Abstract
Brown and beige adipocytes arise from distinct developmental origins. Brown adipose tissue (BAT) develops embryonically from precursors that also give to skeletal muscle. Beige fat develops postnatally and is highly inducible. Beige fat recruitment is mediated by multiple mechanisms, including de novo beige adipogenesis and white-to-brown adipocyte transdifferentiaiton. Beige precursors reside around vasculatures, and proliferate and differentiate into beige adipocytes. PDGFRα+Ebf2+ precursors are restricted to beige lineage cells, while another PDGFRα+ subset gives rise to beige adipocytes, white adipocytes, or fibrogenic cells. White adipocytes can be reprogramed and transdifferentiated into beige adipocytes. Brown and beige adipocytes display many similar properties, including multilocular lipid droplets, dense mitochondria, and expression of UCP1. UCP1-mediated thermogenesis is a hallmark of brown/beige adipocytes, albeit UCP1-independent thermogenesis also occurs. Development, maintenance, and activation of BAT/beige fat are guided by genetic and epigenetic programs. Numerous transcriptional factors and coactivators act coordinately to promote BAT/beige fat thermogenesis. Epigenetic reprograming also influences expression of brown/beige adipocyte-selective genes. BAT/beige fat is regulated by neuronal, hormonal, and immune mechanisms. Hypothalamic thermal circuits define the temperature setpoint that guides BAT/beige fat activity. Metabolic hormones, paracrine/autocrine factors, and various immune cells also play a critical role in regulating BAT/beige fat functions. BAT and beige fat defend temperature homeostasis, and regulate body weight and glucose and lipid metabolism. Obesity is associated with brown/beige fat deficiency, and reactivation of brown/beige fat provides metabolic health benefits in some patients. Pharmacological activation of BAT/beige fat may hold promise for combating metabolic diseases.
INTRODUCTION
Brown adipose tissue (BAT) and beige fat defend body temperature homeostasis, regulate energy balance and body weight, and influence glucose and lipid metabolism in rodents. BAT develops embryonically, while beige fat is induced in white adipose tissue (WAT), termed browning of WAT, in postnatal life (239, 338). Albeit they arise from distinct developmental origins and are located in different anatomic regions, brown adipocytes and beige adipocytes (also called brite adipocytes) exhibit many common morphologic and metabolic characteristics, including multilocular lipid droplets, dense mitochondria, and expression of uncoupling protein 1 (UCP1). UCP1 resides in the inner mitochondrial membrane, and UCP1-mediated mitochondrial thermogenesis is a hallmark of both brown and beige adipocytes (339). In humans, BAT is abundant in infancy, but regresses in adulthood. However, a considerable number of bioactive brown/beige adipocytes, which are scattered in broad regions, are detected in human adults and increases after cold exposure (57, 317, 321). Brown and beige adipocytes are speculated to have similar metabolic functions in humans as in rodents (58, 140, 338, 339). In this review article, I summarize recent advances about brown and beige adipocytes, and speculate on their therapeutic potential in combating obesity, type 2 diabetes, and nonalcoholic fatty liver disease (NAFLD).
BROWN AND BEIGE FAT FUNCTIONS
BAT and beige fat have distinct functions from WAT. WAT serves as an energy store for the body where excess chemical energy is stored as triacylglycerol (TAG). In contrast, both BAT and beige fat are highly metabolically active and utilize chemical energy for heat production. BAT and beige fat thermogenesis play a critical role in body temperature homeostasis, energy homeostasis, and body weight control. Recent findings also highlight the metabolic function of BAT and beige fat, raising the possibility that they may serve as a potential therapeutic target for metabolic diseases.
Body temperature homeostasis.
Warm-blooded animal species, such as mammals, evolve a complex thermoregulatory mechanism to maintain their own internal body temperature within a narrow range, regardless of fluctuating external temperatures. Body temperature homeostasis is critical for survival of these animal species, because vital physiological processes, particularly those involving enzyme-mediated biochemical reactions, are temperature-sensitive and disrupted when body core temperature is aberrantly high or low. In rodents, BAT and beige fat are indispensable for the maintenance of body temperature homeostasis. In humans, BAT is abundant in infancy and likely to regulate temperature homeostasis in a similar fashion; however, BAT declines in adulthood, and behavior adaptations (e.g. clothing) become a predominant way to maintain temperature homeostasis.
Cold exposure is the most powerful stimuli for heat production to keep the body core temperature at a relative stable level. Skeletal muscle shivering and nonshivering thermogenesis (also called adaptive thermogenesis) account for heat production during cold exposure. Using genetic approaches, it was demonstrated that ablation of both BAT and beige fat completely abolishes adaptive thermogenesis, rendering BAT- and beige fat-deficient mice fatal hypothermia upon cold exposure (192). These findings underscore the essential role of BAT and beige fat in the maintenance of rodent body temperature homeostasis. BAT has special physiological characteristics facilitating its thermogenesis function. It has dense vasculatures that allow an efficient delivery of metabolic fuel to BAT and a rapid heat dissemination. BAT is extensively innervated by the sympathetic nervous system (SNS) (211). Cold exposure rapidly activates the SNS and increases SNS inputs into the BAT, thereby stimulating BAT thermogenesis (211). Both brown and beige adipocytes express uncoupling protein 1 (UCP1), their signature mark. UCP1 is a proton channel resided in the inner mitochondrial membrane. Activated UCP1 allows energy-charged protons to leak across the inner mitochondrial membrane, thereby uncoupling oxidative phosphorylation from ATP synthesis and dissipating chemical energy as heat. Genetic deletion of UCP1, like ablation of BAT and beige fat, severely inhibits cold adaptive thermogenesis, and UCP1−null mice develop fatal hypothermia upon cold exposure (75, 98). These results underscore the importance of UCP1-mediated thermogenesis in the maintenance of temperature homeostasis. Notably, UCP1−null mice gradually gain the ability to defend their body temperature after cold acclimation (75, 98), suggesting that chronic cold stress activates an adaptive UCP1-independent thermogenic mechanism that compensates for loss of UCP1. Importantly, cold exposure similarly activates brown and beige adipocytes in human adults, leading to an increase in energy expenditure (132, 232, 355). These exciting findings suggest that a SNS-BAT/beige fat axis also operate in human adults to promote energy expenditure.
Energy homeostasis and body weight.
Energy expenditure counterbalances energy intake, thus maintaining body weight at a relatively stable level. Energy imbalance results in obesity, which becomes a global epidemic (264). Mounting evidence supports the notion that BAT- and beige fat-mediated thermogenesis contributes to energy expenditure and protects against obesity. In line with this notion, a variety of metabolic hormones, metabolites, and nutrients are able to stimulate brown and/or beige adipogenesis (276); BAT and beige fat in turn mediate, at least in part, diet-induced thermogenesis, thus counteracting weight gain (85, 262, 270, 340). Using either pharmacological or genetic approaches, activation of BAT and beige fat thermogenesis considerably induces weight loss in rodents (275, 319). Conversely, ablation of BAT and beige fat results in severe obesity in mice (192). Importantly, activating brown and beige adipocytes, by either chronic cold exposure or pharmacological interventions, also decreases body weight and adiposity in human adults in a similar fashion (284, 355). It is becoming increasingly appreciated that recruitment and thermogenic activation of brown and beige adipocytes provide metabolic health benefits for both rodents and humans in the context of obesity and metabolic diseases.
Glucose and lipid homeostasis.
Body weight and adiposity profoundly influence glucose and lipid metabolism, and obesity is a primary risk factor for metabolic diseases, including type 2 diabetes, dyslipidemia, nonalcoholic fatty liver diseases (NAFLD). Given their important role in regulating energy expenditure and body weight, it is not surprising that BAT and beige fat are involved in the regulation of metabolic homeostasis. Interestingly, BAT and beige fat are also able to regulate glucose and lipid metabolism by a body weight-independent mechanism. Adoptive transplantation of BAT grafts normalizes hyperglycemia and substantially improves glucose intolerance in recipient mice with either streptozotocin-induced or genetic type 1 diabetes (105, 106). BAT transplantation also attenuates insulin resistance in recipient mice with high fat diet (HFD)-induced obesity (287). However, the underlying mechanism of BAT-induced improvement in insulin sensitivity and glucose metabolism remains elusive.
BAT and beige fat primarily use fatty acids to fuel their thermogenesis. Lippids are delivered to BAT and beige fat eitehr as nonestified free fatty acids (FFAs) or in TAG-rich lipoprotein particles. Notably, BAT and beige fat abundantly express and secrete lipoprotein lipase (LPL), which is upregulated by cold exposure (17, 38). LDL hydrolyzes TAG-rich lipoproteins to release FFAs which are taken into brown and beige adipocytes via CD36, a plasma membrane FFA transporter (17, 38, 66). BAT also expresses angiopoietin-like protein 4 (ANGPTL4), an endogenous LDL inhibitor; cold exposure downregulates BAT ANGPTL4, thereby further increasing LPL activity, LDL-mediated catabolism of lipoprotein particles (66). It is emerging that BAT and beige fat play a critical role in the maintenance of blood TAG homeostasis. Importantly, human brown and beige fat are likely to regulate glucose and lipid metabolism in a similar fashion (25, 46, 47, 117).
Endocrine functions.
It is well established that WAT secretes numerous metabolic hormones and mediators (collectively called adipokines), including leptin and adiponectin, and these adipokines help govern energy and nutriment metabolism. Likewise, brown and beige adipocytes also secrete leptin and adiponectin. Given their small mass relative to WAT, brown and beige fat is unlikely to be an important source of circulating leptin and adiponectin in humans. Additionally, brown and beige adipocytes secrete several specific adipokines, including neuregulin 4, IGF-1, FGF21, and interleukin (IL) 6 (62, 87, 105, 149, 287, 324). Neuregulin 4 suppresses hepatic lipogenesis as an endocrine hormone (324). FGF21 and IL6 promotes brown and beige adipocyte thermogenesis in a paracrine/autocrine fashion (87, 128, 158). IGF-1 is believed to be involved in reducing hyperglycemia by BAT in mice with type 1 diabetes (105). BAT and beige fat-derived metabolic factors are gaining increasing attention for their anti-obesity, anti-hyperglycemia, and/or anti-insulin resistance activities.
BROWN AND BEIGE FAT THERMOGENSIS
BAT and beige fat are distinguished from WAT by their high levels of metabolic rates and thermogenic capability (17, 287). BAT and beige fat possess multiple characteristic and thermogenic-promoting properties, including dense mitochondria, thermogenic UCP1, and multilocular lipid droplets that supply chemical fuel. UCP1-mediated thermogenesis is a hallmark of BAT and beige fat; however, recent findings indicate that brown and beige adipocytes also carry out thermogenesis by additional UCP1-independent mechanisms. Furthermore, mitochondrial dynamics, lipid droplet dynamics, and metabolic fuel mobilizations all profoundly influence brown and beige adipocyte thermogenesis.
UCP1-dependent thermogenesis.
UCP1 is commonly used as molecular marker to identify brown and beige adipocytes. It resides in the inner mitochondrial membrane and binds to cardiolipin, a mitochondrial-restricted membrane lipid species (175). UCP1 is absent in brown and beige progenitor cells, and its expression increases progressively during brown and beige adipogenesis under the control of brown/beige adipocyte-selective genetic programs (258, 339). Notably, cold exposure dramatically increases UCP1 expression in beige fat and substantially increases UCP1 in BAT through the SNS-β adrenergic signaling axis in mice (145). Thyroid hormones also potently stimulate UCP1 expression, increasing BAT thermogenesis (249). In addition to its levels, UCP1 thermogenic activity is also regulated by multiple factors. In quiescent brown and beige adipocytes, purine nucleotides (di- and triphosphates) bind to UCP1 and inhibit its thermogenic activity (279). In contrast, polyunsaturated fatty acids, either internally generated from lipid droplets via lipolysis or externally obtained through CD36-meidated FFA uptake, bind to UCP1 and activate its thermogenic activity (19, 81). Cold exposure rapidly increases the levels of reactive oxygen species (ROS) in BAT, and ROS in turn induces sulfenylation of UCP1 at Cys253, enhancing UCP1 thermogenic activity (48). Notably, UCP1 activation reduces mitochondrial superoxide production in BAT (226), suggesting that UCP1 functions as a component of the oxidative defense system. Indeed, chronic cold exposure markedly increases oxidative stress in UCP1 knockout (KO) but wild type (WT) mice (288). Albeit UCP1 KO mice develop fatal hypothermia when exposed to cold temperature for the first time (75, 97, 98, 125, 202), they progressively regain their abilities to defend the core body temperature against external cold environments after a chronic cold acclimation (98, 208, 313). These observations indicate that UCP1-independent thermogenic mechanisms exist and are activated to compensate for loss of UCP1 in UCP1 KO mice during cold acclimation.
UCP1-independent thermogenesis.
Several UCP1-indepemdent mitochondrial processes have been demonstrated in brown and beige adipocytes to dissipate chemical energy as heat, contributing to adaptive thermogenesis. HFD feeding increases adipose expression of adenine nucleotide translocase 2 (ANT2), an inner mitochondrial membrane conduct that allows energy-charged protons to leak across, thus promoting diet-induced thermogenesis (31, 179). BAT, beige fat, and liver secrete peptidase M20 domain containing 1 (PM20D1), an enzyme that catalyzes condensation of fatty acids and amino acids to produce N−acyl amino acid products (189). N−acyl amino acids (e.g. Phe and Leu) directly bind to mitochondria and function as endogenous uncouplers to promote mitochondrial respiration and thermogenesis (189). ATP-Mg2+/P(i) solute transporters (Slc25a25) dissipate chemical energy as heat during shuttling ATP-Mg2+ and P(i) across the inner mitochondrial membrane (8). Cold exposure stimulates mitochondrial creatine kinase activity in beige adipocytes, and a mitochondrial creatine kinase-mediated futile cycle of creatine metabolism promotes thermogenesis independently of UCP1- (148).
Mitochondrial fission and fusion.
Brown and beige adipocytes have a high level of mitochondrial content, contributing to high metabolic rates in these cells. Mitochondrial respiration is profoundly influenced by mitochondrial dynamics (i.e. fission and fusion). Mitochondrial fission and fusion are controlled by distinct molecular machinery. Drp1 is a master regulator of mitochondrial fission and fragmentations (139, 356); in contrast, mitofusin 1 (Mfn1), Mfn2, and Opa1 promote mitochondrial fusion and branching (285). Cold exposure rapidly stimulates Drp1-mediated mitochondrial fission and fragmentations in BAT via the SNS (335). Inhibition of mitochondrial fission, by either overexpressing dominant negative Drp1 or blocking OMA1-mediated degradation of Opa1, substantially decreases brown adipocyte thermogenesis (248, 335). Conversely, forcing mitochondrial fission through silencing Mfn2 increases brown adipocyte thermogenesis (335). However, molecular connections between mitochondrial dynamics and the UCP1-dependent and UCP1-independent thermogenic machinery remain unknown. Perhaps, mitochondrial fission increases mitochondrial number as well as mitochondrial respiration capability, thereby enhancing thermogenesis. In line with this notion, blocking adipose mitophagy (increase mitochondria), using adipocyte-specific deletion of ATG7, increases beige adipogenesis and BAT thermogenesis, and protects against HFD-induced insulin resistance and glucose intolerance in mice (283). In contrast, increasing mitophagy is likely to be a driving force for re-whitening (inactivation) of beige adipocytes (5).
Lipid droplets and fuel mobilization.
Lipid droplet size and morphology are drastically different between WAT and BAT. White adipocytes have a large unilocular lipid droplet per cell; in contrast, brown and beige adipocytes have multilocular lipid droplets, and their sizes vary, depending on cell thermogenic states. Lipid droplets are coated with lipid droplet proteins, including perilipin (Plin) family members and Cide family members. Brown and beige adipocyte lipid droplets contain abundant Plin1, Plin2, Plin5, and cidea, and these lipid droplet proteins play a critical role in regulating lipolysis (59, 223, 336, 348, 357).
Lipid droplet lipolysis has been extensively examined in white adipocytes. It is composed of a chain of biochemical reactions catalyzed by cytoplasmic lipases. Adipose TAG lipase (ATGL) catalyzes the first step of lipolysis to generate diacylglycerol (DAG) and FFAs from TAG in lipid droplets (366). Hormone-sensitive lipase (HSL) hydrolyzes DAG to generate monoacylglycerol (MAG) which is completely hydrolyzed by MAG lipase (MAGL) to release free glycerol and FFAs. Interestingly, adipocyte-specific deletion of ATGL impairs thermogenesis in mice (2). Conversely, adipocyte-specific overexpression of ATGL increases both body core temperature and energy expenditure (3). These findings suggest that ATGL-mediated lipolysis is a regulatory node of BAT and beige fat thermogenesis and energy expenditure. We recently reported that Snail1, a transcriptional repressor, potently suppresses ATGL expression and ATGL-mediated lipolysis in adipocytes (292). ATGL activity is regulated by both positive (e.g. CGI-58) and negative (e.g. G0S2) regulators as well as by lipid droplet proteins (101, 168, 349, 352). It is very important to fully delineate a lipid droplet-lipolysis-mitochondrial thermogenic machinery axis in brown and beige adipocytes in the context of energy imbalance and obesity in the future.
FFAs are the primary products of lipid droplet lipolysis, and are likely to promote BAT and beige fat thermogenesis by multiple mechanisms. FFAs serve as critical chemical fuel to drive mitochondrial respiration and thermogenesis. Interestingly, lipid droplets directly bind to mitochondria in brown adipocytes (357), likely facilitating delivery of FFAs to mitochondria. FFAs are transported by carnitine palmitoyltransferase (CPT) 1 and CPT2 across the inner mitochondrial membrane into the mitochondrial matrix where FFAs are metabolized through β oxidation to release chemical energy. Adipocyte-specific ablation of CPT2, which blocks FFA utilization by adipocytes, severely impairs cold-induced adaptive thermogenesis in mice (170). Furthermore, genetic inactivation of either the long chain acyl CoA dehydrogenase or the short chain acyl CoA dehydrogenase, two enzymes that catalyze FFA β oxidation, also markedly impairs cold adaptive thermogenesis in mice (102). SIRT3 is a mitochondrial enzyme that deacetylates and stimulates the long chain acyl CoA dehydrogenase, and deletion of SIRT3 impairs cold adaptive thermogenesis in mice (123). Together, these findings strongly argue for the notion that FFA delivery to mitochondria and subsequent β oxidation are a determinant of BAT and beige fat thermogenesis and energy expenditure.
FFAs activate UCP1 as discussed above, further stimulating BAT and beige fat thermogenesis. In addition, FFAs are known by their ability to stimulate nuclear hormone receptors PPARα and PPARδ as endogenous ligands. PPARα and PPARδ activate genomic programs that promote mitochondrial biogenesis and mitochondrial thermogenesis (212). Thus, FFAs connect lipid droplets, mitochondria, and the nucleus, coordinating and promoting thermogenic programs in brown and beige adipocytes.
Glucose uptake and FFA uptake.
BAT is one of the organs with the highest glucose and FFA uptake per cell in rodents and humans (17, 57, 287, 317, 321). Lipid droplets directly provide FFAs to fuel mitochondrial thermogenesis in brown and beige adipocytes, and need to be recharged with lipid fuel that is obtained from the blood. Brown adipocyte glucose uptake is mediated by plasma membrane Glut1 and Glut4 (136, 341). Glucose is converted into FFAs through lipogenesis. FFA uptake is mediated by plasma membrane CD36 and FATP1 in brown and beige adipocytes. Deletion of CD36 or FATP1 impairs cold adaptive thermogenesis in mice (7, 243, 342), indicating that FFAs serve as predominant chemical fuel for BAT and beige fat. Interestingly, cold exposure increases expression of genes responsible for replenishing lipid droplets in BAT, including the genes that mediate glucose uptake, lipogenesis, and TAG synthesis (Glut1, Glut4, hexokinase, PFK1, PDHa1, FAS, glycerol kinase, PEPCK, GPAT3, Dgat1, Dgat2) (166). PPARα or PPARγ agonists, which promote differentiation of human mesenchymal stem cells into brown/beige adipocytes, also stimulate expression of genes responsible for lipolysis, de novo lipogenesis, TAG synthesis, and lipid droplet formation (16). Therefore, lipid droplet dynamics, including lipid droplet lipolysis and replenishment, are likely to play an important role in regulating BAT and beige fat thermogenesis.
BAT AND BEIGE FAT DEVELOPMENT
BAT and beige fat development is best understood in rodents. BAT develops embryonically while beige fat develops postnatally (263). BAT mass is maintained at relatively stable levels by a homeostatic mechanism in adulthood; in contrast, beige fat is barely detectable at thermoneutrality and highly induced by cold exposure (18, 103, 338, 339). Furthermore, brown and beige adipocytes arise from completely different progenitor/stem cell origins.
BAT.
Mouse or rat interscapular BAT develops between E15–16, and markedly increases between P15–21 (130, 331). Functional transformation occurs two days before birth (E18–19), generating thermogenic-competent UCP1+ brown adipocytes (94, 130). A common Myf5+ (and Pax7+) progenitor population is believed to gives rise to both BAT and skeletal muscle in mice (180, 274). In line with this notion, the gene expression profiles of BAT contain a skeletal muscle gene expression signature (301). A further differentiated Myf5+PDGFα+ subset is committed to the BAT lineage during fetal development (331). In adults, BAT homeostasis is maintained by proliferation and differentiation of BAT progenitors, which appear to reside at the dorsal edge of iBAT (177).
Beige fat.
Beige adipocytes are distributed in broad areas, including sWAT, perivascular adipose tissue, and skeletal muscle in mice (4, 43, 55, 88). They are detected in the cervical-supraclavicular, shoulder-blades, cervical, axillary, mediastinal, paravertebral, perirenal, and peri-aortic regions in humans (256, 338). Notably, beige adipocytes are heterogeneous populations with regard to their metabolic properties and gene expression profiles (176). Beige adipocyte heterogeneity is profoundly influenced by the nature of its inducers (e.g. β3 adrenergic agonists vs PPARγ agonists) (325). The mechanism of beige fat development (also referred to as beige adipocyte recruitments or browning of WAT) remains elusive, and three models receive great attention.
De novo beige adipogenesis.
In this model, beige fat inducers are believed to stimulate beige fat progenitor expansion and differentiation into mature beige adipocytes. Beige progenitor cells are believed to reside in adipose vasculatures (a progenitor/stem cell niche) where the local microenvironments help preserve the stemness of beige progenitor/stem cells and facilitate de novo beige adipogenesis upon stimulation (23, 190, 205, 307). Indeed, cold exposure or β3 adrenergic agonist stimulation increases beige precursor proliferation and differentiation into beige adipocytes in mice (329, 338). Human beige precursors are also associated with adipose vasculatures (205). Albeit the precise identity of beige progenitor/stem cells remains elusive, a number of molecular markers has been shown to be linked to beige precursors. Adipose vasculature mural cells expressing smooth muscle actin (SMA), Myh-11, or PDGFRα are able to proliferate and differentiate into beige adipocytes in vitro and in vivo (23, 178, 190). Interestingly, beige fat displays a smooth muscle-like signature of gene expression profiles (190), suggesting a potential lineage relationship between beige adipocytes and smooth muscle cells. Diverse beige progenitor lineages may exist; in mice, both beige adipocyte-specific progenitors and beige/white adipocyte-bipotent PDGFRα+ progenitors have been reported (60, 178, 338). Notably, a subpopulation of PDGFRα+ mesenchymal progenitors (PDGFRα+CD9high) also differentiates into fibrogenic cells in obesity, promoting adipose tissue fibrosis (201).
White-to-beige adipocyte transdifferentiation.
It has long been believed that mature white adipocytes and beige adipocytes can be converted between each other (i.e. transdifferentiation) under certain conditions. Cold exposure or β adrenergic agonist stimulation was reported to induce white-to-beige adipocyte transdifferentiation in mice and rats (15, 121). Lineage tracing studies reveal that the majority of UCP1+ beige adipocytes in inguinal WAT, induced by a short-term cold exposure or β adrenergic agonist stimulation, arise from pre-existing white adipocytes that express adiponectin, a molecular marker of mature adipocytes (177, 322). Moreover, UCP1+ beige adipocytes can be reverted back into UCP1− white adipocytes in mice housed at thermoneutrality following cold exposure (5, 259), and beige-to-white adipocyte transdifferentiation is facilitated by mitophagy (5).
Activation of dormant beige adipocytes.
Beige adipocytes may also exist in inactive (dormant) and active states. Dormant beige adipocytes resemble white adipocytes in morphology and some chemical and metabolic properties (e.g. unilocular lipid droplets, lacking UCP1), but they are distinct in developmental origins from white adipocytes. Dormant beige adipocytes can be rapidly activated to become active UCP1+ beige adipocytes upon cold exposure temperature or β adrenergic stimulation (259), accounting for, at least in part, recruitments of beige fat. It is worth mentioning that current methods are unable to distinguish white-to-beige adipocyte transdifferentiation from dormant beige adipocyte activation.
GENETIC AND EPIGENETIC REGULATION OF BROWN AND BEIGE FAT THERMOGENESIS
BAT development and beige fat recruitment are controlled by linage-specific genetic programs. Notably, many transcription factors regulate the develoment, maintenance, and/or activation of both BAT and beige fat in a similar fashion. Recent research also underscores the importance of epigenetics in BAT and beige fat biology.
Zfp423.
Zfp423 is a transcription factor highly expressed in adipocyte precursors (109, 322). It activates expression of PPARγ, thereby promoting commitment of progenitors to white or brown preadipcyte (108). Notably, Zfp423 also binds to early B-cell factor 2 (Ebf2) and inhibits the ability of Ebf2 to stimulate Prdm16 expression and beige adipogenesis (281). Zfp423 is also expressed in mature adipocytes, and its levels are higher in WAT than in BAT (281). Downregulation of Zfp423 induces a white-to-beige adipocyte conversion in mice (281).
Zfp516.
Cold exposure stimulates expression of Zfp516 in BAT that binds to Prdm16 and stimulates UCP1 transcription (64, 267). In agreement with these findings, adipocyte-specific overexpression of Zfp516 increases brown adipogenesis in mice (64). Global deletion of Zfp516 results in embryonic lethality, and BAT mass is dramatically reduced in Zfp516−null fetuses (64).
Ebf2.
Ebf2, in contrast to Zfp432, is expressed at higher levels in BAT than in WAT (250). Efb2 stimulates, in conjunction with PPARγ, expression of both Prdm16 and UCP1 in brown adipocyte cultures (250). In line with these findings, adipocyte-specific overexpression of Ebf2 promotes beige adipogenesis and protects Ebf2−transgenic mice from HFD-induced obesity (289). Mice with global deletion of Ebf2 die soon after birth (53), and brown preadipocytes derived from Ebf2 null embryos have reduced ability to differentiat into brown adipocytes (250). Several factors are known to regulate the development and function of BAT and/or beige fat through Ebf2. Zfp432 suppresses beige fat funciton by binding and inhibiting Ebf2 transcriptional activity as discussed above. Similarly, inhibitor of DNA binding 1 (Id1) also binds to Ebf2 and suppresses Efb2 transcriptional activity, and adipocyte-specific overexpression of Id1 exacerbates age-induced or HFD-induced obesity (237). In contrast, a long noncoding RNA Blnc1 binds to Efb2 and enhances Efb2-stimualted expression of thermogenic genes in brown and beige adipocytes (363).
PPAR family members (γ, α, and δ).
PPARγ is a core transcription factor required for white adipogenesis (258). Interestingly, PPARγ agonists potently increase de novo beige adipogenesis and white-to-beige adipocyte transdifferentiation in both rodents and humans (16, 188, 229, 239). The ability of PPARγ to stimulate preadipocyte differentiation into either white or brown/beige adipocytes appears to be determined by its associated coactivators. When associated with Prdm16, PPARγ stimulates expression of brown/beige adipocyte-selective genes and represses white adipocyte-specific genes (245). Interestingly, Sirt1 deacetylates PPARγ (Lys268 and Lys293), which promotes binding of PPARγ to Prdm16 in brown/beige adipocytes (245). In contrast, binding to TLE3 allows PPARγ to suppress expression of brown/beige adipocyte-selective genes and stimulate white adipocyte-selective genes (320). Moreover, TLE3 and Prdm16 compete for the same binding sites in PPARγ (320).
PPARα is well known to stimulate fatty acid β oxidation and mitochondrial respiration (212). Activation of PPARα has been reported to promote a white-to-beige adipocyte conversion and BAT and beige fat thermogenesis (16, 127). Consistently, PPARα directly stimulates expresion of PGC1α and Prdm16, two coactivators that promote brown and beige adipocyte thermogenesis (127). PPARα binds to period 2 (Per2), a circadian clock protein that acts as a PPARα coactivator to enhance brown/beige adipocyte activity (44), likely linking to a circadian control of BAT and beige fat activities.
PPARδ, like PPARα, also promotes BAT and beige fat thermogenesis in mice (332). Of notice, PPARγ, PPARα, and PPARδ can be activated by lipid ligands produced from lipid droplet lipolysis (212); therefore, these nuclear hormone receptors are likely to be involved in mediating crosstalk between lipid droplets, the nucleus, and mitochondria to coordinate brown/beige adipocyte thermogenic processes.
Additional nuclear hormone receptors.
Estrogen-related receptor β (ERRβ), ERRγ, and nuclear receptor-4A member NOR-1 all are able to stimulate UCP1 expression and brown/beige adipocyte thermogenesis (67, 90, 164). Cold exposure activates the protein kinase A (PKA)/p38 cascade, and p38 activates ERRβ and ERRγ to stimulate expression of UCP1 and other thermogenic genes, increasing BAT thermogenesis (90). ERRγ binds a corepressor SHP that inhibits the ability of ERRγ to activate the PGC1α promoter, and deletion of SHP increases PGC1α levels in BAT and energy expenditure and protects SHP null mice from diet-induced obesity (327). Activation of the retinoic acid recetpor (RAR) by retinaldehydes stimulates beige adipogenesis and adaptive thermogenesis in mice (151). In contrast to the above nuclear receptors that stimulate BAT and beige fat thermogenesis, activation of LXRα or Vitamin D receptors inhibits UCP1 expression and brown and beige adipocyte thermogenesis (200, 326). LXRa binds to receptor-interacting protein 140 (RIP140) corepressor and recruits RIP140 to the UCP1 promoter, inhibiting UCP1 expression in BAT (326).
C/EBP family members (α, β, and δ).
C/EBP family members are core adipogenic transcription factors (258). Deletion of C/EBPα blocks WAT but not BAT development (184), suggesting that C/EBPα is dispensable for BAT. Using an inducible, adipocyte-specific knockout system, C/EBPα was reported to be required for white adipogenesis in adult mice; surprisingly it is dispensable for fetal/early postnatal WAT development (330). Cold-induced beige adipogenesis is also independent of C/EBPα (330).
Deletion of both C/EBPβ and C/EBPδ, in contrast to C/EBPα, blocks BAT development in mice (298), indicating that these two transcription factors are indispensable for BAT and beige fat development, maintenance, and/or function. C/EBPβ binds to Prdm16 that acts as an coactivator for C/EBPβ to promote brown and beige adipogenesis (143). C/EBPβ is likely to serve as a regulatory hub for BAT and beige fat thermogenesis. In line with this notion, Plac8 activates C/EBPβ expression, and deletion of Plac8 impairs beige adipogenesis and adaptive thermogenesis, promoting obesity (142). In contrast, Hoxc8 suppresses C/EBPβ expression and brown adipogenesis (210). C/EBPβ is also inovlved in mediating microRNA regulation of BAT and beige fat activities. miR-196a upregulates C/EBPβ by suppressing expression of Hoxc8, increasing beige adipogenesis (210). miR-155 suppresses C/EBPβ expression, and ablation of miR-155 increases brown and beige adipogenesis in mice (45).
Circadian clock proteins.
Cold exposure stimulates Per2 expression in BAT via heat shock factor 1 (HSF1), and Per2 null mice are cold sensitive (44). Per2 increases UCP1 expression by acting as a PPARα coactivator as discussed above. Rev-erbα levels are higher in BAT than in WAT, and deletion of Rev-erbα impairs BAT development in mice (217). Surprisingly, the other group reported that Rev-erbα directly represses UCP1 expression, and deletion of Rev-erbα improves cold tolerance in mice (92). Rev-erbα deficiency also abolishes normal rhythms of body temperature and BAT activity (92).
Prdm16.
Prdm16 has gained great attention by its ability to promote commitment of Myf5+ progenitor cells to brown adipocyte lineage (274). Mechanistically, Prdm16 acts as a cofactor for multiple transcription factors (e.g. PPARγ, C/EBPβ, Zfp516) to stimulate UCP1 expression and thermogenesis in brown and beige adipocytes (64, 143, 245). Prdm16 is able to suppress expression of negative regulators of BAT and beige fat, thereby promoting BAT and beige fat activities. A Rb family member p107 is expressed in adipose precursors and represses their differentiation into brown adipocytes, and p107-deficient stem cells uniformly give rise to brown adipocytes (63). PRDM16 binds to the p107 promoter and suppresses p107 expression (63). Additionally, Prdm16 also represses expression of white adipocyte-selective genes (118), likely preserving brown and beige adipocyte phenotypes. In line with these findings, adipocyte-specific overexpression of Prdm16 robustly induces beige adipogenesis and protects Prdm16 transgenic mice from HFD-induced obesity and insulin resistance (275).
Numerous factors regulate brown and beige adipocyte thermogenesis through modulating Prdm16 expression and/or its transcriptional activity. Euchromatic histone-lysine N− methyltransferase 1 (EHMT1) and MED1 bind to Prdm16 and activate expression of UCP1 and other brown/beige adipocyte-selective genes (118, 119, 133, 228). Deletion of EHMT1 in either Myf5+ brown adipocyte precursors or adiponectin+ adipocytes impairs brown and beige adipogenesis and cold adaptive thermongenesis in mice (228). PPARα, PPARγ, and IRF4 stimulate PRDM16 expression, which explains, at least in part, the ability of these proteins to stimulate brown and/or beige adipogenesis and thermogenesis (127, 160, 229). In contrast, CtBP1, CtBP2, and TLE3 bind to Prdm16 and inhibit its ability to stimulate expression of brown and beige adipocyte-selective genes and thermogenesis (144, 320). miR-133 binds to the 3’ UTR of Prdm16 and downregulates Prdm16 expression (305, 354), Cold exposure decreases miR-133 expression, thereby contributing to upregulation of Prdm16 and recruitments of BAT and beige fat in mice (305, 354).
Given its roles in promoting commitment to the brown preadipocyte lineage and brown adipocyte thermogenesis, it is surprising that Prdm16 deficiency either in Myf5+ brown precursors and their progeny brown adipocytes or in adiponectin+ adipocytes (e.g. white, brown, and beige adipocytes) does not alter BAT development and BAT thermogenesis in mice (51, 118). Notably, adipocyte-specific deletion of Prdm16 completely blocks cold-induced recruitment of UCP1+ beige fat in mice, leading to the notion that Prdm16 is required for beige adipogenesis but not for brown adipogenesis (51). Additionally, Prdm16-deficiencyt brown adipocytes progressively lose their thermogenic capability during aging and become white adipocyte-like cells, suggesting that Prdm16 plays an important role in preserving brown and beige adipocyte phenotypes in aged mice (118).
PPARγ coactivator-1α (PGC1α).
The PGC1 coactivator family contains three members of PGC1α, PGC1β and PRC (also called PPRC1) which are known to promote mitochondrial biogenesis and respiration (183). The PGC1α gene encodes a long and a short (NT-PGC1α254) variant via mRNA alternative splicing, and both variants are able to promote brown adipocyte thermogenesis (42). Cold exposure increases PGC1α expression in BAT in mice and in subcutaneous fat in humans (150, 170). PGC1α stimulates UCP1 expression and thermogenesis in brown and beige adipocytes by acting as a coactivator for many transcription factors, including PPAR family members, C/EBP family members, Tbx15, N-arginine dibasic convertase (Nrd1/NRDc, also called nardilysin), and IRF4 (91, 122, 160). It also binds to Prdm16, stimulating expression of brown and beige adipocyte-selective genes (144).
Many cellular factors are believed to regulate brown and beige fat thermogenesis through modulating, at least in part, PGC1α expression and/or its transcriptional activity. CREB, ATF2, and ERRγ directly stimulate PGC1α expression in brown and/or beige adipocytes (34, 197, 327). In contrast, Foxa3, pRb, and SHP bind to the PGC1α promoter and suppress PGC1α transcription, thereby inhibiting brown and beige adipocyte activity (197, 272, 327). Smad3 also suppresses PGC1α expression, and deletion of Smad3 enhances beige adipogenesis and protects Smad3 null mice from diet-induced obesity and insulin resistance (347). Twist-1 and RIP140 directly bind to PGC1α and inhibit the ability of PGC1α to stimulate expression of UCP1 and other brown and beige adipocyte-selective genes (49, 113, 234). PGC1α activity is also regulated through posttranslational modifications. SIRT2 deacetylates and activates PGC1α, promoting mitochondrial biogenesis and β oxidation (163). HIF1α directly suppresses expression of SIRT2 and SIRT2-mediated activation of PGC1α (163), and adipocyte-specific deletion of HIF1α or ARNT (the HIF1α partner) increases energy expenditure, contributing to protection against diet-induced obesity and insulin resistance in the mutant mice (141, 163). p38 MAPK phosphorylates PGC1α, which increases PGC1α stability as well as the ability of PGC1α to stimulate UCP1 expression and mitochondrial respiration in brown adipocytes (34, 242).
Given the profound effects of PGC1α on UCP1 expression and mitochondrial respiration, it is surprising that adipocyte-specific deletion of PGC1α only slightly impairs BAT function (157). Perhaps PGC1β, PRC, and/or other related molecules compensate for loss of PGC1α in PGC1α null mice.
Epigenetic regulation of BAT and beige fat functions.
Recent research highlights the importance of epigenetics in the regulation of the development, maintenance, and activation of BAT and beige fat. Brown adipocyte differentiation is associated with increased expression of Jmjd3 (a H3K27me3 demethylase), ubiquitously transcribed tetratricopeptide repeat on chromosome X (UTX, demethylating H3K27me2–3), and lysine-specific histone demethylase 1 (LSD1, demethylating both H3K4me1–3 and H3K9me1–3) (235, 267, 359). Jmjd3 and UTX demethylate H3K27me3, a repressive epigenetic mark, at the promoters of UCP1 and other brown adipocyte-selective genes, thereby upregulating these genes (235, 359). Cold exposure or β3 agonist stimulation increase LSD1 expression in iWAT (71). LSD1 appears to directly promote recruitments/activation of BAT and beige fat; in line with this notion, brown/beige adipocyte-specific deletion of LSD1 impairs both brown and beige adipogenesis, decreases energy expenditure, and increases adiposity in mice (72, 267), whereas global overexpression of LSD1 increases browning of WAT and protects LSD1 transgenic mice from HFD-induced obesity (71). Cold exposure stimulates PKA-mediated phosphorylation of Jmjd1a (also known as Jhdm2a or Kdm3a) and activates Jmjd1a (1). Activated Jmjd1a binds to the UCP1 promoter and demethylates repressive H3K9me2, and deletion of Jmjd1a impairs BAT lipolysis and oxygen consumption, resulting in reduced energy expenditure, adult onset obesity, and hyperlipidemia in mice (299). MLL4 (also called KMT2D) is a H3K4me1–2 methyltransferase, and deletion of MLL4 in Myf5+ brown precursors and their progeny brown adipocytes impairs brown adipogenesis in mice (171). MLL4 is recruited to adipogenic enhancers through its interactions with C/EBPβ (171). Rreb1 recruits Jmjd3 to the UCP1 promoter (235). LSD1 is recruited to brown/beige adipogenic enhancers through its interactions with transcription factors Nrf1 and Zfp516 (71, 267). Cold exposure decreases histone deacetylase 1 (Hdac1) levels in BAT, and β adrenergic signaling decreases the binding of Hdac1 to the UCP1 and PGC1α promoters in brown adipocytes, leading to an increase in active epigenetic H3K27ac mark (181). Concurrently, β adrenergic signaling stimulates dissociation of enhancer of zeste homologue (Ezh2) and suppressor of zeste 12 (SUZ12), leading to a decrease in repressive epigenetic mark H3K27me3 at the UCP1 and PGC1α promoters (181).
Aside from histone modifications, DNA methylations are also involved in regulating BAT and beige fat function. Activation of APMKα1, which stimulates brown/beige adipocyte activity as described later, increases α-ketoglutarate (αKG) levels (351). αKG activates Tet DNA demethylases, leading to DNA demethylation at the Prdm16 promoter, thereby enhancing Prdm16 expression and brown adipogenesis (351).
NEURAL REGULATION OF BROWN AND BEIGE FAT THERMOGENESIS
The core body temperature is maintained at a relatively stable level by a homeostatic mechanism, which is guided by a temperature setpoint. The hypothalamic temperature-regulating center is believed to define the body temperature setpoint. A deviation from the temperature setpoint rapidly triggers thermal responses (i.e. heat production vs heat loss) to defend body temperature homeostasis. In rodents, adaptive thermogenesis by BAT and beige fat plays an essential role in combating cold environments to keep the core body temperate close to the temperature setpoint. Information about ambient cold temperature is transmitted into the hypothalamic temperature-regulating center where a complex thermal circuitry integrates thermal information as well as internal inputs related to body metabolism and physiologic states. The hypothalamic outputs determine the activities of the SNS and the hypothalamic-pituitary-thyroid (HPT) axis that in turn control BAT and beige fat functions. It is worth mentioning that the hypothalamic thermal circuits also mediate social stress-induced thermogenesis as well as infection-induced fever (147, 186, 211, 361).
The core thermal circuitry.
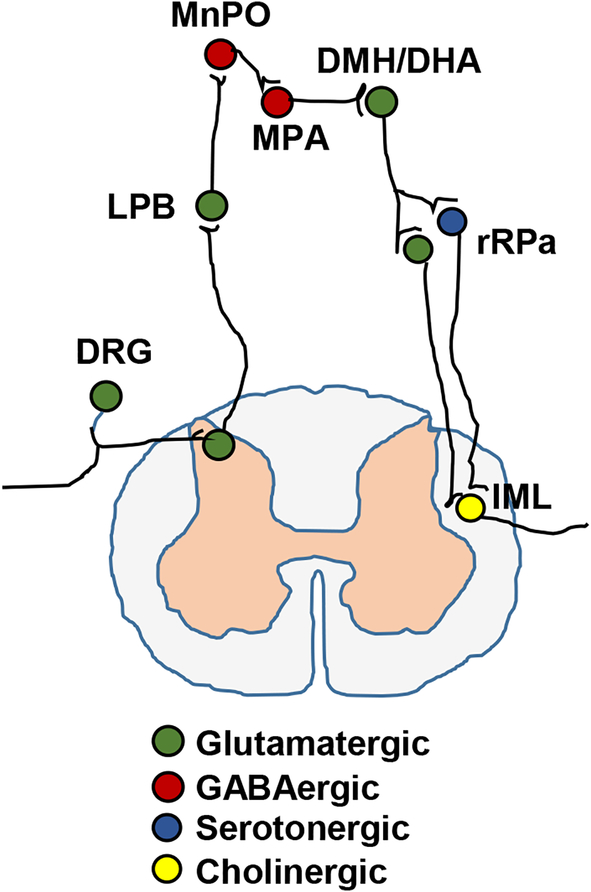
Skin transient receptor potential M8 (TRPM8) cation channels function as cutaneous cold receptors to detect ambient cold temperature and encode cold information (203). TRPV3 and TRPV4 channels are warm receptors and encode warm information (211). Primary temperature-sensing neurons are located in the dorsal root ganglia (DRGs) and transmit thermal information into the central nervous system (CNS) (Fig. 1). Primary thermoreceptive afferent fibers are predominantly myelinated Aδ fibers and synapse on lamina I thermoregulatory glutamatergic neurons in the spinal (or trigeminal) dorsal horns (311). The spinal or trigeminal second-order neurons project to the lateral parabrachial nucleus (LPB) where they synapse on glutamatergic neurons (211, 311). LPB glutamatergic neurons project to in the median preoptic subnucleus (MnPO) of the preoptic area (POA) where they synaptically activate GABAergic neurons (211, 311). MnPO GABAergic neurons innervate and inhibit warm sensitive GABAergic neurons in the medial preoptic area (MPA) (311). MPA GABAergic neurons project to the dorsomedial hypothalamus (DMH)/dorsal hypothalamic area (DHA) where they inhibit glutamatergic neurons (Fig. 1) (311). DMH/DHA glutamatergic neurons project to the rostral raphe pallidus (rRPa), raphe magnus nucleus, and the parapyramidal area (PaPy), and monosynaptically activate BAT premotor neurons in these areas (211, 311). BAT premotor neurons consist of glutamatergic and serotonergic subpopulations, and both project to the intermediolateral nucleus (IML) of the thoracolumbar spinal cord where they activate BAT preganglionic neurons, stimulating BAT thermogenesis (Fig. 1) (211, 216, 311). The DMH/DHA/rRPa circuits also mediate social defeat stress-induced, in addition to cold stress-induced, BAT thermogenesis in rats (147, 186).
Figure 1. A schematic representation of the core thermal circuitry.
DRG: dorsal root ganglia. LPB: lateral parabrachial nucleus. MnPO: median preoptic subnucleus. MPA: medial preoptic area. DMH/DHA: dorsomedial hypothalamus/dorsal hypothalamic area. rRPa: the rostral raphe pallidus. IML: intermediolateral nucleus.
Bacterial infection commonly causes fever by raising the hypothalamic temperature setpoint. The core thermoregulatory circuitry also mediates pyrogen-induced BAT thermogenesis and fever (211). Pyrogens (e.g. TNFα, and IL1β) stimulate production of prostaglandin E2 (PGE2) from the brain vasculature, and PGE2 in turn induces fever by activating ER3 receptors on warm-sensitive GABAergic neurons in the POA (311). Notably, histaminergic inputs to POA neurons also increase BAT thermogenesis in mice by activating H1 and H3 receptors (211, 278).
Hypothalamic thermal circuits.
Aside from the POA and DMH/DHA, many other hypothalamic nuclei, including the arcuate nucleus (ARC), ventromedial hypothalamus (VMH), lateral hypothalamus (LH), and paraventricular hypothalamus (PVH), are also involved in regulating BAT and beige fat thermogenesis (6, 9, 41, 50, 76, 153, 159, 162, 211, 222, 238, 247, 296, 303, 323). ARC POMC neurons and RIP neurons stimulate, while ARC AgRP neurons inhibit, BAT and beige fat energy expenditure (9, 50, 76, 159, 162, 211, 247, 303). RIP neurons and AgRP neurons project to the PVH that mediates the thermal action of these two neuronal subpopulations; in contrast, POMC neurons stimulate BAT and beige fat activities through PVH-independent circuits (14, 159, 261, 282). The PVH contains heterogeneous neuronal subpopulations, and the BDNF, oxytocin, and CRF subsets are able to stimulate BAT and beige fat thermogenesis via the SNS (6, 41, 211, 296, 323). ARC POMC neurons activate MC4R signaling in the LH (209). LH orexin neurons project to the rRPa and the PaPy where they activate BAT premotor neurons, thus increasing BAT thermogenesis (211, 310). It is likely that POMC neurons stimulate BAT and beige fat activities at least in part by stimulating LH orexin neurons (209). Notably, orexin neurons are required for social stress-induced thermogenesis in mice (361).
Multiple hormonal and nutrient signals stimulate BAT and beige fat energy expenditure through the hypothalamic circuitry. Leptin stimulates LepRb neurons in the ARC (e.g. POMC, AgRP, and RIP neurons) (68, 159), DMH (PrRP neurons) (69, 76), and MnPO (warm sensitive glutamatergic neurons) (358, 362). These LepRb neural circuits are likely to act coordinately to mediate leptin-stimulated energy expenditure. Central insulin stimulates ARC neurons and promotes beige fat recruitments (68). Estrogens activate ERα signaling in the VMH, increasing BAT and beige fat thermogenesis (345). Deletion of ERα in Sim1-expressnig neurons (the PVH and medial amygdala) decreases energy expenditure and increases adiposity (343), suggesting that estrogen signaling in these neurons also promote thermogenesis. Central thyroid hormones and urocortin-3 stimulate VMH circuits and increase BAT and beige fat energy expenditure (165, 191). Central FGF21 also increases energy expenditure (268). Thus, it is likely that these hormonal and nutritional signals help determine the CNS temperature setpoints through modulating the activity of the hypothalamic thermal circuits.
Medullary thermal circuits.
The ventrolateral medulla (VLM) and the nucleus tractus solitarius (NTS) also profoundly influence BAT and/or beige fat premotor neuron activity, thereby modulating thermogenesis and energy expenditure. VLM catecholaminergic neurons project to the rRPa and inhibit BAT premotor neurons via α2 adrenergic receptors (198), and stimulation of VLM or A1/C1 catecholaminergic neurons rapidly inhibits sympathetic inputs to BAT and decreases BAT thermogenesis (36, 211).
The NTS is known to integrate satiety signals which are relayed to the brain from the gastrointestinal (GI) tract via vagal afferent nerves. Many satiety hormones stimulate BAT and beige fat thermogenesis (273); moreover, duodenal lipid sensing stimulates BAT thermogenesis via NTS circuits (26). NTS GABAergic neurons directly project to rRPa, inhibit BAT premotor neurons, and decrease BAT thermogenesis (36, 159). Notably, the NTS also contains BAT sympathoexcitatory neurons (311). A GI-NTS-rRPa-BAT axis is emerging and is likely to be involved in mediating diet-induced thermogenesis.
SNS-BAT and SNS-beige fat cascades.
We have discussed that the SNS outflow to BAT is a driving force for BAT activation. Moreover, tonic sympathetic inputs are also required for BAT maintenance and homeostasis, as demonstrated by BAT atrophy when it is denervated (271). Further underscoring the importance of the SNS inputs to BAT, deletion of dopamine β-hydroxylase, which catalyzes neurotransmitter norepinephrine synthesis, blocks BAT thermogenesis (300).
The SNS promotes BAT and beige fat thermogenesis by multiple mechanisms. Norepinephrine stimulates β1, β2, and β3 adrenergic receptors in brown and beige adipocytes. Deletion of β1 or β3 adrenergic receptors partially impairs BAT and beige fat thermogenesis (295, 312), and ablation of all three isoforms causes dysfunction of BAT and beige fat to the highest level relative to ablation of individual isoforms (12). Thus, β1, β2, and β3 adrenergic receptors all are involved in mediating sympathetic stimulation of BAT and beige fat thermogenesis. Activation of β1, β2, or β3 adrenergic receptors stimulates the cAMP/PKA/p38 MAPK pathway, which is responsible for stimulating UCP1 expression, mitochondrial respiration, and thermogenesis in brown/beige adipocytes (34, 35, 242). Activation of the cAMP/PKA pathway also rapidly stimulates phosphorylation and activation of Drp1, thus promoting Drp1-mediated mitochondrial fission (335). Mitochondrial fission further increases brown and beige adipocyte thermogenesis (335). Notably, brown and beige adipocyte β adrenergic sensitivity declines in obesity (37), which may account for, at least in part, a reduction in energy expenditure under these conditions. The cAMP/PKA pathway is negatively regulated by phosphodiesterases (PDEs) that degrade cAMP. Pharmacological inhibition of PDE10A or deletion of PDE10A increases beige adipogenesis and energy expenditure in mice (115, 218). Furthermore, activation of α2 adrenergic receptors, unlike β adrenergic receptors, inhibits brown adipocyte thermogenesis (367). These findings raise the possibility that upregulation of PDE10A or related negative regulators of the adrenergic receptor/cAMP/PKA pathway may contribute to blunted thermal responses of BAT and beige fat to sympathetic inputs in obesity.
Sympathetic postganglionic neurons also release coneurotransmitters into BAT and/or beige fat, including adenosine that activates adenosine A2A receptors (96). A2A receptor agonists stimulate BAT thermogenesis and beige fat recruitment; conversely, pharmacological or genetic inactivation of A2A receptors decreases BAT thermogenesis in mice (96). Connexin 43 is an important gap junction protein; interestingly, brown/beige adipocyte-specific ablation of connexin 43 impairs cold-induced beige adipogenesis (365). These findings raise a possibility that gap junctions may mediate a fast propagation of neuronal signals in BAT to allow a rapid, robust thermal response to cold stress.
Sympathetic inputs are also able to potentiate the thermal responses of brown and beige adipocytes to other thermogenic factors. For instance, activation of the β adrenergic receptor/cAMP/PKA pathway robustly increases expression of type 2 iodothyronine deiodinase (Dio2) (61). Dio2 catalyzes a conversion of prohormone thyroxine T4 to bioactive T3, and deletion of Dio2 impairs cold adaptive thermogenesis in mice (61). Additionally, sympathetic inputs to BAT stimulate de novo brown adipogenesis via β1 adrenergic receptor pathways (177). Sympathetic inputs also stimulate local release of paracrine and/or autocrine factors (e.g. FGF21, prostaglandins) that stimulate brown and beige adipocyte thermogenesis (62, 318). Furthermore, the SNS is likely to mediate cold-stimulated secretion of endocrine hormone natriuretic peptides (NPs) from the heart, and NPs in turn stimulate BAT thermogenesis (28).
The HPT axis.
The HPT axis is known to determine basal metabolic rates (126). Thyrotropin-releasing hormone (TRH) neurons in the PVH receive neural inputs from the NTS, ARC, and DMH (82, 83, 169, 269), and projects to the median eminence where they release TRH. TRH is delivered to the pituitary gland via the hypophyseal portal system and stimulates thyrotrope cells in the anterior pituitary gland to secrete thyroid-stimulating hormone (TSH). TSH is transported in the blood to the thyroid gland where it stimulates production and secretion of thyroid hormones thyroxine (T4) and triiodothyronine (T3). T4 is converted to active T3 by Dio2 inside brown and beige adipocytes. T3 directly stimulates UCP1 expression and mitochondral biogenesis via its nuclear receptors TRα and TRβ in brown and beige adipocytes (172, 249, 255). TRα signaling also augments the thermogenic response of BAT to β adrenergic stimulation (255). Notably, leptin stimulates release of TRH both directly by activating LEPRb in TRH neurons and indirectly by activating ARC POMC neurons that innervate TRH neurons (93, 107, 120, 131). Oxysterols activate LXRα and LXRβ as ligands, and deletion of LXRα and LXRβ enhances TRH neuronal activity and increases beige adipogenesis (204). Thus, the HPT axis appears to link nutrient metabolism to BAT and beige fat energy expenditure. It is worth mentioning that the SNS and the HPT systems are likely to act coordinately/synergistically to promote BAT and beige fat thermogenesis.
HUMORAL REGULATION OF BROWN AND BEIGE FAT THERMOGENESIS
Numerous endocrine, paracrine, and autocrine factors have been demonstrated to regulate the development, maintenance, and function of BAT and beige fat. These factors are secreted by a variety of tissues and organs, including the pancreas, liver, GI tract, heart, adipose tissue, skeletal muscle, and the immune system. These humoral regulators, in conjunction with the SNS system, dynamically regulate BAT and beige fat activities, maintaining body temperature homeostasis and energy balance.
Endocrine regulators.
Insulin.
Insulin receptor expression increases during brown adipocyte differentiation, and deletion of insulin receptors dramatically impairs brown adipocyte differentiation in vitro (77). In line with these findings, deletion of insulin receptors specifically in Myf5+ brown precursor cells substantially decreases BAT mass in mice (196). Furthermore, deletion of insulin receptors in UCP1+ brown/beige adipocytes also decreases BAT mass (104). Deletion of insulin receptors in adiponectin+ adipocytes (white, brown, and beige adipocytes) also decreases BAT mass and impairs cold adaptive thermogenesis in mice (30). These genetic data suggest that insulin signaling is required not only for brown adipogenesis and BAT development but also for the maintenance of the thermogenic and metabolic phenotypes of BAT, and perhaps also beige fat.
Insulin receptors bind to both IRS1 and IRS2 that mediate activation of the PI 3-kinase/Akt pathway; however, IRS1 but not IRS2 appears to mediate stimulation of brown adipogenesis by insulin (79, 80). Insulin stimulates UCP1 expression in brown adipocyte cultures in a PI 3-kinase- and Akt-dependent manner (79, 219, 314). Adipocyte-specific ablation of p110α, a catalytic subunit of PI 3-kinase, decreases BAT UCP1 expression, BAT thermogenesis, and energy expenditure, and promotes obesity, glucose intolerance, and liver steatosis in mice (219). Insulin activates TORC1 via the PI 3-kinase/Akt pathway. Importantly, adipocyte-specific ablation of raptor, an essential component of the TORC1 complex, inhibits β adrenergic-stimulated beige adipogenesis in mice (306). Thus, the IRS1/PI 3-kinase/Akt/TORC1 pathway appears to be indispensable for insulin to promote BAT and beige fat function. TORC1 phosphorylates and inhibits 4E-BP1. Deletion of 4E-BP1 increases beige adipogenesis and metabolic rates and decreases adiposity in mice (309), indicating that 4E-BP1 is a negative regulator of beige fat. It is likely that the insulin/IRS1/PI 3-kinase/Akt/TORC1 pathway disinhibits BAT and/or beige fat by suppressing 4E-BP1. Aside from cell-autonomously promoting brown and beige adipocyte function, insulin is also able to stimulate BAT and beige fat thermogenesis indirectly by regulating hypothalamic circuits (33, 68).
Glucagon.
Deletion of glucagon impairs cold adaptive thermogenesis in mice, suggesting that glucagon promotes BAT thermogenesis in mice (155). (155). Glucagon stimulates FGF21 secretion from the liver in both rodents and humans (10, 112). FGF21 is known to stimulate BAT and beige fat thermogenesis (87, 128), which may be involved in mediating glucagon-stmulated thermogenesis. Notably, deletion of the glucagon gene also abolishes expression of glucagon-like peptide 1 (GLP-1) and GLP-2, (155), and the relative contributions of glucagon, GLP-1, and GLP-2 to BAT and beige fat thermogenesis remain unclear.
Glucocorticoids.
Cold stress activates the hypothalamic-pituitary-adrenal axis, increasing plasma ACTH and corticosterone levels (316). BAT expresses glucocorticoid receptors (84), suggesting that brown adipocytes are regulated directly by stress hormones. Notably, glucocorticoids have the opposite effects on brown and beige adipocytes between rodents and humans. Corticosterone inhibits UCP1 expression in mouse brown adipocyte cultures (316), and a chronic corticosterone treatment inhibits brown/beige fat activity in cold-exposed mice (315). In contrast, glucocorticoids enhance the ability of isoprenaline to stimulate UCP1 expression and mitochondrial respiration in human primary brown adipocyte cultures, and glucocorticoid treatment increases BAT activity in healthy men (252).
Liver-secreted regulators.
The liver is the main source of circulating fibroblast growth factor (FGF) 21 (13, 135). Plasma FGF21 levels correlate with BAT activity in humans (116). FGF21 treatment stimulates BAT and beige fat thermogenesis (74, 128). Conversely, deletion of FGF21 impairs beige adipogenesis and cold adaptive thermogenesis in mice (87). Protein restriction increases energy expenditure in a FGF21-dependent manner in mice (167). Of notice, adipose tissue and skeletal muscle also secrete FGF21 that enhances BAT and beige fat thermogenesis (87, 149, 174). The liver synthesizes bile acids exclusively by hepatocytes. Bile acids increase BAT thermogenesis by activating TGR5 receptors in brown and beige adipocytes (333). Additionally, bile acids also stimulate the GI tract to secrete FGF15/19 (134). FGF15/19 promotes BAT and beige thermogenesis as discussed below. The liver oxidize FFAs to produce ketone bodies, and ketone bodies (e.g. β-hydroxybutyrate) are able to stimulate browning of WAT and BAT/beige fat thermogenesis (39, 286).
Skeletal and cardiac muscle-secreted factors.
Exercise or cold-stimulated shivering stimulates skeletal muscle to release a number of endocrine factors that promote BAT and beige fat activities. Exercise or cold-induced shivering stimulates expression of irisin (derived from FNDC5 mRNA) in skeletal muscle (29, 174, 338). Irisin is able to stimulate BAT thermogenic activity in mice (29, 338). However, it was also reported that exercise does not increase FNDC5 expression, and recombinant irisin does not promote human brown adipocyte differentiation (254). Exercise or cold exposure was reported to stimulate skeletal muscle and adipose tissue to secrete meteorin-like (Metrnl) that stimulates beige fat thermogenesis (253). Surprisingly, overexpression of Metrnl (driven by the aP2 promoter) does not increase beige adipogenesis in Metrnl transgenic mice (182). Skeletal muscle secretes IL6 that enhances beige fat activity, and deletion of IL6 blocks exercise-induced beige adipogenesis (158). Cold exposure increases the circulating levels of cardiac natriuretic peptides that stimulate brown adipocyte mitochondrial biogenesis and BAT thermogenesis through activating the p38 MAPK pathway in brown adipocytes (28).
Exercise was reported to increase brown precursor numbers and BAT thermogenesis in mice (29, 344); however, chroninc exercise was also reported to decrease UCP1 and PGC1α expression and BAT thermogenesis in rats (340). But prolonged exercise training increases browing of WAT and biege fat thermongenesis in both mice and rats (158, 340).
GI-secreted regulators.
The GI tract is like to be involved in mediating diet-induced thermogenesis. It secretes various satiety factors that regulate BAT and beige fat activities via the vagal-brain axis as described above. Small intestines secrete FGF15/19 in the postprandial period (134), and food ingestion increases FGF15/19 secretion and circulating FGF15/19 levels (241). Transgenic overexpression of FGF15/19 increases BAT mass and energy expenditure (302). The GI tract is an important source of circulating serotonin, and fasting increases serotonin secretion from the GI tract (291). Serotonin directly suppresses brown and beige adipocyte activation (54), and pharmacological inhibition of serotonin synthesis induces beige adipogenesis and enhance BAT and beige fat thermogenesis in mice (54, 227). Circulating serotonin levels are lower in the fed state and markedly increase during chronic fasting (291), which may help explain, at least in part, diet-induced thermogenesis. Notably, depletion of gut microbiota increases browning of WAT in both lean and obese mice, leading to improved glucose tolerance and insulin sensitivity (290). These findings suggest that the gut/microbiota/beige fat axis exists and modulates energy and glucose homeostasis.
Renal factors.
Erythropoietin (EPO) is secreted mainly by the kidney and promotes beige adipogenesis (328). Adipocyte-specific deletion of EPO receptors decreases energy expenditure, thereby exacebating HFD-induced obesity and insulin resistance (328). EPO receptors activate the JAK/STAT3 pathway. Interestingly, BAT thermogenesis is impaired in mice with Tyk2-deficiency, and adipocyte-specific expression of constitutively active STAT3 reverts impairment in BAT of Tyk2 null mice (65). Tyk2 dimerizes with JAK1 or JAK2 that in turn phosphorylates and activates STAT3 to promote brown adipogenesis and BAT thermogenesis in mice (251). Thus, EPO stimulates BAT and beige fat thermogenesis, likely by activating the JAK/STAT3 pathway in brown and beige adipose lineage cells. It is worth mentioning that the JAK/STAT3 pathway is also activated by IL6 and other cytokines, raising the possibility that the JAK/STAT3 pathway in BAT and beige fat may also mediate the thermal action of these cytokines.
Paracrine/autocrine factors
Bone morphogenetic proteins (BMPs) and related cytokines.
Both brown preadipocytes and adipocytes express BMP4 and BMP7 (110, 236), and cold exposure or HFD feeding also stimulates expression of BMP8B in BAT (334). BMP4, BMP7, and BMP8B all promote brown and beige adipogenesis in mice (27, 110, 244, 308, 334). BMP7 also stimulates fatty acid uptake into brown adipocytes (304). BMP4 and BMP7 bind to BMPR1A receptors and stimulate the Smad1/5/8 pathway, which mediates their stimulation of brown and beige adipogenesis (271, 308). In contrast, myostatin and TGFβ1, two BMP-related cytokines, inhibit BAT and beige fat function, presumably by activating the ActRIIB/Alk4/5/Smad2/3 pathway (89, 347, 360).
Vasculature-derived factors.
Cold exposure or β adrenergic agonist stimulation induces angiogenesis in both WAT and BAT (277, 346), facilitating fuel delivery, heat dissipation, and thermogenesis. VEGF-A is a key angiogenic growth factor, and overexpression of VEGF-A either in all adipocytes or in UCP1+ brown and beige adipocytes increases adipose vascularization, beige adipogenesis, and BAT and beige fat thermogenesis in mice (73, 293, 294). Conversely, inhibition of adipose angiogenesis by deleting endothelial VEGFR2 impairs β adrenergic-stimulated beige adipogenesis and nonshivering thermogenesis (277, 346). A reduction in vascularization and blood flow is expected to induce hypoxia and activation of HIF1α, and adipocyte-specific ablation of HIF1α or its binding partner ARNT increases energy expenditure in mice (141, 163), suggesting that the hypoxia-HIF1α axis suppresses the recruitments and activities of BAT and/or beige fat.
Endothelial cells are known to sythesize nitric oxide (NO), and NO is able to promote mitochondrial biogenesis in brown adipocytes (221). NO activates soluble guanylyl cyclase (sGC) that catalyzes cGMP synthesis. Genetic ablation of the β1 subunit of sGC impairs BAT thermogenesis in mice (124). cGMP stimulates protein kinase G (PKG). Activation of the cGMP/PKG pathway promotes brown adipogenesis as well as mitochondrial biogenesis and UCP1 expression in brown adipocytes (111, 124, 207). Thus, the endothelial cell/NO/sGC/cGMP/PKG axis may exist and promote BAT and/or beige fat thermogenesis.
Lipid regulators.
Cold exposure or β adrenergic agonist stimulation increases expression of cyclooyxgenate 2 (COX2), the rate-limiting enzyme of the prostaglandin synthesis pathway, in adipose tissue (318). Pharmacological inhibition of COX2 or genetic deletion of COX2 attenuates BAT and beige fat thermogenesis stimulated by either cold exposure or β adrenergic agonist stimulation, and attenuates HFD-induced obesity (199, 318). In line with these findings, adipose PGE2 directly stimulates beige adipogenesis by stimulating EP4 signaling (199). Additionally, central PGE2 acts as a pyrogenic mediator to stimulate hypothalamic thermal circuits via ER3, inducing fever (311).
PDGF-CC and Slit2.
Both brown and beige precursor cells express PDGFRα as discussed above. PDGF-CC is able to stimulate both PDGFRα homodimers and PDGFα/β heterodimers, and it also stimulates differentiation of PDGFRα+ progenitors into beige adipocytes (277). In line with this finding, deletion of PDGF-C, or pharmacological blockage of PDGFRα or PDGRβ, inhibits β adrenergic-stimulated beige adipogenesis in mice (277). However, activation of PDGFRα signaling was also reported to stimulate differentiation of adipose mesenchymal progenitors into fibrotic cells at the expense of suppressing adipogenesis (138). Interestingly, a recently study reported an identification of PDGFRα+CD9high and PDGFRα+CD9low subsets in adipose tissue (201). The PDGFRα+CD9high mesenchymal progenitors differentiate into fibrogenic cells, whereas the PDGFRα+CD9low subset differentiate to adipocytes (201). Thus, the relative abundance of these two subpopulations is likely to a determinant for the outcome of adipose PDGF signaling.
Adipose extracellular matrix (ECM) also influences beige adipogenesis. Adipocytes express and secrete Slit2, an ECM protein (297). A C-terminal fragment of Slit2 is generated endogenously and promotes beige fat recruitment and thermogenesis in mice, presumably by activating the PKA pathway (297).
Wnt, Notch, and hedgehog signaling.
Wnt is well known to suppress white adipocyte differentiation (260). Similarly, overexpression of Wnt10b in UCP1+ cells inhibits BAT activity and blocks beige fat development in Wnt10b transgenic mice (146). Conversely, blocking Wnt signaling enhances differentiation of inguinal preadipocytes into beige adipocyte (187). Notch or hedgehog signaling, as Wnt signaling, also inhibits brown and beige adipogenesis (24, 224).
Diets and metabolic states.
Food intake is well known to increase nonshivering thermogenesis in rodents and humans (262, 270). Ingestion of energy dense food (e.g. HFD) further increases BAT and beige fat thermogenesis (340). Perhaps, diet-induced thermogenesis is a physiological adaptation to combat weight gain and obesity. Notably, dietary constituents greatly influence BAT and beige fat activity. Feeding a ketone ester diet promotes UCP1 expression and mitochondrial biogenesis in BAT, increasing BAT thermogenesis in mice (286). Feeding protein-restricted diets also increases energy expenditure in mice (167). Lactate acts in concert with PPARγ agonists to promote browning of WAT in mice (39).
AMPK is believed to be an intracellular energy sensor to monitor metabolic states. Basal AMPK activity in BAT is high and further increases by chronic cold exposure in mice (215). Prolonged activation of AMPK, by AICAR treatment, induces browning of WAT and body weight loss in mice (319). AMPK is inhibited by folliculin (11), and adipocyte-specific deletion of folliculin increases AMPK activity, browning of WAT, energy expenditure, and protects against HFD-induced obesity and insulin resistance in mice (350). Adipocyte specific deletion of Ip6k1 also increases adipose AMKP activity, browning of WAT, and energy expenditure, and attenuates HFD-induced obesity in mice (364). α-ketoglutarate (αKG) is a TET demethylase cofactor and promotes active DNA methylation at the Prdm16 promoter (351). AMPKα1 increases αKG levels, likely through downregulating IDH2, thereby stimulating Prdm16 expression and brown adipogenesis (351). In line with these findings, adipocyte-specific deletion of both AMPKβ1and AMPKβ2 impairs both BAT thermogenesis and browning of WAT, and exacerbates HFD-induced insulin resistance and liver steatosis (213). Surprisingly, adipocyte-specific deletion of both AMPKα1 and AMPKα2 increases FFA β oxidation and energy expenditure and decreases adiposity in mice (154). The reason for the discrepancy between mice with AMPKβ1/β2 deficiency and mice with AMPKα1/α2 deficiency remains elusive.
REGULATION OF BROWN AND BEIGE FAT THERMOGENESIS BY THE IMMUNE SYSTEM
Metabolic inflammation has been extensively examined in the context of overnutrition states. Chronic, low-grade adipose inflammation is associated with obesity, and is believed to induce insulin resistance (129). Numerous innate immune cells are recruited into WAT where they orchestrate metabolic inflammation (also referred to as metainflammation or sterile inflammation) in obesity (95, 129, 193, 231). It is emerging that immune cells, particularly innate immune cells, play an important role in de novo beige adipogenesis and beige fat thermogenesis. However, metainflammation in BAT remains poorly understood.
Macrophages.
In WAT, M1-polarized macrophages are believed to promote insulin resistance while M2-polarized macrophages counteract the action of M1 macrophages and improve insulin sensitivity (95, 129, 193, 231). Similarly, M1 and M2 macrophages appear to have opposing actions in BAT and/or beige fat. Clodronate liposome-induced depletion of M1 macrophages increases UCP1 expression in BAT and beige fat in mice with HFD-induced obesity (266). M1 macrophages secrete a variety of proinflammatory cytokines and chemokines, including TNFα and IL1β. IL1β directly inhibits brown adipocyte differentiation in vitro (214). TNFα suppresses expression of β3 receptors in mouse adipocytes, thus attenuating β adrenergic signaling (21), and TNFα treatment attenuates cold-induced UCP1 expression in both BAT and iWAT in C57BL mice (265). A chronic treatment with a low dose of lipopolysaccharides (LPS), which stimulate M1 polarization, impairs beige adipogenesis and induces hypothermia in C57BL mice (230). LPS also suppresses BAT activity via Toll-like receptor 4 (TLR4) (214, 230). In stark contrast, M2 macrophages, which are detected in BAT and increase during cold exposure, stimulate BAT thermogenesis in mice by releasing catecholamines (220). Genetically blocking M2 polarization (deleting IL4/13, IL4Rα, or STAT6) impairs cold-induced beige adipogenesis in mice (246). We recently found that the IRE1α branch of unfold protein response (UPR) profoundly influences M1 vs M2 polarization and BAT and beige fat thermogenesis. Myeloid cell-specific deletion of IRE1α promotes M2 polarization while suppressing M1 macrophages in mice with diet-induced obesity, increases BAT and beige fat thermogenesis, and protects against diet-induced obesity and insulin resistance (280).
Eosinophils.
Cold exposure promotes recruitment of eosinophils into mouse adipose tissue (253). Eosinophils express IL4 that stimulates M2 polarization of macrophages by activating the STAT6 pathway (337). Depletion of eosinophils impairs cold-induced beige adipogenesis and adaptive thermogenesis through blocking M2 polarization (246). Additionally, IL4 is also believed to dirctly stimulate PDGFRα+ precursors and induce beige adipogenesis by activating the IL4Rα/STAT6 pathway (173). Notably, chronic caloric restriction, which is known to improve metabolic health conditions and extend lifespan, increases beige adipogenesis in mice, accompanied by increased eosinophil infiltration, type 2 cytokine signaling, and M2 macrophage polarization in WAT (78). Deletion of IL4 or STAT6 prevents caloric restriction-induced browning of WAT and metabolic improvements (78). Interestingly, depletion of gut microbiota, by either antibiotic treatment or housing at germ-free conditions, increases adipose eosinophils, M2 macrophages, and expression of type 2 cytokines (e.g. IL4, IL5, IL13), leading to an increase in browning of WAT and improvement in glucose tolerance and insulin resistance in obese mice (290). These findings raise the intriguing possibility that gut microbiota may modulate energy balance and glucose metabolism through the immune system-BAT/beige fat axis.
Group 2 Innate lymphoid cells (ILC2s).
ILC2s are detected in adipose tissue (32), and activation of adipose ILC2s robustly promotes beige adipogenesis in mice (32, 173). ILC2s secrete IL5 and IL13 that stimulate eosinophils (173). Eosinophils release IL4 that is able to stimulate proliferation and comitment of PDGFRα+ precursors to beige adipocyte lineage as discussed above. The ILC2/eosinophil/IL4 axis is proposed to explain stimulation of de novo beige adipogenesis by ILC2s (173); however, it was also reported that ILC2s elicit beige adipogenesis by an eosinophil- and IL4Rα-independent mechanism (32). ILC2s secrete methionine-enkephalin peptides that are responsible for recruitment of beige fat in mice (32). The reason for the discrepancy between these studies remains unclear. Of notice, adipose ILC2s decrease in obese mice and obese humans (32), which may explain, at least in part, impaired beige adipogenesis in obesity.
iNKT cells.
Adipose tissue contains iNKT cells, and activation of iNKT by αGalCer increases beige adipogenesis and energy expenditure and decreases adiposity in obese mice (194). Adipose iNKT number is lower in obese mice and obese humans relative to lean controls (195), which may also contribute to impaired beige adipogenesis in obesity.
Mast cells.
Mast cells secrete histamine and IL4, both of which are able to stimulate browning of WAT (86). Mast cells are likely to be involved in mediating seasonal beige adipogenesis in the winter in humans (86).
IL33.
IL33 is secreted by a variety of cell types, including fibroblasts, immune cells, endothelial cells, and epithelial cells (206). Deletion of IL33 impairs beige adipogenesis, decreases energy expenditure, and promotes obesity in mice (32). IL33 activates adipose ILC2s which in turn stimulate beige adipogenesis (32, 173). IL33 also directly regulates the behavior of brown and beige lineage cells (225). IL33 signaling appears to be required for UCP1 mRNA splicing, and deletion of IL33 or its receptor ST2 blocks UCP1 protein synthesis in both brown and beige adipocytes (225).
TNFα and IL1β.
These two cytokines serve as key pyrogens in infection, and induce fever through activating the hypothalamic thermal circuits (211, 311).
BROWN AND BEIGE FAT DYSFUNCTION IN METABOLIC DISEASES
Mounting evidence suggests that deficiency and dysfunction of BAT and beige fat increase susceptibility to obesity and metabolic diseases. In mice with obesity, activation of BAT and beige fat, by genetic manipulation, cold exposure, or pharmacological interventions, substantially improves metabolic health conditions, including reducing body weight, insulin resistance, and glucose intolerance. Cold exposure or pharmacological interventions similarly activate brown/beige adipocytes and improve metabolic health in some patients with obesity and metabolic disease (117, 232, 317, 355). It is worth mentioning that the same treatments do not have the metabolic beneficial effects on other patients (150), and the underlying reason for the discrepancy between different individuals remains elusive. Interestingly, cold exposure increases energy expenditure, whole body glucose disposal, fatty acid β oxidation, and insulin sensitivity in BAT-positive, but not BAT-negative, individuals (46). These findings further underscore the metabolic importance of BAT in humans.
Obesity.
The anti-obesity and anti-type 2 diabetes functions of BAT and beige fat are convincingly demonstrated by the findings that genetic ablation of UCP1+ brown and beige adipocytes induces severe obesity, insulin resistance, and hyperglycemia in mice (192). In humans, obesity is associated with lower levels of brown/beige fat activity (25, 57, 317), further supporting the notion that brown/beige fat deficiency and dysfunction are a risk factor for obesity and metabolic disorders.
Protection against obesity by UCP1-dependent and UCP1-independent energy expenditure.
Adipocyte-specific overexpression of UCP1 decreases fat mass in aP2-UCP1 transgenic mice (161). These findings suggest that activation of UCP1-mediated thermogenesis in fat is sufficient to protect against obesity. However, ablation of UCP1, unlike ablation of BAT and beige fat, does not induce obesity and insulin resistance in mice (75, 137). Notably, UCP1−null mice develop more severe obesity relative to wild type mice when housed at thermoneutrality (85). As discussed before, UCP1−null mice develop UCP1-independent thermogenesis that compensates for loss of UCP1 and protects against obesity (185, 313). UCP1-independent thermogenesis is inactive at thermoneutrality, which helps explain why UCP1−null mice develop obesity at thermoneutrality but not in cold-stressed conditions. Thus, defects in both UCP1-dependnent and UCP-independent thermogenesis are likely to pose a risk for obesity and metabolic diseases.
BAT vs beige fat.
In rodents, inactivation of BAT is known to robustly induce a compensatory recruitment of beige fat. BAT atrophy, induced by BAT denervation, potently induces beige adipogenesis in mice (271). Deletion of BMPR1A or insulin receptors in Myf5+ brown progenitor cells impairs BAT development; similarly, BAT paucity markedly increases beige adipogenesis and beige fat recruitment in these mutant mice (196, 271). Beige adipocytes are believed to provide equal or even better metabolic health benefits in BAT-deficient animals (271).
Human BAT was reported to express beige markers (338); however, an independent study suggests that BAT in adult humans resembles classical BAT in rodents (58). Gene expression profile analysis reveals that in adult humans, BAT consists of both classical brown adipocytes and recruitable beige adipocytes (140). Furthermore, beige progenitors was also identified in human skeletal muscle in addition to adipose tissue (55).
Aging-associated dysfunction BAT and beige fat.
BAT activity progressively declines during aging in rodents and humans (100, 256). Aging is also associated with loss of beige regenerative capacity and beige adipocytes (100), and beige adipogenic capability markedly declines in aged mice (257).
Multiple mechanisms are likely to attribute to aging-associated deficiency of BAT and beige fat. Aged beige progenitors display characteristics of cellular senescence in both mice and humans, and beige progenitor senescence contributes to failure of beige adipogenesis in old mice (22). Aging is associated with decreased sympathetic inputs to subcutaneous adipose tissue (257), likely decreasing beige thermogenesis. Furthermore, β adrenergic sensitivivity in WAT and BAT is also impaired in mice with HFD-induced obesity (52). In humans, BAT develop resistance to sympathomimetic ephedrine in obesity (37).
Diabetes.
Obesity is a primary risk factor for insulin resistance and type 2 diabetes. Given that BAT and beige fat have anti-obesity function, it is not surprising that they also protect against insulin resistance and type 2 diabetes. Indeed, cold acclimation, which activate BAT and beige fat as discussed before, was reported to improve insulin sensitivity in some patients with type 2 diabetes (117). BAT mass and activity are lower in patients with type 2 diabetes relative to normal subjects (25), raising the possibility that deficiency of BAT and beige fat may increase susceptibility to insulin resistance and type 2 diabetes. Notably, cold exposure increases whole body glucose disposal and insulin sensitivity only in brown/beige adipocyte-positive men but not in brown/beige adipocyte-deficient men (46). These observations further demonstrate that activation of BAT and beige fat indeed improves insulin resistance and glucose metabolism in some patients with diabetes. Notably, adoptive transplantation of BAT also reverts hyperglycemia and glucose intolerance in recipient mice with type 1 diabetes (106). Thus, BAT and beige fat combats hyperglycemia and glucose intolerance by both insulin-dependent and insulin-independent mechanisms. However, the mechanism by which BAT and beige fat improve glucose metabolism in the absence of insulin remains elusive.
Hyperlipidemia.
Ablation of BAT and beige fat results in hyperlipidemia in both male and female mice (192), indicating that BAT and beige fat play a critical role in maintaining TAG homeostasis in the circulation. Mechanistically, brown and beige adipocytes robustly catabolize TAG-rich lipoproteins in the blood. Cold exposure or β3 adrenergic agonist stimulation increases expression of LDL while suppressing ANGPTL4 (a LPL inhibitor) in BAT, thereby increasing LPL-mediated catabolism of TAG-rich lipoproteins to produce FFAs and LDL remnants (17, 20, 66). Brown and beige adipocytes take up FFAs via CD36 and oxidize them to fuel thermogenesis, while the liver clears plasma LDL remnants (17, 20). Notably, in ApoE−null or Ldlr−null mice that have impaired liver clearance of LDL remnants, cold exposure accelerates atherosclerotic lesions (70). Thus, BAT and beige fat act in concert with the liver to protect against atherosclerosis.
NAFLD.
Given that obesity is a critical risk factor for NAFLD, BAT and beige fat are expected to protect against NAFLD owing to their anti-obesity action. Moreover, BAT and beige fat primarily utilize FFAs to fuel their thermogenesis; hence, activation of BAT and beige fat are expected to decrease both circulating FFA levels and uptake of FFAs into the liver, thereby protecting against liver steatosis. In line with this notion, activation of BAT and beige fat protects against NAFLD in mice, whereas inhibition of BAT and beige fat has the opposite effect (99, 114, 195, 219, 281). Notably, BAT activity is associated with reduced risk for NAFLD (233, 353), suggesting that BAT activation similarly protects against NAFLD in humans. Furthermore, BAT volumes correlate with cold-stimulated lipolysis and FFA oxidation in humans (47), supporting the notion that human BAT and beige fat are also important consumers of circulating FFAs. Thus, the anti-liver steatosis function of BAT and beige fat is likely to be conserved from rodents to humans.
Cancer-associated cachexia.
Cancer is associated with increased beige adipogenesis and energy expenditure, contributing to weight loss (156, 240). Tumor cells secrete parathyroid-hormone related protein (PTHrP), and PTHrP promotes beige adipogenesis and beige fat thermogenesis (156). Cancer is associated with chronic inflammation and elevation of IL6, and IL6 stimulates beige adipogenesis and BAT and beige fat thermogenesis, contributing to cancer-associated cachexia (240).
BAT and beige fat as potential therapeutic targets.
Obesity and metabolic disease are becoming global epidemics; however, treatment options are limited. Recent advances in our understanding of brown and beige adipocytes raise the possiblity that BAT and beige fat may serve as potential therapeutic targets for teating obesity and/or metabolic disorders.
Target brown/beige adipocyte mitochondia.
Mitochondrial respiration (both UCP1- dependent and UCP1-independent) is responsible for thermogenesis. Therefore, mitchondrial biogenesis, dynamics, quality control, and/or metabolic pathways in brown and beige adipocytes may serve as potential targets.
Target brown/beige adipocyte lipid droplets
Lipid droplet lipolysis releases FFAs that directly fuel mitochondrial respiration. FFAs also activate UCP1 and stimulate the genetic programs to further increase thermogenesis in brown and beige adipocytes as discussed before. Thus, molecular regulators, which govern lipolysis, lipid droplet replenishment, and lipid droplet dynamics, may serve as potential targets.
Target reprograming of adipocytes.
It is increasingly recognized that white-to-brown adipocyte transdifferentiations occur under certain conditions (71, 275, 281, 289). Given a large WAT mass in the body, a white-to-brown adipocyte conversion is expected to provide a considerable resource for brown/beige adipocyte recruitments. However, the underlying molecular mechanism of white-to-brown adipocyte transdifferentiations remain elusive, limiting our ability to explore this approach in the translational settings.
Target de novo brown and beige adipogenesis.
Obesity and aging are associated with a decline in the capacity of de novo brown and beige adipogenesis. Brown and beige precursor senescence contributes to reduced beige fat regenerative capability under these conditions as discussed before. Therefore, inhibiting brown and/or beige precursor senescence, preserving brown and/or beige precursor pool size, and facilitating brown and beige adipocyte differentiation are expected to improve metabolic health.
Target adipose β adrenergic signaling.
Chronic β3 adrenergic agonist treatment attenuates HFD-induced obesity in mice (52, 137). Increasing β adrenergic sensitivity, using adipocyte-specific overexpression of FoxC2 (increasing PKA RIα), induces beige adipogenesis and protects FoxC2 transgenic mice from HFD-induced obesity and insulin resistance (40, 152). These findings provide proof of concept evidence for the notion that β adrenergic agonists and/or sensitizers may have translatioal significance in treating obesity and/or metaoblic disorders.
Behavior interventions.
A chronic cold exposure activates brown/beige fat, promoting weight loss and improving insulin sensitivity in both rodents and humans (56, 117, 166, 355). Exercise increases beige adipogenesis in rodents (29, 340, 344). Caloric restriction also increases beige adipogenesis (78). It is worth mentioning that genetic variations may influence the metabolic outcomes of these behavior interventions.
CONCLUSIONS
Albeit they develop from different cellular origins, brown and beige adipocytes display similar thermogenic characteristics, are controlled by similar genetic and epigamic programs, and are regulated by similar neuronal, hormonal, and immune mechanisms. They similarly defend body temperature homeostasis and regulate energy, glucose, and lipid homeostasis. It is emerging that dysfunctions of BAT and beige fat development, maintenance, and/or activation contribute to metabolic disorders, at least in a subpopulation. As such, reactivation of brown and beige adipocytes provides metabolic health benefits in some patients with obesity or type 2 diabetes. We have made a tremendous progress in the understanding of BAT and beige fat biology; however, we are also facing exciting challenging. The nature and the relative contribution of UCP1-independent thermogenesis to energy balance remain elusive. The molecular identities of brown and beige progenitor/stem cells, the chemical niches for brown and beige progenitor/stem cells, and the genetic and epigenetic programs governing individual differentiation steps, are ill-defined. The molecular events that determine brown and beige precursor stemness, regulate brown and beige precursor senescence, preserve brown and beige precursor pool size, and trigger their proliferation and differentiation are poorly understood. The molecular and cellular processes that guide transdifferentiation between white and brown adipocytes are largely unknown. It remains to be determined if and how beige adipocytes, which are intertwined with white adipocytes, influence WAT metabolic and immune homeostasis. We need to answer these exciting questions in order to achieve the goal of targeting BAT and beige fat for obesity, diabetes, and/or NAFLD treatments.
DIDACTIC SYNOPSIS.
Major teaching points:
Understanding of the development, maintenance, and activation of brown fat and beige fat is critical to appreciate body temperature homeostasis, energy balance, and body weight control.
Understanding of the thermogenic machinery is necessary to explain the metabolic function of brown fat and beige fat.
Understanding of neuronal, humoral, and immune regulation of brown fat and beige fat is important to appreciate the role of brown fat and beige fat in obesity and metabolic disease development.
Understanding of adipose signaling pathways, genetic programs, and epigenetic reprograming is critical to appreciate brown and beige adipogenesis.
Understanding of human brown/beige fat characteristics is important to guide development of new obesity and metabolic disease therapies.
CROSS-REFERENCES (5 CHP).
Beige adipose tissue in health and disease
Central nervous system regulation of brown adipose tissue
Contribution of adipose tissue to development of diabetes
Hormonal regulation of adipogenesis
Innate immune system in adipose tissue
ACKNOWLEDGMENTS
This work was supported by grants DK091591 and DK094014 from the National Institutes of Health.
REFERENCES
- 1.Abe Y, Rozqie R, Matsumura Y, Kawamura T, Nakaki R, Tsurutani Y, Tanimura-Inagaki K, Shiono A, Magoori K, Nakamura K, Ogi S, Kajimura S, Kimura H, Tanaka T, Fukami K, Osborne TF, Kodama T, Aburatani H, Inagaki T, and Sakai J. JMJD1A is a signal-sensing scaffold that regulates acute chromatin dynamics via SWI/SNF association for thermogenesis. Nat Commun 6: 7052, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmadian M, Abbott MJ, Tang T, Hudak CS, Kim Y, Bruss M, Hellerstein MK, Lee HY, Samuel VT, Shulman GI, Wang Y, Duncan RE, Kang C, and Sul HS. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab 13: 739–748, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmadian M, Duncan RE, Varady KA, Frasson D, Hellerstein MK, Birkenfeld AL, Samuel VT, Shulman GI, Wang Y, Kang C, and Sul HS. Adipose overexpression of desnutrin promotes fatty acid use and attenuates diet-induced obesity. Diabetes 58: 855–866, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almind K, Manieri M, Sivitz WI, Cinti S, and Kahn CR. Ectopic brown adipose tissue in muscle provides a mechanism for differences in risk of metabolic syndrome in mice. Proc Natl Acad Sci U S A 104: 2366–2371, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altshuler-Keylin S, Shinoda K, Hasegawa Y, Ikeda K, Hong H, Kang Q, Yang Y, Perera RM, Debnath J, and Kajimura S. Beige Adipocyte Maintenance Is Regulated by Autophagy-Induced Mitochondrial Clearance. Cell Metab 24: 402–419, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.An JJ, Liao GY, Kinney CE, Sahibzada N, and Xu B. Discrete BDNF Neurons in the Paraventricular Hypothalamus Control Feeding and Energy Expenditure. Cell Metab 22: 175–188, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson CM, Kazantzis M, Wang J, Venkatraman S, Goncalves RL, Quinlan CL, Ng R, Jastroch M, Benjamin DI, Nie B, Herber C, Van AA, Park MJ, Yun D, Chan K, Yu A, Vuong P, Febbraio M, Nomura DK, Napoli JL, Brand MD, and Stahl A. Dependence of brown adipose tissue function on CD36-mediated coenzyme Q uptake. Cell Rep 10: 505–515, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anunciado-Koza RP, Zhang J, Ukropec J, Bajpeyi S, Koza RA, Rogers RC, Cefalu WT, Mynatt RL, and Kozak LP. Inactivation of the mitochondrial carrier SLC25A25 (ATP-Mg2+/Pi transporter) reduces physical endurance and metabolic efficiency in mice. The Journal of biological chemistry 286: 11659–11671, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aponte Y, Atasoy D, and Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci 14: 351–355, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arafat AM, Kaczmarek P, Skrzypski M, Pruszynska-Oszmalek E, Kolodziejski P, Szczepankiewicz D, Sassek M, Wojciechowicz T, Wiedenmann B, Pfeiffer AF, Nowak KW, and Strowski MZ. Glucagon increases circulating fibroblast growth factor 21 independently of endogenous insulin levels: a novel mechanism of glucagon-stimulated lipolysis? Diabetologia 56: 588–597, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Baba M, Hong SB, Sharma N, Warren MB, Nickerson ML, Iwamatsu A, Esposito D, Gillette WK, Hopkins RF 3rd, Hartley JL, Furihata M, Oishi S, Zhen W, Burke TR Jr., Linehan WM, Schmidt LS, and Zbar B. Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. Proc Natl Acad Sci U S A 103: 15552–15557, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK, and Lowell BB. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science 297: 843–845, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, and Maratos-Flier E. Hepatic Fibroblast Growth Factor 21 Is Regulated by PPARalpha and Is a Key Mediator of Hepatic Lipid Metabolism in Ketotic States. Cell Metab 5: 426–437, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, and Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123: 493–505, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, Giacobino JP, De Matteis R, and Cinti S. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab 298: E1244–1253, 2010. [DOI] [PubMed] [Google Scholar]
- 16.Barquissau V, Beuzelin D, Pisani DF, Beranger GE, Mairal A, Montagner A, Roussel B, Tavernier G, Marques MA, Moro C, Guillou H, Amri EZ, and Langin D. White-to-brite conversion in human adipocytes promotes metabolic reprogramming towards fatty acid anabolic and catabolic pathways. Mol Metab 5: 352–365, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, Eychmuller A, Gordts PL, Rinninger F, Bruegelmann K, Freund B, Nielsen P, Merkel M, and Heeren J. Brown adipose tissue activity controls triglyceride clearance. Nat Med 17: 200–205, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Bartesaghi S, Hallen S, Huang L, Svensson PA, Momo RA, Wallin S, Carlsson EK, Forslow A, Seale P, and Peng XR. Thermogenic activity of UCP1 in human white fat-derived beige adipocytes. Mol Endocrinol 29: 130–139, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beck V, Jaburek M, Demina T, Rupprecht A, Porter RK, Jezek P, and Pohl EE. Polyunsaturated fatty acids activate human uncoupling proteins 1 and 2 in planar lipid bilayers. FASEB J 21: 1137–1144, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Berbee JF, Boon MR, Khedoe PP, Bartelt A, Schlein C, Worthmann A, Kooijman S, Hoeke G, Mol IM, John C, Jung C, Vazirpanah N, Brouwers LP, Gordts PL, Esko JD, Hiemstra PS, Havekes LM, Scheja L, Heeren J, and Rensen PC. Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nat Commun 6: 6356, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berkowitz DE, Brown D, Lee KM, Emala C, Palmer D, An Y, and Breslow M. Endotoxin-induced alteration in the expression of leptin and beta3-adrenergic receptor in adipose tissue. Am J Physiol 274: E992–997, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Berry DC, Jiang Y, Arpke RW, Close EL, Uchida A, Reading D, Berglund ED, Kyba M, and Graff JM. Cellular Aging Contributes to Failure of Cold-Induced Beige Adipocyte Formation in Old Mice and Humans. Cell Metab 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berry DC, Jiang Y, and Graff JM. Mouse strains to study cold-inducible beige progenitors and beige adipocyte formation and function. Nat Commun 7: 10184, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bi P, Shan T, Liu W, Yue F, Yang X, Liang XR, Wang J, Li J, Carlesso N, Liu X, and Kuang S. Inhibition of Notch signaling promotes browning of white adipose tissue and ameliorates obesity. Nat Med 20: 911–918, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blondin DP, Labbe SM, Noll C, Kunach M, Phoenix S, Guerin B, Turcotte EE, Haman F, Richard D, and Carpentier AC. Selective Impairment of Glucose but Not Fatty Acid or Oxidative Metabolism in Brown Adipose Tissue of Subjects With Type 2 Diabetes. Diabetes 64: 2388–2397, 2015. [DOI] [PubMed] [Google Scholar]
- 26.Blouet C, and Schwartz GJ. Duodenal lipid sensing activates vagal afferents to regulate non-shivering brown fat thermogenesis in rats. PLoS One 7: e51898, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boon MR, van den Berg SA, Wang Y, van den Bossche J, Karkampouna S, Bauwens M, De Saint-Hubert M, van der Horst G, Vukicevic S, de Winther MP, Havekes LM, Jukema JW, Tamsma JT, van der Pluijm G, van Dijk KW, and Rensen PC. BMP7 activates brown adipose tissue and reduces diet-induced obesity only at subthermoneutrality. PLoS One 8: e74083, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang C, Takahashi N, Sarzani R, and Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest 122: 1022–1036, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, and Spiegelman BM. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481: 463–468, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boucher J, Softic S, El Ouaamari A, Krumpoch MT, Kleinridders A, Kulkarni RN, O’Neill BT, and Kahn CR. Differential Roles of Insulin and IGF-1 Receptors in Adipose Tissue Development and Function. Diabetes 65: 2201–2213, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brand MD, Pakay JL, Ocloo A, Kokoszka J, Wallace DC, Brookes PS, and Cornwall EJ. The basal proton conductance of mitochondria depends on adenine nucleotide translocase content. Biochem J 392: 353–362, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P, and Artis D. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 519: 242–246, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, and Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science 289: 2122–2125, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Cao W, Daniel KW, Robidoux J, Puigserver P, Medvedev AV, Bai X, Floering LM, Spiegelman BM, and Collins S. p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol Cell Biol 24: 3057–3067, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao W, Medvedev AV, Daniel KW, and Collins S. beta-Adrenergic activation of p38 MAP kinase in adipocytes: cAMP induction of the uncoupling protein 1 (UCP1) gene requires p38 MAP kinase. The Journal of biological chemistry 276: 27077–27082, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Cao WH, Madden CJ, and Morrison SF. Inhibition of brown adipose tissue thermogenesis by neurons in the ventrolateral medulla and in the nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol 299: R277–290, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carey AL, Formosa MF, Van Every B, Bertovic D, Eikelis N, Lambert GW, Kalff V, Duffy SJ, Cherk MH, and Kingwell BA. Ephedrine activates brown adipose tissue in lean but not obese humans. Diabetologia 56: 147–155, 2013. [DOI] [PubMed] [Google Scholar]
- 38.Carneheim C, Nedergaard J, and Cannon B. Beta-adrenergic stimulation of lipoprotein lipase in rat brown adipose tissue during acclimation to cold. Am J Physiol 246: E327–333, 1984. [DOI] [PubMed] [Google Scholar]
- 39.Carriere A, Jeanson Y, Berger-Muller S, Andre M, Chenouard V, Arnaud E, Barreau C, Walther R, Galinier A, Wdziekonski B, Villageois P, Louche K, Collas P, Moro C, Dani C, Villarroya F, and Casteilla L. Browning of White Adipose Cells by Intermediate Metabolites:An Adaptive Mechanism to Alleviate Redox Pressure. Diabetes 2014. [DOI] [PubMed] [Google Scholar]
- 40.Cederberg A, Gronning LM, Ahren B, Tasken K, Carlsson P, and Enerback S. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell 106: 563–573, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Cerri M, and Morrison SF. Corticotropin releasing factor increases in brown adipose tissue thermogenesis and heart rate through dorsomedial hypothalamus and medullary raphe pallidus. Neuroscience 140: 711–721, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Chang JS, Fernand V, Zhang Y, Shin J, Jun HJ, Joshi Y, and Gettys TW. NT-PGC-1alpha protein is sufficient to link beta3-adrenergic receptor activation to transcriptional and physiological components of adaptive thermogenesis. The Journal of biological chemistry 287: 9100–9111, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C, Zhang J, Wu J, Zeng R, and Chen YE. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-gamma deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation 126: 1067–1078, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chappuis S, Ripperger JA, Schnell A, Rando G, Jud C, Wahli W, and Albrecht U. Role of the circadian clock gene Per2 in adaptation to cold temperature. Mol Metab 2: 184–193, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Y, Siegel F, Kipschull S, Haas B, Frohlich H, Meister G, and Pfeifer A. miR-155 regulates differentiation of brown and beige adipocytes via a bistable circuit. Nat Commun 4: 1769, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chondronikola M, Volpi E, Borsheim E, Porter C, Annamalai P, Enerback S, Lidell ME, Saraf MK, Labbe SM, Hurren NM, Yfanti C, Chao T, Andersen CR, Cesani F, Hawkins H, and Sidossis LS. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 63: 4089–4099, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chondronikola M, Volpi E, Borsheim E, Porter C, Saraf MK, Annamalai P, Yfanti C, Chao T, Wong D, Shinoda K, Labbe SM, Hurren NM, Cesani F, Kajimura S, and Sidossis LS. Brown Adipose Tissue Activation Is Linked to Distinct Systemic Effects on Lipid Metabolism in Humans. Cell Metab 23: 1200–1206, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chouchani ET, Kazak L, Jedrychowski MP, Lu GZ, Erickson BK, Szpyt J, Pierce KA, Laznik-Bogoslavski D, Vetrivelan R, Clish CB, Robinson AJ, Gygi SP, and Spiegelman BM. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature 532: 112–116, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Christian M, Kiskinis E, Debevec D, Leonardsson G, White R, and Parker MG. RIP140-targeted repression of gene expression in adipocytes. Mol Cell Biol 25: 9383–9391, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LG, Clements M, Al-Qassab H, Heffron H, Xu AW, Speakman JR, Barsh GS, Viollet B, Vaulont S, Ashford ML, Carling D, and Withers DJ. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest 117: 2325–2336, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ, Lo JC, Zeng X, Ye L, Khandekar MJ, Wu J, Gunawardana SC, Banks AS, Camporez JP, Jurczak MJ, Kajimura S, Piston DW, Mathis D, Cinti S, Shulman GI, Seale P, and Spiegelman BM. Ablation of PRDM16 and Beige Adipose Causes Metabolic Dysfunction and a Subcutaneous to Visceral Fat Switch. Cell 156: 304–316, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Collins S, Daniel KW, Petro AE, and Surwit RS. Strain-specific response to beta 3-adrenergic receptor agonist treatment of diet-induced obesity in mice. Endocrinology 138: 405–413, 1997. [DOI] [PubMed] [Google Scholar]
- 53.Corradi A, Croci L, Broccoli V, Zecchini S, Previtali S, Wurst W, Amadio S, Maggi R, Quattrini A, and Consalez GG. Hypogonadotropic hypogonadism and peripheral neuropathy in Ebf2-null mice. Development 130: 401–410, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Crane JD, Palanivel R, Mottillo EP, Bujak AL, Wang H, Ford RJ, Collins A, Blumer RM, Fullerton MD, Yabut JM, Kim JJ, Ghia JE, Hamza SM, Morrison KM, Schertzer JD, Dyck JR, Khan WI, and Steinberg GR. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nat Med 21: 166–172, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crisan M, Casteilla L, Lehr L, Carmona M, Paoloni-Giacobino A, Yap S, Sun B, Leger B, Logar A, Penicaud L, Schrauwen P, Cameron-Smith D, Russell AP, Peault B, and Giacobino JP. A reservoir of brown adipocyte progenitors in human skeletal muscle. Stem Cells 26: 2425–2433, 2008. [DOI] [PubMed] [Google Scholar]
- 56.Cypess AM, Chen YC, Sze C, Wang K, English J, Chan O, Holman AR, Tal I, Palmer MR, Kolodny GM, and Kahn CR. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc Natl Acad Sci U S A 109: 10001–10005, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, and Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360: 1509–1517, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cypess AM, White AP, Vernochet C, Schulz TJ, Xue R, Sass CA, Huang TL, Roberts-Toler C, Weiner LS, Sze C, Chacko AT, Deschamps LN, Herder LM, Truchan N, Glasgow AL, Holman AR, Gavrila A, Hasselgren PO, Mori MA, Molla M, and Tseng YH. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med 19: 635–639, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dalen KT, Dahl T, Holter E, Arntsen B, Londos C, Sztalryd C, and Nebb HI. LSDP5 is a PAT protein specifically expressed in fatty acid oxidizing tissues. Biochimica et biophysica acta 1771: 210–227, 2007. [DOI] [PubMed] [Google Scholar]
- 60.Daquinag AC, Tseng C, Salameh A, Zhang Y, Amaya-Manzanares F, Dadbin A, Florez F, Xu Y, Tong Q, and Kolonin MG. Depletion of white adipocyte progenitors induces beige adipocyte differentiation and suppresses obesity development. Cell Death Differ 22: 351–363, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Jesus LA, Carvalho SD, Ribeiro MO, Schneider M, Kim SW, Harney JW, Larsen PR, and Bianco AC. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest 108: 1379–1385, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Jong JM, Larsson O, Cannon B, and Nedergaard J. A stringent validation of mouse adipose tissue identity markers. Am J Physiol Endocrinol Metab 308: E1085–1105, 2015. [DOI] [PubMed] [Google Scholar]
- 63.De Sousa M, Porras DP, Perry CG, Seale P, and Scime A. p107 is a crucial regulator for determining the adipocyte lineage fate choices of stem cells. Stem Cells 32: 1323–1336, 2014. [DOI] [PubMed] [Google Scholar]
- 64.Dempersmier J, Sambeat A, Gulyaeva O, Paul SM, Hudak CS, Raposo HF, Kwan HY, Kang C, Wong RH, and Sul HS. Cold-Inducible Zfp516 Activates UCP1 Transcription to Promote Browning of White Fat and Development of Brown Fat. Mol Cell 57: 235–246, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Derecka M, Gornicka A, Koralov SB, Szczepanek K, Morgan M, Raje V, Sisler J, Zhang Q, Otero D, Cichy J, Rajewsky K, Shimoda K, Poli V, Strobl B, Pellegrini S, Harris TE, Seale P, Russell AP, McAinch AJ, O’Brien PE, Keller SR, Croniger CM, Kordula T, and Larner AC. Tyk2 and stat3 regulate brown adipose tissue differentiation and obesity. Cell Metab 16: 814–824, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dijk W, Heine M, Vergnes L, Boon MR, Schaart G, Hesselink MK, Reue K, van Marken Lichtenbelt WD, Olivecrona G, Rensen PC, Heeren J, and Kersten S. ANGPTL4 mediates shuttling of lipid fuel to brown adipose tissue during sustained cold exposure. Elife 4: 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dixen K, Basse AL, Murholm M, Isidor MS, Hansen LH, Petersen MC, Madsen L, Petrovic N, Nedergaard J, Quistorff B, and Hansen JB. ERRgamma enhances UCP1 expression and fatty acid oxidation in brown adipocytes. Obesity (Silver Spring) 21: 516–524, 2013. [DOI] [PubMed] [Google Scholar]
- 68.Dodd GT, Decherf S, Loh K, Simonds SE, Wiede F, Balland E, Merry TL, Munzberg H, Zhang ZY, Kahn BB, Neel BG, Bence KK, Andrews ZB, Cowley MA, and Tiganis T. Leptin and Insulin Act on POMC Neurons to Promote the Browning of White Fat. Cell 160: 88–104, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dodd GT, Worth AA, Nunn N, Korpal AK, Bechtold DA, Allison MB, Myers MG Jr., Statnick MA, and Luckman SM. The thermogenic effect of leptin is dependent on a distinct population of prolactin-releasing Peptide neurons in the dorsomedial hypothalamus. Cell Metab 20: 639–649, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dong M, Yang X, Lim S, Cao Z, Honek J, Lu H, Zhang C, Seki T, Hosaka K, Wahlberg E, Yang J, Zhang L, Lanne T, Sun B, Li X, Liu Y, Zhang Y, and Cao Y. Cold exposure promotes atherosclerotic plaque growth and instability via UCP1-dependent lipolysis. Cell Metab 18: 118–129, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Duteil D, Metzger E, Willmann D, Karagianni P, Friedrichs N, Greschik H, Gunther T, Buettner R, Talianidis I, Metzger D, and Schule R. LSD1 promotes oxidative metabolism of white adipose tissue. Nat Commun 5: 4093, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Duteil D, Tosic M, Lausecker F, Nenseth HZ, Muller JM, Urban S, Willmann D, Petroll K, Messaddeq N, Arrigoni L, Manke T, Kornfeld JW, Bruning JC, Zagoriy V, Meret M, Dengjel J, Kanouni T, and Schule R. Lsd1 Ablation Triggers Metabolic Reprogramming of Brown Adipose Tissue. Cell Rep 17: 1008–1021, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Elias I, Franckhauser S, Ferre T, Vila L, Tafuro S, Munoz S, Roca C, Ramos D, Pujol A, Riu E, Ruberte J, and Bosch F. Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes 61: 1801–1813, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Emanuelli B, Vienberg SG, Smyth G, Cheng C, Stanford KI, Arumugam M, Michael MD, Adams AC, Kharitonenkov A, and Kahn CR. Interplay between FGF21 and insulin action in the liver regulates metabolism. J Clin Invest 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, and Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 387: 90–94, 1997. [DOI] [PubMed] [Google Scholar]
- 76.Enriori PJ, Sinnayah P, Simonds SE, Garcia Rudaz C, and Cowley MA. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J Neurosci 31: 12189–12197, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Entingh AJ, Taniguchi CM, and Kahn CR. Bi-directional regulation of brown fat adipogenesis by the insulin receptor. The Journal of biological chemistry 278: 33377–33383, 2003. [DOI] [PubMed] [Google Scholar]
- 78.Fabbiano S, Suarez-Zamorano N, Rigo D, Veyrat-Durebex C, Stevanovic Dokic A, Colin DJ, and Trajkovski M. Caloric Restriction Leads to Browning of White Adipose Tissue through Type 2 Immune Signaling. Cell Metab 24: 434–446, 2016. [DOI] [PubMed] [Google Scholar]
- 79.Fasshauer M, Klein J, Kriauciunas KM, Ueki K, Benito M, and Kahn CR. Essential role of insulin receptor substrate 1 in differentiation of brown adipocytes. Mol Cell Biol 21: 319–329, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fasshauer M, Klein J, Ueki K, Kriauciunas KM, Benito M, White MF, and Kahn CR. Essential role of insulin receptor substrate-2 in insulin stimulation of Glut4 translocation and glucose uptake in brown adipocytes. The Journal of biological chemistry 275: 25494–25501, 2000. [DOI] [PubMed] [Google Scholar]
- 81.Fedorenko A, Lishko PV, and Kirichok Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 151: 400–413, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fekete C, Kelly J, Mihaly E, Sarkar S, Rand WM, Legradi G, Emerson CH, and Lechan RM. Neuropeptide Y has a central inhibitory action on the hypothalamic-pituitary-thyroid axis. Endocrinology 142: 2606–2613, 2001. [DOI] [PubMed] [Google Scholar]
- 83.Fekete C, Legradi G, Mihaly E, Huang QH, Tatro JB, Rand WM, Emerson CH, and Lechan RM. alpha-Melanocyte-stimulating hormone is contained in nerve terminals innervating thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and prevents fasting-induced suppression of prothyrotropin-releasing hormone gene expression. J Neurosci 20: 1550–1558, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Feldman D Evidence that brown adipose tissue is a glucocorticoid target organ. Endocrinology 103: 2091–2097, 1978. [DOI] [PubMed] [Google Scholar]
- 85.Feldmann HM, Golozoubova V, Cannon B, and Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 9: 203–209, 2009. [DOI] [PubMed] [Google Scholar]
- 86.Finlin BS, Zhu B, Confides AL, Westgate PM, Harfmann BD, Dupont-Versteegden EE, and Kern PA. Mast Cells Promote Seasonal White Adipose Beiging in Humans. Diabetes 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, and Spiegelman BM. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev 26: 271–281, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fitzgibbons TP, Kogan S, Aouadi M, Hendricks GM, Straubhaar J, and Czech MP. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. Am J Physiol Heart Circ Physiol 301: H1425–1437, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fournier B, Murray B, Gutzwiller S, Marcaletti S, Marcellin D, Bergling S, Brachat S, Persohn E, Pierrel E, Bombard F, Hatakeyama S, Trendelenburg AU, Morvan F, Richardson B, Glass DJ, Lach-Trifilieff E, and Feige JN. Blockade of the activin receptor IIb activates functional brown adipogenesis and thermogenesis by inducing mitochondrial oxidative metabolism. Mol Cell Biol 32: 2871–2879, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gantner ML, Hazen BC, Conkright J, and Kralli A. GADD45gamma regulates the thermogenic capacity of brown adipose tissue. Proc Natl Acad Sci U S A 111: 11870–11875, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gburcik V, Cawthorn WP, Nedergaard J, Timmons JA, and Cannon B. An essential role for Tbx15 in the differentiation of brown and “brite” but not white adipocytes. Am J Physiol Endocrinol Metab 303: E1053–1060, 2012. [DOI] [PubMed] [Google Scholar]
- 92.Gerhart-Hines Z, Feng D, Emmett MJ, Everett LJ, Loro E, Briggs ER, Bugge A, Hou C, Ferrara C, Seale P, Pryma DA, Khurana TS, and Lazar MA. The nuclear receptor Rev-erbalpha controls circadian thermogenic plasticity. Nature 503: 410–413, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ghamari-Langroudi M, Vella KR, Srisai D, Sugrue ML, Hollenberg AN, and Cone RD. Regulation of thyrotropin-releasing hormone-expressing neurons in paraventricular nucleus of the hypothalamus by signals of adiposity. Mol Endocrinol 24: 2366–2381, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Giralt M, Martin I, Iglesias R, Vinas O, Villarroya F, and Mampel T. Ontogeny and perinatal modulation of gene expression in rat brown adipose tissue. Unaltered iodothyronine 5’-deiodinase activity is necessary for the response to environmental temperature at birth. Eur J Biochem 193: 297–302, 1990. [DOI] [PubMed] [Google Scholar]
- 95.Glass CK, and Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab 15: 635–645, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gnad T, Scheibler S, von Kugelgen I, Scheele C, Kilic A, Glode A, Hoffmann LS, Reverte-Salisa L, Horn P, Mutlu S, El-Tayeb A, Kranz M, Deuther-Conrad W, Brust P, Lidell ME, Betz MJ, Enerback S, Schrader J, Yegutkin GG, Muller CE, and Pfeifer A. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature 516: 395–399, 2014. [DOI] [PubMed] [Google Scholar]
- 97.Golozoubova V, Cannon B, and Nedergaard J. UCP1 is essential for adaptive adrenergic nonshivering thermogenesis. Am J Physiol Endocrinol Metab 291: E350–357, 2006. [DOI] [PubMed] [Google Scholar]
- 98.Golozoubova V, Hohtola E, Matthias A, Jacobsson A, Cannon B, and Nedergaard J. Only UCP1 can mediate adaptive nonshivering thermogenesis in the cold. FASEB J 15: 2048–2050, 2001. [DOI] [PubMed] [Google Scholar]
- 99.Gomez-Hernandez A, Beneit N, Escribano O, Diaz-Castroverde S, Garcia-Gomez G, Fernandez S, and Benito M. Severe Brown Fat Lipoatrophy Aggravates Atherosclerotic Process in Male Mice. Endocrinology 157: 3517–3528, 2016. [DOI] [PubMed] [Google Scholar]
- 100.Graja A, and Schulz TJ. Mechanisms of aging-related impairment of brown adipocyte development and function. Gerontology 61: 211–217, 2015. [DOI] [PubMed] [Google Scholar]
- 101.Granneman JG, Moore HP, Krishnamoorthy R, and Rathod M. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 (Abhd5) and adipose triglyceride lipase (Atgl). The Journal of biological chemistry 284: 34538–34544, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guerra C, Koza RA, Walsh K, Kurtz DM, Wood PA, and Kozak LP. Abnormal nonshivering thermogenesis in mice with inherited defects of fatty acid oxidation. J Clin Invest 102: 1724–1731, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guerra C, Koza RA, Yamashita H, Walsh K, and Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest 102: 412–420, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Guerra C, Navarro P, Valverde AM, Arribas M, Bruning J, Kozak LP, Kahn CR, and Benito M. Brown adipose tissue-specific insulin receptor knockout shows diabetic phenotype without insulin resistance. J Clin Invest 108: 1205–1213, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gunawardana SC, and Piston DW. Insulin-independent reversal of type 1 diabetes in nonobese diabetic mice with brown adipose tissue transplant. Am J Physiol Endocrinol Metab 308: E1043–1055, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gunawardana SC, and Piston DW. Reversal of type 1 diabetes in mice by brown adipose tissue transplant. Diabetes 61: 674–682, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guo F, Bakal K, Minokoshi Y, and Hollenberg AN. Leptin signaling targets the thyrotropin-releasing hormone gene promoter in vivo. Endocrinology 145: 2221–2227, 2004. [DOI] [PubMed] [Google Scholar]
- 108.Gupta RK, Arany Z, Seale P, Mepani RJ, Ye L, Conroe HM, Roby YA, Kulaga H, Reed RR, and Spiegelman BM. Transcriptional control of preadipocyte determination by Zfp423. Nature 464: 619–623, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gupta RK, Mepani RJ, Kleiner S, Lo JC, Khandekar MJ, Cohen P, Frontini A, Bhowmick DC, Ye L, Cinti S, and Spiegelman BM. Zfp423 expression identifies committed preadipocytes and localizes to adipose endothelial and perivascular cells. Cell Metab 15: 230–239, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gustafson B, Hammarstedt A, Hedjazifar S, Hoffmann JM, Svensson PA, Grimsby J, Rondinone C, and Smith U. BMP4 and BMP Antagonists Regulate Human White and Beige Adipogenesis. Diabetes 64: 1670–1681, 2015. [DOI] [PubMed] [Google Scholar]
- 111.Haas B, Mayer P, Jennissen K, Scholz D, Berriel Diaz M, Bloch W, Herzig S, Fassler R, and Pfeifer A. Protein kinase G controls brown fat cell differentiation and mitochondrial biogenesis. Sci Signal 2: ra78, 2009. [DOI] [PubMed] [Google Scholar]
- 112.Habegger KM, Stemmer K, Cheng C, Muller TD, Heppner KM, Ottaway N, Holland J, Hembree JL, Smiley D, Gelfanov V, Krishna R, Arafat AM, Konkar A, Belli S, Kapps M, Woods SC, Hofmann SM, D’Alessio D, Pfluger PT, Perez-Tilve D, Seeley RJ, Konishi M, Itoh N, Kharitonenkov A, Spranger J, Dimarchi RD, and Tschop MH. Fibroblast growth factor 21 mediates specific glucagon actions. Diabetes 62: 1453–1463, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hallberg M, Morganstein DL, Kiskinis E, Shah K, Kralli A, Dilworth SM, White R, Parker MG, and Christian M. A functional interaction between RIP140 and PGC-1alpha regulates the expression of the lipid droplet protein CIDEA. Mol Cell Biol 28: 6785–6795, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Han YH, Buffolo M, Pires KM, Pei S, Scherer PE, and Boudina S. Adipocyte-Specific Deletion of Manganese Superoxide Dismutase Protects From Diet-Induced Obesity Through Increased Mitochondrial Uncoupling and Biogenesis. Diabetes 65: 2639–2651, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hankir MK, Kranz M, Gnad T, Weiner J, Wagner S, Deuther-Conrad W, Bronisch F, Steinhoff K, Luthardt J, Kloting N, Hesse S, Seibyl JP, Sabri O, Heiker JT, Bluher M, Pfeifer A, Brust P, and Fenske WK. A novel thermoregulatory role for PDE10A in mouse and human adipocytes. EMBO Mol Med 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hanssen MJ, Broeders E, Samms RJ, Vosselman MJ, van der Lans AA, Cheng CC, Adams AC, van Marken Lichtenbelt WD, and Schrauwen P. Serum FGF21 levels are associated with brown adipose tissue activity in humans. Scientific reports 5: 10275, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hanssen MJ, Hoeks J, Brans B, van der Lans AA, Schaart G, van den Driessche JJ, Jorgensen JA, Boekschoten MV, Hesselink MK, Havekes B, Kersten S, Mottaghy FM, van Marken Lichtenbelt WD, and Schrauwen P. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat Med 2015. [DOI] [PubMed] [Google Scholar]
- 118.Harms MJ, Ishibashi J, Wang W, Lim HW, Goyama S, Sato T, Kurokawa M, Won KJ, and Seale P. Prdm16 is required for the maintenance of brown adipocyte identity and function in adult mice. Cell Metab 19: 593–604, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Harms MJ, Lim HW, Ho Y, Shapira SN, Ishibashi J, Rajakumari S, Steger DJ, Lazar MA, Won KJ, and Seale P. PRDM16 binds MED1 and controls chromatin architecture to determine a brown fat transcriptional program. Genes Dev 29: 298–307, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Harris M, Aschkenasi C, Elias CF, Chandrankunnel A, Nillni EA, Bjoorbaek C, Elmquist JK, Flier JS, and Hollenberg AN. Transcriptional regulation of the thyrotropin-releasing hormone gene by leptin and melanocortin signaling. J Clin Invest 107: 111–120, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, and Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol 279: C670–681, 2000. [DOI] [PubMed] [Google Scholar]
- 122.Hiraoka Y, Matsuoka T, Ohno M, Nakamura K, Saijo S, Matsumura S, Nishi K, Sakamoto J, Chen PM, Inoue K, Fushiki T, Kita T, Kimura T, and Nishi E. Critical roles of nardilysin in the maintenance of body temperature homoeostasis. Nat Commun 5: 3224, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV Jr., Alt FW, Kahn CR, and Verdin E. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464: 121–125, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hoffmann LS, Etzrodt J, Willkomm L, Sanyal A, Scheja L, Fischer AW, Stasch JP, Bloch W, Friebe A, Heeren J, and Pfeifer A. Stimulation of soluble guanylyl cyclase protects against obesity by recruiting brown adipose tissue. Nat Commun 6: 7235, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hofmann WE, Liu X, Bearden CM, Harper ME, and Kozak LP. Effects of genetic background on thermoregulation and fatty acid-induced uncoupling of mitochondria in UCP1-deficient mice. The Journal of biological chemistry 276: 12460–12465, 2001. [DOI] [PubMed] [Google Scholar]
- 126.Hollenberg AN. The role of the thyrotropin-releasing hormone (TRH) neuron as a metabolic sensor. Thyroid 18: 131–139, 2008. [DOI] [PubMed] [Google Scholar]
- 127.Hondares E, Rosell M, Diaz-Delfin J, Olmos Y, Monsalve M, Iglesias R, Villarroya F, and Giralt M. Peroxisome proliferator-activated receptor alpha (PPARalpha) induces PPARgamma coactivator 1alpha (PGC-1alpha) gene expression and contributes to thermogenic activation of brown fat: involvement of PRDM16. The Journal of biological chemistry 286: 43112–43122, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hondares E, Rosell M, Gonzalez FJ, Giralt M, Iglesias R, and Villarroya F. Hepatic FGF21 expression is induced at birth via PPARalpha in response to milk intake and contributes to thermogenic activation of neonatal brown fat. Cell Metab 11: 206–212, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hotamisligil GS. Inflammation and metabolic disorders. Nature 444: 860–867, 2006. [DOI] [PubMed] [Google Scholar]
- 130.Houstek J, Kopecky J, Rychter Z, and Soukup T. Uncoupling protein in embryonic brown adipose tissue--existence of nonthermogenic and thermogenic mitochondria. Biochimica et biophysica acta 935: 19–25, 1988. [DOI] [PubMed] [Google Scholar]
- 131.Huo L, Munzberg H, Nillni EA, and Bjorbaek C. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic trh gene expression by leptin. Endocrinology 145: 2516–2523, 2004. [DOI] [PubMed] [Google Scholar]
- 132.Huttunen P, Hirvonen J, and Kinnula V. The occurrence of brown adipose tissue in outdoor workers. Eur J Appl Physiol Occup Physiol 46: 339–345, 1981. [DOI] [PubMed] [Google Scholar]
- 133.Iida S, Chen W, Nakadai T, Ohkuma Y, and Roeder RG. PRDM16 enhances nuclear receptor-dependent transcription of the brown fat-specific Ucp1 gene through interactions with Mediator subunit MED1. Genes Dev 29: 308–321, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, and Kliewer SA. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2: 217–225, 2005. [DOI] [PubMed] [Google Scholar]
- 135.Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, and Kliewer SA. Endocrine Regulation of the Fasting Response by PPARalpha-Mediated Induction of Fibroblast Growth Factor 21. Cell Metab 5: 415–425, 2007. [DOI] [PubMed] [Google Scholar]
- 136.Inokuma K, Ogura-Okamatsu Y, Toda C, Kimura K, Yamashita H, and Saito M. Uncoupling protein 1 is necessary for norepinephrine-induced glucose utilization in brown adipose tissue. Diabetes 54: 1385–1391, 2005. [DOI] [PubMed] [Google Scholar]
- 137.Inokuma K, Okamatsu-Ogura Y, Omachi A, Matsushita Y, Kimura K, Yamashita H, and Saito M. Indispensable role of mitochondrial UCP1 for antiobesity effect of beta3-adrenergic stimulation. Am J Physiol Endocrinol Metab 290: E1014–1021, 2006. [DOI] [PubMed] [Google Scholar]
- 138.Iwayama T, Steele C, Yao L, Dozmorov MG, Karamichos D, Wren JD, and Olson LE. PDGFRalpha signaling drives adipose tissue fibrosis by targeting progenitor cell plasticity. Genes Dev 29: 1106–1119, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.James DI, Parone PA, Mattenberger Y, and Martinou JC. hFis1, a novel component of the mammalian mitochondrial fission machinery. The Journal of biological chemistry 278: 36373–36379, 2003. [DOI] [PubMed] [Google Scholar]
- 140.Jespersen NZ, Larsen TJ, Peijs L, Daugaard S, Homoe P, Loft A, de Jong J, Mathur N, Cannon B, Nedergaard J, Pedersen BK, Moller K, and Scheele C. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab 17: 798–805, 2013. [DOI] [PubMed] [Google Scholar]
- 141.Jiang C, Qu A, Matsubara T, Chanturiya T, Jou W, Gavrilova O, Shah YM, and Gonzalez FJ. Disruption of hypoxia-inducible factor 1 in adipocytes improves insulin sensitivity and decreases adiposity in high-fat diet-fed mice. Diabetes 60: 2484–2495, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jimenez-Preitner M, Berney X, Uldry M, Vitali A, Cinti S, Ledford JG, and Thorens B. Plac8 is an inducer of C/EBPbeta required for brown fat differentiation, thermoregulation, and control of body weight. Cell Metab 14: 658–670, 2011. [DOI] [PubMed] [Google Scholar]
- 143.Kajimura S, Seale P, Kubota K, Lunsford E, Frangioni JV, Gygi SP, and Spiegelman BM. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature 460: 1154–1158, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kajimura S, Seale P, Tomaru T, Erdjument-Bromage H, Cooper MP, Ruas JL, Chin S, Tempst P, Lazar MA, and Spiegelman BM. Regulation of the brown and white fat gene programs through a PRDM16/CtBP transcriptional complex. Genes Dev 22: 1397–1409, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kalinovich AV, de Jong JM, Cannon B, and Nedergaard J. UCP1 in adipose tissues: two steps to full browning. Biochimie 134: 127–137, 2017. [DOI] [PubMed] [Google Scholar]
- 146.Kang S, Bajnok L, Longo KA, Petersen RK, Hansen JB, Kristiansen K, and MacDougald OA. Effects of Wnt signaling on brown adipocyte differentiation and metabolism mediated by PGC-1alpha. Mol Cell Biol 25: 1272–1282, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kataoka N, Hioki H, Kaneko T, and Nakamura K. Psychological stress activates a dorsomedial hypothalamus-medullary raphe circuit driving brown adipose tissue thermogenesis and hyperthermia. Cell Metab 20: 346–358, 2014. [DOI] [PubMed] [Google Scholar]
- 148.Kazak L, Chouchani ET, Jedrychowski MP, Erickson BK, Shinoda K, Cohen P, Vetrivelan R, Lu GZ, Laznik-Bogoslavski D, Hasenfuss SC, Kajimura S, Gygi SP, and Spiegelman BM. A Creatine-Driven Substrate Cycle Enhances Energy Expenditure and Thermogenesis in Beige Fat. Cell 163: 643–655, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Keipert S, Kutschke M, Lamp D, Brachthauser L, Neff F, Meyer CW, Oelkrug R, Kharitonenkov A, and Jastroch M. Genetic disruption of uncoupling protein 1 in mice renders brown adipose tissue a significant source of FGF21 secretion. Mol Metab 4: 537–542, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kern PA, Finlin BS, Zhu B, Rasouli N, McGehee RE Jr., Westgate PM, and Dupont-Versteegden EE. The effects of temperature and seasons on subcutaneous white adipose tissue in humans: evidence for thermogenic gene induction. J Clin Endocrinol Metab 99: E2772–2779, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kiefer FW, Vernochet C, O’Brien P, Spoerl S, Brown JD, Nallamshetty S, Zeyda M, Stulnig TM, Cohen DE, Kahn CR, and Plutzky J. Retinaldehyde dehydrogenase 1 regulates a thermogenic program in white adipose tissue. Nat Med 18: 918–925, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kim JK, Kim HJ, Park SY, Cederberg A, Westergren R, Nilsson D, Higashimori T, Cho YR, Liu ZX, Dong J, Cline GW, Enerback S, and Shulman GI. Adipocyte-specific overexpression of FOXC2 prevents diet-induced increases in intramuscular fatty acyl CoA and insulin resistance. Diabetes 54: 1657–1663, 2005. [DOI] [PubMed] [Google Scholar]
- 153.Kim KW, Zhao L, Donato J Jr., Kohno D, Xu Y, Elias CF, Lee C, Parker KL, and Elmquist JK. Steroidogenic factor 1 directs programs regulating diet-induced thermogenesis and leptin action in the ventral medial hypothalamic nucleus. Proc Natl Acad Sci U S A 108: 10673–10678, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kim SJ, Tang T, Abbott M, Viscarra JA, Wang Y, and Sul HS. AMPK phosphorylates desnutrin/ATGL and HSL to regulate lipolysis and fatty acid oxidation within adipose tissue. Mol Cell Biol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kinoshita K, Ozaki N, Takagi Y, Murata Y, Oshida Y, and Hayashi Y. Glucagon is essential for adaptive thermogenesis in brown adipose tissue. Endocrinology 155: 3484–3492, 2014. [DOI] [PubMed] [Google Scholar]
- 156.Kir S, White JP, Kleiner S, Kazak L, Cohen P, Baracos VE, and Spiegelman BM. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature 513: 100–104, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kleiner S, Mepani RJ, Laznik D, Ye L, Jurczak MJ, Jornayvaz FR, Estall JL, Chatterjee Bhowmick D, Shulman GI, and Spiegelman BM. Development of insulin resistance in mice lacking PGC-1alpha in adipose tissues. Proc Natl Acad Sci U S A 109: 9635–9640, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Knudsen JG, Murholm M, Carey AL, Bienso RS, Basse AL, Allen TL, Hidalgo J, Kingwell BA, Febbraio MA, Hansen JB, and Pilegaard H. Role of IL-6 in exercise training- and cold-induced UCP1 expression in subcutaneous white adipose tissue. PLoS One 9: e84910, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kong D, Tong Q, Ye C, Koda S, Fuller PM, Krashes MJ, Vong L, Ray RS, Olson DP, and Lowell BB. GABAergic RIP-Cre neurons in the arcuate nucleus selectively regulate energy expenditure. Cell 151: 645–657, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kong X, Banks A, Liu T, Kazak L, Rao RR, Cohen P, Wang X, Yu S, Lo JC, Tseng YH, Cypess AM, Xue R, Kleiner S, Kang S, Spiegelman BM, and Rosen ED. IRF4 Is a Key Thermogenic Transcriptional Partner of PGC-1alpha. Cell 158: 69–83, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kopecky J, Clarke G, Enerback S, Spiegelman B, and Kozak LP. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J Clin Invest 96: 2914–2923, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, and Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest 121: 1424–1428, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Krishnan J, Danzer C, Simka T, Ukropec J, Walter KM, Kumpf S, Mirtschink P, Ukropcova B, Gasperikova D, Pedrazzini T, and Krek W. Dietary obesity-associated Hif1alpha activation in adipocytes restricts fatty acid oxidation and energy expenditure via suppression of the Sirt2-NAD+ system. Genes Dev 26: 259–270, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kumar N, Liu D, Wang H, Robidoux J, and Collins S. Orphan nuclear receptor NOR-1 enhances 3’,5’-cyclic adenosine 5’-monophosphate-dependent uncoupling protein-1 gene transcription. Mol Endocrinol 22: 1057–1064, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kuperman Y, Issler O, Regev L, Musseri I, Navon I, Neufeld-Cohen A, Gil S, and Chen A. Perifornical Urocortin-3 mediates the link between stress-induced anxiety and energy homeostasis. Proc Natl Acad Sci U S A 107: 8393–8398, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Labbe SM, Caron A, Bakan I, Laplante M, Carpentier AC, Lecomte R, and Richard D. In vivo measurement of energy substrate contribution to cold-induced brown adipose tissue thermogenesis. FASEB J 29: 2046–2058, 2015. [DOI] [PubMed] [Google Scholar]
- 167.Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, Munzberg H, Hutson SM, Gettys TW, Schwartz MW, and Morrison CD. FGF21 is an endocrine signal of protein restriction. J Clin Invest 124: 3913–3922, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, Kienesberger P, Strauss JG, Gorkiewicz G, and Zechner R. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab 3: 309–319, 2006. [DOI] [PubMed] [Google Scholar]
- 169.Lechan RM, and Fekete C. The TRH neuron: a hypothalamic integrator of energy metabolism. Prog Brain Res 153: 209–235, 2006. [DOI] [PubMed] [Google Scholar]
- 170.Lee J, Ellis JM, and Wolfgang MJ. Adipose Fatty Acid Oxidation Is Required for Thermogenesis and Potentiates Oxidative Stress-Induced Inflammation. Cell Rep 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lee JE, Wang C, Xu S, Cho YW, Wang L, Feng X, Baldridge A, Sartorelli V, Zhuang L, Peng W, and Ge K. H3K4 mono- and di-methyltransferase MLL4 is required for enhancer activation during cell differentiation. Elife 2: e01503, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lee JY, Takahashi N, Yasubuchi M, Kim YI, Hashizaki H, Kim MJ, Sakamoto T, Goto T, and Kawada T. Triiodothyronine induces UCP-1 expression and mitochondrial biogenesis in human adipocytes. Am J Physiol Cell Physiol 302: C463–472, 2012. [DOI] [PubMed] [Google Scholar]
- 173.Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, Yun K, Locksley RM, and Chawla A. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell 160: 74–87, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C, Perron RM, Werner CD, Phan GQ, Kammula US, Kebebew E, Pacak K, Chen KY, and Celi FS. Irisin and FGF21 Are Cold-Induced Endocrine Activators of Brown Fat Function in Humans. Cell Metab 19: 302–309, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lee Y, Willers C, Kunji ER, and Crichton PG. Uncoupling protein 1 binds one nucleotide per monomer and is stabilized by tightly bound cardiolipin. Proc Natl Acad Sci U S A 112: 6973–6978, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lee YH, Kim SN, Kwon HJ, and Granneman JG. Metabolic heterogeneity of activated beige/brite adipocytes in inguinal adipose tissue. Scientific reports 7: 39794, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lee YH, Petkova AP, Konkar AA, and Granneman JG. Cellular origins of cold-induced brown adipocytes in adult mice. FASEB J 29: 286–299, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lee YH, Petkova AP, Mottillo EP, and Granneman JG. In vivo identification of bipotential adipocyte progenitors recruited by beta3-adrenoceptor activation and high-fat feeding. Cell Metab 15: 480–491, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lee YS, Kim JW, Osborne O, Oh da Y, Sasik R, Schenk S, Chen A, Chung H, Murphy A, Watkins SM, Quehenberger O, Johnson RS, and Olefsky JM. Increased Adipocyte O2 Consumption Triggers HIF-1alpha, Causing Inflammation and Insulin Resistance in Obesity. Cell 157: 1339–1352, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lepper C, and Fan CM. Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis 48: 424–436, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Li F, Wu R, Cui X, Zha L, Yu L, Shi H, and Xue B. Histone Deacetylase 1 (HDAC1) Negatively Regulates Thermogenic Program in Brown Adipocytes via Coordinated Regulation of Histone H3 Lysine 27 (H3K27) Deacetylation and Methylation. The Journal of biological chemistry 291: 4523–4536, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Li ZY, Song J, Zheng SL, Fan MB, Guan YF, Qu Y, Xu J, Wang P, and Miao CY. Adipocyte Metrnl Antagonizes Insulin Resistance Through PPARgamma Signaling. Diabetes 64: 4011–4022, 2015. [DOI] [PubMed] [Google Scholar]
- 183.Lin JD. Minireview: the PGC-1 coactivator networks: chromatin-remodeling and mitochondrial energy metabolism. Mol Endocrinol 23: 2–10, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Linhart HG, Ishimura-Oka K, DeMayo F, Kibe T, Repka D, Poindexter B, Bick RJ, and Darlington GJ. C/EBPalpha is required for differentiation of white, but not brown, adipose tissue. Proc Natl Acad Sci U S A 98: 12532–12537, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Liu X, Rossmeisl M, McClaine J, Riachi M, Harper ME, and Kozak LP. Paradoxical resistance to diet-induced obesity in UCP1-deficient mice. J Clin Invest 111: 399–407, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lkhagvasuren B, Nakamura Y, Oka T, Sudo N, and Nakamura K. Social defeat stress induces hyperthermia through activation of thermoregulatory sympathetic premotor neurons in the medullary raphe region. Eur J Neurosci 34: 1442–1452, 2011. [DOI] [PubMed] [Google Scholar]
- 187.Lo KA, Ng PY, Kabiri Z, Virshup D, and Sun L. Wnt inhibition enhances browning of mouse primary white adipocytes. Adipocyte 5: 224–231, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Loft A, Forss I, Siersbaek MS, Schmidt SF, Larsen AS, Madsen JG, Pisani DF, Nielsen R, Aagaard MM, Mathison A, Neville MJ, Urrutia R, Karpe F, Amri EZ, and Mandrup S. Browning of human adipocytes requires KLF11 and reprogramming of PPARgamma superenhancers. Genes Dev 29: 7–22, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Long JZ, Svensson KJ, Bateman LA, Lin H, Kamenecka T, Lokurkar IA, Lou J, Rao RR, Chang MR, Jedrychowski MP, Paulo JA, Gygi SP, Griffin PR, Nomura DK, and Spiegelman BM. The Secreted Enzyme PM20D1 Regulates Lipidated Amino Acid Uncouplers of Mitochondria. Cell 166: 424–435, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X, Rao RR, Lou J, Lokurkar I, Baur W, Castellot JJ Jr., Rosen ED, and Spiegelman BM. A smooth muscle-like origin for beige adipocytes. Cell Metab 19: 810–820, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lopez M, Varela L, Vazquez MJ, Rodriguez-Cuenca S, Gonzalez CR, Velagapudi VR, Morgan DA, Schoenmakers E, Agassandian K, Lage R, Martinez de Morentin PB, Tovar S, Nogueiras R, Carling D, Lelliott C, Gallego R, Oresic M, Chatterjee K, Saha AK, Rahmouni K, Dieguez C, and Vidal-Puig A. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat Med 16: 1001–1008, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lowell BB, SS V, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB, Kozak LP, and Flier JS. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 366: 740–742, 1993. [DOI] [PubMed] [Google Scholar]
- 193.Lumeng CN, and Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest 121: 2111–2117, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lynch L, Hogan AE, Duquette D, Lester C, Banks A, LeClair K, Cohen DE, Ghosh A, Lu B, Corrigan M, Stevanovic D, Maratos-Flier E, Drucker DJ, O’Shea D, and Brenner M. iNKT Cells Induce FGF21 for Thermogenesis and Are Required for Maximal Weight Loss in GLP1 Therapy. Cell Metab 24: 510–519, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lynch L, Nowak M, Varghese B, Clark J, Hogan AE, Toxavidis V, Balk SP, O’Shea D, O’Farrelly C, and Exley MA. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity 37: 574–587, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lynes MD, Schulz TJ, Pan AJ, and Tseng YH. Disruption of insulin signaling in myf5-expressing progenitors leads to marked paucity of brown fat but normal muscle development. Endocrinology 156: 1637–1647, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ma X, Xu L, Gavrilova O, and Mueller E. Role of forkhead box protein A3 in age-associated metabolic decline. Proc Natl Acad Sci U S A 111: 14289–14294, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Madden CJ, Tupone D, Cano G, and Morrison SF. alpha2 Adrenergic receptor-mediated inhibition of thermogenesis. J Neurosci 33: 2017–2028, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Madsen L, Pedersen LM, Lillefosse HH, Fjaere E, Bronstad I, Hao Q, Petersen RK, Hallenborg P, Ma T, De Matteis R, Araujo P, Mercader J, Bonet ML, Hansen JB, Cannon B, Nedergaard J, Wang J, Cinti S, Voshol P, Doskeland SO, and Kristiansen K. UCP1 induction during recruitment of brown adipocytes in white adipose tissue is dependent on cyclooxygenase activity. PLoS One 5: e11391, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Malloy PJ, and Feldman BJ. Cell Autonomous Regulation of Brown Fat Identity Gene UCP1 by Unliganded Vitamin D Receptor. Mol Endocrinol 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Marcelin G, Ferreira A, Liu Y, Atlan M, Aron-Wisnewsky J, Pelloux V, Botbol Y, Ambrosini M, Fradet M, Rouault C, Henegar C, Hulot JS, Poitou C, Torcivia A, Nail-Barthelemy R, Bichet JC, Gautier EL, and Clement K. A PDGFRalpha-Mediated Switch toward CD9high Adipocyte Progenitors Controls Obesity-Induced Adipose Tissue Fibrosis. Cell Metab 25: 673–685, 2017. [DOI] [PubMed] [Google Scholar]
- 202.Matthias A, Ohlson KB, Fredriksson JM, Jacobsson A, Nedergaard J, and Cannon B. Thermogenic responses in brown fat cells are fully UCP1-dependent. UCP2 or UCP3 do not substitute for UCP1 in adrenergically or fatty scid-induced thermogenesis. The Journal of biological chemistry 275: 25073–25081, 2000. [DOI] [PubMed] [Google Scholar]
- 203.McKemy DD, Neuhausser WM, and Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416: 52–58, 2002. [DOI] [PubMed] [Google Scholar]
- 204.Miao Y, Wu W, Dai Y, Maneix L, Huang B, Warner M, and Gustafsson JA. Liver X receptor beta controls thyroid hormone feedback in the brain and regulates browning of subcutaneous white adipose tissue. Proc Natl Acad Sci U S A 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Min SY, Kady J, Nam M, Rojas-Rodriguez R, Berkenwald A, Kim JH, Noh HL, Kim JK, Cooper MP, Fitzgibbons T, Brehm MA, and Corvera S. Human ‘brite/beige’ adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nat Med 22: 312–318, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mirchandani AS, Salmond RJ, and Liew FY. Interleukin-33 and the function of innate lymphoid cells. Trends Immunol 33: 389–396, 2012. [DOI] [PubMed] [Google Scholar]
- 207.Mitschke MM, Hoffmann LS, Gnad T, Scholz D, Kruithoff K, Mayer P, Haas B, Sassmann A, Pfeifer A, and Kilic A. Increased cGMP promotes healthy expansion and browning of white adipose tissue. FASEB J 27: 1621–1630, 2013. [DOI] [PubMed] [Google Scholar]
- 208.Monemdjou S, Hofmann WE, Kozak LP, and Harper ME. Increased mitochondrial proton leak in skeletal muscle mitochondria of UCP1-deficient mice. Am J Physiol Endocrinol Metab 279: E941–946, 2000. [DOI] [PubMed] [Google Scholar]
- 209.Morgan DA, McDaniel LN, Yin T, Khan M, Jiang J, Acevedo MR, Walsh SA, Ponto LL, Norris AW, Lutter M, Rahmouni K, and Cui H. Regulation of Glucose Tolerance and Sympathetic Activity by MC4R Signaling in the Lateral Hypothalamus. Diabetes 64: 1976–1987, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mori M, Nakagami H, Rodriguez-Araujo G, Nimura K, and Kaneda Y. Essential role for miR-196a in brown adipogenesis of white fat progenitor cells. PLoS Biol 10: e1001314, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Morrison SF, Madden CJ, and Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab 19: 741–756, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Mottillo EP, Bloch AE, Leff T, and Granneman JG. Lipolytic products activate peroxisome proliferator-activated receptor (PPAR) alpha and delta in brown adipocytes to match fatty acid oxidation with supply. The Journal of biological chemistry 287: 25038–25048, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Mottillo EP, Desjardins EM, Crane JD, Smith BK, Green AE, Ducommun S, Henriksen TI, Rebalka IA, Razi A, Sakamoto K, Scheele C, Kemp BE, Hawke TJ, Ortega J, Granneman JG, and Steinberg GR. Lack of Adipocyte AMPK Exacerbates Insulin Resistance and Hepatic Steatosis through Brown and Beige Adipose Tissue Function. Cell Metab 24: 118–129, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Mracek T, Cannon B, and Houstek J. IL-1 and LPS but not IL-6 inhibit differentiation and downregulate PPAR gamma in brown adipocytes. Cytokine 26: 9–15, 2004. [DOI] [PubMed] [Google Scholar]
- 215.Mulligan JD, Gonzalez AA, Stewart AM, Carey HV, and Saupe KW. Upregulation of AMPK during cold exposure occurs via distinct mechanisms in brown and white adipose tissue of the mouse. J Physiol 580: 677–684, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Nakamura K, Matsumura K, Hubschle T, Nakamura Y, Hioki H, Fujiyama F, Boldogkoi Z, Konig M, Thiel HJ, Gerstberger R, Kobayashi S, and Kaneko T. Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J Neurosci 24: 5370–5380, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Nam D, Chatterjee S, Yin H, Liu R, Lee J, Yechoor VK, and Ma K. Novel Function of Rev-erbalpha in Promoting Brown Adipogenesis. Scientific reports 5: 11239, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Nawrocki AR, Rodriguez CG, Toolan DM, Price O, Henry M, Forrest G, Szeto D, Keohane CA, Pan Y, Smith KM, Raheem IT, Cox CD, Hwa J, Renger JJ, and Smith SM. Genetic deletion and pharmacological inhibition of phosphodiesterase 10A protects mice from diet-induced obesity and insulin resistance. Diabetes 63: 300–311, 2014. [DOI] [PubMed] [Google Scholar]
- 219.Nelson VL, Jiang YP, Dickman KG, Ballou LM, and Lin RZ. Adipose tissue insulin resistance due to loss of PI3K p110alpha leads to decreased energy expenditure and obesity. Am J Physiol Endocrinol Metab 306: E1205–1216, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, and Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 480: 104–108, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, and Carruba MO. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science 299: 896–899, 2003. [DOI] [PubMed] [Google Scholar]
- 222.Nixon JP, Kotz CM, Novak CM, Billington CJ, and Teske JA. Neuropeptides controlling energy balance: orexins and neuromedins. Handb Exp Pharmacol 77-109, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Nordstrom EA, Ryden M, Backlund EC, Dahlman I, Kaaman M, Blomqvist L, Cannon B, Nedergaard J, and Arner P. A human-specific role of cell death-inducing DFFA (DNA fragmentation factor-alpha)-like effector A (CIDEA) in adipocyte lipolysis and obesity. Diabetes 54: 1726–1734, 2005. [DOI] [PubMed] [Google Scholar]
- 224.Nosavanh L, Yu DH, Jaehnig EJ, Tong Q, Shen L, and Chen MH. Cell-autonomous activation of Hedgehog signaling inhibits brown adipose tissue development. Proc Natl Acad Sci U S A 112: 5069–5074, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Odegaard JI, Lee MW, Sogawa Y, Bertholet AM, Locksley RM, Weinberg DE, Kirichok Y, Deo RC, and Chawla A. Perinatal Licensing of Thermogenesis by IL-33 and ST2. Cell 166: 841–854, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Oelkrug R, Kutschke M, Meyer CW, Heldmaier G, and Jastroch M. Uncoupling protein 1 decreases superoxide production in brown adipose tissue mitochondria. The Journal of biological chemistry 285: 21961–21968, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Oh CM, Namkung J, Go Y, Shong KE, Kim K, Kim H, Park BY, Lee HW, Jeon YH, Song J, Shong M, Yadav VK, Karsenty G, Kajimura S, Lee IK, Park S, and Kim H. Regulation of systemic energy homeostasis by serotonin in adipose tissues. Nat Commun 6: 6794, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ohno H, Shinoda K, Ohyama K, Sharp LZ, and Kajimura S. EHMT1 controls brown adipose cell fate and thermogenesis through the PRDM16 complex. Nature 504: 163–167, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ohno H, Shinoda K, Spiegelman BM, and Kajimura S. PPARgamma agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab 15: 395–404, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Okla M, Wang W, Kang I, Pashaj A, Carr T, and Chung S. Activation of Toll-like receptor 4 (TLR4) attenuates adaptive thermogenesis via endoplasmic reticulum stress. The Journal of biological chemistry 290: 26476–26490, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Olefsky JM, and Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 72: 219–246, 2010. [DOI] [PubMed] [Google Scholar]
- 232.Ouellet V, Labbe SM, Blondin DP, Phoenix S, Guerin B, Haman F, Turcotte EE, Richard D, and Carpentier AC. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest 122: 545–552, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ozguven S, Ones T, Yilmaz Y, Turoglu HT, and Imeryuz N. The role of active brown adipose tissue in human metabolism. Eur J Nucl Med Mol Imaging 43: 355–361, 2016. [DOI] [PubMed] [Google Scholar]
- 234.Pan D, Fujimoto M, Lopes A, and Wang YX. Twist-1 is a PPARdelta-inducible, negative-feedback regulator of PGC-1alpha in brown fat metabolism. Cell 137: 73–86, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Pan D, Huang L, Zhu LJ, Zou T, Ou J, Zhou W, and Wang YX. Jmjd3-Mediated H3K27me3 Dynamics Orchestrate Brown Fat Development and Regulate White Fat Plasticity. Dev Cell 35: 568–583, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Park JH, Kang HJ, Kang SI, Lee JE, Hur J, Ge K, Mueller E, Li H, Lee BC, and Lee SB. A multifunctional protein, EWS, is essential for early brown fat lineage determination. Dev Cell 26: 393–404, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Patil M, Sharma BK, Elattar S, Chang J, Kapil S, Yuan J, and Satyanarayana A. Id1 Promotes Obesity by Suppressing Brown Adipose Thermogenesis and White Adipose Browning. Diabetes 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Perkins MN, Rothwell NJ, Stock MJ, and Stone TW. Activation of brown adipose tissue thermogenesis by the ventromedial hypothalamus. Nature 289: 401–402, 1981. [DOI] [PubMed] [Google Scholar]
- 239.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, and Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. The Journal of biological chemistry 285: 7153–7164, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Petruzzelli M, Schweiger M, Schreiber R, Campos-Olivas R, Tsoli M, Allen J, Swarbrick M, Rose-John S, Rincon M, Robertson G, Zechner R, and Wagner EF. A Switch from White to Brown Fat Increases Energy Expenditure in Cancer-Associated Cachexia. Cell Metab 2014. [DOI] [PubMed] [Google Scholar]
- 241.Potthoff MJ, Boney-Montoya J, Choi M, He T, Sunny NE, Satapati S, Suino-Powell K, Xu HE, Gerard RD, Finck BN, Burgess SC, Mangelsdorf DJ, and Kliewer SA. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1alpha pathway. Cell Metab 13: 729–738, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Puigserver P, Rhee J, Lin J, Wu Z, Yoon JC, Zhang CY, Krauss S, Mootha VK, Lowell BB, and Spiegelman BM. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARgamma coactivator-1. Mol Cell 8: 971–982, 2001. [DOI] [PubMed] [Google Scholar]
- 243.Putri M, Syamsunarno MR, Iso T, Yamaguchi A, Hanaoka H, Sunaga H, Koitabashi N, Matsui H, Yamazaki C, Kameo S, Tsushima Y, Yokoyama T, Koyama H, Abumrad NA, and Kurabayashi M. CD36 is indispensable for thermogenesis under conditions of fasting and cold stress. Biochem Biophys Res Commun 457: 520–525, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Qian SW, Tang Y, Li X, Liu Y, Zhang YY, Huang HY, Xue RD, Yu HY, Guo L, Gao HD, Liu Y, Sun X, Li YM, Jia WP, and Tang QQ. BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proc Natl Acad Sci U S A 110: E798–807, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y, Rosenbaum M, Zhao Y, Gu W, Farmer SR, and Accili D. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Ppargamma. Cell 150: 620–632, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD, and Chawla A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 157: 1292–1308, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Quan W, Kim HK, Moon EY, Kim SS, Choi CS, Komatsu M, Jeong YT, Lee MK, Kim KW, Kim MS, and Lee MS. Role of hypothalamic proopiomelanocortin neuron autophagy in the control of appetite and leptin response. Endocrinology 153: 1817–1826, 2012. [DOI] [PubMed] [Google Scholar]
- 248.Quiros PM, Ramsay AJ, Sala D, Fernandez-Vizarra E, Rodriguez F, Peinado JR, Fernandez-Garcia MS, Vega JA, Enriquez JA, Zorzano A, and Lopez-Otin C. Loss of mitochondrial protease OMA1 alters processing of the GTPase OPA1 and causes obesity and defective thermogenesis in mice. EMBO J 31: 2117–2133, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Rabelo R, Reyes C, Schifman A, and Silva JE. Interactions among receptors, thyroid hormone response elements, and ligands in the regulation of the rat uncoupling protein gene expression by thyroid hormone. Endocrinology 137: 3478–3487, 1996. [DOI] [PubMed] [Google Scholar]
- 250.Rajakumari S, Wu J, Ishibashi J, Lim HW, Giang AH, Won KJ, Reed RR, and Seale P. EBF2 determines and maintains brown adipocyte identity. Cell Metab 17: 562–574, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Raje V, Derecka M, Cantwell M, Meier J, Szczepanek K, Sisler JD, Strobl B, Gamero A, Harris TE, and Larner AC. Kinase inactive Tyrosine kinase (Tyk2) Supports Differentiation of Brown fat Cells. Endocrinology en 20152048, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Ramage LE, Akyol M, Fletcher AM, Forsythe J, Nixon M, Carter RN, van Beek EJ, Morton NM, Walker BR, and Stimson RH. Glucocorticoids Acutely Increase Brown Adipose Tissue Activity in Humans, Revealing Species-Specific Differences in UCP-1 Regulation. Cell Metab 24: 130–141, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, Camera DM, Lachey J, Gygi S, Seehra J, Hawley JA, and Spiegelman BM. Meteorin-like Is a Hormone that Regulates Immune-Adipose Interactions to Increase Beige Fat Thermogenesis. Cell 157: 1279–1291, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Raschke S, Elsen M, Gassenhuber H, Sommerfeld M, Schwahn U, Brockmann B, Jung R, Wisloff U, Tjonna AE, Raastad T, Hallen J, Norheim F, Drevon CA, Romacho T, Eckardt K, and Eckel J. Evidence against a Beneficial Effect of Irisin in Humans. PLoS One 8: e73680, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Ribeiro MO, Carvalho SD, Schultz JJ, Chiellini G, Scanlan TS, Bianco AC, and Brent GA. Thyroid hormone--sympathetic interaction and adaptive thermogenesis are thyroid hormone receptor isoform--specific. J Clin Invest 108: 97–105, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Rogers NH. Brown adipose tissue during puberty and with aging. Ann Med 47: 142–149, 2015. [DOI] [PubMed] [Google Scholar]
- 257.Rogers NH, Landa A, Park S, and Smith RG. Aging leads to a programmed loss of brown adipocytes in murine subcutaneous white adipose tissue. Aging Cell 11: 1074–1083, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Rosen ED, and Spiegelman BM. What we talk about when we talk about fat. Cell 156: 20–44, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Rosenwald M, Perdikari A, Rulicke T, and Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol 15: 659–667, 2013. [DOI] [PubMed] [Google Scholar]
- 260.Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, and MacDougald OA. Inhibition of adipogenesis by Wnt signaling. Science 289: 950–953, 2000. [DOI] [PubMed] [Google Scholar]
- 261.Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE, Choi MJ, Lauzon D, Lowell BB, and Elmquist JK. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab 13: 195–204, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Rothwell NJ, and Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature 281: 31–35, 1979. [DOI] [PubMed] [Google Scholar]
- 263.Rothwell NJ, and Stock MJ. Surgical removal of brown fat results in rapid and complete compensation by other depots. Am J Physiol 257: R253–258, 1989. [DOI] [PubMed] [Google Scholar]
- 264.Rui L Brain regulation of energy balance and body weight. Rev Endocr Metab Disord 14: 387–407, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Sakamoto T, Nitta T, Maruno K, Yeh YS, Kuwata H, Tomita K, Goto T, Takahashi N, and Kawada T. Macrophage infiltration into obese adipose tissues suppresses the induction of UCP1 expression in mice. Am J Physiol Endocrinol Metab ajpendo 00028 02015, 2016. [DOI] [PubMed] [Google Scholar]
- 266.Sakamoto T, Nitta T, Maruno K, Yeh YS, Kuwata H, Tomita K, Goto T, Takahashi N, and Kawada T. Macrophage infiltration into obese adipose tissues suppresses the induction of UCP1 level in mice. Am J Physiol Endocrinol Metab 310: E676–E687, 2016. [DOI] [PubMed] [Google Scholar]
- 267.Sambeat A, Gulyaeva O, Dempersmier J, Tharp KM, Stahl A, Paul SM, and Sul HS. LSD1 Interacts with Zfp516 to Promote UCP1 Transcription and Brown Fat Program. Cell Rep 15: 2536–2549, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Sarruf DA, Thaler JP, Morton GJ, German J, Fischer JD, Ogimoto K, and Schwartz MW. Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes 59: 1817–1824, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Sawchenko PE, Swanson LW, Grzanna R, Howe PR, Bloom SR, and Polak JM. Colocalization of neuropeptide Y immunoreactivity in brainstem catecholaminergic neurons that project to the paraventricular nucleus of the hypothalamus. J Comp Neurol 241: 138–153, 1985. [DOI] [PubMed] [Google Scholar]
- 270.Schlogl M, Piaggi P, Pannacciuli N, Bonfiglio SM, Krakoff J, and Thearle MS. Energy Expenditure Responses to Fasting and Overfeeding Identify Phenotypes Associated With Weight Change. Diabetes 64: 3680–3689, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Schulz TJ, Huang P, Huang TL, Xue R, McDougall LE, Townsend KL, Cypess AM, Mishina Y, Gussoni E, and Tseng YH. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature 495: 379–383, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Scime A, Grenier G, Huh MS, Gillespie MA, Bevilacqua L, Harper ME, and Rudnicki MA. Rb and p107 regulate preadipocyte differentiation into white versus brown fat through repression of PGC-1alpha. Cell Metab 2: 283–295, 2005. [DOI] [PubMed] [Google Scholar]
- 273.Scott R, Tan T, and Bloom S. Gut hormones and obesity: physiology and therapies. Vitam Horm 91: 143–194, 2013. [DOI] [PubMed] [Google Scholar]
- 274.Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, and Spiegelman BM. PRDM16 controls a brown fat/skeletal muscle switch. Nature 454: 961–967, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, and Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest 121: 96–105, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Seale P, Kajimura S, and Spiegelman BM. Transcriptional control of brown adipocyte development and physiological function--of mice and men. Genes Dev 23: 788–797, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Seki T, Hosaka K, Lim S, Fischer C, Honek J, Yang Y, Andersson P, Nakamura M, Naslund E, Yla-Herttuala S, Sun M, Iwamoto H, Li X, Liu Y, Samani NJ, and Cao Y. Endothelial PDGF-CC regulates angiogenesis-dependent thermogenesis in beige fat. Nat Commun 7: 12152, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Sethi J, Sanchez-Alavez M, and Tabarean IV. Loss of histaminergic modulation of thermoregulation and energy homeostasis in obese mice. Neuroscience 217: 84–95, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Shabalina IG, Jacobsson A, Cannon B, and Nedergaard J. Native UCP1 displays simple competitive kinetics between the regulators purine nucleotides and fatty acids. The Journal of biological chemistry 279: 38236–38248, 2004. [DOI] [PubMed] [Google Scholar]
- 280.Shan B, Wang X, Wu Y, Xu C, Xia Z, Dai J, Shao M, Zhao F, He S, Yang L, Zhang M, Nan F, Li J, Liu J, Liu J, Jia W, Qiu Y, Song B, Han JJ, Rui L, Duan SZ, and Liu Y. The metabolic ER stress sensor IRE1alpha suppresses alternative activation of macrophages and impairs energy expenditure in obesity. Nat Immunol 2017. [DOI] [PubMed] [Google Scholar]
- 281.Shao M, Ishibashi J, Kusminski CM, Wang QA, Hepler C, Vishvanath L, MacPherson KA, Spurgin SB, Sun K, Holland WL, Seale P, and Gupta RK. Zfp423 Maintains White Adipocyte Identity through Suppression of the Beige Cell Thermogenic Gene Program. Cell Metab 23: 1167–1184, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Shi YC, Lau J, Lin Z, Zhang H, Zhai L, Sperk G, Heilbronn R, Mietzsch M, Weger S, Huang XF, Enriquez RF, Baldock PA, Zhang L, Sainsbury A, Herzog H, and Lin S. Arcuate NPY Controls Sympathetic Output and BAT Function via a Relay of Tyrosine Hydroxylase Neurons in the PVN. Cell Metab 17: 236–248, 2013. [DOI] [PubMed] [Google Scholar]
- 283.Singh R, Xiang Y, Wang Y, Baikati K, Cuervo AM, Luu YK, Tang Y, Pessin JE, Schwartz GJ, and Czaja MJ. Autophagy regulates adipose mass and differentiation in mice. J Clin Invest 119: 3329–3339, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Snitker S, Fujishima Y, Shen H, Ott S, Pi-Sunyer X, Furuhata Y, Sato H, and Takahashi M. Effects of novel capsinoid treatment on fatness and energy metabolism in humans: possible pharmacogenetic implications. Am J Clin Nutr 89: 45–50, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Song Z, Chen H, Fiket M, Alexander C, and Chan DC. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J Cell Biol 178: 749–755, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Srivastava S, Kashiwaya Y, King MT, Baxa U, Tam J, Niu G, Chen X, Clarke K, and Veech RL. Mitochondrial biogenesis and increased uncoupling protein 1 in brown adipose tissue of mice fed a ketone ester diet. FASEB J 26: 2351–2362, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng YH, and Goodyear LJ. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest 123: 215–223, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Stier A, Bize P, Habold C, Bouillaud F, Massemin S, and Criscuolo F. Mitochondrial uncoupling prevents cold-induced oxidative stress: a case study using UCP1 knockout mice. J Exp Biol 217: 624–630, 2014. [DOI] [PubMed] [Google Scholar]
- 289.Stine RR, Shapira SN, Lim HW, Ishibashi J, Harms M, Won KJ, and Seale P. EBF2 promotes the recruitment of beige adipocytes in white adipose tissue. Mol Metab 5: 57–65, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Suarez-Zamorano N, Fabbiano S, Chevalier C, Stojanovic O, Colin DJ, Stevanovic A, Veyrat-Durebex C, Tarallo V, Rigo D, Germain S, Ilievska M, Montet X, Seimbille Y, Hapfelmeier S, and Trajkovski M. Microbiota depletion promotes browning of white adipose tissue and reduces obesity. Nat Med 21: 1497–1501, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Sumara G, Sumara O, Kim JK, and Karsenty G. Gut-derived serotonin is a multifunctional determinant to fasting adaptation. Cell Metab 16: 588–600, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Sun C, Jiang L, Liu Y, Shen H, Weiss SJ, Zhou Y, and Rui L. Adipose Snail1 Regulates Lipolysis and Lipid Partitioning by Suppressing Adipose Triacylglycerol Lipase Expression. Cell Rep 17: 2015–2027, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Sun K, Kusminski CM, Luby-Phelps K, Spurgin SB, An YA, Wang QA, Holland WL, and Scherer PE. Brown adipose tissue derived VEGF-A modulates cold tolerance and energy expenditure. Mol Metab 3: 474–483, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Sun K, Wernstedt Asterholm I, Kusminski CM, Bueno AC, Wang ZV, Pollard JW, Brekken RA, and Scherer PE. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc Natl Acad Sci U S A 109: 5874–5879, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Susulic VS, Frederich RC, Lawitts J, Tozzo E, Kahn BB, Harper ME, Himms-Hagen J, Flier JS, and Lowell BB. Targeted disruption of the beta 3-adrenergic receptor gene. The Journal of biological chemistry 270: 29483–29492, 1995. [DOI] [PubMed] [Google Scholar]
- 296.Sutton AK, Pei H, Burnett KH, Myers MG Jr., Rhodes CJ, and Olson DP. Control of food intake and energy expenditure by Nos1 neurons of the paraventricular hypothalamus. J Neurosci 34: 15306–15318, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Svensson KJ, Long JZ, Jedrychowski MP, Cohen P, Lo JC, Serag S, Kir S, Shinoda K, Tartaglia JA, Rao RR, Chedotal A, Kajimura S, Gygi SP, and Spiegelman BM. A Secreted Slit2 Fragment Regulates Adipose Tissue Thermogenesis and Metabolic Function. Cell Metab 23: 454–466, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Tanaka T, Yoshida N, Kishimoto T, and Akira S. Defective adipocyte differentiation in mice lacking the C/EBPbeta and/or C/EBPdelta gene. EMBO J 16: 7432–7443, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Tateishi K, Okada Y, Kallin EM, and Zhang Y. Role of Jhdm2a in regulating metabolic gene expression and obesity resistance. Nature 458: 757–761, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Thomas SA, and Palmiter RD. Thermoregulatory and metabolic phenotypes of mice lacking noradrenaline and adrenaline. Nature 387: 94–97, 1997. [DOI] [PubMed] [Google Scholar]
- 301.Timmons JA, Wennmalm K, Larsson O, Walden TB, Lassmann T, Petrovic N, Hamilton DL, Gimeno RE, Wahlestedt C, Baar K, Nedergaard J, and Cannon B. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci U S A 104: 4401–4406, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Tomlinson E, Fu L, John L, Hultgren B, Huang X, Renz M, Stephan JP, Tsai SP, Powell-Braxton L, French D, and Stewart TA. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology 143: 1741–1747, 2002. [DOI] [PubMed] [Google Scholar]
- 303.Tong Q, Ye CP, Jones JE, Elmquist JK, and Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci 11: 998–1000, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Townsend KL, An D, Lynes MD, Huang TL, Zhang H, Goodyear LJ, and Tseng YH. Increased Mitochondrial Activity in BMP7-Treated Brown Adipocytes, Due to Increased CPT1- and CD36-Mediated Fatty Acid Uptake. Antioxid Redox Signal 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Trajkovski M, Ahmed K, Esau CC, and Stoffel M. MyomiR-133 regulates brown fat differentiation through Prdm16. Nat Cell Biol 14: 1330–1335, 2012. [DOI] [PubMed] [Google Scholar]
- 306.Tran CM, Mukherjee S, Ye L, Frederick DW, Kissig M, Davis JG, Lamming DW, Seale P, and Baur JA. Rapamycin blocks induction of the thermogenic program in white adipose tissue. Diabetes 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Tran KV, Gealekman O, Frontini A, Zingaretti MC, Morroni M, Giordano A, Smorlesi A, Perugini J, De Matteis R, Sbarbati A, Corvera S, and Cinti S. The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab 15: 222–229, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, Ahrens MJ, Dudley AT, Norris AW, Kulkarni RN, and Kahn CR. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 454: 1000–1004, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Tsukiyama-Kohara K, Poulin F, Kohara M, DeMaria CT, Cheng A, Wu Z, Gingras AC, Katsume A, Elchebly M, Spiegelman BM, Harper ME, Tremblay ML, and Sonenberg N. Adipose tissue reduction in mice lacking the translational inhibitor 4E-BP1. Nat Med 7: 1128–1132, 2001. [DOI] [PubMed] [Google Scholar]
- 310.Tupone D, Madden CJ, Cano G, and Morrison SF. An orexinergic projection from perifornical hypothalamus to raphe pallidus increases rat brown adipose tissue thermogenesis. J Neurosci 31: 15944–15955, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Tupone D, Madden CJ, and Morrison SF. Autonomic regulation of brown adipose tissue thermogenesis in health and disease: potential clinical applications for altering BAT thermogenesis. Front Neurosci 8: 14, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Ueta CB, Fernandes GW, Capelo LP, Fonseca TL, Maculan FD, Gouveia CH, Brum PC, Christoffolete MA, Aoki MS, Lancellotti CL, Kim B, Bianco AC, and Ribeiro MO. beta(1) Adrenergic receptor is key to cold- and diet-induced thermogenesis in mice. J Endocrinol 214: 359–365, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Ukropec J, Anunciado RP, Ravussin Y, Hulver MW, and Kozak LP. UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1−/− mice. The Journal of biological chemistry 281: 31894–31908, 2006. [DOI] [PubMed] [Google Scholar]
- 314.Valverde AM, Arribas M, Mur C, Navarro P, Pons S, Cassard-Doulcier AM, Kahn CR, and Benito M. Insulin-induced up-regulated uncoupling protein-1 expression is mediated by insulin receptor substrate 1 through the phosphatidylinositol 3-kinase/Akt signaling pathway in fetal brown adipocytes. The Journal of biological chemistry 278: 10221–10231, 2003. [DOI] [PubMed] [Google Scholar]
- 315.van den Beukel JC, Boon MR, Steenbergen J, Rensen PC, Meijer OC, Themmen AP, and Grefhorst A. Cold Exposure Partially Corrects Disturbances in Lipid Metabolism in a Male Mouse Model of Glucocorticoid Excess. Endocrinology 156: 4115–4128, 2015. [DOI] [PubMed] [Google Scholar]
- 316.van den Beukel JC, Grefhorst A, Quarta C, Steenbergen J, Mastroberardino PG, Lombes M, Delhanty PJ, Mazza R, Pagotto U, van der Lely AJ, and Themmen AP. Direct activating effects of adrenocorticotropic hormone (ACTH) on brown adipose tissue are attenuated by corticosterone. FASEB J 28: 4857–4867, 2014. [DOI] [PubMed] [Google Scholar]
- 317.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, and Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med 360: 1500–1508, 2009. [DOI] [PubMed] [Google Scholar]
- 318.Vegiopoulos A, Muller-Decker K, Strzoda D, Schmitt I, Chichelnitskiy E, Ostertag A, Berriel Diaz M, Rozman J, Hrabe de Angelis M, Nusing RM, Meyer CW, Wahli W, Klingenspor M, and Herzig S. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science 328: 1158–1161, 2010. [DOI] [PubMed] [Google Scholar]
- 319.Vila-Bedmar R, Lorenzo M, and Fernandez-Veledo S. Adenosine 5’-monophosphate-activated protein kinase-mammalian target of rapamycin cross talk regulates brown adipocyte differentiation. Endocrinology 151: 980–992, 2010. [DOI] [PubMed] [Google Scholar]
- 320.Villanueva CJ, Vergnes L, Wang J, Drew BG, Hong C, Tu Y, Hu Y, Peng X, Xu F, Saez E, Wroblewski K, Hevener AL, Reue K, Fong LG, Young SG, and Tontonoz P. Adipose subtype-selective recruitment of TLE3 or Prdm16 by PPARgamma specifies lipid storage versus thermogenic gene programs. Cell Metab 17: 423–435, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, and Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med 360: 1518–1525, 2009. [DOI] [PubMed] [Google Scholar]
- 322.Vishvanath L, MacPherson KA, Hepler C, Wang QA, Shao M, Spurgin SB, Wang MY, Kusminski CM, Morley TS, and Gupta RK. Pdgfrbeta(+) Mural Preadipocytes Contribute to Adipocyte Hyperplasia Induced by High-Fat-Diet Feeding and Prolonged Cold Exposure in Adult Mice. Cell Metab 23: 350–359, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Wang C, Bomberg E, Billington C, Levine A, and Kotz CM. Brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus increases energy expenditure by elevating metabolic rate. Am J Physiol Regul Integr Comp Physiol 293: R992–1002, 2007. [DOI] [PubMed] [Google Scholar]
- 324.Wang GX, Zhao XY, Meng ZX, Kern M, Dietrich A, Chen Z, Cozacov Z, Zhou D, Okunade AL, Su X, Li S, Bluher M, and Lin JD. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat Med 20: 1436–1443, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Wang H, Liu L, Lin JZ, Aprahamian TR, and Farmer SR. Browning of White Adipose Tissue with Roscovitine Induces a Distinct Population of UCP1+ Adipocytes. Cell Metab 24: 835–847, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Wang H, Zhang Y, Yehuda-Shnaidman E, Medvedev AV, Kumar N, Daniel KW, Robidoux J, Czech MP, Mangelsdorf DJ, and Collins S. Liver X receptor alpha is a transcriptional repressor of the uncoupling protein 1 gene and the brown fat phenotype. Mol Cell Biol 28: 2187–2200, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Wang L, Liu J, Saha P, Huang J, Chan L, Spiegelman B, and Moore DD. The orphan nuclear receptor SHP regulates PGC-1alpha expression and energy production in brown adipocytes. Cell Metab 2: 227–238, 2005. [DOI] [PubMed] [Google Scholar]
- 328.Wang L, Teng R, Di L, Rogers H, Wu H, Kopp JB, and Noguchi CT. PPARalpha and Sirt1 mediate erythropoietin action in increasing metabolic activity and browning of white adipocytes to protect against obesity and metabolic disorders. Diabetes 62: 4122–4131, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Wang QA, Tao C, Gupta RK, and Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 19: 1338–1344, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Wang QA, Tao C, Jiang L, Shao M, Ye R, Zhu Y, Gordillo R, Ali A, Lian Y, Holland WL, Gupta RK, and Scherer PE. Distinct regulatory mechanisms governing embryonic versus adult adipocyte maturation. Nat Cell Biol 17: 1099–1111, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Wang W, Kissig M, Rajakumari S, Huang L, Lim HW, Won KJ, and Seale P. Ebf2 is a selective marker of brown and beige adipogenic precursor cells. Proc Natl Acad Sci U S A 111: 14466–14471, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Wang YX, Lee CH, Tiep S, Yu RT, Ham J, Kang H, and Evans RM. Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell 113: 159–170, 2003. [DOI] [PubMed] [Google Scholar]
- 333.Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, and Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439: 484–489, 2006. [DOI] [PubMed] [Google Scholar]
- 334.Whittle AJ, Carobbio S, Martins L, Slawik M, Hondares E, Vazquez MJ, Morgan D, Csikasz RI, Gallego R, Rodriguez-Cuenca S, Dale M, Virtue S, Villarroya F, Cannon B, Rahmouni K, Lopez M, and Vidal-Puig A. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell 149: 871–885, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Wikstrom JD, Mahdaviani K, Liesa M, Sereda SB, Si Y, Las G, Twig G, Petrovic N, Zingaretti C, Graham A, Cinti S, Corkey BE, Cannon B, Nedergaard J, and Shirihai OS. Hormone-induced mitochondrial fission is utilized by brown adipocytes as an amplification pathway for energy expenditure. EMBO J 33: 418–436, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Wolins NE, Quaynor BK, Skinner JR, Tzekov A, Croce MA, Gropler MC, Varma V, Yao-Borengasser A, Rasouli N, Kern PA, Finck BN, and Bickel PE. OXPAT/PAT-1 is a PPAR-induced lipid droplet protein that promotes fatty acid utilization. Diabetes 55: 3418–3428, 2006. [DOI] [PubMed] [Google Scholar]
- 337.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, and Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332: 243–247, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerback S, Schrauwen P, and Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150: 366–376, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Wu J, Cohen P, and Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev 27: 234–250, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Wu MV, Bikopoulos G, Hung S, and Ceddia RB. Thermogenic capacity is antagonistically regulated in classical brown and white subcutaneous fat depots by high-fat diet and endurance training in rats: Impact on whole-body energy expenditure. The Journal of biological chemistry 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Wu N, Zheng B, Shaywitz A, Dagon Y, Tower C, Bellinger G, Shen CH, Wen J, Asara J, McGraw TE, Kahn BB, and Cantley LC. AMPK-dependent degradation of TXNIP upon energy stress leads to enhanced glucose uptake via GLUT1. Mol Cell 49: 1167–1175, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Wu Q, Kazantzis M, Doege H, Ortegon AM, Tsang B, Falcon A, and Stahl A. Fatty acid transport protein 1 is required for nonshivering thermogenesis in brown adipose tissue. Diabetes 55: 3229–3237, 2006. [DOI] [PubMed] [Google Scholar]
- 343.Xu P, Cao X, He Y, Zhu L, Yang Y, Saito K, Wang C, Yan X, Hinton AO Jr., Zou F, Ding H, Xia Y, Yan C, Shu G, Wu SP, Yang B, Feng Y, Clegg DJ, DeMarchi R, Khan SA, Tsai SY, DeMayo FJ, Wu Q, Tong Q, and Xu Y. Estrogen receptor-alpha in medial amygdala neurons regulates body weight. J Clin Invest 125: 2861–2876, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Xu X, Ying Z, Cai M, Xu Z, Li Y, Jiang SY, Tzan K, Wang A, Parthasarathy S, He G, Rajagopalan S, and Sun Q. Exercise ameliorates high-fat diet-induced metabolic and vascular dysfunction, and increases adipocyte progenitor cell population in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 300: R1115–1125, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, Zhang X, Zou F, Gent LM, Hahner LD, Khan SA, Elias CF, Elmquist JK, and Clegg DJ. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab 14: 453–465, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Xue Y, Petrovic N, Cao R, Larsson O, Lim S, Chen S, Feldmann HM, Liang Z, Zhu Z, Nedergaard J, Cannon B, and Cao Y. Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell Metab 9: 99–109, 2009. [DOI] [PubMed] [Google Scholar]
- 347.Yadav H, Quijano C, Kamaraju AK, Gavrilova O, Malek R, Chen W, Zerfas P, Zhigang D, Wright EC, Stuelten C, Sun P, Lonning S, Skarulis M, Sumner AE, Finkel T, and Rane SG. Protection from obesity and diabetes by blockade of TGF-beta/Smad3 signaling. Cell Metab 14: 67–79, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Yamaguchi T, Matsushita S, Motojima K, Hirose F, and Osumi T. MLDP, a novel PAT family protein localized to lipid droplets and enriched in the heart, is regulated by peroxisome proliferator-activated receptor alpha. The Journal of biological chemistry 281: 14232–14240, 2006. [DOI] [PubMed] [Google Scholar]
- 349.Yamaguchi T, Omatsu N, Morimoto E, Nakashima H, Ueno K, Tanaka T, Satouchi K, Hirose F, and Osumi T. CGI-58 facilitates lipolysis on lipid droplets but is not involved in the vesiculation of lipid droplets caused by hormonal stimulation. J Lipid Res 48: 1078–1089, 2007. [DOI] [PubMed] [Google Scholar]
- 350.Yan M, Audet-Walsh E, Manteghi S, Rosa Dufour C, Walker B, Baba M, St-Pierre J, Giguere V, and Pause A. Chronic AMPK activation via loss of FLCN induces functional beige adipose tissue through PGC-1alpha/ERRalpha. Genes Dev 30: 1034–1046, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Yang Q, Liang X, Sun X, Zhang L, Fu X, Rogers CJ, Berim A, Zhang S, Wang S, Wang B, Foretz M, Viollet B, Gang DR, Rodgers BD, Zhu MJ, and Du M. AMPK/alpha-Ketoglutarate Axis Dynamically Mediates DNA Demethylation in the Prdm16 Promoter and Brown Adipogenesis. Cell Metab 24: 542–554, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Yang X, Lu X, Lombes M, Rha GB, Chi YI, Guerin TM, Smart EJ, and Liu J. The G(0)/G(1) switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab 11: 194–205, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Yilmaz Y, Ones T, Purnak T, Ozguven S, Kurt R, Atug O, Turoglu HT, and Imeryuz N. Association between the presence of brown adipose tissue and non-alcoholic fatty liver disease in adult humans. Aliment Pharmacol Ther 34: 318–323, 2011. [DOI] [PubMed] [Google Scholar]
- 354.Yin H, Pasut A, Soleimani VD, Bentzinger CF, Antoun G, Thorn S, Seale P, Fernando P, van Ijcken W, Grosveld F, Dekemp RA, Boushel R, Harper ME, and Rudnicki MA. MicroRNA-133 controls brown adipose determination in skeletal muscle satellite cells by targeting Prdm16. Cell Metab 17: 210–224, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y, Iwanaga T, and Saito M. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest 123: 3404–3408, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Yoon Y, Krueger EW, Oswald BJ, and McNiven MA. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol Cell Biol 23: 5409–5420, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Yu J, Zhang S, Cui L, Wang W, Na H, Zhu X, Li L, Xu G, Yang F, Christian M, and Liu P. Lipid droplet remodeling and interaction with mitochondria in mouse brown adipose tissue during cold treatment. Biochimica et biophysica acta 1853: 918–928, 2015. [DOI] [PubMed] [Google Scholar]
- 358.Yu S, Qualls-Creekmore E, Rezai-Zadeh K, Jiang Y, Berthoud HR, Morrison CD, Derbenev AV, Zsombok A, and Munzberg H. Glutamatergic Preoptic Area Neurons That Express Leptin Receptors Drive Temperature-Dependent Body Weight Homeostasis. J Neurosci 36: 5034–5046, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Zha L, Li F, Wu R, Artinian L, Rehder V, Yu L, Liang H, Xue B, and Shi H. The Histone Demethylase UTX Promotes Brown Adipocyte Thermogenic Program Via Coordinated Regulation of H3K27 Demethylation and Acetylation. The Journal of biological chemistry 290: 25151–25163, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Zhang C, McFarlane C, Lokireddy S, Masuda S, Ge X, Gluckman PD, Sharma M, and Kambadur R. Inhibition of myostatin protects against diet-induced obesity by enhancing fatty acid oxidation and promoting a brown adipose phenotype in mice. Diabetologia 55: 183–193, 2012. [DOI] [PubMed] [Google Scholar]
- 361.Zhang W, Sunanaga J, Takahashi Y, Mori T, Sakurai T, Kanmura Y, and Kuwaki T. Orexin neurons are indispensable for stress-induced thermogenesis in mice. J Physiol 588: 4117–4129, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Zhang Y, Kerman IA, Laque A, Nguyen P, Faouzi M, Louis GW, Jones JC, Rhodes C, and Munzberg H. Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. J Neurosci 31: 1873–1884, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Zhao XY, Li S, Wang GX, Yu Q, and Lin JD. A long noncoding RNA transcriptional regulatory circuit drives thermogenic adipocyte differentiation. Mol Cell 55: 372–382, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Zhu Q, Ghoshal S, Rodrigues A, Gao S, Asterian A, Kamenecka TM, Barrow JC, and Chakraborty A. Adipocyte-specific deletion of Ip6k1 reduces diet-induced obesity by enhancing AMPK-mediated thermogenesis. J Clin Invest 126: 4273–4288, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Zhu Y, Gao Y, Tao C, Shao M, Zhao S, Huang W, Yao T, Johnson JA, Liu T, Cypess AM, Gupta O, Holland WL, Gupta RK, Spray DC, Tanowitz HB, Cao L, Lynes MD, Tseng YH, Elmquist JK, Williams KW, Lin HV, and Scherer PE. Connexin 43 Mediates White Adipose Tissue Beiging by Facilitating the Propagation of Sympathetic Neuronal Signals. Cell Metab 24: 420–433, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, and Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306: 1383–1386, 2004. [DOI] [PubMed] [Google Scholar]
- 367.Zylan KD, and Carlisle HJ. Effect of ambient temperature on the paradoxical metabolic responses to norepinephrine. Pharmacol Biochem Behav 43: 577–582, 1992. [DOI] [PubMed] [Google Scholar]