Abstract
Objective:
Intra-cranial pressure (ICP) after mild traumatic brain injury (mTBI) is poorly studied due to lack of sensitive non-invasive methods. The purpose of this review was to summarize the existing knowledge of changes in ICP after mTBI.
Literature selection:
PubMed, Embase, CINAHL, and Scopus were searched by 3 reviewers independently up to December 2016. Inclusion criteria: animal and human studies measuring ICP and brain oedema after an mTBI. Exclusion criteria: Moderate and severe forms of traumatic brain injury, repeat samples, and studies that measured ICP at the time of impact but not after. Study quality was assessed using Downs and Black criteria.
Results:
Of 1067 papers, 9 studies were included. In human studies, 1 provided direct evidence on increased, 1 provided indirect evidence of increased, and 2 provided indirect evidence of decreased ICP. In animal studies, 3 studies provided direct evidence of increased, 1 provided indirect evidence of increased, and 1 provided indirect evidence of no change in ICP.
Conclusion:
The existing research suggests that there may be increased ICP after mTBI and animal studies suggest an elevation for days which returns to baseline, which corresponds with functional and symptomatic recovery. Future human studies using sensitive indirect methods to measure ICP longitudinally after mTBI are needed.
Keywords: concussion, cerebral blood flow
INTRODUCTION
Mild traumatic brain injuries (mTBI) are caused by trauma to the head or neck that results in physiological dysfunction manifest as loss of consciousness, altered mental status, or transient memory loss.1 It is characterized by absence of observable changes on conventional imaging and a Glasgow Coma Scale of 13 to 15.2 It is estimated that 42 million people worldwide suffer some form of mTBI every year and that the majority of them do not seek medical attention.3 Concussion, a subcategory of mTBI, is thought to be reversible and is often caused by sports.4,5 It is estimated that 1.6 to 3.8 million brain injuries occur in sports every year in the USA, the majority of them being mTBI.6 The pathophysiology of mTBI remains poorly understood.7
Intra-cranial pressure (ICP) is the pressure of the cerebrospinal fluid in the subarachnoid space. Normal values are 7–15 mmHg in a healthy supine adult and −10 mmHg in the standing position.8 Increased ICP is well documented in moderate and severe forms of traumatic brain injury (TBI) due to gross swelling or mass effect from bleeding.9 Since the brain exists within a stiff skull, increased ICP can impair cerebral blood flow (CBF) and cause secondary ischemic insult.10,11 Research is confirming cerebrovascular dysfunction that characterizes mTBI, including depressed resting CBF, regional alterations in CBF, and greater CBF for a given level of exercise intensity.12–15 It is hypothesized that these to contribute to the classic symptoms of mTBI.16
The symptoms of increased ICP include but are not limited to headache, behavioural problems, nausea, and vision problems,17 which overlap with the symptoms of mTBI and concussion.5,18 ICP has not been studied in much detail in mTBI. Direct methods such as catheters are invasive, have high risk of complications, and are not justified for mTBI,19 whereas indirect methods have poor sensitivity and reliability.20 Hypertonic saline is used to decrease ICP in severe brain injuries.21 In a randomized double blind study in the emergency department, Lumba-Brown et al.22 showed that intravenous hypertonic (3%) saline was more effective than normal saline in acutely reducing concussion pain in children 4 to 7 years of age with a GCS score greater than 13, suggesting that decreasing ICP may be an effective treatment for mTBI. The purpose of this review was to systematically search the literature and to summarize the existing knowledge about ICP changes after mTBI.
METHODS
PRISMA guidelines for systematic reviews were followed.23 This review was prospectively registered on PROSPERO24 (Registration number CRD42016040140).
Literature search:
PubMed (1946-onwards), Embase (1947-onwards), CINAHL (1937-onwards), and Scopus (1823-onwards) were searched on January 2017 up till December 2016 using combinations of the following terms; mild traumatic brain injury, concussion, Glasgow coma scale 13–15, intra-cranial pressure, intra-cranial hypertension, brain oedema, cerebral pressure, intra-ocular pressure, optic disk, optic nerve sheath diameter, ultrasonography, brain swelling, and papilledema. Human and animal models were included. There was no limit to the year of publication. The exact search syntax is provided online. Title/abstract screen and data extraction were performed by three independent reviewers.
Study selection criteria:
Inclusion criteria: Animal and human articles that directly or indirectly measured ICP after mTBI. Articles whose main focus was not measurement of ICP but suggested the presence or absence of a change in ICP were included. Additionally, the articles had to explain how ICP was measured. Some articles, specifically animal studies, were confusing since no universal criteria of TBI severity were used. These articles were assessed by an expert on animal traumatic brain injuries and were included if he agreed they could be considered mTBI on the basis of mortality rate, pressure, and/or method of injury.
Exclusion criteria: Articles that did not directly or indirectly measure ICP after mTBI, articles whose severity of brain injury was greater than mild, non-brain injury, and review articles. For those studies using the same sample population, the article that described ICP was used. If both described ICP then the earlier article was used. Articles that measured only ICP during impact of injury but not after were excluded.
Data extraction:
Full texts of screened articles were read in their entirety, the following data was extracted using a uniform data extraction form: author, year of publication, study design, animal or human, sample size, method of ICP measurement, cause of mTBI, time interval from injury to measurement, and results of ICP measurement. Conflicting results were resolved in a meeting of the reviewers. Pairwise Cohen’s Kappa of inter-rater reliability (non-weighted) after title/abstract screen was calculated.25
Risk of bias and level of evidence:
Study Quality Assessment was performed using the Downs and Black Criteria26, this is a validated checklist that assess risk of bias in randomized and non-randomized studies. Level of Evidence was determined using guidelines set forth by Melnyk et al.27, this system uses a seven level grading system that begins with systematic review of randomized control trials (Level 1) on down to expert opinion (Level 7).
RESULTS
Literature search:
The results on of the literature search is presented in Figure 1. PubMed yielded 591, Embase yielded 621, CINAHL yielded 117, and Scopus yielded 814 results. After excluding the duplicate articles, a total of 1067 non-duplicate studies were retrieved. Title and abstract screen was performed and the full text of 55 articles were read. Thirty five articles were excluded because they did not measure ICP after an mTBI. Eleven animal studies were excluded during data extraction for reasons explained below. Nine articles met inclusion criteria for this systematic review. Pairwise Cohen’s Kappa of inter-rater reliability of after title/abstract screen was 0.61. Study characteristics of included studies are presented in Table 1. One abstract/poster presented in 2015, Serrador et al.28, had interesting findings. They showed that there was a significant increase of indirectly measured ICP 2 hours after a sports-related concussion when compared with controls (14±3 vs. 7±1 mmHg, P=0.032). We contacted the author but, at the time of submission of this review, no original article had been published.
Figure 1.
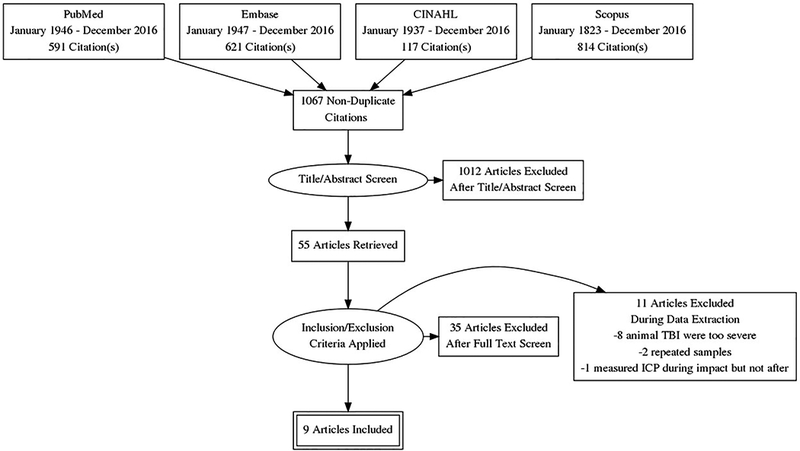
PRISMA flow chart.
Table 1.
Study Characteristics
| First Author | Year | Study Design | Animal/ Human | Sample Size | Cause of mTBI | Age |
|---|---|---|---|---|---|---|
| Jarrett41 | 2016 | Prospective Cohort | Human | 45 athletes, 15 controls | Sports related concussion, hockey. | 21.2 ± 3.1 years |
| Lumba-Brown 22 | 2014 | RCT; intent-to-treat | Human | 21 injured, 23 controls | Fall or direct blow. | 12.1 ± 2.5 years |
| Pomschar 50 | 2013 | Case-control | Human | 15 injured, 15 controls | Fall or motor vehicle accident, possible whiplash. | Mean 27.2 years |
| Toth 42 | 2013 | Case-control | Human | 14 injured, 14 controls | Not mentioned, but all were uncomplicated mTBI i.e a GCS of 15, loss of consciousness for less than 1 min, posttraumatic amnesia for less than 30 min, and a normal posttraumatic CT read by a board-certified neuroradiologist. | Injured: 34.9 ± 18.4, controls: 35.8 ± 18.5 |
| Bolouri 38 | 2012 | Case-Control | Animal | 42 rats (33 intervention, 9 control) | Three impacts of a 50g projectile at a velocity of 11.2m/s to a helmet-protected rat head. | Not mentioned |
| Donovan51 | 2012 | Case-control | Animal | 50 male Sprague-Dawley rats | Mild controlled cortical impact (MCCI) either 3 or 7 days apart. Craniotomy and MCCI was delivered to cortical surface using a piston. | 2–4 months rats |
| Kane43 | 2012 | Case-control | Animal | 327 mice | Focal impact by dropping a weight onto mouse head. | Not mentioned |
| Saljo 53 | 2010 | Case-control | Animal | 180 male Wistar rats | Blast exposure was generated with a 3.1 meter long, 0.2 meter diameter shock tube. Pulses with a Pmax of 10, 30, and 60 kPa were generated with pressures of 0.2, 0.6, and 1.2 atm, respectively, and at a duration of 4–6 msec. | Not mentioned |
| Lewelt 47 | 1980 | Experimental | Animal | 24 cats | Cerebral trauma caused by apparatus inducing fluid percussion injury. | Adult cats |
Excluded articles during data extraction:
Saljo et al.29 only measured ICP during simulated blast injury and not after. Cernak et al.30, Gurdjian et al.31 Prins et al.32, Engelborghs et al.33, Chandra et al.34, Abdul-Muneer et al.35, Obenaus et al.36, and Millen et al.37 could not be equated to human mTBI as assessed by an expert in animal traumatic brain injury based upon force of injury, cognitive impairment, or fatality rate. Bolouri et al.38 and Hamberger et al.39 used the same sample and Saljo et al.40 summarized previous data.
Study quality assessment and level of evidence:
Table 2 includes the level of evidence and study quality assessment. All the articles except Lumba-Brown22 were either case-control or cohort studies with a Level of Evidence of 4 according to the criteria set forth by Melnyk et al..27 Study quality, according to the Downs and Black Criteria26, was low to moderate for most of the studies. Studies in general had well defined objectives (Q1) and main outcomes (Q2). Some animal studies did not specify their sample characteristics (Q3) and only mentioned the species. Interventions or investigation (Q4), principal confounders (Q5), main findings (Q6), and the random variability (Q7) were explained in almost all studies. Adverse events (Q8) did not apply to most of the studies since they were observational. Patient follow-up (Q9), probability values (Q10), and source population (Q11 and 12) were clearly documented in almost all studies. Treatment facilities (Q13) and blinding (Q14 and 15) did not apply to the majority of the studies since they were specific to treatment. Studies were well planned (Q16 and 17) and used appropriate analysis (Q18). Compliance and follow-up (Q19 and 26) was not reported well in human studies. Internal validity (Q20–22) was appropriately documented in all the studies except that there was very little adjustment for confounding variables (Q25). Randomization (Q23 and 24) and power (Q27) were applicable only to randomized control trials.
Table 2.
Study Quality Assessment and Level of Evidence
| First author | LOE | Downs and Black Question | Total | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | |||
| Jarrett41 | 4 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | - | 0 | 1 | 1 | 0 | 1 | - | - | 1 | 1 | 1 | 0 | 1 | 1 | 1 | - | - | 0 | 0 | - | 17 |
| Lumba-Brown 22 | 2 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 5 | 28 |
| Pomschar 50 | 4 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | - | 0 | 1 | 1 | 1 | 1 | - | - | 1 | 0 | 1 | 0 | 1 | 0 | 0 | - | - | 0 | 0 | - | 14 |
| Toth 42 | 4 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | - | 0 | 1 | 1 | 1 | 1 | - | - | 1 | 1 | 1 | 0 | 1 | 1 | 1 | - | - | 1 | 0 | - | 19 |
| Bolouri 38 | 4 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | - | 1 | 1 | 1 | 1 | 1 | - | - | 0 | 1 | 1 | 1 | 1 | 1 | 1 | - | - | 0 | 1 | - | 19 |
| Donovan51 | 4 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | - | 1 | 1 | 1 | 1 | 1 | - | - | 1 | 1 | 1 | 1 | 1 | 1 | 1 | - | - | 1 | 1 | - | 22 |
| Kane43 | 4 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | - | 1 | 0 | 1 | 1 | 1 | - | - | 1 | 0 | 1 | 1 | 1 | 0 | 1 | - | - | 0 | 1 | - | 16 |
| Saljo 53 | 4 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | - | 1 | 1 | 1 | 1 | 1 | - | - | 1 | 1 | 1 | 1 | 1 | 1 | 1 | - | - | 0 | 1 | - | 21 |
| Lewelt 47 | 4 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | - | 1 | 0 | 1 | 1 | 1 | - | - | 1 | 0 | 0 | 1 | 1 | - | - | - | - | 0 | 1 | - | 12 |
Intra-cranial pressure and measurement methods:
In human studies, 1 study provided direct evidence using MRI and 3 studies provided indirect evidence using either MRI or changes in FACES (Visual Analogue Scale) pain chart to measure symptoms after administration of 3% saline. In animal studies, 3 studies provided direct evidence using invasive ICP monitoring and 2 studies provided indirect evidence using either MRI for volume analysis or post-mortem brain weight to measure oedema. Two out of 4 human studies suggest an increase in ICP and 2 did not (Jarrett et al.41 and Toth et al.42 identified reduced brain volume which suggests a decrease in ICP). Four out of 5 animal studies suggest an increase in ICP and 1 did not (Kane et al.43 found no formation of oedema which suggest no change in ICP). The individual study details on ICP and its measurement are presented in Table 3.
Table 3.
Details of ICP
| Study | Increase/ decrease of ICP | Method of measurement | ICP details | Position | Time Intervals |
|---|---|---|---|---|---|
| Human Studies (Direct Evidence / Measured) | |||||
| Pomschar 50 | Increase | MR equivalent ICP based on compliance; Compliance: ratio of volume and pressure changes; venous outflow patterns. | MR ICP: 12.5±2.9 mmHg in injured versus 8.8±2.0mmHg in controls | Supine | Mean 11.4 years. |
| Human Studies (Indirect Evidence / Inferred) | |||||
| Jarrett41 | Decrease in brain volume (correlates to decreased ICP) | Two time point global brain volume changes were estimated based on the three-dimensional T1-weighted MRI scans of the brain and skull. Images from the two time points was used to measure percent brain volume change (PBVC). All PBVC measurements were made against each subject’s baseline scan. | Compared to controls, PBVC of the concussed players were not significantly reduced 3 days after concussion (−0.11%, p = 0.15). Two weeks and 2 months after concussion, PBVC of −0.08% (p = 0.027) and −0.23% (p = 0.035) were significant compared to controls. No differences were seen at the end of the season between concussed and non-concussed athletes. | Supine | 72 hours, 2 weeks and 2 months after concussion. |
| Lumba-Brown 22 | Increase | Change in Faces Pain Scale upon discharge and follow up phone call (mean 5 days). | Mean reduction of 3.5 points in hypertonic saline group vs 1.1 points in the normal saline group on the Faces Pain Scale. | Cannot be determined | Average of 2–3 days after treatment. |
| Toth 42 | Decrease in brain volume (correlates to decreased ICP) | MRI. Volume analysis was done using tensor-based morphometry on T-1 weighted scan on concussed patients longitudinally and compared with healthy controls. | A significant (p < 0.05) decrease in cortical volumes (mean 1%) and increase in ventricular volumes (mean 3.4%) appeared at 1 month after injury in the mTBI group. No statistically significant differences were seen in control group. | Supine | Mean 2 days from injury (range 12 – 72 hours) and 35 days from injury (range 28 – 43 days) |
| Animal Studies (Direct Evidence / Measured) | |||||
| Bolouri 38 | Increase | A miniature optic probe (diameter 0.42 mm), the probe was inserted through a 1.3-mm hole drilled in the skull 2 mm behind the bregma and 2 mm from the midline, and into the brain parenchyma to a depth of 6 mm from the surface of the skull. | Baseline ICP was 5.7mmHg which maximally increased 10 h after impact to 14mmHg which returned to normal 7 days later. | Cannot be determined | 6 hours, 10 hours, Day 1, Day 2, Day 3, Day 5, Day 7 after injury. |
| Saljo 53 | Increase | A miniature optic probe (diameter 0.42 mm), the probe was inserted through a 1.3-mm hole drilled in the skull 2 mm behind the bregma and 2 mm from the midline, and into the brain parenchyma to a depth of 6 mm from the surface of the skull. | The mean value in the control groups ranged between 5.8 and 6.6 mmHg. In the standard-feed animals exposed at 30 kPa, the mean ICP had increased by 90% at 10h (p<0.0001). The corresponding peak value in the animals on processed cereal feed showed an increase of 60%. In rats exposed to 60 kPa, the group on standard feed had an ICP elevation of 145% at 10h, while those on processed cereal feed had increases of only 50% (p < 0.0001). The ICP continued to be elevated till day 7 and functional recovery occurred on day 8. | Cannot be determined | 6 hours, 10 hours, Day 1, Day 2, Day 3, Day 5, Day 7 after injury. |
| Lewelt 47 | Increase | Animal fitted with a stent and wells stereotaxic frame with a catheter accommodation to measure ICP. | In the low injury group (which is similar to mTBI) the ICP rose from a rest value of 0–10mmHg to 33±11 mmHg which normalized over the next hour. | Cannot be determined | 1 hour intervals. |
| Animal Studies (Indirect Evidence / Inferred) | |||||
| Donovan51 | Increase | Enhanced MRI quantification methods with regions of interest manually drawn. | Total brain volume increase by 1.4.002% after first injury. In the repeated injury participants, the first injury was still edematous 3 days after but not 7 days after first injury. No observable difference could be seen after 14 days. | Supine | Day 3, Day 7, Day 14, and Day 60 after injury. |
| Kane43 | No formation of edema | Edema determined as brain water content; brains of decapitated mice were weighed using a wet/dry method. | There was no significant increase in brain weight in the repetitive mTBI group in post-mortem analysis when compared to controls. | Cannot be determined. | 24 hours and Day 7. |
DISCUSSION
We systematically reviewed the literature to search for studies that measured ICP after mTBI. Since this is an understudied area, it wasn’t surprising that we found a limited, heterogeneous group of studies. Increased ICP during severe or moderate TBI is a well-known phenomenon due to the mass effect of bleeding or gross swelling of the brain but it hasn’t been studied to great extent in mTBI. The majority of the articles that met our inclusion criteria suggested increased ICP after mTBI, but majority of them were animal studies whose results cannot be directly compared with human studies. The pathophysiology of this increase is not known. Changes in ICP could be due to alterations in CBF and autonomic nervous system (ANS) seen in mTBI patients.44 The primary ANS control center located in the brainstem may be damaged particularly if there is a rotational force applied to the upper cervical spine as seen in head injuries.45 Consistent with this, brainstem DTI changes have been reported in patients with post-concussion syndrome, a form of mTBI.46 More research in this area is required.
Level of Evidence and Study Quality:
Most studies had a moderate Level of Evidence and were low to moderate quality. Lewelt et al.47 scored relatively low, perhaps because it is a much older study that used different criteria to minimize and report bias. Lumba-Brown22 scored relatively high because it is the only randomized control trial and all the components of the Downs and Black criteria were applicable to it.
Intra-Cranial Pressure and Measurement:
The four human studies are heterogeneous. The results of Lumba-Brown et al.22 suggested increased ICP after a significant reduction in acute post-concussive headache with 3% hypertonic saline. Hypertonic saline is a commonly used and effective pharmacotherapy for increased ICP.11,48,49 This study is important because it suggests we can treat some of the symptoms of mTBI by pharmacologically decreasing ICP. However, other explanations should also be investigated. Normal saline (NS) administration has beneficial properties of plasma volume expansion, and this study demonstrated that patients who received NS had an acute improvement of pain immediately following administration, but at a lesser value. A limitation of the study was not including a control arm which received no treatment.
Pomschar et al.50 used MRI to indirectly measure ICP via changes in compliance and venous outflow. They showed a pressure of 12.5 ± 2.9 mmHg in people with a history of concussion versus 8.8 ± 2.0 mmHg in people with no history of concussion. Unfortunately the mean time of measurement from injury was 11 years and the study population had long since recovered clinically. Toth et al.42 measured oedema formation in a uniform sample. Jarrett et al.41 indirectly measured ICP after mTBI via a reduction in global brain volumes, which correlates to reduced ICP.9 They longitudinally monitored hockey players who had a concussion, hockey players who did not have a concussion, and controls who did not play contact sports. The results of this study are controversial because they found reduced global brain volumes both in concussed and non-concussed athletes compared to controls with no significant difference between the two athlete groups. The reasons for the decreased brain volumes in non-concussed athletes are unknown but theoretically could be due to undiagnosed concussive or sub-concussive injuries, or other lifestyle or genetic factors apart from brain injury may be at work.
Animal studies have the advantage of measuring ICP directly, which is more reliable and sensitive than indirect methods. Bolouri et al.38 simulated sports-related concussions by giving helmeted rats three impacts and longitudinally monitored ICP. Pre-injury ICP was 5.7 mmHg, increased with time, peaked at 14 mmHg after multiple impacts 10 hours after injury, and returned to pre-injury levels by 7 days. In human concussed athletes, it can take hours for the development of concussive symptoms while clinical recovery generally requires 7 to 10 days.5 Thus, increased ICP in this animal study correlated with the delayed onset of concussive symptoms and short recovery duration seen in most humans after sports-related concussion. Donovan et al.51 gave single and multiple impacts either 3 or 7 days apart using a direct mild cortical impact injury and collected MRI data at baseline, 1 day post first injury, 1 day post last injury, 7 days post last injury, 14 days post last injury and 60–68 days post first injury. They measured haemorrhagic lesions and incidentally reported brain oedema formation. They found oedema formation 1 day post first injury (1.4 ± 0.002% increase in total brain volume, p<0.05). In the 7 day multiple injury group, oedema at the site of initial injury had subsided at day 7 post-injury while there was still oedema formation at the site of second impact. There was no observable difference in oedema formation at day 14 from first injury in all the groups. These data suggest that brain oedema happens early after mTBI, resolves within 14 days, and that multiple impacts have a cumulative effect, which could explain why Second Impact Syndrome may be a complication of repetitive head injuries.52 Kane et al.43 measured brain oedema formation 4 hours after single and multiple injuries using a post-mortem brain weight method but found no significant oedema formation after multiple mTBI. This is the only animal study that did not report increased ICP even though there were temporary functional deficits in balance and co-ordination, which is consistent with mTBI. Lewelt et al.47 reported increased ICP in adult cats using a direct catheter method after low impact brain injury that normalized over the first hour. Saljo et al.53 longitudinally measured variations in ICP at regular intervals after mTBI, similar to Bolouri et al.38. ICP increased, peaked at 10 hours, and remained significantly elevated at day 7, after which there was no significant difference. The animals were functionally impaired during the time of increased ICP but recovered by day 7.
Limitations:
A limitation of this study is the inability to perform a meta-analysis with the data. Table 1 shows the heterogeneity in the study designs, species studied, mechanism and timing of injuries, and ICP measurement methods, which make a meaningful meta-analysis impossible. Another potential limitation of this study is publication bias. There may be valuable data available only from non-peer reviewed sources and the grey literature. Even though language was not an exclusion criteria, all of the full text articles we read were in English. Articles in other languages had abstracts that were translated into English but did not pass the title/abstract screening step. Since direct ICP measurement after mTBI is not standard of care, the indirect measurements of ICP in humans are limited to estimated values. Also, even thought we did not have a limit to the publication year, only one study published more than 10 years ago was included. This is because the older animal and human studies had a very different definition of mild traumatic brain injury, typically including haemorrhages in humans and high rates of fatalities in animals. Some studies, for example Gurdjian et al.,31 published in 1954, claimed “concussion” after a brain impact of 120 pounds per square inch (approximately 8.1 atm), which is significantly greater than the current definition of a mild TBI. Lastly, the agreement score between reviewers is relatively low (0.61). We suspect it was due to this being an understudied topic with a variety of endpoints/variables and we had to identify if the animal studies could be comparable to mTBI.
Future directions:
Future studies should measure ICP longitudinally in acute mTBI human patients until resolution of symptoms using sensitive, modern indirect measurement methods. Unfortunately indirect/non-invasive are less sensitive and less reliable than direct/invasive methods.20 Some methods, like tonometry, require a large increase in ICP to identify any change and their correlation coefficient with direct measurement is only 0.44 for moderately increased ICP.54 Since it is hard to measure small changes in ICP indirectly using traditional methods, Cartwright et al.55 hypothesized that they could effectively diagnose concussions using ultrasonic parameters. They studied indirect ICP measurement in healthy controls and presented a formula to accurately and sensitively measure ICP using optic nerve sheath diameter. They also hypothesized an increase in ICP after concussion and recommend further investigation. The Two-Depth Trans-orbital Doppler is an effective method of detecting small increases in ICP. It is the only non-invasive method that gives absolute values of ICP as opposed to the estimated values from other indirect methods but currently is used only for limited research purposes.56 Finally, a novel method using a sensitive Transcranial Doppler can produce cerebral blood flow velocity waveforms. Each of these waveforms correlate with a different mean ICP.57 This Morphological Clustering and Analysis of continuous Intracranial Pressure (MOCAIP)58 method could be used in the future to indirectly monitor ICP after mTBI.
Conclusion
ICP is important to study because the symptoms of intracranial hypertension include but are not limited to headache, behavioural problems, nausea, and vision problems, which overlap with the symptoms of mTBI and concussion.5,18 The limited human data support that there may be increased ICP after mTBI and animal studies suggest that this increase can remain elevated for several days after injury which is similar to the symptom recovery time reported in humans after sports-related concussion.59–62 Future research should use sensitive, modern indirect methods to monitor ICP longitudinally after human mTBI and correlate it with clinical signs, symptoms and functional outcomes.
References
- 1.Head J Definition of mild traumatic brain.. Injury. J Head Trauma Rehabil 1993;8(3):86–87. [Google Scholar]
- 2.Shenton ME, Hamoda H, Schneiderman J, Bouix S, Pasternak O, Rathi Y, Vu M-A, Purohit MP, Helmer K, Koerte I. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain imaging and behavior 2012;6(2):137–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cassidy JD, Carroll L, Peloso P, Borg J, Von Holst H, Holm L, Kraus J, Coronado V. Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. Journal of rehabilitation medicine 2004;36(0):28–60. [DOI] [PubMed] [Google Scholar]
- 4.Anderson T, Heitger M, Macleod A. Concussion and mild head injury. Practical Neurology 2006;6(6):342–357. [Google Scholar]
- 5.McCrory P, Meeuwisse WH, Aubry M, Cantu B, Dvořák J, Echemendia RJ, Engebretsen L, Johnston K, Kutcher JS, Raftery M. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. British journal of sports medicine 2013;47(5):250–258. [DOI] [PubMed] [Google Scholar]
- 6.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. The Journal of head trauma rehabilitation 2006;21(5):375–378. [DOI] [PubMed] [Google Scholar]
- 7.Thompson J, Sebastianelli W, Slobounov S. EEG and postural correlates of mild traumatic brain injury in athletes. Neuroscience Letters 2005;377(3):158–163. [DOI] [PubMed] [Google Scholar]
- 8.Czosnyka M, Pickard JD. Monitoring and interpretation of intracranial pressure. Journal of Neurology, Neurosurgery & Psychiatry 2004;75(6):813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn LT. Raised intracranial pressure. Journal of Neurology, Neurosurgery & Psychiatry 2002;73(suppl 1):i23–i27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steiner L, Andrews P. Monitoring the injured brain: ICP and CBF. British journal of anaesthesia 2006;97(1):26–38. [DOI] [PubMed] [Google Scholar]
- 11.Czosnyka M, Pickard J, Steiner L. Principles of intracranial pressure monitoring and treatment. Handbook of clinical neurology 2017;140:67. [DOI] [PubMed] [Google Scholar]
- 12.Maugans TA, Farley C, Altaye M, Leach J, Cecil KM. Pediatric sports-related concussion produces cerebral blood flow alterations. Pediatrics 2012;129(1):28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meier TB, Bellgowan PS, Singh R, Kuplicki R, Polanski DW, Mayer AR. Recovery of cerebral blood flow following sports-related concussion. JAMA Neurol 2015;72(5):530–8. [DOI] [PubMed] [Google Scholar]
- 14.Mutch WAC, Ellis MJ, Ryner LN, Ruth Graham M, Dufault B, Gregson B, Hall T, Bunge M, Essig M, Fisher JA. Brain magnetic resonance imaging CO2 stress testing in adolescent postconcussion syndrome. Journal of neurosurgery 2016;125(3):648–660. [DOI] [PubMed] [Google Scholar]
- 15.Clausen M, Pendergast DR, Wilier B, Leddy J. Cerebral Blood Flow During Treadmill Exercise Is a Marker of Physiological Postconcussion Syndrome in Female Athletes. Journal of Head Trauma Rehabilitation 2016;31(3):215–224. [DOI] [PubMed] [Google Scholar]
- 16.Marshall S, Bayley M, McCullagh S, Velikonja D, Berrigan L, Ouchterlony D, Weegar K. Updated clinical practice guidelines for concussion/mild traumatic brain injury and persistent symptoms. Brain injury 2015;29(6):688–700. [DOI] [PubMed] [Google Scholar]
- 17.Sheth K, McCullough M, Kazmi S, Lazaridis C, O’Phelan K, Shepherd SA, DeJesus-Alvelo I, Marshall SA, Willis AM, Durrant JC. Cerebral Herniation Syndromes and Intracranial Hypertension. Rutgers University Press; 2016. [Google Scholar]
- 18.McCrory P, Meeuwisse W, Johnston K, Dvorak J, Aubry M, Molloy M, Cantu R. Consensus statement on Concussion in Sport–the 3rd International Conference on Concussion in Sport held in Zurich, November 2008. South African Journal of Sports Medicine 2009;21(2). [DOI] [PubMed] [Google Scholar]
- 19.Muralidharan R External ventricular drains: Management and complications. Surgical neurology international 2015;6(Suppl 6):S271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kristiansson H, Nissborg E, Bartek J Jr, Andresen M, Reinstrup P, Romner B. Measuring elevated intracranial pressure through noninvasive methods: a review of the literature. Journal of neurosurgical anesthesiology 2013;25(4):372–385. [DOI] [PubMed] [Google Scholar]
- 21.Johnson AL, Criddle LM. Pass the salt indications for and implications of using hypertonic saline. Critical care nurse 2004;24(5):36–48. [PubMed] [Google Scholar]
- 22.Lumba-Brown A, Harley J, Lucio S, Vaida F, Hilfiker M. Hypertonic saline as a therapy for pediatric concussive pain: a randomized controlled trial of symptom treatment in the emergency department. Pediatr Emerg Care 2014;30(3):139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of internal medicine 2009;151(4):264–269. [DOI] [PubMed] [Google Scholar]
- 24.University of York CfRaD. 2/19. PROSPERO: International prospective register of systematic reviews. <http://www.crd.york.ac.uk/PROSPERO/>. Accessed 2016 2/19.
- 25.McHugh ML. Interrater reliability: the kappa statistic. Biochemia medica 2012;22(3):276–282. [PMC free article] [PubMed] [Google Scholar]
- 26.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. Journal of epidemiology and community health 1998;52(6):377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melnyk BM, Fineout-Overholt E. Evidence-based practice in nursing & healthcare: A guide to best practice. Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 28.Serrador J, Tosto J, Reyes L, Blatt M, Ghobreal B, Falvo M. Cerebrovascular regulation is impaired immediately post concussion and associated with increased estimated ICP. FASEB Journal 2015;29. [Google Scholar]
- 29.Säljö A, Arrhén F, Bolouri H, Mayorga M, Hamberger A. Neuropathology and pressure in the pig brain resulting from low-impulse noise exposure. Journal of neurotrauma 2008;25(12):1397–1406. [DOI] [PubMed] [Google Scholar]
- 30.Cernak I, Chang T, Ahmed FA, Cruz MI, Vink R, Stoica B, Faden AI. Pathophysiological response to experimental diffuse brain trauma differs as a function of developmental age. Dev Neurosci 2010;32(5–6):442–53. [DOI] [PubMed] [Google Scholar]
- 31.Gurdjian ES, Lissner HR, Webster JE, Latimer FR, Haddad BF. Studies on experimental concussion: relation of physiologic effect to time duration of intracranial pressure increase at impact. Neurology 1954;4(9):674–81. [DOI] [PubMed] [Google Scholar]
- 32.Prins ML, Lee SM, Cheng CL, Becker DP, Hovda DA. Fluid percussion brain injury in the developing and adult rat: a comparative study of mortality, morphology, intracranial pressure and mean arterial blood pressure. Brain Res Dev Brain Res 1996;95(2):272–82. [DOI] [PubMed] [Google Scholar]
- 33.Engelborghs K, Verlooy J, Van Deuren B, Van Reempts J, Borgers M. Intracranial pressure in a modified experimental model of closed head injury. Acta Neurochir Suppl 1997;70:123–5. [DOI] [PubMed] [Google Scholar]
- 34.Chandra N, Skotak M, Wang F, Ganpule SG, Haorah J. Do primary blast-shock waves cause mild TBI? Experimental evidence based on animal models and human cadaveric heads. Journal of Neurotrauma 2013;30(15):A80. [Google Scholar]
- 35.Abdul-Muneer PM, Schuetz H, Wang F, Skotak M, Jones J, Gorantla S, Zimmerman MC, Chandra N, Haorah J. Induction of oxidative and nitrosative damage leads to cerebrovascular inflammation in an animal model of mild traumatic brain injury induced by primary blast. Free Radical Biology and Medicine 2013;60:282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Obenaus A, Robbins M, Blanco G, Galloway NR, Snissarenko E, Gillard E, Lee S, Currás-Collazo M. Multi-modal magnetic resonance imaging alterations in two rat models of mild neurotrauma. Journal of neurotrauma 2007;24(7):1147–1160. [DOI] [PubMed] [Google Scholar]
- 37.Millen JE, Glauser FL, Zimmerman M. Physiological effects of controlled concussive brain trauma. J Appl Physiol Respir Environ Exerc Physiol 1980;49(5):856–62. [DOI] [PubMed] [Google Scholar]
- 38.Bolouri H, Saljo A, Viano DC, Hamberger A. Animal model for sport-related concussion; ICP and cognitive function. Acta Neurol Scand 2012;125(4):241–7. [DOI] [PubMed] [Google Scholar]
- 39.Hamberger A, Viano DC, Saljo A, Bolouri H. Concussion in professional football: morphology of brain injuries in the NFL concussion model--part 16. Neurosurgery 2009;64(6):1174–82; discussion 1182. [DOI] [PubMed] [Google Scholar]
- 40.Saljo A, Mayorga M, Bolouri H, Svensson B, Hamberger A. Mechanisms and pathophysiology of the low-level blast brain injury in animal models. Neuroimage 2011;54 Suppl 1:S83–8. [DOI] [PubMed] [Google Scholar]
- 41.Jarrett M, Tam R, Hernandez-Torres E, Martin N, Perera W, Zhao Y, Shahinfard E, Dadachanji S, Taunton J, Li DK and others. A Prospective Pilot Investigation of Brain Volume, White Matter Hyperintensities, and Hemorrhagic Lesions after Mild Traumatic Brain Injury. Front Neurol 2016;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toth A, Kovacs N, Perlaki G, Orsi G, Aradi M, Komaromy H, Ezer E, Bukovics P, Farkas O, Janszky J. Multi-modal magnetic resonance imaging in the acute and sub-acute phase of mild traumatic brain injury: can we see the difference? Journal of neurotrauma 2013;30(1):2–10. [DOI] [PubMed] [Google Scholar]
- 43.Kane MJ, Angoa-Pérez M, Briggs DI, Viano DC, Kreipke CW, Kuhn DM. A mouse model of human repetitive mild traumatic brain injury. Journal of Neuroscience Methods 2012;203(1):41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leddy JJ, Kozlowski K, Fung M, Pendergast DR, Willer B. Regulatory and autoregulatory physiological dysfunction as a primary characteristic of post concussion syndrome: implications for treatment. NeuroRehabilitation 2007;22(3):199–205. [PubMed] [Google Scholar]
- 45.Geets W, de Zegher F. EEG and brainstem abnormalities after cerebral concussion. Short term observations. Acta Neurol Belg 1985;85(5):277–83. [PubMed] [Google Scholar]
- 46.Polak P, Leddy JJ, Dwyer MG, Willer B, Zivadinov R. Diffusion tensor imaging alterations in patients with postconcussion syndrome undergoing exercise treatment: a pilot longitudinal study. J Head Trauma Rehabil 2015;30(2):E32–42. [DOI] [PubMed] [Google Scholar]
- 47.Lewelt W, Jenkins L, Miller JD. Autoregulation of cerebral blood flow after experimental fluid percussion injury of the brain. Journal of neurosurgery 1980;53(4):500–511. [DOI] [PubMed] [Google Scholar]
- 48.Maguigan KL, Dennis BM, Hamblin SE, Guillamondegui OD. Method of Hypertonic Saline Administration: Effects on Osmolality in Traumatic Brain Injury Patients. Journal of Clinical Neuroscience 2017. [DOI] [PubMed] [Google Scholar]
- 49.Sokhal N, Rath GP, Chaturvedi A, Singh M, Dash HH. Comparison of 20% mannitol and 3% hypertonic saline on intracranial pressure and systemic hemodynamics. Journal of Clinical Neuroscience 2017. [DOI] [PubMed] [Google Scholar]
- 50.Pomschar A, Koerte I, Lee S, Laubender RP, Straube A, Heinen F, Ertl-Wagner B, Alperin N. MRI evidence for altered venous drainage and intracranial compliance in mild traumatic brain injury. PLoS One 2013;8(2):e55447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Donovan V, Bianchi A, Hartman R, Bhanu B, Carson MJ, Obenaus A. Computational analysis reveals increased blood deposition following repeated mild traumatic brain injury. NeuroImage: Clinical 2012;1(1):18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bowen AP. Second impact syndrome: a rare, catastrophic, preventable complication of concussion in young athletes. J Emerg Nurs 2003;29(3):287–9. [DOI] [PubMed] [Google Scholar]
- 53.Saljo A, Bolouri H, Mayorga M, Svensson B, Hamberger A. Low-level blast raises intracranial pressure and impairs cognitive function in rats: prophylaxis with processed cereal feed. J. Neurotrauma 2010;27(2):383–389. [DOI] [PubMed] [Google Scholar]
- 54.Yavin D, Luu J, James MT, Roberts DJ, Sutherland GR, Jette N, Wiebe S. Diagnostic accuracy of intraocular pressure measurement for the detection of raised intracranial pressure: meta-analysis: a systematic review. J Neurosurg 2014;121(3):680–7. [DOI] [PubMed] [Google Scholar]
- 55.Cartwright MS, Dupuis JE, Bargoil JM, Foster DC. Can a combination of ultrasonographic parameters accurately evaluate concussion and guide return-to-play decisions? Med Hypotheses 2015;85(3):262–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ragauskas A, Matijosaitis V, Zakelis R, Petrikonis K, Rastenyte D, Piper I, Daubaris G. Clinical assessment of noninvasive intracranial pressure absolute value measurement method. Neurology 2012;78(21):1684–1691. [DOI] [PubMed] [Google Scholar]
- 57.Kim S, Hamilton R, Pineles S, Bergsneider M, Hu X. Noninvasive intracranial hypertension detection utilizing semisupervised learning. IEEE Transactions on Biomedical Engineering 2013;60(4):1126–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu X, Xu P, Scalzo F, Vespa P, Bergsneider M. Morphological clustering and analysis of continuous intracranial pressure. IEEE Transactions on Biomedical Engineering 2009;56(3):696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leddy JJ, Baker JG, Kozlowski K, Bisson L, Willer B. Reliability of a graded exercise test for assessing recovery from concussion. Clinical Journal of Sport Medicine 2011;21(2):89–94. [DOI] [PubMed] [Google Scholar]
- 60.Leddy JJ, Willer B. Use of graded exercise testing in concussion and return-to-activity management. Curr Sports Med Rep 2013;12(6):370–6. [DOI] [PubMed] [Google Scholar]
- 61.Matuszak JM, McVige J, McPherson J, Willer B, Leddy J. A Practical Concussion Physical Examination Toolbox Evidence-Based Physical Examination for Concussion. Sports Health: A Multidisciplinary Approach 2016;8(3):260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Willer B, Leddy JJ. Management of concussion and post-concussion syndrome. Curr Treat Options Neurol 2006;8(5):415–26. [DOI] [PubMed] [Google Scholar]


