This review summarizes the literature about the characteristics of epideral growth factor receptor (EGFR) brain metastases and describes the central nervous system activity of various EGFR inhibitors. Key literature about the sequencing of tyrosine kinase inhibitors and radiotherapy for EGFR‐mutant patients with newly diagnosed brain metastases.
Keywords: Brain metastases, Lung cancer, Epidermal growth factor receptor mutation, Radiation therapy, Targeted therapy
Abstract
The growth of genotype‐directed targeted therapies, such as inhibitors of the epidermal growth factor receptor (EGFR), has revolutionized treatment for some patients with oncogene‐addicted lung cancer. However, as systemic control for these patients has improved, brain metastases remain an important source of morbidity and mortality. Traditional treatment for brain metastases has been radiotherapy, either whole‐brain radiation or stereotactic radiosurgery. The growing availability of drugs that can cross the blood‐brain barrier and have activity in the central nervous system (CNS) has led to many studies investigating whether targeted therapy can be used in combination with or in lieu of radiation. In this review, we summarize the key literature about the incidence and nature of EGFR‐mutant brain metastases (EGFR BMs), the data about the activity of EGFR inhibitors in the CNS, and whether they can be used as front‐line therapy for brain metastases. Although initial use of tyrosine kinase inhibitors for EGFR BMs can often be an effective treatment strategy, multidisciplinary evaluation is critical, and prospective studies are needed to clarify which patients may benefit from early radiotherapy.
Implications for Practice.
Management of brain metastases in epidermal growth factor receptor (EGFR) mutant lung cancer is a common clinical problem. The question of whether to start initial therapy with an EGFR inhibitor or radiotherapy (either whole‐brain radiotherapy or stereotactic radiosurgery) is controversial. The development of novel EGFR inhibitors with enhanced central nervous system (CNS) penetration is an important advance in the treatment of CNS disease. Multidisciplinary evaluation and evaluation of extracranial disease status are critical to choosing the best treatment option for each patient.
摘要
表皮生长因子受体(EGFR)抑制剂等基因靶向治疗的发展为某些癌基因依赖性肺癌的治疗带来了革命性的变化。然而,尽管这些患者的系统控制已经有所改善,但脑转移仍然是病损和死亡的一个重要原因。放疗包括全脑放疗或立体定向放射手术是脑转移的传统治疗方法。可透过血脑屏障并且作用于中枢神经系统 (CNS)的药物越来越多,因此,许多研究探索了靶向治疗是否可与放疗结合或者替代放疗。在本篇综述中,我们总结了关于EGFR突变脑转移(EGFR BMs)的发病率及其性质的关键文献、关于CNS中EGFR抑制剂活性的数据、以及EGFR抑制剂是否可用作脑转移的一线疗法。尽管初始使用酪氨酸激酶抑制剂治疗EGFR BMs通常是一种有效治疗策略,但多学科评估是关键,未来需要展开前瞻性研究以明确哪些患者可获益于早期放疗。
实践意义:表皮生长因子受体(EGFR)突变肺癌脑转移的治疗是一个常见的临床问题。应当用EGFR抑制剂还是放疗(全脑放疗或立体定向放射手术)作为初始治疗尚存在争议。具有更强中枢神经系统(CNS)渗透力的新型EGFR抑制剂的开发是治疗CNS疾病的一个重要进展。多学科评估和颅外疾病状态评估对于为每位患者选择最佳治疗方案至关重要
Introduction
Identification of activating mutations in the epidermal growth factor receptor (EGFR) in lung cancer was a monumental advance in our understanding of the molecular basis of carcinogenesis [1], [2]. The activity of EGFR‐specific tyrosine kinase inhibitors (TKIs) against such cancers ushered in an era of genotype‐directed targeted therapy that fundamentally changed the overall approach to lung cancer. Despite these advances, the central nervous system (CNS) remains a common site of metastatic spread and morbidity for patients with EGFR‐mutant lung cancer.
Management of EGFR‐mutant brain metastases (EGFR BMs) presents multiple challenges. Radiotherapy (RT), either with whole‐brain radiotherapy (WBRT) or stereotactic radiosurgery (SRS), has been the mainstay of treatment for brain metastases due to the poor blood‐brain barrier penetration of most systemic therapies. However, there has recently been increasing concern about the potential long‐term impact of cranial RT, especially WBRT, upon neurocognitive function and quality of life [3]. Given the prolonged survival of patients with EGFR BMs [4], the late toxicities of cranial RT have become a growing concern. Furthermore, EGFR inhibitors have consistently shown some degree of activity in the brain even without RT [5], [6], [7], [8], [9], [10]. In this context, there has been interest in omitting or delaying cranial radiation for EGFR BMs by using TKIs. In this review, we will summarize the literature about the characteristics of EGFR BMs as well as describe the CNS activity of various EGFR inhibitors. We will also review the key literature about the sequencing of TKIs and RT for EGFR‐mutant patients with newly diagnosed brain metastases.
Materials and Methods
We conducted a computerized literature search in PubMed to identify publications relevant to the topic of EGFR, EGFR inhibitors, lung cancer, and brain metastases. We included only full text publications written in English and selected the studies based on methodological design and sample sizes. We prioritized prospective studies or large retrospective studies. We did include publications in abstract form if the data included were of exceptional relevance to this review and were not published in a full‐length format. We also examined the references of selected studies to identify additional references that did not appear in our original searches. The following data were extracted from the published articles, as available: characteristics of the study population, length of follow‐up, incidence of extracranial disease, rates of salvage therapy, CNS overall response rate (CNS ORR), CNS progression‐free survival (CNS PFS), and overall survival (OS).
Results
Characteristics of Patients with EGFR Brain Metastases
Many studies have shown that patients with EGFR driver mutations appear to have a similar incidence of brain metastases when compared with the overall lung cancer population. In a review of 93 patients with lung adenocarcinoma brain metastases treated at our institution from 2004 to 2008, 44% carried an EGFR mutation and 56% did not [11]. Similarly, in a genotyped cohort of 209 patients from Colorado, there was a similar distribution of brain metastases among patients with EGFR, KRAS, ALK, and wild‐type lung cancers [12]. A retrospective case control study from The Netherlands [13] identified 62 patients with stage IV EGFR mutant lung cancer and paired them with consecutive patients with KRAS mutant and wild‐type lung cancer. A similar incidence of brain metastases was identified in each group.
However, several studies, mostly from Asia, have suggested an increased risk of BMs in EGFR mutant lung cancer. In a cohort of 314 lung adenocarcinoma patients from Korea [14], 51 patients had brain metastases. In a multivariable model, EGFR mutation was associated with an increased risk of brain metastasis (adjusted OR 3.83, p = .001) but no increased risk of extracranial metastasis. A study from the People's Republic of China [15] showed that among 234 patients with lung adenocarcinoma, 108 carried EGFR mutations. Seventy‐six of these patients developed brain metastasis, which was significantly higher than the rate among those without EGFR mutation. There was no difference seen in the rate of extracranial metastatic disease. On multivariable analysis, EGFR mutation was associated with an independent increased risk of brain metastases (OR 2.52, p = .022).
There are some data that EGFR mutation may impact the clinical presentation of BMs. The Chinese study suggested that patients with EGFR mutation had larger and more numerous BMs compared with Wild Type patients [15]. Another study of 57 patients with lung adenocarcinoma with synchronous brain metastasis [16] reported that EGFR exon 19 deletion was associated with more numerous brain metastases compared with wild‐type cancers, although that was not true of cases with EGFR exon 21 mutations. In addition, the median size of the tumors in patients with exon 19 deletion was smaller compared with wild‐type cancers, as was the associated peri‐tumoral edema. Radiographic analysis of 200 patients with brain metastases treated in Japan [17] demonstrated that EGFR L858R was associated with more superficial lesions, preferentially located in the caudate, cerebellum, and temporal lobes compared with exon 19 patients. These data, while still preliminary, suggest that different EGFR mutations may result in somewhat different patterns of CNS metastasis. Analysis of patterns of EGFR BMs remains a nascent area of investigation, and hopefully future studies will shed more light on the differences in presentation among different genotypes.
One important point to emphasize is that patients with EGFR BMs can have a prolonged survival. The Graded Prognostic Assessment for Lung Cancer (dsGPA), a system used to define prognosis of brain metastases from lung cancer, was recently updated to included presence of mutations in EGFR or ALK, along with the traditional risk factors of age, Karnofsky performance status, extracranial metastases, and number of brain metastases. The investigators found that patients in the best prognosis group, which includes patients with targetable mutations, had a median survival of 46.8 months [18]. These data emphasize that therapeutic considerations for patients with EGFR BMs must consider the short‐term and long‐term risks of treatment.
In summary, EGFR BMs are a common problem, although it remains unclear whether there is truly an increased incidence compared with other genotypically defined lung cancer groups. However, as more EGFR inhibitors enter clinical practice, the resultant increased survival of patients with EGFR‐mutant lung cancer will translate into a longer time in which brain metastases may develop. Thus, identifying the optimal strategy for CNS control of EGFR BMs remains an important area of study.
The Activity of TKIs in the CNS
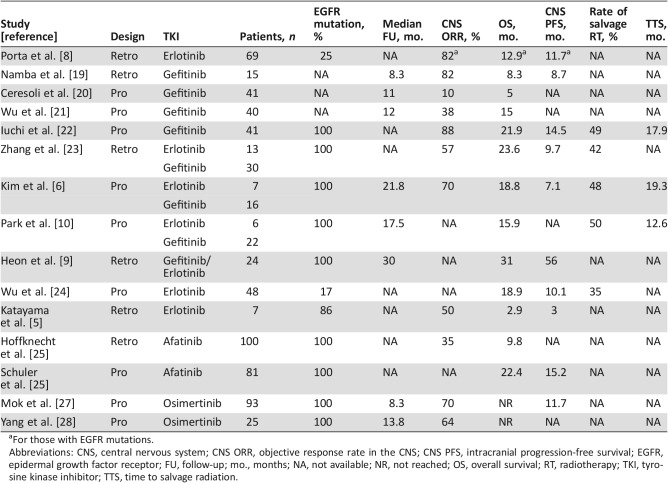
The early availability and widespread use of the first‐generation EGFR inhibitors, erlotinib and gefitinib, led many to investigate whether these drugs had any activity in the CNS. These data, summarized in Table 1, generally demonstrate a high overall CNS response rate to first‐generation EGFR TKIs. For example, a retrospective study from Spain [8] examined 69 patients with brain metastases who were treated with erlotinib on prospective trials, including 17 with EGFR mutations. Half of the patients with EGFR mutation did not receive brain radiation prior to starting erlotinib. Among these patients, erlotinib yielded an 82% objective response rate (ORR) including a 47% complete response (CR) rate. A larger study from the People's Republic of China [23] of 43 patients with confirmed EGFR BMs treated with erlotinib or gefitinib showed a 57% ORR in the CNS compared with 91% for extracranial disease (ECD). None of the patients had received CNS RT prior to treatment. Although this ORR is lower than that in many of the other studies, this study did include patients receiving TKI as second‐line therapy. Additionally, the disease control rate was 91% for the CNS versus 95% for ECD, suggesting many patients had at least stable disease in the CNS. The median progression free survival (PFS) was 9.7 months for intracranial disease versus 13.7 months for extracranial disease, indicating that CNS progression occurred earlier than systemic progression. Consistent with these clinical data, pharmacologic studies have shown that both gefitinib and erlotinib are present in the CNS with a cerebrospinal fluid (CSF) penetration rate of 1.13% and 2.77%, respectively [29]. Although this degree of CNS penetration is low, the extreme sensitivity of EGFR‐addicted cancers to EGFR inhibitors allows responses to occur even with a low concentration of drug in the CNS; in addition, the presence of CNS metastases themselves can disrupt the blood‐brain barrier more than predicted by pharmacologic studies [30].
Table 1. List of selected studies examining the efficacy of EGFR TKIs for patients with EGFR brain metastases.
For those with EGFR mutations.
Abbreviations: CNS, central nervous system; CNS ORR, objective response rate in the CNS; CNS PFS, intracranial progression‐free survival; EGFR, epidermal growth factor receptor; FU, follow‐up; mo., months; NA, not available; NR, not reached; OS, overall survival; RT, radiotherapy; TKI, tyrosine kinase inhibitor; TTS, time to salvage radiation.
Afatinib, an irreversible second‐generation EGFR TKI, was evaluated for activity in the CNS of patients who received it under a compassionate access program [25]. Of 541 patients receiving the drug, 100 patients had either parenchymal brain metastases or leptomeningeal disease, with 74% having documented EGFR mutation. In the 31 patients evaluated for CNS response, the CNS ORR was 35%. This was an impressive result given these patients had been previously treated with chemotherapy and at least one other TKI. The disease control duration was approximately 4 months. CSF analysis of one patient showed a CSF penetration rate of 0.7% and a concentration of 1 nM, which is in the range of Ki of the drug. Retrospective analysis of patients with EGFR BMs in the LUX‐Lung 3 and LUX‐Lung 6 randomized trials of afatinib compared with front‐line chemotherapy (cisplatin and pemetrexed or gemcitabine) [26] showed that, as in the overall study population, the PFS was longer for EGFR BM patients on afatinib compared with chemotherapy (8.2 months and 5.4 months for afatinib and chemotherapy, respectively; hazard ratio [HR] 0.50, 95% confidence interval [CI]: 0.27–0.95, p = .0297). Interestingly, the PFS benefit of afatinib was higher in those who received prior WBRT (n = 24) than those who did not (n = 57). Although CNS ORR was not reported, the overall ORR was 82% for those with exon 19 deletion and 60% for those with L858R mutations. In patients with common EGFR mutations, the afatinib group had a longer median time to CNS progression (LUX‐Lung3: 15.2 months vs. 5.7 months; LUX‐Lung 6: 15.2 months vs. 7.3 months).
The third‐generation EGFR TKI osimertinib is specifically designed to overcome the common T790M resistance mutation, and emerging data suggest that it is very effective against EGFR BMs, including those that developed on another EGFR TKI.
The third‐generation EGFR TKI osimertinib is specifically designed to overcome the common T790M resistance mutation, and emerging data suggest that it is very effective against EGFR BMs, including those that developed on another EGFR TKI. Our group published a case series of patients with dramatic response to osimertinib after progression on rociletinib, an experimental third‐generation EGFR TKI, including three patients who developed new BM on rociletinib, which subsequently responded to osimertinib [31]. Pharmacokinetic data from a patient in the Aura phase II extension cohort [32] found a CSF concentration of 3.44 nM, higher than analogous data for earlier TKIs and comparable to the predicted plasma free circulating concentration. More recent direct measurements suggest osimertinib has a CSF/free plasma ratio of ∼16% [33]. Analysis of 25 patients with asymptomatic EGFR BMs from this cohort [28] showed a CNS ORR of 64% (4 CR, 12 partial response [PR]), with 2 patients having lesion growth. In the AURA3 randomized trial of osimertinib versus chemotherapy for T790M‐positive patients progressing on first‐line EGFR TKIs [34], 144 of the 490 patients had CNS metastatic disease. Among these patients, osimertinib was associated with an improved PFS compared with chemotherapy (8.5 months for osimertinib and 4.2 months for chemotherapy; HR 0.32, 95% CI 0.21–0.49). It remains to be seen whether other T790M‐specific EGFR inhibitors currently in development will match osimertinib's CNS activity.
There are a few newer EGFR TKIs being specifically designed for enhanced CNS penetration. For example, AZD3759, a novel first‐generation‐like EGFR TKI, has been shown to accumulate in pharmacologic doses in the CSF and have activity in preclinical models. In two patients treated on the phase I BLOOM trial [35], the concentration of drug in the CSF was approximately equal to that in the serum, with some evidence of CNS response. In addition, tesavatinib may have enhanced CNS penetration, although limited clinical data are available at this time [36].
Synthesizing the data, it appears that the response rates of EGFR BMs to CNS‐penetrant TKIs are in the range of 60%–80%, which is similar to that of WBRT. The duration of intracranial response is more variable, with a range of 3–14.5 months, likely depending in part on the extent of prior treatment of the patients in each study. Approximately 25%–50% [10], [22], [37] of patients receive salvage RT. Thus, a significant proportion of patients who are treated with initial TKI with documented EGFR BMs never go on to receive cranial RT. It remains unclear whether RT is not given in these cases due to long‐term control in the brain, extensive intracranial recurrence (e.g., leptomeningeal dissemination), or lack of sufficient follow‐up in these small reports.
Combining EGFR TKIs and radiotherapy
With the promising results of EGFR TKIs in EGFR BMs, there has been a lot of interest in combining EGFR TKIs with brain RT. A prospective U.S.‐based phase II trial tested the combination of erlotinib and WBRT in 40 patients with brain metastases from lung adenocarcinoma [38]. The combination was well tolerated without an increase in CNS or skin toxicity. Only nine of the patients were confirmed to carry an EGFR mutation, and the CNS ORR was 89% for those with EGFR mutations compared with 63% for those known to lack EGFR mutations. These data were supported by a U.K. study [39] that randomized patients with non‐small cell lung cancer and BMs to erlotinib or placebo concurrently with WBRT. Only one patient had a documented EGFR mutation. There was no difference between the groups in terms of response, PFS, or OS, nor was there a difference in toxicity, other than lower levels of fatigue in the group receiving erlotinib/WBRT. These two prospective studies show that combining EGFR TKIs with WBRT is reasonably well tolerated and is an option for those with EGFR mutations, but the low number of patients with documented EGFR mutations in these trials negates the ability to perform efficacy analyses. Furthermore, it should be noted that the majority of these patients did not have EGFR mutations, and the survival was poor. Thus, very few data regarding potential late neurocognitive toxicity of EGFR inhibitors combined with WBRT are available.
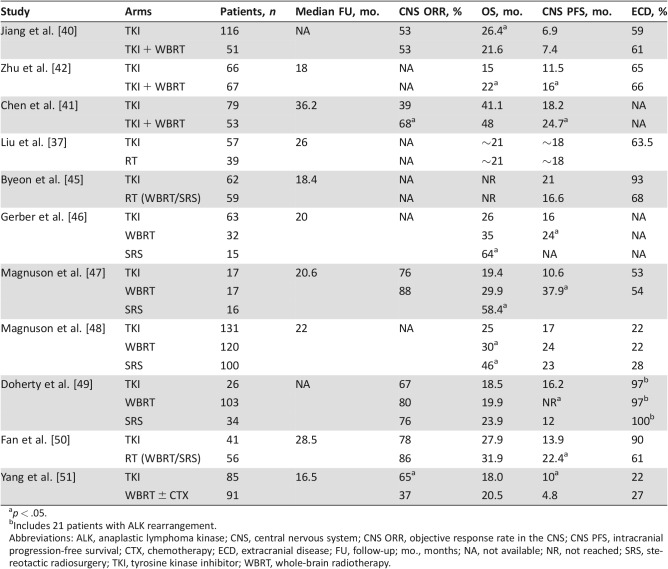
Retrospective studies specific to EGFR BMs also suggest that combining brain RT and EGFR TKIs is tolerable but have yielded conflicting data on efficacy. For example, a series of 230 patients with EGFR BMs from Shanghai had 116 patients who received TKI alone, whereas 51 patients received both TKI and WBRT [40]. The patients receiving WBRT were more likely to be symptomatic from their BMs but were otherwise well matched. The CNS ORR was equal in both groups at 53%, and the CNS PFS was also similar (6.9 months for TKI alone and 7.4 months for TKI/WBRT). However, overall survival was worse in the group receiving TKI/WBRT (21.6 months vs. 26.4 months, p = .049). There was no analysis of causes of death, but this observation raises the question as to whether excess toxicity secondary to WBRT may have played a role in the worse outcome in this group.
In contrast, a cohort of 132 patients with EGFR BMs from Wuhan included 79 patients treated with TKI alone and 43 treated with TKI/WBRT [41]. Again, the TKI/WBRT patients were more likely to be symptomatic but were otherwise similar. The CNS ORR for TKI alone was 39% versus 68% for TKI/WBRT (p = .001). The median CNS time‐to‐progression was 18.2 months for TKI and 24.7 months for TKI/WBRT (p = .004). Neurocognitive assessment showed that at 6 months, there was significant impairment in memory and learning with TKI/WBRT compared with the TKI alone group, although these differences lessened at the 2‐year follow‐up. Similarly, another cohort of 133 patients from Zhengzhou [42] also showed a longer CNS PFS (16 months vs. 11.5 months) among the 67 patients who received TKI/WBRT compared with the 66 patients who got TKI alone, although this was seen mostly in patients with L858R mutations.
Combining EGFR TKIs and SRS is generally thought to be safe and well tolerated. Given the limited schedule of SRS and the small volume involved, there are few reports of increased toxicity with EGFR inhibitors. For example, a series from the Cleveland Clinic showed no increase in radiation necrosis among lung adenocarcinoma patients receiving SRS with EGFR TKI compared with those receiving SRS alone (10.3% vs. 7.9%) [43].
Can TKIs Be Used Instead of RT?
With the excellent response rates seen with TKIs in the CNS and the potential for neurotoxicity with WBRT [3], [44], investigators have asked whether patients with EGFR BMs can be treated with TKIs upfront, delaying or perhaps eliminating the need for cranial radiotherapy. A number of retrospective studies have addressed this question, with varying results (Table 2). For example, a study from Korea reviewed 121 patients with EGFR BMs at diagnosis [45]. Of these 121 patients, 59 received RT upfront (32 SRS, 26 WBRT, 1 both) and 62 were treated with initial TKI (primarily gefitinib). The patients who received initial RT were more likely to be symptomatic, whereas the group receiving TKIs had more patients with >5 metastases and were more likely to have extracranial disease. The intracranial disease control rate (CR, PR, or stable disease) was higher with brain RT (80% vs. 60%, p = .019), but there was no difference in median CNS PFS (16.6 months for RT and 21.0 months for the TKI group). There was no difference in 3‐year landmark survival, with the RT group achieving 72% 3‐year OS and the TKI group 68%. Again, only 24% of patients in the TKI alone group required salvage RT. These data suggest that initial TKI in lieu of radiotherapy is a reasonable approach for selected groups of patients.
Table 2. Listing of studies comparing initial TKI therapy with RT.
p < .05.
Includes 21 patients with ALK rearrangement.
Abbreviations: ALK, anaplastic lymphoma kinase; CNS, central nervous system; CNS ORR, objective response rate in the CNS; CNS PFS, intracranial progression‐free survival; CTX, chemotherapy; ECD, extracranial disease; FU, follow‐up; mo., months; NA, not available; NR, not reached; SRS, stereotactic radiosurgery; TKI, tyrosine kinase inhibitor; WBRT, whole‐brain radiotherapy.
Recently, a randomized trial from the People's Republic of China evaluated 176 patients who were assigned to receive icotinib (a first‐generation EGFR inhibitor) or WBRT with or without chemotherapy [51]. The primary endpoint of CNS PFS was significantly longer for the icotinib group compared with WBRT (10.0 months vs. 4.8 months, p = .014). The CNS ORR was significantly higher for icotinib (65%) compared with WBRT (37%). This low ORR for WBRT is unusual and may have contributed to the inferior outcomes in this study. Additionally, 20% of the patients in the WBRT group dropped out, lessening the power of the study and likely affecting the efficacy of randomization. Furthermore, the WBRT arm received chemotherapy rather than TKI, and thus the study cannot directly address whether initial TKI is a better choice than initial RT followed by TKI. However, this is one of the only randomized trials that have attempted to shed light on the optimal management of EGFR BMs.
Several studies have argued that omitting RT from upfront treatment may be associated with inferior outcomes. A systematic review and meta‐analysis from 2015 examined 12 studies including 363 patients with EGFR BMs who received TKI, WBRT, SRS, or some combination [52]. There was no significant difference in CNS ORR between TKI monotherapy upfront or concurrent/sequential RT/TKI. There was a small but significant improvement in CNS PFS at 4 months with WBRT upfront compared with TKI monotherapy (relative risk [RR] 1.05, 95% CI 0.98–1.12, p = .03). The OS rate at 2 years was higher with sequential upfront WBRT (RR 1.33, p = .05) or SRS (RR 2.08) compared with TKI alone. There were more neurological adverse events in arms receiving RT [52]. One concern about this study is how the apparently small improvement in intracranial disease control at 4 months translates to a larger benefit in overall survival at 2 years. However, this meta‐analysis suggests that there may be some advantage to pursuing upfront RT in select groups of patients.
Multiple recent single institutional series have tried to clarify whether there is a benefit to initial RT. The majority of studies (summarized in Table 2) demonstrate that initial brain RT results in improved intracranial control, but this may not necessarily translate to improved survival. For example, a study of 110 patients with EGFR BMs from Memorial Sloan Kettering Cancer Center included 63 patients treated initially with erlotinib and 47 who had initial RT (32 WBRT, 15 SRS) [46]. The patients receiving RT were more likely to be symptomatic. The SRS group tended to have fewer tumors and a higher dsGPA score [53]. In this cohort, there was no difference in survival between the erlotinib group and the WBRT group. However, the WBRT group did have a longer time to intracranial progression (24 months vs. 16 months, p = .04). The SRS group demonstrated improved survival compared with the erlotinib group, which is not unexpected given the higher dsGPA score in this group. In the cohort who received initial erlotinib, 38% of patients ultimately received brain RT at a median of 17 months after the diagnosis of brain metastasis. Thus, the majority of patients receiving initial TKI never went on to receive RT.
Another recent retrospective cohort study from Yale provocatively suggested that patients with EGFR BMs may derive a survival benefit from early cranial radiotherapy [47]. A total of 50 patients who were TKI‐naïve and received different therapies were examined; 17 had initial TKI, 17 had WBRT then TKI, and 16 had SRS then TKI. These groups were similar in terms of dsGPA score, but the patients receiving RT first were more likely to be symptomatic from their CNS disease. These groups had similar rates of extracranial disease as well as similar performance status. The CNS ORR was similar for both groups (88% and 76% for RT and TKI, respectively), akin to other studies discussed above. OS was significantly longer in the initial RT group compared with the initial erlotinib group (34.1 months vs. 19.4 months, p = .01). When separating by type of RT, those treated with initial SRS had the best survival (median 58.4 months), and the initial WBRT group was intermediate (29.9 months). On multivariable analysis, initial RT and fewer than two organs with metastatic disease were significantly associated with overall survival. Interestingly, of 24 patients for whom a cause of death was identified, only 5 died of intracranial disease, whereas 15 died of extracranial disease. However, four of the five patients who died of intracranial disease were treated with initial erlotinib. Of the 15 patients in the RT group who died, only 1 died of intracranial disease. Although the numbers are small, these data raise the possibility that at least some of the patients receiving initial TKI may succumb to intracranial disease, which may have been prevented with initial radiotherapy.
Expanding on this finding, an updated analysis including data from several institutions demonstrated similar results [48]. They analyzed 351 patients with TKI‐naive EGFR BMs, easily the largest cohort of patients with EGFR BMs analyzed to date. The patients were evenly divided, as 37% received initial TKI, 34% received WBRT followed by TKI, and 29% received SRS followed by TKI. Again, patients receiving initial TKI were less likely to be symptomatic and had smaller brain metastases. More patients with a poor prognosis (dsGPA 0–1.5) received initial WBRT. The SRS patients had a better dsGPA, had fewer brain metastases, and were more likely to have been early stage at initial diagnosis of disease. There was no difference in performance status or presence of extracranial disease among the three groups. As might be expected based on dsGPA, the best overall survival was in the SRS group (46 months). The WBRT group had a longer OS (36 months) compared with the group that received initial TKI (25 months). Furthermore, restricting the analysis to the most favorable group (dsGPA 2–4), the WBRT group again had a longer OS (52 months) compared with the TKI group (32 months). Interestingly, only 52% of patients receiving initial TKI received RT at some point in their course (43% WBRT, 43% SRS, 14% both). It is unclear whether the OS of the group that received salvage RT was similar to those who received RT upfront. It would be very interesting to know if close surveillance and early salvage for the TKI patients would result in similar outcomes to using RT upfront. There was no difference in the use of second‐line systemic therapy among the groups. Initial radiotherapy (WBRT and SRS) remained significantly associated with improved survival in multivariable analysis. One notable difference in this study from many of the others was the relatively low burden of extracranial disease in this cohort. Only 24% of patients had extracranial metastases, compared with 50%–100% of patients in the other studies. It seems plausible that for patients with limited or no extracranial disease, improved intracranial control takes on additional importance and might translate into better overall survival.
Key Take‐Home Points.
Patients with EGFR BMs can have a long survival
EGFR TKIs have activity in the CNS, especially newer agents like osimertinib
Multidisciplinary evaluation is essential to select an optimal strategy tailored for the patient
Selected patients with small/asymptomatic lesions may delay or avoid radiation with TKI upfront and close surveillance
There may be a benefit to early radiotherapy in some patients, especially those with limited extracranial disease, but avoiding the toxicity of conventional WBRT is important
One possible explanation for the worsened survival in the TKI upfront group would be that patients may progress in such a way that renders them unfit for salvage therapy. From the Yale study, it appears that patients receiving TKI upfront are more likely to have the CNS as an isolated site of first failure (46% vs. 15%, p = .01). As noted above, this failure was associated with an increased risk of death from intracranial disease. The most likely concern would be the development of leptomeningeal disease, which is associated with a very poor prognosis. However, the study of 110 EGFR BM patients treated at Memorial Sloan Kettering found that 16% of patients developed leptomeningeal disease at a median of 15 months from diagnosis. There was no difference in incidence between the erlotinib, whole brain, and SRS groups. Thus, the data are mixed on whether using a TKI upfront may result in potentially unsalvageable intracranial progression.
Discussion
Brain metastases in lung cancer remain a vexing problem for clinicians. In patients with EGFR mutations, where systemic therapy has resulted in prolonged survival, treatment of intracranial disease has become an especially challenging problem. The desire to balance long‐term CNS control with potential detrimental neurocognitive effects of early cranial RT makes the initial management of these patients controversial. As described above, many of the EGFR TKIs have activity in the CNS that is comparable to WBRT. Both erlotinib and gefitinib are present in the CSF, although at lower levels than in plasma. Exciting new data regarding novel third‐generation TKIs, particularly osimertinib, suggest that such drugs may have an even greater efficacy for EGFR BMs. Furthermore, newer agents still in early phase studies have the potential for further improvements in CSF penetrance and CNS activity.
Review of the literature regarding the choice of initial therapy for EGFR BMs demonstrates significant heterogeneity and potential for selection bias, but it appears that the majority of studies show an improvement in intracranial control with upfront brain RT compared with TKI alone, without a difference in overall survival. Perhaps this is not entirely surprising, as multiple studies of brain metastases from many cancer types have shown that RT‐mediated improvement in intracranial control does not necessarily translate into improved overall survival [3], [54], [55].
Most of the studies discussed above show a similar intracranial response rate for either EGFR TKIs or radiotherapy, which raises the question of why there may be a difference in outcome in some subsets of patients. Most of the deaths reported were due to extracranial disease, which was unlikely to be influenced by the choice of initial RT. However, one possibility is that a subset of patients with EGFR BMs progress in the CNS after initial response to TKIs in a way that makes subsequent salvage WBRT much less effective (e.g., leptomeningeal dissemination). However, the data supporting this idea are limited. Another possibility is that initial brain RT may result in increased efficacy of TKIs in the CNS due to disruption of the blood‐brain barrier or some other mechanism. These studies suffer from their retrospective nature, as the choice between TKI and RT is likely driven by multiple factors, not all of which can be accounted for by propensity score, multivariable regression, or other correction techniques.
Conclusion
Moving forward, the choice of initial therapy for EGFR BMs must integrate these data along with the potential toxicity of WBRT, which was not adequately addressed in most of these studies. The preliminary data regarding the efficacy of newer TKIs against EGFR BMs (especially osimertinib) suggest that these drugs may be better initial therapies than erlotinib and gefitinib and thus may obviate the prior data comparing initial TKI versus RT. This is especially pertinent because standards of care are currently transitioning to upfront osimertinib. The FLAURA trial, a phase III randomized comparison of osimertinib versus either erlotinib or gefitinib, recently showed a significant PFS benefit for osimertinib, including among patients with CNS metastases [56]. A prospective randomized study in patients presenting with EGFR BMs comparing, for example, osimertinib followed by RT at progression to RT followed by osimertinib and examining key outcomes including intracranial control, quality of life, and neurocognitive measures would be the best way to address this question. Retrospectively, a more detailed analysis of patterns of CNS failure in patients treated with TKI as well as an analysis of causes of death would also help shed light on these questions.
In the absence of that data, we favor a multidisciplinary evaluation with consideration of initial TKI or SRS for those with limited intracranial disease. We favor initial TKI for lesions that are small, with minimal edema, and in areas where tumor growth is unlikely to result in neurologic symptoms. For patients with more diffuse disease, we generally recommend WBRT for those who are symptomatic or have larger lesions or significant vasogenic edema. We also consider investigational cognitive sparing strategies including hippocampal avoidance WBRT [57], as well as the use of memantine [58] to attempt to mitigate the long‐term adverse toxicity associated with WBRT. In selected patients, we use upfront SRS (single dose or occasionally fractionated) to individual lesions that are located in areas likely to produce symptoms upon progression, or those approaching 1 cm or greater. In these patients, we might consider treating only those most concerning lesions and rely on TKI to control the remainder of the lesions in an attempt to avoid the toxicity of WBRT. In some patients, the need for achieving extracranial control is the driving factor to start TKI therapy before radiation. In patients on TKI with diffuse CNS progression, we typically hold TKI for the duration of WBRT. If patients need SRS, the TKI can be continued, or held for a day or so surrounding SRS depending on the preference of the treatment team. Patients who are started on initial TKI therapy must be closely monitored to ensure that there is no symptomatic progression and to ensure that if there is intracranial progression it is caught at a time when salvage radiotherapy can still be entertained.
The preliminary data regarding the efficacy of newer TKIs against EGFR BMs (especially osimertinib) suggest that these drugs may be better initial therapies than erlotinib and gefitinib and thus may obviate the prior data comparing initial TKI versus RT. This is especially pertinent because standards of care are currently transitioning to upfront osimertinib.
Footnotes
For Further Reading: Maikel Verduin, Jaap D. Zindler, Hanneke M.A. Martinussen et al. Use of Systemic Therapy Concurrent With Cranial Radiotherapy for Cerebral Metastases of Solid Tumors. The Oncologist 2017;22:222–235.
Implications for Practice: The treatment of symptomatic brain metastases diagnosed while patients are receiving systemic therapy continues to pose a dilemma to clinicians. Will concurrent treatment with cranial radiotherapy and systemic therapy (chemotherapeutics, molecular targeted agents, and monoclonal antibodies), used to control intra‐ and extracranial tumor load, increase the risk for neurotoxicity? This review addresses this clinically relevant question and evaluates the toxicity of combining systemic therapies with cranial radiotherapy, based on currently available literature, in order to determine the need to and interval to interrupt systemic treatment.
Author Contributions
Conception/design: Melin J. Khandekar, Zofia Piotrowska, Henning Willers, Lecia V. Sequist
Collection and/or assembly of data: Melin J. Khandekar, Zofia Piotrowska, Henning Willers, Lecia V. Sequist
Data analysis and interpretation: Melin J. Khandekar, Zofia Piotrowska, Henning Willers, Lecia V. Sequist
Manuscript writing: Melin J. Khandekar, Zofia Piotrowska, Henning Willers, Lecia V. Sequist
Final approval of manuscript: Melin J. Khandekar, Zofia Piotrowska, Henning Willers, Lecia V. Sequist
Disclosures
Zofia Piotrowska: AstraZeneca, Ariad/Takeda, Novartis, Guardant Health, AbbVie, Boehringer‐Ingelheim (C/A), Novartis (to institution) (RF); Lecia V. Sequist: AstraZeneca, Pfizer, Novartis, Boehringer‐Ingelheim, Merrimack (C/A), AstraZeneca, Pfizer, Novartis, Boehringer‐Ingelheim, Merrimack, Incyte (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Lynch TJ, Bell DW, Sordella R et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non‐small‐cell lung cancer to gefitinib. N Engl J Med 2004;350:2129–2139. [DOI] [PubMed] [Google Scholar]
- 2. Paez JG, Janne PA, Lee JC et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science 2004;304:1497–1500. [DOI] [PubMed] [Google Scholar]
- 3. Brown PD, Jaeckle K, Ballman KV et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: A randomized clinical trial. JAMA 2016;316:401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mak KS, Gainor JF, Niemierko A et al. Significance of targeted therapy and genetic alterations in EGFR, ALK, or KRAS on survival in patients with non‐small cell lung cancer treated with radiotherapy for brain metastases. Neuro Oncol 2015;17:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Katayama T, Shimizu J, Suda K et al. Efficacy of erlotinib for brain and leptomeningeal metastases in patients with lung adenocarcinoma who showed initial good response to gefitinib. J Thorac Oncol 2009;4:1415–1419. [DOI] [PubMed] [Google Scholar]
- 6. Kim JE, Lee DH, Choi Y et al. Epidermal growth factor receptor tyrosine kinase inhibitors as a first‐line therapy for never‐smokers with adenocarcinoma of the lung having asymptomatic synchronous brain metastasis. Lung Cancer 2009;65:351–354. [DOI] [PubMed] [Google Scholar]
- 7. Maemondo M, Inoue A, Kobayashi K et al. Gefitinib or chemotherapy for non‐small‐cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380–2388. [DOI] [PubMed] [Google Scholar]
- 8. Porta R, Sanchez‐Torres JM, Paz‐Ares L et al. Brain metastases from lung cancer responding to erlotinib: The importance of EGFR mutation. Eur Respir J 2011;37:624–631. [DOI] [PubMed] [Google Scholar]
- 9. Heon S, Yeap BY, Lindeman NI et al. The impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non‐small cell lung cancer with EGFR mutations. Clin Cancer Res 2012;18:4406–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park SJ, Kim HT, Lee DH et al. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non‐small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer 2012;77:556–560. [DOI] [PubMed] [Google Scholar]
- 11. Eichler AF, Kahle KT, Wang DL et al. EGFR mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro Oncol 2010;12:1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Doebele RC, Lu X, Sumey C et al. Oncogene status predicts patterns of metastatic spread in treatment‐naive nonsmall cell lung cancer. Cancer 2012;118:4502–4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hendriks LE, Smit EF, Vosse BA et al. EGFR mutated non‐small cell lung cancer patients: More prone to development of bone and brain metastases? Lung Cancer 2014;84:86–91. [DOI] [PubMed] [Google Scholar]
- 14. Shin DY, Na, II, Kim CH et al. EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J Thorac Oncol 2014;9:195–199. [DOI] [PubMed] [Google Scholar]
- 15. Han G, Bi J, Tan W et al. A retrospective analysis in patients with EGFR‐mutant lung adenocarcinoma: Is EGFR mutation associated with a higher incidence of brain metastasis? Oncotarget 2016;7:56998–57010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sekine A, Kato T, Hagiwara E et al. Metastatic brain tumors from non‐small cell lung cancer with EGFR mutations: Distinguishing influence of exon 19 deletion on radiographic features. Lung Cancer 2012;77:64–69. [DOI] [PubMed] [Google Scholar]
- 17. Takano K, Kinoshita M, Takagaki M et al. Different spatial distributions of brain metastases from lung cancer by histological subtype and mutation status of epidermal growth factor receptor. Neuro Oncol 2016;18:716–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sperduto PW, Yang TJ, Beal K et al. Estimating survival in patients with lung cancer and brain metastases: An update of the graded prognostic assessment for lung cancer using molecular markers (Lung‐molGPA). JAMA Oncol 2017;3:827–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Namba Y, Kijima T, Yokota S et al. Gefitinib in patients with brain metastases from non‐small‐cell lung cancer: Review of 15 clinical cases. Clin Lung Cancer 2004;6:123–128. [DOI] [PubMed] [Google Scholar]
- 20. Ceresoli GL, Cappuzzo F, Gregorc V et al. Gefitinib in patients with brain metastases from non‐small‐cell lung cancer: A prospective trial. Ann Oncol 2004;15:1042–1047. [DOI] [PubMed] [Google Scholar]
- 21. Wu C, Li YL, Wang ZM et al. Gefitinib as palliative therapy for lung adenocarcinoma metastatic to the brain. Lung Cancer 2007;57:359–364. [DOI] [PubMed] [Google Scholar]
- 22. Iuchi T, Shingyoji M, Sakaida T et al. Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR‐mutant lung adenocarcinoma. Lung Cancer 2013;82:282–287. [DOI] [PubMed] [Google Scholar]
- 23. Zhang Q, Zhang X, Yan H et al. Effects of epidermal growth factor receptor‐tyrosine kinase inhibitors alone on EGFR‐mutant non‐small cell lung cancer with brain metastasis. Thorac Cancer 2016;7:648–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu YL, Zhou C, Cheng Y et al. Erlotinib as second‐line treatment in patients with advanced non‐small‐cell lung cancer and asymptomatic brain metastases: A phase II study (CTONG‐0803). Ann Oncol 2013;24:993–999. [DOI] [PubMed] [Google Scholar]
- 25. Hoffknecht P, Tufman A, Wehler T et al. Efficacy of the irreversible ErbB family blocker afatinib in epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI)‐pretreated non‐small‐cell lung cancer patients with brain metastases or leptomeningeal disease. J Thorac Oncol 2015;10:156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schuler M, Wu YL, Hirsh V et al. First‐line afatinib versus chemotherapy in patients with non‐small cell lung cancer and common epidermal growth factor receptor gene mutations and brain metastases. J Thorac Oncol 2016;11:380–390. [DOI] [PubMed] [Google Scholar]
- 27. Mok T, Ahn MJ, Han JY et al. CNS response to osimertinib in patients (pts) with T790M‐positive advanced NSCLC: Data from a randomized phase III trial (AURA3). Paper presented at: 2017 ASCO Annual Meeting; 2017; Chicago.
- 28. Yang JC, Ahn MJ, Kim DW et al. Osimertinib in pretreated T790M‐positive advanced non‐small‐cell lung cancer: AURA study phase II extension component. J Clin Oncol 2017;35:1288–1296. [DOI] [PubMed] [Google Scholar]
- 29. Togashi Y, Masago K, Fukudo M et al. Efficacy of increased‐dose erlotinib for central nervous system metastases in non‐small cell lung cancer patients with epidermal growth factor receptor mutation. Cancer Chemother Pharmacol 2011;68:1089–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kodack DP, Askoxylakis V, Ferraro GB et al. Emerging strategies for treating brain metastases from breast cancer. Cancer Cell 2015;27:163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sequist LV, Piotrowska Z, Niederst MJ et al. Osimertinib responses after disease progression in patients who had been receiving rociletinib. JAMA Oncol 2016;2:541–543. [DOI] [PubMed] [Google Scholar]
- 32. Ahn MJ, Tsai CM, Yang JCH, et al. 3083 AZD9291 activity in patients with EGFR‐mutant advanced non‐small cell lung cancer (NSCLC) and brain metastases: Data from phase II studies. Eur J Cancer 2015;51:S625–S626. [Google Scholar]
- 33. Yang JC, Cho BC, Kim DW et al. Osimertinib for patients (pts) with leptomeningeal metastases (LM) from EGFR‐mutant non‐small cell lung cancer (NSCLC): Updated results from the BLOOM study. J Clin Oncol 2017;35(suppl 15):2020a. [Google Scholar]
- 34. Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum‐pemetrexed in EGFR T790M‐positive lung cancer. N Engl J Med 2017;376:629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang Z, Guo Q, Wang Y et al. AZD3759, a BBB‐penetrating EGFR inhibitor for the treatment of EGFR mutant NSCLC with CNS metastases. Science Transl Med 2016;8:368ra172. [DOI] [PubMed] [Google Scholar]
- 36. Berz D, Subrananiam D, Tonra J et al. Tesevatinib in NSCLC patients with EGFR activating mutations and brain metastases or leptomeningeal metastases. Paper presented at: IASLC 17th World Conference on Lung Cancer; 2016; Vienna, Austria.
- 37. Liu S, Qiu B, Chen L et al. Radiotherapy for asymptomatic brain metastasis in epidermal growth factor receptor mutant non‐small cell lung cancer without prior tyrosine kinase inhibitors treatment: A retrospective clinical study. Radiat Oncol 2015;10:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Welsh JW, Komaki R, Amini A et al. Phase II trial of erlotinib plus concurrent whole‐brain radiation therapy for patients with brain metastases from non‐small‐cell lung cancer. J Clin Oncol 2013;31:895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee SM, Lewanski CR, Counsell N et al. Randomized trial of erlotinib plus whole‐brain radiotherapy for NSCLC patients with multiple brain metastases. J Natl Cancer Inst 2014;106:dju151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jiang T, Su C, Li X et al. EGFR TKIs plus WBRT demonstrated no survival benefit other than that of TKIs alone in patients with NSCLC and EGFR mutation and brain metastases. J Thorac Oncol 2016;11:1718–1728. [DOI] [PubMed] [Google Scholar]
- 41. Chen Y, Yang J, Li X et al. First‐line epidermal growth factor receptor (EGFR)‐tyrosine kinase inhibitor alone or with whole‐brain radiotherapy for brain metastases in patients with EGFR‐mutated lung adenocarcinoma. Cancer Sci 2016;107:1800–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhu Q, Sun Y, Cui Y et al. Clinical outcome of tyrosine kinase inhibitors alone or combined with radiotherapy for brain metastases from epidermal growth factor receptor (EGFR) mutant non small cell lung cancer (NSCLC). Oncotarget 2017;8:13304–13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim JM, Miller JA, Kotecha R et al. The risk of radiation necrosis following stereotactic radiosurgery with concurrent systemic therapies. J Neurooncol 2017;133:357–368. [DOI] [PubMed] [Google Scholar]
- 44. Gondi V, Paulus R, Bruner DW et al. Decline in tested and self‐reported cognitive functioning after prophylactic cranial irradiation for lung cancer: Pooled secondary analysis of radiation therapy oncology group randomized trials 0212 and 0214. Int J Radiat Oncol Biol Phys 2013;86:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Byeon S, Ham JS, Sun JM et al. Analysis of the benefit of sequential cranial radiotherapy in patients with EGFR mutant non‐small cell lung cancer and brain metastasis. Med Oncol 2016;33:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gerber NK, Yamada Y, Rimner A et al. Erlotinib versus radiation therapy for brain metastases in patients with EGFR‐mutant lung adenocarcinoma. Int J Radiat Oncol Biol Phys 2014;89:322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Magnuson WJ, Yeung JT, Guillod PD et al. Impact of deferring radiation therapy in patients with epidermal growth factor receptor‐mutant non‐small cell lung cancer who develop brain metastases. Int J Radiat Oncol Biol Phys 2016;95:673–679. [DOI] [PubMed] [Google Scholar]
- 48. Magnuson WJ, Lester‐Coll NH, Wu AJ et al. Management of brain metastases in tyrosine kinase inhibitor‐naive epidermal growth factor receptor‐mutant non‐small‐cell lung cancer: A retrospective multi‐institutional analysis. J Clin Oncol 2017;35:1070–1077. [DOI] [PubMed] [Google Scholar]
- 49. Doherty MK, Korpanty GJ, Tomasini P et al. Treatment options for patients with brain metastases from EGFR/ALK‐driven lung cancer. Radiother Oncol 2017;123:195–202. [DOI] [PubMed] [Google Scholar]
- 50. Fan Y, Xu Y, Gong L et al. Effects of icotinib with and without radiation therapy on patients with EGFR mutant non‐small cell lung cancer and brain metastases. Sci Rep 2017;7:45193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang JJ, Zhou C, Huang Y et al. Icotinib versus whole‐brain irradiation in patients with EGFR‐mutant non‐small‐cell lung cancer and multiple brain metastases (brain): A multicentre, phase 3, open‐label, parallel, randomised controlled trial. Lancet Respir Med 2017;5:707–716. [DOI] [PubMed] [Google Scholar]
- 52. Soon YY, Leong CN, Koh WY et al. EGFR tyrosine kinase inhibitors versus cranial radiation therapy for EGFR mutant non‐small cell lung cancer with brain metastases: A systematic review and meta‐analysis. Radiother Oncol 2015;114:167–172. [DOI] [PubMed] [Google Scholar]
- 53. Sperduto PW, Kased N, Roberge D et al. Summary report on the graded prognostic assessment: An accurate and facile diagnosis‐specific tool to estimate survival for patients with brain metastases. J Clin Oncol 2012;30:419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Patchell RA, Tibbs PA, Regine WF et al. Postoperative radiotherapy in the treatment of single metastases to the brain: A randomized trial. JAMA 1998;280:1485–1489. [DOI] [PubMed] [Google Scholar]
- 55. Aoyama H, Shirato H, Tago M et al. Stereotactic radiosurgery plus whole‐brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA 2006;295:2483–2491. [DOI] [PubMed] [Google Scholar]
- 56. Soria JC, Ohe Y, Vansteenkiste J et al. Osimertinib in untreated EGFR‐mutated advanced non‐small‐cell lung cancer. N Engl J Med 2018;378:113–125. [DOI] [PubMed] [Google Scholar]
- 57. Gondi V, Pugh SL, Tome WA et al. Preservation of memory with conformal avoidance of the hippocampal neural stem‐cell compartment during whole‐brain radiotherapy for brain metastases (RTOG 0933): A phase II multi‐institutional trial. J Clin Oncol 2014;32:3810–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Brown PD, Pugh S, Laack NN et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole‐brain radiotherapy: A randomized, double‐blind, placebo‐controlled trial. Neuro Oncol 2013;15:1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]