Deficiencies in the DNA mismatch repair (MMR) system cause errors during DNA replication, which in turn give rise to microsatellite instability (MSI). This article reports a retrospective cohort study conducted within the Mayo Clinic Rochester campus from 1992 to 2016, which compared patients with high MSI metastatic colorectal cancer with patients with microsatellite‐stable tumors.
Keywords: High microsatellite instability, Metastatic colorectal cancer, Metastasectomy, Microsatellite‐stable
Abstract
Background.
Deficiencies in the DNA mismatch repair system cause errors during DNA replication, which in turn give rise to microsatellite instability (MSI). The impact of MSI on survival in metastatic colorectal cancer (mCRC) is unclear. This cohort study aims to investigate the prognostic and predictive value of MSI in mCRC prior to the immune therapy era.
Materials and Methods.
A total of 75 MSI‐high (MSI‐H) mCRC patients (pts) and 75 matched (age, gender, disease sidedness, metachronous/synchronous) microsatellite‐stable (MSS) mCRC pts were identified from 1,268 mCRC pts who had MSI/mismatch repair test results at Mayo Clinic Rochester between January 1992 and July 2016. A retrospective review was conducted by using data from electronic medical records. Statistical analyses utilized the Kaplan‐Meier method, log‐rank test, and Cox proportional hazards models.
Results.
The MSS group was well matched to the MSI‐H group based on age, gender, location, and chronicity of metastatic disease. MSI‐H mCRC pts had earlier disease recurrence (median time from initial diagnosis to metastatic disease diagnosis, MSI‐H group 12.9 vs. MSS group 20.9 months, p = .034). Median overall survival (OS) was 28.1 and 37.4 months for MSI‐H and MSS pts, respectively (p = .99). In total, 94.7% of MSI‐H pts and 98.7% of MSS pts had fluoropyrimidine‐based chemotherapy for metastatic disease, and there was no difference in OS between these two groups (32.3 vs. 37.4 months, p = .91). Forty‐three MSI‐H and thirty‐nine MSS pts had metastasectomy and/or ablation of metastases (p = .51) with longer median OS compared with pts without metastasectomy (MSI‐H: 82.0 vs. 13.9, p < .001; MSS: 69.9 vs. 19.7, p < .001). Age <65 years, BRAF wild type, and metastasectomy were associated with better OS in univariate analysis. Only metastasectomy remained statistically significant in multivariate analysis (p < .001).
Conclusion.
In mCRC, patients with MSI‐H tumors have similar, but numerically shorter, median overall survival compared with those with MSS tumors. In both groups, metastasectomy and ablation of metastatic disease should be considered to optimize OS.
Implications for Practice.
This study clearly demonstrated the survival benefits that aggressive metastasectomy provides in selected microsatellite instability‐high metastatic colorectal cancer patients. This could be meaningful practice‐changing information that has been long awaited.
Introduction
Deficiencies in the DNA mismatch repair (MMR) system cause errors during DNA replication, which in turn give rise to microsatellite instability (MSI). Microsatellites, which are simple repeat sequences of one to six base pairs (also known as short tandem repeats), are particularly prone to DNA replication errors [1]. MSI manifests as small increases or decreases (“instability”) in the number of repeats in microsatellites throughout the genome because of defects in MMR genes [1]. MMR proteins including MLH1, PMS2, MSH2, and MSH6 can form heterodimers to correct these mismatches. Mutations in any of these MMR genes, as well as in EpCAM (which leads to loss of MSH2 expression), can cause truncated protein products that result in accumulation of mismatch mutations and, subsequently, tumorigenesis [2]. These unrepaired alterations contribute to carcinogenesis along a distinct pathway (the MSI pathway) that differs from the chromosomal instability pathway.
About 15% of colorectal cancers harbor MMR defects [3], [4]. Among these cases, about 20% are caused by Lynch syndrome, which is characterized by germline mutations in the mismatch repair genes. The majority of cases (80%) are caused by sporadic hypermethylation of the MLH1 gene promoter [2], [3], [4], [5], [6], [7]. As the result of deficient MMR (dMMR), mismatched nucleotides are incorporated into the cell DNA so that as many as 10–100 times more somatic mutations are detected in dMMR colorectal cells compared with those with normal MMR [8], [9]. Somatic mosaicism and double somatic hits can also lead to MSI colorectal cancer [10].
The standard diagnostic procedure recommended by the National Cancer Institute/International Collaborative Group‐hereditary non‐polyposis colorectal cancer (HNPCC) occupies a set of five microsatellite markers. Accordingly, tumors with presence of MSI are classified as either MSI‐high (MSI‐H) or MSI‐low (MSI‐L) depending on the extent of instability in the markers tested, whereas tumors without this characteristic are classified as microsatellite‐stable (MSS). Meanwhile, immunohistochemistry staining for MMR proteins is widely used and is also recommended by current guidelines for the diagnosis of dMMR colorectal cancer. Tumors that have dMMR status are biologically the same population as those with MSI‐H status [11], [12].
Prognostic Value of MSI‐H
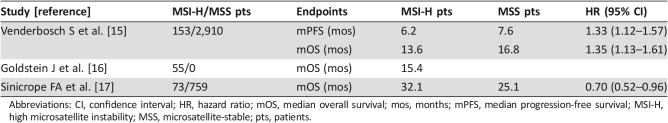
MSI‐H status in colorectal cancer is associated with improved stage‐specific survival in localized disease [13], [14]; however, its prognostic value in the metastatic setting is not clear (Table 1). One pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies revealed that 5% of metastatic colorectal cancer (mCRC) patients had dMMR, and 34.6% of these cancers had a BRAF V600E mutation (suggesting sporadic MSI‐H). Median progression‐free survival and overall survival (OS) were significantly worse for patients with dMMR cancers [15]. However, a retrospective review of 55 patients with MSI‐H mCRC from two institutes suggested that there is no statistically significant difference in OS when compared with historical data [16]. Most recently, a secondary analysis of two randomized clinical studies suggested that dMMR was associated with better survival after recurrence [17].
Table 1. Prognostic value of MSI‐H status in metastatic CRC.
Abbreviations: CI, confidence interval; HR, hazard ratio; mOS, median overall survival; mos, months; mPFS, median progression‐free survival; MSI‐H, high microsatellite instability; MSS, microsatellite‐stable; pts, patients.
Predictive Value of MSI‐H
The predictive value of MSI‐H in colorectal cancer is also debated [18], [19], [20], [21], [22]. In stage II and III CRC patients, adjuvant fluorouracil treatment in MSI‐H patients was not associated with an increase but possibly even a decrease in OS [23]. Further analysis from 165 MSI‐H stage II and III CRC patients revealed that adjuvant fluorouracil treatment did not show disease‐free survival benefit, and subgroup analysis from this study showed a trend of detrimental effect of adjuvant fluorouracil treatment in stage II CRC (p = .09) and no benefit in stage III CRC (p = .98) compared with surgery alone [24]. However, in the stage II adjuvant CRC (QUASAR) study, only the prognostic value but not predictive value of MSI status was confirmed [25]. Furthermore, a large study reported that fluorouracil‐based adjuvant treatment was associated with better clinical outcomes (reduced distant and all‐site recurrence) in a group of 2,141 stage II/III CRC patients. Moreover, these data suggested that the benefit of fluorouracil‐based adjuvant treatment was restricted to CRC in which MSI originated from a germline defect [21].
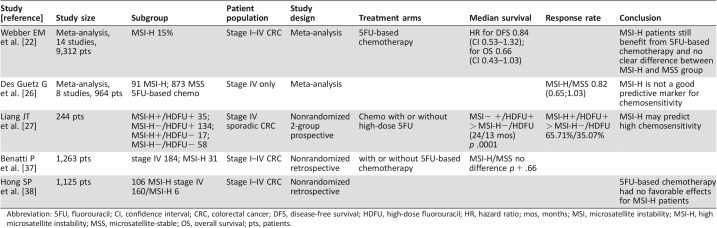
The data regarding predictive value of MSI status in mCRC are sparse and inconsistent (Table 2) [20], [24], [25], [26], [27]. A meta‐analysis showed that MSI did not predict the efficacy of chemotherapy in patients with mCRC [26]. But in a nonrandomized prospective study in which fluorouracil chemotherapy use was determined by each individual patient, there was an improved median OS for the 35 MSI‐H patients and the 134 MSS patients who received chemotherapy (24 vs. 13 months, p < .001, respectively) [27].
Table 2. Predictive value of MSI‐H status in metastatic CRC patients who received fluorouracil‐based chemotherapy.
Abbreviation: 5FU, fluorouracil; CI, confidence interval; CRC, colorectal cancer; DFS, disease‐free survival; HDFU, high‐dose fluorouracil; HR, hazard ratio; mos, months; MSI, microsatellite instability; MSI‐H, high microsatellite instability; MSS, microsatellite‐stable; OS, overall survival; pts, patients.
A better understanding of the prognostic and predictive value of dMMR mCRC is warranted to help us adjust our treatment approach for these patients with better clinical outcome. In this study, we sought to determine the prognostic and predictive value of dMMR/MSI‐H and other molecular alterations associated with outcome in mCRC patients treated at Mayo Clinic.
Materials and Methods
Patient Selection
We identified 1,268 patients with mCRC who had testing for MSI or dMMR between January 1, 1992, and July 1, 2016. Of those, 75 patients were found to have tumors with MSI‐H mCRC. These patients were matched with 75 patients with MSS mCRC based on age (±10 years), gender, primary CRC side of origin, and timing of presentation (synchronous or metachronous, which is defined as no metastatic disease detected at the time of primary cancer diagnosis). Matching limitations between groups were as follows: six patients with age disparity of more than 10 years, two for gender, and one for metachronous mCRC.
Data Collection
An orienting medical records review was first done for 1,268 patients to record the MSI/MSS status, age, gender, disease sidedness, and synchronous/metachronous timing of metastatic disease. A detailed medical records review was done in the identified matched 150 patients to collect demographic data, tumor characteristics, treatment types, treatment responses, and follow‐up information. Response evaluation was based on the treating physician's assessment. This study was approved by the Mayo Clinic Institutional Review Board.
Molecular Testing
The MMR protein expression of MLH1, MSH2, PMS2, and MSH6 were analyzed in formalin‐fixed, paraffin‐embedded tumor tissues, and in some patients with MLH1 loss, a methylation‐specific polymerase chain reaction (PCR) MLH1 promoter methylation assay was conducted. MSI analysis was carried out using the five National Cancer Institute‐recommended microsatellite markers [12]. MSI‐H was defined as the presence of two or more (or >30%) loci showing instability, MSI‐L as the presence of one (or <30%) loci showing instability, and MSS as no loci of instability. Patients with MSI‐L or MSS tumors were grouped together and called MSS in this study. BRAF mutational status was tested by a mutation‐specific real‐time polymerase chain reaction assay in a subset of cases.
Statistical Analysis
The OS was estimated using the Kaplan‐Meier method, and differences in OS were evaluated by the log‐rank test. Point estimates of median OS and the associated Brookmeyer and Crowley 95% confidence interval (CI) estimates are reported when appropriate. Cox proportional hazards models were used to assess the effect of variables on OS. Bivariate associations of categorical variables were investigated using Pearson's chi‐square test or Fisher's exact test as appropriate. All statistical analyses were carried out using SAS 9.2 (SAS Institute, Inc., Cary, NC), and statistical significance was defined as p < .05.
Results
Patient Characteristics
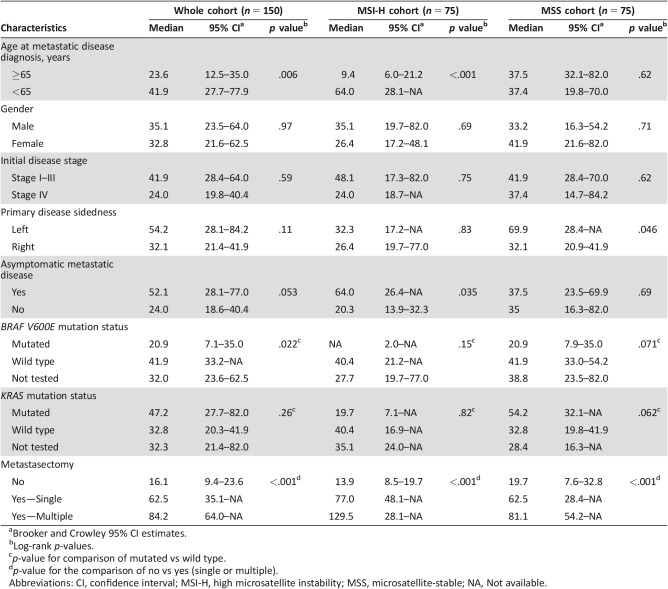
Patient characteristics are shown in Table 3. Seventy‐five patients had MSI‐H mCRC. Median age was 54 years (interquartile range: 41.8–66.8), and 48% were female. Seventy‐five control patients had MSS mCRC and were well matched by age, gender, disease sidedness, and metachronous/synchronous disease, as shown in Table 3. The majority of the patients had high‐grade cancers (81.4% in MSI‐H and 78.7% in MSS patients). The median time from initial localized disease diagnosis to metastases for MSI‐H patients was shorter than that for MSS patients (12.9 vs. 20.9 months, p = .034).
Table 3. Patient characteristics (n = 75 for both groups).
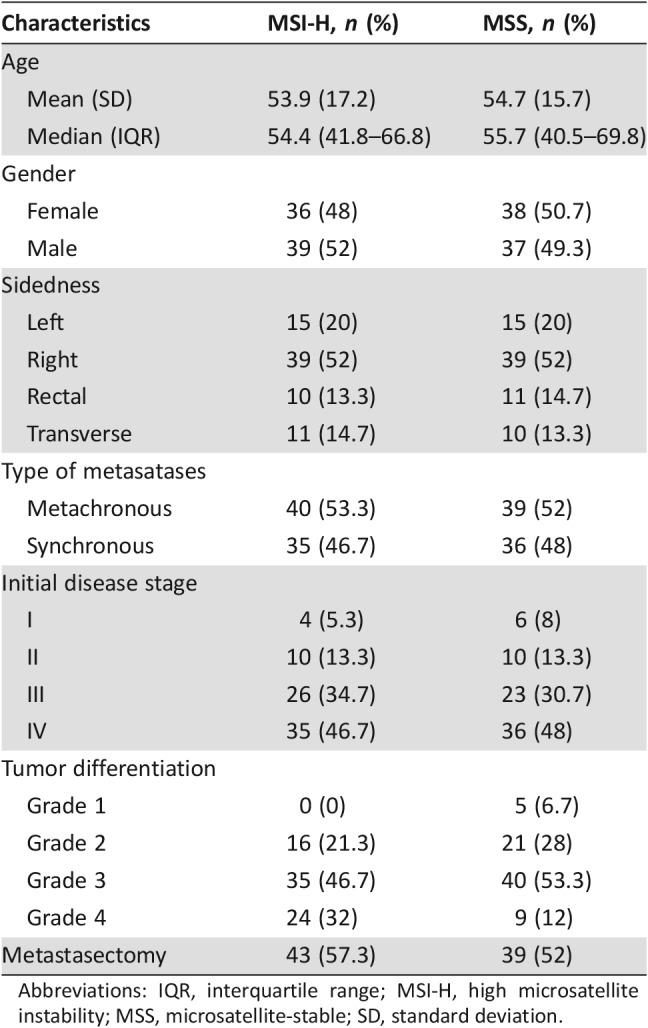
Abbreviations: IQR, interquartile range; MSI‐H, high microsatellite instability; MSS, microsatellite‐stable; SD, standard deviation.
Among the 75 dMMR/MSI‐H patients, 72 were tested and had dMMR by immunohistochemistry staining. Thirty of these patients were also tested and had MSI‐H by PCR. Only three patients had MSI‐H confirmed by MSI PCR alone. Forty‐four patients had MLH‐1/PMS‐2 protein deficiency (58.7%). Eight of fifteen patients had MLH‐1 promotor hypermethylation (53.3%). Of 27 patients with MSI‐H mCRC, 7 had BRAF V600E mutations (25.9%), and of 22 with MSS mCRC, 7 had a BRAF V600E mutation (31.8%). A BRAF D594G mutation was detected in one MSS mCRC patient. KRAS mutation was tested in 90 patients. KRAS mutation occurred in 13 of 42 (31%) MSI‐H patients and in 19 of 48 (40%) MSS patients. HRAS/NRAS were tested in 37 patients, and no mutation was detected.
Overall Survival Analysis
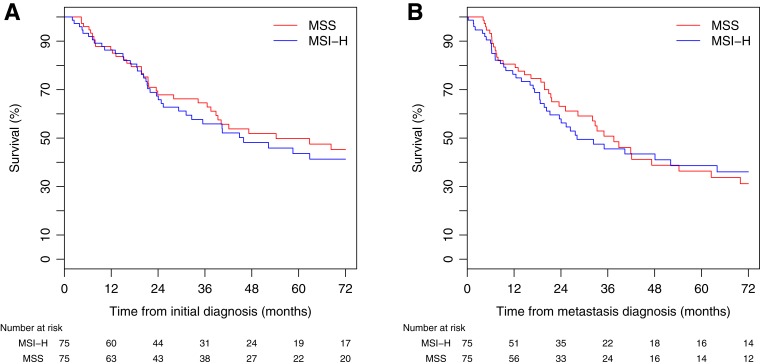
Median follow‐up was 30.0 months (range 1.6–271 months). There were 87 deaths (43 in the MSI‐H group and 44 in the MSS group) during follow‐up. Median OS from diagnosis of mCRC was 35.0 months (95% CI 25.2–48.1) and 52.3 months from the date of initial CRC diagnosis (95% CI 37.4–84.2). There was no statistically significant difference between MSI‐H and MSS patients for both median OS from the date of metastatic disease diagnosis (MSI‐H 28.1 vs. MSS 37.4 months, p = .99) and median OS from the date of initial diagnosis (MSI‐H 45.9 vs. MSS 54.2 months, p = .98; Fig. 1). We used OS from the date of metastatic disease diagnosis in the subsequent survival analysis.
Figure 1.
(A): Median OS from the date of metastatic disease diagnosis. (B): Median OS from the date of initial diagnosis.
Abbreviations: MSI‐H, high microsatellite instability; MSS, microsatellite‐stable.
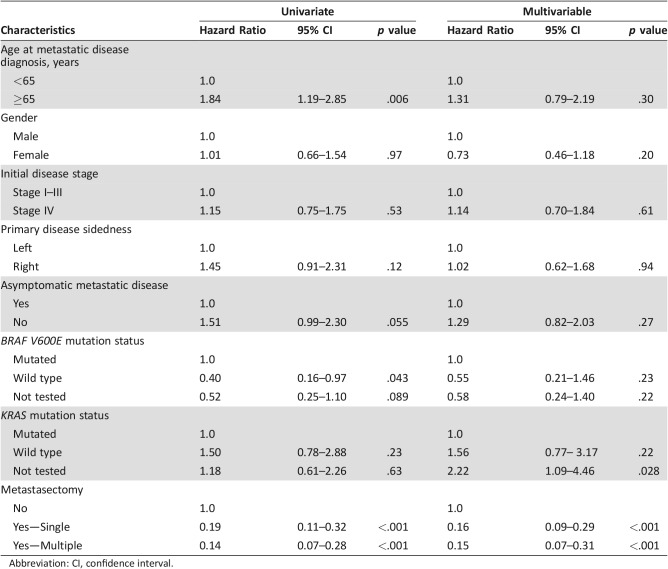
Among all patients, older age at diagnosis of mCRC (≥65 vs. <65) was associated with lower OS (23.6 vs. 41.9 months, p = .006). Median OS was greater for patients with BRAF wild‐type than BRAF V600E mCRC (41.9 vs. 20.9 months, p = .022). Median OS of patients with asymptomatic mCRC detected by surveillance showed a trend toward statistical significance compared with symptomatic patients (52.1 vs. 24.0 months, p = .053). Primary disease location was not associated with OS (54.2 vs. 32.1 months for left‐sided disease vs. right‐sided disease, p = .11). For patients who had metachronous metastatic disease, there was no association between initial disease stage and OS (p = 0.61). Similarly, KRAS mutation status did not correlate with OS (median OS 47.24 months for KRAS mutated group vs. 32.8 months for KRAS wild‐type group, p = .26; Table 4).
Table 4. Univariate analysis for survival.
Brooker and Crowley 95% CI estimates.
Log‐rank p‐values.
p‐value for comparison of mutated vs wild type.
p‐value for the comparison of no vs yes (single or multiple).
Abbreviations: CI, confidence interval; MSI‐H, high microsatellite instability; MSS, microsatellite‐stable; NA, Not available.
Metastasectomy Cohort
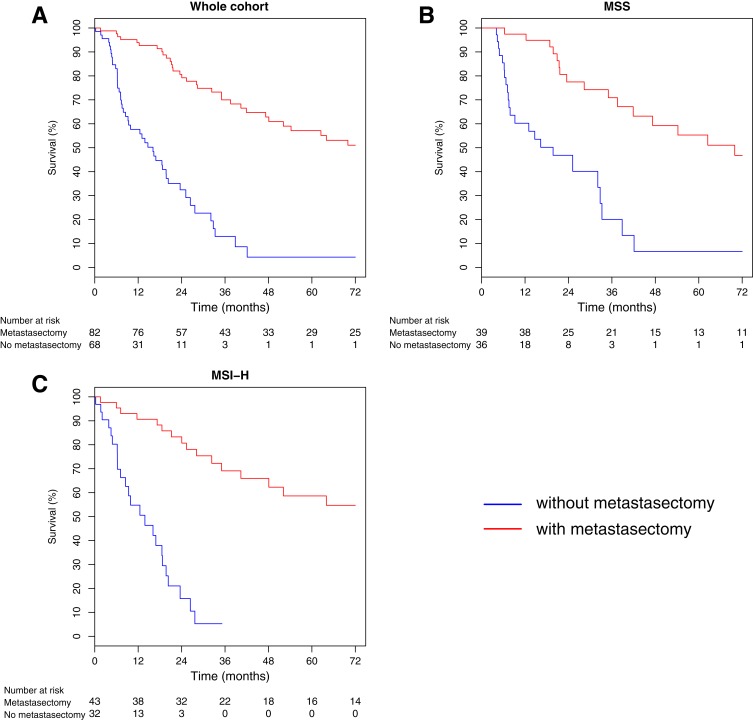
A total of 44 patients with MSI‐H mCRC and 39 patients with MSS mCRC had resection or ablation of metastases. OS was significantly greater among patients who received surgical intervention (median OS 77.0 months) compared with those who did not (median OS 16.1 months, p < .001). The clinical characteristics of both groups are listed in Table 5. The association of surgical therapy and survival was similar for both MSI‐H and MSS patients. Median OS of MSI‐H patients who did or did not have metastasectomy was 82.0 months and 13.9 months, respectively (p < .001), and median OS of MSS patients was 69.9 months and 19.7 months, respectively (p < .001; Fig. 2; Table 4).
Table 5. Clinical characteristics of patients who underwent metastasectomy.
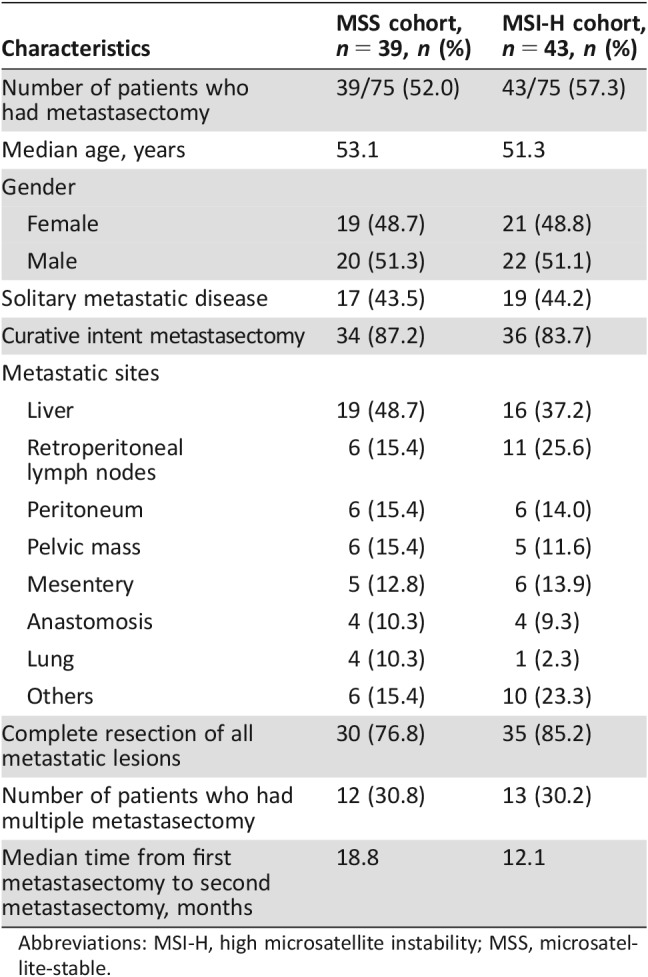
Abbreviations: MSI‐H, high microsatellite instability; MSS, microsatellite‐stable.
Figure 2.
(A): Median overall survival among patients who received surgery versus no surgical intervention. (B): Median overall survival of MSS patients who underwent metastasectomy versus those who did not. (C): Median overall survival of MSI‐H patients who underwent metastasectomy versus those who did not.
Abbreviations: MSI‐H, high microsatellite instability; MSS, microsatellite‐stable.
A total of 25 patients (12 in the MSS group and 13 in the MSI‐H group) had repeated metastasectomy either for locally or distantly recurrent mCRC. Median OS of these patients (84.2 months) was significantly greater than that of those patients who did not have repeated metastasectomy for recurrent mCRC (25.3 months, p = .006).
Fifteen patients presented with mCRC and had upfront surgery with metastasectomy and primary cancer resection; the median OS for these patients was 32.3 months, and no statistical significance was seen when comparing them with stage IV patients who had metastasectomy later during their disease treatment (median OS not reached yet, p = .40).
Chemotherapy Cohort
A total of 145 patients (71 MSI‐H patients and 74 MSS patients) had systemic chemotherapy for mCRC. Poor performance status at disease diagnosis of mCRC (two patients), surgical complication (two patients), or lost to follow‐up (one patient) precluded chemotherapy. All patients who had systemic chemotherapy had fluoropyrimidine‐based treatment (either fluoropyrimidine alone or in combination with oxaliplatin or irinotecan, with or without a biologic agent). Six patients with MSI‐H disease received pembrolizumab and were included in the analysis. The OS for patients treated with fluoropyrimidine‐based therapy regimens was not different between the MSI‐H and MSS groups (32.3 vs. 37.4 months, p = .91).
Multivariable Analysis
A Cox proportional hazards model was used to assess the effect of multiple factors on OS from the date of metastatic disease (Table 6). Undergoing a metastasectomy (single or multiple) was associated with improved OS (single vs. no: p < .001, hazard ratio [HR] 0.16, 95% CI 0.09–0.29; multiple vs. no: p < .001, HR 0.15, 95% CI 0.07–0.33; Table 6).
Table 6. Cox proportional hazards models.
Abbreviation: CI, confidence interval.
Discussion
The currently available data regarding the prognostic and predictive value of MSI status in mCRC are very limited. Here we provided one of the largest studies to date to try to address these critical clinical questions. We represent data from a single institute; therefore, most patients were tested and treated in a similar fashion so that we were able to use the same patient pool to get a matched MSS patient for each MSI‐H mCRC patient (matched by age, gender, disease sidedness, and metachronous metastatic lesion) to better investigate the effects of MSI status on clinical outcome.
Previous studies revealed that MSI‐H CRC is more common in stage II disease compared with stage III and metastatic CRC, which suggests that MSI‐H CRCs are less likely to metastasize [28], [29]. However, we noticed that in patients with metachronous disease diagnosis (40 in the MSI‐H group and 39 in the MSS group), the median time from initial limited‐stage CRC diagnosis to metastatic CRC diagnosis was significantly shorter in the MSI‐H group (12.9 vs. 20.9 months, p = .034). This phenomenon could suggest that MSI‐H CRCs metastasize earlier once the tumor cells evade the host immune surveillance and are destined to metastasize.
The median OS for the whole study group was 35.0 months from the date of diagnosis of metastatic disease and 52.3 months from the date of initial diagnosis. The median OS (from initial disease diagnosis) was 45.2 and 54.3 months for the MSI‐H and MSS groups, respectively (p = .98). The median OS (from metastatic disease diagnosis) was 28.1 and 37.4 months for the MSI‐H and MSS groups respectively (p = .99). Although there was a numerical difference in the OS between the MSI‐H and MSS groups, it was not statistically significant. The median OS is similar to the most recently reported study for mCRC patients (median OS of 25.1–32.1 months from disease recurrence) and was also within the range of previous studies [9], [17], [30]. Our further study focused on OS from metastatic disease diagnosis unless otherwise specified.
Our study showed that metastasectomy plays an important role in the MSI‐H patient population. The median OS for MSI‐H patients who did not have metastasectomy (32 patients, 42.7%) was 13.9 months, whereas the median OS was 19.7 months for the MSS group (36 patients, 48%). This difference trends toward statistical significance (p = .054). However, the median OS was 82.0 months for the 43 MSI‐H patients (57.3%) who had metastasectomy, and the median OS for their MSS counterpart group (39 patients, 52%) was 69.9 months (p = .59). It is conceivable that by aggressive metastasectomy with maximal cytoreduction, the minimal residual disease (if there is still any after the surgery) in MSI‐H patients may be able to be eradicated by the host's active immune surveillance system, the similar mechanism that the MSI‐H patients have with lower disease recurrence when these patients have stage I–III colorectal cancer [21], [31].
We noticed that the median OS of MSI‐H patients in our study is longer than that in a previous study [18]. In the published report, the median OS for MSI‐H patients was 15.4 months. We believe this could be largely due to two main reasons: First, our study population was younger (median age of 54.8), whereas the prior study patients’ median age was 67 years. Second, a high percentage of patients in our study underwent metastasectomy (57.3% in the MSI‐H group and 52% in the MSS group), which we believe significantly improved OS, whereas in the previous study, only 23.6% of patients had metastasectomy, and this may also reflect the difference in patient population (amount of disease, performance status, etc.). The median OS in our patients who did not have metastasectomy (13.9 vs. 19.7 months in MSI‐H and MSS patients, respectively) is similar to this study and another previous study in this specific population [15].
A BRAF V600E mutation is associated with sporadic MSI‐H cancers and is clinically used as a biomarker to help differentiate between germline MSI‐H (Lynch syndrome) and sporadic MSI‐H CRC [32]. The presence of a BRAF V600E mutation is correlated with poor clinical outcome in both localized and metastatic CRC [33], [34]. A recent report showed that patients with MSI‐H and BRAF V600E mutation had poor outcome in a secondary analysis of two randomized adjuvant clinical trials [17]. In our study, we only had 49 patients tested for BRAF mutation, and 14 patients were found to harbor a BRAF V600E mutation in the tumor. These 14 patients with BRAF V600E had a median OS of 20.9 months compared with a median OS of 41.9 months for patients who were tested and confirmed to not be harboring a BRAF V600E mutation (p = .022), which is consistent with the recent reports [17], [18].
KRAS mutation analysis was performed in 90 mCRC patients, 32 of whom were found to harbor a KRAS mutation (13/42, 31% in the MSI‐H group; and 19/48, 40% in the MSS group). The presence of a KRAS mutation did not influence OS (47.2 months for the KRAS mutated group vs. 32.8 months for the KRAS wild‐type group, p = .26). This could be due to the sample size of the study and the limited use of epidermal growth factor receptor (EGFR) inhibitors in the whole patient population (only 3 patients received EGFR antibodies in first line, and only 15 patients had exposure during their whole treatment).
Another question we were trying to address when we initially designed this study was the potential predictive value of MSI status. Almost all the patients (94.7% in the MSI‐H group and 98.7% in the MSS group) had fluoropyrimidine‐based chemotherapy as part of their treatment, and we did not see any significant OS difference between the MSI‐H group and the MSS group (32.3 vs. 37.4 months, p = .91).
The main limitation inherent to our study is its retrospective nature and relatively small sample size of patients with metastatic MSI‐H CRC. The small sample size and limited test results (such as pan‐RAS panel study, BRAF V600E mutation study, etc.) limit the interpretation of subgroup analyses. This study was conducted over an extended period; thus, tumor response assessment could only be collected based on the treating physician's assessment. The choice of therapy for each patient was also dependent upon the treating physician and the available agents at that time. In addition, the results reflect our institution's aggressive surgical treatment approach toward limited metastatic disease, with close to 50% of patients undergoing metastasectomy. Despite these limitations, this study does contain one of the largest reported cohorts of metastatic MSI‐H CRC.
Conclusion
Patients with MSI‐H metastatic CRC appear to have similar overall survival and treatment benefit as patients with MSS tumors. However, these patients may have a greater benefit from aggressive management of metastases including ablation and multiple metastasectomy. As with MSS mCRC, BRAF V600E mutation is a poor prognostic factor in metastatic MSI‐H mCRC.
In 2017, National Comprehensive Cancer Network guidelines recommended screening all patients with CRC for MSI status, independent of stage and age. Recently, therapy with immune checkpoint inhibition showed very encouraging results in MSI‐H mCRC patients [35], [36]. The role of metastasectomy in the immune treatment era and the role of immunotherapy after metastasectomy in MSI‐H mCRC will need to be investigated. We anticipate that the number of MSI‐H/dMMR mCRC detected by general screening will rapidly increase in the coming years, and studies for this unique subset of CRC patients will help to further delineate the prognostic and predictive impact of MSI.
Author Contributions
Conception/design: Axel Grothey, Joleen M. Hubbard
Provision of study material or patients: Axel Grothey, Joleen M. Hubbard
Collection and/or assembly of data: Zhaohui Jin, Cristobal T. Sanhueza, Benny Johnson, David M. Nagorney, David W. Larson, Kristin C. Mara, William C. Harmsen, Thomas C. Smyrk, Axel Grothey, Joleen M. Hubbard
Data analysis and interpretation: Zhaohui Jin, Cristobal T. Sanhueza, Benny Johnson, David M. Nagorney, David W. Larson, Kristin C. Mara, William C. Harmsen, Thomas C. Smyrk, Axel Grothey, Joleen M. Hubbard
Manuscript writing: Zhaohui Jin, Cristobal T. Sanhueza, Benny Johnson, David M. Nagorney, David W. Larson, Kristin C. Mara, William C. Harmsen, Thomas C. Smyrk, Axel Grothey, Joleen M. Hubbard
Final approval of manuscript: Zhaohui Jin, Cristobal T. Sanhueza, Benny Johnson, David M. Nagorney, David W. Larson, Kristin C. Mara, William C. Harmsen, Thomas C. Smyrk, Axel Grothey, Joleen M. Hubbard
Disclosures
The authors indicated no financial relationships.
References
- 1. Geiersbach KB, Samowitz WS. Microsatellite instability and colorectal cancer. Arch Pathol Lab Med 2011;135:1269–1277. [DOI] [PubMed] [Google Scholar]
- 2. Ligtenberg MJ, Kuiper RP, Chan TL et al. Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3' exons of TACSTD1. Nat Genet 2009;41:112–117. [DOI] [PubMed] [Google Scholar]
- 3. Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med 2003;348:919–932. [DOI] [PubMed] [Google Scholar]
- 4. Aaltonen LA, Salovaara R, Kristo P et al. Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. N Engl J Med 1998;338:1481–1487. [DOI] [PubMed] [Google Scholar]
- 5. Hampel H, Frankel WL, Martin E et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med 2005;352:1851–1860. [DOI] [PubMed] [Google Scholar]
- 6. Halvarsson B, Anderson H, Domanska K et al. Clinicopathologic factors identify sporadic mismatch repair‐defective colon cancers. Am J Clin Pathol 2008;129:238–244. [DOI] [PubMed] [Google Scholar]
- 7. Hendriks YM, de Jong AE, Morreau H et al. Diagnostic approach and management of Lynch syndrome (hereditary nonpolyposis colorectal carcinoma): A guide for clinicians. CA Cancer J Clin 2006;56:213–225. [DOI] [PubMed] [Google Scholar]
- 8. Timmermann B, Kerick M, Roehr C et al. Somatic mutation profiles of MSI and MSS colorectal cancer identified by whole exome next generation sequencing and bioinformatics analysis. PLoS One 2010;5:e15661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cancer Genome Atlas Network . Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487:330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sourrouille I, Coulet F, Lefevre JH et al. Somatic mosaicism and double somatic hits can lead to MSI colorectal tumors. Fam Cancer 2013;12:27–33. [DOI] [PubMed] [Google Scholar]
- 11. Perucho M. Correspondence re: Boland C.R. et al., A National Cancer Institute workshop on microsatellite instability for cancer detection and familial predisposition: Development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res., 58: 5248‐5257, 1998. Cancer Res 1999;59:249–256. [PubMed] [Google Scholar]
- 12. Boland CR, Thibodeau SN, Hamilton SR et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: Development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 1998;58:5248–5257. [PubMed] [Google Scholar]
- 13. Lothe RA, Peltomaki P, Meling GI et al. Genomic instability in colorectal cancer: Relationship to clinicopathological variables and family history. Cancer Res 1993;53:5849–5852. [PubMed] [Google Scholar]
- 14. Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science 1993;260:816–819. [DOI] [PubMed] [Google Scholar]
- 15. Venderbosch S, Nagtegaal ID, Maughan TS et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: A pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res 2014;20:5322–5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goldstein J, Tran B, Ensor J et al. Multicenter retrospective analysis of metastatic colorectal cancer (CRC) with high‐level microsatellite instability (MSI‐H). Ann Oncol 2014;25:1032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sinicrope FA, Shi Q, Allegra CJ et al. Association of DNA mismatch repair and mutations in BRAF and KRAS with survival after recurrence in stage III colon cancers: A secondary analysis of 2 randomized clinical trials. JAMA Oncol 2017;3:472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goldstein J, Tran B, Ensor J et al. Multicenter retrospective analysis of metastatic colorectal cancer (CRC) with high‐level microsatellite instability (MSI‐H). Ann Oncol 2014;25:1032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 2005;23:609–618. [DOI] [PubMed] [Google Scholar]
- 20. Ribic CM, Sargent DJ, Moore MJ et al. Tumor microsatellite‐instability status as a predictor of benefit from fluorouracil‐based adjuvant chemotherapy for colon cancer. N Engl J Med 2003;349:247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sinicrope FA, Foster NR, Thibodeau SN et al. DNA mismatch repair status and colon cancer recurrence and survival in clinical trials of 5‐fluorouracil‐based adjuvant therapy. J Natl Cancer Inst 2011;103:863–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Webber EM, Kauffman TL, O'Connor E et al. Systematic review of the predictive effect of MSI status in colorectal cancer patients undergoing 5FU‐based chemotherapy. BMC Cancer 2015;15:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ribic CM, Sargent DJ, Moore MJ et al. Tumor microsatellite‐instability status as a predictor of benefit from fluorouracil‐based adjuvant chemotherapy for colon cancer. N Engl J Med 2003;349:247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil‐based adjuvant therapy in colon cancer. J Clin Oncol 2010;28:3219–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hutchins G, Southward K, Handley K et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol 2011;29:1261–1270. [DOI] [PubMed] [Google Scholar]
- 26. Des Guetz G, Uzzan B, Nicolas P et al. Microsatellite instability does not predict the efficacy of chemotherapy in metastatic colorectal cancer. A systematic review and meta‐analysis. Anticancer Res 2009;29:1615–1620. [PubMed] [Google Scholar]
- 27. Liang JT, Huang KC, Lai HS et al. High‐frequency microsatellite instability predicts better chemosensitivity to high‐dose 5‐fluorouracil plus leucovorin chemotherapy for stage IV sporadic colorectal cancer after palliative bowel resection. Int J Cancer 2002;101:519–525. [DOI] [PubMed] [Google Scholar]
- 28. Van Cutsem E, Labianca R, Bodoky G et al. Randomized phase III trial comparing biweekly infusional fluorouracil/leucovorin alone or with irinotecan in the adjuvant treatment of stage III colon cancer: PETACC‐3. J Clin Oncol 2009;27:3117–3125. [DOI] [PubMed] [Google Scholar]
- 29. Koopman M, Kortman GA, Mekenkamp L et al. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br J Cancer 2009;100:266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ogino S, Shima K, Meyerhardt JA et al. Predictive and prognostic roles of BRAF mutation in stage III colon cancer: results from intergroup trial CALGB 89803. Clin Cancer Res 2012;18:890–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tejpar S, Saridaki Z, Delorenzi M et al. Microsatellite instability, prognosis and drug sensitivity of stage II and III colorectal cancer: More complexity to the puzzle. J Natl Cancer Inst 2011;103:841–844. [DOI] [PubMed] [Google Scholar]
- 32. Sinicrope FA. The role of microsatellite instability testing in management of colorectal cancer. Clin Adv Hematol Oncol 2016;14:476–479. [PubMed] [Google Scholar]
- 33. Tran B, Kopetz S, Tie J et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer 2011;117:4623–4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Samowitz WS, Sweeney C, Herrick J et al. Poor survival associated with the BRAF V600E mutation in microsatellite‐stable colon cancers. Cancer Res 2005;65:6063–6069. [DOI] [PubMed] [Google Scholar]
- 35. Le DT, Uram JN, Wang H et al. PD‐1 blockade in tumors with mismatch‐repair deficiency. N Engl J Med 2015;372:2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee V, Murphy A, Le DT et al. Mismatch repair deficiency and response to immune checkpoint blockade. The Oncologist 2016;21:1200–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Benatti P, Gafa R, Barana D et al. Microsatellite instability and colorectal cancer prognosis. Clin Cancer Res 2005;11:8332–8340. [DOI] [PubMed] [Google Scholar]
- 38. Hong SP, Min BS, Kim TI et al. The differential impact of microsatellite instability as a marker of prognosis and tumour response between colon cancer and rectal cancer. Eur J Cancer 2012;48:1235–1243. [DOI] [PubMed] [Google Scholar]