This article describes a web‐based survey of the Alliance for Clinical Trials in Oncology membership, including physicians, nurses, patient advocates, project managers, statisticians, leadership, and administrative staff. The goal of the survey was to identify new strategies to effect change in accrual for older patients with cancer by eliciting the opinions of a national sample of the oncology clinical research workforce and patient advocates who participate in high‐impact cancer clinical research.
Keywords: Older adults, Cancer, Clinical trials, Accrual
Abstract
Background.
There are multiple known individual‐ and practice‐level barriers to enrollment of older patients with cancer to clinical trials, but little is known about how the clinical research workforce feels about potential higher‐level strategy changes aimed to promote increased enrollment of older patients.
Subjects, Materials, and Methods.
We invited all 11,351 Alliance for Clinical Trials in Oncology (“Alliance”) members to participate in an anonymous, web‐based survey to examine awareness of current accrual patterns for older patients to clinical trials, to ascertain consensus on how to tackle enrollment challenges, and to provide the impetus for high‐level changes to improve clinical trial accrual of older patients with cancer.
Results.
During the period from February 28, 2017, to June 16, 2017, 1,146 Alliance members participated (response rate = 10%), including a national diverse sample of physicians, nurses, administrative/clinical research staff, and patient advocates with representation from community, academic, and rural sites. Overall, one third felt that >50% of clinical trial enrollees should be age ≥65, and 64.9% felt the Alliance could improve upon enrollment of older patients. The four most commonly ranked strategies to improve enrollment of older patients were creating more dedicated trials for this population (36.3%), minimizing exclusion criteria focused on comorbidity (35.5%), developing independent strategies for those aged ≥65 and for those aged ≥70 (33.2%), and requiring that most/all Alliance trials have a specific expansion cohort of older patients (30.0%).
Conclusion.
We anticipate that the recommendations from >1,000 Alliance members will continue to propel important strategy changes aimed to improve accrual of older patients with cancer to clinical trials.
Implications for Practice.
This survey of the Alliance for Clinical Trials membership sought opinions on potential, large‐scale, national strategies to improve accrual of older adults with cancer. Consensus was found around multiple strategies, including creating more dedicated trials for older patients, developing less stringent eligibility criteria, and mandating expansion cohorts of older patients within broader Alliance trials. It is anticipated that the recommendations from >1,000 Alliance members will continue to propel important strategy changes aimed to improve accrual of older patients with cancer to clinical trials.
Introduction
Cancer is a disease of aging, and the current median age at cancer diagnosis in the U.S. is 66 years, with 53.3% of all new cancer cases diagnosed in those aged ≥65 [1]. With an anticipated increase in U.S. life expectancy over time [2], there will be a concomitant increasing number of older adults who will develop cancer [3], yet accrual of older patients to cancer clinical trials remains challenging and stagnant [4], [5]. Approximately 25% of all trial participants for National Cancer Institute trials during 2000–2011 were aged ≥65 years, and 10% were aged ≥75 [4], [6].
Although older patients have been shown to enroll on research protocols as frequently as younger patients if a cancer clinical trial is offered [7], multiple individual‐ and practice‐level barriers to accrual have been identified; these include comorbidity and toxicity concerns, physician/patient preferences, socioeconomic factors, access to care, concerns about losing continuity with primary oncologists, distance and time considerations, caregiver and transportation factors, and age itself [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19]. Thus far, specific efforts to improve enrollment of under‐represented subgroups with cancer to clinical trials have included educational interventions [20], improved processes for consenting [21], [22], [23], [24], conferences and policy statements to promote change [4], [25], [26], [27], [28], and the development of trials dedicated to older adults [29], [30], [31]. None of these strategies, however, has had a major impact on accrual of older patients. In addition, previous studies have examined the impact of doctor communication skills training [32], oncology nurse navigation [33], and improved tracking systems [34] with mixed success.
Although multiple studies have described potential barriers to accrual of patients in practice, there are limited data on how providers feel about potential strategies to effect change in accrual. In one relevant survey of 156 oncologists from 10 high‐accruing cancer centers within the Cancer and Leukemia Group B (CALGB) [8], providers were asked about barriers to accrual of older patients with breast cancer in practice, how they would rank their importance, and their opinions about seven possible interventions to improve accrual, including education of staff and patients and personnel issues. In this survey, 25% of providers endorsed making personnel available in the clinic to explain clinical trials to elderly breast cancer patients and their families as the most important intervention. Additionally, 13% felt that providing patients with better educational materials concerning clinical trials was most important, and 14% felt that providing transportation was most important [8]. Although this survey was informative on a provider and practice level, it only surveyed physicians and did not provide clear direction regarding what larger‐scale intervention changes might facilitate accrual of elderly patients. In addition, none of the above‐mentioned intervention studies has led to clear improvements in accrual or policy changes around accrual of older adults to clinical trials.
In this study, to gain perspective on potential changes in strategies that would effect change in accrual for older patients with cancer, we conducted a web‐based survey of the entire Alliance for Clinical Trials in Oncology (“Alliance”) membership, including physicians, nurses, patient advocates, project managers, statisticians, leadership, and administrative staff. CALGB is now part of the Alliance for Clinical Trials in Oncology, a network group in the NIH National Clinical Trials Network. Our goal was to further propel implementation of new strategies by eliciting the opinions of a national sample of the oncology clinical research workforce and patient advocates who participate in high‐impact cancer clinical research.
Subjects, Materials, and Methods
Survey Content
Through our survey, we aimed to assess the awareness of the current accrual of older patients with cancer to clinical trials, to ascertain whether there is consensus on how to tackle enrollment challenges on a national level, and to provide an impetus to apply new, large‐scale strategy changes to improve clinical trial accrual of older patients within the National Clinical Trials Network. We developed a 26‐question survey (full survey provided in supplemental online data) after obtaining input on questions from the Alliance Cancer in the Elderly Committee. The survey asked participants to report their opinions on the current state of accrual of older patients with cancer to clinical trials in the Alliance, at what age(s) they consider a person to be “elderly,” and whether they think we can impact accrual of older patients within the Alliance, as well as demographics about their practice setting, gender, what Alliance committee(s) they participate in, their professional position, and years in practice (when relevant). We also asked about individual‐ and practice‐level barriers to enrolling older patients in clinical practice, and we asked participants to rank up to four of these barriers, with number 1 identifying the most common/important barrier to accrual. Similarly, we asked participants to select and rank up to four large‐scale interventions that they felt would promote enrollment of older patients with cancer. At the end of the survey, participants were asked if they would like to enter a drawing for a chance to win a $100 Amazon gift card as a token of their appreciation. Five of these gift cards were distributed randomly to survey participants. The survey was delivered in a web‐based link using Qualtrics (www.qualtrics.com), allowing for sophisticated survey design and analysis. Because of the nature of our study, we received exemption from review by the Dana‐Farber Cancer Institute Office for Human Research Studies.
Survey Administration
We invited all rostered Alliance members to participate in this survey using a blast e‐mail invitation sent centrally from the Alliance Central Protocol Operations Office in Chicago, IL. This e‐mail was sent four times, asking members to complete a brief online, one‐time, anonymous, and confidential survey. We provided a direct link to the survey in the invitation with the ability to complete it on a computer or a mobile device. The initial e‐mail invitation was sent twice to 11,351 members on February 28, 2017, with e‐mail reminders sent on March 9, 2017, and March 16, 2017. We also provided a one‐page paper survey invitation/reminder with the registration materials at the in‐person Alliance meeting in Chicago, IL, in May 2017. All answers were automatically tabulated by the Qualtrics program software and aggregated for analyses; analyses were conducted by the Alliance Statistics and Data Center.
Statistical Analysis
Responses for the questions and demographics were analyzed descriptively. Due to the high frequency and variety of Alliance member type and disease specialty, we collapsed some of these categories for ease of analyses (see Table 1 for how these were defined). We compared the frequencies of responses by demographic factors (supplemental online Table 1) for the following questions using a chi‐square test or Fisher's exact test: (a) At what age do you consider a patient to be “elderly”? (select all that apply). (b) What do you think should be the “right” or appropriate proportion of trial enrollees who are age 65 or older? (c) Do you think accrual of older patients to clinical trials in the Alliance is something we need to improve upon? (d) Do you feel that the geriatric assessment should be incorporated into all Alliance trials?
Table 1. Participant demographics (n = 1,146).
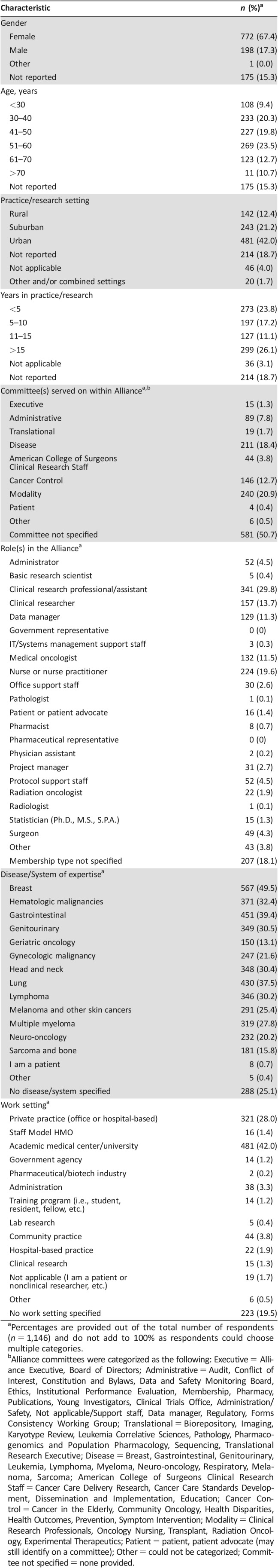
Percentages are provided out of the total number of respondents (n = 1,146) and do not add to 100% as respondents could choose multiple categories.
Alliance committees were categorized as the following: Executive = Alliance Executive, Board of Directors; Administrative = Audit, Conflict of Interest, Constitution and Bylaws, Data and Safety Monitoring Board, Ethics, Institutional Performance Evaluation, Membership, Pharmacy, Publications, Young Investigators, Clinical Trials Office, Administration/Safety, Not applicable/Support staff, Data manager, Regulatory, Forms Consistency Working Group; Translational = Biorepository, Imaging, Karyotype Review, Leukemia Correlative Sciences, Pathology, Pharmacogenomics and Population Pharmacology, Sequencing, Translational Research Executive; Disease = Breast, Gastrointestinal, Genitourinary, Leukemia, Lymphoma, Myeloma, Neuro‐oncology, Respiratory, Melanoma, Sarcoma; American College of Surgeons Clinical Research Staff = Cancer Care Delivery Research, Cancer Care Standards Development, Dissemination and Implementation, Education; Cancer Control = Cancer in the Elderly, Community Oncology, Health Disparities, Health Outcomes, Prevention, Symptom Intervention; Modality = Clinical Research Professionals, Oncology Nursing, Transplant, Radiation Oncology, Experimental Therapeutics; Patient = patient, patient advocate (may still identify on a committee); Other = could not be categorized; Committee not specified = none provided.
The frequencies of rankings for the perceived barriers to accrual in practice and the potential strategies for improved accrual of older adults were summarized and differences in rankings were also compared by demographic factors utilizing a chi‐square test or Fisher's exact test (supplemental online Table 1). Demographic factors used for these analyses included the following: gender (male vs. female), participant role within the Alliance (clinician vs. nonclinician), age group (≤50 vs. >50 years of age), years of clinical/research experience (≤10 vs. >10 years of experience), and practice/research setting (rural vs. suburban vs. urban).
Results
Survey Participants (Table 1)
Among the 11,351 Alliance members initially contacted, 1,146 participated in the survey (response rate = 10%) during the period from February 28, 2017, to June 16, 2017. Respondents (Table 1) were mostly female (67.4%); 29.7% were aged ≤40 years, and 23.4% were aged ≥61. Overall, 42.0% reported practicing in an urban setting and 12.4% in a rural setting, with 17.2% and 26.1% of all participants reporting being in practice or research for 5–10 years and >15 years, respectively. Overall, 581 participants did not report that they served on a specific committee within the Alliance, and there were many who reported membership on multiple committees. With regard to their roles, the most common responses were the following: 29.8% were clinical research professionals, 19.6% were nurses or nurse practitioners, 13.7% were clinical researchers, and 11.5% were medical oncologists (overlap of responses allowed). Most worked in academic (42.0%) or private practice (28.0%) settings, and the most commonly reported disease areas of expertise included breast (49.5%), gastrointestinal (39.4%), lung (37.5%), and hematologic malignancies (32.4%).
Current State of Clinical Trial Accrual for Older Patients with Cancer (Table 2)
Table 2. Opinions on current state of accrual for older patients with cancer (n = 1,146).
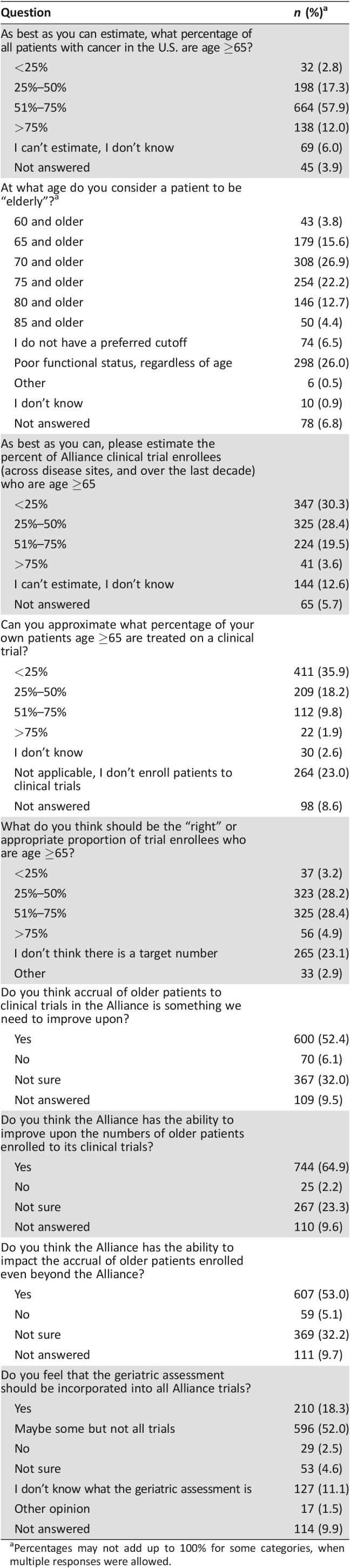
Percentages may not add up to 100% for some categories, when multiple responses were allowed.
Participants acknowledged that most cancers occur in older patients, with 57.9% responding correctly that 51%–75% of U.S. cancer diagnoses occur in individuals age 65 and older. There was variability in responses for the age cutoffs for when participants consider a patient to be “elderly” (categories were not mutually exclusive), with 179 (15.6%) and 308 (26.9%) participants reporting that this should include patients age ≥65 and ≥70 and older, respectively. In addition, 298 (26.0%) felt that “elderly” should be defined by functional age rather than chronological age. Approximately 35.9% of participants reported that <25% of their own patients ages ≥65 participate in cancer clinical trials, whereas 1.9% reported that >75% of their older patients participate; 28.4% felt that 51%–75% of cancer clinical trials enrollees should be age ≥65. Overall, 64.9% felt that accrual of older patients to cancer clinical trials in the Alliance is something we can improve upon, and 18.3% felt that the geriatric assessment [35] should be included in all Alliance trials. However, 11.1% of respondents reported that they did not know what a geriatric assessment is.
Barriers to Accrual (Table 3)
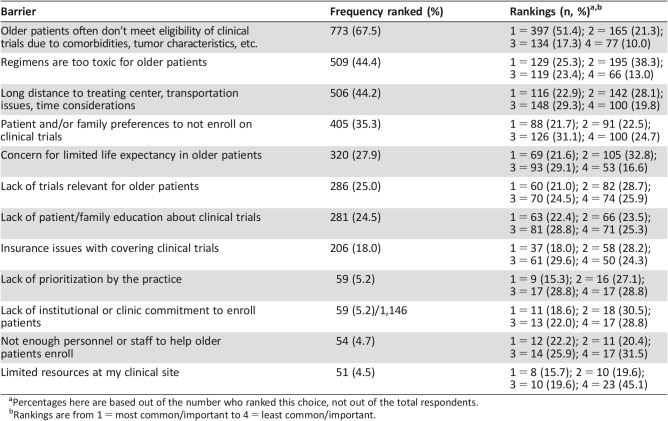
Table 3. Barriers to accrual of older patients to clinical trials in practice in order of frequency ranked.
Percentages here are based out of the number who ranked this choice, not out of the total respondents.
Rankings are from 1 = most common/important to 4 = least common/important.
The most commonly reported barriers to accrual of older patients in practice included “older patients often don't meet eligibly requirements due to comorbidities or tumor characteristics” (n = 773 [67.5%], with 397 ranking this as the number 1 barrier); “regimens are too toxic for older patients” (n = 509 [44.4%], with 129 ranking this as the number 1 barrier); “long distance to treating center, transportation issues, time considerations” (n = 506 [44.2%], with 116 participants ranking this as the number 1 barrier); and “patient and/or family preferences to not enroll on clinical trials” (n = 405 [35.3%], with 88 ranking this as the number 1 barrier).
In a separate question, we asked participants to expand on barriers in their practice and within the Alliance beyond the options provided, and we received 158 written‐in responses (data not shown). Recurrent themes from these responses include the following concerns/suggestions: cost and insurance, exclusions with regard to past history of cancer and other medical conditions, complex and lengthy protocol consents, the intensity of schedules and visits, education gaps for patients and their families, fear or anxiety related to clinical trials, discrimination within the health care system, the feeling that trials are not publicized enough and should be offered in partnership with relevant organizations such as the American Association of Retired Persons to increase publicity of trials, and the need for more supportive care trials and trials aimed at quality of life and function.
Strategies to Improve Older Patient Accrual (Table 4)
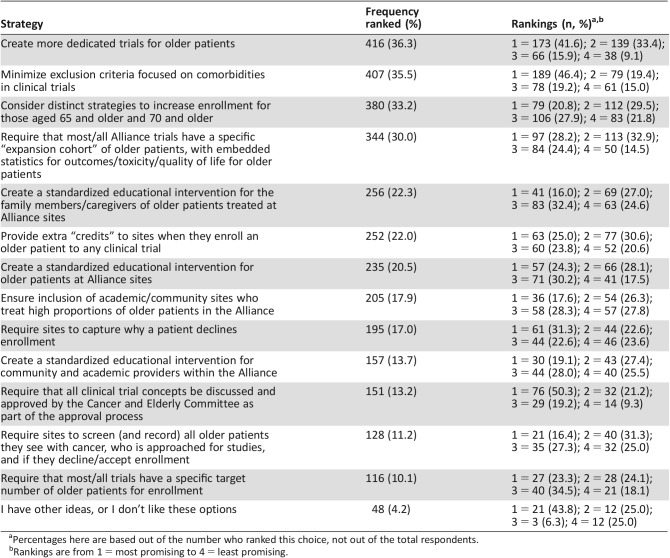
Table 4. Strategies to improve older patient accrual in order of frequency ranked.
Percentages here are based out of the number who ranked this choice, not out of the total respondents.
Rankings are from 1 = most promising to 4 = least promising.
The most commonly ranked strategies for improvement of enrollment of older patients included the following: “create more dedicated trials for older patients” (n = 416 [36.3%], with 173 ranking it as the number 1 strategy); “minimize exclusion criteria focused on comorbidities in clinical trials” (n = 407 [35.5%], with 189 ranking it as the number 1 strategy); “consider distinct strategies to increase enrollment for those aged 65 and older and 70 and older” (n = 380 [33.2%], with 79 ranking it as number 1); and “require that most/all Alliance trials have a specific ‘expansion cohort’ of older patients, with embedded statistics for outcomes/toxicity/quality of life for older patients” (n = 344 [30.0%], with 97 ranking it as number 1).
Associations of Responses with Participant Demographics (Supplemental Online Table 1)
Regarding responses by participants’ gender, we observed significant differences in the responses for the appropriate percentage of clinical trial enrollees who should be aged ≥65 (p = .015; e.g., 27.2% of women vs. 16.2% of men felt there was no target proportion of older patients who should enroll). In addition, most men (68.2%) and women (56.5%) responded that we need to improve upon accrual of older patients. Men more frequently (23.2% vs. 19.8% in women) responded that the geriatric assessment should be included in all clinical trials, although it is of note that <25% of either gender responded this way. Differences between men and women for reported barriers to clinical trial enrollment in practice included “not enough trials relevant for older patients” (38.4% of men vs. 26.3% of women, p = .0008) and “not enough personnel to help patients enroll” (10.1% of men vs. 4.3% of women, p = .001; data not shown). There were no gender differences in opinions on strategies to improve accrual except the suggestion of providing extra credits to sites when they enroll older patients (37.4% of men vs. 23.1% of women, p < .0001; data not shown).
With regard to clinicians versus nonclinicians, clinicians more frequently responded that we need to improve upon accrual of older patients (73.0% vs. 48.9%, p < .0001) and were more likely to respond that we should include geriatric assessment in all trials (23.5% vs. 18.2%, p = .026). Nonclinicians more frequently reported that “elderly” are those aged ≥65 and less frequently stated that “elderly” is determined by poor functional status. There were also significant differences in preferred strategies to improve accrual, with 48.3% of clinicians selecting the option to “create more dedicated trials for older patients” versus 39.2% of nonclinicians (p = .005). Additional findings for differences in responses by participants’ age, years of experience, and practice setting are summarized in supplemental online Table 1.
Discussion
In this study of 1,146 participants representing a national and diverse sample of clinical researchers, including providers, nurses, scientists, patient advocates, clinical and administrative support staff, and leadership who treat multiple cancers in a wide array of practice settings, we observed that most felt that the Alliance has the power to effect change with regard to clinical trial enrollment of older patients with cancer and agreed that older patients should be enrolled on cancer trials more frequently. Similar to what has been suggested in prior calls to action [26], participants felt that the ideal large‐scale strategies to improve accrual include a specific focus on this older cancer population: creating more dedicated trials for older patients, relaxing eligibility criteria so that older patients are not excluded from trials as frequently (reported as the limiting barrier to accrual in practice for over two thirds of survey participants), and recommending that clinical trials include expansion cohorts to specifically accrue older patients. Providing a standardized education tool for patients and family members/caregivers was also appealing for many survey participants. Participants also differed in their opinions based on demographic characteristics, with some differences noted by gender, position/role, age, and years of experience.
Our survey addressed important knowledge gaps and is the first‐of‐its‐kind in its execution of a “needs assessment” for high‐level strategies by those in the trenches of clinical research, protocol design, and patient care. Although Kornblith and colleagues asked providers about their preferences for potential interventions within their own breast cancer clinical setting [8], to our knowledge, no prior study has examined the opinions of all Alliance members, including patient advocates and nonclinicians, about potential structured high‐level changes that could more globally promote enrollment of older patients with cancer to clinical trials across multiple disease sites. Through this survey, we harnessed the opinions of over 1,000 Alliance leaders, patient advocates, statisticians, clinicians, and clinical trials support staff providing a wealth of information on how the Alliance can effect change, further reinforcing ongoing initiatives and prior pleas for action [4], [36]. For example, the American Society of Clinical Oncology (ASCO) assembled a working group [27], [28] to address the issues surrounding stringent eligibility criteria for older patients, and we anticipate that their recommendations to relax criteria for organ dysfunction in particular will be widely adopted, disseminated, and implemented across the National Clinical Trials Network. Further, the U.S. Food and Drug Administration and ASCO recently led a Geriatric Oncology Workshop focused on these issues, which we hope will move the needle on accrual issues for older adults with cancer.
Aside from the efforts to relax eligibility described above and despite multiple calls to action to improve the evidence base for older patients with cancer [4], [5], [17], [25], [26], [37], little has been accomplished on a policy level to effect change. As a next step, a multipronged strategy will be required if we want to make increased accrual a reality for older patients with cancer on a national level. This will include earnest cooperation, commitment, and prioritization from funding agencies, industry, the U.S. Food and Drug Administration, the National Cancer Institute, the National Clinical Trials Network, and clinical and research leadership, particularly because of the high anticipated costs of implementing large‐scale strategies to improve accrual. Our survey results promote the implementation of a more standardized process for protocol development by which each clinical trial undergoes a specific review by a geriatric‐focused committee, including relevant statisticians. With this, eligibility criteria can be scrutinized to promote optimal inclusion of older patients with cancer and endpoints can be assured to be relevant to older adults. In addition, recommendations can be made to create an expansion cohort of older patients if a cancer treatment is found to be efficacious with broad implementation expected. Creating more education tools for this patient population and their caregivers is also warranted based on our results.
We acknowledge study limitations. First, although we obtained responses from a large sample of participants, we recognize that our member response rate was only 10% and that some subgroups were small and had potential for nonresponse bias. Because the survey was anonymous, we could not compare the demographics of those who participated to those who did not. However, participants came from a wide array of research backgrounds, ages, practice sites, and positions, all strengthening our findings and providing important data for clinical trial leadership and policy makers in the U.S. Further, we surveyed Alliance members only, although we had representation and inclusion of both academic and community sites as well as rural and urban centers. It is reassuring that the opinions of Alliance membership mirror those of the current national conversations to improve accrual of older patients.
Conclusion
The results from our survey of national stakeholders should catalyze change, with concrete strategies provided by the Alliance membership that can, we hope, translate into significant improvements in the evidence base for treatments of this growing subgroup of patients who are in urgent need of level I evidence to inform their care.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
We thank all the study participants as well as Jamilah Owens and the Alliance for Clinical Trials Central Protocol Operations Office for their assistance with survey administration. We also thank the Alliance Cancer in the Elderly Committee for their assistance in survey development and for their support for this study. This study was supported by the National Cancer Institute of the National Institutes of Health under the Award Number UG1CA189823 (Alliance for Clinical Trials in Oncology NCORP Grant). R.A.F. is supported by the American Cancer Society (125912‐MRSG‐14‐240‐01‐CPPB) and Susan G. Komen (CCR CCR14298143).
Author Contributions
Conception/design: Rachel A. Freedman, Travis J. Dockter, Jacqueline M. Lafky, Arti Hurria, Hyman J. Muss, Harvey J. Cohen, Aminah Jatoi, M. Margaret Kemeny, Kathryn J. Ruddy
Provision of study material or patients: Rachel A. Freedman
Collection and/or assembly of data: Rachel A. Freedman, Travis J. Dockter, Kathryn J. Ruddy
Data analysis and interpretation: Rachel A. Freedman, Travis J. Dockter, Jacqueline M. Lafky, Arti Hurria, Hyman J. Muss, Harvey J. Cohen, Aminah Jatoi, M. Margaret Kemeny, Kathryn J. Ruddy
Manuscript writing: Rachel A. Freedman, Travis J. Dockter, Jacqueline M. Lafky, Arti Hurria, Hyman J. Muss, Harvey J. Cohen, Aminah Jatoi, M. Margaret Kemeny, Kathryn J. Ruddy
Final approval of manuscript: Rachel A. Freedman, Travis J. Dockter, Jacqueline M. Lafky, Arti Hurria, Hyman J. Muss, Harvey J. Cohen, Aminah Jatoi, M. Margaret Kemeny, Kathryn J. Ruddy
Disclosures
Arti Hurria: Pierian Biosciences, MJH Healthcare Holdings, LLC, Boehringer Ingelheim Pharmaceuticals (C/A), Celgene, Novartis (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.National Cancer Institute . Surveillance, Epidemiology, and End Results Program. Cancer Fast Facts: Cancer of Any Site. Statistics at a Glance. Available at https://seer.cancer.gov/statfacts/html/all.html. Accessed September 14, 2017.
- 2.U.S. Census Bureau. Available at http://www.census.gov/. Accessed July 17, 2015.
- 3. Smith BD, Smith GL, Hurria A et al. Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J Clin Oncol 2009;27:2758–2765. [DOI] [PubMed] [Google Scholar]
- 4. Hurria A, Dale W, Mooney M et al. Designing therapeutic clinical trials for older and frail adults with cancer: U13 conference recommendations. J Clin Oncol 2014;32:2587–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Freedman RA, Foster JC, Seisler DK et al. Accrual of older patients with breast cancer to Alliance Systemic Therapy Trials over time: Protocol A151527. J Clin Oncol 2017;35:421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mohile S, Hurria A, Dale W. Introduction to U13 supplement. J Geriatr Oncol 2016;7:223–224. [DOI] [PubMed] [Google Scholar]
- 7. Kemeny MM, Peterson BL, Kornblith AB et al. Barriers to clinical trial participation by older women with breast cancer. J Clin Oncol 2003;21:2268–2275. [DOI] [PubMed] [Google Scholar]
- 8. Kornblith AB, Kemeny M, Peterson BL et al. Survey of oncologists' perceptions of barriers to accrual of older patients with breast carcinoma to clinical trials. Cancer 2002;95:989–996. [DOI] [PubMed] [Google Scholar]
- 9. Javid SH, Unger JM, Gralow JR et al. A prospective analysis of the influence of older age on physician and patient decision‐making when considering enrollment in breast cancer clinical trials (SWOG S0316). The Oncologist 2012;17:1180–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lara PN Jr, Higdon R, Lim N et al. Prospective evaluation of cancer clinical trial accrual patterns: Identifying potential barriers to enrollment. J Clin Oncol 2001;19:1728–1733. [DOI] [PubMed] [Google Scholar]
- 11. Lewis JH, Kilgore ML, Goldman DP et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol 2003;21:1383–1389. [DOI] [PubMed] [Google Scholar]
- 12. Hutchins LF, Unger JM, Crowley JJ et al. Underrepresentation of patients 65 years of age or older in cancer‐treatment trials. N Engl J Med 1999;341:2061–2067. [DOI] [PubMed] [Google Scholar]
- 13. Simon MS, Du W, Flaherty L et al. Factors associated with breast cancer clinical trials participation and enrollment at a large academic medical center. J Clin Oncol 2004;22:2046–2052. [DOI] [PubMed] [Google Scholar]
- 14. Gross CP, Filardo G, Mayne ST et al. The impact of socioeconomic status and race on trial participation for older women with breast cancer. Cancer 2005;103:483–491. [DOI] [PubMed] [Google Scholar]
- 15. Unger JM, Hershman DL, Albain KS et al. Patient income level and cancer clinical trial participation. J Clin Oncol 2013;31:536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Basche M, Baron AE, Eckhardt SG et al. Barriers to enrollment of elderly adults in early‐phase cancer clinical trials. J Oncol Pract 2008;4:162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kimmick G. Clinical trial accrual in older cancer patients: The most important steps are the first ones. J Geriatr Oncol 2016;7:158–161. [DOI] [PubMed] [Google Scholar]
- 18. Ayodele O, Akhtar M, Konenko A et al. Comparing attitudes of younger and older patients towards cancer clinical trials. J Geriatr Oncol 2016;7:162–168. [DOI] [PubMed] [Google Scholar]
- 19. Avis NE, Smith KW, Link CL et al. Factors associated with participation in breast cancer treatment clinical trials. J Clin Oncol 2006;24:1860–1867. [DOI] [PubMed] [Google Scholar]
- 20. Kimmick GG, Peterson BL, Kornblith AB et al. Improving accrual of older persons to cancer treatment trials: A randomized trial comparing an educational intervention with standard information: CALGB 360001. J Clin Oncol 2005;23:2201–2207. [DOI] [PubMed] [Google Scholar]
- 21. Coyne CA, Xu R, Raich P et al. Randomized, controlled trial of an easy‐to‐read informed consent statement for clinical trial participation: A study of the Eastern Cooperative Oncology Group. J Clin Oncol 2003;21:836–842. [DOI] [PubMed] [Google Scholar]
- 22. Treweek S, Lockhart P, Pitkethly M et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta‐analysis. BMJ Open 2013;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Treweek S, Pitkethly M, Cook J et al. Strategies to improve recruitment to randomised controlled trials. Cochrane Database Syst Rev 2010:MR000013. [DOI] [PubMed] [Google Scholar]
- 24. Hack TF, Whelan T, Olivotto IA et al. Standardized audiotape versus recorded consultation to enhance informed consent to a clinical trial in breast oncology. Psychooncology 2007;16:371–376. [DOI] [PubMed] [Google Scholar]
- 25. Hurria A, Cohen HJ, Extermann M. Geriatric oncology research in the cooperative groups: A report of a SIOG special meeting. J Geriatr Oncol 2010;1:40–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hurria A, Levit LA, Dale W et al. Improving the evidence base for treating older adults with cancer: American Society of Clinical Oncology statement. J Clin Oncol 2015;33:3826–3833. [DOI] [PubMed] [Google Scholar]
- 27. Lichtman SM, Harvey RD, Damiette Smit MA et al. Modernizing clinical trial eligibility criteria: Recommendations of the American Society of Clinical Oncology‐Friends of Cancer Research Organ Dysfunction, Prior or Concurrent Malignancy, and Comorbidities Working Group. J Clin Oncol 2017;35:3753–3759. [DOI] [PubMed] [Google Scholar]
- 28. Kim ES, Bruinooge SS, Roberts S et al. Broadening Eligibility Criteria to Make Clinical Trials More Representative: American Society of Clinical Oncology and Friends of Cancer Research Joint Research Statement. J Clin Oncol 2017;35:3737–3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Muss HB, Berry DA, Cirrincione CT et al. Adjuvant chemotherapy in older women with early‐stage breast cancer. N Engl J Med 2009;360:2055–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hughes KS, Schnaper LA, Bellon JR et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: Long‐term follow‐up of CALGB 9343. J Clin Oncol 2013;31:2382–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hughes KS, Schnaper LA, Berry D et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med 2004;351:971–977. [DOI] [PubMed] [Google Scholar]
- 32. Brown RF, Butow PN, Boyle F et al. Seeking informed consent to cancer clinical trials; evaluating the efficacy of doctor communication skills training. Psychooncology 2007;16:507–516. [DOI] [PubMed] [Google Scholar]
- 33. Holmes DR, Major J, Lyonga DE et al. Increasing minority patient participation in cancer clinical trials using oncology nurse navigation. Am J Surg 2012;203:415–422. [DOI] [PubMed] [Google Scholar]
- 34. Carpenter WR, Tyree S, Wu Y et al. A surveillance system for monitoring, public reporting, and improving minority access to cancer clinical trials. Clin Trials 2012;9:426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hurria A, Cirrincione CT, Muss HB et al. Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. J Clin Oncol 2011;29:1290–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wildiers H, Mauer M, Pallis A et al. End points and trial design in geriatric oncology research: A joint European Organisation for Research and Treatment of Cancer–Alliance for Clinical Trials in Oncology–International Society Of Geriatric Oncology position article. J Clin Oncol 2013;31:3711–3718. [DOI] [PubMed] [Google Scholar]
- 37. Hurria A, Naylor M, Cohen HJ. Improving the quality of cancer care in an aging population: Recommendations from an IOM report. JAMA 2013;310:1795–1796. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.