This article summarizes the review and rationale for the 2017 approval of gemtuzumab ozogamicin by the U.S. Food and Drug Administration, focusing on known serious toxicities and dose recommendations.
Keywords: Acute myeloid leukemia, Gemtuzumab ozogamicin, Mylotarg, U.S. Food and Drug Administration approval
Abstract
On September 2, 2017, the U.S. Food and Drug Administration approved gemtuzumab ozogamicin (GO; Mylotarg; Pfizer, New York City, NY) for treatment of relapsed or refractory (R/R) CD33‐positive acute myeloid leukemia (AML) in patients 2 years of age and older. GO is a CD33‐directed antibody drug conjugate linked to the cytotoxic antibiotic calicheamicin. It originally received accelerated approval for treatment of older patients with relapsed CD33‐positive AML in 2000, but it was withdrawn from the market in 2010 when the confirmatory trial failed to demonstrate clinical benefit among safety concerns, such as a higher rate of induction fatalities on the GO combination arm compared with chemotherapy alone. In addition, GO was associated with hepatic veno‐occlusive disease (VOD), which has substantial morbidity and mortality. Pharmacokinetic analyses suggested a lower maximum concentration of GO would result in less VOD without affecting target saturation or efficacy. A meta‐analysis across dose schedules of GO in patients with R/R AML showed that a lower‐dose “fractionated” schedule of 3 mg/m2 days 1, 4, and 7 was associated with less early mortality, hemorrhage, and VOD, without an apparent decrease in complete remission (CR) rate. MyloFrance 1 was a single‐arm study evaluating response rates in patients with relapsed CD33‐positive AML treated with the lower‐dose fractionated GO regimen. The CR rate was 26% (95% confidence interval 16%–40%). Common adverse reactions were fever, infections, nausea, vomiting, constipation, bleeding, increased liver enzymes, and mucositis. There were no cases of VOD. These results supported the approval of GO as monotherapy for R/R CD33‐positive AML using the lower‐dose fractionated regimen.
Implications for Practice.
Gemtuzumab ozogamicin (GO) 3 mg/m2 days 1, 4, and 7 is an active regimen for induction of remission when used to treat patients with relapsed or refractory CD33‐positive acute myeloid leukemia without curative intent. The risks of hepatic veno‐occlusive disease and early mortality with this regimen appear to be lower than reported previously for GO 9 mg/m2 days 1 and 15. The data were not sufficient to enable conclusions about the safety of GO in children younger than 2 years of age.
Introduction
Acute myeloid leukemia (AML) is a potentially fatal disorder, particularly in patients with relapsed or refractory (R/R) disease. For those in first relapse, prognosis varies based on duration of first complete remission (CR), cytogenetic risk, age, and prior hematopoietic stem cell transplantation (HSCT), with 5‐year overall survival (OS) rate ranging from 4% to 46% depending on these factors [1]. Patients who can tolerate intensive chemotherapy may achieve CR rates of 30%–70% [2]. However, patients treated with low‐intensity regimens, including hypomethylating agents and low‐dose cytarabine, achieve CR rates of only 10%–17%, with a median OS of 6–8 months [3], [4]. Patients with refractory AML or those with multiple relapses have an even poorer prognosis [5]. Thus, there remains a need for additional effective therapies for R/R AML.
Gemtuzumab ozogamicin (GO, also known as CMA‐676) is a humanized anti‐CD33 monoclonal antibody linked covalently to the cytotoxic agent N‐acetyl gamma calicheamicin. GO binds to the CD33 antigen, which is expressed on the surface of most AML cells and on immature cells of the myelomonocytic lineage. Binding of GO to its cognate antigen leads to internalization and release of calicheamicin, which intercalates DNA, inducing double‐strand DNA breaks and cell death.
GO received accelerated approval on May 17, 2000, for treatment of CD33‐positive AML in first relapse in patients 60 years of age or older who were not considered candidates for cytotoxic chemotherapy. The recommended GO regimen for this approval was 9 mg/m2 for two doses 14 days apart. Myelosuppression and infusion‐related reactions were identified as major safety concerns at the time [6]. In the postmarketing period, fatal hepatotoxicity and veno‐occlusive disease (VOD) were added as boxed warnings, highlighting an especially increased risk of VOD in patients who received GO before or after hematopoietic stem cell transplantation (HSCT) [7].
The accelerated approval of GO was issued with a postmarketing requirement to confirm clinical benefit. The confirmatory study, S0106, was a randomized clinical trial evaluating GO 6 mg/m2 in combination with induction chemotherapy in patients ≤60 years with newly diagnosed AML. Study S0106 failed to demonstrate clinical benefit for GO, and a higher rate of fatal induction toxicities was observed in the GO combination arm compared with chemotherapy alone [8]. GO was withdrawn voluntarily from the U.S. market in 2010. Recent pharmacokinetic (PK) analyses indicate that the originally approved dose of GO may have been too high, leading to excess toxicity. More recent studies have assessed the safety and efficacy of repeated lower doses of GO, referred to as the “fractionated” dosing schedule, for treatment of R/R AML [9], [10], [11], [12].
Herein, we provide a summary of the review and rationale for the U.S. Food and Drug Administration's (FDA) 2017 approval of GO at 3 mg/m2 days 1, 4 and 7, for treatment of patients with R/R CD33‐positive AML. Given the known serious toxicities of the previously approved 9 mg/m2 dose of GO, the rationale for the dose selected for use in the Prescribing Information was a major focus of this review.
Dose Selection
Clinical Pharmacology
FDA performed analyses on the PK and pharmacodynamics (PD) of GO in patients with AML treated on a phase I dose‐escalation trial testing GO 0.25–9 mg/m2 for three doses 14 days apart [13]. PK analyses demonstrated that exposure increased more than proportionally as the dose of GO increased. That is, small increases in dose resulted in larger than proportional increases in maximum concentration (Cmax) and area under the time‐concentration curve (AUC). Furthermore, there was a notable decrease in clearance after subsequent doses 14 days apart, resulting in an increase in Cmax and AUC with subsequent doses. In simulation studies for regimens of 9 mg/m2 days 1 and 15 or 3 mg/m2 days 1, 4, and 7, Cmax and total AUC were reduced by approximately 75% with the latter fractionated regimen, although the total dose was half that of the original regimen (i.e., 9 vs. 18 mg/m2). The PD analysis showed that GO binding to CD33 was saturated at doses above 2 mg/m2.
FDA explored exposure‐response relationships for safety and efficacy outcomes using data from three single‐arm trials of GO 9 mg/m2 for two or three doses 14 days apart in patients with AML [13]. Increase in Cmax was significantly correlated with a higher risk of VOD. There was, however, no remarkable association between Cmax or AUC and CR, suggesting that CR did not differ across a range of exposures. Given that data came from a single dose level, the conclusions regarding the lack of an exposure‐efficacy relationship were limited by the fact that the range of exposure was driven primarily by individual variability rather than dose. Therefore, evidence from clinical trials was required to establish a safe and effective dose.
Clinical Safety and Efficacy by Dose Schedule
Safety and efficacy outcomes were reviewed across trials of single‐agent GO in R/R AML using various unfractionated and fractionated doses and schedules. Meta‐analyses were performed across multiple studies using VOD and CR as outcomes by dose‐schedule when data were available, and a pooled analysis was used to assess the early mortality by dose‐schedule. All studies used are listed in supplemental online Table 1. Methods for identification of the relevant publications and for the meta‐analysis are described in supplemental online Appendix 1.
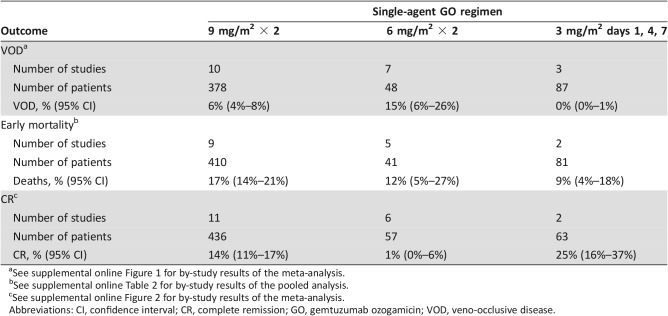
As shown in Table 1, the incidence of VOD differed by dose‐schedule. For the 9 mg/m2 dose subgroup, the VOD incidence was 6% (95% confidence interval [CI] 4%–8%). This is consistent with the results of a large U.S. postmarketing observational study, which showed a VOD incidence of 9% (95% CI 7%–12%); 3% were fatal [14]. By contrast, there were no cases of VOD in 87 patients treated with GO 3 mg/m2 days 1, 4, and 7, including a high‐risk subgroup of 19 patients who underwent HSCT before or after treatment with GO [9], [10], [11]. A similar trend for less VOD with lower doses of GO was seen in Study 100374 using GO for treatment of post‐HSCT relapse, in which the reported VOD incidence was 33%, 17%, and 0% following GO doses of 6 mg/m2 (n = 6), 4 mg/m2 (n = 18), and 2 mg/m2 (n = 13), respectively. Acknowledging the limitations of small sample size, it appears that the risk of VOD was reduced with lower doses of GO. This is consistent with the conclusion of the pharmacometrics analysis that the risk of VOD is related to Cmax of GO.
Table 1. Clinical outcomes by single‐agent GO regimen for treatment of relapsed or refractory acute myeloid leukemia.
See supplemental online Figure 1 for by‐study results of the meta‐analysis.
See supplemental online Table 2 for by‐study results of the pooled analysis.
See supplemental online Figure 2 for by‐study results of the meta‐analysis.
Abbreviations: CI, confidence interval; CR, complete remission; GO, gemtuzumab ozogamicin; VOD, veno‐occlusive disease.
Table 1 also shows early mortality rates pooled by dose‐schedule. The point estimates suggest lower early mortality with the GO 3 mg/m2 days 1, 4, and 7 fractionated regimen at 9% (95% CI 4%–18%) compared with 17% (95% CI 14%–21%) using the 9 mg/m2 regimen. Further, where cause of death information was available, treatment‐related early mortality was also lower with the fractionated regimen (2/57; 4%) than with the 9 mg/m2 regimen (39/351; 11%). Infection and hemorrhage comprised the most common causes of treatment‐related early mortality with the 9 mg/m2 regimen. Coincident with this, for patients who achieved CR or CR with incomplete platelet recovery (CRp), the times to neutrophil and platelet recovery were shorter with the fractionated regimen than with the 9 mg/m2 regimen (supplemental online Table 3).
Lastly, the meta‐analysis (Table 1) showed that the CR rate was no worse using the GO 3 mg/m2 days 1, 4, and 7 fractionated regimen (25%) than with 9 mg/m2 days 1 and 15 (14%) in patients with R/R AML. In a pooled analysis excluding studies limited to AML in first relapse, CR rates were 14% across trials using GO 9 mg/m2 (supplemental online Table 1; supplemental online Fig. 2) and 17% in a small retrospective study using the fractionated regimen [10]. Cross‐trial comparisons of efficacy outcomes can be confounded by differences between studies in the proportions of patients in prognostic subgroups, but the meta‐analysis confirmed that responses were seen across a wide range of GO dose‐schedules, and the results are consistent with the conclusion of the pharmacometrics exposure‐response analysis. Thus, based on the available safety and efficacy data, the GO 3 mg/m2 days 1, 4, and 7 fractionated regimen was expected to have a more favorable risk/benefit than the 9 mg/m2 regimen.
Trial Design
MyloFrance 1 [9] was identified by FDA as an adequate and well‐controlled trial, testing an appropriate dose of GO. The single‐arm, open‐label trial tested fractionated dosing of GO in adults with CD33‐positive de novo AML in first relapse. Key eligibility criteria included duration of first remission 3–18 months, CD33 positivity in >80% of cells, no prior autologous or allogeneic HSCT, and no proposed HSCT within 3 months following GO. Induction consisted of GO 3 mg/m2 days 1, 4, and 7. In the absence of toxicity, responding patients were eligible to receive consolidation with high‐dose cytarabine. Patients could undergo HSCT following GO, but it was recommended to delay this by at least 90 days. The primary endpoint was overall response rate (ORR; CR plus CRp) within 43 days from starting GO. The study targeted a 30% ORR and aimed to exclude 15%. Secondary endpoints included CR rate, OS, and relapse‐free survival (RFS).
Results
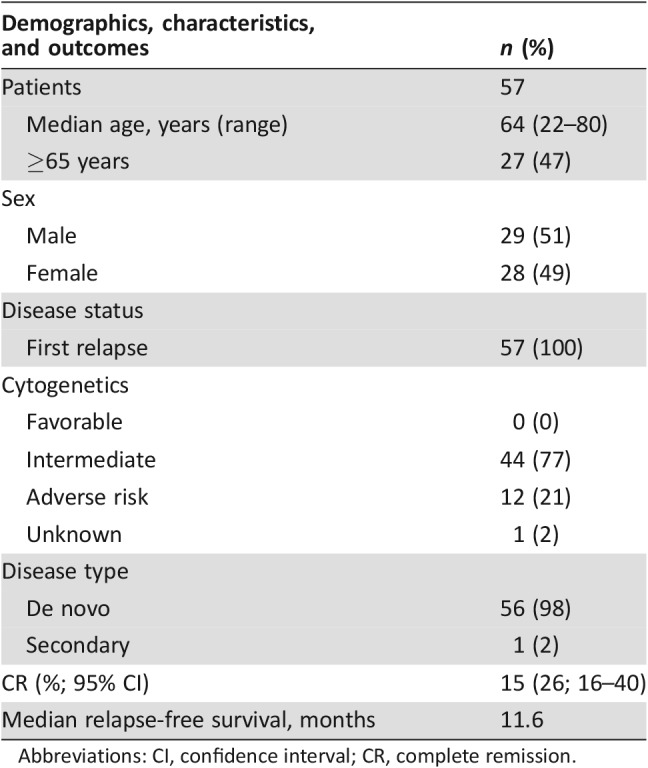
Fifty‐seven patients were treated with GO. Table 2 lists baseline demographics and disease characteristics. All 57 patients received the three planned doses of GO, and 18 of 19 patients achieving CR/CRp received consolidation with high‐dose cytarabine. Seven patients underwent HSCT a mean of 152 days (range 112–220 days) after GO.
Table 2. MyloFrance 1: Patient characteristics and outcomes.
Abbreviations: CI, confidence interval; CR, complete remission.
Efficacy
The efficacy of GO was established on the basis of CR and duration of CR. Fifteen (26%; 95% CI 16%–40%) patients achieved a CR following GO, and the median relapse‐free survival was 11.6 months. Remission rates were similar across the subpopulations examined, including cytogenetics, age, and duration of first remission, although some subgroups were small. The investigators reported that ORR correlated with P‐glycoprotein and multidrug resistance protein 1 expression in leukemia cells [9], but these data were not submitted for FDA review, so the conclusion could not be confirmed.
Safety
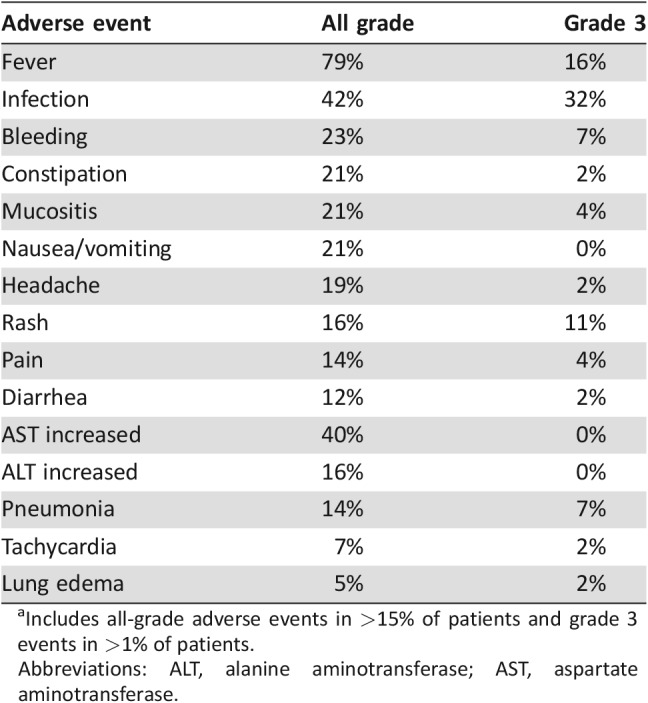
Four (7%) patients died before day 43. Two died of persistent AML and two died suddenly at home. Common adverse reactions are shown in Table 3. There were no grade 4 adverse reactions observed, no fatal infections, and no cases of VOD. For patients who achieved a CR/CRp, the median time to absolute neutrophil count (ANC) >0.5 Gi/L was 23 days and median time to platelets >50 Gi/L was 20 days (supplemental online Table 3).
Table 3. Common adverse events on MyloFrance 1a.
Includes all‐grade adverse events in >15% of patients and grade 3 events in >1% of patients.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Pediatric Experience
The safety and efficacy of GO as a single agent for treatment of pediatric patients with R/R AML was supported by the single‐arm, dose‐escalation study in 29 patients (Study 102) using unfractionated dose‐schedules. Median age was 12 years (range 1–16). A literature review included 96 additional patients ages 0.2–21 years [10], [15], [16], [17], [18]. Across 52 pediatric patients with R/R AML and CR data available, the pooled CR rate was 8%. The only pediatric trial that looked at the fractionated regimen was a small retrospective trial that included six patients treated with GO 3 mg/m2 days 1, 4, and 7 [10]; the CR rate was 17%.
The safety profile of GO in children in Study 102 was similar to that seen in adults, with the exception of a higher incidence of VOD (31% overall). However, almost half of the pediatric patients underwent HSCT after treatment with the unfractionated GO regimen, accounting for the high risk for VOD. From the literature review, rates of VOD ranged from 0% to 18% in children [10], [15], [16], [17], [18], with no reported cases using the fractionated regimen [10]. There was not, however, sufficient experience to confirm safety in children younger than 2 years of age using body surface area‐based dosing.
Discussion
MyloFrance 1 served as the pivotal trial assessing the activity of GO for treatment of CD33‐positive R/R AML. The CR rate was 26% and the median RFS was 11.6 months in this first relapse patient population. Additional studies of GO demonstrated CR rates of 0%–32% in patients with CD33‐positive refractory AML or with later relapses (supplemental online Table 1; supplemental online Fig. 2). Although these are relatively low response rates, the clinical benefit of GO was confirmed by the ALFA‐0701 trial, which demonstrated an event‐free survival benefit of GO 3 mg/m2 on days 1, 4, and 7 when added to 7 + 3 induction therapy in patients with newly diagnosed AML [19], [20], [21]. The details of this trial, a meta‐analysis of five randomized trials using GO in addition to induction chemotherapy, and the randomized monotherapy trial in elderly patients with newly diagnosed AML are discussed in a separate manuscript dedicated to the newly diagnosed CD33‐positive AML indication [19]. Based on the entirety of the data, regular approval was warranted. Nonetheless, given that treatment with GO alone is not curative, how best to incorporate it into the R/R AML treatment paradigm remains to be determined.
FDA approved GO for the treatment of R/R CD33‐positive AML. Although CD33 is expressed in approximately 90% of AML patients [22], expression levels vary [23], [24], [25]. Nearly all clinical studies investigating GO monotherapy in patients with R/R AML enrolled patients with CD33 expression levels >80%. However, some smaller studies suggested a benefit with a lower cutoff or no cutoff for CD33 expression [10], [26]. Given a lack of data regarding the optimal expression level for maximal treatment benefit, FDA did not specify a threshold for CD33 positivity in the drug label. Further study in this area may help to elucidate R/R AML patients most likely to benefit from GO therapy.
Notably, as opposed to the original review [6], FDA did not consider CRp responses in the current assessment of the activity of GO. The pooled analysis in the original approval showed that CRps accounted for about half of the responses, and the CR rate was only 16% [6]. Data published since that time indicate that CRp and CR with incomplete hematologic recovery responses are associated with higher levels of minimal residual disease and shorter disease control than with CR responses [27], [28]. For nonintensive therapies with a tolerable toxicity profile, FDA has considered durable CR with partial hematologic recovery (defined as meeting CR criteria but with lower count thresholds of ANC >0.5 Gi/L and platelet count >50 Gi/L) as a palliative benefit to patients with R/R AML, when accompanied by data on transfusion independence [29]. In the case of GO, CRp responses on MyloFrance 1 only required a platelet count of >20 Gi/L, which could still represent a bleeding risk to patients. Thus, the approval relied only on durable CR rate, which remains a preferred endpoint for assessment of new therapies for R/R AML [30].
FDA extended the GO indication for treatment of CD33‐positive R/R AML to pediatric patients 2 years and older. Pediatric safety and preliminary efficacy outcomes were comparable to adults, with the exception of a higher incidence of VOD with the unfractionated regimen, likely a consequence of higher rates of HSCT post‐GO. Based on mechanism of action of GO, the similar biology of AML in adults and children [31], and supported by the reviewed clinical trial experience, the efficacy of GO could be extrapolated to the pediatric population. Although the studies of GO included some children as young as 2 months old, safety and PK data were limited for children below the age of 2 years. Therefore, in the absence of adequate data for review, FDA could not extend the indication to infants.
In the current approval, the recommended GO regimen of 3 mg/m2 days 1, 4, and 7 differs substantially from the regimen of 9 mg/m2 for two doses 14 days apart proposed originally. Altogether, the pharmacometrics analyses, the meta‐analyses of trials using various doses of GO, and the clinical efficacy and safety data provided a strong case for use of the lower‐dose fractionated schedule. The results are somewhat counter to the traditional approach of using the maximal tolerated dose for cancer drug development, but they are clearly consistent with the current recommendation for targeted therapies to avoid unnecessary toxicity by using the lowest biologically effective dose [32]. Ultimately, this experience demonstrates the importance of optimizing the dose of novel targeted agents in early stages of clinical development.
Conclusion
Treatment with GO was associated with notable toxicities, including myelosuppression, infusion reactions, infection, bleeding, and liver toxicity. Overall, however, the available data indicate that the expected clinical benefit outweighs the safety concerns for patients with CD33‐positive R/R AML treated with GO 3 mg/m2 days 1, 4, and 7.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
We thank Professor Sylvie Castaigne for generously sharing the data from MyloFrance 1 for review and Dr. Kristopher Kolibab for expert review management.
Footnotes
For Further Reading: Maria Nieto, Pierre Demolis, Eliane Béhanzin et al. The European Medicines Agency Review of Decitabine (Dacogen) for the Treatment of Adult Patients With Acute Myeloid Leukemia: Summary of the Scientific Assessment of the Committee for Medicinal Products for Human Use. The Oncologist 2016;21:692‐700.
Implications for Practice: Acute myeloid leukemia (AML) remains an area of significant unmet need, especially in older patients. Older patients and those with comorbidities are often considered ineligible for standard induction therapy, and outcome for these patients is poor. Decitabine has favorable effects in terms of overall survival, which were considered clinically meaningful in the context of a manageable toxicity profile and after consideration of the lack of therapeutic alternatives for these patients. Decitabine is widely used in the treatment of AML in patients aged >60 years, as per current guidelines, including the European LeukemiaNet and the U.S. National Cancer Comprehensive Network.
Author Contributions
Conception/design: Kelly J. Norsworthy, Donna Przepiorka
Provision of study material or patients: Kelly J. Norsworthy, Donna Przepiorka
Collection and/or assembly of data: Kelly J. Norsworthy, Jee Eun Lee
Data analysis and interpretation: Kelly J. Norsworthy, Chia‐Wen Ko, Jee Eun Lee, Jiang Liu, Christy S. John
Manuscript writing: Kelly J. Norsworthy, Chia‐Wen Ko, Donna Przepiorka, with edits by Jee Eun Lee, Jiang Liu, Christy S. John, Ann T. Farrell
Final approval of manuscript: Kelly J. Norsworthy, Chia‐Wen Ko, Jee Eun Lee, Jiang Liu, Christy S. John, Donna Przepiorka, Ann T. Farrell, Richard Pazdur
Disclosures
The authors indicated no financial relationships.
References
- 1. Breems DA, Van Putten WL, Huijgens PC et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol 2005;23:1969–1978. [DOI] [PubMed] [Google Scholar]
- 2. Thol F, Schlenk RF, Heuser M et al. How I treat refractory and early relapsed acute myeloid leukemia. Blood 2015;126:319–327. [DOI] [PubMed] [Google Scholar]
- 3. Sarkozy C, Gardin C, Gachard N et al. Outcome of older patients with acute myeloid leukemia in first relapse. Am J Hematol 2013;88:758–764. [DOI] [PubMed] [Google Scholar]
- 4. Itzykson R, Thepot S, Berthon C et al. Azacitidine for the treatment of relapsed and refractory AML in older patients. Leuk Res 2015;39:124–130. [DOI] [PubMed] [Google Scholar]
- 5. Roboz GJ, Rosenblat T, Arellano M et al. International randomized phase III study of elacytarabine versus investigator choice in patients with relapsed/refractory acute myeloid leukemia. J Clin Oncol 2014;32:1919–1926. [DOI] [PubMed] [Google Scholar]
- 6. Bross PF, Beitz J, Chen G et al. Approval summary: Gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin Cancer Res 2001;7:1490–1496. [PubMed] [Google Scholar]
- 7.Mylotarg label 2006 [old Mylotarg drug label]. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/021174s020lbl.pdf. Accessed April 2, 2018.
- 8. Petersdorf SH, Kopecky KJ, Slovak M et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood 2013;121:4854–4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taksin AL, Legrand O, Raffoux E et al. High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: A prospective study of the alfa group. Leukemia 2007;21:66–71. [DOI] [PubMed] [Google Scholar]
- 10. Brethon B, Auvrignon A, Galambrun C et al. Efficacy and tolerability of gemtuzumab ozogamicin (anti‐CD33 monoclonal antibody, CMA‐676, Mylotarg) in children with relapsed/refractory myeloid leukemia. BMC Cancer 2006;6:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thomas X, Le QH, Tavernier E et al. Gemtuzumab ozogamicin (Mylotarg®) as single agent treatment for adult patients with acute myeloid leukemia (AML). Blood 2005;106:4598. 16174759 [Google Scholar]
- 12. Yahagi Y, Usui N, Yamaguchi Y et al. Standard administration and fractionated administration of gemtuzumab ozogamicin for patients with relapsed and refractory acute myeloid leukemia. Blood 2012;120:4342. [Google Scholar]
- 13.FDA Briefing Document: Oncologic Drugs Advisory Committee Meeting, BLA 761060, Mylotarg (gemtuzumab ozogamicin) 2017. Available at https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/UCM566013.pdf. Accessed April 2, 2018.
- 14. Tallman MS, McDonald GB, DeLeve LD et al. Incidence of sinusoidal obstruction syndrome following Mylotarg (gemtuzumab ozogamicin): A prospective observational study of 482 patients in routine clinical practice. Int J Hematol 2013;97:456–464. [DOI] [PubMed] [Google Scholar]
- 15. Reinhardt D, Diekamp S, Fleischhack G et al. Gemtuzumab ozogamicin (Mylotarg) in children with refractory or relapsed acute myeloid leukemia. Onkologie 2004;27:269–272. [DOI] [PubMed] [Google Scholar]
- 16. Reinhardt D, Zwaan CM, Sander A et al. Gemtuzumab ozogamicin in refractory childhood acute myeloid leukemia. Blood 2010;116:1075. [Google Scholar]
- 17. Zwaan CM, Reinhardt D, Corbacioglu S et al. Gemtuzumab ozogamicin: First clinical experiences in children with relapsed/refractory acute myeloid leukemia treated on compassionate‐use basis. Blood 2003;101:3868–3871. [DOI] [PubMed] [Google Scholar]
- 18. Zwaan CM, Reinhardt D, Zimmerman M et al. Salvage treatment for children with refractory first or second relapse of acute myeloid leukaemia with gemtuzumab ozogamicin: Results of a phase II study. Br J Haematol 2010;148:768–776. [DOI] [PubMed] [Google Scholar]
- 19. Jen EY, Ko CW, Lee JE et al. FDA Approval: Gemtuzumab ozogamicin for the treatment of adults with newly‐diagnosed CD33 positive AML. Clin Cancer Res 2018. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 20. Castaigne S, Pautas C, Terre C et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de‐novo acute myeloid leukaemia (ALFA‐0701): A randomised, open‐label, phase 3 study. Lancet 2012;379:1508–1516. [DOI] [PubMed] [Google Scholar]
- 21. Castaigne S, Pautas C, Terré C et al. Final analysis of the ALFA 0701 study. Blood 2014;124:376. [Google Scholar]
- 22. Dinndorf PA, Andrews RG, Benjamin D et al. Expression of normal myeloid‐associated antigens by acute leukemia cells. Blood 1986;67:1048–1053. [PubMed] [Google Scholar]
- 23. Amadori S, Suciu S, Selleslag D et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: Results of the randomized phase III EORTC‐GIMEMA AML‐19 trial. J Clin Oncol 2016;34:972–979. [DOI] [PubMed] [Google Scholar]
- 24. Pollard JA, Loken M, Gerbing RB et al. CD33 expression and its association with gemtuzumab ozogamicin response: Results from the randomized phase III children's oncology group trial AAML0531. J Clin Oncol 2016;34:747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khan N, Hills RK, Virgo P et al. Expression of CD33 is a predictive factor for effect of gemtuzumab ozogamicin at different doses in adult acute myeloid leukaemia. Leukemia 2017;31:1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van der Heiden PL, Jedema I, Willemze R et al. Efficacy and toxicity of gemtuzumab ozogamicin in patients with acute myeloid leukemia. Eur J Haematol 2006;76:409–413. [DOI] [PubMed] [Google Scholar]
- 27. Chen X, Xie H, Wood BL et al. Relation of clinical response and minimal residual disease and their prognostic impact on outcome in acute myeloid leukemia. J Clin Oncol 2015;33:1258–1264. [DOI] [PubMed] [Google Scholar]
- 28. Walter RB, Kantarjian HM, Huang X et al. Effect of complete remission and responses less than complete remission on survival in acute myeloid leukemia: A combined Eastern Cooperative Oncology Group, Southwest Oncology Group, and M. D. Anderson Cancer Center Study. J Clin Oncol 2010;28:1766–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.IDHIFA Prescribing Information 2017. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209606s000lbl.pdf. Accessed April 2, 2018.
- 30.Guidance for Industry: Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics 2007. Available at https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071590.pdf. Accessed April 2, 2018.
- 31. Advani AS, Hunger SP, Burnett AK. Acute leukemia in adolescents and young adults. Semin Oncol 2009;36:213–226. [DOI] [PubMed] [Google Scholar]
- 32. Bullock JM, Rahman A, Liu Q. Lessons learned: Dose selection of small molecule‐targeted oncology drugs. Clin Cancer Res 2016;22:2630–2638. [DOI] [PubMed] [Google Scholar]
- 33. Roboz GJ, Knovich MA, Bayer RL et al. Efficacy and safety of gemtuzumab ozogamicin in patients with poor‐prognosis acute myeloid leukemia. Leuk Lymphoma 2002;43:1951–1955. [DOI] [PubMed] [Google Scholar]
- 34. Piccaluga PP, Martinelli G, Rondoni M et al. Gemtuzumab ozogamicin for relapsed and refractory acute myeloid leukemia and myeloid sarcomas. Leuk Lymphoma 2004;45:1791–1795. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.