Abstract
Diabetes mellitus causes secondary osteoporosis and muscle atrophy. The ability of alfacalcidol (ALF) and exercise (Exe) to inhibit osteoporosis and muscle atrophy in type 2 diabetes mellitus (T2DM) model rats was examined. Twenty-week-old Otsuka Long-Evans Tokushima Fatty rats were randomized to ALF (orally 0.1 μg/kg/day), Exe (treadmill exercise at 10 m/min, 60 min/day, 5 days/week), Comb (ALF and Exe), and Cont (T2DM control treated with vehicle and no exercise) groups (n = 8–10 per group). Sedentary Long-Evans Tokushima Otsuka rats were used as a non-hyperphagic control. After treatment for 2 or 6 weeks, blood glucose (BG) levels, cross-sectional area (CSA) of tibialis anterior muscle fibers, femoral bone mineral density (BMD), and relative quantities of muscle anabolic markers (Pax7, MyoD, and myogenin) and catabolic markers (Atrogin-1, MuRF1, and REDD1) of the soleus muscle assessed by real-time polymerase chain reaction assays were measured. Exe and Comb treatments for 6 weeks decreased BG levels compared with those of the Cont group. ALF, Exe, and Comb treatments for 2 and 6 weeks recovered the CSA compared with that of the Cont group. ALF and Comb treatments for 6 weeks increased femoral BMDs compared with those of the Cont group. After 2 weeks of treatment, Comb treatment increased MyoD expression and decreased MuRF1 expression. ALF or Exe monotherapy significantly decreased Atrogin-1 or MuRF1 expression after 2 weeks of treatment, respectively. After 6 weeks of treatment, ALF and Comb treatments decreased Atrogin-1 and REDD1. These results demonstrate that a combination of ALF and Exe improved CSA from the early phase of treatment by stimulating skeletal muscle differentiation and suppressing muscle catabolic genes. Improvements in BG, BMD, and CSA were observed as long-term effects of the combination therapy. Continued suppression of muscle catabolic genes was observed as a background to these effects.
Introduction
In recent years, population aging has become a global public health issue. Aging societies face a number of issues, such as an increase in aging-associated diseases, multiple comorbidities, and decrease in activities of daily living (ADLs) of elderly persons, who eventually become bedridden. In addition, the prevalence of age-related musculoskeletal disorders, especially osteoporosis and sarcopenia, is also increasing. Several recent reports demonstrated that osteoporotic fractures directly decrease ADLs of elderly persons, as well as increase their mortality risk [1–3]. Sarcopenia also decreases ADLs of elderly persons [4] and is strongly correlated with osteoporosis [5–8]. Therefore, in aging societies, management of aging-associated diseases, osteoporosis and sarcopenia, is imperative to maintain ADLs and improve the life expectancy of elderly persons.
Diabetes mellitus (DM) is one of the most common aging-associated diseases. A recent report showed that 8.4% of global all-cause mortality could be attributed to DM [9]. DM is associated with not only mortality, but also a decrease in activity of patients. Recent meta-analyses showed that patients with type 2 DM (T2DM), which accounts for most cases of DM, have an increased risk of fracture, even though they have normal or high bone mineral density (BMD) [10, 11]. T2DM is associated with decreased balance and increased risk of falls [12, 13]. Furthermore, T2DM patients have an increased risk of muscle atrophy, especially elderly patients [14, 15]. Thus, elderly T2DM patients have a very high risk of fracture, which is directly associated with a reduction in ADLs.
Vitamin D is a fundamental drug for the treatment of osteoporosis. A meta-analysis showed that both native and activated vitamin D prevent osteoporosis and reduce the risk of falls [16]. Alfacalcidol, activated vitamin D, also increased muscle strength in ovariectomized and glucocorticoid-treated rats [17, 18]. An in vivo study showed that 1,25-dihydroxyvitamin D3 stimulated the expression of muscle anabolic markers as a mechanism of the beneficial effects on muscle [19]. Vitamin D is essential for calcium and phosphorus homeostasis, and it also has an important role in controlling blood glucose (BG) levels. Several papers have reported the interaction between vitamin D and DM. Vitamin D deficiency inhibited insulin secretion by 48% [20], and there was an inverse association between serum 25-hydroxy vitamin D [25(OH)D] and the onset of T2DM [21]. Thus, vitamin D has the potential to reduce the risk of fracture and to improve muscle atrophy and BG levels in T2DM patients.
On the other hand, exercise has been used as a basic treatment to control BG levels and prevent the complications of T2DM [22–24]. Furthermore, exercise is also important in the management of osteoporosis. Recent studies have shown the benefits of exercise, for example, increased BMD [25–27], improved balance [28–30], and fewer falls [31]. Exercise-induced mediators, such as peroxisome proliferator-activated receptor-γ coactivator 1α [32–34], Akt [35], and insulin-like growth factor-1[36], are associated with these effects. Exercise is also an important factor in preventing muscle atrophy in T2DM patients. Specific training was shown to be effective in improving gait speed, balance, muscle strength, and joint mobility in DM patients [37, 38].
Based on these findings, we hypothesized that the combination therapy of vitamin D and exercise improves BMD and muscle weakness in T2DM. Although a meta-analysis investigated the effects of vitamin D on the neuromuscular remodeling following exercise in non-diabetic controls, the effect is still inconclusive, and there has been no investigation of bone properties [39]. Furthermore, no studies have investigated the effects of the combination therapy in T2DM. Therefore, the purpose of this study was to elucidate the effects of combination therapy of activated vitamin D and exercise on bone and skeletal muscle in T2DM model rats.
Materials and methods
Animals and experimental protocol
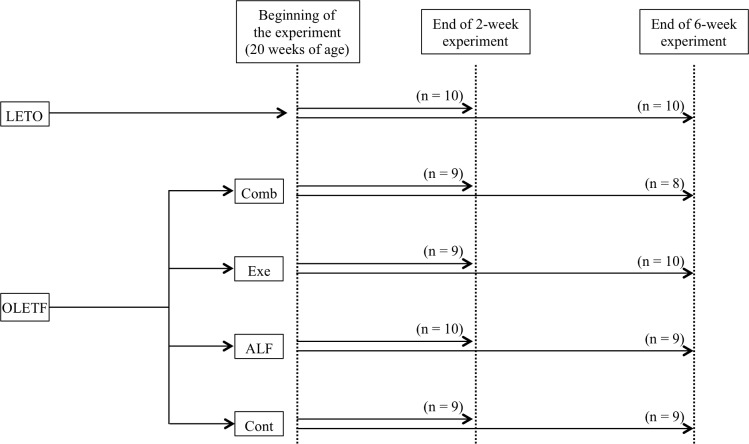
Six-week-old, male Otsuka Long-Evans Tokushima Fatty (OLETF) rats and Long-Evans Tokushima Otsuka (LETO) rats (Hoshino Laboratory Animals, Ibaraki, Japan) were housed in a controlled environment (temperature 23 ± 2°C, humidity 40% ± 20%) with a 12-h light-dark cycle. The details were described in the previous study [40]. OLETF rats are a model of T2DM, while LETO rats are a control model of T2DM (non-T2DM).[41] Rats were allowed ad libitum access to standard rodent chow (CE-7; Clea Japan, Tokyo, Japan) and remained sedentary until 20 weeks of age. OLETF rats have been reported to show increased levels of blood glucose from 20 weeks of age [41]. OLETF rats were randomly assigned to one of the following groups at the age of 20 weeks (n = 8-10/group): (1) ALF group, administered alfacalcidol; (2) Exe group, low-intensity aerobic exercise training; (3) Comb group, administered alfacalcidol and low-intensity aerobic exercise training; and (4) T2DM control group (Cont group), administered vehicle. LETO rats were kept in a sedentary cage condition as non-hyperphagic controls. Each treatment began at 20 weeks of age and continued for 2 or 6 weeks (Fig 1). The following parameters were analyzed in LETO rats to check the changes of these parameters with T2DM and the treatment effects on these parameters to determine whether they recovered to the level of non-T2DM control (LETO) rats. The protocols for all animal experiments were approved in advance by the Animal Experimentation Committee at Akita University (permit No. a-1-2729), and all subsequent animal experiments adhered to the “Guidelines for Animal Experimentation” of Akita University.
Fig 1. Diagram of the experimental protocol.
OLETF rats were assigned to each treatment group. Each treatment began at the age of 20 weeks. OLETF = Otsuka Long-Evans Tokushima Fatty; LETO = Long-Evans Tokushima Otsuka (non-diabetic control); Cont = control (diabetic control); ALF = alfacalcidol; Exe = exercise; Comb = combination of alfacalcidol and exercise.
Body weight measurement
Body weight (BW) was measured at the beginning (20 weeks of age) and end of the experiment. Changes in BW were compared among and within the groups at 2 or 6 weeks.
ALF administration and treadmill exercise
Alfacalcidol (Chugai Pharmaceutical, Tokyo, Japan) at a dose of 0.1 μg/kg/day or vehicle (medium-chain triglyceride) was administered orally every day for 2 or 6 weeks. This dose was selected based on previous studies demonstrating that it did not increase serum calcium levels [17, 18].
Low-intensity treadmill exercise was performed at a speed of 10 m/min, 5% incline, 60 min/day for 2 or 6 weeks (MK-680; Muromachi Kikai, Tokyo, Japan).
Blood glucose measurement
Blood samples were taken from the tail vein for blood glucose measurement. BG (mg/dl) was measured using an automatic analyzer (Antsense III; Horiba, Kyoto, Japan) at the beginning (20 weeks of age) and end of the experiment. Changes in BG levels were compared among and within the groups at 2 or 6 weeks.
Tissue preparation
Rats were euthanized by an injection of sodium pentobarbital (150 mg/kg body weight) (Dainippon Sumitomo Pharma Co. Ltd., Osaka, Japan), and the right femur, left tibialis anterior muscle, and soleus muscle were harvested for evaluations after 2 or 6 weeks of treatment.
The right femur was dipped in 10% formalin neutral buffer (Wako Pure Chemical Industries, Osaka, Japan) for BMD measurement. The left tibialis anterior muscle was immediately frozen in liquid nitrogen and stored at -80°C for histological analyses. The soleus muscle was stored in RNAlater solution (Qiagen, Hilden, Germany) at -80°C for gene expression analysis.
BMD measurement
BMD of the femur was measured using dual-energy X-ray absorptiometry (QDR-4500 Delphi; Hologic, Bedford, MA, USA). The total length of the femur was trisected into proximal, middle, and distal regions. Each region was scanned in the “small animal” mode, with the “regional high-resolution” scan option.
Histological analysis of muscle
The left tibialis anterior muscle was analyzed histologically. Samples were cut into 10-μm-thick transverse serial sections at the thickest part of the muscle belly using a cryostat maintained at -18°C. Sections were stained histochemically (hematoxylin and eosin, H&E).
To measure the cross-sectional area (CSA) of muscle fibers, microscopic images at a magnification of 200× were captured digitally (BX-50; Olympus, Tokyo, Japan), and individual muscle fibers were traced on-screen using ImageJ (National Institutes of Health, Bethesda, MD, USA). Areas were calculated using the ImageJ software based on a calibrated pixel-to-actual size (micrometer) ratio. Fifty fibers per muscle were randomly chosen, and the mean CSA for one muscle fiber was calculated.
Intra-observer variation, as assessed by the coefficient of variation for three corresponding measurements in 50 randomly selected fibers, ranged from 0.8% to 2.6%. Inter-observer variation among the three investigators, as assessed by the coefficient of variation of measurements in 50 randomly selected images, ranged from 2.8% to 6.3%.
Gene expression analysis of skeletal muscle
The soleus muscle was prepared using a homogenizer (MS-100R; Tomy, Tokyo, Japan). Total RNA was extracted and reverse transcribed using an Omniscript Reverse Transcription kit (Qiagen), according to the manufacturer’s instructions. The gene expressions of the following were examined: paired box protein-7 (Pax7), MyoD, and myogenin as muscle anabolic markers; Atrogin-1 and muscle ring finger 1 (MuRF1) as muscle catabolic markers; and regulated in development and DNA damage responses 1 (REDD1). The real-time polymerase chain reaction (PCR) was carried out with TaqMan probes specific for rat Pax7 (Taqman probe ID: Rn00834076_m1), MyoD (Taqman probe ID: Rn01457527_g1), myogenin (Taqman probe ID: Rn01490689_g1), Atrogin-1 (Taqman probe ID: Rn00591730_m1), MuRF1 (Taqman probe ID: Rn00590197_m1), and REDD1 (Taqman probe ID: Rn01433735_g1). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control for sample normalization (Taqman probe ID: Rn01775763_g1).
Statistical analyses
All data are expressed as means ± standard deviation (SD). The results of gene expression did not show a normal distribution; therefore, the nonparametric gene expression data were analyzed using the Kruskal-Wallis test and Dunn’s method as a post hoc test. All other data were parametric and analyzed using one-way analysis of variance (ANOVA) and Scheffé’s post hoc test. The paired t-test was used for the analyses of body weight and BG changes within the groups. All statistical analyses were performed using Statistical Package for the Biosciences Software (SPBS v 9.6) [42]. Values of p < 0.05 were considered significant.
Results
Combined treatment with ALF and exercise prevented the increase of body weight
The BW of LETO rats was significantly lower than of the OLETF groups at any time point (p < 0.0001, respectively). There were no significant differences between the OLETF groups at any time points.
Comparing BW before and after treatment within the groups, the BWs of the Cont and LETO groups after 2 weeks of treatment were significantly increased from before treatment (all p < 0.001). After 6 weeks of treatment, only the Comb group suppressed the increase of BW, while the other groups had significant increases of BW (all p < 0.01; Table 1).
Table 1. Body weight (g) of each experimental group.
| LETO | Cont | ALF | Exe | Comb | ANOVA | |
|---|---|---|---|---|---|---|
| 2 weeks | ||||||
| Start | 476 ± 21 | 608 ± 23* | 600 ± 41* | 595 ± 29* | 604 ± 28* | p < 0.0001 |
| Sacrifice | 490 ± 23† | 622 ± 26†* | 600 ± 38* | 594 ± 30* | 586 ± 40* | p < 0.0001 |
| 6 weeks | ||||||
| Start | 488 ± 20 | 599 ± 32* | 602 ± 20* | 602 ± 28* | 601 ± 17* | p < 0.0001 |
| Sacrifice | 518 ± 18† | 636 ± 37†* | 627 ± 27†* | 624 ± 31†* | 606 ± 21* | p < 0.0001 |
mean ± SD
n = 8–10 in each group
*: p < 0.01 vs. LETO group by Scheffé’s method
†: significantly increased from the start of the experiment within the group
Exercise and combined treatment with ALF and exercise decreased blood glucose levels
Although BG levels did not differ significantly among the groups at the start of the experiment and after 2 weeks of treatment, the BG level of T2DM model rats (Cont group) was significantly higher than that of non-T2DM rats (LETO group) (p < 0.001) after 6 weeks. Exercise and combined treatment with exercise and ALF significantly decreased BG levels (all p < 0.05) compared with that of the Cont group after 6 weeks of treatment.
Comparing BG levels before and after treatment within the groups, the BG levels of the Cont group after 2 and 6 weeks of treatment were significantly increased from before treatment (p = 0.00245, p = 0.0083, respectively; Table 2). In the Exe and Comb groups, the BG level tended to decrease compared to that at the start after 6 weeks of treatment, but not significantly.
Table 2. Blood glucose level (mg/dL) of each experimental group.
| LETO | Cont | ALF | Exe | Comb | ANOVA | |
|---|---|---|---|---|---|---|
| 2 weeks | ||||||
| Start | 169.7 ± 18.0 | 181.9 ± 17.1 | 180.7 ± 18.4 | 214.2 ± 45.7 | 213.2 ± 45.1 | |
| Sacrifice | 157.1 ± 19.7 | 225.6 ± 39.6† | 198.2 ± 23.3 | 220.7 ± 60.0 | 206.0 ± 60.2 | p = 0.0655 |
| 6 weeks | ||||||
| Start | 172.1 ± 11.4 | 211.6 ± 41.9 | 206.3 ± 25.6 | 203.1 ± 33.5 | 215.3 ± 52.4 | |
| Sacrifice | 167.5 ± 22.1* | 288.0 ± 87.2† | 239.8 ± 39.6 | 191.9 ± 29.3* | 204.6 ± 40.8** | p = 0.0001 |
n = 8–10 in each group
*: p < 0.01 vs. Cont group by Scheffé’s method
**: p < 0.05 vs. Cont group by Scheffé’s method
†: significantly increased from the start of the experiment within the group
Both ALF monotherapy and combined treatment with ALF and exercise increased femoral BMD
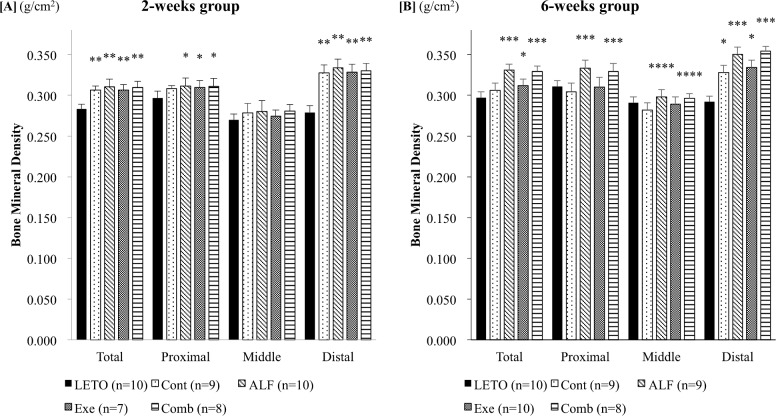
Total and distal femoral BMDs of T2DM model rats (Cont groups) were significantly higher than of non-T2DM rats (LETO group, all p < 0.0001) after 2 weeks of treatment. Two weeks of treatment with ALF with or without exercise significantly increased the total, proximal, and distal femoral BMDs compared with those of non-T2DM rats (LETO group, all p < 0.05 - < 0.0001; Fig 2A), but not with T2DM control rats (Cont group). However, there was no significant difference in cortical (middle femoral) BMD among the groups. ALF and combined treatment with ALF and exercise for 6 weeks significantly increased the total, proximal, and distal femoral BMDs compared with those of T2DM control rats (Cont group), non-T2DM rats (LETO group), and rats treated with only exercise (Exe group) (all p < 0.05). ALF and combined treatment significantly increased cortical (middle femoral) BMD compared with that of T2DM control rats (Cont group, all p < 0.05). Treadmill exercise significantly increased total and distal femoral BMDs compared with those of non-T2DM rats (LETO group, all p < 0.05). Distal femoral BMD was significantly higher in T2DM control rats (Cont group) than in non-T2DM rats (LETO group, p < 0.05; Fig 2B).
Fig 2. Bone mineral density of the femur.
[A] 2-week group BMD values in T2DM model rats (Cont, ALF, Ex, and Comb groups) are significantly higher than those in the non-T2DM rats (LETO group). However, there is no significant difference in cortical (middle femoral) BMD among the groups. [B] 6-week group Both ALF monotherapy and combined treatment with ALF and exercise increase femoral BMD. *: p < 0.05 vs. LETO group by Scheffé’s method **: p < 0.0001 vs. LETO group by Scheffé’s method ***: p < 0.05 vs. LETO, Cont, and Exe groups by Scheffé’s method ****: p < 0.05 vs. Cont group by Scheffé’s method LETO = Long-Evans Tokushima Otsuka (non-diabetic control); Cont = control (diabetic control); ALF = alfacalcidol; Exe = exercise; Comb = combination of alfacalcidol and exercise.
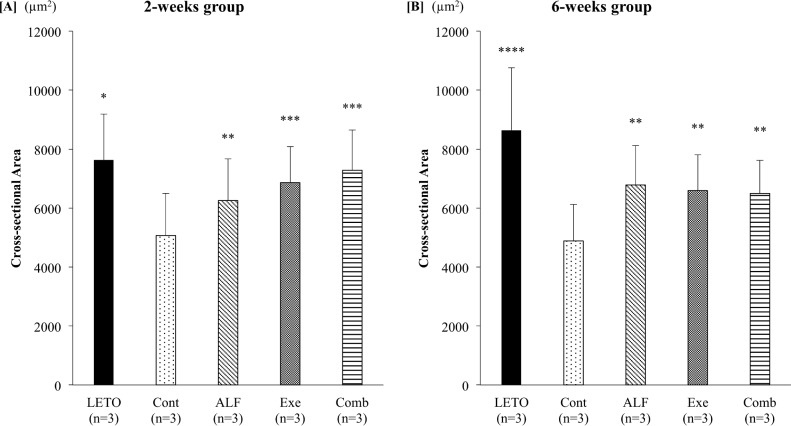
Combined treatment with ALF and exercise for 2 weeks recovered CSA to that of non-T2DM rats in the tibialis anterior muscle
ALF and/or treadmill exercise for 2 weeks significantly increased the CSA compared with that of T2DM control rats (Cont group, all p < 0.01). Although monotherapy with ALF or treadmill exercise for 2 weeks could not recover the CSA to that of non-T2DM control rats (LETO), combined treatment with ALF and exercise significantly increased the CSA compared with that of ALF monotherapy (p < 0.01) and significantly recovered the CSA to that of non-T2DM control rats (LETO group). Exercise monotherapy significantly increased CSA compared with ALF monotherapy (p < 0.01) (Fig 3A).
Fig 3. Cross-sectional area of the tibialis anterior muscle.
[A] 2-week group Only combined treatment with ALF and treadmill exercise for 2 weeks recovers the CSA to that of non-T2DM rats. [B] 6-week group ALF, treadmill exercise, and combined treatment significantly increase the CSA compared with that of T2DM control rats. *: p < 0.01 vs. Cont, ALF, and Ex groups by Scheffé’s method **: p < 0.01 vs. Cont group by Scheffé’s method ***: p < 0.01 vs. Cont and ALF groups by Scheffé’s method ****: p < 0.01 vs. Cont, ALF, Exe, and Comb groups by Scheffé’s method LETO = Long-Evans Tokushima Otsuka (non-diabetic control); Cont = control (diabetic control); ALF = alfacalcidol; Exe = exercise; Comb = combination of alfacalcidol and exercise.
After 6 weeks of treatment, ALF, treadmill exercise, and combined treatment significantly increased the CSA compared with that of T2DM control rats (Cont group, all p < 0.01); however, the CSAs in those groups were still significantly smaller than of non-T2DM rats (LETO group, all p < 0.01; Fig 3B).
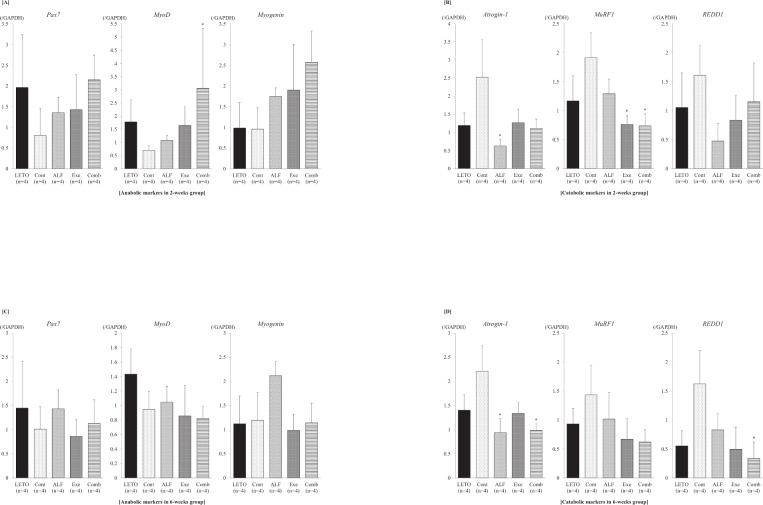
Combined treatment with ALF and exercise increased MyoD expression and decreased MuRF1 expression of the soleus muscle
Combined treatment with ALF and treadmill exercise for 2 weeks significantly increased MyoD expression (Fig 4A) and decreased MuRF1 expression (Fig 4B) compared with those of T2DM control rats (Cont group, all p < 0.05). ALF or treadmill exercise monotherapy for 2 weeks significantly decreased the expression of Atrogin-1 (p < 0.05) or MuRF1 (p < 0.05) compared with that of T2DM control rats (Cont group)(Fig 4B).
Fig 4. Gene expressions of muscle anabolic and catabolic markers.
[A] Muscle anabolic markers in the 2-week group Combined treatment with ALF and treadmill exercise increases MyoD expression. [B] Muscle catabolic markers in the 2-week group Combined treatment with ALF and treadmill exercise decreases MuRF1 expression, and ALF or treadmill exercise monotherapy for 2 weeks significantly decreases Atrogin-1 or MuRF1 expression compared with that of T2DM control rats. [C] Muscle anabolic markers in the 6-week group There are no significant differences among the groups. [D] Muscle catabolic markers in 6-weeks group Combined treatment with ALF and treadmill exercise significantly decreases Atrogin-1 and REDD1 expressions compared with that of T2DM control rats. ALF monotherapy also significantly decreases Atrogin-1 expression. *: p < 0.05 vs. Cont group by Dunn’s method LETO = Long-Evans Tokushima Otsuka (non-diabetic control); Cont = control (diabetic control); ALF = alfacalcidol; Exe = exercise; Comb = combination of alfacalcidol and exercise.
After 6 weeks of treatment, there were no significant differences in muscle anabolic markers (Pax7, MyoD, and myogenin) among the groups (Fig 4C). On the other hand, combined treatment with ALF and treadmill exercise significantly decreased Atrogin-1 and REDD1 expressions compared with those of T2DM control rats (Cont group, all p < 0.05). ALF monotherapy also significantly decreased Atrogin-1 expression (p < 0.05; Fig 4D).
Discussion
In this study, T2DM model rats (OLEFT rats) showed increased total and distal femoral BMDs at 2 weeks and decreased CSA of the tibialis anterior muscle at 2 and 6 weeks compared with non-T2DM control rats (LETO rats). However, there were no significant changes of muscle anabolic and catabolic-related gene expressions in the soleus muscle compared with those of non-T2DM control rats (LETO rats). The effects of combination treatment with ALF and treadmill exercise on bone and skeletal muscle were examined in T2DM model rats. As a short-term effect, combination therapy enhanced muscle anabolic marker (MyoD) expression and recovered the CSA of the tibialis anterior muscle compared with T2DM control rats. As a long-term effect, combined treatment significantly increased femoral BMDs, decreased BG levels, and suppressed muscle catabolic marker (REDD1) expression.
Effect of ALF on blood glucose levels
ALF monotherapy did not inhibit the increase in BG levels in T2DM rats in the present study. Several previous studies have documented the relationship between vitamin D and BG levels or insulin resistance in subjects with lower serum vitamin D, 25(OH)D, levels [43]. However, recent meta-analyses have shown that there is insufficient evidence of a beneficial effect of vitamin D supplementation on improving glycemia or insulin resistance in patients with diabetes, normal fasting glucose, or impaired glucose tolerance [44, 45]. Further investigation is likely needed to elucidate the effects of vitamin D on BG levels or insulin resistance, especially in patients with vitamin D deficiency and T2DM.
BMD in T2DM
A meta-analysis has demonstrated that T2DM is a risk factor for proximal femoral fractures compared to patients without DM [46]. However, a decrease in BMD is not necessarily seen in T2DM when compared with non-DM, even though the risk of fractures is higher [11, 47]. Distal metaphyseal femoral BMD in OLETF rats as a model of T2DM was also increased compared with the control T2DM (LETO) rats in the present study. On the other hand, cortical (middle femoral) BMD in OLETF rats did not show a significant change compared with LETO rats. A previous study demonstrated that cortical BMD of the tibia, measured by peripheral quantitative computed tomography, at 20 weeks was significantly higher in OLETF rats than in LETO rats [48]. Differences in the measurement equipment used or the site selected for cortical BMD determination may have contributed to the difference in cortical BMD in T2DM OLETF rats.
Effects of ALF and treadmill exercise on BMD in T2DM
In T2DM model rats, 6 weeks of treatment with ALF increased proximal femoral BMD. A randomized, placebo-controlled study reported that ALF increased BMD of the lumbar spine by 2.3% in osteoporotic women [49]. However, few studies have investigated the effect of ALF on BMD in T2DM.
On the other hand, treadmill exercise did not exert a significant effect on BMD in T2DM in the present study. A recent study reported that treadmill running exercise increased femoral BMD in T2DM mice [50]. Another study showed that moderate treadmill running increased femoral BMD in diabetic obese Zucker rats [51]. The duration of treadmill exercise in these studies was greater than 10 weeks. Thus, a longer duration of treadmill exercise may show significant results for BMD in T2DM model rats.
Combined treatment with ALF and treadmill exercise significantly increased the femoral BMDs, including both cancellous and cortical bone-dominant regions. This is the first study to investigate the effect of combined treatment with ALF and treadmill exercise on BMD.
Effects of ALF and treadmill exercise on muscle
Muscle anabolic and catabolic genes were evaluated in this study. In the differentiation process of skeletal muscle, Pax7 is essential for the activation of satellite cells [52]. MyoD plays a crucial role in the differentiation of satellite cells into myoblasts [53], and myogenin controls the differentiation of myoblasts to myotubes [54]. On the other hand, myostatin is a major autocrine inhibitor of muscle growth, and one of the important factors that promotes the expression of muscle catabolic genes such as MuRF1 and Atrogin-1 [55]. These two muscle-specific ubiquitin ligases are now widely used as markers of accelerated muscle atrophy [56, 57] and are related to many types of skeletal muscle atrophy. REDD1, which is a strong repressor of the mammalian target of rapamycin (mTOR) [58], is another important muscle catabolic gene stimulated by glucocorticoids.
In the present study, activated vitamin D and exercise monotherapy repressed Atrogin1 and MuRF1, respectively. A previous report showed that activated vitamin D stimulates the myogenic differentiation process by inhibiting myostatin [59]. A recent report also showed that exercise downregulates myostatin [36]. Thus, activated vitamin D or exercise monotherapy appeared to suppress the expression of Atrogin-1 or MuRF1 via the downregulation of myostatin. However, each single treatment did not increase the expressions of muscle anabolic genes, and only the combination therapy increased MyoD expression and repressed REDD1.
A previous study reported that the factors associated with muscle atrophy in T2DM are increased myostatin, endogenous glucocorticoids, and insulin resistance [55].
Since activated vitamin D and exercise therapy both inhibit myostatin, as described above, it is highly likely that the combination therapy similarly suppressed myostatin. Additionally, although the mechanisms are unclear, suppression of REDD1 means that the combined treatment decreased the endogenous glucocorticoid level. While the mechanisms of these results require further clarification, combined treatment may suppress the muscle catabolic pathway multidirectionally, which finally enables the increase of MyoD.
As a limitation, treadmill exercise was performed at a speed of 10 m/min. This setting is relatively low compared to that of a previous report [60]. However, the appropriate exercise setting differs depending on the type of animal model and the age of the animal. Alterations in the speed or duration of treadmill exercise may demonstrate other effects on BMD or muscle properties and related gene expressions.
In conclusion, the combination of ALF and exercise improved the CSA of the tibialis anterior muscle from the early phase (2 weeks) of treatment by stimulating MyoD expression and suppressing atrogenes. Improvements in BG levels, BMD, and muscle volume were observed as long-term effects of the combination therapy. Continued suppression of atrogenes and REDD1 was observed as a background to these long-term effects. BMD and muscle-related results of this study indicate that combined treatment is recommended for the treatment of osteoporosis and muscle atrophy caused by T2DM.
The results of this study are expected to contribute to the treatment of muscle wasting and osteoporosis, which will lead to a reduction of the fracture risk and an improvement of ADLs in patients with T2DM. Since no previous reports investigated the effects of combination therapy with activated vitamin D and exercise on blood glucose levels, bone, and skeletal muscle in patients with T2DM, we would like to apply the results of the present study to clinical research and establish clinical evidence in the future study.
Supporting information
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
The authors would like to thank Ms. Matsuzawa for her support in performing the experiments.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant No. 17K10920), https://www.jsps.go.jp/index.html.
References
- 1.Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301(5):513–21. 10.1001/jama.2009.50 [DOI] [PubMed] [Google Scholar]
- 2.Cauley JA, Thompson DE, Ensrud KC, Scott JC, Black D. Risk of mortality following clinical fractures. Osteoporos Int. 2000;11(7):556–61. 10.1007/s001980070075 [DOI] [PubMed] [Google Scholar]
- 3.Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353(9156):878–82. 10.1016/S0140-6736(98)09075-8 [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiology. 1998;147(8):755–63. [DOI] [PubMed] [Google Scholar]
- 5.Hars M, Biver E, Chevalley T, Herrmann F, Rizzoli R, Ferrari S, et al. Low lean mass predicts incident fractures independently from FRAX: a prospective cohort study of recent retirees. J Bone Miner Res. 2016;31(11):2048–56. 10.1002/jbmr.2878 [DOI] [PubMed] [Google Scholar]
- 6.He H, Liu Y, Tian Q, Papasian CJ, Hu T, Deng HW. Relationship of sarcopenia and body composition with osteoporosis. Osteoporos Int. 2016;27(2):473–82. 10.1007/s00198-015-3241-8 [DOI] [PubMed] [Google Scholar]
- 7.Kawao N, Kaji H. Interactions between muscle tissues and bone metabolism. J Cell Biochem. 2015;116(5):687–95. 10.1002/jcb.25040 [DOI] [PubMed] [Google Scholar]
- 8.Miyakoshi N, Hongo M, Mizutani Y, Shimada Y. Prevalence of sarcopenia in Japanese women with osteopenia and osteoporosis. J Bone Miner Metab. 2013;31(5):556–61. 10.1007/s00774-013-0443-z [DOI] [PubMed] [Google Scholar]
- 9.Group IDFDA. Update of mortality attributable to diabetes for the IDF Diabetes Atlas: Estimates for the year 2013. Diabetes Res Clin Pract. 2015;109(3):461–5. 10.1016/j.diabres.2015.05.037 [DOI] [PubMed] [Google Scholar]
- 10.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis. Osteoporos Int. 2007;18(4):427–44. 10.1007/s00198-006-0253-4 [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto M, Yamaguchi T, Yamauchi M, Kaji H, Sugimoto T. Diabetic patients have an increased risk of vertebral fractures independent of BMD or diabetic complications. J Bone Miner Res. 2009;24(4):702–9. 10.1359/jbmr.081207 [DOI] [PubMed] [Google Scholar]
- 12.Gregg EW, Beckles GL, Williamson DF, Leveille SG, Langlois JA, Engelgau MM, et al. Diabetes and physical disability among older U.S. adults. Diabetes Care. 2000;23(9):1272–7. 10.2337/diacare.23.9.1272 [DOI] [PubMed] [Google Scholar]
- 13.Wallace C, Reiber GE, LeMaster J, Smith DG, Sullivan K, Hayes S, et al. Incidence of falls, risk factors for falls, and fall-related fractures in individuals with diabetes and a prior foot ulcer. Diabetes Care. 2002;25(11):1983–6. 10.2337/diacare.25.11.1983 [DOI] [PubMed] [Google Scholar]
- 14.Kim TN, Park MS, Yang SJ, Yoo HJ, Kang HJ, Song W, et al. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: the Korean Sarcopenic Obesity Study (KSOS). Diabetes Care. 2010;33(7):1497–9. 10.2337/dc09-2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leenders M, Verdijk LB, van der Hoeven L, Adam JJ, van Kranenburg J, Nilwik R, et al. Patients with type 2 diabetes show a greater decline in muscle mass, muscle strength, and functional capacity with aging. J Am Med Dir Assoc. 2013;14(8):585–92. 10.1016/j.jamda.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 16.Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ. 2009;339:b3692 10.1136/bmj.b3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasukawa Y, Miyakoshi N, Maekawa S, Nozaka K, Noguchi H, Shimada Y. Effects of alfacalcidol on muscle strength, muscle fatigue, and bone mineral density in normal and ovariectomized rats. Biomed Res. 2010;31(5):273–9. [DOI] [PubMed] [Google Scholar]
- 18.Miyakoshi N, Sasaki H, Kasukawa Y, Kamo K, Shimada Y. Effects of a vitamin D analog, alfacalcidol, on bone and skeletal muscle in glucocorticoid-treated rats. Biomed Res. 2010;31(6):329–36. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka K, Kanazawa I, Yamaguchi T, Yano S, Kaji H, Sugimoto T. Active vitamin D possesses beneficial effects on the interaction between muscle and bone. Biochem Biophys Res Commun. 2014;450(1):482–7. 10.1016/j.bbrc.2014.05.145 [DOI] [PubMed] [Google Scholar]
- 20.Norman A, Frankel J, Heldt A, Grodsky G. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science. 1980;209(4458):823–5. 10.1126/science.6250216 [DOI] [PubMed] [Google Scholar]
- 21.Song Y, Wang L, Pittas AG, Del Gobbo LC, Zhang C, Manson JE, et al. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2013;36(5):1422–8. 10.2337/dc12-0962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boule NG, Kenny GP, Haddad E, Wells GA, Sigal RJ. Meta-analysis of the effect of structured exercise training on cardiorespiratory fitness in Type 2 diabetes mellitus. Diabetologia. 2003;46(8):1071–81. 10.1007/s00125-003-1160-2 [DOI] [PubMed] [Google Scholar]
- 23.Kelley GA, Kelley KS. Effects of aerobic exercise on lipids and lipoproteins in adults with type 2 diabetes: A meta-analysis of randomized-controlled trials. Public Health. 2007;121(9):643–55. 10.1016/j.puhe.2007.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Umpierre D, Ribeiro PA, Kramer CK, Leitao CB, Zucatti AT, Azevedo MJ, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2011;305(17):1790–9. 10.1001/jama.2011.576 [DOI] [PubMed] [Google Scholar]
- 25.Bonaiuti D, Shea B, Iovine R, Negrini S, Robinson V, Kemper HC, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2002;(3):CD000333 10.1002/14651858.CD000333 [DOI] [PubMed] [Google Scholar]
- 26.Wallace BA, Cumming RG. Systematic review of randomized trials of the effect of exercise on bone mass in pre- and postmenopausal women. Calcif Tissue Int. 2000;67(1):10–8. [DOI] [PubMed] [Google Scholar]
- 27.Wolff I, van Croonenborg JJ, Kemper HC, Kostense PJ, Twisk JW. The effect of exercise training programs on bone mass: a meta-analysis of published controlled trials in pre- and postmenopausal women. Osteoporos Int. 1999;9(1):1–12. 10.1007/s001980050109 [DOI] [PubMed] [Google Scholar]
- 28.Binder EF, Brown M, Sinacore DR, Steger-May K, Yarasheski KE, Schechtman KB. Effects of extended outpatient rehabilitation after hip fracture: a randomized controlled trial. JAMA. 2004;292(7):837–46. 10.1001/jama.292.7.837 [DOI] [PubMed] [Google Scholar]
- 29.Papaioannou A, Adachi JD, Winegard K, Ferko N, Parkinson W, Cook RJ, et al. Efficacy of home-based exercise for improving quality of life among elderly women with symptomatic osteoporosis-related vertebral fractures. Osteoporos Int. 2003;14(8):677–82. 10.1007/s00198-003-1423-2 [DOI] [PubMed] [Google Scholar]
- 30.Sherrington C, Lord SR. Home exercise to improve strength and walking velocity after hip fracture: a randomized controlled trial. Arch Phys Med Rehabil. 1997;78(2):208–12. [DOI] [PubMed] [Google Scholar]
- 31.Sakamoto K, Endo N, Harada A, Sakada T, Tsushita K, Kita K, et al. Why not use your own body weight to prevent falls? A randomized, controlled trial of balance therapy to prevent falls and fractures for elderly people who can stand on one leg for ≤15 s. J Orthop Sci. 2013;18(1):110–20. 10.1007/s00776-012-0328-3 [DOI] [PubMed] [Google Scholar]
- 32.Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454(7203):463–9. 10.1038/nature07206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miura S, Kai Y, Kamei Y, Ezaki O. Isoform-specific increases in murine skeletal muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) mRNA in response to beta2-adrenergic receptor activation and exercise. Endocrinology. 2008;149(9):4527–33. 10.1210/en.2008-0466 [DOI] [PubMed] [Google Scholar]
- 34.Vega RB, Huss JM, Kelly DP. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol Cell Biol.2000;20(5):1868–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nader GA, Esser KA. Intracellular signaling specificity in skeletal muscle in response to different modes of exercise. J Appl Physiol (1985). 2001;90(5):1936–42. 10.1152/jappl.2001.90.5.1936 [DOI] [PubMed] [Google Scholar]
- 36.Ruas JL, White JP, Rao RR, Kleiner S, Brannan KT, Harrison BC, et al. A PGC-1alpha isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell. 2012;151(6):1319–31. 10.1016/j.cell.2012.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allet L, Armand S, de Bie RA, Golay A, Monnin D, Aminian K, et al. The gait and balance of patients with diabetes can be improved: a randomised controlled trial. Diabetologia. 2010;53(3):458–66. 10.1007/s00125-009-1592-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mavros Y, Kay S, Anderberg KA, Baker MK, Wang Y, Zhao R, et al. Changes in insulin resistance and HbA1c are related to exercise-mediated changes in body composition in older adults with type 2 diabetes: interim outcomes from the GREAT2DO trial. Diabetes Care. 2013;36(8):2372–9. 10.2337/dc12-2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minshull C, Biant LC, Ralston SH, Gleeson N. A systematic review of the role of vitamin D on neuromuscular remodelling following exercise and injury. Calcif Tissue Int. 2015;98(5):426–37. 10.1007/s00223-015-0099-x [DOI] [PubMed] [Google Scholar]
- 40.Kinoshita H, Miyakoshi N, Kasukawa Y, Sakai S, Shiraishi A, Segawa T, et al. Effects of eldecalcitol on bone and skeletal muscles in glucocorticoid-treated rats. J Bone Miner Metab. 2016;34(2):171–8. 10.1007/s00774-015-0664-4 [DOI] [PubMed] [Google Scholar]
- 41.Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications: Otsuka Long-Evans Tokushima fatty (OLETF) strain. Diabetes. 1992;41(11):1422–8. 10.2337/diab.41.11.1422 [DOI] [PubMed] [Google Scholar]
- 42.Murata K, Yano E. Medical statistics for evidence-based medicine with SPBS user’s guide. Tokyo: Nankodo; 2002. [Google Scholar]
- 43.Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007;30(4):980–6. 10.2337/dc06-1994 [DOI] [PubMed] [Google Scholar]
- 44.George PS, Pearson ER, Witham MD. Effect of vitamin D supplementation on glycaemic control and insulin resistance: a systematic review and meta-analysis. Diabet Med. 2012;29(8):e142–50. 10.1111/j.1464-5491.2012.03672.x [DOI] [PubMed] [Google Scholar]
- 45.Poolsup N, Suksomboon N, Plordplong N. Effect of vitamin D supplementation on insulin resistance and glycaemic control in prediabetes: a systematic review and meta-analysis. Diabet Med. 2016;33(3):290–9. 10.1111/dme.12893 [DOI] [PubMed] [Google Scholar]
- 46.Janghorbani M, Van Dam RM, Willett WC, Hu FB. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol. 2007;166(5):495–505. 10.1093/aje/kwm106 [DOI] [PubMed] [Google Scholar]
- 47.Schwartz AV, Vittinghoff E, Bauer DC, Hillier TA, Strotmeyer ES, Ensrud KE, et al. Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA. 2011;305(21):2184–92. 10.1001/jama.2011.715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hinton PS, Shankar K, Eaton LM, Rector RS. Obesity-related changes in bone structural and material properties in hyperphagic OLETF rats and protection by voluntary wheel running. Metabolism. 2015;64(8):905–16. 10.1016/j.metabol.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 49.Shiraki M, Kushida K, Yamazaki K, Nagai T, Inoue T, Orimo H. Effects of 2 years' treatment of osteoporosis with 1 a-hydroxy vitamin D3 on bone mineral density and incidence of fracture: A placebo-controlled, double-blind prospective study. Endocrine Journal. 1996;43(2):211–20. 10.1507/endocrj.43.211 [DOI] [PubMed] [Google Scholar]
- 50.Takagi S, Yamashita T, Miura T. Does a treadmill running exercise contribute to preventing deterioration of bone mineral density and bone quality of the femur in KK-Ay mice, a type 2 diabetic animal model? Calcif Tissue Int. 2017;101(6):631–40. 10.1007/s00223-017-0310-3 [DOI] [PubMed] [Google Scholar]
- 51.Mathey J, Horcajada-Molteni MN, Chanteranne B, Picherit C, Puel C, Lebecque P, et al. Bone mass in obese diabetic Zucker rats: influence of treadmill running. Calcif Tissue Int. 2002;70(4):305–11. 10.1007/s00223-001-2077-8 [DOI] [PubMed] [Google Scholar]
- 52.Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102(6):777–86. 10.1016/s0092-8674(00)00066-0 [DOI] [PubMed] [Google Scholar]
- 53.Smith CK 2nd, Janney MJ, Allen RE. Temporal expression of myogenic regulatory genes during activation, proliferation, and differentiation of rat skeletal muscle satellite cells. J Cell Physiol. 1994;159(2):379–85. 10.1002/jcp.1041590222 [DOI] [PubMed] [Google Scholar]
- 54.Nabeshima Y, Hanaoka K, Hayasaka M, Esumi E, Li S, Nonaka I, et al. Myogenin gene disruption results in perinatal lethality because of severe muscle defect. Nature. 1993;364(6437):532–5. 10.1038/364532a0 [DOI] [PubMed] [Google Scholar]
- 55.Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov. 2015;14(1):58–74. 10.1038/nrd4467 [DOI] [PubMed] [Google Scholar]
- 56.Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA. 2001;98(25):14440–5. 10.1073/pnas.251541198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294(5547):1704–8. 10.1126/science.1065874 [DOI] [PubMed] [Google Scholar]
- 58.Wang H, Kubica N, Ellisen LW, Jefferson LS, Kimball SR. Dexamethasone represses signaling through the mammalian target of rapamycin in muscle cells by enhancing expression of REDD1. J Biol Chem. 2006;281(51):39128–34. 10.1074/jbc.M610023200 [DOI] [PubMed] [Google Scholar]
- 59.Garcia LA, King KK, Ferrini MG, Norris KC, Artaza JN. 1,25(OH)2vitamin D3 stimulates myogenic differentiation by inhibiting cell proliferation and modulating the expression of promyogenic growth factors and myostatin in C2C12 skeletal muscle cells. Endocrinology. 2011;152(8):2976–86. 10.1210/en.2011-0159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iwamoto J, Takeda T, Sato Y. Effect of treadmill exercise on bone mass in female rats. Exp Anim. 2005;54(1):1–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.