Abstract
Verbal and physical aggression begin early in life and steadily decline thereafter in normal development. As a result, elevated aggressive behavior in adolescence may signal atypical development and greater vulnerability for negative mental and health outcomes. Converging evidence suggests that brain disturbances in regions involved in impulse control, emotional regulation, and sensation seeking may contribute to heightened aggression. However, little is known regarding the neural mechanisms underlying subtypes of aggression (i.e., proactive and reactive aggression) and whether they differ between males and females. Using a sample of 106 14-year-old adolescent twins, this study found that striatal enlargement was associated with both proactive and reactive aggression. We also found that volumetric alterations in several frontal regions including smaller middle frontal and larger orbitofrontal cortex were correlated with higher levels of aggression in adolescent twins. In addition, cortical thickness analysis showed that thickness alterations in many overlapping regions including middle frontal, superior frontal, and anterior cingulate cortex and temporal regions were associated with aggression in adolescent twins. Results support the involvement of fronto-limbic-striatal circuit in the etiology of aggression during adolescence.
Keywords: aggression, cortical thickness, frontal cortex, striatum, adolescence
Introduction
Aggression begins early in life and steadily declines thereafter in normal development (Coie & Dodge, 1998; Nagin & Tremblay, 1999). Aggressive behavior, both verbal and physical, during adolescence poses greater risk for negative mental and health outcomes including criminal offending, low socioeconomic status, higher unemployment, and marital problems (Buchmann, Hohmann, Brandeis, Banaschewski, & Poustka, 2014). Aggression is also a prominent feature in many children with neurodevelopmental disorders including attention deficit/hyperactivity disorder, conduct disorder, oppositional defiant disorder, and autistic spectrum disorders (Matson & Cervantes, 2014; Murray, Obsuth, Zirk-Sadowski, Ribeaud, & Eisner, 2016). Therefore, it is of critical importance to understand the neurobiological underpinnings of aggression during adolescence to guide future development of targeted prevention and intervention.
Multiple brain regions have been implicated in aggression, with the strongest evidence to date pointing to the involvement of the fronto-limbic-striatal circuitry. The majority of existing brain imaging studies on aggression focus on adults with antisocial personality disorder and psychopathy, showing potential links between aggressive behavior and lower gray matter volumes in the orbitofrontal cortex, middle frontal cortex, and the temporal lobe (Boccardi et al., 2013; Boccardi et al., 2010; Dolan, Deakin, Roberts, & Anderson, 2002; Gregory et al., 2012; Raine, Lencz, Bihrle, LaCasse, & Colletti, 2000; Yang & Raine, 2009; Yang, Raine, Colletti, Toga, & Narr, 2009; Yang et al., 2005; Yang, Raine, Narr, Colletti, & Toga, 2009). Studies of criminal offenders also reported structural abnormalities in the amygdala, hippocampus, caudate, putamen, and the nucleus accumbens (Boccardi et al., 2013; Boccardi et al., 2011; Yang, et al., 2010; Yang, et al., 2012a). However, increasing evidence begins to suggest that brain abnormalities observed in violent offenders may differ across diagnostic groups (Bertsch et al., 2013; Howner et al., 2012). Furthermore, despite controlling for substance abuse in most of these studies (Gregory et al., 2012), an argument remains that substance misuse may contribute, at least partially, to brain abnormalities observed in violent, antisocial individuals (Schiffer et al., 2011).
The examination of neural correlates of aggression in younger populations provides many advantages including limited exposure to the effects of substance abuse and the potential to establish a neurodevelopmental pathway to violence. However, brain imaging studies on aggression in young adolescents are scarce. Our understanding of neural correlates of aggression in adolescents largely came from studies of children with conduct disorders, in which aggressive behavior is one of the prominent features. Children with conduct disorders were found to show lower volumes and cortical thickness in the frontal cortex, cingulate, and insula and lower volumes in the amygdala and the hippocampus (Fahim et al., 2011; Fairchild et al., 2012; G. Fairchild et al., 2011; Huebner et al., 2008; Sterzer, Stadler, Poustka, & Kleinschmidt, 2007; Walhovd, Tamnes, Ostby, Due-Tonnessen, & Fjell, 2012). Two recent studies found that incarcerated male adolescents who committed homicide showed lower gray matter volumes in the medial and lateral temporal lobes, including the hippocampus and posterior insula, than non-homicide offenders (Cope et al., 2014; Kolla, Gregory, Attard, Blackwood, & Hodgins, 2014). Taken together with adult findings, smaller frontal and temporal regions and enlarged striatum are likely to contribute to impaired impulse control and emotional regulation, leading to the use of aggressive acts as a means to resolve conflict or obtain desirable outcomes.
Despite these efforts, the knowledge regarding the neurodevelopmental underpinning of aggression remains limited for several reasons. First, findings from clinical samples in prior studies are influenced by potential confounds such as treatment status, age-of-onset, and comorbid psychiatric disorders. Second, most studies focused on only one brain morphological feature (e.g., volume, thickness, shape), making it difficult to have a more comprehensive understanding of how disturbances in brain architecture lead to elevated aggression. Third, the majority of studies on aggression to date have focused on males exclusively, partially attributable to large disparities between males and females in the prevalence of violent crimes, and the diagnosis of antisocial personality disorder and conduct disorders (Israel et al., 2014; Loukas, Paulos, & Robinson, 2005). Fourth, studies to date have largely focused on neural mechanisms underlying pathological forms of aggression by comparing violent to non-violent individuals. This categorical approach to the underlying neuropathology of aggression goes against empirical evidence indicating that aggression is a continuous trait present in all populations (Hudziak et al., 2003; Ligthart, Bartels, Hoekstra, Hudziak, & Boomsma, 2005). Last, increasing evidence supports further distinction between two subtypes of aggression, the controlled-instrumental subtype (proactive) and the impulse subtypes (reactive). However, no study to our knowledge has identified differences and/or overlaps in the neural correlates between these two subtypes of aggression in adolescents.
Using a sample of 106 14-year-old typically developing adolescent twins (49.06% females), the current study extends the existing literature by examining the neural underpinnings of both proactive and reactive aggression and identifying any potential sex specificity. Using two independent but complementary methods, namely tensor-based morphometry (TBM) and cortical thickness mapping, we conducted a thorough investigation on both cortical and subcortical volumes and cortical thickness to determine how variations in these features associate with elevated aggressive behavior in adolescents. These two methods were chosen because they provide complementary and comprehensive assessment of the brain structural integrity. Specifically, tensor-based morphometry (TBM) provides automated and relatively unbiased voxel-wise assessment of brain tissue-specific changes (gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF)) across the entire brain. Specifically, global and regional variations in brain tissue volume are estimated by applying localized deformations to adjust the anatomy of each individual to match a population-specific group-average template and then by linking these deformation fields at the voxel-level to behavioral scores. Cortical thickness was analyzed using FreeSurfer software package for identifying cortical thickness variations associated with behavioral measures across the brain surface on a vertex-by-vertex basis. It has been argued that gray matter volume is a combination of several measures including cortical thickness and surface area; however, few studies assess contributions of different architectural components of the brain to behavioral problems in adolescents. Thus, in this study, we examine potential unique and overlapping contributions of both volumetric and thickness disturbances to adolescent aggression.
Here, we predicted that lower volume and cortical thickness in frontal regions, particularly those that are involved in impulse control (e.g., dorsolateral prefrontal cortex, anterior cingulate cortex) and emotional regulation (e.g., orbitofrontal cortex), would be associated with elevated aggression in adolescents. We also predicted that greater striatal volumes will be linked to higher levels of aggression in adolescents.
Methods and Materials
Subjects
The 106 14-year-old adolescent twins (54 males and 52 females) included in this study were drawn from participants in the University of Southern California (USC) Risk Factors for Antisocial Behavior Twin Study (Baker, Barton, Lozano, Raine, & Fowler, 2006; Baker et al., 2013). The adolescent twins and their families were originally recruited from Los Angeles County at ages 9–10, and were followed up at age 14 to obtain additional imaging and behavioral assessments. Participants were excluded if they had a history of significant head injury, major neurological, psychiatric illness, substance abuse, or contraindication to MRI (Baker et al., 2013; Yang, et al., 2012). The adolescent twins and their primary caregivers participated in 6–8 hours of laboratory assessment at USC including a one-hour scan. Both caregivers and twins gave written informed consent/assent prior to the study. The study was approved by the CHLA and USC Institutional Review Boards.
Behavioral Measurements
To measure aggressive behavior in adolescent twins, the child’s caregivers completed the Reactive and Proactive Aggression Questionnaire (RPQ) as previously reported (Baker, Raine, Liu, & Jacobson, 2008; Tuvblad, Raine, Zheng, & Baker, 2009). The RPQ is a 23-item validated questionnaire designed to assess both verbal and physical aggression in young children and adolescents (Raine et al., 2006). The RPQ includes 11 reactive items (e.g., hitting others when teased, gets angry when frustrated) and 12 proactive items (e.g., damages things for fun, uses force to get things from others). Because of the characteristic of the RPQ, it is worth mentioning that the term “aggression” used throughout this article refers to both verbal and physical aggression. The RPQ for the item responses were computed to form reactive and proactive aggression subscales. The RPQ scores of the total, male, and female sample are described in Table 1.
Table 1.
Description of Reactive and Proactive Aggression Questionnaire (RPQ) Scores.
| RPQ | Total (N = 106) | Males (N = 54) | Females (N = 52) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | S.D. | Range | Mean | S.D. | Range | Mean | S.D. | Range | |
| Total Score | 7.28 | 4.82 | 0–24 | 7.13 | 5.10 | 0–24 | 7.44 | 4.56 | 0–17 |
| Sub-scale Score (Averaged) | |||||||||
| Proactive Aggression | .08 | .12 | 0–.58 | .08 | .14 | 0–.58 | .08 | .10 | 0–.42 |
| Reactive Aggression | .58 | .35 | 0–1.55 | .56 | .35 | 0–1.55 | .59 | .36 | 0–1.55 |
MRI Acquisition and Preprocessing
All participants were scanned using a 3 Tesla Siemens Magnetom Trio whole-body scanner at the USC Dornsife Cognitive Neuroscience Imaging Center. 3D high-resolution T1-weighted images were acquired with a magnetization prepared rapid gradient echo (MP-RAGE) protocol with the following parameters: inversion time (TI)/repetition time (TR)/echo time (TE) = 800/2530/3.09 msec, slice thickness = 1 mm without gap, matrix = 256 × 256, and field of view (FOV) = 256 mm × 256 mm. Image preprocessing (e.g. skull-stripping, correction of signal intensity and inhomogeneity artifacts) was conducted using FreeSurfer processing streams where outputs were visually inspected and manually corrected for accuracy (Yang, et al., 2010; Yang, et al., 2012b).
Brain tissue volume analysis
To detect local differences in brain cortical and subcortical structures associated with aggression scores, TBM processing streams were implemented using methods similar to those described in prior investigations (Yang et al., 2015; Yang et al., 2011). In brief, TBM relies on matching structures with similar intensity patterns, and measures volumetric differences in a population by analyzing the gradients of the non-linear deformation fields required to align individual images to an anatomical template specific to the population studied (Yang et al., 2015; Yang et al., 2011). TBM processing steps include a 9-parameter registration, the creation of an anatomical template or minimal deformation target (MDT) using a mutual information-based inverse-consistent algorithm, and the alignment of image volumes from all subjects to the MDT by nonlinearly deforming the anatomy of each individual image to match the anatomical template. The Jacobian operator was then applied to the deformation fields to produce univariate Jacobian determinants (i.e., Jacobian maps) that index the extent of local expansion or contraction required to non-linearly warp each brain to match each subject’s anatomy to the MDT. These 3D Jacobian maps represent relative tissue volume differences between each individual and the MDT, and were used to characterize local differences in brain tissue structure associated with the targeted behavioral measure across the sample.
Cortical thickness analysis
For each participant, cortical thickness was estimated using the FreeSurfer software v5.1.0 (Fischl, 2012) at each vertex over the entire cortical surface, a process that has been validated against postmortem histological analysis (Rosas et al., 2002) and manual measurements (Kuperberg et al., 2003; Salat et al., 2004). FreeSurfer processing streams included skull-stripping, tissue segmentation, and spatial normalization of each image volume (Yang, et al., 2012a; Yang, et al., 2012b). After intensity normalization, gray-white tissue segmentation was used to extract the pial and gray-white cortical surface to estimate cortical thickness. The pial and gray-white cortical surface of each subject was visually inspected and manually corrected to ensure accuracy (Yang, et al., 2012a; Yang, et al., 2012b). To boost the signal to noise ratio, we applied a 25-mm full-width at half-maximum Gaussian surface-based smoothing kernel to the estimated thickness values (Panizzon et al., 2009). Each individual cortical surface was then registered to an averaged template to allow statistical analysis across the sample.
Statistical Analysis
The R statistical package (version 2.9.2; http://www.r-project.org/) was used to compute the correlation between RPQ scores and 1) the TBM Jacobian determinant at each voxel across the entire brain and 2) the FreeSurfer thickness value at each surface point using the mixed effects model while controlling for sex, whole brain volume, and subject relatedness. Random intercepts were included for each family to account for relatedness of the subjects. The analysis was implemented using the ‘nlme’ library in the R statistical package. Since comparisons were made at thousands of voxels/vertices, results were thresholded using False Discovery Rate (FDR) (q-value = 0.05)(Chu, Cui, & Dinov, 2009). For descriptive purposes and to confirm the location of the observed effects, we further identified clusters showing significant structural deformations associated with aggression scores using the software FEAT Cluster (http://fsl.fmrib.ox.ac.uk/fsl/) for clusters with > 3000 voxels. The coordinates are converted from MNI (FSL) space to Talaraich using software GingerALE (https://www.brainmap.org/) and the closest gray matter structures are identified using software Talaraich Daemon (http://talairach.org/). The clusters and coordinates are reported in Table S1 (Supplementary Material).
Post-Hoc Analyses
To confirm that local striatal expansions observed in the TBM analyses reflect volumetric changes associated with aggression, gray matter volumes of left and right caudate nuclei, putamen and nucleus accumbens were extracted using FreeSurfer (Fischl et al., 2002). For these procedures, any small topographical errors in segmentation were corrected manually on a case-by-case basis. The same statistical models described above were used to identify striatal volumes that are correlated with aggression scores, and the results are provided in Table S2 (see the Supplementary Material).
Results
Volumetric disturbances associated with aggression
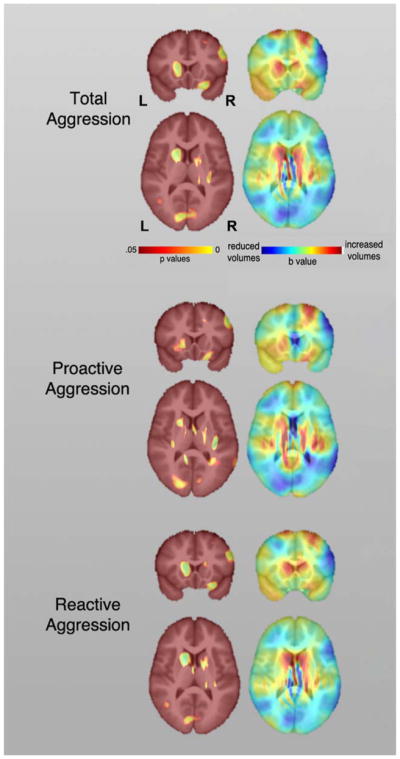
Across the entire sample, higher total aggression was correlated with larger volumes in the right lateral orbitofrontal cortex (OFC) and striatal regions, particularly the left caudate nuclei and bilateral putamen, and smaller volumes in the right middle frontal cortex (MFC) and bilateral occipital lobes (Figure 1, Table S1 and S2). The coordinates of effects are reported in Table S1 in the Supplementary Material. The post-hoc analyses on gray matter volumes of caudate nuclei, putamen, and nucleus accumbens correlated with aggression scores further confirmed the observed effects (Table S2 & Figure S1; Supplementary Material). Higher proactive aggression was found to correlate with larger volumes in the left caudate, left putamen, and the right OFC and smaller volumes in the right MFC and bilateral occipital lobes. On the other hand, higher reactive aggression was found to correlate with larger volumes in the bilateral caudate, bilateral putamen, and the right OFC and smaller volumes in the right MFC and bilateral occipital lobes. Although post-hoc analyses showed that the unique contributions of brain volumetric variations in these regions to subtypes of aggression did not survive FDR corrections (Figure S2, Supplementary Material), findings indicated that lower lateral and medial frontal volumes may contribute more strongly to proactive aggression, whereas increased left putamen may contribute more strongly to reactive aggression.
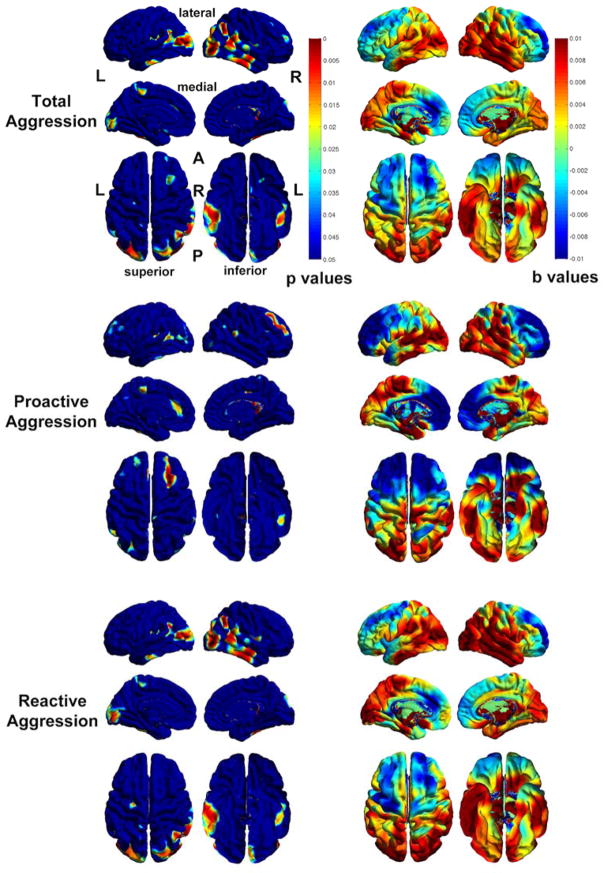
Figure 1.
Probability maps showing significant correlations between brain tissue volumes and total (top), proactive (middle) and reactive aggression (bottom) across the sample. The corresponding beta maps indicates the direction of the effects, with cold colors indicate negative and hot colors positive correlations.
To determine whether there is sex-specific effect on the correlations between brain volumes and aggression, we further mapped the “sex by aggression” interaction against brain tissue volumes. The interaction effect was most significant in the left putamen (uncorrected p < .05), indicating that the positive correlation between higher aggression and increased left putamen volume was stronger in adolescent males than females (Figure S3, Supplementary Material). However, the interaction effect did not survive FDR correction.
Thickness disturbances associated with aggression
Across the entire sample, higher total aggression was found to correlate with thinner frontal cortex and thicker temporal and parietal lobes (Figure 2). Specifically, higher total aggression was correlated with thinner right MFC and thicker cortices in the right superior temporal gyrus (STG), right middle temporal gyrus (MTG), bilateral inferior temporal gyri (ITG), bilateral inferior parietal lobes, right superior parietal lobe, and bilateral occipital lobes (Figure 2). Higher proactive aggression was found to correlate with thinner bilateral SFC, bilateral MFC, and left anterior cingulate cortex (ACC) and thicker left paracentral gyrus, left ITG and right STG. On the other hand, higher reactive aggression was correlated with thicker left paracentral gyrus, left precentral gyrus, right STG, right MTG, bilateral ITG, right inferior and superior parietal lobe, and bilateral occipital lobes (Figure 2). Although post-hoc analyses for unique contributions of thickness variations to subtypes of aggression did not survive FDR correction (Figure S4, Supplementary Material), the maps indicated that reduced cortical thickness across the lateral and medial frontal cortex, bilateral ACC, and left anterior temporal regions may contribute uniquely to proactive aggression, whereas increased cortical thickness in right ACC, medial frontal cortex, and inferior and medial temporal cortex may contribute uniquely to higher proactive aggression.
Figure 2.
Probability maps showing significant correlations between cortical thickness and total (top), proactive (middle) and reactive aggression (bottom) across the sample. The corresponding beta maps indicates the direction of the effects, with cold colors indicate negative and hot colors positive correlations.
Additional analyses mapping the “sex by aggression” interaction against cortical thickness showed a significant interaction effect in the lateral left orbitofrontal, bilateral middle frontal and right posterior cingulate cortex (uncorrected p < .05), indicating that the correlations between higher aggression and thicker cortex in these regions were greater in adolescent males than females (Figure S5, Supplementary Material). However, the interaction results did not survive FDR correction.
Discussion
To our knowledge, this is the first study to show support for both volumetric and thickness disturbances underlying aggression in a typically developing population of adolescent twins. Specifically, we found striatal enlargement to be most strongly associated with both proactive and reactive aggression. We also found volumetric alterations in several frontal regions including smaller MFC and larger OFC to be correlated with higher aggression in adolescent twins. In addition to volumetric findings, cortical thickness analysis also showed thickness alterations in many overlapping regions including MFC, SFC, as well as ACC and temporal regions to be associated with aggression in adolescent twins.
One of the most prominent findings was the correlation between higher aggression and larger volume in the striatum, particularly the caudate nuclei, putamen, and the nucleus accumbens. This is consistent with previous reports showing that enlarged caudate and putamen are associated with: (1) aggressive behavior in children (Ducharme et al., 2012); (2) elevated psychopathic traits in both adults and children (Glenn, Raine, Yaralian, & Yang, 2009; Yang et al., 2015); and (3) violent offending in adults (Schiffer et al., 2011). Larger caudate size has also been linked to higher levels of aggression in patients with schizophrenia (Hoptman et al., 2006). The present findings are supported by functional imaging studies showing that atypical caudate activation in response to both reward and non-reward stimuli in children with externalizing behavioral disorders compared to healthy children, who showed caudate activation only to reward stimuli (Gatzke-Kopp et al., 2009). The striatal regions have long been associated with the mesolimbic dopamine system that governs the regulation of motivated behavior (Mogenson, Jones, & Yim, 1980). Animal research showed that mesolimbic dopamine is critical for the expression of aggression, and dopamine transporter (DAT) knock-out mice showed increased rates of reactivity and aggression following mild social contact (Rodriguiz, Chu, Caron, & Wetsel, 2004). In line with these findings, our study showed that enlarged striatum in adolescents may be a potential biomarker for elevated aggression.
We also observed higher levels of aggression to be associated with structural variations in widespread areas of the frontal cortex, particularly MFC and OFC, but also IFC and SFC. These volumetric findings are complemented by the independent surface-based findings, which showed a thinner cortex in the MFC, a thicker cortex in the OFC, and an thinner cortex in the SFC, supporting the hypothesis that frontal lobe dysfunction is one of the key predispositions towards aggression (Anderson & Kiehl, 2014; Brower & Price, 2001; Fahim et al., 2011; Viding, Seara-Cardoso, & McCrory, 2014; Wallace et al., 2014; Yang & Raine, 2009). Findings also support the argument that both increased and decreased regional volume/thickness may indicate a deviation from typical developmental trajectories and may reflect impaired cognitive functioning of that region (Gautam, Warner, Kan, & Sowell, 2015). The OFC, MFC, and the SFC are interactively involved in an extensive variety of crucial psychosocial and cognitive functions from directing and controlling attention, emotion regulation, decision-making, to executive function. Thus, it is expected that, if the morphological development of these intertwined frontal nodes is disrupted at any given point, the person may be at risk of poor inhibitory control, executive deficits, impaired response perseveration, and social inappropriateness (Hawkins & Trobst, 2000). Thus, the findings of this study provide evidence that disturbances in the frontal cortex may increase aggressive behavior even among typically developing adolescents.
In this study, we also found evidence that thinner ACC is associated with aggression, particularly proactive aggression. These findings are consistent with prior studies showing decreased right ACC volume and abnormal ACC asymmetry to be associated with aggression in adolescents (Boes, Tranel, Anderson, & Nopoulos, 2008; Gorka, Norman, Radtke, Carre, & Hariri, 2015; Visser et al., 2014). The only study to our knowledge that examined the relationship between cortical thickness and aggression found a negative trend between cortical thickness in the right ACC and aggressive behavior (assessed using Child Behavior Checklist) in adolescent males, but a positive trend in the subgenual ACC in adolescent females (Ducharme et al., 2012). The inconsistency in these findings may be due to sample characteristics (e.g., age, gender distributions, levels of aggression). Nonetheless, it is clear that the ACC plays a crucial role in aggression. The ACC is involved in the regulation of emotions and social behavior including conflict monitoring and empathy, and is densely connected to the limbic system and the prefrontal cortex (Botvinick, 2007; Devinsky, Morrell, & Vogt, 1995). Studies have found increased activity in the ACC in aggressive adolescents with disruptive behavior disorders (Cohn et al., 2013) and thinner ACC in psychopathic inmates (Ly et al., 2012), although one recent study failed to find differences in ACC activity during social decision-making between severely antisocial adolescents and normal controls (van den Bos et al., 2014). Despite this, the findings may be supported by the argument that, while reactive aggression is impulsive in nature, proactive aggression is more closely associated with delinquency and psychopathic traits (Raine et al., 2006). Therefore, findings of this study provide further evidence that ACC disturbances may contribute to impaired socialization and emotional dysregulation, leading to higher levels of proactive aggression.
We also found that a thicker cortex in the insula was correlated with higher aggression. Various brain architectural components of a developing brain are influenced by on-going myelination and pruning processes during adolescence that improve the efficiency of cognitive functioning. Thicker cortices may thus reflect a delay in maturation in those brain regions. The insula is strongly implicated in empathy (Preston & de Waal, 2002) and is activated by pain and distress signals (Fehr, Achtziger, Roth, & Struber, 2014). Thus, delayed maturation in the insula may contribute to poorer functioning of this brain region. Low empathy is one of the most robust indicators for later aggression (Van der Graaff, Branje, De Wied, & Meeus, 2012). Previous studies of conduct disorders also reported reduced gray matter volume in anterior insula in adolescents (Fairchild et al., 2013), which may be linked to their facial emotion recognition impairment, particularly for expressions of disgust (Fairchild, Stobbe, van Goozen, Calder, & Goodyer, 2010).
Despite the advantage of a relatively large, homogeneously aged sample of both male and female adolescents, this study has a few limitations. First, the range of aggression in this study may be limited because it is a community-based sample of relatively healthy adolescents. Also, personality traits such as lack of self-control, high sensation-seeking and narcissism may also influence the level of aggression in adolescents. Future studies may further test for potential contributions of personality traits that are often comorbid with aggression. Second, volumetric findings from TBM whole-brain analysis were not fully consistent with thickness findings from cortical thickness mapping. This may simply reflect the nature of the assessed brain features, that they are related yet distinct brain architectural features that contribute to different neuropathological underpinnings of aggression. Third, as a cross-sectional imaging study, it is unclear whether the observed structural disturbances associated with aggression reflect normal variations in brain maturation among typically developing adolescents or are precursors to future, more severe forms of aggression. Last, although the findings may suggest that structural brain changes associated with aggression observed in this study contribute to functional impairments in those regions, future studies are required to clarify the structure-function relationship in the neural mechanisms underlying adolescent aggression. Despite these limitations, this study demonstrates that volumetric and thickness variations in the frontal cortex, ACC, striatum, and the temporal lobe are associated with elevated aggression among adolescents. Overall, these results advance our knowledge of the neural mechanisms underlying aggression and pave the way for future unraveling of the neuropathological etiology of aggression in youth.
Conclusion
Results from this study of adolescent twins suggest critical involvement of cortico-limbic-striatal circuits in aggression during adolescence. We found initial evidence for unique brain structural disturbances associated with different subtypes of aggression, suggesting potential etiological differences between proactive and reactive aggression that require further investigation. Future studies incorporating longitudinal brain scans and an enriched sample will help further elucidate the dynamic relationship between brain maturation and the developmental trajectory of aggressive behavior in both male and female adolescents.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute of Mental Health (R00 MH093388 to Y.Y., R01MH58354 to L.A.B., and R01 AG040060 and P41 RR013642 to P.M.T.), and by a grant from the Brain and Creativity Institute, University of Southern California. The authors thank Anand Joshi, Ph.D. and Jason Stein, Ph.D. for their contributions to the data processing in this study.
References
- Anderson NE, Kiehl KA. Psychopathy and aggression: when paralimbic dysfunction leads to violence. Current Topics in Behavioral Neurosciences. 2014;17:369–393. doi: 10.1007/7854_2013_257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker LA, Barton M, Lozano DI, Raine A, Fowler JH. The Southern California Twin Register at the University of Southern California: II. Twin Research Human Genetics. 2006;9(6):933–940. doi: 10.1375/183242706779462912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker LA, Raine A, Liu J, Jacobson KC. Differential genetic and environmental influences on reactive and proactive aggression in children. Journal of Abnormal Child Psychology. 2008;36:1265–1278. doi: 10.1007/s10802-008-9349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker LA, Tuvblad C, Wang P, Gomez K, Bezdjian S, Niv S, Raine A. The Southern California Twin Register at the University of Southern California: III. Twin Research Human Genetics. 2013;16(1):336–343. doi: 10.1017/thg.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertsch K, Grothe M, Prehn K, Vohs K, Berger C, Hauenstein K, … Herpertz SC. Brain volumes differ between diagnostic groups of violent criminal offenders. European Archives of Psychiatry and Clinical Neuroscience. 2013 doi: 10.1007/s00406-013-0391-6. [DOI] [PubMed] [Google Scholar]
- Boccardi M, Bocchetta M, Aronen HJ, Repo-Tiihonen E, Vaurio O, Thompson PM, … Frisoni GB. Atypical nucleus accumbens morphology in psychopathy: another limbic piece in the puzzle. International Journal of Law and Psychiatry. 2013;36(2):157–167. doi: 10.1016/j.ijlp.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccardi M, Frisoni GB, Hare RD, Cavedo E, Najt P, Pievani M, … Tiihonen J. Cortex and amygdala morphology in psychopathy. Psychiatry Research. 2011;193(2):85–92. doi: 10.1016/j.pscychresns.2010.12.013. [DOI] [PubMed] [Google Scholar]
- Boccardi M, Ganzola R, Rossi R, Sabattoli F, Laakso MP, Repo-Tiihonen E, … Tiihonen J. Abnormal hippocampal shape in offenders with psychopathy. Human Brain Mapping. 2010;31(3):438–447. doi: 10.1002/hbm.20877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boes AD, Tranel D, Anderson SW, Nopoulos P. Right anterior cingulate: a neuroanatomical correlate of aggression and defiance in boys. Behavavioral Neuroscience. 2008;122(3):677–684. doi: 10.1037/0735-7044.122.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cognitive, Affective, & Behavioral Neuroscience. 2007;7(4):356–366. doi: 10.3758/CABN.7.4.356. [DOI] [PubMed] [Google Scholar]
- Brower MC, Price BH. Neuropsychiatry of frontal lobe dysfunction in violent and criminal behaviour: a critical review. Journal of Neurolology, Neurosurgery, & Psychiatry. 2001;71(6):720–726. doi: 10.1136/jnnp.71.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmann A, Hohmann S, Brandeis D, Banaschewski T, Poustka L. Aggression in children and adolescents. Current Topics in Behavioral Neurosciences. 2014;17:421–442. doi: 10.1007/7854_2013_261. [DOI] [PubMed] [Google Scholar]
- Chu A, Cui J, Dinov ID. SOCR Analyses: Implementation and Demonstration of a New Graphical Statistics Educational Toolkit. Journal of Statistical Software. 2009;30(3):1–19. doi: 10.18837/jss.v030.i03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn MD, Popma A, van den Brink W, Pape LE, Kindt M, van Domburgh L, … Veltman DJ. Fear conditioning, persistence of disruptive behavior and psychopathic traits: an fMRI study. Translational Psychiatry. 2013;3:e319. doi: 10.1038/tp.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coie J, Dodge K. Aggression and antisocial behavior. In: Eisenberg WDN, editor. Handbook of Child psychology. Vol. 3. Toronto: Wiley; 1998. pp. 779–862. [Google Scholar]
- Cope LM, Ermer E, Gaudet LM, Steele VR, Eckhardt AL, Arbabshirani MR, … Kiehl KA. Abnormal brain structure in youth who commit homicide. Neuroimage: Clinical. 2014;4:800–807. doi: 10.1016/j.nicl.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dolan M, Deakin JFW, Roberts N, Anderson IM. Quantitative frontal and temporal structural MRI studies in personality-disordered offenders and control subjects. Psychiatry Research Neuroimaging. 2002;116:133–149. doi: 10.1016/S0925-4927(02)00085-9. [DOI] [PubMed] [Google Scholar]
- Ducharme S, Hudziak JJ, Botteron KN, Albaugh MD, Nguyen TV, Karama S, Evans AC. Decreased regional cortical thickness and thinning rate are associated with inattention symptoms in healthy children. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(1):18–27. e12. doi: 10.1016/j.jaac.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim C, He Y, Yoon U, Chen J, Evans A, Perusse D. Neuroanatomy of childhood disruptive behavior disorders. Aggressive Behavior. 2011;37(4):326–337. doi: 10.1002/ab.20396. [DOI] [PubMed] [Google Scholar]
- Fairchild G, Hagan CC, Walsh ND, Passamonti L, Calder AJ, Goodyer IM. Brain structure abnormalities in adolescent girls with conduct disorder. Journal of Child Psychology and Psychiatry. 2012 doi: 10.1111/j.1469-7610.2012.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild G, Hagan CC, Walsh ND, Passamonti L, Calder AJ, Goodyer IM. Brain structure abnormalities in adolescent girls with conduct disorder. Journal of Child Psychology and Psychiatry. 2013;54(1):86–95. doi: 10.1111/j.1469-7610.2012.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild G, Passamonti L, Hurford G, Hagan CC, von dem Hagen EA, van Goozen SH, … Calder AJ. Brain structure abnormalities in early-onset and adolescent-onset conduct disorder. American Journal of Psychiatry. 2011;168(6):624–633. doi: 10.1176/appi.ajp.2010.10081184. [DOI] [PubMed] [Google Scholar]
- Fairchild G, Stobbe Y, van Goozen SH, Calder AJ, Goodyer IM. Facial expression recognition, fear conditioning, and startle modulation in female subjects with conduct disorder. Biological Psychiatry. 2010;68(3):272–279. doi: 10.1016/j.biopsych.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr T, Achtziger A, Roth G, Struber D. Neural correlates of the empathic perceptual processing of realistic social interaction scenarios displayed from a first-order perspective. Brain Research. 2014;1583:141–158. doi: 10.1016/j.brainres.2014.04.041. [DOI] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, … Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/S0896-6273(02)00569-X. [DOI] [PubMed] [Google Scholar]
- Gatzke-Kopp LM, Beauchaine TP, Shannon KE, Chipman J, Fleming AP, Crowell SE, … Aylward E. Neurological correlates of reward responding in adolescents with and without externalizing behavior disorders. Journal of Abnormal Psychology. 2009;118(1):203–213. doi: 10.1037/a0014378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam P, Warner TD, Kan EC, Sowell ER. Executive function and cortical thickness in youths prenatally exposed to cocaine, alcohol and tobacco. Developmental Cognitive Neuroscience. 2015;16:155–165. doi: 10.1016/j.dcn.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn AL, Raine A, Yaralian PS, Yang Y. Increased volume of the striatum in psychopathic individuals. Biological Psychiatry. 2009;67:52–58. doi: 10.1016/j.biopsych.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka AX, Norman RE, Radtke SR, Carre JM, Hariri AR. Anterior cingulate cortex gray matter volume mediates an association between 2D:4D ratio and trait aggression in women but not men. Psychoneuroendocrinology. 2015;56:148–156. doi: 10.1016/j.psyneuen.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory S, ffytche D, Simmons A, Kumari V, Howard M, Hodgins S, Blackwood N. The antisocial brain: psychopathy matters. Archives of General Psychiatry. 2012;69(9):962–972. doi: 10.1001/archgenpsychiatry.2012.222. [DOI] [PubMed] [Google Scholar]
- Hawkins KA, Trobst KK. Frontal lobe dysfunction and aggression: conceptual issues and research findings. Aggression and Violent Behavior. 2000;5:147–157. doi: 10.1016/S1359-1789(98)00033-0. [DOI] [Google Scholar]
- Hoptman MJ, Volavka J, Czobor P, Gerig G, Chakos M, Blocher J, … Bilder RM. Aggression and quantitative MRI measures of caudate in patients with chronic schizophrenia or schizoaffective disorder. The Journal of Neuropsychiatry of Clinical Neurosciences. 2006;18(4):509–515. doi: 10.1176/appi.neuropsych.18.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howner K, Eskildsen SF, Fischer H, Dierks T, Wahlund LO, Jonsson T, … Kristiansson M. Thinner cortex in the frontal lobes in mentally disordered offenders. Psychiatry Research. 2012;203(2–3):126–131. doi: 10.1016/j.pscychresns.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Hudziak JJ, van Beijsterveldt CE, Bartels M, Rietveld MJ, Rettew DC, Derks EM, Boomsma DI. Individual differences in aggression: genetic analyses by age, gender, and informant in 3-, 7-, and 10-year-old Dutch twins. Behavioural Genetics. 2003;33(5):575–589. doi: 10.1023/A:1025782918793. [DOI] [PubMed] [Google Scholar]
- Huebner T, Vloet TD, Marx I, Konrad K, Fink GR, Herpertz SC, Herpertz-Dahlmann B. Morphometric brain abnormalities in boys with conduct disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(5):540–547. doi: 10.1097/CHI.0b013e3181676545. [DOI] [PubMed] [Google Scholar]
- Israel S, Moffitt TE, Belsky DW, Hancox RJ, Poulton R, Roberts B, … Caspi A. Translating personality psychology to help personalize preventive medicine for young adult patients. Journal of Personality and Social Psychology. 2014;106(3):484–498. doi: 10.1037/a0035687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolla NJ, Gregory S, Attard S, Blackwood N, Hodgins S. Disentangling possible effects of childhood physical abuse on gray matter changes in violent offenders with psychopathy. Psychiatry Research. 2014;221(2):123–126. doi: 10.1016/j.pscychresns.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, … Fischl B. Regionally localized thinning of the cerebral cortex in schizophrenia. Archives of General Psychiatry. 2003;60(9):878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Ligthart L, Bartels M, Hoekstra RA, Hudziak JJ, Boomsma DI. Genetic contributions to subtypes of aggression. Twin Research and Human Genetics. 2005;8(5):483–491. doi: 10.1375/183242705774310169. [DOI] [PubMed] [Google Scholar]
- Loukas A, Paulos S, Robinson S. Early Adolescent Social and Overt Aggression: Examining the Roles of Social Anxiety and Maternal Psychological Control. Journal of Youth and Adolescence. 2005;34(4):335–345. doi: 10.1007/s10964-005-5757-2. [DOI] [Google Scholar]
- Ly M, Motzkin JC, Philippi CL, Kirk GR, Newman JP, Kiehl KA, Koenigs M. Cortical thinning in psychopathy. American Journal of Psychiatry. 2012;169(7):743–749. doi: 10.1176/appi.ajp.2012.11111627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson JL, Cervantes PE. Assessing aggression in persons with autism spectrum disorders: an overview. Research in Devevlopmental Disabilities. 2014;35(12):3269–3275. doi: 10.1016/j.ridd.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Progress in Neurobiology. 1980;14(2–3):69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Murray AL, Obsuth I, Zirk-Sadowski J, Ribeaud D, Eisner M. Developmental Relations Between ADHD Symptoms and Reactive Versus Proactive Aggression Across Childhood and Adolescence. Journal of Attention Disorders. 2016 doi: 10.1177/1087054716666323. [DOI] [PubMed] [Google Scholar]
- Nagin D, Tremblay RE. Trajectories of boys’ physical aggression, opposition, and hyperactivity on the path to physically violent and nonviolent juvenile delinquency. Child Development. 1999;70(5):1181–1196. doi: 10.1111/1467-8624.00086. [DOI] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, … Kremen WS. Distinct genetic influences on cortical surface area and cortical thickness. Cerebral Cortex. 2009;19(11):2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston SD, de Waal FB. Empathy: Its ultimate and proximate bases. Behavioral and Brain Sciences. 2002;25(1):1–20. doi: 10.1017/S0140525X02000018. discussion 20–71. [DOI] [PubMed] [Google Scholar]
- Raine A, Dodge K, Loeber R, Gatzke-Kopp L, Lynam D, Reynolds C, … Liu J. The Reactive-Proactive Aggression Questionnaire: Differential Correlates of Reactive and Proactive Aggression in Adolescent Boys. Aggressive Behavior. 2006;32(2):159–171. doi: 10.1002/ab.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Archives of General Psychiatry. 2000;57(2):119–127. doi: 10.1001/archpsyc.57.2.119. discussion 128–119. [DOI] [PubMed] [Google Scholar]
- Rodriguiz RM, Chu R, Caron MG, Wetsel WC. Aberrant responses in social interaction of dopamine transporter knockout mice. Behavioural Brain Research. 2004;148(1–2):185–198. doi: 10.1016/S0166-4328(03)00187-6. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, … Fischl B. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology. 2002;58(5):695–701. doi: 10.1212/WNL.58.5.695. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, … Fischl B. Thinning of the cerebral cortex in aging. Cerebral Cortex. 2004;14(7):721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Schiffer B, Muller BW, Scherbaum N, Hodgins S, Forsting M, Wiltfang J, … Leygraf N. Disentangling structural brain alterations associated with violent behavior from those associated with substance use disorders. Archieves of General Psychiatry. 2011;68(10):1039–1049. doi: 10.1001/archgenpsychiatry.2011.61. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Stadler C, Poustka F, Kleinschmidt A. A structural neural deficit in adolescents with conduct disorder and its association with lack of empathy. Neuroimage. 2007;37(1):335–342. doi: 10.1016/j.neuroimage.2007.04.043. [DOI] [PubMed] [Google Scholar]
- Tuvblad C, Raine A, Zheng M, Baker LA. Genetic and environmental stability differs in reactive and proactive aggression. Aggressive Behavior. 2009;35(6):437–452. doi: 10.1002/ab.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos W, Vahl P, Guroglu B, van Nunspeet F, Colins O, Markus M, … Crone EA. Neural correlates of social decision-making in severely antisocial adolescents. Social Cognitive & Affective Neuroscience. 2014;9(12):2059–2066. doi: 10.1093/scan/nsu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Graaff J, Branje S, De Wied M, Meeus W. The moderating role of empathy in the association between parental support and adolescent aggressive and delinquent behavior. Aggressive Behavior. 2012;38(5):368–377. doi: 10.1002/ab.21435. [DOI] [PubMed] [Google Scholar]
- Viding E, Seara-Cardoso A, McCrory EJ. Antisocial and callous behaviour in children. Current Topics in Behavioral Neurosciences. 2014;17:395–419. doi: 10.1007/7854_2013_266. [DOI] [PubMed] [Google Scholar]
- Visser TA, Ohan JL, Whittle S, Yucel M, Simmons JG, Allen NB. Sex differences in structural brain asymmetry predict overt aggression in early adolescents. Social Cognitive & Affective Neuroscience. 2014;9(4):553–560. doi: 10.1093/scan/nst013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Tamnes CK, Ostby Y, Due-Tonnessen P, Fjell AM. Normal variation in behavioral adjustment relates to regional differences in cortical thickness in children. European Child & Adolescenct Psychiatry. 2012 doi: 10.1007/s00787-012-0241-5. [DOI] [PubMed] [Google Scholar]
- Wallace GL, White SF, Robustelli B, Sinclair S, Hwang S, Martin A, Blair RJ. Cortical and subcortical abnormalities in youths with conduct disorder and elevated callous-unemotional traits. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53(4):456–465. e451. doi: 10.1016/j.jaac.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Joshi AA, Joshi SH, Baker LA, Narr KL, Raine A, … Damasio H. Genetic and environmental influences on cortical thickness among 14-year-old twins. Neuroreport. 2012;23(12):702–706. doi: 10.1097/WNR.0b013e328355a62a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Narr KL, Baker LA, Joshi SH, Jahanshad N, Raine A, Thompson PM. Frontal and striatal alterations associated with psychopathic traits in adolescents. Psychiatry Research. 2015;231(3):333–340. doi: 10.1016/j.pscychresns.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Nuechterlein KH, Phillips O, Hamilton LS, Subotnik KL, Asarnow RF, … Narr KL. The contributions of disease and genetic factors towards regional cortical thinning in schizophrenia: the UCLA family study. Schizophrenia Research. 2010;123(2–3):116–125. doi: 10.1016/j.schres.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Nuechterlein KH, Phillips OR, Gutman B, Kurth F, Dinov I, … Narr KL. Disease and genetic contributions toward local tissue volume disturbances in schizophrenia: A tensor-based morphometry study. Human Brain Mapping. 2011 doi: 10.1002/hbm.21349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Raine A. Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: a meta-analysis. Psychiatry Research. 2009;174(2):81–88. doi: 10.1016/j.pscychresns.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Raine A, Colletti P, Toga AW, Narr KL. Abnormal temporal and prefrontal cortical gray matter thinning in psychopaths. Molecular Psychiatry. 2009;14(6):561–562. 555. doi: 10.1038/mp.2009.12. [DOI] [PubMed] [Google Scholar]
- Yang Y, Raine A, Han C, Schug RA, Toga AW, Narr KL. Reduced hippocampal and parahippocampal volumes in murderers with schizophrenia. Psychiatry Research: Neuroimaging. 2010;182(1):9–13. doi: 10.1016/j.pscychresns.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Raine A, Joshi AA, Joshi S, Chang YT, Schug RA, … Narr KL. Frontal information flow and connectivity in psychopathy. British Journal of Psychiatry. 2012a;201(5):408–409. doi: 10.1192/bjp.bp.111.107128. [DOI] [PubMed] [Google Scholar]
- Yang Y, Raine A, Joshi AA, Joshi S, Chang YT, Schug RA, … Narr KL. Frontal information flow and connectivity in psychopathy. British Journal of Psychiatry. 2012b doi: 10.1192/bjp.bp.111.107128. [DOI] [PubMed] [Google Scholar]
- Yang Y, Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Volume reduction in prefrontal gray matter in unsuccessful criminal psychopaths. Biological Psychiatry. 2005;57(10):1103–1108. doi: 10.1016/j.biopsych.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Yang Y, Raine A, Narr KL, Colletti P, Toga AW. Localization of deformations within the amygdala in individuals with psychopathy. Archives of General Psychiatry. 2009;66(9):986–994. doi: 10.1001/archgenpsychiatry.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Roussotte F, Kan E, Sulik KK, Mattson SN, Riley EP, … Sowell ER. Abnormal cortical thickness alterations in fetal alcohol spectrum disorders and their relationships with facial dysmorphology. Cerebral Cortex. 2012;22(5):1170–1179. doi: 10.1093/cercor/bhr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.