TM00199/LG0703). All tumor-bearing mice (n = 5/group) received an injection of either [89Zr]Zr-DFO-cetuximab or [89Zr]Zr-DFO-IgG (9.3–10.4 MBq in 170 μL 0.25 M NaOAc containing 0.5 mg·mL−1 NAC) via the tail vein. Mice were anesthetized with 1–2% isoflurane and imaged at 24, 72 and 144 h p.i. Images were reconstructed using ordered subset expectation maximum (OSEM) algorithms and then coregistered with the CT image. The percent injected dose of activity per gram of tissue (%ID/g = Ct × Vt/Wt × 1/Dinj) was determined at every time point from regions of interest drawn on all animals in the study. In this equation, Ct (MBq/cc) is the radioactivity in the region of interest, Vt/Wt (~1cc/g) is the density of tissue, and Dinj (MBq) is the injected dose. Immediately after the 144 h imaging time point, mice were euthanized. Organs and tissues of interest were excised, weighed, and counted on a Perkin Elmer 2480 Wizard® gamma counter (Waltham, MA). The percent injected dose per gram (%ID/g) was calculated by comparison to a weighed, counted standard for each group.
Statistical Methods.
Data are presented as mean ± SD or mean (95% confidence intervals). For statistical classification a Student’s t test (two-tailed, unpaired) or one-way Anova (with Tukey’s multiple comparison post-test) was performed using GraphPad Prism 5.0 software (San Diego, CA). A p < 0.05 was considered significant.
Results and discussion
Since the conjugation efficiency of p-SCN-Bz-DFO to solvent accessible primary amine groups on the mAb surface varies considerably in the literature, we chose to investigate this parameter first, and prepared DFO-cetuximab and DFO- trastuzumab as model conjugates. These monoclonal antibodies are clinically approved therapies used to treat epidermal growth factor receptor (EGFR) and receptor tyrosine kinase erbB-2 (HER2/neu) positive malignancies, respectively.40–45 Furthermore, numerous clinical trials, which have goals of detecting disease, performing patient stratification before therapy or monitoring response to therapy are being conducted with these 89Zr-labeled mAbs.5, 46–49 In our hands, a five molar excess of p-SCN-Bz-DFO yielded an average of 3.5 ± 0.2 and 2.9 ± 0.1 chelators per cetuximab and trastuzumab, respectively after 30 minutes (Table S1). While we tested several other concentrations of this reagent, a 5-molar excess seemed to offer a meaningful compromise since an excellent conjugation efficiency could be obtained while limiting the loss of unreactive bifunctional chelator (BFC) reagent. Additionally, it minimized mAb aggregation that is often observed when larger concentrations of p-SCN-Bz-DFO are used during mAb conjugation. While our ratios demonstrate literature agreement,50 they are larger than that reported by Knight et al.,36 and this discrepancy may result from increased NCS hydrolysis, which can occur during the extended reaction period used by them.
We then examined the radiochemistry conditions used by both groups to generate radiopharmaceuticals with clinically relevant specific activities, but found that we could further increase specific activity (As) without compromising radiochemical yield or radiochemical purity. Table 1 and Table S2 compare our optimized conditions with those reports.21, 34, 36 We were able to radiolabel cetuximab (Figure S1) and trastuzumab (Figure S2) quantitatively with a radiochemical purity greater than 97 % and an As that was 40–70% greater than that achieved by the other protocols. Furthermore, we reduced the mass of DFO-mAb conjugates used in the radiochemical synthesis 2.9- and 1.5- fold when compared to the procedure published by Vosjan et al. and Knight et al., respectively. Additionally, we observed that HEPES buffer was the most suitable reaction solvent for radiopharmaceutical preparation since large concentrations of aqueous chloride anions are absent. Interestingly, its buffering capacity was sufficient to maintain reaction pH, which allowed for oxalic acid removal without having to neutralize it with Na2CO3. Eliminating this acid neutralization step reduced preparation time by 75% without compromising radiochemical yield, radiochemical purity or As (Table 1), and represents a significant improvement over the current methodologies used to prepare these agents.
Table 1.
Summarized comparison of conditions used to prepare 89Zr-immuno-PET agents
| Parameter | Reference 21, 34 | Reference 36 | This work |
|---|---|---|---|
| mAb (mg)51 | 0.7–3.0 | 0.1 | 0.33 |
| 89Zr added (MBq) | 37–185 | 10 | 50–55 |
| Oxalic acid neutralization method | 2 M Na2CO3 | 1 M Na2CO3 | None |
| Reaction buffer | 0.5 M HEPES52 | PBS53 | 0.5 M HEPES52 |
| Final reaction pH | 6.8–7.2 | 7–8 | 6.8–7.2 |
| Reaction temperature (°C) | 21–24 | 21–24 | 21–24 |
| Reaction time (h) | 1 | 1 | 0.25 |
| Radiolabeling yield (%) | > 85 | 96.9 ± 3.3 | ≥ 9754 |
| Radiochemical purity (%) | > 95 | > 95 | ≥ 9754 |
| Specific activity (As; MBq·μg−1) | 0.067–0.086 | 0.1 ± 0.03 | 0.144 ± 0.003 (n = 10)55 |
Radiochemical purity and radiochemical yield were determined by radio-ITLC and radio-SEC.
A second goal of this work was to improve quality control and stability study strategies used to quantify radiochemical purity. In contrast to centrifugal filtration and radio-ITLC analysis, size exclusion chromatography (SEC) methods adequately resolve high molecular weight protein aggregates and low molecular weight impurities from the product in SEC chromatogram. Since these species may constitute as much as 15% of the total reaction mixture and can impact the in vivo performance of the radiopharmaceutical, accurately quantifying them becomes a necessity. Using a Superdex 200 Increase 10/300 GL column, and an isocratic mobile phase consisting of phosphate buffered saline (DPBS), we could resolve the high and low molecular weight impurities from the radiochemical product. This allowed us to determine radiochemical purity with greater accuracy when compared to centrifugal filtration analysis, which often overestimated this parameter. Adoption of this method should improve existing analytical measures necessary to determine the initial purity and long-term stability of 89Zr-immuno-PET agents within the clinical setting.
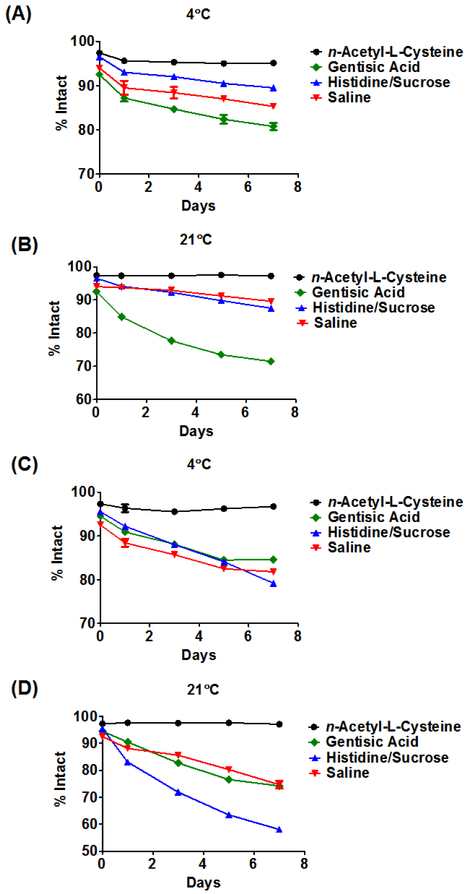
The final goal of this work was to improve the long-term stability of 89Zr-immuno-PET agents. Literature protocols have described storage in 0.9% saline, 20 mM histidine/240 mM sucrose or 0.25 M sodium acetate (NaOAc) buffer supplemented with 5 mg·mL−1 2,5,-dihydroxbenzoic acid (gentisic acid; GA).25 Although formulation in 0.9% saline is nearly ideal for clinical injection, storage in this media is not advised since the radiolysis of water in the presence of a large chloride anion (Cl-) concentration generates hydroxyl radical (·OH) and hypochlorous acid (HOCl), which are believed to be detrimental to radiopharmaceutical integrity. A 20 mM histidine/ 240 mM sucrose solution has been suggested, but this buffer-excipient combination is not perceived to be an effective radioprotectant and the exact mechanism by which it protects the radiolabelled mAb in solution remains unknown.25, 56 Finally, even though 0.25 M NaOAc/GA is more effective at protecting the radiolabelled mAb in solution compared to the prior two, 25 it is still sub-optimal since the calculated rate constant describing the reaction between GA and ·OH is smaller than the rate constant describing the latter’s reaction with thiourea, which is a common functional group linking DFO to the mAb.57 Including a more powerful antioxidant in the radiopharmaceutical formulation would be beneficial. While many antioxidants are available, we focused on n-acetyl-L-cysteine (NAC) since 1) the rate constant describing its reaction with ·OH is larger than the rate constant describing the reaction of ·OH and thiourea, 2) it is a powerful HOCl scavenger and 3) it is approved by the Food and Drug Administration (FDA) for clinical use.58–62 Accordingly, we compared the stability of [89Zr]Zr-DFO-cetuximab and [89Zr]Zr-DFO-trastuzumab over seven days while being stored in 0.9% saline, 20 mM histidine/240 mM sucrose, 0.25 M NaOAc containing 5 mg·mL−1 GA or 0.25 M NaOAc containing 0.5 mg·mL−1 NAC. The results of these studies are presented in Figure 1, in Figures S4-S9 and Tables S3-S8. After 24h at 21◦C, [89Zr]Zr-DFO-trastuzumab formulated with 0.25 M NaOAc containing 0.5 mg·mL−1 NAC exhibited a radiochemical purity that was significantly greater than when formulated in all other buffer-excipient combinations (average % intact; saline vs. 20 mM histidine/240 mM sucrose vs. 0.25 M NaOAc/5 mg·mL−1 GA vs. 0.25 M NaOAc/0.5 mg·mL−1 NAC; p value: 87.4 ± 0.1 vs. 82.7 ± 0.7 vs. 89.9 ± 0.1 vs. 97.6 ± 0.2; F(348,0.96) = 967, p < 0.0001). This result was recapitulated with [89Zr]Zr-DFO-cetuximab (average % intact; saline vs. 20mM histidine/240 mM sucrose vs. 0.25 M NaOAc/5 mg·mL−1 GA vs. 0.25 M NaOAc/0.5 mg·mL−1 NAC; p value: 93.7 ± 0.2 vs. 94.1 ± 0.2 vs. 84.9 ± 0.3 vs. 97.3 ± 0.2; F(256,0.31) = 2229, p < 0.0001).
Figure 1. Comparative stability of 89Zr-labelled antibodies at 4ºC or 21ºC in the presence of different buffer-excipient combinations.
Stability was determined using the size exclusion chromatography. [89Zr]Zr-DFO-cetuximab (A, B) or [89Zr]Zr-DFO-trastuzumab (C, D) formulated in 0.25 M sodium acetate containing 0.5 mg·mL−1 n-acetyl-L-cysteine underwent minimal degradation over the experimental time course, while the same radiopharmaceuticals formulated with 0.25 M sodium acetate containing 5 mg·mL−1 gentisic acid, 20 mM histidine/240 mM sucrose or 0.9% saline were observed to be less stable over time. Each data point is the average of three SEC runs.
Moreover, even after seven days the radiochemical purities of both agents were still greater than 97%. Conversely SEC revealed increasing amounts of high and low molecular weight impurities in samples of these agents formulated with the other three buffer-excipient combinations over the same study period. This suggests that NAC may provide superior in vitro protection from radical-induced damage during the transport and storage of 89Zr-immuno-PET agents. Finally, in accord with the literature both radiopharmaceuticals were more than 98% intact after seven days at 37◦C in human serum (Table S9)25, 34.
Although in vitro stability studies indicated that NAC had a preservative effect on radiopharmaceutical integrity, they could not provide proof of antigen reactivity in vivo. To assess this parameter, [89Zr]Zr-DFO-cetuximab was evaluated using small animal PET/CT and a clinically relevant, patient-derived xenograft (PDX) murine model of epidermal growth factor receptor positive (EGFR+) lung cancer.63 The PDX tumors, which were implanted in NOD-SCIDγ mice, were derived from a 44 year old female who presented with treatment resistant, grade 2 non-small cell lung adenocarcinoma. Cells and subsequent tumors were characterized according to published guidelines with EGFR expression confirmed at the gene and protein levels by PCR and histology, respectively.64 This model was chosen for several reasons. Foremost, lung cancer is the leading cause of cancer deaths among men and women in the United States so this model is salient and clinically relevant.65 Secondly, it has been used to investigate the effects of novel antibody drug conjugates successfully.66 Additionally, several reports have shown that cetuximab therapy in combination with chemotherapy increases survival of non-small cell lung cancer patients, and efficacy is not affected by the presence of somatic mutations such as L858R, which is one of the most common mutations located within the tyrosine kinase domain of the EGFR gene.65, 67 Finally, using [89Zr]Zr-DFO-cetuximab to identify patients that might respond favorably to EGFR-based therapies has already shown promise in clinical trials.68
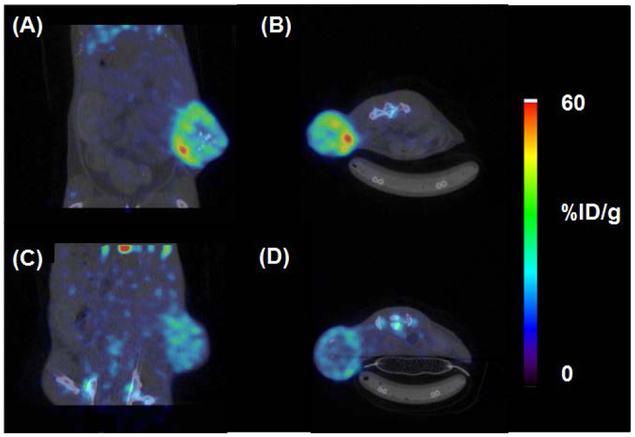
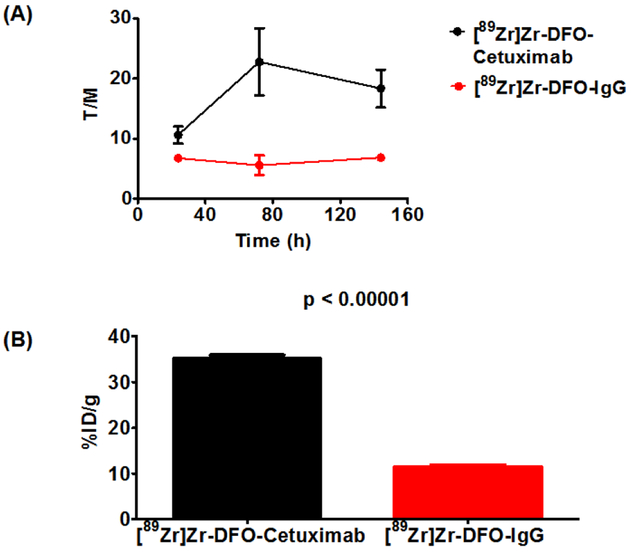
Once tumors reached a palpable mass, animals were randomly divided and injected with either [89Zr]Zr-DFO-cetuximab or [89Zr]Zr-DFO-IgG, which functioned as a negative control in these studies. Animals tolerated [89Zr]Zr-DFO-cetuximab formulated with NAC extremely well showing no signs of distress even 24 hours after injection, and small animal PET/CT imaging was conducted over the subsequent six days. Representative images from both cohorts are found in Figure 2. Tumor necrosis, which was independent of tumor size, contributed to the non-homogeneous radiotracer distribution and accumulation within the tumors of both cohorts. Despite this observation, image quantification revealed that animals receiving [89Zr]Zr-DFO-cetuximab retained approximately 10-fold more activity in tumor tissue compared to animals receiving [89Zr]Zr-DFO-IgG. Figure 3A describes the average tumor-to-muscle (T/M) ratios for both cohorts over the experimental time course of this study, and provides an assessment of image contrast. Animals injected with [89Zr]Zr-DFO-cetuximab demonstrated T/M ratios that doubled by 72 hours p.i. without significantly decreasing at 144 hours. In contrast, animals injected with [89Zr]Zr-DFO-IgG demonstrated low T/M ratios that remained low over the experimental time course of this study. This suggests that [89Zr]Zr-DFO-cetuximab accumulated in the EGFR+ tumor by a receptor mediated mechanism while undergoing efficient clearance from tissues expressing low levels of EGFR. In contrast [89Zr]Zr-DFO-IgG, which is non-specific for the EGFR could not bind to this antigen and localize at the tumor during the study, and is consistent with a non-targeted agent. A post-PET biodistribution study was also conducted after the imaging study. Results describing the average tumor associated activity from both cohorts are depicted in Figure 3B, while a complete biodistribution of all tissues can be found in Table S10. The biodistribution of [89Zr]Zr-DFO-cetuximab was consistent with literature.69–75 Animals injected with [89Zr]Zr-DFO-cetuximab demonstrated significantly less radioactivity associated with blood and non-target tissues such as the liver, spleen, lung, and kidney at 144 h post-injection ([89Zr]Zr-DFO-cetuximab vs. [89Zr]Zr-DFO-IgG; %ID/g ± SD, p value) (blood, 0.4 ± 0.1 vs. 4.5 ± 0.3, p < 0.0001; liver, 11.8 ± 0.9 vs. 13.7 ± 0.9, p = 0.005; spleen, 5.4 ± 0.4 vs. 6.8 ± 0.4, p = 0.0001; lung, 2.5 ± 0.2 vs. 5.2 ± 0.4, p < 0.0001; kidney 3.2 ± 0.3 vs. 4.7 ± 0.3, p < 0.0001). Conversely, more radioactivity was associated with the tumors of animals injected with [89Zr]Zr-DFO-cetuximab at the same time point ([89Zr]Zr-DFO-cetuximab vs. [89Zr]Zr-DFO-IgG; %ID/g ± SD, p value) (tumor, 35.1 ± 1.8 vs. 11.4 ± 1.2, p < 0.0001). This elevated level of radioactivity associated within the EGFR+ tumor is consistent with the receptor-mediated interaction between cetuximab and the EGF receptor, and published literature.69–75 Contrarily, the elevated levels of radioactivity in the blood and other highly perfused organs such as the liver, lung and spleen of animals injected with [89Zr]Zr-DFO-IgG are consistent with the non-targeted nature of this radiopharmaceutical. Additionally, given the increased level of radioactivity in the blood pool, it is probable that radioactivity retained in the tumors of mice receiving [89Zr]Zr-DFO-IgG results from perfusion rather than receptor mediated interactions between it and the EGF receptor. Finally, since bone marrow is often considered a dose-limiting organ and radioactivity levels in bone tissue are considered a surrogate indicator for radiopharmaceutical integrity and [89Zr]Zr-DFO stability,1, 31, 76 the amount of radioactivity in the bone tissues of both cohorts was examined at 144 hours post-injection. Mice injected with [89Zr]Zr-DFO-cetuximab had less radioactivity associated with their bone tissue than did mice receiving [89Zr]Zr-DFO-IgG (bone, 12.8 ± 1.7 vs. 15.2 ± 1.9, p = 0.047). Moreover, the amount of radioactivity associated with the bone tissues of mice that received the former radiopharmaceutical was consistent with the literature suggesting that the use of NAC did not have a significant effect on radiopharmaceutical stability in vivo and corroborates the in vitro results.77, 78
Figure 2. Representative images of EGFR+, PDX-mice injected with [89Zr]Zr-DFO-cetuximab (A, B) or [89Zr]Zr-DFO-IgG (C, D).
After 144 h p.i., significantly more radioactivity was retained in the EGFR+ tumors of mice injected with [89Zr]Zr-DFO-cetuximab. Images were acquired in one bed position. Coronal and axial images are from the same animals, taken from the same anatomical location and equally scaled.
Figure 3. Quantified results from PET/CT imaging studies suggest that the retention of [89Zr]Zr-DFO-cetuximab is receptor mediated.
(A) Tumor-to-muscle (T/M) ratios of PDX mice injected with [89Zr]Zr-DFO-cetuximab or [89Zr]Zr-DFO-IgG. T/M ratios improved over the experimental time course of this study for animals injected with [89Zr]Zr-DFO-cetuximab. In contrast, animals injected with [89Zr]Zr-DFO-IgG demonstrated non-increasing T/M ratios over the experimental time course of this study. (B) Post-PET biodistribution result depicting tumor associated activity at 144 hours p.i. Animals injected with 89Zr]Zr-DFO-cetuximab retained more radioactivity in EGFR+ tumors than animals injected with [89Zr]Zr-DFO-IgG.
Our revised strategy offers several advantages. It reduces the number of preparative steps and time necessary to synthesize 89Zr-immuno-PET agents, which should translate into reduced production costs. Additionally, these changes may facilitate the development of standardized kit technology that can be utilized at clinical sites where radiochemistry resources or technical expertise are unavailable and provide greater access to clinical immuno-PET. N-acetyl-L-cysteine’s superior protection of radiopharmaceutical integrity represents an additional improvement since enhanced stability should yield better image quality, and provide clinicians with greater confidence in the data obtained from the clinical imaging enterprise. Finally and most importantly, our revised protocol allows radiopharmaceuticals to be generated with significantly improved As, which is an important outcome criterion in radiochemistry.79–84 Injection of a radiopharmaceutical that exhibits a high amount of radioactivity per unit mass reduces the possibility of a host response after agent injection and ensures favorable imaging contrast can be achieved during the clinical imaging session.79 Furthermore, radiopharmaceuticals with high and non-variable specific activity and formulated with clinically approved antioxidants should face less scrutiny by regulatory agencies responsible for ensuring patient safety and agent efficacy in the clinical setting.84
Conclusions
In summary, this report describes a comprehensively revised strategy to produce clinically relevant 89Zr-labelled antibodies that should have broad appeal to industrial, academic and clinical laboratories since it improves production and specific activity. Additionally, data demonstrate that n-acetyl-L-cysteine provides superior protection against radiolysis-induced degradation. Its use in 89Zr-immuno-PET agent radiochemistry improves the long-term stability of these radiopharmaceuticals without compromising antigen reactivity in vivo. It is anticipated that the adoption of this revised strategy will improve the quality of care for patients needing PET-guided interventions, which are rapidly being integrated into precision medicine strategies.
Supplementary Material
Acknowledgements
The authors acknowledge financial support from Wake Forest University Health Sciences, the Comprehensive Cancer Center of Wake Forest (NIH P30 CA012197), Wake Forest Innovations, and the North Carolina Biotechnology Center (2016-BIG-6524). 89Zr was provided by Sophie, Inc. The authors gratefully acknowledge use of the services and facilities of the Translational Imaging Program and the editorial assistance of Karen Klein, M.A., funded by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UL1TR001420. The authors thank Jason Lewis, Ph.D. of Memorial Sloan Kettering Cancer Center for insightful discussions.
Footnotes
Conflicts of interest
Relating to this research, NBB, DNP and TJW have filed the provisional patent application 62/622,332 with the United States Patent and Trademark Office. All other authors declare no conflicts.
Electronic Supplementary Information (ESI) available: See DOI: 10.1039/x0xx00000x
Notes and references
- 1.Bhatt NB, Pandya DN and Wadas TJ, Molecules, 2018, 23, 638–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vugts DJ, Visser GW and van Dongen GA, Curr. Top. Med. Chem, 2013, 13, 446–457. [DOI] [PubMed] [Google Scholar]
- 3.Bernard-Gauthier V, Collier TL, Liang SH and Vasdev N, Drug Discov. Today Technol, 2017, 25, 19–26. [DOI] [PubMed] [Google Scholar]
- 4.Wu AM, Methods, 2014, 65, 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jauw YW, Menke-van der Houven van Oordt CW, Hoekstra OS, Hendrikse NH, Vugts DJ, Zijlstra JM, Huisman MC and van Dongen GA, Front. Pharmacol, 2016, 7, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams CJ, Wilson JJ and Boros E, Mol. Pharm, 2017, 14, 2831–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allott L, Da Pieve C, Meyers J, Spinks T, Ciobota DM, Kramer-Marek G and Smith G, Chem. Commun, 2017, 53, 8529–8532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatt NB, Pandya DN, Xu J, Tatum D, Magda D and Wadas TJ, PLoS One, 2017, 12, e0178767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boros E, Holland JP, Kenton N, Rotile N and Caravan P, ChemPlusChem, 2016, 81, 274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Briand M, Aulsebrook ML, Mindt TL and Gasser G, Dalton Trans., 2017, 46, 16387–16389. [DOI] [PubMed] [Google Scholar]
- 11.Buchwalder C, Rodriguez-Rodriguez C, Schaffer P, Karagiozov SK, Saatchi K and Hafeli UO, Dalton Trans., 2017, 46, 9654–9663. [DOI] [PubMed] [Google Scholar]
- 12.Deri MA, Ponnala S, Kozlowski P, Burton-Pye BP, Cicek HT, Hu C, Lewis JS and Francesconi LC, Bioconjugate Chem., 2015, 26, 2579–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deri MA, Ponnala S, Zeglis BM, Pohl G, Dannenberg JJ, Lewis JS and Francesconi LC, J. Med. Chem, 2014, 57, 4849–4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guerard F, Lee YS, Tripier R, Szajek LP, Deschamps JR and Brechbiel MW, Chem. Commun, 2013, 49, 1002–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.J NT, Pandya DN, Pailloux SL, Ogasawara A, Vanderbilt AN, Gill HS, Williams SP, Wadas TJ, Magda D and Marik J, Theranostics, 2016, 6, 511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma MT, Meszaros LK, Paterson BM, Berry DJ, Cooper MS, Ma Y, Hider RC and Blower PJ, Dalton Trans., 2015, 44, 4884–4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meijs WE, Herscheid JDM, Haisma HJ and Pinedo HM, Appl. Radiat. Isot, 1992, 43, 1443–1447. [DOI] [PubMed] [Google Scholar]
- 18.Pandya DN, Bhatt N, Yuan H, Day CS, Ehrmann BM, Wright M, Bierbach U and Wadas TJ, Chem. Sci, 2017, 8, 2309–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pandya DN, Pailloux S, Tatum D, Magda D and Wadas TJ, Chem. Commun, 2015, 51, 2301–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patra M, Bauman A, Mari C, Fischer CA, Blacque O, Haussinger D, Gasser G and Mindt TL, Chem. Commun, 2014, 50, 11523–11525. [DOI] [PubMed] [Google Scholar]
- 21.Perk LR, Vosjan MJ, Visser GW, Budde M, Jurek P, Kiefer GE and van Dongen GA, Eur. J. Nucl. Med. Mol. Imaging, 2010, 37, 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richardson-Sanchez T, Tieu W, Gotsbacher MP, Telfer TJ and Codd R, Org. Biomol. Chem, 2017, 15, 5719–5730. [DOI] [PubMed] [Google Scholar]
- 23.Rousseau J, Zhang Z, Dias GM, Zhang C, Colpo N, Benard F and Lin KS, Bioorg. Med. Chem. Lett, 2017, 27, 708–712. [DOI] [PubMed] [Google Scholar]
- 24.Rudd SE, Roselt P, Cullinane C, Hicks RJ and Donnelly PS, Chem. Commun, 2016, 52, 11889–11892. [DOI] [PubMed] [Google Scholar]
- 25.Vugts DJ, Klaver C, Sewing C, Poot AJ, Adamzek K, Huegli S, Mari C, Visser GW, Valverde IE, Gasser G, Mindt TL and van Dongen GA, Eur. J. Nucl. Med. Mol. Imaging, 2017, 44, 286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhai C, Summer D, Rangger C, Franssen GM, Laverman P, Haas H, Petrik M, Haubner R and Decristoforo C, Mo.l Pharm, 2015, 12, 2142–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price EW and Orvig C, Chem Soc Rev, 2014, 43, 260–290. [DOI] [PubMed] [Google Scholar]
- 28.Price TW, Greenman J and Stasiuk GJ, Dalton Trans, 2016, 45, 15702–15724. [DOI] [PubMed] [Google Scholar]
- 29.Ikotun OF and Lapi SE, Future Med. Chem, 2011, 3, 599–621. [DOI] [PubMed] [Google Scholar]
- 30.Wadas TJ, Wong EH, Weisman GR and Anderson CJ, Chem. Rev, 2010, 110, 2858–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deri MA, Zeglis BM, Francesconi LC and Lewis JS, Nucl. Med. Biol, 2013, 40, 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen R, Vugts DJ, Stigter-van Walsum M, Visser GW and van Dongen GA, Nat. Protoc, 2013, 8, 1010–1018. [DOI] [PubMed] [Google Scholar]
- 33.Verel I, Visser GW, Boellaard R, Stigter-van Walsum M, Snow GB and van Dongen GA, J. Nucl. Med, 2003, 44, 1271–1281. [PubMed] [Google Scholar]
- 34.Vosjan MJ, Perk LR, Visser GW, Budde M, Jurek P, Kiefer GE and van Dongen GA, Nat. Protoc, 2010, 5, 739–743. [DOI] [PubMed] [Google Scholar]
- 35.Dijkers EC, Kosterink JG, Rademaker AP, Perk LR, van Dongen GA, Bart J, de Jong JR, de Vries EG and Lub-de Hooge MN, J. Nucl. Med, 2009, 50, 974–981. [DOI] [PubMed] [Google Scholar]
- 36.Knight JC, Paisey SJ, Dabkowski AM, Marculescu C, Williams AS, Marshall C and Cornelissen B, Dalton Trans., 2016, 45, 6343–6347. [DOI] [PubMed] [Google Scholar]
- 37.Lapi SE and Welch MJ, Nucl. Med. Biol, 2013, 40, 314–320. [DOI] [PubMed] [Google Scholar]
- 38.Perk L and Rispens SI, www.knowledgeatwork.edu. The future of immuno-PET in drug development. 2012, 1–16. [Google Scholar]
- 39.Kukis DL, DeNardo GL, DeNardo SJ, Mirick GR, Miers LA, Greiner DP and Meares CF, Cancer Res., 1995, 55, 878–884. [PubMed] [Google Scholar]
- 40.Lambertini M, Ponde NF, Solinas C and de Azambuja E, Expert Rev. Anticancer Ther, 2017, 17, 61–74. [DOI] [PubMed] [Google Scholar]
- 41.Wahid M, Mandal RK, Dar SA, Jawed A, Lohani M, Areeshi MY, Akhter N and Haque S, Crit. Rev. Oncol. Hematol, 2016, 104, 124–130. [DOI] [PubMed] [Google Scholar]
- 42.Haq R and Gulasingam P, Curr Oncol, 2016, 23, 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Granados-Garcia M, Aguilar-Ponce JL, Maldonado-Magos F and De la Garza-Salazar JG, ORL J. Otorhinolaryngol. Relat. Spec, 2016, 78, 320–333. [DOI] [PubMed] [Google Scholar]
- 44.Gracia-Cazana T, Salazar N, Zamarron A, Mascaraque M, Lucena SR and Juarranz A, Actas Dermosifiliogr., 2016, 107, 740–750. [DOI] [PubMed] [Google Scholar]
- 45.Wen F and Li Q, World J. Gastroenterol, 2016, 22, 5332–5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Even AJ, Hamming-Vrieze O, van Elmpt W, Winnepenninckx VJ, Heukelom J, Tesselaar ME, Vogel WV, Hoeben A, Zegers CM, Vugts DJ, van Dongen GA, Bartelink H, Mottaghy FM, Hoebers F and Lambin P, Oncotarget, 2017, 8, 3870–3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menke-van der Houven van Oordt CW, Gootjes EC, Huisman MC, Vugts DJ, Roth C, Luik AM, Mulder ER, Schuit RC, Boellaard R, Hoekstra OS, van Dongen GA and Verheul HM, Oncotarget, 2015, 6, 30384–30393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dijkers EC, Oude Munnink TH, Kosterink JG, Brouwers AH, Jager PL, de Jong JR, van Dongen GA, Schroder CP, Lub-de Hooge MN and de Vries EG, Clin. Pharmacol. Ther, 2010, 87, 586–592. [DOI] [PubMed] [Google Scholar]
- 49.Ulaner GA, Hyman DM, Ross DS, Corben A, Chandarlapaty S, Goldfarb S, McArthur H, Erinjeri JP, Solomon SB, Kolb H, Lyashchenko SK, Lewis JS and Carrasquillo JA, J. Nucl. Med, 2016, 57, 1523–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marquez BV, Ikotun OF, Zheleznyak A, Wright B, Hari-Raj A, Pierce RA and Lapi SE, Mol. Pharm, 2014, 11, 3988–3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mass of mAb used in radiochemical synthesis.
- 52.pH 7.1–7.3
- 53.pH 7.4
- 54.Unchelated 89Zr was not present in the original reaction mixture as determined by Radio-ITLC. Final purity and yield reflects the presence of high and low-molecular weight species, which were additionally determined by SE-HPLC.
- 55.[89Zr]Zr-DFO-trastuzumab (n=5) and [89Zr]Zr-DFO-cetuximab (n=5).
- 56.Baek Y, Singh N, Arunkumar A and Zydney AL, Pharm. Res, 2017, 34, 629–639. [DOI] [PubMed] [Google Scholar]
- 57.Gonzalez A, Galano A and Idaboy J, New J. Chem, 2014, 38, 2639–2652. [Google Scholar]
- 58.Wang W, Schuchmann M, Schuchmann H, Knolle W, von Sonntag J and vonSonntag C, J. Am. Chem. Soc, 1999, 121, 238–245. [Google Scholar]
- 59.Sansone RA and Sansone LA, Innov. Clin. Neurosci, 2011, 8, 10–14. [PMC free article] [PubMed] [Google Scholar]
- 60.Shankle WR, Hara J, Barrentine LW and Curole MV, J. Alzheimer’s Dis, 2016, 54, 1073–1084. [DOI] [PubMed] [Google Scholar]
- 61.Dhouib IE, Jallouli M, Annabi A, Gharbi N, Elfazaa S and Lasram MM, Life Sci., 2016, 151, 359–363. [DOI] [PubMed] [Google Scholar]
- 62.Aruoma OI, Halliwell B, Hoey BM and Butler J, Free Radic. Biol. Med, 1989, 6, 593–597. [DOI] [PubMed] [Google Scholar]
- 63.Jackson Laboratory, http://tumor.informatics.jax.org/mtbwi/pdxDetails.do?modelID=TM00199).
- 64.Meehan TF, Conte N, Goldstein T, Inghirami G, Murakami MA, Brabetz S, Gu ZP, Wiser JA, Dunn P, Begley DA, Krupke DM, Bertotti A, Bruna A, Brush MH, Byrne AT, Caldas C, Christie AL, Clark DA, Dowst H, Dry JR, Doroshow JH, Duchamp O, Evrard YA, Ferretti S, Frese KK, Goodwin NC, Greenawalt D, Haendel MA, Hermans E, Houghton PJ, Jonkers J, Kemper K, Khor TO, Lewis MT, Lloyd KCK, Mason J, Medico E, Neuhauser SB, Olson JM, Peeper DS, Rueda OM, Seong JK, Trusolino L, Vinolo E, Wechsler-Reya RJ, Weinstock DM, Welm A, Weroha SJ, Amant F, Pfister SM, Kool M, Parkinson H, Butte AJ and Bult CJ, Cancer Res., 2017, 77, E62–E66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arora R and Krishnan V, Front. Oncol, 2017, 7, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Phillips AC, Boghaert ER, Vaidya KS, Mitten MJ, Norvell S, Falls HD, DeVries PJ, Cheng D, Meulbroek JA, Buchanan FG, McKay LM, Goodwin NC and Reilly EB, Mol. Cancer Ther, 2016, 15, 661–669. [DOI] [PubMed] [Google Scholar]
- 67.Cho J, Chen L, Sangji N, Okabe T, Yonesaka K, Francis JM, Flavin RJ, Johnson W, Kwon J, Yu S, Greulich H, Johnson BE, Eck MJ, Janne PA, Wong KK and Meyerson M, Cancer Res., 2013, 73, 6770–6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Loon J, Even AJG, Aerts HJWL, Ollers M, Hoebers F, van Elmpt W, Dubois L, Dingemans AMC, Lalisang RI, Kempers P, Brans B, Winnepenninckx V, Speel EJ, Thunnissen E, Smits KM, Boellaard R, Vugts DJ, De Ruysscher D and Lambin P, Radiother. Oncol, 2017, 122, 267–273. [DOI] [PubMed] [Google Scholar]
- 69.Aerts HJ, Dubois L, Perk L, Vermaelen P, van Dongen GA, Wouters BG and Lambin P, J. Nucl. Med, 2009, 50, 123–131. [DOI] [PubMed] [Google Scholar]
- 70.Cai W, Chen K, He L, Cao Q, Koong A and Chen X, Eur. J. Nucl. Med. Mol. Imaging, 2007, 34, 850–858. [DOI] [PubMed] [Google Scholar]
- 71.Eiblmaier M, Meyer LA, Watson MA, Fracasso PM, Pike LJ and Anderson CJ, J. Nucl. Med, 2008, 49, 1472–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoeben BA, Molkenboer-Kuenen JD, Oyen WJ, Peeters WJ, Kaanders JH, Bussink J and Boerman OC, Int. J. Cancer, 2011, 129, 870–878. [DOI] [PubMed] [Google Scholar]
- 73.Ping Li W, Meyer LA, Capretto DA, Sherman CD and Anderson CJ, Cancer Biother. Radiopharm, 2008, 23, 158–171. [DOI] [PubMed] [Google Scholar]
- 74.Schechter NR, Wendt RE 3rd, Yang DJ, Azhdarinia A, Erwin WD, Stachowiak AM, Broemeling LD, Kim EE, Cox JD, Podoloff DA and Ang KK, J. Nucl. Med, 2004, 45, 1683–1687. [PubMed] [Google Scholar]
- 75.van Dongen GA, Visser GW, Lub-de Hooge MN, de Vries EG and Perk LR, Oncologist, 2007, 12, 1379–1389. [DOI] [PubMed] [Google Scholar]
- 76.Abou DS, Ku T and Smith-Jones PM, Nucl. Med. Biol, 2011, 38, 675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zeglis BM, Davis CB, Aggeler R, Kang HC, Chen A, Agnew BJ and Lewis JS, Bioconjugate Chem., 2013, 24, 1057–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chang AJ, De Silva RA and Lapi SE, Mol. Imaging, 2013, 12, 17–27. [PMC free article] [PubMed] [Google Scholar]
- 79.Lapi SE and Welch MJ, Nucl. Med. Biol, 2012, 39, 601–608. [DOI] [PubMed] [Google Scholar]
- 80.Wessels BW and Rogus RD, Med. Phys, 1984, 11, 638–645. [DOI] [PubMed] [Google Scholar]
- 81.Wessels BW and Rogus R, J. Nucl. Med, 1983, 24, P95–P95. [Google Scholar]
- 82.Mausner LF and Srivastava SC, Med. Phys, 1993, 20, 503–509. [DOI] [PubMed] [Google Scholar]
- 83.Bonardi ML and de Goeij JJM, J. Radioanal. Nucl. Ch, 2005, 263, 87–92. [Google Scholar]
- 84.de Goeij JJM and Bonardi ML, J. Radioanal. Nucl. Ch, 2005, 263, 13–18. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.