Background
The taxonomy of the genus Burkholderia is continually growing and includes over 75 species of gram negative bacteria1. Ubiquitous in soil and water, Burkholderia cepacia complex (Bcc) emerged in the healthcare setting as a significant and transmissible pathogen among pediatric patients with cystic fibrosis.2,3 Many outbreaks among non-cystic fibrosis patients, including immunocompromised and immunocompetent patients have been linked to intrinsic and extrinsic Bcc contamination of medical products such as temperature probes, ultrasound gels, nasal sprays, nebulized or intravenous solutions, mouthwashes, prefabricated wet wipes or washcloths, antiseptics, and disinfectant solutions.4–10
In February 2016, a pediatric hospital in Texas began an internal investigation of a cluster of patients in the critical care unit with Bcc positive cultures.11 Clinical isolates from the hospital were submitted to the University of Michigan Burkholderia cepacia Research Laboratory and Repository for molecular analysis. In May 2016, the Centers for Disease Control and Prevention (CDC) was notified by public health officials in Texas and Illinois that the Burkholderia cepacia Research Laboratory had found that the isolates submitted by the pediatric hospital in Texas were indistinguishable from a cluster of Bcc positive cultures taken from critically ill, non-cystic fibrosis, pediatric patients in Illinois, and that the isolates belonged to a previously undescribed species of Bcc. Within days, the California Department of Health notified CDC of two additional clusters occurring among patients in pediatric critical care units. This suggested that contamination of a widely distributed product could be the common source for these clusters of infections. This report describes the investigation by multiple hospitals, state and local health departments, CDC, and the Food and Drug Administration (FDA).
Methods
Identification of Clusters
Following the initial reports about a possible outbreak of Bcc infections among hospitalized children, CDC used the Epidemic Information Exchange and the Emerging Infections Network as well as email distribution lists of clinical professional organizations to request reports of clusters of Bcc infections (i.e., ≥2 Bcc infections) among critically ill pediatric non-cystic fibrosis patients. This call was broadened to include adult patients following the report of a new cluster among critically ill adult non-cystic fibrosis patients. State public health authorities further disseminated requests for reporting of cases within their jurisdictions. During the investigation the affected pediatric institution in Texas used a reference laboratory to test environmental cultures of specific products and medications used in the care of patients.11
Data Collection
Data collection was conducted using a standardized linelist. Information about medical devices, procedures, products used for respiratory, oral and skin care, and intranasal, inhaled and oral medications was collected. Product information, such as brand and manufacturer, was not always available for respiratory, oral and skin care products that were thought to have been administered to patients because these types of products are often not charged to patients and their use is often not documented in the medical record. Medication administration and pharmacy records were used to compare the National Drug Code (NDC) of medications.
Case Definition
Based on the evolution of epidemiologic and laboratory strain typing data during the outbreak, the case definition was refined during the initial investigation and ultimately included 2 strains of Bcc that were recovered from patient clinical specimens, referred to as strain A and strain B. A confirmed case was defined as the first clinical culture of Bcc matching one of the outbreak strains by molecular typing methods, collected from a hospitalized patient on or after January 1, 2016. A suspect case was defined as a clinical culture obtained since January 1, 2016, yielding Bcc of an unknown strain type in a patient located in a healthcare facility with confirmed cases or who had exposure to the implicated product.
FDA Laboratory Methods
The FDA identified the manufacturing site of the suspected product using the NDC code. FDA collected environmental samples from the purified water system used for drug production at Manufacturer X as well as product samples obtained at the production site and affected health care facilities. The FDA’s, Office of Regulatory Affairs (ORA) within the Office of Regulatory Science (ORS), utilized both the United States Pharmacopeial Convention (USP) and the Bacteriological Analytical Manual methodology for testing medical products and cosmetics, respectively. 12, 13 Organisms of interest were Bcc and Pseudomonas aeruginosa. A detailed description of FDA microbiologic laboratory methods used for the specific identification of Bcc is available online in supplementary materials.
CDC Laboratory Methods
The University of Michigan Burkholderia cepacia Research Laboratory and Repository identified the initial isolates among suspect case-patients as indistinguishable and noted a wide geographic dispersion. The CDC was notified and state health departments shared these and additional isolates from suspect case-patients for confirmatory culture and molecular analysis.
Thirty-one products from affected healthcare facilities, including liquid docusate, sterile water for inhalation, ventilator circuits, oral care products, bathing wipes, and others were submitted by State Health Departments and cultured. Molecular analysis of isolates yielded by clinical and environmental cultures was performed using pulsed field gel lectrophoresis (PFGE) and whole genome sequencing (WGS). A detailed description of methods is available in online supplementary materials. The Tenover criteria were used to interpret the relatedness of the PFGE patterns; patterns were classified as indistinguishable (100% similarity), closely related (1–3 bands difference), possibly related (4–6 band difference) or unrelated (>7 band difference).14
Results
Epidemiologic Investigation
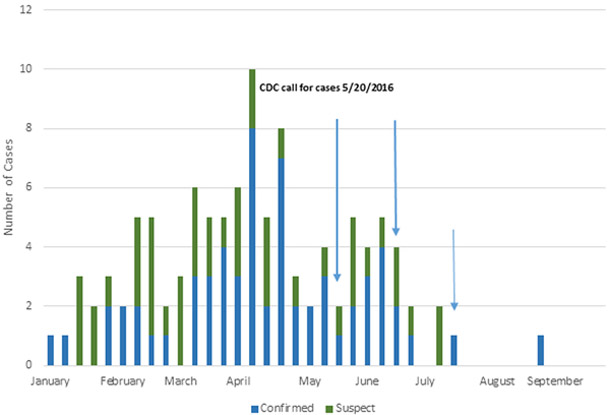
As a result of initial reporting and case finding efforts, CDC investigated >300 reports of positive Bcc cultures and identified 108 cases (63 confirmed and 45 suspect cases) in 12 states (Figure 1). The age of confirmed and suspect cases ranged from 2 months to 85 years (median 10 years), with 58 (53%) of cases occurring among infants and children (ages 2 months- 12 years). Clinical cultures yielding Bcc were collected from a variety of body sites including respiratory, blood, abdominal organ space, urine, stool, and the access site of a peripheral intravenous catheter. Forty-three of the 108 (40%) patients had Bcc cultured from multiple sites (Table 1). Four of the 12 (33%) Bcc bloodstream infections were classified as secondary bloodstream infections. Fourteen (22%) individuals with cultures confirmed to be indistinguishable or closely related by PFGE to strain A or B were deceased at the time the report was received.
Figure 1.
Epidemiologic Curve, January to September, 2016.
Note: Epidemiologic curve by the date of the patient’s first culture yielding Bcc.
Table 1.
Characteristics of case-patients with B. cepacia complex Infections Associated with Contaminated Oral Liquid Docusate Sodium
| n (%) | |||
|---|---|---|---|
| Characteristic | Confirmed (n=63) | Suspect (n=45) | Total (n=108) |
| Age | |||
| Infant (2 mo. – 23 mo.) | 29 (46.0) | 8 (17.8) | 37 (34.3) |
| Child (2-17 years) | 15 (23.8) | 7 (15.6) | 22 (20.4) |
| Adult (18-64 years) | 15 (23.8) | 24 (53.3) | 39 (36.1) |
| Older Adult (≥ 65 years) | 4 (16.3) | 6 (13.3) | 10 (9.3) |
| Site of cultures yielding B. cepacia complex (includes cases with more than one culture positive site) | |||
| Confirmed (n=89) | Suspect (n=63) | Total (n=152) | |
| Respiratory Tract | 50 (69.4) | 34 (73.9) | 84 (71.2) |
| Blood | 12 (16.7) | 4 (8.7) | 16 (13.6) |
| Abdominal, Organ Space | 1 (1.4) | 2 (4.3) | 3 (2.5) |
| Urine | 5 (6.9) | 6 (13.0) | 11 (9.3) |
| Stool | 3 (4.2) | 0 | 3 (2.5) |
| Wound (Peripheral IV Site) | 1 (1.4) | 0 | 1 (0.8) |
| Admission unit | Confirmed (n=63) | Suspect (n= 45) | Total (n=108) |
| Pediatric Critical Care | 40 (63.4) | 13 (28.9) | 53 (49.1) |
| Adult Critical Care | 16 (25.4) | 22 (48.9)) | 38 (35.2) |
| Other Inpatient Care Units | 7 (11.1) | 10 (22.2) | 17 (15.7) |
| Disposition (at time of reporting) | Confirmed (n=63) | Suspect (n= 45) | Total (n=108) |
| Inpatient | 37 (58.7) | 12 (26.7) | 49 (45.4) |
| Discharged or Transferred | 12 (19.8) | 26 (57.8) | 38 (35.2) |
| Deceased | 14 (22.2) | 7 (15.6) | 21 (19.4) |
| Use of medical devices | Confirmed (n=57) | Suspect (n=27) | Total (n=84) |
| Mechanical Ventilation | 53 (93.0) | 27 (100) | 80 (95.2) |
| Central Venous Catheter | 39 (68.4) | 7 (25.9) | 46 (54.8) |
| Feeding Tube | 34 (59.6) | 7 (25.9) | 41 (48.8) |
| Urinary Catheter | 33 (57.9) | 7 (25.9) | 41 (48.8) |
| Patient exposure to oral, liquid docusate | |||
| Product | Confirmed (n= 63) | Suspect (n=45) | Total (n=108) |
| NDC of implicated product | 58 (92.0) | 37 (82.2) | 95 (87.9) |
| NDC of implicated product + additional formulations | 11 (17.4) | 14 (31.1) | 25 (23.1) |
Cases occurred among patients hospitalized at 16 facilities; 89/108 (82%) received care in an adult or pediatric critical care unit. Information about medical device use among 84 case patients revealed that 80 (95%) were mechanically ventilated and 41 (48%) had feeding tubes. Other case patient characteristics are presented in Table 1. Review of products used for oral and skin care did not reveal any common products in use across facilities. The review of oral care products was problematic due to post-production repackaging of supplies with inclusion of multiple lot numbers by several distributors. Products used in the care of ventilated patients revealed that ventilator circuits and sterile water for ventilation were produced by a common manufacturer but differences in product models and a lack of microbiologic evidence resulted in none being implicated.
Review of medication administration records revealed that 58 of the 63 (92%) case-patients had received liquid docusate sodium bearing the National Drug Code (NDC) of the implicated product. Thirty-nine (36 %) cases were exposed to more than one docusate formulation. Besides docusate, no other common medication exposures were identified.
Clinical and Public Health Laboratory Testing
On the evening of June 22, 2016, the Texas Department of State Health Services notified the CDC that the reference laboratory for the affected hospital in Texas had preliminarily identified Bcc in a culture obtained from a pre-filled oral syringe containing docusate sodium. The Texas Department of State Health Services submitted isolates from the affected hospital’s reference laboratory to CDC, which confirmed the presence of Bcc in the liquid docusate sodium.
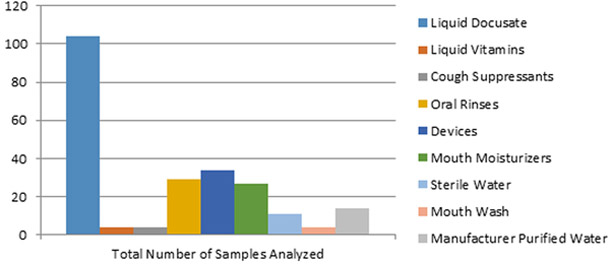
During the course of the investigation, FDA laboratories tested over 200 samples (Figure 2). Bcc was isolated from 24 samples of liquid docusate finished product made by Manufacturer X and from their purified water system. Furthermore, 22 samples of the liquid docusate produced by Manufacturer X also contained high levels of other microbial organisms, including Candida spp., mold, and Enterobacter spp., with total aerobic microbial counts as high as 40,000 colony forming units per milliliter. Data about the effectiveness of the preservatives used by Manufacturer X to control microbial growth in the implicated liquid docusate product were not available. FDA testing did not detect Bcc or related species from any of the other products analyzed during the investigation.
Figure 2:
Summary of Samples Analyzed by FDA Laboratories
Note: Several products from multiple manufacturers were initially tested for contamination. As docusate samples from Manufacturer X began testing positive for Bcc, more docusate samples were collected and tested. This led to docusate forming a higher proportion of samples tested.
FDA shared isolates obtained from environmental cultures with CDC for comparison to clinical isolates. Molecular analysis by CDC confirmed that isolates obtained from the purified water system at Manufacturer X’s production facility were closely related to strain A. Isolates yielded by the samples of liquid docusate revealed that they were indistinguishable or closely related to strain A or strain B. Thus, isolates from Manufacturer X’s purified water, from the liquid docusate and from case patient clinical cultures were all closely related.
Among the isolates collected from the 63 confirmed cases, 49 (77%) were indistinguishable or closely related to strain A, and fourteen (23%) isolates were indistinguishable or closely related to strain B. Each strain was identified from cases from multiple states and from multiple lots of Manufacturer X’s liquid docusate product (Figure 3). Of the 31 products cultured at CDC, including oral and respiratory care kits, and bath wipes from the affected pediatric hospitals, only liquid docusate yielded Bcc.
Figure 3.
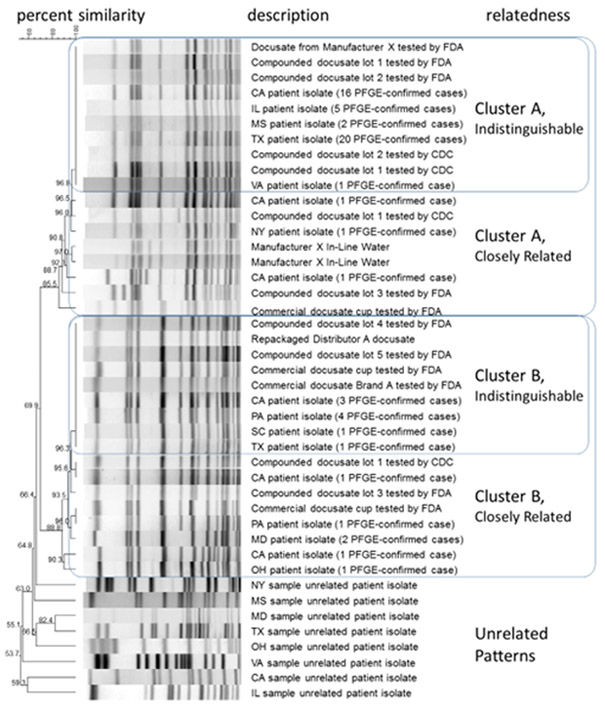
Dendrogram: Percent Similarity of PFGE Patterns of Bcc isolates
Note: Figure 3 includes a representative isolate from each of the affected states, that is indistinguishable or closely related by PFGE to Strain A or Strain B; the comment indicates the total number of isolates confirmed in that state. Products and environmental samples are indicated within strain clusters.
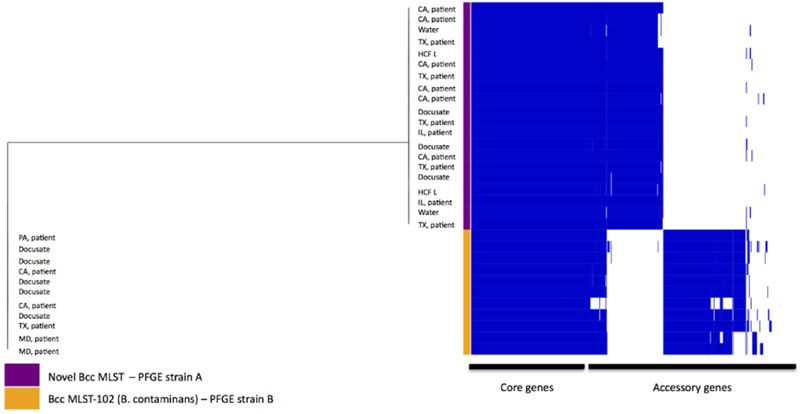
Whole Genome Sequence (WGS) data were consistent with PFGE results and indicated two distinct clusters of B. cepacia complex (Figure 4). The average SNP count between both clusters and PFGE species was widely divergent with 122,355 SNPs over a core genome of 4.1 Mb, which equals 49% of the mapping reference genome. WGS data also revealed a novel Bcc clade (strain A), the genetic diversity of which ranged between 0 and 11 SNPs, identified over a core genome of 5.1 Mb which equals 62% of the mapping reference genome and translated to a maximum core genome difference of 0.0002% . Isolates from the other cluster represented a B. contaminans clade (ST102 [strain B]) and had a larger genetic diversity range, from 0 to 49 SNPs , identified over a core genome of 5 Mb which equals 61 % of the mapping reference genome and translated to a maximum core genome difference of 0.00098%. Additionally, functional pangenome analysis displayed extensive gene content differences between the two clades, indicative of two distinct Bcc strains.
Figure 4:
Phylogenetic Tree
Note: Phylogenetic tree based on Single Nucleotide Polymorphism (SNP) data of all sequenced isolates, displaying two distinct clusters of Burkholderia cepacia complex species. Average SNP count between both clusters, an ST102 and a novel MLST cluster, was 122355 SNPs. The genetic diversity of the novel Bcc strain cluster ranged between 0 and 11 SNPs, however a larger diversity range, from 0 to 49 SNPs, was observed for the B. contaminans clade. The first column to the right of the tree corresponds to Multilocus Sequence Typing (MLST) and Pulsed-Field Gel Electrophoresis (PFGE) data for each isolate. Columns in the gene matrix represent homologous gene clusters and are ordered by frequency of gene presence. Dark blue bars indicate gene presence and white gene absence.
Termination of Outbreak
On June 24, 2016, in response to the initial finding of Bcc in a prefilled syringe containing liquid docusate, CDC recommended that healthcare providers not use any liquid docusate products for critically ill, ventilated, or immunosuppressed patients until more information became available.15 On July 16, 2016 the FDA announced that Manufacturer X was voluntarily recalling liquid docusate sodium.16 Because Manufacturer X used the same water system in the production of all of their liquid products, on August 9, 2016 they expanded the recall to include all liquid products made at the site. Manufacturer X also recalled the majority of their solid products due to separate product quality issues and safety complaints. The last confirmed case of Bcc associated with the outbreak occurred on September 4, 2016. In an effort to fully understand the scope and cause of the contamination issue, the FDA conducted inspections at multiple domestic and international drug manufacturing facilities. On October 12, 2016 the FDA completed their investigation, concluding that poor manufacturing practices and contamination of the purified water supply at Manufacturer X were the root causes of the docusate contamination.16 Identification and removal of the intrinsically contaminated product from the market were essential in terminating the outbreak.
Discussion
We investigated a large, multistate, multi-hospital outbreak associated with liquid docusate sodium that was contaminated with two distinct strains of Bcc. The recognition of unusual clusters of Bcc infections among non-cystic fibrosis patients at multiple hospitals followed by identification and recall of intrinsically contaminated liquid docusate was essential to the detection and control of the outbreak. This and other recent outbreaks highlight Bcc as a frequent and problematic pathogen in healthcare due to its environmental persistence and tendency to contaminate many types of aqueous solutions. 17,18 Water is the most common raw material used in the manufacture of non-sterile liquid drugs which may pose an underappreciated risk for exposure to pathogens resulting in healthcare associated infections.19
The FDA requirements for carefully designed and controlled manufacturing operations that proactively prevent contamination with objectionable microbes are the first line of defense against the contamination of non-sterile drugs. A risk-based approach to evaluating each isolated organism, rather than an exhaustive list of objectionable organisms, is used by manufacturers to determine the safety of non-sterile pharmaceutical products.20 Manufacturers must assess relevant factors, which include the nature of the raw materials, the processing these will undergo, and the ability of the finished product to support microbial growth. The intended use, the route of administration, and the susceptibility of the population who will use the drug should also be considered. During production and prior to release of the product, it is the responsibility of the manufacturer to perform validated microbiologic testing on batches of liquid pharmaceuticals to ensure the absence of objectionable microorganisms, such as Bcc. Manufacturers of non-sterile water-based pharmaceuticals must pay attention to the possibility of contamination and proactively limit bioburden levels, prevent objectionable contamination, and establish sufficient microbial quality standards for finished product. This multi-state Bcc outbreak among susceptible patients underscores the importance of the manufacturer’s responsibility to establish strict specifications for non-sterile drugs that ensure the safety of the full breadth of patient populations using the product. 21 Lapses in current good manufacturing practices and quality control can have serious consequences.
Preservatives used by Manufacturer X may not have been sufficient to prevent the proliferation of Bcc or other microorganisms in the finished drug product. This problem could have enabled contaminants to proliferate in the liquid docusate product after release prior to use. Administration of the contaminated liquid docusate to critically ill patients via feeding tubes, coupled with mechanical ventilation, and the risk of aspiration among such patients, may have increased the ability of the organisms to colonize and infect the respiratory tract and spread to other body sites.22
The intrinsic tolerance of Bcc to commonly used antiseptics and disinfectants is problematic in the healthcare setting. 23,24 Bcc can be spread via the hands of healthcare personnel or mobile medical equipment. Transmission via indirect contact was suspected in one facility in which patients had overlapping stays in the same unit and bed space and in whom exposure to implicated docusate could not be verified. Transmission-based precautions are not routinely recommended for Bcc infections in most settings; however, in an outbreak setting where patient to patient transmission is suspected or confirmed, strict adherence to Contact Precautions accompanied by thorough environmental cleaning may assist in limiting transmission.25
Several limitations should be noted. Case finding was conducted based on clinical cultures among hospitalized, critically ill patients only and may not have identified all patients who acquired the organism. Reports of infections were submitted on a voluntary basis by acute care facilities and it is likely that patients in other settings were exposed to contaminated docusate. Clinical information was collected at the time of the report and did not always include the final disposition of the patient; therefore it is not known how many deaths were directly or indirectly attributed to this outbreak. Finally, confirmed cases among infants and children may have been detected at an increased rate because this organism is readily recognized as a threat among pediatric patients. A higher proportion of adults were classified as suspect cases; isolates were not retained by reporting facilities for confirmation.
Clinician recognition and reporting of unusual clusters of infections is an important component of identifying contaminated drug products and their removal from the market. This outbreak investigation was aided by ongoing surveillance to detect clusters of common strains within the B. cepacia complex at the University of Michigan Burkholderia cepacia Research Laboratory and Repository. Prompt reporting to the CDC and collaboration among private and public, regulatory and non-regulatory agencies resulted in the removal of the implicated product from patient care, preventing additional cases.
Members of the B. cepacia complex investigation workgroup include: Jannifer Anderson, Geoffrey Brousseau, Deborah Baker, Alison Laufer-Halpin, Bonnie Herring, Lindsay Montoya, Rebecca Perlmutter, Imran Shakih, Rolieria Deadwyler-West
Supplementary Material
Acknowledgments
*Financial Support: none reported.
Footnotes
*Disclaimer:The findings and conclusions in this report are those of the author and do not necessarily represent the official positions of the Centers for Disease Control and Prevention and the Food and Drug Administration.
*The authors report no conflicts of interest.
References
- 1.List of parokaryotic names with standing in nomenclature. LPSNbacterio.net. http://www.bacterio.net/burkholderia.html Accessed February 14, 2017. [Google Scholar]
- 2.Mahenthiraglingam E, Urban TA, Goldberg JB. The multifarious, multireplicon Burkholderia cepacia complex. Nat Rev Microbiol. 2005: 2:144–56. [DOI] [PubMed] [Google Scholar]
- 3.Isles A, Mclusky I, Corey M, et al. Pseudomonas cepacia infection in cystic fibrosis: An emerging problem. J Pediatr. 1984;104: 206–10. [DOI] [PubMed] [Google Scholar]
- 4.Ghazal S, Al-Mudalmeegh K, Fakihl E, Aserv A. Outbreak of Burkholderia cepacia bacteremia in immunocompetent children caused by contaminated nebulized salbutamol in Saudi Arabia. Am J Infect Control. 2006; 34: 394–8. [DOI] [PubMed] [Google Scholar]
- 5.Hutchinson J, Runge W, Mulvey M, et al. Burkholderia cepacia infections associated with intrinsically contaminated ultrasound gel: the role of microbial degradation of parabens. Infect Control Hosp Epidemiol. 2004; 25:291–6. [DOI] [PubMed] [Google Scholar]
- 6.Dolan S, Dowell E, LiPuma J, et al. An outbreak of Burkholderia cepacia complex associated with intrinsically contaminated nasal spray. Infect Control Hosp Epidemiol. 2011; 32: 804–10. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Manufacturer’s recall of nasal spray contaminated with Burkholderia cepacia complex. MMWR Dispatch. March 24, 2004: Volume 54. [Google Scholar]
- 8.Doit C, Simon A, Ferroni A, et al. Outbreak of Burkholderia cepacia bacteremia in a pediatric hospital due to contamination of lipid emulsion stoppers. J Clin Microbiol. 2004; 42: 2227–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metcalf P, Newman K, Siegel J, et al. Nosocomial Burkholderia cepacia infections associated with exposure to sublingual probes, Texas 2004. MMWR Weekly. 2004; 53: 796. [Google Scholar]
- 10.Lee S, Han SW, Kim G, et al. An outbreak of Burkholderia cenocepaica associated with contaminated chlorhexidine solutions prepared in the hospital. Am J Infect Control. 2013; 41:e93–6. [DOI] [PubMed] [Google Scholar]
- 11.Marquez L, Jones K, Whaley E, et al. An outbreak of Burkholderia cepacia Complex Infections Associated with contaminated liquid docusate. Infect Control Hosp Epi. 2017; 43:42. [DOI] [PubMed] [Google Scholar]
- 12.United States Pharmacopeial Convention. General Methods. Available at http://www.uspnf.com/ Published June 27, 2013. Accessed June 21, 2017. [Google Scholar]
- 13.Food and Drug Administration. Bacteriologic Analytic Manual. Available at https://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm071363.htm Updated June 9, 2017. Accessed June 21, 2017. [Google Scholar]
- 14.Tenover FC, Arbeit RD, Goerling RV, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. of Clin. Micro. 1995;33:2233–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Multistate Outbreak of Burkholderia cepacia Infections Associated with Oral Liquid Docusate Sodium. https://www.cdc.gov/hai/outbreaks/b-cepacia/ Published June 24, 2016. Accessed March 24, 2017.
- 16.FDA Updates on Multistate Outbreak of Burkholderia cepacia Infections. https://www.fda.gov/Drugs/DrugSafety/ucm511527.htm Published July 16, 2016. Accessed March 24, 2017.
- 17.Cunha B, Gian J, Dieguez B, et al. Burkholderia contaminans colonization from contaminated liquid docusate (Colace) in an immunocompetent adult with Legionnaire’s disease: Infection control implications and the potential role of Candida pellucosa. J. Clin Med; 2016, 5: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kutty P, Moody B, Gullion J, et al. Multistate outbreak of Burkholderia cenocepaica colonization and infection associated with the use of intrinsically contaminated alcohol-free mouthwash. Chest. 2007;132:1825–31. [DOI] [PubMed] [Google Scholar]
- 19.Torbeck L, Raccasi D, Guilfoyle D, et al. Burkholderia cepacia: This decision is overdue. J Pharm Sci and Tech. 2011;65:535–43. [DOI] [PubMed] [Google Scholar]
- 20.Code of Federal Regulations Title 21. US Food and Drug Administration website. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=211 Published April 1, 2017. Accessed August 30, 2017. [Google Scholar]
- 21.FDA advises drug manufacturers that Burkholderia cepacia complex poses a contamination risk in non-sterile, water-based drug products. https://www.fda.gov/Drugs/DrugSafety/ucm559508.htm Published May 22, 2017. Accessed August 2, 2017. [Google Scholar]
- 22.Williams D, Lewis M, Marino P, Wise M. The oral cavity, biofilms and ventilator-associated pneumonia. Curr Respir Med Rev. 2012; 8:161–9. [Google Scholar]
- 23.Kim J, Ahn Y, LiPuma J, Cerniglia C. Survival and susceptibility of Burkholderia cepacia complex in chlorhexidine gluconate and benzalkonium chloride. J Ind Microbiol Biotechnol. 2015; 42:905–13. [DOI] [PubMed] [Google Scholar]
- 24.Chapman J Characterizing bacterial resistance to preservatives and disinfectants. Int. Biodeterior Biodegradation. 1998; 41:241–45. [Google Scholar]
- 25.Seigel J, Rhinehart E, Jackson M, et al. Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings 2007. CDC: Atlanta. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.