This article assesses the current practice of radiotherapy for cancer of the uterine cervix. The outcomes of patients who adhered to radiotherapy guidelines were compared with patients who discontinued radiotherapy, and conclusions were drawn about whether and to what extent patients benefited from adherence.
Keywords: Cervical cancer, Radiotherapy, Sub‐Saharan Africa, Survival, Adherence
Abstract
Background.
Discontinuation of radiotherapy (RT) for cervical cancer (CC) in sub‐Saharan Africa is common because of patient‐ and health service‐related reasons. This analysis describes toxicities and the effect of adherence on survival.
Materials and Methods.
A total of 788 patients with CC (2008–2012) who received RT at Addis Ababa University Hospital were included. External beam RT without brachytherapy was performed according to local guidelines. We previously described survival and prognostic factors. Now we analyzed adherence and survival according to total doses received. Adjustment via multivariate cox regression analysis was done.
Results.
One‐year overall survival (OS) after radical RT (n = 180) for International Federation of Gynecology and Obstetrics (FIGO) stages IIA–IIIA was 89% for discontinuation (<72 Gy) and 96% for adherence (≥72 Gy; hazard ratio [HR], 1.3; 95% confidence interval [CI], 0.5–3.3). One‐year OS after nonradical RT (n = 389) for FIGO stages IIIB–IVA was 71% for discontinuation (<40 Gy) and 87% for adherence (44–50 Gy; HR, 3.1; 95% CI, 1.4–6.9). One‐year OS for FIGO stages IIIB–IVB (n = 219) after one compared with two or more palliative single fractions of 10 Gy were 14% and 73% respectively (HR, 7.3; 95% CI, 3.3–16). Reasons for discontinuation were toxicities, economic background, and RT machine breakdown. Grade 1–2 late toxicities were common (e.g., 30% proctitis, 22% incontinence). Grade 3 early and late toxicities were seen in 5% and 10% respectively; no grade 4 toxicities occurred.
Conclusion.
Patients who adhered to guideline‐conforming RT had optimum survival. Better supportive care, brachytherapy to reduce toxicities, socioeconomic support, and additional radiation capacities could contribute to better adherence and survival.
Implications for Practice.
This study presents the effect of adherence on survival of 788 patients with cervical cancer receiving external beam radiotherapy without brachytherapy in Ethiopia. Discontinuation of planned radiotherapy according to local guidelines considerably reduced survival for all International Federation of Gynecology and Obstetrics (FIGO) stages treated (hazard ratios were 1.3, 3.1, and 7.3 for FIGO stages IIA–IIIA and IIIB–IVA and the palliative approach, respectively). Early toxicity (5% grade 3) should be treated to improve adherence. Economic difficulties and machine breakdown should also be addressed to reduce discontinuation and improve survival.
Introduction
Cancer of the uterine cervix (CC) is the leading cause of cancer death among women in sub‐Saharan Africa [1] and other parts of the economically developing world, largely because of limited access to early detection and treatment services [2]. CC tragically serves as a symbol for global health disparity. The age‐standardized death rate per 100,000 women in east Africa is 12 times as high as in western Europe (25.3% vs. 2%) [3].
Patients with CC diagnosed with early stage disease (stage IIA or lower) are primarily treated with surgery, whereas those patients diagnosed with advanced stage disease (stage IIB or higher) are treated with chemoradiation [4]. Because of the lack of early detection services, most patients with CC in sub‐Saharan Africa are diagnosed at a late stage of the disease [5], [6], and thus they are candidates for radiotherapy (RT). However, the availability of RT services is limited in this part of the world. In Africa, 28 countries lack any RT facility [7]. Even in those countries with RT, the number of RT machines is woefully inadequate. Furthermore, 30% of RT machines in Africa are Cobalt‐60 units rather than intensity‐modulated RT using linear accelerators (LINACs), which are the standard of care because they reduce adverse effects from unnecessary irradiation of surrounding tissues [8]. However, Cobalt‐60 units with more superficial and less sharp beam penetration are cheaper to install and maintain, easier to operate, much less dependent on reliable electrical power, and less vulnerable to changes in humidity or temperature [9]. Regardless of the type of RT, the combination of external beam RT (EBRT) with intracavitary brachytherapy (ICBT) is strongly better re‐commended for International Federation of Gynecology and Obstetrics (FIGO) stages IIB–IVA [4]. In these cases, the tumor center should receive a total radiation dose of 80–95 gray (Gy) [4]. Despite the importance of ICBT in the treatment of CC, in 2010 brachytherapy services were available in merely 20 of 52 African countries, Ethiopia not among them [10].
Ethiopia is the second most populated African country, preceded by Nigeria, with over 44 million women and girls [11]. According to GLOBOCAN 2012 estimates, 7,095 women are newly diagnosed with CC every year in Ethiopia [12]. Access to adequate treatment for these women is severely limited, as only one RT machine exists in the country, hosted by the Radiotherapy Center of Tikur Anbessa University Hospital (TAHRC). The staff of the center comprises the only four radiation oncologists in the whole country. Until the closing date of this study (August 7, 2013), RT at TAHRC was performed solely as EBRT by a Theratron Equinox 80 Cobalt‐60 unit (Best Theratronics Ltd., Ottawa, Canada) with a source‐to‐surface distance of 80 cm without additional ICBT. The Cobalt‐60 unit is in daily use from 8 am to 5 pm and is subject to monthly maintenance procedures during which no patients are treated. In 2014, we reported long waiting times for patients treated in the years 2008 to 2012, which resulted in a considerable stage migration. Data on patient baseline characteristics, waiting times, and overall survival have been previously published [13]. However, regarding RT guidelines for patients with CC in Ethiopia, there are no published data on adherence and outcome. In order to guarantee reliable health care standards, treatment guidelines need to be transparent and comparable throughout centers [14]. In terms of oncological RT, underdosing, resulting in residual disease or recurrence, might be as harmful as overdosing. Radiation toxicities or secondary cancers similarly lead to decreased quality of life and even lower survival.
The primary purpose of this study is to assess the current practice of RT for CC at TAHRC by means of a dose‐specific survival analysis. The outcomes of patients who adhered to guideline‐conforming RT were compared with those of patients who discontinued RT in order to draw conclusions about whether and to what extent patients benefit from adherence to guideline‐conforming RT.
Materials and Methods
Patients and Methods
All women with histologically verified cancer of the cervix uteri (International Classification of Diseases for Oncology codes C53.0–9) who were diagnosed and treated with RT between September 11, 2008, and September 11, 2012, at TAHRC, were screened for inclusion to the study. Patients whose cervix uteri was surgically removed, patients who did not receive RT to the pelvis, and patients who were not assigned to RT according to TAHRC guidelines were excluded. All patient and tumor characteristics, diagnostic results, and therapy information were extracted from patients’ files. Regarding data on human immunodeficiency virus (HIV) status, regular screening started in the third year of data collection on September 10, 2011. If several analgesics were used, only the most potent analgesic was documented.
Patients were followed up every 6 months after the end of RT at TAHRC. For information on survival, adverse effects, and state of disease, the files’ last date of personal contact and additional information from patients or their relatives via phone calls were used. For differentiation of acute and late adverse effects, data collected within the first 3 months after the last day of RT were documented separately from those collected afterwards. The Common Terminology Criteria for Adverse Events, version 4.0 [15] were applied for grading the toxicities.
Staging
Tumors were classified according to the FIGO staging system [16] by at least one of the four radiation oncologists at TAHRC. In cases of discrepancy between two physicians, another radiation oncologist was consulted. An additional radiologic or sonographic suspicion of distant metastasis or hydronephrosis resulted in upstaging at the time of RT planning. Histological results were documented according to pathology reports.
Radiotherapy
Indications for RT were stages of FIGO higher than IIA or lower stages for inoperable patients. Renal failure and FIGO stage IVB were contraindications to curative RT. Planning of RT consisted of reassessment of FIGO stage and marking the optimal beam entry 3 cm cranially from the pubis with an intradermal ink injection. Body imaging was not available. For calculation of tumor‐to‐skin distance for anterior‐posterior field and lateral field size, the sagittal and transversal diameters at the marked localization were measured and bisected.
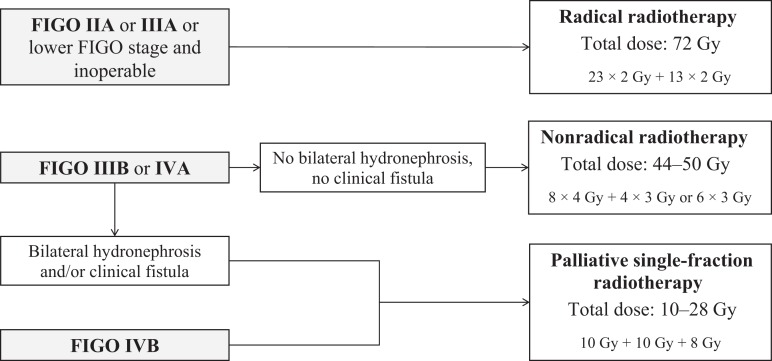
RT was performed either curatively as radical and nonradical RT or as palliation with monthly single fractions (Fig. 1). For details, see Kantelhardt et al. [13]. Radical and nonradical RT were applied in two phases. Patients with FIGO stage IIB–IIIA were planned to receive a total dose of 72 Gy (radical RT), which were distributed in 23 fractions at 2 Gy each within 5 to 6 weeks in the first phase and 13 fractions at 2 Gy each within 2 to 4 weeks in the second phase. Patients with FIGO stage IIIB or IVA without bilateral hydronephrosis or vesicovaginal fistula received a nonradical RT pattern with a total dose of 44–50 Gy and larger doses per fraction: 8 fractions of 4 Gy within 4 weeks in the first phase, followed by a second phase of 12 or 18 Gy (4 or 6 fractions of 3 Gy each) within 2 to 3 weeks. Patients with FIGO stage IVA or IIIB with bilateral hydronephrosis, IVA with clinical fistula, or IVB received palliative RT: monthly single fractions of 10 Gy each with a maximum 28 Gy in total, with a third single fraction of 8 Gy.
Figure 1.
Criteria for therapeutic decision making at the Radiotherapy Center of Tikur Anbessa Hospital, Addis Ababa, Ethiopia.
Abbreviation: FIGO, International Federation of Gynecology and Obstetrics.
Statistical Analysis
The primary endpoint of this study was overall survival. Person time equaled the time from the first day of RT to death, censoring, or closing date (August 7, 2013), whichever came first. Probabilities of overall survival were estimated using the Kaplan‐Meier method. The 95% confidence intervals (95% CIs) at years one and two were shown. The Cox proportional hazards model [17] was used to describe differences between patients who discontinued and those who adhered. Confounders were identified by directed acyclic graphs: grade of anemia, the respective RT schedule, performance status, estimated glomerular filtration rate and HIV status. Analyses were conducted using SPSS Statistics, version 24 (IBM, Armonk, NY). The median follow‐up time for patients was 9.6 months. Right censoring was assumed to be unrelated to the risk of death. As most (81%) of the 788 patients were censored, an additional worst‐case analysis based on the follow‐up intervals of 6 months was performed: all patients who were neither seen nor reached by telephone calls within 6 months after last contact were assumed to have died 1 day after last contact.
Ethics
Ethical approval was obtained from the Addis Ababa University of Health Science and the Medical Faculty of Martin‐Luther University Halle‐Wittenberg. The study was conducted without individual informed consent, as the study relied on retrospective data collected as part of routine patient care. For follow‐up interviews by telephone, patients or relatives gave oral consent.
Results
Description of the Study Population
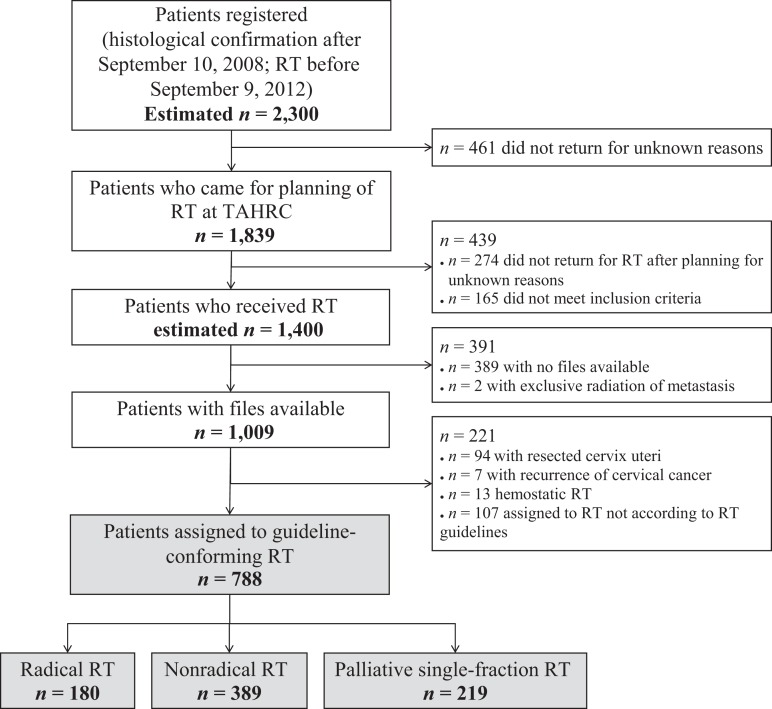
As displayed in Figure 2, an estimated 1,009 patients at TAHRC had treatment records available. Out of those, 788 were correctly assigned to RT according to local guidelines. Dose‐specific survival analysis was performed based on the assigned RT schedule. One hundred eighty patients received radical RT, 389 patients were assigned to nonradical RT, and 219 patients received palliative single fractions.
Figure 2.
CONSORT diagram for study at Tikur Anbessa Hospital Radiotherapy Center.
Abbreviations: RT, radiotherapy; TAHRC, Tikur Anbessa Hospital Radiotherapy Center.
Patient Characteristics
As shown in Table 1, most patients originated from rural Ethiopia (58%). The mean age was 49 years (22–91 years). Parity ranged from 0 to 17 children. One sexual partner in the patient's lifetime and early marriage at the age of 18 years or younger were reported in the majority of cases (52% and 84%, respectively). Contraception was used by 29% of patients. More than one tenth of the patients were known to be HIV positive. The high rate of 74% of patients with unknown HIV status is because within the first 3 years of data collection, screening was only done for patients with high risk history (e.g., HIV‐positive family members or other HIV‐defining diseases). Most patients presented with lightly restricted performance status (56%) and histologically confirmed squamous cell carcinoma (95%). FIGO stages IIIB–IVA at the time of RT were very common (71%).
Table 1. Basic demographic and disease‐specific data of all 788 patients.
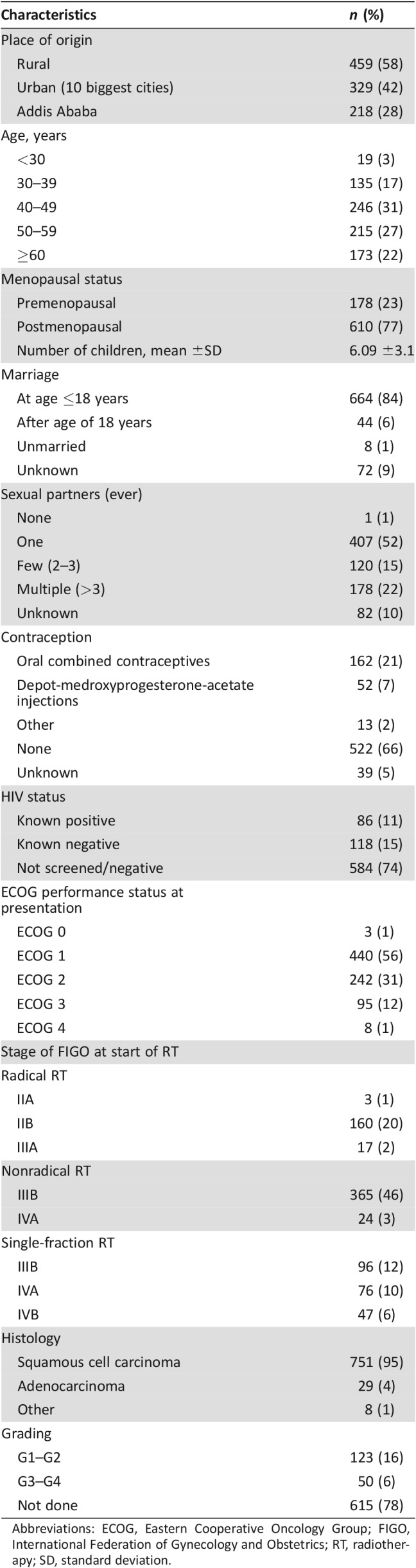
Abbreviations: ECOG, Eastern Cooperative Oncology Group; FIGO, International Federation of Gynecology and Obstetrics; RT, radiotherapy; SD, standard deviation.
Adherence and Supportive Treatment
Among patients assigned to radical RT, 49% received the recommended dose of 72 Gy or more. In case of nonradical RT, the rate of adherence was higher, and 83% received the recommended dose of 44 Gy. However, 10% received a higher dose of radiation than the recommended maximum of 50 Gy. Most palliative patients received two single fractions of 10 Gy each (53%).
For the whole cohort, reasons for discontinuation of an ongoing RT schedule were reported in 32 out of 159 cases of discontinuation. Half of these 32 patients discontinued because of radiation toxicities. Twenty‐two percent did not return for RT for financial or logistical reasons. One patient with FIGO stage IIB discontinued her second phase of radical RT because of breakdown of the Cobalt‐60 unit.
Only 15% of all patients received at least one cycle of chemotherapy (50–60 mg/m2 cisplatin and 500 mg/m2 5‐fluoruracil). Out of the 219 palliative patients, chemotherapy was administered in only 12 cases.
At the time of presentation, 90% of all patients suffered from abdominal pain, which was severe in 121 cases. However, morphine injections were given to only 14% of all patients, and tramadol was administered to 43% of all patients. Four percent of all patients received paracetamol only. Two hundred twenty‐one patients with abdominal pain did not receive any analgesic.
Toxicities
As shown in Table 2, radiation dermatitis and diarrhea occurred in 9% and 12%, respectively, of the 610 patients who returned for a first follow‐up within 3 months after RT ended. Half of the patients who suffered from vomiting previously received chemotherapy.
Table 2. Incidence of early and late adverse effects graded according to Common Terminology Criteria for Adverse Events [15].
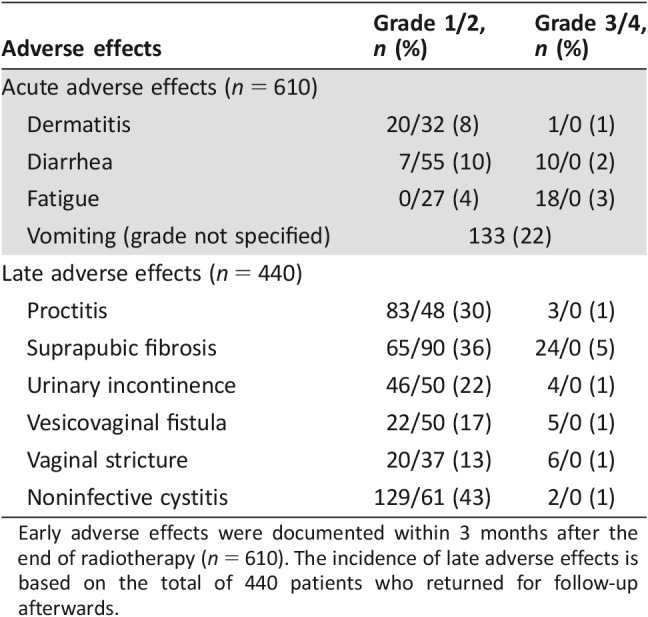
Early adverse effects were documented within 3 months after the end of radiotherapy (n = 610). The incidence of late adverse effects is based on the total of 440 patients who returned for follow‐up afterwards.
Late adverse effects were documented more often, partly because of additional information via phone calls. Among 440 patients with available follow‐up data, 31% suffered from radiation proctitis. Subcutaneous fibrosis of the suprapubic tissue was very common (41%), and vaginal strictures occurred in 14% of the cases. Twenty‐three percent of patients who returned for follow‐up after 3 months after RT ended were incontinent, mostly because of vesicovaginal fistula (18%). Almost half of all patients with late follow‐up information suffered from dysuria. Severe acute toxicities were observed in 5% of the 610 patients at the time of first follow‐up, and severe late toxicities were seen in 10% of the 440 cases with late follow‐up information. No life‐threatening events because of radiation were reported.
Survival According to Adherence
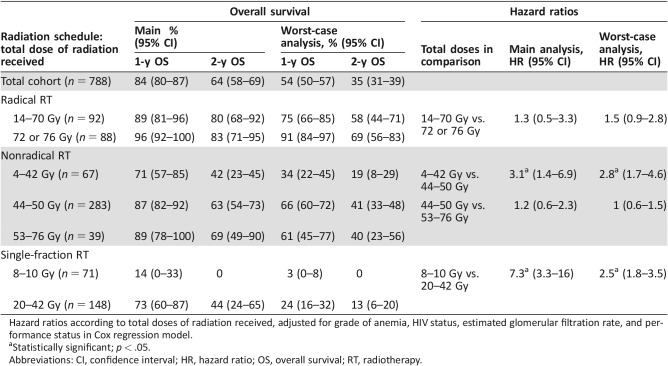
A total of 154 deaths were registered within the cohort of 788 patients. For the worst‐case scenario, the number increased to 448. As displayed in Table 3, the estimated overall survival after 1 and 2 years was 84% and 64%, respectively, and decreased to 54% and 35% in the assumption of worst‐case scenario. The median survival time of 33 months in the main analysis declined to 14 months in the worst‐case analysis.
Table 3. Overall survival of patients according to total doses of radiation received in main and worst‐case Kaplan‐Meier analyses.
Hazard ratios according to total doses of radiation received, adjusted for grade of anemia, HIV status, estimated glomerular filtration rate, and performance status in Cox regression model.
Statistically significant; p < .05.
Abbreviations: CI, confidence interval; HR, hazard ratio; OS, overall survival; RT, radiotherapy.
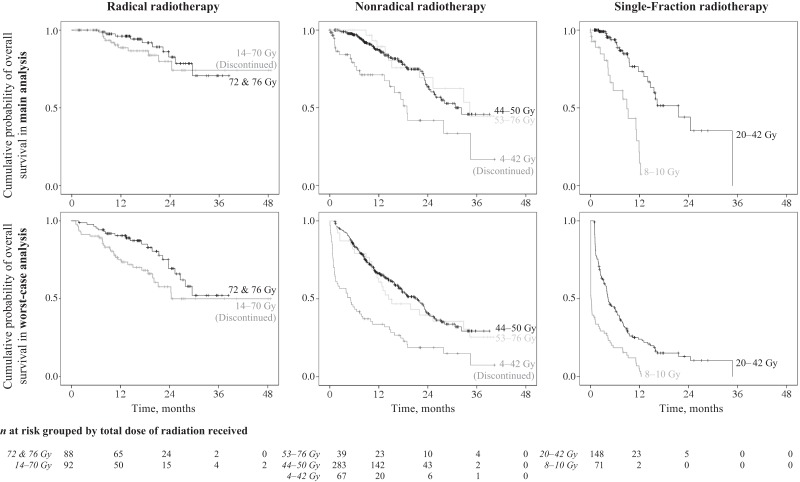
Regarding adherence, patients had lower survival probabilities in the case of discontinuation (see Fig. 3). The most favorable 1‐year overall survival was seen in patients who completed radical RT (96%). For those who discontinued, it decreased to 89% (HR, 1.3; 95% CI, 0.5–3.3, and HR, 1.5; 95% CI, 0.9–2.8) in the main and worst‐case analyses, respectively). Patients who received the recommended dose of 44–50 Gy for nonradical RT had 1‐ and 2‐year survival rates of 87% and 63%. In cases of discontinuation, survival dropped to 71% and 42%, respectively (HR, 3.1; 95%, CI 1.4–6.9; p < .01) However, for nonradical RT, survival did not differ significantly with a further increase of the total dose of radiation up to 76 Gy. For palliation, single fractions of 10 Gy each were administered. The estimated 1‐year overall survival for patients with one single fraction was the least favorable within the whole cohort (14% in the main and 3% in the worst‐case analysis). These patients were 7.3 times (95% CI, 3.3–16) more likely to die compared with patients with more single fractions.
Figure 3.
Overall survival of patients according to their radiotherapy schedules, grouped by total doses of radiation received, in the main and worst‐case Kaplan‐Meier analyses.
Among the other variables included in the Cox model, only the comparison of no anemia and grade 1 anemia for radical (HR, 0.3; 95% CI, 0.1–0.9) and nonradical RT (HR, 0.4; 95% CI, 0.2–0.7) and the comparison of grade 1 and grade 2 anemia for nonradical RT (HR, 0.5; 95% CI, 0.3–0.9) were significantly relevant for prognosis in the worst‐case analysis.
Discussion
This is the first study from sub‐Saharan Africa to report on the discontinuation of RT affecting outcome in a large cohort of patients with CC in Ethiopia. In the case of adherence to guideline‐conforming RT, patients at TAHRC had the best outcome.
Among patients assigned to radical and nonradical RT, 49% and 17%, respectively, discontinued their RT schedules and had lower chances of survival. Most known discontinuations were because of radiation toxicities. Hence, supportive therapies and brachytherapy are urgently needed. However, a larger number of patients discontinued for undocumented reasons, most likely financial and logistical obstacles [18], [19] or breakdown of the RT machine. Socioeconomic support for patients in need, reliable maintenance services with available spare parts, and more RT machines are necessary for better therapy adherence.
The advantage in survival of adherent patients might reflect a possible dose effect of RT. However, we assume that there was a certain selection bias toward the nonadherence group because of prognosis‐relevant conditions that may have led to discontinuation of RT, which were not consistently reported (e.g., tumor progression, socioeconomic background of the patients). Unexpectedly, patients treated with nonradical RT seemingly did not benefit from a further dose increase above the recommended threshold. A possible explanation is that higher doses were administered in case of more aggressive and thus radiotherapy‐resistant tumors.
Mostly because of methodological shortcomings, studies on a radiation dose‐response relation for patients with CC have had controversial outcomes [20]. However, in the case of RT for prostate cancer, there is clear proof for better survival when a certain dose threshold (78–80 Gy) is reached [21]. Correspondingly, Beskow et al. demonstrated better survival along with dose increase for patients with CC [22].
We found very few studies reporting on the survival of patients with CC after sole EBRT because the standard of care is a combination of EBRT and ICBT for FIGO stages IB2 or IIB–IVA [23]. Several authors describe a RT pattern with Cobalt‐60 similar to the radical RT schedule at TAHRC; however, this was not restricted to FIGO stages IIA–IIIA [24], [25], [26], [27]. A prognostic benefit for patients with FIGO stage IIIB by increasing the total dose up to 85 Gy has been demonstrated [20], [28]. A large Brazilian study on RT for patients with CC at FIGO stage IIIB (n = 202) compared different doses of radiation delivered by a LINAC without IBRT. The authors reported a 5‐year survival of almost 30% after RT with a total dose of 60 or 70 Gy, compared with significantly lower survival rates for patients who received EBRT with lower doses [29]. However, access to RT at TAHRC is limited. Hypofractionation, as in the nonradical schedule for patients with FIGO stages IIIB and IVA, comes with the advantage of a lower number of treatment days (a maximum of 14 fractions compared with 36 fractions for radical RT) and allows a larger number of patients to be treated.
Regarding the adverse effects that occurred, no grade 4 toxicity was seen, and grade 3 toxicities occurred in 5% of patients within 3 months after RT and in 10% afterward. Compared with the respective highest rates reported by other studies, late adverse effects at TAHRC were seen more frequently and acute adverse effects less frequently.
Other studies have reported lower rates for any‐grade radiation proctitis (17% [26]), urinary incontinence (20% [30]), vesicovaginal fistulae (13% [30]), noninfective cystitis (20% [26]), and suprapubic fibrosis (24% [31]) than the respective 31%, 23%, 18%, 44%, and 41% from our study sample. Vaginal strictures appear to be associated with higher age and treatment with ICBT [31], [32]. As ICBT was not available at TAHRC, a lower rate of 14% was found, compared with the 34% reported by Saibishkumar et al. [31].
Regarding acute adverse effects, the highest rate for radiation dermatitis found in literature was 17% [32], which is twofold the rate of dermatitis occurring at TAHRC. For radiation diarrhea, rates up to 65% have been reported [27], whereas we found a low rate of 12%, most probably because of underdiagnosing at TAHRC and the less frequent administration of chemotherapy.
Measured by international guidelines [4], [23], RT for CC at TAHRC was suboptimal in four respects in addition to the described negative effect of nonadherence on outcome: (a) no ICBT was used, (b) no LINAC was available for EBRT, (c) advanced stages of CC received lower doses than internationally recommended because of the lack of capacities, and (d) the generally recommended addition of chemotherapy to radiation was only realized in 15% of all patients.
The relevance of ICBT for primary endpoints such as survival and toxicity has been well established by numerous studies. A large cohort study by Han et al. included 7,359 patients with CC and reported significantly higher survival rates for patients who received ICBT compared with those who did not (58% vs. 46%, respectively) [33]. Similarly, ICBT has been applied successfully in low‐resource settings [32], [34], [35], [36]. At the time of publishing, brachytherapy services have finally been installed and used daily at TAHRC.
Concerning the lack of LINACs at TAHRC, their role as the optimal and most targeted RT modality for deep‐seated tumors such as CC is generally accepted. The advantageous role of LINACs is marked by higher percent depth dose and dose rate, lower skin dose, sharper beam, and therefore fewer radiation‐associated adverse effects. However, we should question the feasibility of LINACs in a setting without reliable electricity and with a small number of well‐trained staff members.
At TAHRC, most patients included in the present study were FIGO stage IIIB, which has been shown to respond well to high‐dosage EBRT [20], [28]. However, these patients received a low‐dose nonradical RT, which allowed more patients to be treated by saving radiation capacities. To that end, the limited availability of RT, regardless of its modality, is the main obstacle for adequate RT in Ethiopia and needs to be addressed. In Africa, Ethiopia has the second largest gap, after Nigeria, between the availability of and demand for RT machines. Judging from the WHO recommendations, there are 73 RT units missing in Ethiopia [10]. This serious quantitative deficit should be addressed first, before insularly enhancing RT quality with complex techniques.
Regarding chemoradiation, most patients at TAHRC did not receive the recommended concurrent or palliative chemotherapy along with RT. The facts that a larger proportion of patients with cancer in sub‐Saharan Africa might not be fit for platin‐based chemotherapy [37] and that adverse effects can be difficult to control do not sufficiently explain the lack of chemotherapy in 85% of all patients observed. Our findings point toward mainly financial obstacles that interfere with clinical indications. Hence, we emphasize the importance of available and affordable chemotherapy for all patients with CC. For palliation, chemotherapy including cisplatin, paclitaxel and/or bevacizumab is the standard of care according to international guidelines [4], [23]. At TAHRC, in a setting of limited availability and finances, only 6% of palliative patients received additional chemotherapy. The more affordable monthly palliative single‐fraction RT has proved effective since the late 1970s in cessation of bleeding and pain, although serious complications have occurred [38].
Our results are limited by the retrospective nature of the study. First, we were unable to ascertain whether patients discontinued RT because of substandard care, and correspondingly survival tragically decreased, or whether patients discontinued RT because of fatal progression of the disease, and survival inevitably decreased. A prospective interventional study to compare adherence with discontinuation (substandard care) is ethically not feasible. Hence, this kind of retrospective data will be the only source of information for observing the effects of substandard care. Second, AIDS not only leads to significantly lower survival rates in patients with CC [39] but also has negative effects on therapy adherence [40]. As only 26% of patients included were screened for HIV, we were not able to fully control for HIV status in the multivariate analysis. Third, adverse effects, as a possible reason for discontinuation, were not documented in a prospective, standardized way. We expect that the actual number of adverse effects was higher than documented. Physicians did not precisely inquire about and document side effects because therapeutic measures were unavailable. Fourth, the low median follow‐up time of 9.6 months, because of the 81% of patients who were lost to follow‐up, limited our results. Most patients came from rural areas, where only 12% have access to electricity [41]. Hence, even if patients were alive, contact via telephone was rarely possible. The time and financial resources of this study did not allow us to contact each patient personally.
However, this study is the first large cohort study in the African continent to report in detail on RT schedules, adherence, survival, and adverse effects. Findings on the outcome of EBRT alone for CC without ICBT are of high interest for the still‐numerous countries lacking ICBT.
Conclusion
In this study, data from 788 patients with cervical cancer treated according to the Ethiopian RT guidelines of 2008–2012 were retrospectively analyzed. Nonradical RT for late stages with total doses of 44–50 Gy was administered in most cases. We noted higher rates of late toxicities than comparable studies reported. Chemotherapy was administered to only 15% of all patients. Discontinuation of RT was associated with lower chances of survival. To enhance therapy adherence, socioeconomic support for patients in need, reliable maintenance services, and supportive therapies are necessary. Chemotherapy and analgesics need to be available and affordable. Besides guideline‐conforming treatment, mere access to RT is extremely limited. To assure standard radical RT for all FIGO stages up to IVA, more RT facilities are needed to provide more radiation time. Additionally, ICBT must be implemented to reduce the adverse effects associated with Cobalt‐60 alone.
Acknowledgments
The authors are grateful for the dedicated work of the entire staff at the oncology ward at Tikur Anbessa Hospital. The authors would especially like to thank their study nurse, Tinsae Gelatae, for conducting follow‐up phone calls in Amharic and Oromo; their colleague Timotewos Genebo for logistical support far beyond his responsibilities, and Mrs. Mulu and Mr. Neme for searching and retrieving the archived patient files. This study was supported by the Federal Ministry of Research and Education of Germany, grant 01DG12006.
Footnotes
For Further Reading: Kathleen R. Ragan, Natasha Buchanan Lunsford, Judith Lee Smith et al. Perspectives of Screning‐Eligible Women and Male Partners on Benefits of and Barriers to Treatment for Precancerous Lesions and Cervical Cancer in Kenya. The Oncologist 2018;23:35‐43.
Implications for Practice: This article provides important insight into female and male partner perspectives regarding benefits, facilitators, and barriers to treatment for precancerous lesions and cervical cancer. These novel research findings can inform the development of targeted community health interventions, educational messages, and resources and aid stakeholders in strengthening strategic plans regarding treatment coverage and cervical cancer prevention. Because several treatment barriers identified in this study are similar to barriers associated with cervical cancer screening in low‐ and midle‐resourced countries, effective messaging interventions could address barriers to receipt of both screening and treatment.
Author Contributions
Conception/design: Ulrike Moelle, Adamu Addissie, Christoph Thomssen, Dirk Vordermark; Eva J. Kantelhardt
Provision of study material or patients: Assefa Mathewos, Abreha Aynalem, Tigeneh Wondemagegnehu, Bekuretsion Yonas
Collection and/or assembly of data: Ulrike Moelle, Mathias Begoihn
Data analysis and interpretation: Ulrike Moelle, Assefa Mathewos, Susanne Unverzagt, Ahmedin Jemal, Eva J. Kantelhardt
Manuscript writing: Ulrike Moelle, Ahmedin Jemal, Eva J. Kantelhardt
Final approval of manuscript: Ulrike Moelle, Assefa Mathewos, Abreha Aynalem, Tigeneh Wondemagegnehu, Bekuretsion Yonas, Adamu Addissie, Susanne Unverzagt, Ahmedin Jemal, Christoph Thomssen, Dirk Vordermark; Eva J. Kantelhardt
Disclosures
The authors indicated no financial relationships.
References
- 1. Jemal A, Bray F, Forman D et al. Cancer burden in Africa and opportunities for prevention. Cancer 2012;118:4372–4384. [DOI] [PubMed] [Google Scholar]
- 2. Torre LA, Siegel RL, Ward EM et al. Global cancer incidence and mortality rates and trends–An update. Cancer Epidemiol Biomarkers Prev 2016;25;16–27. [DOI] [PubMed] [Google Scholar]
- 3. Arbyn M, Castellsagué X, de Sanjosé S et al. Worldwide burden of cervical cancer in 2008. Ann Oncol 2011;22:2675–2686. [DOI] [PubMed] [Google Scholar]
- 4. Oaknin A, Rubio MJ, Redondo A et al. SEOM guidelines for cervical cancer. Clin Transl Oncol 2015;17:1036–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chokunonga E, Ramanakumar AV, Nyakabau AM et al. Survival of cervix cancer patients in Harare, Zimbabwe, 1995–1997. Int J Cancer 2004;109:274–277. [DOI] [PubMed] [Google Scholar]
- 6. Sankaranarayanan R, Ferlay J. Worldwide burden of gynaecological cancer: The size of the problem. Best Pract Res Clin Obstet Gynaecol 2006;20:207–225. [DOI] [PubMed] [Google Scholar]
- 7. Zubizarreta EH, Fidarova E, Healy B et al. Need for radiotherapy in low and middle income countries – The silent crisis continues. Clin Oncol (R Coll Radiol) 2015;27:107–114. [DOI] [PubMed] [Google Scholar]
- 8. Portelance L, Chao KC, Grigsby PW et al. Intensity‐modulated radiation therapy (IMRT) reduces small bowel, rectum, and bladder doses in patients with cervical cancer receiving pelvic and para‐aortic irradiation. Int J Radiat Oncol Biol Phys 2001;51:261–266. [DOI] [PubMed] [Google Scholar]
- 9. van Dyk J, Battista JJ. Cobalt‐60: An old modality, a renewed challenge. Curr Oncol 1996;3:8–17. [Google Scholar]
- 10. Abdel‐Wahab M, Bourque JM, Pynda Y et al. Status of radiotherapy resources in Africa: An International Atomic Energy Agency analysis. Lancet Oncol 2013;14:e168–e175. [DOI] [PubMed] [Google Scholar]
- 11.Population projection values of 2015 at zonal and wereda levels by urban and rural residence and by sex. In: Population Projection of Ethiopia for All Regions at Wereda Level from 2014–2017. Addis Ababa, Ethiopia: Central Statistical Agency, Federal Democratic Republic of Ethiopia; August 2013:60.
- 12.Ethiopia (2012): Estimated incidence and prevalence, adult population: Female. In: Ferlay J, Soerjomataram I, Ervik M et al. GLOBOCAN 2012 version 1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer; 2013. Available at http://globocan.iarc.fr. Accessed on March 2, 2016.
- 13. Kantelhardt EJ, Moelle U, Begoihn M et al. Cervical cancer in Ethiopia: Survival of 1,059 patients who received oncologic therapy. The Oncologist 2014;19:727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sharma V, Gaye PM, Wahab SA et al. Patterns of practice of palliative radiotherapy in Africa, Part 1: Bone and brain metastases. Int J Radiat Oncol Biol Phys 2008;70:1195–1201. [DOI] [PubMed] [Google Scholar]
- 15.National Cancer Institute . Common Terminology Criteria for Adverse Events. Version 4.0. Rockville, MD: National Cancer Institute, National Institutes of Health, U.S. Department of Health and Human Services; May 29, 2009:3–194.
- 16. Pecorelli S, Zigliani L, Odicino F. Revised FIGO staging for carcinoma of the cervix. Int J Gynecol Obstet 2009;105:107–108. [DOI] [PubMed] [Google Scholar]
- 17. Cox DR, Oakes D. Analysis of Survival Data. Boca Raton, LA: Chapman & Hall/CRC Press; 1984. [Google Scholar]
- 18. Hailu A, Mariam DH. Patient side cost and its predictors for cervical cancer in Ethiopia: A cross sectional hospital based study. BMC Cancer 2013;13:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Akinlade BI, Folasire AM, Elumelu‐Kupoluyi TN et al. Radiation therapy interruption in a poor resource setting: Causes and management. Afr J Med Med Sci 2014;43:333–337. [PubMed] [Google Scholar]
- 20. Petereit DG, Pearcey R. Literature analysis of high dose rate brachytherapy fractionation schedules in the treatment of cervical cancer: Is there an optimal fractionation schedule? Int J Radiat Oncol Biol Phys 1999;43:359–366. [DOI] [PubMed] [Google Scholar]
- 21. Eade TN, Hanlon AL, Horwitz EM et al. What dose of external‐beam radiation is high enough for prostate cancer? Int J Radiat Oncol Biol Phys 2007;68:682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beskow C, Agren‐Cronqvist AK, Lewensohn R et al. Biological effective dose evaluation and assessment of rectal and bladder complications for cervical cancer treated with radiotherapy and surgery. J Contemp Brachytherapy 2012;4:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Colombo N, Carinelli S, Colombo A et al. Cervical cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2012;23(suppl 7):vii27–vii32. [DOI] [PubMed] [Google Scholar]
- 24. Logsdon MD, Eifel PJ. FIGO IIIB squamous cell carcinoma of the cervix: An analysis of prognostic factors emphasizing the balance between external beam and intracavitary radiation therapy. Int J Radiat Oncol Biol Phys 1999;43:763–775. [DOI] [PubMed] [Google Scholar]
- 25. Akine Y, Hashida I, Kajiura Y et al. Carcinoma of the uterine cervix treated with external irradiation alone. Int J Radiat Oncol Biol Phys 1986;12:1611–1616. [DOI] [PubMed] [Google Scholar]
- 26. Lei ZZ, He FZ. External cobalt 60 irradiation alone for stage IIB carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys 1989;16:339–341. [PubMed] [Google Scholar]
- 27. Koeck GP, Jacobson LE, Hillsinger WR. Results of cobalt 60 rotation therapy in carcinoma of the cervix. Am J Roentgenol Radium Ther Nucl Med 1966;96:81–91. [DOI] [PubMed] [Google Scholar]
- 28. Lanciano RM, Won M, Coia LR et al. Pretreatment and treatment factors associated with improved outcome in squamous cell carcinoma of the uterine cervix: A final report of the 1973 and 1978 Patterns of Care Studies. Int J Radiat Oncol Biol Phys 1991;20:667–676. [DOI] [PubMed] [Google Scholar]
- 29. Ferreira PR, Braga‐Filho A, Barletta A et al. Radiation therapy alone in stage III‐B cancer of the uterine cervix–A 17‐year experience in southern Brazil. Int J Radiat Oncol Biol Phys 1999;45:441–446. [DOI] [PubMed] [Google Scholar]
- 30. Elghamrawi KA, Haggag MH, Habib EE. Treatment complications among long‐term survivors of cervical cancer: Treated by surgery or radiotherapy. Oncol Rev 2011;5:261–266. [Google Scholar]
- 31. Saibishkumar EP, Patel FD, Sharma SC. Evaluation of late toxicities of patients with carcinoma of the cervix treated with radical radiotherapy: An audit from India. Clin Oncol (R Coll Radiol) 2006;18:30–37. [DOI] [PubMed] [Google Scholar]
- 32. Arulponni TR, Janaki MG, Nirmala S et al. Carcinoma cervix treated with radiotherapy ‐ Our experience with emphasis on our concerns. J Obstet Gynaecol India 2010;60:61–65. [Google Scholar]
- 33. Han K, Milosevic M, Fyles A et al. Trends in the utilization of brachytherapy in cervical cancer in the Unitd States. Int J Radiat Oncol Biol Phys 2013;87:111–119. [DOI] [PubMed] [Google Scholar]
- 34. Wabinga H, Ramanakumar AV, Banura C et al. Survival of cervix cancer patients in Kampala, Uganda: 1995–1997. Br J Cancer 2003;89:65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ferrigno R, Campos de Oliveira Faria SL, Weltman E et al. Radiotherapy alone in the treatment of uterine cervix cancer with telecobalt and low‐dose‐rate brachytherapy: Retrospective analysis of results and variables. Int J Radiat Oncol Biol Phys 2003;55:695–706. [DOI] [PubMed] [Google Scholar]
- 36. Levin CV, El Gueddari B, Meghzifene A. Radiation therapy in Africa: Distribution and equipment. Radiother Oncol 1999;52:79–83. [DOI] [PubMed] [Google Scholar]
- 37. McArdle O, Kigula‐Mugambe JB. Contraindications to cisplatin based chemotherapy in the treatment of cervical cancer in sub‐Saharan Africa. Radiother Oncol 2007;83:94–96. [DOI] [PubMed] [Google Scholar]
- 38. Smith SC, Koh WJ. Palliative radiation therapy for gynaecological malignancies. Best Pract Res Clin Obstet Gynaecol 2001;15:265–278. [DOI] [PubMed] [Google Scholar]
- 39. Maiman M, Fruchter RG, Guy L et al. Human immunodeficiency virus infection and invasive cervical carcinoma. Cancer 1993;71:402–406. [DOI] [PubMed] [Google Scholar]
- 40. Ngugi P. Response and Adherence of HIV Positive Women to Cervical Cancer Treatment [master's thesis]. Port Elizabeth, South Africa: Nelson Mandela Metropolitan University; 2011.
- 41.Access to electricity (% of population): Ethiopia, 1991–2014. Sustainable Energy for All (SE4ALL) database from the SE4ALL Global Tracking Framework led jointly by the World Bank, International Energy Agency, and the Energy Sector Management Assistance Program. In: World Bank Open Data. Washington, D.C.: World Bank. Available at https://data.worldbank.org/indicator/EG.ELC.ACCS.ZS?locations=ET. Accessed November 19, 2017.