Primary and metastatic tumors of the central nervous system present a difficult clinical challenge and are a common cause of disease progression and death. Treatment traditionally consists of surgery and/or radiotherapy or stereotactic radiosurgery; however, systemic therapies have become available or are under investigation for patients whose tumors are driven by specific genetic alterations. This article considers this rapidly advancing field with a focus on recently uncovered gene fusions and the brain‐penetrant systemic therapies targeting them.
Keywords: Gene rearrangement, Primary brain tumor, Brain metastasis, Entrectinib, Non‐small cell lung cancer
Abstract
Primary and metastatic tumors of the central nervous system present a difficult clinical challenge, and they are a common cause of disease progression and death. For most patients, treatment consists primarily of surgery and/or radiotherapy. In recent years, systemic therapies have become available or are under investigation for patients whose tumors are driven by specific genetic alterations, and some of these targeted treatments have been associated with dramatic improvements in extracranial and intracranial disease control and survival. However, the success of other systemic therapies has been hindered by inadequate penetration of the drug into the brain parenchyma. Advances in molecular characterization of oncogenic drivers have led to the identification of new gene fusions driving oncogenesis in some of the most common sources of intracranial tumors. Systemic therapies targeting many of these alterations have been approved recently or are in clinical development, and the ability to penetrate the blood‐brain barrier is now widely recognized as an important property of such drugs. We review this rapidly advancing field with a focus on recently uncovered gene fusions and brain‐penetrant systemic therapies targeting them.
Implications for Practice.
Driver gene fusions involving receptor tyrosine kinases have been identified across a wide range of tumor types, including primary central nervous system (CNS) tumors and extracranial solid tumors that are associated with high rates of metastasis to the CNS (e.g., lung, breast, melanoma). This review discusses the systemic therapies that target emerging gene fusions, with a focus on brain‐penetrant agents that will target the intracranial disease and, where present, also extracranial disease.
Introduction
Central nervous system (CNS) malignancies, including primary tumors and metastatic tumors of extracranial origin, continue to be a clinical challenge. Although some primary CNS tumors are considered low grade, they can be associated with significant morbidity. Higher‐grade CNS tumors, such as glioblastoma (GBM), are aggressive and associated with an approximate overall survival (OS) of 12–17 months after multimodal treatment [1], [2], [3]. The poor prognosis of these cancers reflects, in part, the lack of systemic therapies that target the oncogenic mechanisms from which they arise. Recently, gene fusions have emerged as oncogenic drivers in GBM and other primary CNS tumors, including the neurotrophic tropomyosin receptor kinase (NTRK) family, the fibroblast growth factor receptor (FGFR) family, c‐ros oncogene 1 (ROS1), and v‐Raf murine sarcoma viral oncogene homolog B (BRAF) [4], [5], [6], [7]. Thus, although rare, these tyrosine kinase gene fusions are attractive targets for systemic therapies for primary CNS cancers, and efforts are underway to specifically develop small molecule tyrosine kinase inhibitors (TKIs) that penetrate the blood‐brain barrier (BBB) [8].
Metastatic Disease to the CNS
Central nervous system metastases are the most common intracranial tumors in adult cancer patients [9], and they are one of the most feared complications of systemic cancer. The most common primary sources are non‐small cell lung cancer (NSCLC), breast cancer, colorectal cancer, and melanoma [10], [11], which combined account for about 67%–80% of CNS metastases [11]. Although recent advances have extended survival in select patient groups, CNS metastases are still associated with poor prognosis, with median survival of about 7 months [12], reflecting the difficulty of disease control in these patients. In a retrospective analysis of patients with CNS metastases, intracranial progression was the direct cause of death in 57% of patients with NSCLC [13]. In NSCLC, therapies targeting epidermal growth factor receptor (EGFR) aberrations or anaplastic lymphoma kinase (ALK) fusions improve intracranial response rates and overall outcomes in patients whose tumors arise from these genetic alterations [14]. Similarly, activating mutations of BRAF occur in about half of melanomas, and BRAF inhibitors are now a standard part of the therapeutic sequence for patients with BRAF‐related melanoma, including those with CNS metastases [15]. As with primary CNS malignancies, recent studies have identified recurrent gene fusions involving tropomyosin receptor kinase (TRK) and ROS1 tyrosine kinases in patients with NSCLC, breast cancer, and melanoma [16], [17], [18], [19]. Because of the frequent occurrence of CNS metastases in these patients, intensive efforts are focused on the development of brain‐penetrant therapies against these targets.
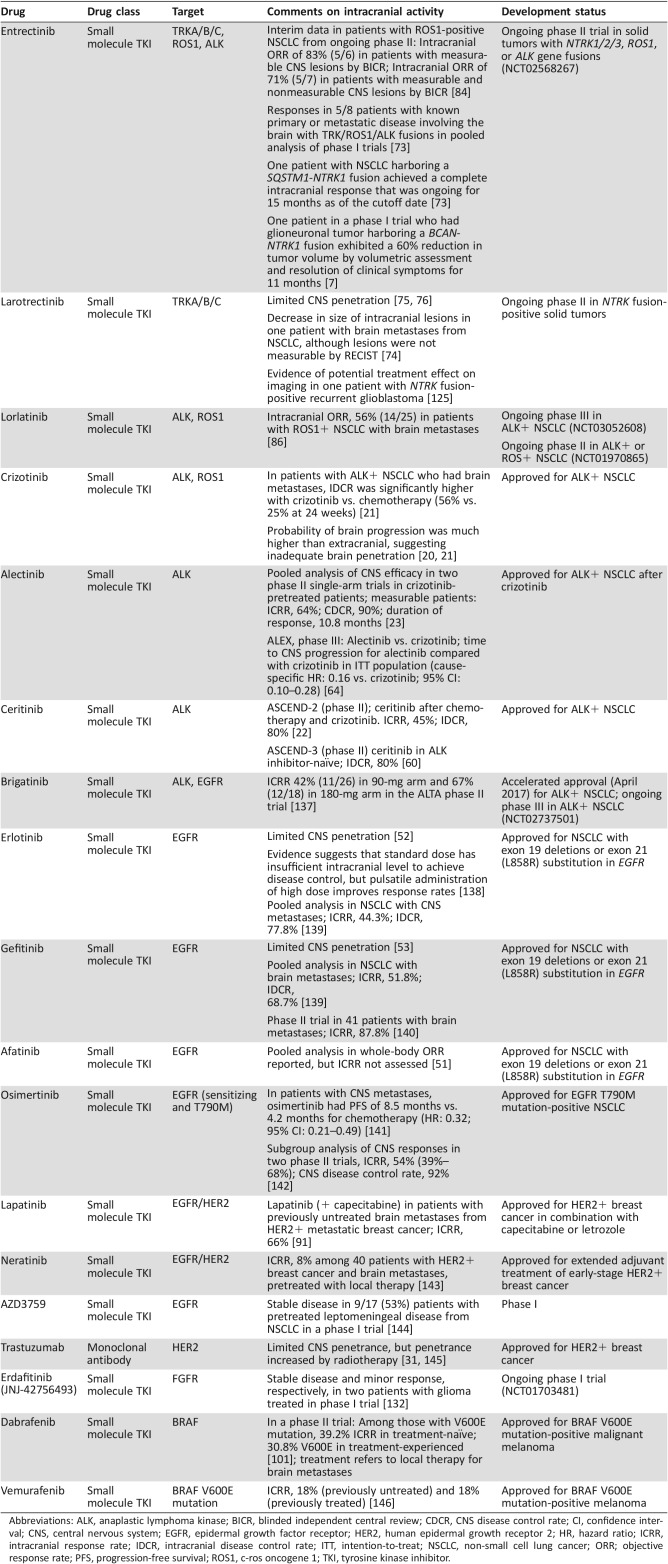
The evolution of ALK inhibitors for the treatment of NSCLC has illustrated the importance of considering CNS efficacy during drug development. Although the first U.S. approved ALK inhibitor, crizotinib, improved intracranial disease control rate versus chemotherapy, patients commonly developed new CNS metastases or intracranial progression, often attributed to the limited brain penetrance of crizotinib [20], [21]. Newer ALK inhibitors such as alectinib and ceritinib have increased CNS penetrance and can re‐establish intracranial disease control in many patients [22], [23]. Thus, as research evolves in the development of TKIs targeting FGFR, TRK, and ROS1 for metastatic cancer, the ability of molecules to penetrate the brain and have resulting CNS activity is an important aspect to evaluate in the clinic. As such, clinical trials are increasingly designed to include patients with CNS metastases, whereas these patients were often excluded from older trials. A list of select TKIs studied for the treatment of CNS malignancies, with key observations regarding intracranial efficacy, is shown in Table 1.
Testing for gene fusions should be considered when there is access to relevant drugs and tumor tissue for patients with primary CNS tumors with good performance status who have exhausted standard therapy. Furthermore, in patients with CNS metastases, we recommend testing for gene fusions from the metastatic site if the patient is progressing in the brain and if there is brain metastasis tissue available for patients who have undergone surgery as part of clinical care.
Table 1. Intracranial activity of select tyrosine kinase inhibitors approved or in development for treatment of NSCLC, breast cancer, or melanoma.
Abbreviations: ALK, anaplastic lymphoma kinase; BICR, blinded independent central review; CDCR, CNS disease control rate; CI, confidence interval; CNS, central nervous system; EGFR, epidermal growth factor receptor; HER2, human epidermal growth receptor 2; HR, hazard ratio; ICRR, intracranial response rate; IDCR, intracranial disease control rate; ITT, intention‐to‐treat; NSCLC, non‐small cell lung cancer; ORR; objective response rate; PFS, progression‐free survival; ROS1, c‐ros oncogene 1; TKI, tyrosine kinase inhibitor.
It is important to appropriately identify patients who may be eligible for targeted therapies. Testing for gene fusions should be considered when there is access to relevant drugs and tumor tissue for patients with primary CNS tumors with good performance status who have exhausted standard therapy. Furthermore, in patients with CNS metastases, we recommend testing for gene fusions from the metastatic site if the patient is progressing in the brain and if there is brain metastasis tissue available for patients who have undergone surgery as part of clinical care. A number of methods are available for the identification of gene fusions, including immunohistochemistry, fluorescence in situ hybridization, next‐generation sequencing, and RNA‐Seq [24], [25], [26]. Each of these testing methods have specific attributes and applicable clinical scenarios that have been previously reviewed [24], [25], [26].
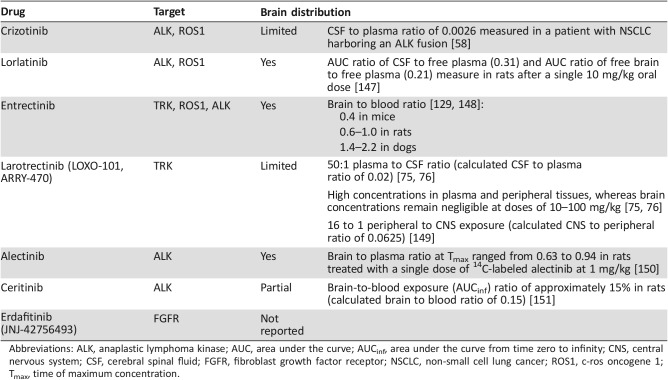
Several factors contribute to the challenges of developing targeted systemic therapies that are effective against CNS metastases. One factor is that mutational status may be different between primary and metastatic sites [27], [28], although the heterogeneity of gene fusions has yet to be firmly established and is an area of continued research. Complicating these differences is the fact that brain biopsy specimens are rarely available, depriving investigators and clinicians of knowledge about the molecular characteristics of CNS metastases [27]. Another factor is the brain penetrance of drugs in development, as detailed in Table 2, which can be difficult to predict from physicochemical properties or preclinical models [29]. It is also important to consider that CNS active drugs may be associated with on‐target neurological side effects, such as cognitive disturbances [30]. There is a delicate balance in the development of new targeted therapies to have a high level of CNS activity as well as a safety and tolerability profile without these concerning neurologic adverse events (AEs). This review will summarize the current challenges of treating primary and metastatic CNS malignancies, and emerging targets for systemic therapies, primarily those that target gene fusions in clinical development.
Table 2. Blood‐brain barrier penetration of select agents.
Abbreviations: ALK, anaplastic lymphoma kinase; AUC, area under the curve; AUCinf, area under the curve from time zero to infinity; CNS, central nervous system; CSF, cerebral spinal fluid; FGFR, fibroblast growth factor receptor; NSCLC, non‐small cell lung cancer; ROS1, c‐ros oncogene 1; Tmax, time of maximum concentration.
The BBB and Assessment of Intracranial Responses
When the BBB is fully intact, it forms a highly restrictive barrier to the CNS for most chemotherapy drugs and large molecules [29]. Multiple factors may disrupt the BBB and affect the CNS penetrance of systemic therapies for brain malignancies, such as the formation of the blood‐tumor barrier, neovascularization, and prior localized therapies allowing for altered access of drugs to the intracranial space [31], [32], [33]. Therefore, clinical trials assessing intracranial responses must take into account variables such as number, origin, and size of CNS metastases, prior therapies including radiotherapy, and traditional factors affecting the integrity of the BBB, such as patient age and performance status [34], [35].
Given the strong influence of CNS malignancies on patient survival, evaluation of intracranial responses is an essential component of clinical trials of systemic targeted therapies for primary CNS tumors and CNS metastases. Although response evaluation criteria in solid tumors (RECIST) criteria are well established for assessment of responses to oncology treatment, it is recognized that RECIST criteria have significant limitations with respect to their applicability in patients with CNS metastases [34]. As a result, international multidisciplinary efforts have led to the development of criteria for assessing intracranial responses, including Response Assessment in Neuro‐Oncology criteria (RANO) and Response Assessment in Neuro‐Oncology Brain Metastases (RANO‐BM) [34], [35]. Although these criteria continue to evolve, they are important for standardizing the evaluation of response rates across clinical trials. The current criteria for assessment of brain metastases from the RANO‐BM group provide guidance on the selection of target and nontarget lesions and methods for imaging and measurement of tumor dimensions. They also discuss the potential confounding effects of prior treatments, such as stereotactic radiosurgery (SRS), and the preference for target lesions not previously treated with local therapy [34].
CNS Metastases
Treatment options for brain metastases generally include surgical resection, SRS, whole‐brain radiation therapy (WBRT), and chemotherapy [9]. However, short‐term and long‐term neurological concerns associated with these therapies, including cognitive side effects and the long‐term side effects of radiation [30], have prompted a search for other treatment modalities. In recent years, targeted therapies that penetrate the BBB have gained in prominence because of their ability to selectively inhibit molecular pathways promoting growth in cancer cells, while minimizing off‐target side effects to normal tissues [36].
NSCLC
Among patients with NSCLC, up to 22% will have CNS metastases at initial presentation [37], [38], [39], [40], and up to 40% will develop CNS metastases during the course of disease [41]. Gain‐of‐function mutations in the EGFR gene are found in about 10%–20% of NSCLC cases [42], [43]. Tyrosine kinase inhibitors directed at EGFR are effective and recommended for treatment of NSCLC patients with EGFR mutations [44], but they have had limited success in patients who also have brain metastases [45]. The first‐ and second‐generation EGFR TKIs (erlotinib, gefitinib, afatinib) administered either as monotherapy or in combination with WBRT have exhibited variable efficacy. Response rates ranging from 10% to 86% and median progression‐free survival (PFS) of 1.6–11.1 months have been reported in patients with NSCLC, depending on the drug, study methodology, and whether overall or intracranial effects were assessed [46], [47], [48], [49], [50], [51]. Furthermore, evidence suggests that erlotinib and gefitinib have limited penetration into the CNS (Table 1) [52], [53]. The irreversible third‐generation EGFR TKI osimertinib, which targets activating EGFR mutations (EGFRm) and resistance mutations (T790M), has activity in the CNS both preclinically and clinically [54], [55]. The same trial also found evidence of tumor shrinkage in patients with brain metastases treated with the reversible EGFRm‐inhibitor AZD3759 [56].
Another common targetable genetic alteration reported in NSCLC is a gene fusion involving ALK, which is present in approximately 3%–7% of NSCLC tumors (Table 3) [57]. Retrospective data suggest that crizotinib, a TKI targeting ALK, can initially achieve some tumor control in the CNS. However, intracranial disease progression has been frequently reported in NSCLC patients with brain metastasis treated with this agent (Table 1) [20], [58], [59], which is likely due to the limited BBB penetration of crizotinib (Table 2) [58]. Next‐generation ALK TKIs such as ceritinib and alectinib have shown promising results in terms of intracranial tumor control in patients with ALK‐positive NSCLC brain metastases [22], [60], [61], [62], [63]. The recently reported results of the ALEX trial, a head‐to‐head study of alectinib versus crizotinib in previously untreated patients with ALK‐positive NSCLC, demonstrated that time to CNS progression was significantly longer with alectinib compared with crizotinib (hazard ratio [HR]: 0.16; 95% confidence interval [CI]: 0.10–0.28) [64]. These agents also shed light on the importance of therapies that can penetrate the BBB and the benefit of using these agents in the first‐line setting to treat or prevent CNS metastases and extend response to treatment. It is important to note that many patients develop acquired resistance to targeted therapies. For example, the majority of patients with NSCLC treated with crizotinib will develop disease progression within 1 year, likely due to acquired resistance [65]. The molecular mechanisms and management of secondary resistance to targeted therapies have been reviewed previously [66].
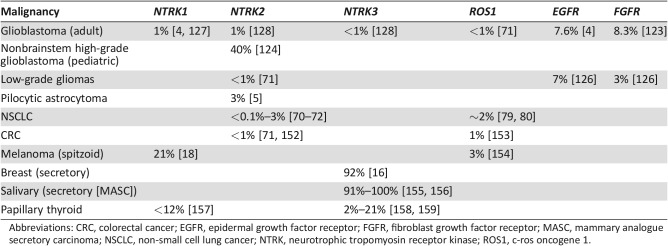
Table 3. Estimated prevalence of driver gene fusions in selected malignancies.
Abbreviations: CRC, colorectal cancer; EGFR, epidermal growth factor receptor; FGFR, fibroblast growth factor receptor; MASC, mammary analogue secretory carcinoma; NSCLC, non‐small cell lung cancer; NTRK, neurotrophic tropomyosin receptor kinase; ROS1, c‐ros oncogene 1.
Emerging Gene Fusions as Targets.
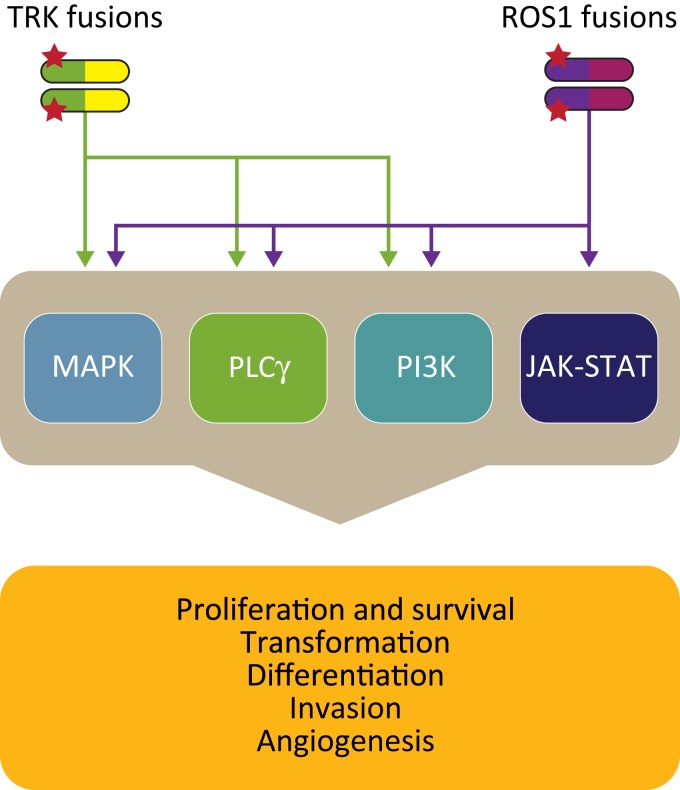
Beyond the ALK fusion pathway, other gene fusions involving tyrosine kinases are emerging as oncogenic drivers in NSCLC and other cancers with brain metastases (Table 3). Inhibitors targeting those tyrosine kinases are in development, with intracranial activity being recognized as a critical drug feature. One emerging set of genetic alterations involves the TRK family. Members of this family include the three transmembrane receptor tyrosine kinases TRKA, TRKB, and TRKC, which are encoded by the NTRK genes (Fig. 1; NTRK1, NTRK2, and NTRK3) [67], [68]. The TRK receptor tyrosine kinases are involved in development of the peripheral nervous system and in cell survival (Fig. 2) [69]. However, several aberrations of the TRK pathway have been associated with the initiation and progression of various cancers. Of those, NTRK gene fusions are currently the best‐characterized aberrations (Table 3) [70], [71].
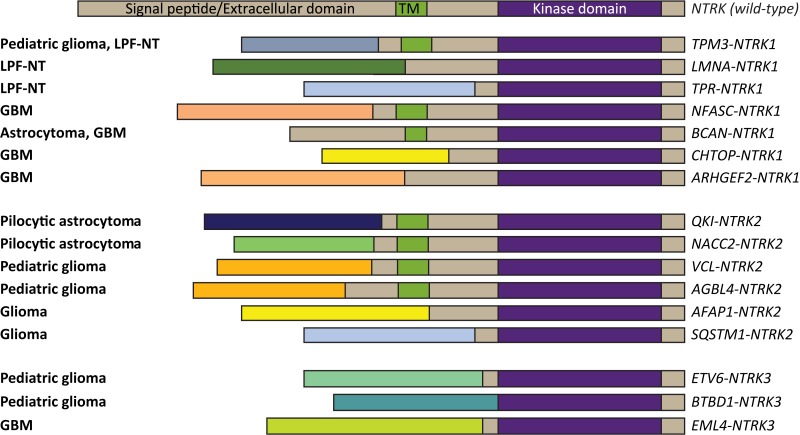
Figure 1.
NTRK gene fusions in CNS malignancy. A depiction of NTRK gene fusions in various primary CNS malignancies [4], [5], [71], [124], [128], [134], [135], [136]. NTRK gene fusions have also been identified in CNS metastases from extracranial solid tumors, including lung, breast, and melanoma. In this case, NTRK is used as an illustrative example of the breadth of gene fusions that have been identified in CNS malignancies. CNS, central nervous system; GBM, glioblastoma; LPF‐NT, lipofibromatosis‐like neural tumor.
Figure 2.
Cell signaling pathways activated by TRK and ROS1 kinases. Ligand‐independent signaling through TRK and ROS1 fusion proteins leads to activation of multiple pathways that stimulate proliferation and survival of tumor cells.
NTRK gene fusions have been detected at a frequency of 0.1% overall in patients with NSCLC and up to 3% in patients with NSCLC and no known oncogenic drivers [19], [70], [71], [72]. In a recent study of 1,378 patients [19], 1 of the 2 NTRK1‐positive patients had multiple NSCLC brain metastases and was subsequently treated with entrectinib in a phase I dose‐escalation study. Entrectinib is a CNS‐active, potent, and selective TRK and ROS1 inhibitor. The patient had a rapid and clinically significant response and exhibited complete resolution of all brain metastases. Entrectinib was well tolerated, and the patient continued on treatment for over 6 months with an ongoing response as of the date of publication [19]. The safety profile and antitumor activity of entrectinib has been evaluated more extensively in two phase I studies (ALKA‐372‐001, n = 54 and STARTRK‐1, n = 65) in patients with advanced solid tumors, including patients with brain metastases [73]. The predominant tumor types in these two studies were NSCLC (60%; n = 71/119) and gastrointestinal tumors (15%; n = 18/119), and 60/119 patients in the pooled cohort had gene fusions involving NTRK1/2/3, ROS1, or ALK. Of those 60 patients, 30 had received no prior TKI targeting TRK fusions, and 24 of those patients were evaluable and had tumors of extracranial origin. Entrectinib was well tolerated; the majority of treatment‐related AEs (TRAEs) were less than grade 2, and all related AEs were reversible upon dose modification. The most common TRAEs were fatigue/asthenia (46%), dysgeusia (42%), paresthesias (29%), nausea (28%), and myalgias (23%) [73]. Objective responses were observed in 3/3 (100%) patients with NTRK1/2/3‐positive tumors (NSCLC, mammary analog secretory carcinoma, colorectal cancer), 12/14 (86%) patients with ROS1‐positive tumors (NSCLC and melanoma), and 4/7 (57%) patients with ALK‐positive tumors (NSCLC, colorectal cancer, renal cell carcinoma). In this phase I cohort of 24 patients, 8 (32%) had known primary or secondary lesions in the brain, and intracranial responses to entrectinib were observed in 5 (63%) patients [73]. A phase II basket trial investigating entrectinib in the treatment of patients with solid tumors harboring NTRK 1/2/3, ROS1, or ALK gene fusions (STARTRK‐2) is currently ongoing and recruiting participants, including those with CNS involvement (NCT02568267).
Another compound that targets the TRK family is the small molecule inhibitor larotrectinib (LOXO‐101, ARRY‐470). An ongoing phase I trial is evaluating the safety and efficacy of larotrectinib in patients with solid tumors (NCT02122913). A preliminary analysis of six patients with NTRK gene fusions in extracranial tumors found partial responses in five (83%) patients according to RECIST criteria [74]. The efficacy of larotrectinib for treatment of intracranial metastases will be an important endpoint, given that preclinical studies showed that the drug has limited brain penetrance (Table 2) [75], [76]. One patient in this preliminary analysis had brain metastases from NSCLC and exhibited an 18% reduction and stable disease in the primary tumor along with decreases in the size of intracranial lesions during treatment, although the lesions were not measurable by RECIST criteria [74]. A phase II basket trial evaluating larotrectinib in patients with solid tumors harboring NTRK gene fusions is ongoing (NCT02576431). Results from an integrated dataset of three studies demonstrated an objective response rate of 76% (38/50); however, only the one patient with NSCLC noted above had CNS metastases, and updated response data were not provided for this patient [77]. Larotrectinib was well tolerated, and the most common treatment‐emergent AEs included fatigue (38%), dizziness (27%), nausea (26%), and anemia (26%).
Another promising target in NSCLC is the ROS1 receptor tyrosine kinase, which is encoded by the ROS1 oncogene [78]. ROS1 gene fusions are present in approximately 2% of patients with NSCLC (Table 3) [79], [80], 19% of whom may have brain metastases at diagnosis [81]. The ALK TKI crizotinib also inhibits ROS1 and was associated with objective responses in 36 of 50 patients (72%) who had advanced NSCLC harboring a ROS1 gene fusion [82]. However, no evidence was provided that crizotinib was active against brain metastases in ROS1‐positive NSCLC, and, as detailed above, the CNS was a preferential site of disease progression in ALK‐positive NSCLC brain metastases treated with crizotinib [83]. Regarding the previously mentioned pooled results of the phase I studies ALKA‐372‐001 and STARTRK‐1, an overall response rate of 86% to treatment with entrectinib was detected in a total of 14 patients, of whom 13 patients had ROS1‐positive NSCLC and 1 patient had ROS1‐positive melanoma [73]. Interim results in patients with ROS1‐positive NSCLC were recently reported from the ongoing STARTRK‐2 phase II trial. Among 32 total patients, the objective response rate was 78% (n = 25) as assessed by the investigator and 69% (n = 22) as assessed by blinded‐independent central review (BICR). The median duration of response was 28.6 months (95% CI: 6.8–34.8) and median PFS was 29.6 months (95% CI: 7.7–36.6) with a median follow‐up of 12.9 and 8.5 months, respectively. In patients with measurable CNS lesions, there was an 83% intracranial objective response rate (five of six patients), and in those with measurable and nonmeasurable CNS lesions, there was a 71% intracranial objective response rate (five of seven patients) as assessed by BICR [84]. The updated tolerability profile of patients treated with entrectinib at the recommended phase 2 dose was consistent with previous reports [84]. Of note, the more concerning neurologic AEs such as mood swings associated with other inhibitors [85] have not been observed with entrectinib treatment at the recommended phase 2 dose to date [84]. The ALK/ROS1 inhibitor lorlatinib, which is also able to cross the BBB (Table 3), recently demonstrated an overall response rate of 36.2% in 47 patients with ROS1‐positive NSCLC [86]. Lorlatinib exhibited clinically meaningful intracranial activity, with an intracranial overall response rate of 56% in 25 patients with brain metastases, but was also associated with neurologic AEs, including cognitive effects (17%) and mood effects (13%) [86]. Of note, many of these targeted therapies for gene fusions have demonstrated clinical efficacy regardless of fusion partner [73], [77], [82].
Breast Cancer.
Breast cancer is the second most common primary tumor that leads to brain metastasis. Approximately 10%–30% of all breast cancer patients will develop brain metastases during the course of disease [45]. Among subtypes of breast cancer, advanced triple‐negative breast cancer and human epidermal growth factor receptor 2 (HER2)‐positive breast cancer have the highest propensity to metastasize to the brain, accounting for 25%–46% and 30%–55%, respectively, of brain metastases in breast cancer [87], [88]. Those same subtypes are also associated with shorter median OS after first diagnosis [89]. In comparison with patients with brain metastases from HER2‐positive disease, patients with triple‐negative brain metastases are more likely to die from progression of systemic disease than from CNS progression only, underscoring the need for drugs simultaneously targeting intra‐ and extracranial disease [90].
A number of targeted therapies are approved for the treatment of breast cancer and can be administered to patients with brain metastases. These include lapatinib, a dual HER2 and EGFR TKI, and the antibody‐drug conjugate trastuzumab‐emtansine (T‐DM1), which both have some CNS penetration. The combination of lapatinib and capecitabine was associated with an objective CNS response rate of 65.9% (95% CI: 50.1–79.5) and a median time to progression of 5.5 months (95% CI: 4.3–6.0) in previously untreated patients with brain metastases studied in the phase II trial [91]. In a retrospective, exploratory analysis of the phase III trial, T‐DM1 was associated with a significant increase in OS among patients with HER2‐positive brain metastases (26.8 months) compared with those treated with lapatinib plus capecitabine (12.9 months; p = .008); however, there was no significant difference in PFS (5.9 months vs. 5.7 months) [92]. The utility of these therapies, and other promising agents, has been previously reviewed. Furthermore, a number of agents are in development for the treatment of breast cancer, and an important distinction with this new generation of drugs is the ability to cross the BBB [45], [93], [94].
Several gene fusions such as ESR1‐CCDC170, SEC16A‐NOTCH1, SEC22B‐NOTCH2, and ESR1‐YAP1 have recently been identified in breast cancer [95], with multiple reports of a gene fusion involving the TRKC tyrosine kinase (ETV6‐NTRK3) in secretory breast cancer [16], [96], [97]. However, no data are yet available describing targeted therapy for treatment of brain metastases in patients whose cancers are driven by such gene fusions.
Melanoma.
Melanoma is the third most common origin of brain metastases [98], and it has the highest propensity of all solid tumors to cause metastatic brain lesions [99]. In patients with newly diagnosed advanced melanoma, brain metastases are present in approximately 20% of patients [100], [101], and up to 75% of melanoma patients will develop brain metastases during the course of disease [100], [102]. Melanoma brain metastasis is associated with a poor prognosis, with a median survival of approximately 7 months [12]. Inhibitors targeting BRAF, its primary downstream target, mitogen‐activated protein kinase (MEK), and immune checkpoint inhibitors have changed the landscape of melanoma treatment in recent years. Although these agents have demonstrated initial responses in the brain, CNS metastases are often the first site of progression [15]. Targeted therapies for the treatment of brain metastases arising from melanoma have previously been reviewed [15], [28], [103].
Emerging targeted therapies for patients with melanoma brain metastases also focus on NTRK, ROS1, ALK, or BRAF gene fusions. A small percentage of melanoma patients, and particularly those with spitzoid melanomas, have been shown to harbor fusions of NTRK1 (16%), ROS1 (17%), ALK (10%), or BRAF (5%) [18]. There was a report of a patient with an extracranial spitzoid melanoma harboring BRAF fusion responding to the MEK inhibitor trametinib [104]. Furthermore, response to treatment with the TKI entrectinib has been observed in patients with GOPC‐ROS1‐positive melanoma [73], [105], but data on patients with melanoma brain metastasis treated with this agent remain unreported (NCT02568267).
Primary CNS Tumors
Glioblastoma is the most common and aggressive primary malignancy of CNS origin, accounting for 47% of such tumors [106]. Survival remains poor with the current standard of care—maximal debulking followed by radiotherapy and temozolomide—with median OS ranging from 14.6 to 16.7 months [1], [2], [3], [107]. Multiple studies of novel therapies for newly diagnosed patients have failed to improve OS [2], [108], [109], with the exception of the NovoTTF device combined with chemoradiation [110]. However, the clinical availability of NovoTTF is limited at this time.
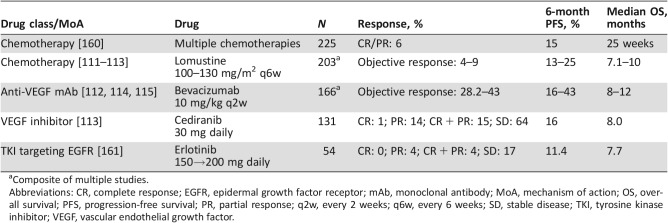
Tumor relapse after primary treatment is nearly universal, and outcomes in recurrent GBM are poor with current treatments (Table 4). Treatment with chemotherapy has generally poor outcomes, with lomustine as the most common drug used after temozolomide failure. The 6‐month PFS (PFS‐6) rate with lomustine is reported to be 13%–19%, objective response rates 4%–5%, and median OS of 7–8 months (Table 4) [111], [112]. Several recent studies of promising agents have not improved efficacy versus standard of care chemotherapy in the recurrent tumor setting [111], [113]. Bevacizumab treatment was associated with improved response rates (28%–38%) and PFS‐6 (16%–43%) compared with other treatments, but this effect was partly due to the radiologic artifact of pseudo‐normalization of tumor vasculature; bevacizumab did not improve median OS (range 7–8 months) [112], [114], [115]. Small molecule inhibitors against a variety of targets, including EGFR, have also failed in trials for recurrence of GBM. Focusing on EGFR illustrates some of the issues that might explain these failures. Brain penetration of erlotinib is poor (brain tissue‐to‐plasma ratio of 5%–11% for the active metabolite) and insufficient to reliably reduce EGFR signaling [116], [117]. Furthermore, EGFR alterations can occur concurrently with alterations in other tyrosine kinases or signaling pathways in GBM, which may provide a pathway for continued tumor growth that is resistant to EGFR TKIs [118], [119], [120], [121].
Table 4. Outcomes in the treatment of recurrent primary central nervous system tumors.
Composite of multiple studies.
Abbreviations: CR, complete response; EGFR, epidermal growth factor receptor; mAb, monoclonal antibody; MoA, mechanism of action; OS, overall survival; PFS, progression‐free survival; PR, partial response; q2w, every 2 weeks; q6w, every 6 weeks; SD, stable disease; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor.
Emerging Gene Fusions in GBM and Other Primary CNS Malignancies.
Clearly, effective treatments for relapsed GBMs and other gliomas are required. Similar to CNS metastases, drugs that target gene fusions should explored. Individually, such alterations are of low prevalence, but collectively they are quite common. For example, gene fusions were present in 30%–50% of tumor samples from 185 patients with GBM [6]. In addition to gene fusions involving EGFR [4], other gene fusions reported in patients with GBM have involved the tyrosine kinases ROS1 [122], PDGFRA [122], FGFR [123], TRKA, TRKB, and TRKC [4], [124], [125] (Table 3). EGFR and FGFR3 fusions have been reported in 7% and 3%, respectively, of low‐grade, isocitrate dehydrogenase wild‐type gliomas [126]. Gene fusions observed for NTRK include NFASC‐NTRK1, BCAN‐NTRK1, AGBL4‐NTRK2, VCL‐NTRK2, ETV6‐NTRK3, and EML4‐NTRK3 [4], [124], [125]. NTRK fusions are present in 1% of tumors from adult patients with GBM [4], [127], [128], and they were found in 40% of nonbrainstem high‐grade GBM in children younger than 3 years (Table 3) [124]. In a recent analysis of 404 gliomas, 8 were identified with NTRK fusions, and 6 of these fusions involved NTRK2 [128]. In this series, 5 of the NTRK fusions (GKAP1‐NTRK2, KCTD8‐NTRK2, TBC1D2‐NTRK2, SQSTM1‐NTRK2, EML4‐NTRK3) were in GBM and the remainder in lower‐grade gliomas (BCAN‐NTRK1 in pilocytic astrocytoma; NOS1AP‐NTRK2 in anaplastic astrocytoma; VCAN‐NTRK2 in grade 2 astrocytoma) [128]. Fusions have also been found in pilocytic astrocytomas, the most common childhood brain tumor. The most frequently reported gene fusion in these tumors is KIAA1549‐BRAF, but fusions involving NTRK2 (QKI‐NTRK2 and NACC2‐NTRK2) have also been reported [5]. In a recent genomic study of 26 glioneuronal tumors, fusions were detected in 30% of patients and involved NTRK, FGFR1, and BRAF fusions, among others [7].
Clinically, a 54‐year‐old patient with an unresectable pontine glioneuronal tumor harboring BCAN‐NTRK1 fusion was treated with entrectinib, and a 60% reduction in tumor volume was observed using 3‐dimensional volumetric assessment.
Although early, the data about targeting such gene fusion abnormalities are encouraging. Entrectinib has been reported to have efficient brain penetration in preclinical models (Table 2) [129]. A recent report demonstrated that a BCAN‐NTRK1 fusion was a potent driver of high‐grade gliomas, and entrectinib demonstrated effective inhibition of tumor growth and increased survival compared with control treatment in a mouse model of BCAN‐NTRK1‐driven glioma [130]. Clinically, a 54‐year‐old patient with an unresectable pontine glioneuronal tumor harboring BCAN‐NTRK1 fusion was treated with entrectinib, and a 60% reduction in tumor volume was observed using 3‐dimensional volumetric assessment [7]. The radiologic response was associated with resolution of clinical symptoms of diplopia and ataxia, and the response was maintained for the 11 months on treatment [7].
The ALK/ROS1 inhibitor lorlatinib has been shown to inhibit tumor growth in a FIG‐ROS1 mouse model of malignant glioma [131]. The ROS1/TRK inhibitor DS‐6051b is currently being evaluated in a phase I study in Japanese patients with advanced solid malignancies harboring either a ROS1 or NTRK gene fusion (NCT02675491). The FGFR inhibitor erdafitinib (JNJ‐42756493) has been shown to inhibit growth of glioma cells harboring FGFR3‐TACC3 fusions. Two patients with glioma harboring this genetic alteration exhibited stable disease and minor response when treated with JNJ‐42756493 in a phase I trial [132].
Conclusion
Central nervous system malignancies, including primary tumors and metastatic tumors of extracranial origin, continue to be a clinical challenge. The incidence of CNS metastases is increasing as new therapies achieve better systemic control and patients are living longer [133]. Gene fusions are an important class of oncogenic drivers and have been identified in a variety of primary CNS malignancies and CNS metastases originating from extracranial tumors. We recommend that testing for gene fusions be considered in patients with primary CNS tumors and CNS metastases where clinically relevant. A number of targeted therapies are approved or under investigation for the treatment of patients with certain gene fusions, and an ability to penetrate the BBB is an important attribute for many of these agents. Investigational therapies that can cross the BBB may treat both the primary tumor and CNS metastases and have the potential to prevent progression to the CNS. It will be interesting to monitor the development of these agents and their ability to provide intracranial and extracranial disease control.
Acknowledgments
We thank Nick Cianciola, Ph.D., of The Lockwood Group (Stamford, Connecticut, USA), and Ken Scholtz, Ph.D., for providing medical writing support, which was in accordance with Good Publication Practice (GPP3) guidelines and funded by Ignyta (San Diego, California, USA).
Footnotes
For Further Reading: Adrienne Johnson, Eric Severson, Laurie Gay et al. Comprehensive Genomic Profiling of 282 Pediatric Low‐ and High‐ Grade Gliomas Reveals Genomic Drivers, Tumor Mutational Burden, and Hypermutation Signatures. The Oncologist 2017;22:1478–1490.
Implications for Practice: By providing objective data to support diagnostic, prognostic, and therapeutic decision‐making, comprehensive genomic profiling is necessary for advancing care for pediatric neuro‐on cology patients. This article presents the largest cohort of pediatric low‐ and high‐grade gliomas profiled by next‐generation sequencing. Reportable alterations were detected in 95% of patients, including diagnostically relevant lesions as well as novel oncogenic fusions and mutations. Additionally, tumor mutational burden (TMB) is reported, which identifies a subpopulation of hypermutated glioblastomas that harbor deleterious mutations in DNA repair genes. This provides support for TMB as a potential biomarker to identify patients who may preferentially benefit from immune checkpoint inhibitors.
Author Contributions
Conception/design: Priscilla K. Brastianos, Umbreen Hafeez, Hui K. Gan
Provision of study material or patients: Priscilla K. Brastianos, Hui K. Gan
Data analysis and interpretation: Franziska Maria Ippen
Manuscript writing: Priscilla K. Brastianos, Franziska Maria Ippen, Umbreen Hafeez, Hui K. Gan
Final approval of manuscript: Priscilla K. Brastianos, Franziska Maria Ippen, Umbreen Hafeez, Hui K. Gan
Disclosures
Priscilla K. Brastianos: Merck, Genentech (H), Lilly, Genentech‐Roche, Angiochem, Merck (C/A), Merck (RF); Umbreen Hafeez: Roche (travel, accommodations, or expenses); Hui K. Gan: AbbVie, Bristol‐Myers Squibb, Ignyta (C/A), AbbVie (RF, travel, accommodations, or expenses). Franzisca Maria Ippen indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Stupp R, Hegi ME, Mason WP et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5‐year analysis of the EORTC‐NCIC trial. Lancet Oncol 2009;10:459–466. [DOI] [PubMed] [Google Scholar]
- 2. Chinot OL, Wick W, Mason W et al. Bevacizumab plus radiotherapy‐temozolomide for newly diagnosed glioblastoma. N Engl J Med 2014;370:709–722. [DOI] [PubMed] [Google Scholar]
- 3. Gilbert MR, Dignam JJ, Armstrong TS et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 2014;370:699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frattini V, Trifonov V, Chan JM et al. The integrated landscape of driver genomic alterations in glioblastoma. Nat Genet 2013;45:1141–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jones DT, Hutter B, Jager N et al. Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat Genet 2013;45:927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shah N, Lankerovich M, Lee H et al. Exploration of the gene fusion landscape of glioblastoma using transcriptome sequencing and copy number data. BMC Genomics 2013;14:818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alvarez‐Breckenridge C, Miller JJ, Nayyar N et al. Clinical and radiographic response following targeting of BCAN‐NTRK1 fusion in glioneuronal tumor. NPJ Precis Oncol 2017;1:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miller JJ, Wen PY. Emerging targeted therapies for glioma. Expert Opin Emerg Drugs 2016;21:441–452. [DOI] [PubMed] [Google Scholar]
- 9. Eichler AF, Loeffler JS. Multidisciplinary management of brain metastases. The Oncologist 2007;12:884–898. [DOI] [PubMed] [Google Scholar]
- 10. Smedby KE, Brandt L, Backlund ML et al. Brain metastases admissions in Sweden between 1987 and 2006. Br J Cancer 2009;101:1919–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep 2012;14:48–54. [DOI] [PubMed] [Google Scholar]
- 12. Sperduto PW, Kased N, Roberge D et al. Summary report on the graded prognostic assessment: An accurate and facile diagnosis‐specific tool to estimate survival for patients with brain metastases. J Clin Oncol 2012;30:419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lagerwaard FJ, Levendag PC, Nowak PJ et al. Identification of prognostic factors in patients with brain metastases: A review of 1292 patients. Int J Radiat Oncol Biol Phys 1999;43:795–803. [DOI] [PubMed] [Google Scholar]
- 14. Wong A. The emerging role of targeted therapy and immunotherapy in the management of brain metastases in non‐small cell lung cancer. Front Oncol 2017;7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Berghoff AS, Preusser M. Targeted therapies for melanoma brain metastases. Curr Treat Options Neurol 2017;19:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tognon C, Knezevich SR, Huntsman D et al. Expression of the ETV6‐NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell 2002;2:367–376. [DOI] [PubMed] [Google Scholar]
- 17. Takeuchi K, Soda M, Togashi Y et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med 2012;18:378–381. [DOI] [PubMed] [Google Scholar]
- 18. Wiesner T, He J, Yelensky R et al. Kinase fusions are frequent in Spitz tumours and spitzoid melanomas. Nat Commun 2014;5:3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Farago AF, Le LP, Zheng Z et al. Durable clinical response to entrectinib in NTRK1‐rearranged non‐small cell lung cancer. J Thorac Oncol 2015;10:1670–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Costa DB, Shaw AT, Ou SH et al. Clinical experience with crizotinib in patients with advanced ALK‐rearranged non‐small‐cell lung cancer and brain metastases. J Clin Oncol 2015;33:1881–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Solomon BJ, Cappuzzo F, Felip E et al. Intracranial efficacy of crizotinib versus chemotherapy in patients with advanced ALK‐positive non‐small‐cell lung cancer: results from PROFILE 1014. J Clin Oncol 2016;34:2858–2865. [DOI] [PubMed] [Google Scholar]
- 22. Crino L, Ahn MJ, De Marinis F et al. Multicenter phase II study of whole‐body and intracranial activity with ceritinib in patients with ALK‐rearranged non‐small‐cell lung cancer previously treated with chemotherapy and crizotinib: Results from ASCEND‐2. J Clin Oncol 2016;34:2866–2873. [DOI] [PubMed] [Google Scholar]
- 23. Gadgeel SM, Shaw AT, Govindan R et al. Pooled analysis of CNS response to alectinib in two studies of pretreated patients with ALK‐positive non‐small‐cell lung cancer. J Clin Oncol 2016;34:4079–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Serrati S, De Summa S, Pilato B et al. Next‐generation sequencing: Advances and applications in cancer diagnosis. Onco Targets Ther 2016;9:7355–7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mertens F, Johansson B, Fioretos T et al. The emerging complexity of gene fusions in cancer. Nat Rev Cancer 2015;15:371–381. [DOI] [PubMed] [Google Scholar]
- 26. Costa V, Aprile M, Esposito R et al. RNA‐Seq and human complex diseases: Recent accomplishments and future perspectives. Eur J Hum Genet 2013;21:134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brastianos PK, Carter SL, Santagata S et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov 2015;5:1164–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chukwueke UN, Brastianos PK. Sequencing brain metastases and opportunities for targeted therapies. Pharmacogenomics 2017;18:585–594. [DOI] [PubMed] [Google Scholar]
- 29. Banks WA. Characteristics of compounds that cross the blood‐brain barrier. BMC Neurol 2009;9(suppl 1):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lamba N, Muskens IS, DiRisio AC et al. Stereotactic radiosurgery versus whole‐brain radiotherapy after intracranial metastasis resection: A systematic review and meta‐analysis. Radiat Oncol 2017;12:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stemmler HJ, Schmitt M, Willems A et al. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2‐positive breast cancer patients with brain metastases and impairment of blood‐brain barrier. Anticancer Drugs 2007;18:23–28. [DOI] [PubMed] [Google Scholar]
- 32. Gerstner ER, Fine RL. Increased permeability of the blood‐brain barrier to chemotherapy in metastatic brain tumors: Establishing a treatment paradigm. J Clin Oncol 2007;25:2306–2312. [DOI] [PubMed] [Google Scholar]
- 33. Tamura K, Kurihara H, Yonemori K et al. 64Cu‐DOTA‐trastuzumab PET imaging in patients with HER2‐positive breast cancer. J Nucl Med 2013;54:1869–1875. [DOI] [PubMed] [Google Scholar]
- 34. Lin NU, Lee EQ, Aoyama H et al. Response assessment criteria for brain metastases: Proposal from the RANO group. Lancet Oncol 2015;16:e270–e278. [DOI] [PubMed] [Google Scholar]
- 35. Huang RY, Wen PY. Response assessment in neuro‐oncology criteria and clinical endpoints. Magn Reson Imaging Clin N Am 2016;24:705–718. [DOI] [PubMed] [Google Scholar]
- 36. Weidle UH, Niewohner J, Tiefenthaler G. The blood‐brain barrier challenge for the treatment of brain cancer, secondary brain metastases, and neurological diseases. Cancer Genomics Proteomics 2015;12:167–177. [PubMed] [Google Scholar]
- 37. Salbeck R, Grau HC, Artmann H. Cerebral tumor staging in patients with bronchial carcinoma by computed tomography. Cancer 1990;66:2007–2011. [DOI] [PubMed] [Google Scholar]
- 38. Yokoi K, Kamiya N, Matsuguma H et al. Detection of brain metastasis in potentially operable non‐small cell lung cancer: A comparison of CT and MRI. Chest 1999;115:714–719. [DOI] [PubMed] [Google Scholar]
- 39. Shi AA, Digumarthy SR, Temel JS et al. Does initial staging or tumor histology better identify asymptomatic brain metastases in patients with non‐small cell lung cancer? J Thorac Oncol 2006;1:205–210. [DOI] [PubMed] [Google Scholar]
- 40. Villano JL, Durbin EB, Normandeau C et al. Incidence of brain metastasis at initial presentation of lung cancer. Neuro Oncol 2015;17:122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Duell T, Kappler S, Knöferl B et al. Prevalence and risk factors of brain metastases in patients with newly diagnosed advanced non‐small‐cell lung cancer. Cancer Treat Commun 2015;4:106–112. [Google Scholar]
- 42. Li T, Kung HJ, Mack PC et al. Genotyping and genomic profiling of non‐small‐cell lung cancer: Implications for current and future therapies. J Clin Oncol 2013;31:1039–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kawaguchi T, Koh Y, Ando M et al. Prospective analysis of oncogenic driver mutations and environmental factors: Japan Molecular Epidemiology for Lung Cancer Study. J Clin Oncol 2016;34:2247–2257. [DOI] [PubMed] [Google Scholar]
- 44. Juan O, Popat S. Treatment choice in epidermal growth factor receptor mutation‐positive non‐small cell lung carcinoma: Latest evidence and clinical implications. Ther Adv Med Oncol 2017;9:201–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brastianos HC, Cahill DP, Brastianos PK. Systemic therapy of brain metastases. Curr Neurol Neurosci Rep 2015;15:518. [DOI] [PubMed] [Google Scholar]
- 46. Ceresoli GL, Cappuzzo F, Gregorc V et al. Gefitinib in patients with brain metastases from non‐small‐cell lung cancer: A prospective trial. Ann Oncol 2004;15:1042–1047. [DOI] [PubMed] [Google Scholar]
- 47. Kim JE, Lee DH, Choi Y et al. Epidermal growth factor receptor tyrosine kinase inhibitors as a first‐line therapy for never‐smokers with adenocarcinoma of the lung having asymptomatic synchronous brain metastasis. Lung Cancer 2009;65:351–354. [DOI] [PubMed] [Google Scholar]
- 48. Ma S, Xu Y, Deng Q et al. Treatment of brain metastasis from non‐small cell lung cancer with whole brain radiotherapy and Gefitinib in a Chinese population. Lung Cancer 2009;65:198–203. [DOI] [PubMed] [Google Scholar]
- 49. Welsh JW, Komaki R, Amini A et al. Phase II trial of erlotinib plus concurrent whole‐brain radiation therapy for patients with brain metastases from non‐small‐cell lung cancer. J Clin Oncol 2013;31:895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee SM, Lewanski CR, Counsell N et al. Randomized trial of erlotinib plus whole‐brain radiotherapy for NSCLC patients with multiple brain metastases. J Natl Cancer Inst 2014;106:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schuler M, Wu YL, Hirsh V et al. First‐line afatinib versus chemotherapy in patients with non‐small cell lung cancer and common epidermal growth factor receptor gene mutations and brain metastases. J Thorac Oncol 2016;11:380–390. [DOI] [PubMed] [Google Scholar]
- 52. Broniscer A, Panetta JC, O'Shaughnessy M et al. Plasma and cerebrospinal fluid pharmacokinetics of erlotinib and its active metabolite OSI‐420. Clin Cancer Res 2007;13:1511–1515. [DOI] [PubMed] [Google Scholar]
- 53. Zhao J, Chen M, Zhong W et al. Cerebrospinal fluid concentrations of gefitinib in patients with lung adenocarcinoma. Clin Lung Cancer 2013;14:188–193. [DOI] [PubMed] [Google Scholar]
- 54. Ballard P, Yates JW, Yang Z et al. Preclinical comparison of osimertinib with other EGFR‐TKIs in EGFR‐mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res 2016;22:5130–5140. [DOI] [PubMed] [Google Scholar]
- 55. Yang JC, Kim DW, Kim SW et al. Osimertinib activity in patients (pts) with leptomeningeal (LM) disease from non‐small cell lung cancer (NSCLC): Updated results from BLOOM, a phase I study. J Clin Oncol 2016;34(suppl 15):9002a. [Google Scholar]
- 56. Ahn MJ, Kim DW, Kim TM et al. Phase I study of AZD3759, a CNS penetrable EGFR inhibitor, for the treatment of non‐small‐cell lung cancer (NSCLC) with brain metastasis (BM) and leptomeningeal metastasis (LM). J Clin Oncol 2016;34(suppl 15):9003a. [Google Scholar]
- 57. Shaw AT, Hsu PP, Awad MM et al. Tyrosine kinase gene rearrangements in epithelial malignancies. Nat Rev Cancer 2013;13:772–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Costa DB, Kobayashi S, Pandya SS et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol 2011;29:e443–e445. [DOI] [PubMed] [Google Scholar]
- 59. Maillet D, Martel‐Lafay I, Arpin D et al. Ineffectiveness of crizotinib on brain metastases in two cases of lung adenocarcinoma with EML4‐ALK rearrangement. J Thorac Oncol 2013;8:e30–e31. [DOI] [PubMed] [Google Scholar]
- 60. Felip E, Orlov S, Park K et al. ASCEND‐3: A single‐arm, open‐label, multicenter phase II study of ceritinib in ALKi‐naïve adult patients (pts) with ALK‐rearranged (ALK+) non‐small cell lung cancer. J Clin Oncol 2015;33(suppl 15):8060a. [Google Scholar]
- 61. Ou SH, Ahn JS, De Petris L et al. Alectinib in crizotinib‐refractory ALK‐rearranged non‐small‐cell lung cancer: A phase II global study. J Clin Oncol 2016;34:661–668. [DOI] [PubMed] [Google Scholar]
- 62. Shaw AT, Gandhi L, Gadgeel S et al. Alectinib in ALK‐positive, crizotinib‐resistant, non‐small‐cell lung cancer: A single‐group, multicentre, phase 2 trial. Lancet Oncol 2016;17:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sharma J, Shum E, Chau V et al. The evolving role of biomarkers in personalized lung cancer therapy. Respiration 2017;93:1–14. [DOI] [PubMed] [Google Scholar]
- 64. Peters S, Camidge DR, Shaw AT et al. Alectinib versus crizotinib in untreated ALK‐positive non‐small‐cell lung cancer. N Engl J Med 2017;377:829–838. [DOI] [PubMed] [Google Scholar]
- 65. Dagogo‐Jack I, Shaw AT. Crizotinib resistance: Implications for therapeutic strategies. Ann Oncol 2016;27(suppl 3):iii42–iii50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lovly CM, Iyengar P, Gainor JF. Managing resistance to EFGR‐ and ALK‐targeted therapies. Am Soc Clin Oncol Educ Book 2017;37:607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Amatu A, Sartore‐Bianchi A, Siena S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. ESMO Open 2016;1:e000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Khotskaya YB, Holla VR, Farago AF et al. Targeting TRK family proteins in cancer. Pharmacol Ther 2017;173:58–66. [DOI] [PubMed] [Google Scholar]
- 69. Nakagawara A. Trk receptor tyrosine kinases: A bridge between cancer and neural development. Cancer Lett 2001;169:107–114. [DOI] [PubMed] [Google Scholar]
- 70. Vaishnavi A, Capelletti M, Le AT et al. Oncogenic and drug‐sensitive NTRK1 rearrangements in lung cancer. Nat Med 2013;19:1469–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Stransky N, Cerami E, Schalm S et al. The landscape of kinase fusions in cancer. Nat Commun 2014;5:4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Suh JH, Johnson A, Albacker L et al. Comprehensive genomic profiling facilitates implementation of the National Comprehensive Cancer Network Guidelines for Lung Cancer Biomarker Testing and identifies patients who may benefit from enrollment in mechanism‐driven clinical trials. The Oncologist 2016;21:684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Drilon A, Siena S, Ou SI et al. Safety and antitumor activity of the multitargeted pan‐TRK, ROS1, and ALK inhibitor entrectinib: Combined results from two phase I trials (ALKA‐372‐001 and STARTRK‐1). Cancer Discov 2017;7:400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hong DS, Farago AF, Brose MS et al. Clinical safety and activity from a phase I study of LOXO‐101, a selective TRKA/B/C inhibitor, in solid‐tumor patients with NTRK gene fusions. Cancer Res 2016;76(suppl 14):CT008a. [Google Scholar]
- 75. Ghilardi JR, Freeman KT, Jimenez‐Andrade JM et al. Administration of a tropomyosin receptor kinase inhibitor attenuates sarcoma‐induced nerve sprouting, neuroma formation and bone cancer pain. Mol Pain 2010;6:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ghilardi JR, Freeman KT, Jimenez‐Andrade JM et al. Sustained blockade of neurotrophin receptors TrkA, TrkB and TrkC reduces non‐malignant skeletal pain but not the maintenance of sensory and sympathetic nerve fibers. Bone 2011;48:389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hyman DM, Laetsch TW, Kummar S et al. The efficacy of larotrectinib (LOXO‐101), a selective tropomyosin receptor kinase (TRK) inhibitor, in adult and pediatric TRK fusion cancers. J Clin Oncol 2017;35(suppl 18):LBA2501a. [Google Scholar]
- 78. Acquaviva J, Wong R, Charest A. The multifaceted roles of the receptor tyrosine kinase ROS in development and cancer. Biochim Biophys Acta 2009;1795:37–52. [DOI] [PubMed] [Google Scholar]
- 79. Bergethon K, Shaw AT, Ou SH et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol 2012;30:863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Li S, Choi YL, Gong Z et al. Comprehensive characterization of oncogenic drivers in Asian lung adenocarcinoma. J Thorac Oncol 2016;11:2129–2140. [DOI] [PubMed] [Google Scholar]
- 81. Gainor JF, Tseng D, Yoda S et al. Patterns of metastatic spread and mechanisms of resistance to crizotinib in ROS1‐positive non–small‐cell lung cancer. JCO Precis Oncol 2017;1:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Shaw AT, Ou SH, Bang YJ et al. Crizotinib in ROS1‐rearranged non‐small‐cell lung cancer. N Engl J Med 2014;371:1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yoshida T, Oya Y, Tanaka K et al. Clinical impact of crizotinib on central nervous system progression in ALK‐positive non‐small lung cancer. Lung Cancer 2016;97:43–47. [DOI] [PubMed] [Google Scholar]
- 84. Ahn MJ, Cho BC, Siena S et al. Entrectinib in patients with locally advanced or metastatic ROS1 fusion‐positive non‐small cell lung cancer (NSCLC). Presented at: IASLC 18th World Conference on Lung Cancer; October 15–18, 2017; Yokohama, Japan.
- 85. Shaw AT, Ou SI, Felip E et al. Efficacy and safety of lorlatinib in ALK+ non‐small cell lung cancer (NSCLC) patients (pts) with >1 prior ALK tyrosine kinase inhibitor (TKI): A phase 1/2 study. J Clin Oncol 2017;35(suppl 15):9006a. [Google Scholar]
- 86. Besse B, Shaw AT, Solomon BJ et al. Preliminary efficacy and safety of lorlatinib in patients (Pts) with ROS1‐positive non‐small cell lung cancer (NSCLC). Ann Oncol 2017;28(suppl 5):1308PDa. [Google Scholar]
- 87. Kennecke H, Yerushalmi R, Woods R et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol 2010;28:3271–3277. [DOI] [PubMed] [Google Scholar]
- 88. Lin NU, Amiri‐Kordestani L, Palmieri D et al. CNS metastases in breast cancer: Old challenge, new frontiers. Clin Cancer Res 2013;19:6404–6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sperduto PW, Kased N, Roberge D et al. The effect of tumor subtype on the time from primary diagnosis to development of brain metastases and survival in patients with breast cancer. J Neurooncol 2013;112:467–472. [DOI] [PubMed] [Google Scholar]
- 90. Lin NU, Claus E, Sohl J et al. Sites of distant recurrence and clinical outcomes in patients with metastatic triple‐negative breast cancer: High incidence of central nervous system metastases. Cancer 2008;113:2638–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bachelot T, Romieu G, Campone M et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2‐positive metastatic breast cancer (LANDSCAPE): A single‐group phase 2 study. Lancet Oncol 2013;14:64–71. [DOI] [PubMed] [Google Scholar]
- 92. Krop IE, Lin NU, Blackwell K et al. Trastuzumab emtansine (T‐DM1) versus lapatinib plus capecitabine in patients with HER2‐positive metastatic breast cancer and central nervous system metastases: A retrospective, exploratory analysis in EMILIA. Ann Oncol 2015;26:113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Venur VA, Leone JP. Targeted therapies for brain metastases from breast cancer. Int J Mol Sci 2016;17:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Dagogo‐Jack I, Gill CM, Cahill DP et al. Treatment of brain metastases in the modern genomic era. Pharmacol Ther 2017;170:64–72. [DOI] [PubMed] [Google Scholar]
- 95. Veeraraghavan J, Ma J, Hu Y et al. Recurrent and pathological gene fusions in breast cancer: Current advances in genomic discovery and clinical implications. Breast Cancer Res Treat 2016;158:219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Arce C, Cortes‐Padilla D, Huntsman DG et al. Secretory carcinoma of the breast containing the ETV6‐NTRK3 fusion gene in a male: Case report and review of the literature. World J Surg Oncol 2005;3:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Del Castillo M, Chibon F, Arnould L et al. Secretory breast carcinoma: A histopathologic and genomic spectrum characterized by a joint specific ETV6‐NTRK3 gene fusion. Am J Surg Pathol 2015;39:1458–1467. [DOI] [PubMed] [Google Scholar]
- 98. Goulart CR, Mattei TA, Ramina R. Cerebral melanoma metastases: A critical review on diagnostic methods and therapeutic options. ISRN Surg 2011;2011:276908:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Westphal D, Glitza Oliva IC, Niessner H. Molecular insights into melanoma brain metastases. Cancer 2017;123:2163–2175. [DOI] [PubMed] [Google Scholar]
- 100. Gibney GT, Gauthier G, Ayas C et al. Treatment patterns and outcomes in BRAF V600E‐mutant melanoma patients with brain metastases receiving vemurafenib in the real‐world setting. Cancer Med 2015;4:1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Long GV, Trefzer U, Davies MA et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF‐mutant melanoma metastatic to the brain (BREAK‐MB): A multicentre, open‐label, phase 2 trial. Lancet Oncol 2012;13:1087–1095. [DOI] [PubMed] [Google Scholar]
- 102. Lotze M, Dallal R, Kirkwood JM et al. Cutaneous melanoma (section 42.2) In: DeVita VT, Hellman S, Rosenburg SA, eds. Cancer: Principles & Practice of Oncology. Philadelphia: Lippincott Williams and Wilkins, 2001:1492–1533. [Google Scholar]
- 103. Cohen JV, Tawbi H, Margolin KA et al. Melanoma central nervous system metastases: Current approaches, challenges, and opportunities. Pigment Cell Melanoma Res 2016;29:627–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ross JS, Wang K, Chmielecki J et al. The distribution of BRAF gene fusions in solid tumors and response to targeted therapy. Int J Cancer 2016;138:881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Couts KL, McCoach CE, Murphy D et al. Acral lentiginous melanoma harboring a ROS1 gene fusion with clinical response to entrectinib. JCO Precis Oncol 2017;10.1200/po.16.00013:1–7. [DOI] [PubMed] [Google Scholar]
- 106. Ostrom QT, Gittleman H, Xu J et al. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro Oncol 2016;18(suppl 5):v1–v75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Stupp R, Mason WP, van den Bent MJ et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–996. [DOI] [PubMed] [Google Scholar]
- 108. Stupp R, Hegi ME, Gorlia T et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071‐22072 study): A multicentre, randomised, open‐label, phase 3 trial. Lancet Oncol 2014;15:1100–1108. [DOI] [PubMed] [Google Scholar]
- 109. Gilbert MR, Wang M, Aldape KD et al. Dose‐dense temozolomide for newly diagnosed glioblastoma: A randomized phase III clinical trial. J Clin Oncol 2013;31:4085–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Stupp R, Wong E, Scott C et al. Interim analysis of the EF‐14 trial: A prospective, multi‐center trial of NovoTTF‐100A together with temozolomide compared to temozolomide alone in patients with newly diagnosed GBM. Neuro Oncol 2014;16(suppl 5):v167. [Google Scholar]
- 111. Wick W, Puduvalli VK, Chamberlain MC et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol 2010;28:1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Taal W, Oosterkamp HM, Walenkamp AM et al. Single‐agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): A randomised controlled phase 2 trial. Lancet Oncol 2014;15:943–953. [DOI] [PubMed] [Google Scholar]
- 113. Batchelor TT, Mulholland P, Neyns B et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol 2013;31:3212–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Friedman HS, Prados MD, Wen PY et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 2009;27:4733–4740. [DOI] [PubMed] [Google Scholar]
- 115. Kreisl TN, Zhang W, Odia Y et al. A phase II trial of single‐agent bevacizumab in patients with recurrent anaplastic glioma. Neuro Oncol 2011;13:1143–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lassman AB, Rossi MR, Raizer JJ et al. Molecular study of malignant gliomas treated with epidermal growth factor receptor inhibitors: Tissue analysis from North American Brain Tumor Consortium Trials 01‐03 and 00‐01. Clin Cancer Res 2005;11:7841–7850. [DOI] [PubMed] [Google Scholar]
- 117. Raizer JJ, Abrey LE, Lassman AB et al. A phase II trial of erlotinib in patients with recurrent malignant gliomas and nonprogressive glioblastoma multiforme postradiation therapy. Neuro Oncol 2010;12:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Huang PH, Mukasa A, Bonavia R et al. Quantitative analysis of EGFRvIII cellular signaling networks reveals a combinatorial therapeutic strategy for glioblastoma. Proc Natl Acad Sci U S A 2007;104:12867–12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Stommel JM, Kimmelman AC, Ying H et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science 2007;318:287–290. [DOI] [PubMed] [Google Scholar]
- 120. Brennan CW, Verhaak RG, McKenna A et al. The somatic genomic landscape of glioblastoma. Cell 2013;155:462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Wykosky J, Hu J, Gomez GG et al. A urokinase receptor‐Bim signaling axis emerges during EGFR inhibitor resistance in mutant EGFR glioblastoma. Cancer Res 2015;75:394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ozawa T, Brennan CW, Wang L et al. PDGFRA gene rearrangements are frequent genetic events in PDGFRA‐amplified glioblastomas. Genes Dev 2010;24:2205–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Parker BC, Annala MJ, Cogdell DE et al. The tumorigenic FGFR3‐TACC3 gene fusion escapes miR‐99a regulation in glioblastoma. J Clin Invest 2013;123:855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Wu G, Diaz AK, Paugh BS et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non‐brainstem high‐grade glioma. Nat Genet 2014;46:444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Schram AM, Taylor BS, Hechtman JF et al. Potential role of larotrectinib (LOXO‐101), a selective pan‐TRK inhibitor, in NTRK fusion‐positive recurrent glioblastoma. Cancer Res 2017;77(suppl 13):LB–302a. [Google Scholar]
- 126.Cancer Genome Atlas Research Network , Brat DJ, Verhaak Rg et al. Comprehensive, integrative genomic analysis of diffuse lower‐grade gliomas. N Engl J Med 2015;372:2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kim J, Lee Y, Cho HJ et al. NTRK1 fusion in glioblastoma multiforme. PLoS One 2014;9:e91940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Subramaniam DS, Xiu J, Mehta S et al. RNA‐Seq analysis of glioma tumors reveal targetable gene fusions. J Clin Oncol 2017;35(suppl 15):2019a. [Google Scholar]
- 129. Ardini E, Menichincheri M, Banfi P et al. Entrectinib, a pan‐TRK, ROS1, and ALK inhibitor with activity in multiple molecularly defined cancer indications. Mol Cancer Ther 2016;15:628–639. [DOI] [PubMed] [Google Scholar]
- 130. Cook PJ, Thomas R, Kannan R et al. Somatic chromosomal engineering identifies BCAN‐NTRK1 as a potent glioma driver and therapeutic target. Nat Commun 2017;8:15987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zou HY, Li Q, Engstrom LD et al. PF‐06463922 is a potent and selective next‐generation ROS1/ALK inhibitor capable of blocking crizotinib‐resistant ROS1 mutations. Proc Natl Acad Sci U S A 2015;112:3493–3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Di Stefano AL, Fucci A, Frattini V et al. Detection, characterization, and inhibition of FGFR‐TACC fusions in IDH wild‐type glioma. Clin Cancer Res 2015;21:3307–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Arvold ND, Lee EQ, Mehta MP et al. Updates in the management of brain metastases. Neuro Oncol 2016;18:1043–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Agaram NP, Zhang L, Sung YS et al. Recurrent NTRK1 gene fusions define a novel subset of locally aggressive lipofibromatosis‐like neural tumors. Am J Surg Pathol 2016;40:1407–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Vaishnavi A, Le AT, Doebele RC. TRKing down an old oncogene in a new era of targeted therapy. Cancer Discov 2015;5:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Zheng Z, Liebers M, Zhelyazkova B et al. Anchored multiplex PCR for targeted next‐generation sequencing. Nat Med 2014;20:1479–1484. [DOI] [PubMed] [Google Scholar]
- 137. Kim DW, Tiseo M, Ahn MJ et al. Brigatinib in patients with crizotinib‐refractory anaplastic lymphoma kinase‐positive non‐small‐cell lung cancer: A randomized, multicenter phase II trial. J Clin Oncol 2017;35:2490–2498. [DOI] [PubMed] [Google Scholar]
- 138. Grommes C, Oxnard GR, Kris MG et al. “Pulsatile” high‐dose weekly erlotinib for CNS metastases from EGFR mutant non‐small cell lung cancer. Neuro Oncol 2011;13:1364–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Fan Y, Xu X, Xie C. EGFR‐TKI therapy for patients with brain metastases from non‐small‐cell lung cancer: A pooled analysis of published data. Onco Targets Ther 2014;7:2075–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Iuchi T, Shingyoji M, Sakaida T et al. Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR‐mutant lung adenocarcinoma. Lung Cancer 2013;82:282–287. [DOI] [PubMed] [Google Scholar]
- 141. Mok TS, Wu YL, Ahn MJ et al. Osimertinib or platinum‐pemetrexed in EGFR T790M‐positive lung cancer. N Engl J Med 2017;376:629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Goss G, Tsai C‐M, Shepherd F et al. CNS response to osimertinib in patients with T790M‐positive advanced NSCLC: pooled data from two phase II trials. J Thoracic Oncol 2017;12(suppl 15):S440–S441. [DOI] [PubMed] [Google Scholar]
- 143. Freedman RA, Gelman RS, Wefel JS et al. Translational Breast Cancer Research Consortium (TBCRC) 022: A phase II trial of neratinib for patients with human epidermal growth factor receptor 2‐positive breast cancer and brain metastases. J Clin Oncol 2016;34:945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Cho BC, Ahn M‐J, Lee J‐S et al. Phase I study (BLOOM) of AZD3759, a BBB penetrable EGFR inhibitor, in EGFRm NSCLC patients with leptomeningeal metastasis (LM) who progressed after other anti‐cancer therapy. J Clin Oncol 2017;35(suppl 15):2069a. [Google Scholar]
- 145. van Vulpen M, Kal HB, Taphoorn MJ et al. Changes in blood‐brain barrier permeability induced by radiotherapy: Implications for timing of chemotherapy? Oncol Rep 2002;9:683–688. [PubMed] [Google Scholar]
- 146. McArthur GA, Maio M, Arance A et al. Vemurafenib in metastatic melanoma patients with brain metastases: An open‐label, single‐arm, phase 2, multicentre study. Ann Oncol 2017;28:634–641. [DOI] [PubMed] [Google Scholar]
- 147. Johnson TW, Richardson PF, Bailey S et al. Discovery of (10R)‐7‐amino‐12‐fluoro‐2,10,16‐trimethyl‐15‐oxo‐10,15,16,17‐tetrahydro‐2H‐8,4‐(m etheno)pyrazolo[4,3‐h][2,5,11]‐benzoxadiazacyclotetradecine‐3‐carbonitrile (PF‐06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (ALK) and c‐ros oncogene 1 (ROS1) with preclinical brain exposure and broad‐spectrum potency against ALK‐resistant mutations. J Med Chem 2014;57:4720–4744. [DOI] [PubMed] [Google Scholar]
- 148. De Braud FG, Pilla L, Niger M et al. Phase 1 open label, dose escalation study of RXDX101, an oral pan‐trk, ROS1, and ALK inhibitor, in patients with advanced solid tumors with relevant molecular alterations. J Clin Oncol 2014;32(suppl 5):2502a. [Google Scholar]
- 149. Andrews SW. Allosteric small molecule inhibitors of the NGF/TrkA pathway: A new approach to treating inflammatory pain. Poster presented at: International Association for the Study of Pain World Congress on Pain; August 27–31, 2012; Milan, Italy.
- 150. Kodama T, Hasegawa M, Takanashi K et al. Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases. Cancer Chemother Pharmacol 2014;74:1023–1028. [DOI] [PubMed] [Google Scholar]
- 151.Zykadia (ceritinib) capsules for oral use [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation, 2017. [Google Scholar]
- 152. Ardini E, Bosotti R, Borgia AL et al. The TPM3‐NTRK1 rearrangement is a recurring event in colorectal carcinoma and is associated with tumor sensitivity to TRKA kinase inhibition. Mol Oncol 2014;8:1495–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Aisner DL, Nguyen TT, Paskulin DD et al. ROS1 and ALK fusions in colorectal cancer, with evidence of intratumoral heterogeneity for molecular drivers. Mol Cancer Res 2014;12:111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Davies KD, Doebele RC. Molecular pathways: ROS1 fusion proteins in cancer. Clin Cancer Res 2013;19:4040–4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Bishop JA, Yonescu R, Batista DA et al. Cytopathologic features of mammary analogue secretory carcinoma. Cancer Cytopathol 2013;121:228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Balanza R, Arrangoiz R, Cordera F et al. Mammary analog secretory carcinoma of the parotid gland: A case report and literature review. Int J Surg Case Rep 2015;16:187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Greco A, Miranda C, Pierotti MA. Rearrangements of NTRK1 gene in papillary thyroid carcinoma. Mol Cell Endocrinol 2010;321:44–49. [DOI] [PubMed] [Google Scholar]
- 158. Ricarte‐Filho JC, Li S, Garcia‐Rendueles ME et al. Identification of kinase fusion oncogenes in post‐Chernobyl radiation‐induced thyroid cancers. J Clin Invest 2013;123:4935–4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Prasad ML, Vyas M, Horne MJ et al. NTRK fusion oncogenes in pediatric papillary thyroid carcinoma in northeast United States. Cancer 2016;122:1097–1107. [DOI] [PubMed] [Google Scholar]
- 160. Wong ET, Hess KR, Gleason MJ et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol 1999;17:2572–2578. [DOI] [PubMed] [Google Scholar]
- 161. van den Bent MJ, Brandes AA, Rampling R et al. Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J Clin Oncol 2009;27:1268–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]