Patients who develop non‐small cell lung cancer at a young age have unknown risk factors and are poorly studied. In this study, broad, hybrid capture‐based next‐generation sequencing assays were used to identify targeted genetic alterations in young patients with lung adenocarcinoma, which could provide comprehensive knowledge of the genomics of this population.
Keywords: Lung adenocarcinoma, Young age, Next‐generation sequencing, Genetic profile
Abstract
Background.
Occurrence at a younger age has been demonstrated to be associated with a distinct biology in non‐small cell lung cancer. However, genomics and clinical characteristics among younger patients with lung adenocarcinoma remain to be determined. Here we studied the potentially targetable genetic alterations by next‐generation sequencing (NGS) assay in young Chinese patients with lung adenocarcinoma.
Materials and Methods.
Seventy‐one surgically resected lung adenocarcinoma tissue samples from patients aged less than 45 years were collected with informed consent from all patients. Targeted NGS assays were used to identify actionable genetic alterations in the cancer tissues. Additionally, the genomic and clinicopathologic characteristics of 106 patients with lung adenocarcinoma who received NGS testing over the same period were analyzed retrospectively.
Results.
The frequencies of targetable genetic alterations in 177 patients with lung adenocarcinoma were analyzed by defined age categories, which unveiled a distinctive molecular profile in the younger group, aged less than 45 years. Notably, higher frequency of ALK and HER2 genetic alterations were associated with young age. However, a reverse trend was observed for KRAS, STK11 and EGFR exon 20 mutations, which were more frequently identified in the older group, aged more than 46 years. Furthermore, concurrent EGFR/TP53 mutations were much more prevalent in the younger patients (81.6% vs. 46.8%), which might have a poor response to treatment with epidermal growth factor receptor tyrosine kinase inhibitor.
Conclusion.
In this study, NGS assay revealed a distinctive genetic profile in younger patients with adenocarcinoma. High frequency of concurrent EGFR/TP53 mutations was found in the younger patients, which especially warranted personalized treatment in this population.
Implications for Practice.
Further investigation is needed to understand the genomics and clinical characteristics of young patients with lung adenocarcinoma. In the present study, hybrid capture‐based next‐generation sequencing assays were used to identify targeted genetic alterations in young lung adenocarcinoma patients. Young patients with lung adenocarcinoma, aged less than 45 years, harbored a higher frequency of ALK and HER2 genetic alterations compared with patients aged more than 46 years. Dramatically, concurrent EGFR/TP53 mutations were much more prevalent in younger patients, which had a poor response to treatment with epidermal growth factor receptor kinase inhibitor. These results reveal a distinctive genetic profile in younger patients with adenocarcinoma, which might improve the treatment of this subpopulation.
摘要
背景。非小细胞肺癌发生在年轻的人群已经被证实与独特生物学相关。然而,年纪较轻的肺腺癌患者的基因组和临床特征尚未确定。在本次研究中,我们通过对中国年轻肺腺癌患者实施下一代测序(NGS)试验分析,来探索可能的靶向基因改变。
材料和方法。在所有患者均签署知情同意书的前提下,从年龄小于45岁的患者中共采集了71份手术切除的肺腺癌组织样本。用靶向NGS检测确定癌组织中可能有用的基因改变。此外,还回顾性地分析了同一时期接受NGS检测的106例肺腺癌患者的基因组和临床病理特征。
结果。按规定的年龄组别分析了177例肺腺癌患者的靶向基因改变频率,揭示了45岁以下年轻患者的独特分子谱。值得注意的是,较高的ALK和HER2基因变化发生频率与年纪较轻有关。然而,KRAS、STK11和EGFR外显子20突变的趋势则相反,这些突变在46岁以上的年纪较大人群中更为常见。而且,年纪较轻患者中并发EGFR/TP53突变的发生率要高得多(81.6% vs. 46.8%),这可能对表皮生长因子受体酪氨酸激酶抑制剂治疗的反应较差。
结论。在本次研究中,NGS试验揭示了年纪较轻腺癌患者的独特基因谱。并发EGFR/TP53突变在年轻患者中的发生率较高,这说明此类人群尤其需要个性化的治疗方案。
实践意义: 未来需要展开深入研究,以理解年轻肺腺癌患者的基因组和临床特征。在本次研究中,使用基于杂交捕获的下一代测序试验确定年轻肺腺癌患者的靶向基因改变。45岁以下的年轻肺腺癌患者的ALK和HER2基因改变发生频率高于46岁以上患者。而且,并发EGFR/TP53突变在年轻患者中的发生率明显更高。此类患者对表皮生长因子受体酪氨酸激酶抑制剂治疗的反应较差。这些结果揭示了年纪较轻腺癌患者的独特基因谱,这也许能改善对此类亚组人群的治疗。
Introduction
Non‐small cell lung cancer (NSCLC) is the leading cause of cancer mortality worldwide [1]. Prior studies have clearly demonstrated that occurrence at a younger age is associated with a distinct biology that guides treatment in a number of cancers [2], [3]. NSCLC is considered to be a disease of the elderly, and young adult patients represent only a small proportion of patients [4], [5]. Notably, NSCLC among young adults has been found to have an increasing incidence rate [6].
Patients who developed NSCLC at a young age have unknown risk factors and are poorly studied [7], [8]. Controversy persists regarding the relative prognosis of young patients compared with older patients with NSCLC. Study from the National Cancer Data Base showed that overall survival in younger patients with NSCLC is better than in older patients, with greater benefit seen in only earlier stages [5]. A retrospective analysis found that the prognosis of younger patients with NSCLC was poor, especially when the patients were not screened for driver mutations and the platinum‐based doublet was the most frequently used treatment scheme [4]. Consequently, the molecular signature and clinical behavior of NSCLC in young patients must be defined, and a unique treatment approach can then be developed to improve the outcome of this population.
Few reports have focused on the incidence of targeted genetic alterations in younger patients with NSCLC, including lung adenocarcinoma, yet it now draws increasing research. Recent data have suggested that younger patients with lung adenocarcinoma had distinctive molecular features and harbored a high prevalence of targeted genetic alterations such as ALK rearrangement [9], [10], [11], [12], [13]. However, no difference of oncogenic mutations was found between Chinese younger and older patients with lung adenocarcinoma in a prior small study [14]. In terms of the prevalence of EGFR mutations in young patients with lung adenocarcinoma, the findings of several recent studies were controversial [9], [10], [13]. Consequently, multiple factors, including definition of young age, race, the relative rarity of young patients with lung adenocarcinoma, and the different genetic testing assays used, complicate and challenge this study.
Despite the larger number of potentially targeted genetic alterations identified in lung adenocarcinoma, only a few driven genes were studied in the aforementioned research in young patients via low throughput assays. In this study, broad, hybrid capture‐based next generation sequencing (NGS) assays were used to identify targeted genetic alterations in young patients with lung adenocarcinoma, which could provide comprehensive knowledge of the genomics of this population.
Material and Methods
Patients and Samples
The diagnosis of adenocarcinoma was confirmed by hematoxylin and eosin staining. All formalin‐fixed, paraffin‐embedded tissue samples were reviewed by qualified pathologists to confirm the diagnosis of adenocarcinoma and ensure >30% tumor content. Staging of patients was assessed according to the 8th edition of the tumor, node and metastasis classification for lung cancer [15]. This study was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University. Signed informed consent was obtained from all patients included in the study. All the experiments were carried out in accordance with the guidelines of the Ethics Committee of the Affiliated Hospital of Qingdao University and the National Health and Family Planning Commission of the People's Republic of China.
NGS‐Based Assay
In this study, tissue samples from 177 patients were analyzed by NGS assay using three versions of capture‐based targeted sequencing panel. The details of this NGS assay may be found in the supplemental online Material and Methods, and gene lists of three versions of capture‐based targeted sequencing panel are shown in supplemental online Table 1. Targeted genetic alterations were found in 28 genes in all 177 patients. However, only 16 of the 28 genes covered by all the three versions of NGS assay were selected for further analysis (supplemental online Fig. 1). This analysis focused on targetable genetic alterations annotated by categories of evidence Level 1–3 and Level R1 in OncoKB (Memorial Sloan Kettering Cancer Center, New York, NY, http://oncokb.org/).
Statistical Analysis
The experimental data are presented as mean ± standard error. Pearson's chi‐squared test, Fisher's exact test and Student's t test were used to analyze the gene mutation status and patient characteristics between the two groups. For all the tests, p values were two tailed. Statistical significance was defined as p < .05.
Results
Design of the Study and Patient Characteristics
Between January 2013 and June 2016, we consecutively screened 2,535 patients who had surgically resected lung cancer diagnosed as adenocarcinoma. Among these patients, 106 were aged less than 45 years and accounted for 3.9% of the total cases. Tissue samples were collected from 72 patients and analyzed by NGS assay. Additionally, 105 consecutive adenocarcinoma cases that had received NGS assay during the above period were retrospectively collected. The design of the study may be found in supplemental online Figure 1.
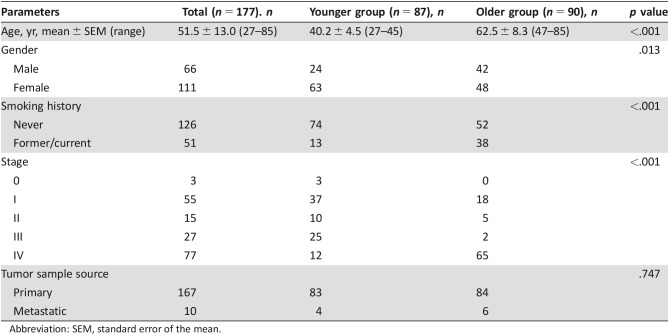
The clinical characteristics of all 177 patients with lung adenocarcinoma with NGS assay are shown in Table 1. The average age of all patients was 51.5 years (range, 27–85 years); 62.7% (111/177) patients were women, and 71.2% (126/177) were never smokers. There were 1.7% (3/177) patients with stage 0 disease, 31.1% (55/177) with stage I disease, 8.5% (15/177) with stage II disease, 15.3% (27/177) with stage III disease, and 43.5% (77/177) with stage IV disease. One hundred sixty‐seven primary tumor tissue samples and 10 metastasis biopsies were collected for NGS analysis.
Table 1. Clinical characteristics of 177 patients with lung adenocarcinoma with next‐generation sequencing assay.
Abbreviation: SEM, standard error of the mean.
Association Between Targeted Genetic Profile and Age
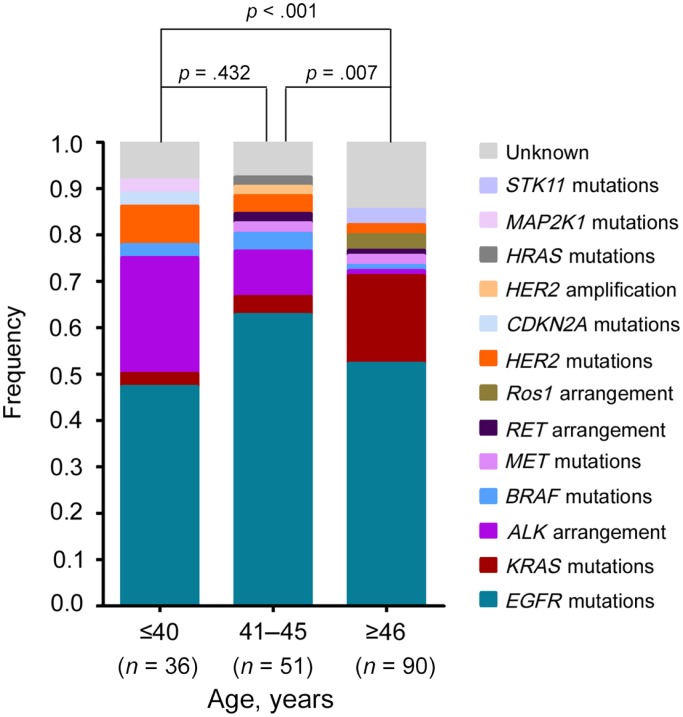
In prior studies, age cutoff points of 40 [10–12, 14, 16], 45 [17], 46 [5], and 50 [13, 18] years have all been identified as young age at NSCLC diagnosis. In this study, the frequency of targeted genetic alterations across three age groupings was compared to determine a reasonable age cutoff point. As shown in Figure 1, the difference of targeted genetic alterations between the first two groups was statistically insignificant (p = .566). However, the first two groups were statistically different from the third group (aged more than 46 years). Specifically, patients aged less than 40 years and patients between 41 to 45 years of age harbored a much higher frequency of ALK arrangement (25.0% vs 9.8%, p = .077) than patients aged more than 46 years (1.1%). We therefore identified an age at diagnosis of 45 years as a cutoff point to differentiate the younger and the older patients.
Figure 1.
The genetic profiles in different age groups of patients with lung adenocarcinoma. One hundred seventy‐seven patients with lung adenocarcinoma patients were enrolled, and tissue samples were analyzed by NGS assays. The frequency of targeted genetic alterations across three age groupings was compared to determine an age cutoff point that could differentiate young patients with a distinctive molecular profile. Statistical significance was defined as p < .05.
Prevalence of Targeted Genetic Alterations in Younger Patients with Lung Adenocarcinoma
Of the 177 lung adenocarcinoma patients, 87 less than 45 years of age were assigned to the younger group, and 90 more than 46 years of age were enrolled in the older group. There was significant difference in the distribution of age, gender, smoking history, and stage between the younger and older age groups (Table 1). More women, fewer smokers, and more early adenocarcinoma were found in the younger group. The difference of tumor sample source between the two groups was not significant.
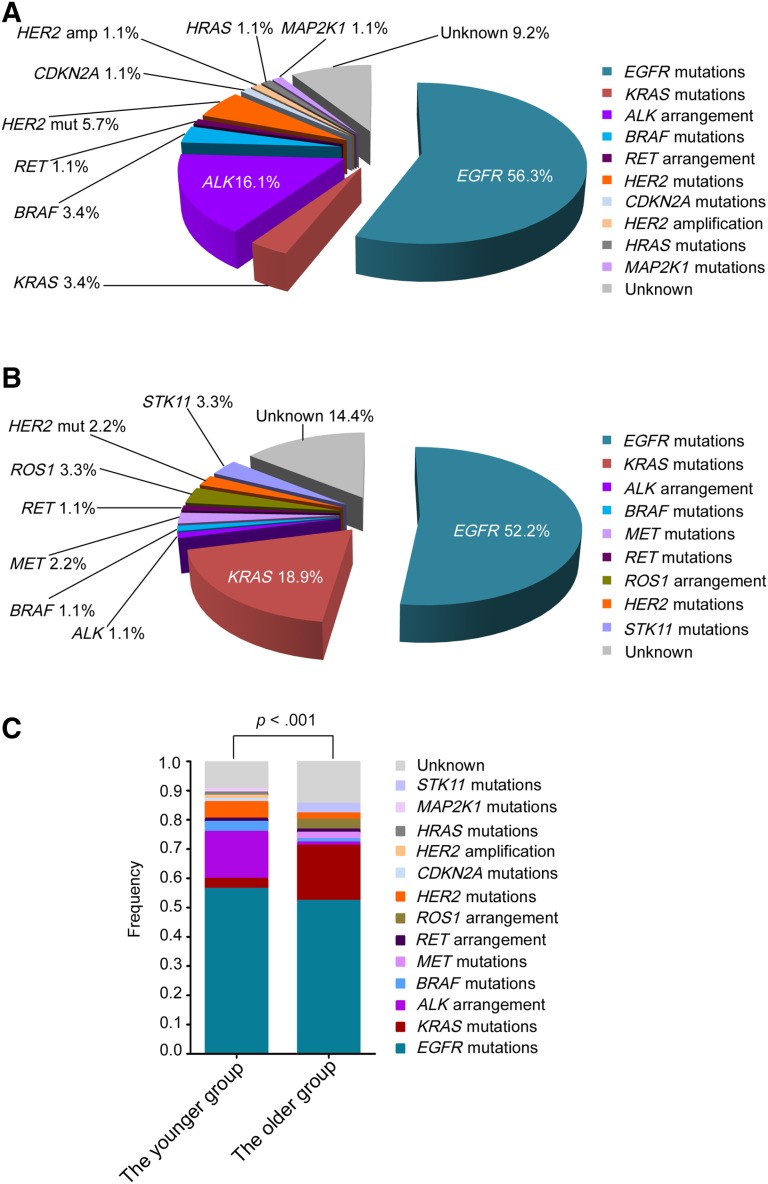
Potentially targetable genetic alterations were detected in 90.8% of younger patients. The specific alterations that were observed in the most common actionable targets included the following: EGFR (n = 49, 56.3%), KRAS (n = 3, 3.4%), ALK (n = 14, 16.1%), BRAF (n = 3, 3.4%), RET (n = 1, 1.1%), HER2 mutations (n = 5, 5.7%), CDKN2A (n = 1, 1.1%), HER2 amplification (n = 1, 1.1%), HRAS (n = 1, 1.1%), and MAP2K1 (n = 1, 1.1%; Fig. 2A). Additionally, potentially targetable genetic alterations were identified in 85.6% of older patients and included the following: EGFR (n = 47, 52.2%), KRAS (n = 17, 18.9%), ALK (n = 1, 1.1%), BRAF (n = 1, 1.1%), MET (n = 2, 2.2%), RET (n = 1, 1.1%), ROS1 (n = 3, 3.3%), HER2 mutations (n = 2, 2.2%), and STK11 (n = 3, 3.3%; Fig. 2B). A significant difference was seen between the younger and the older groups in targeted genetic profile identified by NGS (p < .001; Fig. 2C). Most importantly, 80.5% (70/87) of younger patients harbored genetic alterations mapping to therapies approved by the National Comprehensive Cancer Network (NCCN) Guidelines (version 1.2018 updates, Non‐Small Cell Lung Cancer) approved therapies, compared with 54.4% (49/90) of older patients.
Figure 2.
The targeted genetic profiles of younger and older patients with lung adenocarcinoma. (A): The targeted genetic profile of the younger patients, aged less than 45 years. (B): The targeted genetic profile of the older patients, aged more than 45 years. (C): The comparative analysis of the frequency of targeted genetic alterations between the younger and the older group. Statistical significance was defined as p < .05.
Abbreviations: amp, amplification; mut, mutation.
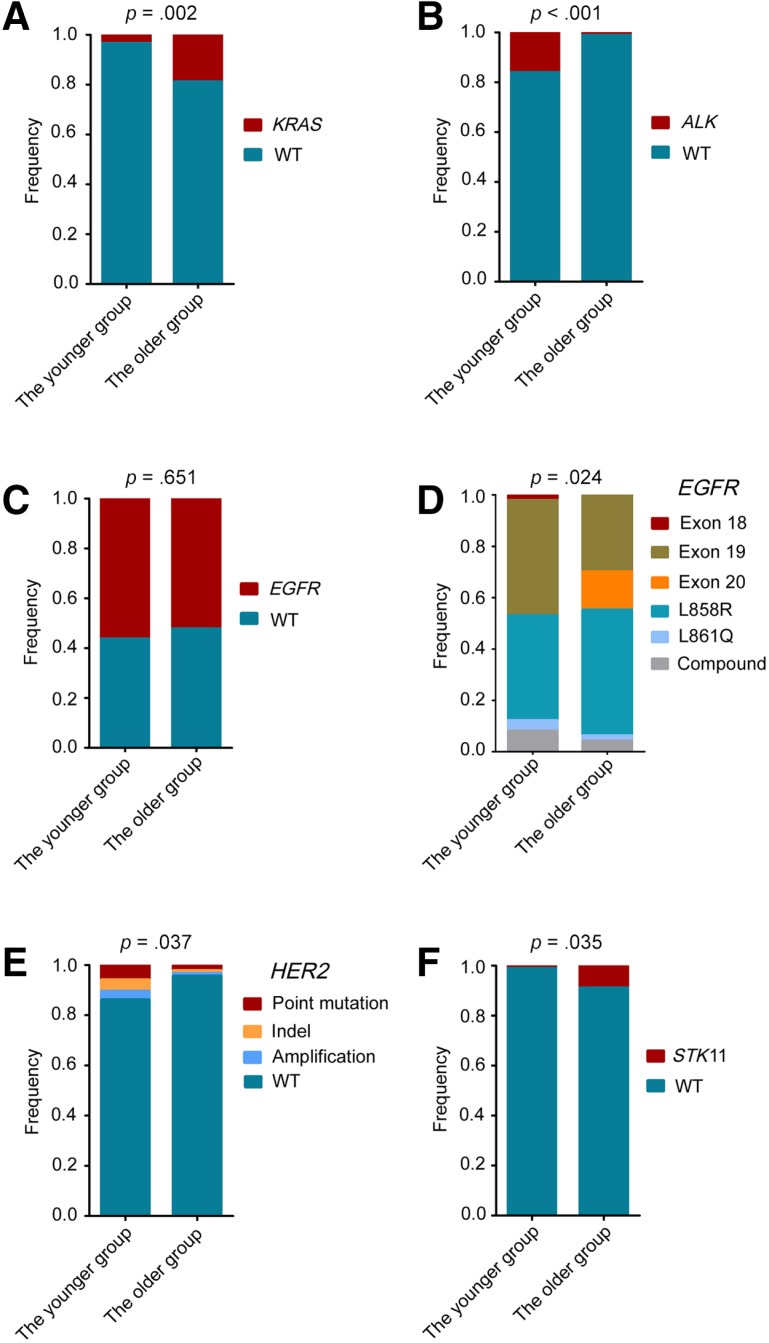
In terms of all the identified targeted genetic alterations, only the distribution of KRAS mutations and ALK arrangement differed significantly between the younger and older age groups (supplemental online Table 2). Compared with the older group, KRAS mutation was less frequent in the younger population (3.4% vs. 18.9%; p = .002), whereas ALK arrangement was significantly higher in the younger group (16.1% vs. 1.1%; p < .001; Fig. 3A, B). A comparable prevalence of EGFR mutations was identified between the younger and the older groups (56.3% vs. 52.2%; p = .653), whereas the distribution of EGFR mutation subtypes between the two groups was significantly different (p = .024; Fig. 3C, D). Among patients with EGFR‐mutant lung adenocarcinoma, EGFR exon 19 del and L858R were most frequently detected (Fig. 3D). Of all the mutation subtypes, exon 20 insertion was less frequent in the younger group (p = .005), whereas the frequencies of the remaining mutation subtypes were similar between the two groups (supplemental online Table 3).
Figure 3.
The distribution of representative targeted genetic alterations between the younger and older patients with lung adenocarcinoma. The comparative analysis of KRAS mutations (A), ALK arrangements (B), EGFR mutations (C), EGFR mutation subtypes (D), HER2‐targeted genetic alterations (E), and STK11 mutations (F) between the younger and the older groups. Statistical significance was defined as p < .05.
Abbreviation: WT, wild type.
For some patients, both approved drugs and experimental agents were simultaneously available because the patients harbored several different actionable alterations (supplemental online Fig. 2). HER2 mutations, HER2 amplification and STK11 mutations commonly overlapped with other targeted alterations. When only the genetic alterations with the optimal clinical option were exhibited, no significant difference was identified in these genes between the younger and the older groups (Table 2). However, taking into account all the single and concurrent genetic alterations, a high frequency of HER2‐targeted alterations and a low frequency of STK11 mutations were found in the younger group (HER2 13.8% vs. 4.4%; p = .037; STK11 1.1% vs. 8.9%; p = .035; Fig. 3E, 3F).
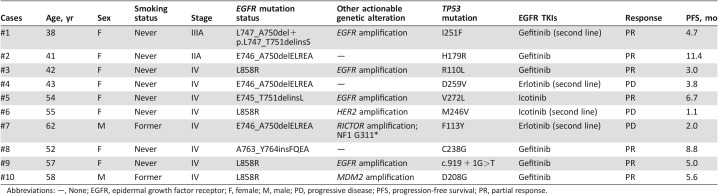
Table 2. Clinical data for partial patients with concurrent EGFR and TP53 mutations.
Abbreviations: —, None; EGFR, epidermal growth factor receptor; F, female; M, male; PD, progressive disease; PFS, progression‐free survival; PR, partial response.
TP53 Mutations in Younger Patients with Lung Adenocarcinoma
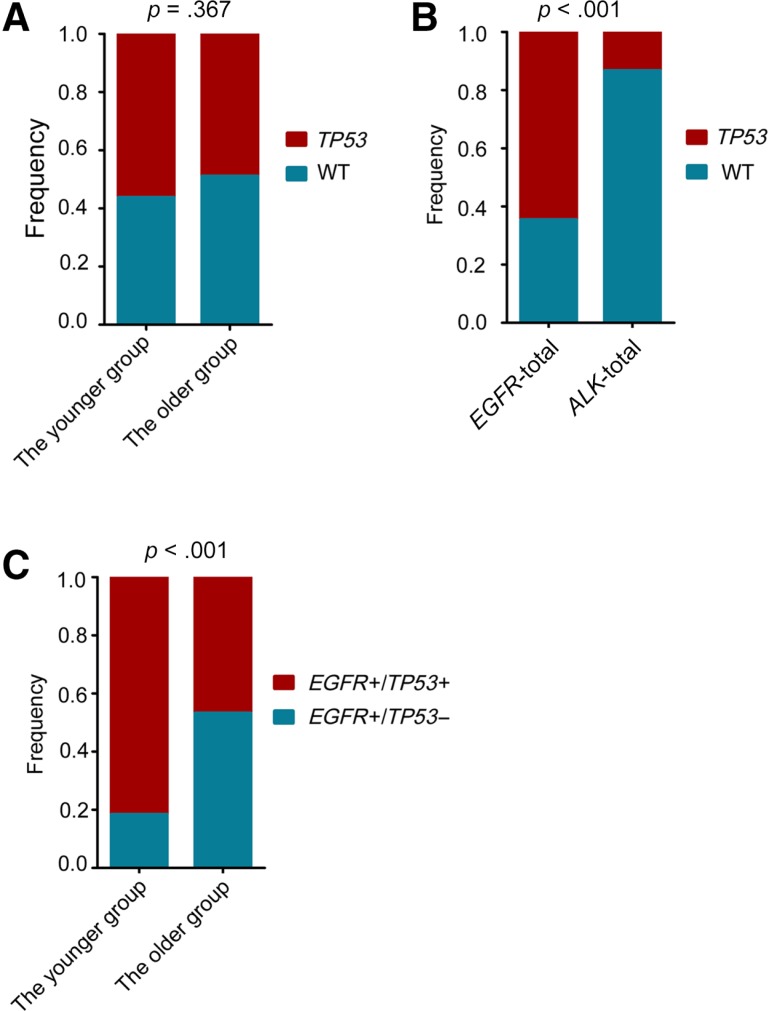
Among the genetic alterations without available targeted agents identified by NGS assay, the most frequently mutated gene was TP53. TP53 mutations were comparably identified in the younger and the older group (56.3% vs. 48.9%; p = .367; Fig. 4A). However, concurrent TP53 mutations were more frequent in EGFR‐mutated patients than ALK arrangement patients (64.6% vs. 13.3%, p < .001; Fig. 4B). Notably, among the EGFR‐mutant patients, concurrent TP53 mutations were identified more frequently in the younger group compared with the older patients (81.6% vs. 46.8%; p < .001; Fig. 4C). Progression‐free survival (PFS) data were available for 10 patients harboring concurrent EGFR and TP53 mutations who had a median PFS of 5.2 months after treatment with epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs; Table 2). One patient (case #4) harboring EGFR exon 19del and TP53 D259V experienced disease progression after 3.8 months of erlotinib maintenance treatment (Table 2, supplemental online Fig. 3).
Figure 4.
Mutation analysis of the TP53 gene. (A): The comparative analysis of TP53 mutations between the younger and the older groups. (B): Analysis of TP53 mutations between patients with EGFR mutant and ALK‐arranged lung adenocarcinoma. (C): Analysis of concurrent EGFR/TP53 mutations between the younger and the older groups. Statistical significance was defined as p < .05.
Abbreviation: WT, wild type.
Discussion
NSCLC in young patients attracts increasing attention and accounts for less than 5.0% of all patients [5], [16], [17], [19]. Many studies have suggested that NSCLC in the young has unique characteristics, with greater percentages of female patients, adenocarcinoma, and never smokers [5], [7], [14], [17]. Younger patients with lung adenocarcinoma, especially those at an advanced stage, had no significantly better overall survival than older patients despite undergoing more aggressive treatment [14]. Although care of lung adenocarcinoma is rapidly changing with advances in genomic testing and the development of next‐generation targeted agents, the molecular pathologic features of young patients have not been well investigated. A recent report demonstrated that younger age was associated with an increased likelihood of harboring a targetable genotype in NSCLC, including lung adenocarcinoma [13]. Most of the previous data on molecular features in young patients with lung adenocarcinoma have been discordant, and only a few driven genes have been studied using non‐NGS assays [9], [10], [12], [13], [14]. Comprehensive genomic profiling using NGS could detect potentially targetable alterations missed by non‐NGS assays and accelerate the development of targeted agents for rare mutational events [20], [21]. In this study, the genetic profile in young patients with lung adenocarcinoma was comprehensively identified by broadly targeted NGS testing. Patients under the age of 45 years harbored a distinctive genetic profile, and age 45 might be a useful age cutoff by which lung adenocarcinoma in the young could be defined. Notably, younger patients harbored a much higher rate of genetic alterations with NCCN‐approved targeted agents than older patients. This is the first study demonstrating an association between young age and comprehensive genetic profile in lung adenocarcinoma.
Specifically, a higher frequency of ALK and a lower frequency of KRAS were identified in younger patients with lung adenocarcinoma, which were consistent with previous studies [9], [10], [13]. However, there have also been studies finding a similar prevalence of these genetic mutations, which might attributed to the small number of young cases [14], [22] Additionally, our NGS study suggested an association between age and uncommon genetic alterations usually missed by standard genotyping. A higher frequency of HER2‐targeted alterations and a low frequency of STK11 mutations were identified in the younger patients in this study. A previous report by Kosuke Tanaka et al. also found that the frequency of HER2 mutations was significantly higher in younger patients with adenocarcinomas compared with the older group [10]. However, a nonsignificant trend toward younger age at diagnosis was seen for HER2 in a recent study, in which only HER2 in‐frame insertion in exon 20 was studied [13]. The above findings highlighted that all types of HER2‐targeted alterations, including amplification, could be a valuable target in young patients with lung adenocarcinoma.
Compared with ALK arrangements, the prevalence of EGFR mutations in young patients with lung adenocarcinoma were highly controversial, as shown in supplemental online Table 4. Both a higher and a lower frequency of EGFR mutations have been identified in younger patients with lung adenocarcinoma, compared with their older counterparts [10], [13], [18]. In this study, no difference of all types of EGFR mutations or EGFR‐sensitive mutations was found between the younger and the older patients. Our findings were consistent with another three studies in young patients with lung adenocarcinoma [9], [14], [22]. Interestingly, the EGFR exon 20 insertions, which predict resistance to clinically available EGFR TKIs, were less frequent in younger patients in our study. The conflicting data in the aforementioned research on EGFR prevalence in young patients with lung cancer might be attributed to race, age cutoffs, and pathology, which calls for further study.
Younger age was associated with an increased likelihood of harboring a targetable genotype, yet the survival of young patients with NSCLC was found to be unexpectedly poor compared with other age groups, suggesting more aggressive disease biology [13]. Prior research also implied that patients with lung adenocarcinoma of young age had poorer efficacy of EGFR TKIs [18]. The reason why young patients with EGFR‐mutant adenocarcinoma had poor prognosis under EGFR TKI therapy is not clear, partially because of more brain metastases in this subpopulation [23]. Many mechanisms have been found to be involved in primary resistance to EGFR TKIs in advanced NSCLC with activating EGFR mutations [24]. Apart from these mechanisms, the commonly concurrent TP53 mutations have been proved to reduce responsiveness to EGFR TKIs and worsen prognosis in patients with EGFR‐mutated NSCLC [25], [26]. In this study, no higher prevalence of TP53 mutations was found in the younger patients compared with older patients, which was not consistent to one prior finding [14]. However, we identified much higher concurrent EGFR/TP53 mutations in the younger patients than in their older counterparts. More importantly, follow‐up data from partial patients harboring concurrent EGFR/TP53 mutations patients have shown poor response to EGFR TKI treatment. Considering that concurrent EGFR/TP53 mutations account for over 80% of the EGFR‐mutant young patients, our report might disclose an important mechanism for poor prognosis and low efficiency of EGFR TKIs in this specific population. It has been reported young patients with lung adenocarcinoma tended to have a poorer prognosis and that angiogenesis of lung adenocarcinoma in young patients was more closely correlated with p53 expression than in elderly patients [27]. Consequently, the combination of erlotinib plus bevacizumab might be an optimal regimen in young patients with concurrent EGFR/TP53‐mutant lung adenocarcinoma, warranting further well‐conducted investigation to validate this deduction [28].
Although this is the first large NGS study on the molecular features of young patients with lung adenocarcinoma, several disadvantages must be considered. The patients included in this study were from a single institution and were all Chinese. Follow‐up data are only available for a small number of the patients. Three versions of NGS testing were performed in this study, which resulted in complexity of sequence data analysis. In addition, the number of patients between 46 and 50 years of age enrolled in this research (only four patients) was too small to be analyzed as an age group. Therefore, further study is needed to define the age cutoff point for young patients with NSCLC who have a unique genetic profile.
Conclusion
Young patients with lung adenocarcinoma are an understudied population who harbor high prevalence of ALK arrangement, HER2‐targeted alterations, and concurrent EGFR/TP53 mutations. The distinctive molecular profile identified in young patients with lung adenocarcinoma calls for more attention to personalized treatment of this population.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
This work was supported by the Taishan Scholar Foundation (grant tshw201502061 to X.Z.), Qingdao People's Livelihood Science and Technology Program (grant 16‐6‐2‐3‐nsh to X.Z.), Chinese Postdoctoral Science Foundation (2017M622143 to H.H.), and Qingdao Postdoctoral Application Research Funded Project (2016052 to H.H.).
Footnotes
For Further Reading: Zong‐Han Yao, Wei‐Yu Liao, Chao‐Chi Ho et al. Real‐World Data on Prognostic Factors for Overall Survival in EGFR Mutation‐Positive Advanced Non‐Smal Cell Lung Cancer Patients Treated with First‐Line Gefitinib. The Oncologist 2017;22:1075‐1083.
Implications for Practice: The finding that chronic hepatitis C virus (HCV) infection might predict poor overall survival (OS) in epidermal growth factor receptor mutation‐positive advanced non‐smal cell lung cancer (NSCLC) patients treated with first‐line gefitinib may raise awareness of benefit from anti‐HCV treatment in this patient population. Brain metastasis in the initial diagnosis or intracranial progression during gefitinib treatment is not a prognostic factor for OS. This study, which enrolled a real‐world population of NSCLC patients, including sicker patients who were not eligible for a clinical trial, may have impact on guiding usual clinical practice.
Author Contributions
Conception/design: Helei Hou, Xiaochun Zhang
Provision of study material or patients: Hua Zhu, Han Zhao, Weihua Yan, Yongjie Wang, Man Jiang, Dong Liu, Na Zhou, Chuantao Zhang
Collection and/or assembly of data: Helei Hou, Bin Liu, Pansong Li, Lianpeng Chang, Yanfang Guan, Zhe Wang, Xiaoping Zhang, Zhuokun Li
Data analysis and interpretation: Helei Hou, Bin Liu, Pansong Li, Lianpeng Chang, Yanfang Guan, Zhe Wang, Xiaoping Zhang, Zhuokun Li
Manuscript writing: Helei Hou, Bingliang Fang, Xiaochun Zhang
Final approval of manuscript: Helei Hou, Hua Zhu, Han Zhao, Weihua Yan, Yongjie Wang, Man Jiang, Bin Liu, Dong Liu, Na Zhou, Chuantao Zhang, Pansong Li, Lianpeng Chang, Yanfang Guan, Zhe Wang, Xiaoping Zhang, Zhuokun Li, Bingliang Fang, Xiaochun Zhang
Disclosures
The authors indicated no financial relationships.
References
- 1. Soo RA, Stone ECA, Cummings KM et al. Scientific advances in thoracic oncology 2016. J Thorac Oncol 2017;12:1183–1209. [DOI] [PubMed] [Google Scholar]
- 2. Cancello G, Maisonneuve P, Rotmensz N et al. Prognosis and adjuvant treatment effects in selected breast cancer subtypes of very young women (<35 years) with operable breast cancer. Ann Oncol 2010;21:1974–1981. [DOI] [PubMed] [Google Scholar]
- 3. Gryfe R, Kim H, Hsieh ET et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med 2000;342:69–77. [DOI] [PubMed] [Google Scholar]
- 4. Gomes R, Dabó H, Queiroga H et al. Non‐small cell lung cancer in young patients–A retrospective analysis of 10 years in a tertiary university hospital. Rev Port Pneumol 2016;22:125–126. [DOI] [PubMed] [Google Scholar]
- 5. Arnold BN, Thomas DC, Rosen JE et al. Lung cancer in the very young: Treatment and survival in the National Cancer Data Base. J Thorac Oncol 2016;11:1121–1131. [DOI] [PubMed] [Google Scholar]
- 6. Zhang J, Chen SF, Zhen Y et al. Multicenter analysis of lung cancer patients younger than 45 years in Shanghai. Cancer 2010;116:3656–3662. [DOI] [PubMed] [Google Scholar]
- 7. Subramanian J, Morgensztern D, Goodgame B et al. Distinctive characteristics of non‐small cell lung cancer (NSCLC) in the young: A surveillance, epidemiology, and end results (SEER) analysis. J Thorac Oncol 2010;5:23–28. [DOI] [PubMed] [Google Scholar]
- 8. Polo V, Zago G, Frega S et al. Non‐small cell lung cancer in a very young woman: A case report and critical review of the literature. Am J Case Rep 2015;16:782–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scarpino S, Rampioni Vinciguierra GL, Di Napoli A et al. High prevalence of ALK+/ROS1+ cases in pulmonary adenocarcinoma of adolescents and young adults. Lung Cancer 2016;97:95–98. [DOI] [PubMed] [Google Scholar]
- 10. Tanaka K, Hida T, Oya Y et al. Unique prevalence of oncogenic genetic alterations in young patients with lung adenocarcinoma. Cancer 2017;123:1731–1740. [DOI] [PubMed] [Google Scholar]
- 11. Catania C, Botteri E, Barberis M et al. Molecular features and clinical outcome of lung malignancies in very young people. Future Oncol 2015;11:1211–1221. [DOI] [PubMed] [Google Scholar]
- 12. Nagashima O, Ohashi R, Yoshioka Y et al. High prevalence of gene abnormalities in young patients with lung cancer. J Thorac Dis 2013;5:27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sacher AG, Dahlberg SE, Heng J et al. Association between younger age and targetable genomic alterations and prognosis in non‐small‐cell lung cancer. JAMA Oncol 2016;2:313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ye T, Pan Y, Wang R et al. Analysis of the molecular and clinicopathologic features of surgically resected lung adenocarcinoma in patients under 40 years old. J Thorac Dis 2014;6:1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goldstraw P, Chansky K, Crowley J et al. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016;11:39–51. [DOI] [PubMed] [Google Scholar]
- 16. Corrales‐Rodríguez L, Arrieta O, Mas L et al. An international epidemiological analysis of young patients with non‐small cell lung cancer (AduJov‐CLICaP). Lung Cancer 2017;113:30–36. [DOI] [PubMed] [Google Scholar]
- 17. Hsu CH, Tseng CH, Chiang CJ et al. Characteristics of young lung cancer: Analysis of Taiwan's nationwide lung cancer registry focusing on epidermal growth factor receptor mutation and smoking status. Oncotarget 2016;7:46628–46635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu SG, Chang YL, Yu CJ et al. Lung adenocarcinoma patients of young age have lower EGFR mutation rate and poorer efficacy of EGFR tyrosine kinase inhibitors. ERJ Open Res 2017;3:00092–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thomas A, Chen Y, Yu T et al. Trends and characteristics of young non‐small cell lung cancer patients in the United States. Front Oncol 2015;5:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Drilon A, Wang L, Arcila ME et al. Broad, hybrid capture‐based next‐generation sequencing identifies actionable genomic alterations in lung adenocarcinomas otherwise negative for such alterations by other genomic testing approaches. Clin Cancer Res 2015;21:3631–3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jordan EJ, Kim HR, Arcila ME et al. Prospective comprehensive molecular characterization of lung adenocarcinomas for efficient patient matching to approved and emerging therapies. Cancer Discov 2017;7:596–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim L, Kim KH, Yoon YH et al. Clinicopathologic and molecular characteristics of lung adenocarcinoma arising in young patients. J Korean Med Sci 2012;27:1027–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jain A, Lim C, Gan EM et al. Impact of smoking and brain metastasis on outcomes of advanced EGFR mutation lung adenocarcinoma patients treated with first line epidermal growth factor receptor tyrosine kinase inhibitors. PLoS One 2015;10:e123587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang J, Wang B, Chu H et al. Intrinsic resistance to EGFR tyrosine kinase inhibitors in advanced non‐small‐cell lung cancer with activating EGFR mutations. Onco Targets Ther 2016;9:3711–3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Canale M, Petracci E, Delmonte A et al. Impact of TP53 mutations on outcome in EGFR‐mutated patients treated with first‐line tyrosine kinase inhibitors. Clin Cancer Res 2017;23:2195–2202. [DOI] [PubMed] [Google Scholar]
- 26. VanderLaan PA, Rangachari D, Mockus SM et al. Mutations in TP53, PIK3CA, PTEN and other genes in EGFR mutated lung cancers: Correlation with clinical outcomes. Lung Cancer 2017;106:17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kondou M, Nagayasu T, Hidaka S et al. Correlation between angiogenesis and p53 expression in lung adenocarcinoma of young patients. Tohoku J Exp Med 2009;217:101–107. [DOI] [PubMed] [Google Scholar]
- 28. Seto T, Kato T, Nishio M et al. Erlotinib alone or with bevacizumab as first‐line therapy in patients with advanced non‐squamous non‐small‐cell lung cancer harbouring EGFR mutations (JO25567): An open‐label, randomised, multicentre, phase 2 study. Lancet Oncol 2014;15:1236–1244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.