A bacterial enzyme that eats nicotine reverses addiction-like behaviors and offers a new strategy to help smokers quit.
Abstract
Tobacco use disorder is the leading cause of disease and preventable death worldwide, but current medications that are based on pharmacodynamics have low efficacy. Novel pharmacokinetic approaches to prevent nicotine from reaching the brain have been tested using vaccines, but these efforts have failed because antibody affinity and concentration are not sufficient to completely prevent nicotine from reaching the brain. We provide preclinical evidence of the efficacy of an enzymatic approach to reverse nicotine dependence, reduce compulsive-like nicotine intake, and prevent relapse in rats with a history of nicotine dependence. Chronic administration of NicA2-J1, an engineered nicotine-degrading enzyme that was originally isolated from Pseudomonas putida S16, completely prevented nicotine from reaching the brain and reversed somatic signs of withdrawal, hyperalgesia, and irritability-like behavior in nicotine-dependent rats with a history of escalation of nicotine self-administration. NicA2-J1 also decreased compulsive-like nicotine intake, reflected by responding despite the adverse consequences of contingent footshocks, and prevented nicotine- and stress (yohimbine)–induced relapse. These results demonstrate the efficacy of enzymatic therapy in treating nicotine addiction in advanced animal models and provide a strong foundation for the development of biological therapies for smoking cessation in humans.
INTRODUCTION
Tobacco use disorder is the leading cause of disease and preventable death worldwide. It is responsible for over 400,000 deaths annually in the United States. Three of five people who try one cigarette become daily smokers (1), and nearly all smokers who lapse experience the rapid escalation of smoking behavior, followed by full-blown relapse despite the adverse health consequences that are associated with smoking (2–5). Converging evidence indicates that such an escalation and compulsive-like pattern of smoking is mediated by nicotine (6–11), the main psychoactive ingredient of tobacco that is also responsible for the development of tobacco dependence (12, 13). Despite the existence of several approved medications, the rate of relapse in abstinent smokers remains high after 12 months (~75 to 80%) (14). Therefore, it is critical to develop novel approaches to reduce the psychoactive effects of nicotine, decrease craving, and prevent relapse. An alternative strategy to treat tobacco use disorder is to reduce the psychoactive effects of nicotine by preventing nicotine from reaching the brain. Such an approach may allow a progressive reduction of the level of dependence, leading to a decrease in craving and preventing relapse that is induced by reexposure to nicotine. Pharmacokinetic approaches to prevent nicotine from reaching the brain have been tested using vaccines, but these efforts have failed because antibody titers are not sufficient to prevent nicotine from reaching the brain.
We recently reported the characterization and therapeutic potential of NicA2-J1 (15), a reengineered nicotine-degrading enzyme that was originally isolated from Pseudomonas putida S16 (16–18). This enzyme blocked the access of nicotine to the brain and prevented the development of nicotine dependence in a simple model of passive induction of nicotine dependence in rats (19). However, it is not yet known whether such an enzymatic approach could also reverse nicotine dependence in individuals that are already dependent on nicotine. In humans, nicotine withdrawal is characterized by somatic and affective symptoms, including irritability and hyperalgesia (20, 21), leading to powerful craving for tobacco (20) and associated with smoking to relieve negative effects (22). Abstinence from chronic nicotine also leads to a withdrawal syndrome in rodents (13) that can be characterized by the emergence of somatic signs, irritability-like behavior, and hyperalgesia (7, 23, 24), leading to an increase in craving and the escalation of nicotine self-administration once access to nicotine is renewed. Even more compelling from a translational perspective would be to demonstrate that such an enzymatic approach also reduces compulsive-like responding for nicotine and prevents nicotine- and stress-induced relapse.
We conducted a series of studies to determine whether chronic NicA2-J1 administration (i) prevents nicotine from accessing the brain in an animal model of escalation of nicotine self-administration, (ii) reverses nicotine dependence, reflected by the emergence of somatic signs of withdrawal, irritability-like behavior, and hyperalgesia at different time points after acute NicA2-J1 treatment, (iii) decreases compulsive-like responding for nicotine, and (iv) reduces nicotine- and stress (yohimbine)–induced relapse in rats with a history of escalation of nicotine self-administration. Moreover, we allowed rats to self-administer nicotine under long-access conditions with various protocols for ~2 weeks to test the effects of NicA-J1 on nicotine dependence and the escalation of nicotine self-administration with long access (21 hours/day) and intermittent access (every 48 hours), compulsive-like intake (i.e., responding in the presence of contingent footshocks), and stress- and nicotine-induced reinstatement after the extinction of nicotine seeking. We previously showed that intermittent access to extended (21 hours/day) nicotine self-administration produced robust escalation of nicotine intake in rats, with high irritability-like behavior, hyperalgesia, and somatic signs of withdrawal (8).
RESULTS
NicA2-J1 decreases blood nicotine levels
To evaluate the effect of NicA2-J1 on blood nicotine levels, we trained two groups of rats (n = 8 per group) for 12 consecutive days to self-administer nicotine (0.03 mg/kg per injection) for 21 hours daily. Once a stable baseline of nicotine intake was reached, both groups were exposed to intermittent nicotine intake (every 48 hours) in four sessions. Both groups exhibited robust escalation of nicotine intake. From this point onward, one group of rats continued the escalation phase for an additional five sessions, with the only difference that NicA2-J1 (2 mg/kg, intraperitoneally) was administered 60 min before each nicotine exposure. The other group was administered phosphate-buffered saline (PBS; intraperitoneally) and run in parallel, serving as a control group. At the end of the fifth session, tail blood was collected for the detection of blood nicotine levels (Fig. 1A). NicA2-J1 (2 mg/kg) did not produce a consistent decrease in blood nicotine levels (Fig. 1B). The rats were then given another five sessions of access to nicotine with a higher dose of NicA2-J1 (10 mg/kg), and blood was collected again at the end of the fifth session. NicA2-J1 at 10 mg/kg significantly reduced blood nicotine levels (Fig. 1C).
Fig. 1. Blood nicotine levels after escalation of nicotine intake.
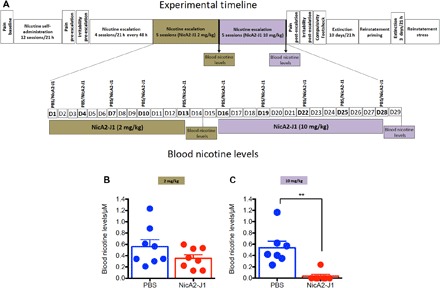
(A) Detailed timeline of the experiments. (B) After the last session of escalation of nicotine intake, blood nicotine levels were detected in rats that were pretreated with NicA2-J1 (2 mg/kg) (red circles) and rats that were pretreated with PBS (blue circles). Student’s t test showed no difference in blood nicotine levels between groups (t = 1.15, df = 14, P > 0.05). (C) At the termination of escalation of nicotine intake, rats that were pretreated with NicA2-J1 (10 mg/kg) exhibited nearly undetectable levels of nicotine in blood compared with the PBS-pretreated group (Student’s t test; t = 4.198, df = 14, P < 0.001). **P < 0.01.
NicA2-J1 reverses somatic and affective signs of withdrawal in dependent rats
Individuals who attempt to cease smoking experience somatic and affective withdrawal symptoms. Some of the most prominent symptoms are irritability and nociception. Thus, we tested whether NicA2-J1 reverses irritability-like behavior and hyperalgesia in withdrawn, nicotine-dependent rats with a history of escalation of nicotine self-administration. To determine whether NicA2-J1 prevents hyperalgesia during withdrawal, we tested the effect of 5 days of treatment with NicA2-J1 or PBS on mechanical sensitivity thresholds at three time points: (i) prior to nicotine exposure to establish naive animals’ baseline, (ii) after completion of the self-administration phase, 48 hours into nicotine withdrawal (pre-escalation), and (iii) again 48 hours into nicotine withdrawal after chronic treatment with NicA2-J1 (post-escalation phase). To determine the effect of NicA2-J1 on irritability-like behavior, aggressive and defensive behaviors were evaluated in the bottle-brush test (25) after 48 hours of nicotine withdrawal following the escalation of nicotine intake (Fig. 2A). We found a significant decrease in mechanical sensitivity thresholds in both groups during nicotine withdrawal compared with the rats’ baseline hyperalgesia thresholds in their nicotine-naive state. After 5 days of treatment with NicA2-J1 (10 mg/kg), NicA2-J1 completely reversed hyperalgesia compared with the group that was treated with PBS, normalizing hyperalgesia thresholds to nicotine-naive baseline levels (Fig. 2B). The NicA2-J1 group exhibited lower defensive and aggressive responses compared with their level of irritability before treatment and compared with the PBS-treated group (Fig. 2C). One possible explanation for these results is that withdrawal in NicA2-J1–treated rats may have occurred before the withdrawal test was performed. To evaluate this possibility, we performed the same experiment as described above, with the only difference that after a single injection of NicA2-J1 (10 mg/kg) or PBS (as a control), hyperalgesia was measured 3, 11, 22, 46, and 70 hours after the NicA2-J1 injection, corresponding to 2, 10, and 21 hours after nicotine self-administration and 24 and 48 hours into spontaneous nicotine withdrawal (Fig. 3, A to D). No difference in pain threshold was observed before and after treatment with NicA2-J1 (Fig. 3D) or PBS (Fig. 3C) at the three time points (2, 10, and 21 hours) during nicotine self-administration, demonstrating that NicA2-J1 did not precipitate withdrawal. After 24 hours of withdrawal, both NicA2-J1– and vehicle-treated rats exhibited a significant decrease in pain thresholds, but the animals that were treated with NicA2-J1 exhibited less of a decrease in pain thresholds and recovered after 48 hours, whereas the control animals continued to exhibit significant hyperalgesia.
Fig. 2. NicA2-J1 prevents nicotine addiction–like behavior during withdrawal.

(A) Detailed timeline of the experiments. (B) NicA2-J1 suppressed hyperalgesia during nicotine withdrawal. The two-way mixed-factorial analysis of variance (ANOVA), with group (PBS versus NicA2-J1) as the between-subjects factor and time (baseline, pre-escalation hyperalgesia, and post-escalation hyperalgesia) as the within-subjects factor, revealed significant effects of group (F1,14 = 4.91, P = 0.04) and time (F2,14 = 10.89, P = 0.0003) and a significant group × time interaction (F2,28 = 5.11, P = 0.012). The Newman-Keuls post hoc analysis revealed that mechanical sensitivity significantly decreased during withdrawal (pre-escalation) in PBS-treated rats compared with their mechanical sensitivity before nicotine exposure (P = 0.017) and compared with the NicA2-J1 group (P = 0.007). Mechanical sensitivity after pretreatment with NicA2-J1 was comparable to baseline sensitivity before nicotine exposure (P > 0.05), suggesting that NicA2-J1 treatment completely reversed withdrawal-induced hyperalgesia (pre-escalation versus post-escalation, P = 0.043), whereas this effect was not detected in the PBS group, which exhibited more severe hyperalgesia during withdrawal (post-escalation versus baseline, P = 0.0018). Moreover, the Newman-Keuls post hoc analysis showed that hyperalgesia during post-escalation was completely reversed in the NicA2-J1 group (post-escalation in the PBS group versus post-escalation in the NicA2-J1 group, P = 0.041). (C) Effect of NicA2-J1 on irritability-like behavior, reflected by defensive and aggressive responses. The baseline of defensive and aggressive responses was measured during 48 hours of spontaneous nicotine withdrawal before treatment with NicA2-J1 or PBS. All the other measures were performed during 48 hours of spontaneous nicotine withdrawal after the last escalation phase after the completion of NicA2-J1 treatment (10 mg/kg). A significant decrease in defensive responses (n = 8; t = 4.5, df = 7, P < 0.01) and aggressive responses (n = 8; t = 5.22, df = 7, P < 0.01) was observed in NicA2-J1–pretreated rats. No changes from baseline were observed in the PBS group. Student’s paired t test revealed a significant reduction of aggressive responses (t = 2.27, df = 14, P < 0.05) but not defensive responses (t = 1.85, df = 14, P > 0.05) in NicA2-J1–pretreated rats compared with PBS-pretreated rats. *P < 0.05, **P < 0.01, versus baseline; #P < 0.05, versus pre-escalation; &P < 0.05, post-escalation comparisons between the PBS and NicA2-J1 groups.
Fig. 3. Acute administration of NicA2-J1 decreased withdrawal-induced hyperalgesia.
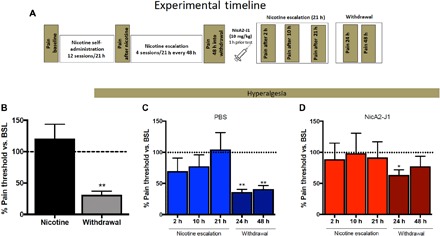
(A) Detailed timeline of the experiments. (B) Mechanical nociceptive thresholds immediately after nicotine escalation and 48 hours into withdrawal. During withdrawal, a significant decrease in hyperalgesia thresholds was observed compared with baseline (BSL) thresholds (n = 11; t = 11.9, df = 10, P < 0.01). (C) In PBS-treated animals, a significant decrease in hyperalgesia thresholds was observed compared with baseline thresholds at 24 hours into withdrawal (n = 11; t = 13.89, df = 10, P < 0.01) and 48 hours into withdrawal (n = 11; t = 9.96, df = 10, P < 0.01) but not during nicotine self-administration. (D) In NicA2-J1–treated rats, a decrease in hyperalgesia thresholds was observed at 24 hours into withdrawal (n = 11; t = 13.89, df = 10, P < 0.05). *P < 0.05, **P < 0.01.
NicA2-J1 does not affect nicotine self-administration in dependent rats
In both groups of rats, no difference in nicotine self-administration was observed after NicA2-J1 (2 mg/kg) or PBS treatment during escalation compared with treatment prior to escalation (Fig. 4, D and H). After completing this phase, blood was collected immediately after the cessation of nicotine intake to measure blood nicotine levels, which did not differ between groups (P > 0.05; Fig. 1B). To determine whether the escalation of nicotine intake in NicA2-J1–treated animals was dose dependent, we treated the rats with a fivefold higher dose of NicA2-J1 (10 mg/kg) for another 5 days, in parallel with PBS treatment in the control group. No difference in nicotine intake was observed between groups, but a significant decrease in blood nicotine levels was observed in NicA2-J1–treated animals compared with the control group (P < 0.05; Fig. 1C). These results indicate that NicA2-J1 (10 mg/kg) degraded nicotine in blood to levels that were too low to produce nicotine dependence but still sufficiently high to serve as a discriminative stimulus to maintain nicotine self-administration.
Fig. 4. Effect of NicA2-J1 on the escalation of nicotine intake (1 and 21 hours).

(A) Detailed timeline of the experiments. (B) Nicotine self-administration and escalation of nicotine intake during the first hour of nicotine exposure. Two separate one-way ANOVAs showed that the animals significantly escalated their nicotine intake in the first hour of the session during escalation in the PBS group (F15,105 = 9.92, P = 0.0001) and NicA2-J1 group (F15,105 = 51.691, P = 0.0001). The Newman-Keuls post hoc analysis revealed significant escalation on the last three intermittent-access days compared with the last three continuous-access days (P = 0.001) in both groups. (C) Inactive lever responses did not change over time in either the PBS group (F15,105 = 0.62, P = 0.54) or NicA2-J1 group (F15,105 = 0.79, P = 0.32). (D) The two-way mixed-factorial ANOVA, with treatment (PBS and NicA2-J1) as the between-subjects factor and time as the within-subjects factor, did not show a significant treatment × time interaction (F10,140 = 0.991, P = 0.45). (E) Inactive lever responding was unaffected by NicA2-J1 treatment (F10,140 = 0.782, P = 0.55). (F) Nicotine self-administration and escalation of nicotine intake during the 21 hours of nicotine exposure. Two separate one-way ANOVAs showed that the animals significantly escalated their nicotine intake in the PBS group (F15,105 = 5.53, P = 0.0001) and NicA2-J1 group (F15,105 = 4.186, P = 0.001). The Newman-Keuls post hoc analysis indicated significant escalation on the last three intermittent-access days compared with the last three continuous-access days (P = 0.01) in both groups. (G) Inactive lever responses did not change over time in either the PBS group (F15,105 = 0.32, P = 0.23) or NicA2-J1 group (F15,105 = 0.49, P = 0.41). (H) The two-way mixed-factorial ANOVA, with treatment (PBS and NicA2-J1) as the between-subjects factor and time as the within-subjects factor, showed a significant effect of time (F10,14 = 2.37, P = 0.013) but no treatment × time interaction (F10,140 = 0.991, P = 0.45). (I) Inactive lever responding was unaffected by NicA2-J1 treatment (F10,140 = 0.65, P = 0.76).
NicA2-J1 reduces compulsive-like responding for nicotine in dependent rats
We next sought to determine whether NicA2-J1 decreases compulsive-like responding for nicotine. Blood nicotine levels may be sufficiently high to serve as a discriminative stimulus but not sufficiently high to maintain nicotine self-administration in the face of adverse consequences, which is a hallmark of tobacco use disorder. We recorded the number of nicotine rewards that were obtained by the rats during 1 hour of nicotine self-administration when 30% of the nicotine rewards were paired with contingent footshocks (0.1 and 0.2 mA). The results were compared with the number of rewards that were obtained in the first hour of nicotine intake on the previous days (i.e., without footshocks). Nicotine-dependent animals that exhibited the escalation of nicotine intake and were treated with PBS continued to respond for nicotine despite the adverse consequences of footshocks (Fig. 5, A to C), whereas animals that were treated with NicA2-J1 exhibited a significant reduction of nicotine intake when footshocks were introduced. These results indicate that NicA2-J1 decreased compulsive-like responding for nicotine, in addition to reversing symptoms of nicotine dependence. These results suggest that NicA2-J1 may facilitate smoking cessation by reducing the symptoms of nicotine dependence and the motivation to smoke, but its effect on stress- and nicotine-induced relapse after protracted abstinence remains to be demonstrated.
Fig. 5. NicA2-J1 reduces compulsive-like responding for nicotine in dependent rats.
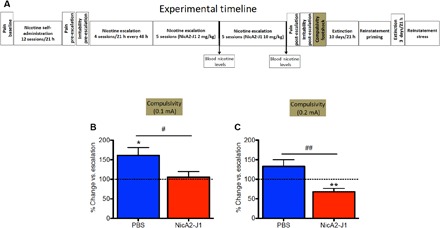
(A) Detailed timeline of the experiments. (B) When footshock (0.1 mA) was introduced, the results showed that nicotine-dependent animals that exhibited escalation of nicotine intake and were pretreated with PBS continued responding for nicotine despite the adverse consequences of footshocks at a higher level compared with their intake prior to footshock exposure (n = 8; t = 3.063, df = 7, P = 0.028). No change in responding was observed in NicA2-J1 (10 mg/kg)–pretreated rats (n = 8; t = 0.39, df = 7, P = 0.7). Student’s t test revealed a significant difference between groups (t = 2.315, df = 14, P = 0.04). (C) When a higher footshock intensity (0.2 mA) was introduced, the results showed that nicotine-dependent animals that were pretreated with PBS exhibited no changes in responding for nicotine despite the adverse consequences of footshocks compared with their intake prior to footshock exposure (t = 1.88, df = 7, P = 0.118), whereas a significant reduction was observed in NicA2-J1–pretreated rats (t = 3.78, df = 7, P = 0.0091). Student’s t test revealed a significant difference between groups (t = 3.5, df = 14, P = 0.0048). *P < 0.05, **P < 0.01, versus baseline; #P < 0.05, ##P < 0.01, comparison between the PBS and NicA2-J1 groups.
NicA2-J1 prevents stress- and nicotine-induced relapse in nicotine-dependent rats
Rats that previously escalated their nicotine intake underwent an extinction phase for 21 hours/day for 10 consecutive days. During this phase, the operant program was identical to the one that was previously used for nicotine self-administration, with the exception that responses at the drug lever did not result in nicotine delivery. After the extinction criterion was met (< 5 total responses in the first hour; Fig. 6B), stress- and nicotine-induced reinstatement was assessed using a within-subjects design. Animals with a history of PBS treatment exhibited robust reinstatement of nicotine seeking after a single intravenous injection of nicotine (0.03 mg/kg), whereas animals with a history of NicA2-J1 treatment did not exhibit reinstatement (Fig. 6C). The rats were then left undisturbed in their home cages for 2 days, followed by three additional extinction sessions to reestablish the extinction criterion (< 5 total responses in the first hour) before being tested in the stress-induced reinstatement paradigm. In this test, the day after the last extinction phase, the rats intraperitoneally received yohimbine (2 mg/kg) in a Latin-square design 1 hour before the reinstatement test. A 3-day interval, during which the animals were subjected to daily extinction sessions, interspersed yohimbine/vehicle testing. Yohimbine significantly reinstated responding on the lever that was previously associated with nicotine delivery only in PBS-treated rats, whereas NicA2-J1 prevented yohimbine-induced reinstatement (Fig. 6E). Finally, we found that inactive lever responses were unaffected by all of the treatments, demonstrating selectivity of the effects of nicotine and yohimbine in eliciting the reinstatement of nicotine seeking. These results demonstrate that a history of NicA2-J1 treatment in dependent animals was sufficient to prevent stress- and nicotine-induced relapse after protracted abstinence.
Fig. 6. NicA2-J1 prevents nicotine- and stress-induced reinstatement after extinguished nicotine intake.
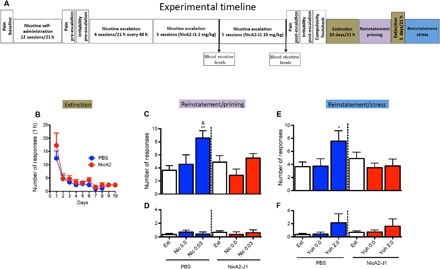
(A) Detailed timeline of the experiments. (B) Extinction phase. (C) Pretreatment with NicA2-J1 (10 mg/kg) prevented nicotine prime-induced reinstatement of nicotine-seeking behavior. The two-way mixed-factorial ANOVA, with group (PBS versus NicA2-J1) as the between-subjects factor and treatment (extinction, vehicle, and 0.03 mg/kg per injection of nicotine) as the within-subjects factor, showed a significant effect of treatment (F2,14 = 9.14, P = 0.0008) and a significant group × treatment interaction (F2,28 = 3.48, P = 0.044). The Newman-Keuls post hoc analysis revealed that one injection of nicotine (0.03 mg/kg per injection) significantly increased the number of lever presses compared with the extinction phase in PBS-treated rats (P < 0.0008). Nicotine priming did not induce the reinstatement of nicotine-seeking behavior in rats that were pretreated with NicA2-J1 during escalation (nicotine priming versus extinction, P = 0.61). The post hoc analysis showed that nicotine-induced priming was abolished in the NicA2-J1 group compared with the PBS group (nicotine priming in the PBS group versus nicotine priming in the NicA2-J1 group, P = 0.047). (D) Inactive lever responses were unaffected by nicotine infusions in either group (F2,28 = 0.64, P = 0.53). (E) NicA2-J1 (10 mg/kg) prevented the stress (yohimbine)–induced reinstatement of nicotine-seeking behavior in the PBS-pretreated group. The two-way mixed-factorial ANOVA, with group (PBS versus NicA2-J1) as the between-subjects factor and treatment (extinction, vehicle, and yohimbine) as the within-subjects factor, showed a significant effect of treatment (F2,14 = 3.31, P = 0.05) and a significant group × treatment interaction (F2,28 = 5.12, P = 0.0127). The Newman-Keuls post hoc analysis revealed that yohimbine significantly increased the number of lever presses in the PBS-pretreated group compared with the extinction phase (P = 0.011). No effect of yohimbine-induced reinstatement was observed in the NicA2-J1–pretreated group. (F) Inactive lever responses were unaffected by yohimbine in either group (F2,28 = 0.15, P = 0.85). *P < 0.05, **P < 0.01, versus extinction; &P < 0.05, nicotine priming–induced reinstatement between the PBS and NicA2-J1 groups.
DISCUSSION
We previously showed that the NicA2-J1 enzyme degrades nicotine and prevents the development of nicotine dependence in a passive model of nicotine dependence in rats. However, whether NicA2-J1 has translational relevance and prevents addiction-like behaviors in animals with a history of compulsive-like nicotine self-administration remains to be demonstrated. The present study found that NicA2-J1 decreased blood nicotine levels and had remarkable preclinical efficacy in reducing addiction-like behaviors in nicotine-dependent rats. NicA2-J1 administration reduced blood nicotine levels, reversed somatic and emotional signs of nicotine withdrawal in dependent rats, reduced compulsive-like responding for nicotine, and prevented nicotine- and stress-induced relapse.
NicA2-J1 dose-dependently affected nicotine clearance in blood. At a low dose (2 mg/kg), blood nicotine levels did not significantly decrease, which may reflect a compensatory effect of repeated nicotine intake. However, at a higher dose (10 mg/kg), NicA2-J1 degraded nicotine in blood to undetectable levels in all of the rats (< 5 nM), with the exception of one rat that still had 25% of blood nicotine relative to controls. Such a robust decrease in blood nicotine levels is remarkable and vastly superior to previous approaches that used immunopharmacotherapies (26). Moreover, the results were consistent with our previous study that observed a > 95% decrease in nicotine in both blood and brain at this high dose (19). Thus, the decrease in nicotine levels was associated with a reversal of key behavioral symptoms of nicotine dependence.
Rats with intermittent (every 48 hours) and extended (21 hours/day) access to nicotine self-administration exhibit significant somatic signs of withdrawal, hyperalgesia, irritability-like behavior, the escalation of nicotine intake, and greater motivation for nicotine (6, 8). The vehicle-treated group exhibited a similar pattern of nicotine-dependent behavior, replicating our previous studies. Nicotine-dependent rats that were treated with NicA2-J1 exhibited a lack of hyperalgesia and a robust decrease in irritability-like behavior after 2 weeks of treatment. Moreover, acute NicA2-J1 treatment did not precipitate withdrawal during nicotine self-administration and decreased withdrawal-induced hyperalgesia when administered acutely. These findings suggest that although NicA2-J1 degraded most of the nicotine in blood, a slight amount of nicotine likely remained in blood that was sufficient to prevent withdrawal in rats while decreasing the level of dependence. These data further confirm and extend our previous study (19), demonstrating that the animals that were treated with NicA2-J1 did not exhibit withdrawal symptoms compared with the control group after 5 days of treatment.
NicA2-J1 prevented the development of nicotine dependence and reversed nicotine dependence by normalizing somatic and emotional signs of nicotine withdrawal in only 2 weeks. This is a critical result because irritability during abstinence is often mentioned by users as one of the primary reasons why they relapse. Even more compelling from a translational perspective, NicA2-JI decreased compulsive-like responding for nicotine, reflected by nicotine intake despite the adverse consequences of contingent footshocks. This suggests that treatment with the NicA2-J1 enzyme reduced symptoms of nicotine withdrawal and diminished the incentive value of nicotine, thereby decreasing the motivation to take nicotine when confronted with adverse consequences. NicA2-J1 did not affect nicotine self-administration when no adverse consequences were presented, suggesting that the very low blood nicotine levels were sufficient to serve as a discriminative stimulus but not sufficient to have incentive value, including responding despite adverse consequences. Similar observations have been made in humans after a ~95% reduction of nicotine content in cigarettes (27). Blood nicotine levels rapidly decreased, but the number of cigarettes smoked remained stable for months; however, the subjects reported lower nicotine withdrawal symptoms, a greater interest in quitting, and lower motivation to smoke (28, 29). Moreover, NicA2-J1 reduced compulsive-like nicotine seeking, which is highly relevant to the human condition, in which smoking is often associated with significant adverse social consequences (e.g., conflicts with partners) and health consequences (e.g., coughing, pulmonary disease, and cancer) that are often ignored by smokers because of the high incentive value of nicotine and the ability of nicotine to provide relief from withdrawal symptoms. The present results suggest that chronic NicA2-J1 treatment may help reduce withdrawal symptoms and reduce compulsive nicotine seeking and taking.
In humans, two of the most common causes of relapse are stress (social or physiological) and reexposure to nicotine (initial lapse leading to relapse). Rats that received NicA2-J1 did not exhibit stress- or nicotine-induced reinstatement of nicotine seeking after extinction or during protracted abstinence. Responding at the lever that was previously paired with nicotine was unaltered and similar to extinction levels in rats that were treated with NicA2-J1. Inactive lever responding was unaffected, providing strong evidence against nonspecific effects of NicA2-J1 and yohimbine. The α2 adrenergic receptor antagonist yohimbine is considered a pharmacological stressor because it increases hypothalamic-pituitary-adrenal axis hormones (30). Yohimbine has been frequently used in preclinical studies to model stress-induced relapse to nicotine, cocaine, alcohol, and opioid intake (31–33). The activation of nicotinic acetylcholine receptors is also crucial for nicotine reinforcement and is hypothesized to mediate the transition from a single lapse to a full relapse in humans. In the present study, pretreatment with NicA2-J1 significantly reduced nicotine seeking following a priming injection of nicotine. No effect was observed on the inactive lever, again excluding possible nonspecific effects of the enzyme and nicotine treatments. These results suggest that chronic NicA2-J1 treatment reversed symptoms of nicotine withdrawal and reduced compulsive-like responding for nicotine and may have also facilitated the normalization of brain stress and nicotinic systems to a naive state that is no longer abnormally sensitive to small stress or nicotine challenges. These results suggest that the level of dependence is a critical driving force that contributes to both stress- and nicotine-induced reinstatement. This interpretation is consistent with previous work that showed that rats with a higher level of dependence also had higher vulnerability to stress- and drug-induced relapse (34–36).
In summary, the present study found that a nicotine-degrading enzyme can reverse nicotine dependence, decrease compulsive-like intake, and prevent relapse in a translational animal model of nicotine addiction. Moreover, as we have previously reported (19), NicA2-J1 has a favorable pharmacokinetic profile, including a relatively long half-life and simple route of administration. We highlight its ability to lower compulsive-like nicotine intake, decrease relapse, and decrease signs of withdrawal. Further investigations should evaluate its efficacy for smoking cessation therapy.
MATERIALS AND METHODS
Subjects
Male Wistar rats (n = 27, Charles River Laboratories) were used, weighing 250 to 275 g at the beginning of the experiments. The animals were housed in standard cages in a room with artificial lightning (12-hour/12-hour light/dark cycle, lights off at 8:00 a.m.) at constant temperature (20° to 22°C) and humidity (45° to 55°) with food and water available ad libitum. The rats were handled once daily for 5 min during the first week after arrival to the vivarium. The animal procedures met the guidelines of the National Institutes of Health and were approved by The Scripps Research Institute Institutional Animal Care and Use Committee (protocol no. 08-0015). All of the surgical procedures were performed under isoflurane anesthesia, and all necessary steps were taken to minimize suffering of the animals.
Drugs
Nicotine hydrogen tartrate salt was dissolved in 0.9% sterile physiological sodium chloride and prepared fresh every other day (pH 7.3). The dose of nicotine for self-administration was 0.03 mg/kg per 100 μl. Yohimbine HCl was dissolved in distilled water and injected intraperitoneally at a dose of 2 mg/kg. NicA2-J1 was synthesized as previously reported (19) and administered intraperitoneally at doses of 2 and 10 mg/kg.
Mechanical nociceptive von Frey test
Mechanical sensitivity was evaluated using von Frey filaments (Stoelting) that ranged from 3.63 to 125.89 g as previously reported (37). The choice of the standard von Frey test instead of the electronic von Frey test or Hargreaves test was based on the ease with which comparisons can be made with our previous studies (8, 38, 39). Briefly, the series of von Frey filaments was applied from below the wire mesh to the central region of the plantar surface of the left and right hindpaws in ascending order, beginning with the lowest filament (3.63 g) after 10 min of habituation to the testing environment. A withdrawal response was considered valid only if the hindpaw was completely removed from the platform, indicating a positive response. The stimulus was incrementally increased until a positive response was observed and then decreased until a negative response was observed. The ascending and descending testing protocol was used to determine the paw withdrawal threshold as previously reported (39). Paw withdrawal thresholds were recorded before self-administration (serving as an individual baseline), before the initiation of escalation (pre-escalation), and after 14 days of escalation (post-escalation). The data are expressed as grams of applied force. In a separate cohort of animals, we performed the same experiment, with the only difference that after a single injection of NicA2-J1, hyperalgesia was measured after 2, 10, and 21 hours of nicotine self-administration and then 24 and 48 hours into withdrawal, corresponding to 3, 11, 22, 46, and 70 hours after NicA2-J1 treatment.
Irritability-like behavior
The bottle-brush test was used to test irritability-like behavior during nicotine withdrawal, based on the methods of Kimbrough et al. (25). This test was performed after 48 hours of nicotine withdrawal and measured aggressive and defensive responses. Testing consisted of 10 trials per rat in plastic cages (26.67 cm by 48.26 cm by 20.32 cm; Ancare, Bellmore, NY, USA) with fresh bedding. Three observers who were blinded to treatment scored behavior, and the average scores of the three observers for each behavior were calculated: aggressive responses (smelling, biting, boxing, following, and exploring the target) and defensive responses (escaping, burying, jumping, climbing, grooming, and vocalization). The test was performed in the pre-escalation and post-escalation phases.
Intravenous catheter surgery
Chronic intravenous jugular catheter implantation was performed as previously described (40). Briefly, the rats were anesthetized with 1 to 3% isoflurane in an oxygen mixture. Incisions were made to expose the right jugular vein. A catheter that was made from silicon tubing (inner diameter, 0.5 mm; outer diameter, 0.9 mm) was subcutaneously positioned. After insertion into the vein, the proximal end of the catheter was anchored with surgical silk to the muscles under the vein. The distal end of the catheter was attached to a stainless steel cannula that was bent at a 90° angle. The cannula was inserted in a support that was made with dental cement on the back of the rats. For 1 week after surgery, the rats were treated daily with 0.2 ml of the antibiotic cefazolin (262 mg/ml). For the duration of the experiments, the catheters were flushed daily with 0.2 to 0.3 ml of heparinized saline solution. Body weights were monitored every day, and catheter patency was confirmed approximately every 15 days with an injection of 0.2 to 0.3 ml of Brevital sodium solution (10 mg/ml). Catheter patency was assumed if there was an immediate loss of reflexes. The self-administration experiments began 1 week after surgery.
Operant training and nicotine self-administration
The self-administration chambers consisted of operant conditioning chambers (Med Associates, St. Albans, VT, USA) that were enclosed in sound-attenuating, ventilated environmental cubicles. Each chamber was equipped with two retractable levers that were located in the front panel and two nosepoke holes in the back panel for food and water delivery. A plastic tube that was connected to the catheter before beginning the session delivered nicotine. An infusion pump was activated by responses on the right (“active”) lever, and responses on the left (“inactive”) lever were recorded but did not result in any programmed consequences. Activation of the pump resulted in the delivery of 0.1 ml of nicotine solution (0.03 mg/kg per 0.1 ml) under a fixed-ratio 1 (FR1) schedule of reinforcement, paired with illumination of the chamber by the cue light. The delivery of nicotine solution was followed by a 20-s timeout (TO) period, during which further lever presses did not result in any consequences. The duration of nicotine delivery was 21 hours/day. Rats had access to food and water in the operant chambers for the duration of the test. Nicotine solution was prepared fresh every other day from (−)nicotine hydrogen tartrate salt (Sigma-Aldrich) on the basis of the animals’ body weight, and the pH was adjusted to 7.3 with 1 M NaOH.
Nicotine self-administration despite adverse consequences
The day after the last extended access to nicotine self-administration, the animals were placed in the self-administration chamber for a 1-hour session, and they were tested for compulsive-like behavior. In this experiment, the rats were allowed to self-administer nicotine on an FR1 schedule of reinforcement, in which 30% of the reinforced responses were paired with a contingent footshock (0.1 mA, 0.5 s). After completion of this phase, the same procedure was used the next day, with the only difference that the intensity of the footshock was increased to 0.2 mA, 0.5 s.
Reinstatement of nicotine-seeking behavior
This experiment consisted of different stages.
Self-administration: The nicotine self-administration procedure was performed as described previously (7). Drug self-administration occurred under an FR1 schedule of reinforcement, with a 20-s TO period after each drug infusion, for 21 hours/day. Every drug delivery was associated with illumination of the cue light, which lasted for the duration of the TO period. During the TO period, responses were recorded but did not lead to drug delivery. Self-administration sessions (21 hours/day, 7 days/week) were performed in operant chambers that were equipped with two levers and two nosepoke holes. Responses on one of the levers (active lever) resulted in drug delivery, whereas responses on the other lever (inactive lever, a measure of nonspecific activity) were recorded but not reinforced. Food and water delivery occurred after each nosepoke.
Escalation: The escalation phase was identical to the self-administration phase, with the exception that the rats were introduced to the operant chambers every 48 hours (Monday, Thursday, Sunday, Wednesday, and so on).
Extinction: This phase was similar to the self-administration procedure, with the exception that presses on the active lever resulted in saline instead of nicotine infusions.
Prime-induced reinstatement: After the extinction criterion of nicotine-taking behavior was reached, the effects of priming injections of nicotine (0.03 mg/kg per injection; one infusion of nicotine solution at the same concentration as the one that was used during self-administration) or saline on the reinstatement of drug seeking were determined in separate daily 1-hour sessions. The rats were tested after intravenous drug priming injections. During the tests for reinstatement, lever presses resulted in saline infusions. Priming injections were administered immediately before the test sessions began. The experiment was performed using a Latin-square design. The extinction sessions were continued between priming sessions.
Yohimbine-induced reinstatement: After completion of the test of prime-induced reinstatement, the rats were left undisturbed in their home cages for 2 days. They then underwent the extinction phase again for three consecutive days. Once the extinction criterion was met, the rats received an intraperitoneal injection of yohimbine (2 mg/kg) 1 hour before the reinstatement test. Responses on the active and inactive levers were recorded. During the test, lever presses resulted in saline infusions for the duration of the session. Data were recorded using a computer that controlled the operant chambers. For the reinstatement experiments, only responses during the first hour of extinction and reinstatement were analyzed.
Blood sample preparation
Blood was collected and immediately mixed with four volumes of methanol (with 1 μM nicotine D3 as an internal standard) to quench the enzyme. The samples were centrifuged at 10,000 rpm for 30 min, and the supernatant was transferred to clean tubes and evaporated in a rotatory evaporator Genevac. The residual was redissolved in 5% NH4OH in water and extracted by an Oasis HLB 96-well μElution Plate (Waters). The elution was evaporated in a rotatory evaporator Genevac and redissolved in Hepes buffer and 2% trifluoroacetic acid for liquid chromatography–mass spectrometry (LC-MS).
LC-MS for nicotine detection
Nicotine concentrations were determined by LC-MS using the Agilent 1260 Infinity liquid chromatography system with 6130 quadrupole MS. Aliquots (20 μl) of each sample were injected into a Poroshell 120 EC-C8 column (4.6 mm by 50 mm, 2.7 μm; Agilent Technologies) and subjected to a gradient (A to B, where A = 0.1% formic acid in water and B = 0.1% formic acid in acetonitrile) of 0% B for 3 min, 0% B to 100% B from 3 to 7 min, and 100% B from 7 to 10 min at a constant flow rate of 0.5 ml/min. A column-solvent equilibration time of 3 min was implemented before the next sample was analyzed. The following were the MS operational parameters: API-ES (atmospheric pressure ionization electrospray) mode, channel 1 (90%) positive single ion monitoring of mass/charge ratios 166 (50%, nicotine D3) and 163 (50%, nicotine) and channel 2 (10%) scan for positive ions, nitrogen as a nebulizing and drying gas (35 psi, 12 L/min), high capillary voltage of 4 kV, and drying gas temperature to 300°C. To protect the detector from salts in the buffer, MS was turned on with a delay of 1.4 min after the injection.
Statistical analysis
The effects of NicA2-J1 on blood nicotine levels, irritability-like behavior, and compulsive-like responding were analyzed using unpaired two-tailed Student’s t test. The effect of NicA2-J1 or PBS on hyperalgesia at different time points was analyzed using one-sample t test. The effects of NicA2-J1 on hyperalgesia and yohimbine- and nicotine-induced priming were analyzed using two-way mixed-factorial ANOVA. The effect of NicA2-J1 on nicotine intake during escalation was analyzed using one-way ANOVA. Significant effects in the ANOVA were followed by the Newman-Keuls multiple-comparison post hoc test. Values of P < 0.05 were considered statistically significant.
Acknowledgments
We thank G. de Guglielmo, D. Conlisk, A. Kimbrough, J. Kononoff, K. Contreras, and M. Brennan for assistance with the behavioral experiments and M. Arends for manuscript editing. Funding: This study was funded by the National Institute on Drug Abuse (DA036691 to O.G. and DA041839 to K.D.J.). Author contributions: Conceptualization: M.K., O.G., K.D.J., and S.X. Investigation: M.K., S.X., and B.Z. Writing: M.K., S.X., and O.G. Competing interests: K.D.J. is an inventor on two provisional patent applications related to this work (TSRI case 1848.0P TSR 2193P, U.S. serial no. 62/607,423, filed 19 December 2017; and TSRI case 1848.1P TSR 2254P, U.S. serial no. not yet available, filed 20 August 2018). The other authors declare no competing interests. Data and material availability: All data that are needed to evaluate the conclusions in this paper are present in the paper. Additional data related to this paper may be requested from the authors.
REFERENCES AND NOTES
- 1.Birge M., Duffy S., Miler J. A., Hajek P., What proportion of people who try one cigarette become daily smokers? A meta analysis of representative surveys. Nicotine Tob. Res. 2017, ntx243 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Brandon T. H., Tiffany S. T., Obremski K. M., Baker T. B., Postcessation cigarette use: The process of relapse. Addict. Behav. 15, 105–114 (1990). [DOI] [PubMed] [Google Scholar]
- 3.Garvey A. J., Bliss R. E., Hitchcock J. L., Heinold J. W., Rosner B., Predictors of smoking relapse among self-quitters: A report from the Normative Aging Study. Addict. Behav. 17, 367–377 (1992). [DOI] [PubMed] [Google Scholar]
- 4.Chornock W. M., Stitzer M. L., Gross J., Leischow S., Experimental model of smoking re-exposure: Effects on relapse. Psychopharmacology (Berl) 108, 495–500 (1992). [DOI] [PubMed] [Google Scholar]
- 5.Conklin C. A., Perkins K. A., Sheidow A. J., Jones B. L., Levine M. D., Marcus M. D., The return to smoking: 1-Year relapse trajectories among female smokers. Nicotine Tob. Res. 7, 533–540 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Cohen A., Koob G. F., George O., Robust escalation of nicotine intake with extended access to nicotine self-administration and intermittent periods of abstinence. Neuropsychopharmacology 37, 2153–2160 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen A., Soleiman M. T., Talia R., Koob G. F., George O., Mandyam C. D., Extended access nicotine self-administration with periodic deprivation increases immature neurons in the hippocampus. Psychopharmacology (Berl) 232, 453–463 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen A., Treweek J., Edwards S., Leão R. M., Schulteis G., Koob G. F., George O., Extended access to nicotine leads to a CRF1 receptor dependent increase in anxiety-like behavior and hyperalgesia in rats. Addict. Biol. 20, 56–68 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.George O., Ghozland S., Azar M. R., Cottone P., Zorrilla E. P., Parsons L. H., O’Dell L. E., Richardson H. N., Koob G. F., CRF–CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc. Natl. Acad. Sci. U.S.A. 104, 17198–17203 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valentine J. D., Hokanson J. S., Matta S. G., Sharp B. M., Self-administration in rats allowed unlimited access to nicotine. Psychopharmacology (Berl) 133, 300–304 (1997). [DOI] [PubMed] [Google Scholar]
- 11.Harris A. C., Pentel P. R., Lesage M. G., Prevalence, magnitude, and correlates of an extinction burst in drug-seeking behavior in rats trained to self-administer nicotine during unlimited access (23 h/day) sessions. Psychopharmacology (Berl) 194, 395–402 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Epping-Jordan M. P., Watkins S. S., Koob G. F., Markou A., Dramatic decreases in brain reward function during nicotine withdrawal. Nature 393, 76–79 (1998). [DOI] [PubMed] [Google Scholar]
- 13.Malin D. H., Lake J. R., Newlin-Maultsby P., Roberts L. K., Lanier J. G., Carter V. A., Cunningham J. S., Wilson O. B., Rodent model of nicotine abstinence syndrome. Pharmacol. Biochem. Behav. 43, 779–784 (1992). [DOI] [PubMed] [Google Scholar]
- 14.Taylor G. M. J., Taylor A. E., Thomas K. H., Jones T., Martin R. M., Munafò M. R., Windmeijer F., Davies N. M., The effectiveness of varenicline versus nicotine replacement therapy on long-term smoking cessation in primary care: A prospective cohort study of electronic medical records. Int. J. Epidemiol. 46, 1948–1957 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue S., Schlosburg J. E., Janda K. D., A new strategy for smoking cessation: Characterization of a bacterial enzyme for the degradation of nicotine. J. Am. Chem. Soc. 137, 10136–10139 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Tang H., Wang L., Wang W., Yu H., Zhang K., Yao Y., Xu P., Systematic unraveling of the unsolved pathway of nicotine degradation in Pseudomonas. PLOS Genet. 9, e1003923 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu H., Tang H., Wang L., Yao Y., Wu G., Xu P., Complete genome sequence of the nicotine-degrading Pseudomonas putida strain S16. J. Bacteriol. 193, 5541–5542 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu H., Tang H., Xu P., Green strategy from waste to value-added-chemical production: Efficient biosynthesis of 6-hydroxy-3-succinoyl-pyridine by an engineered biocatalyst. Sci. Rep. 4, 5397 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xue S., Kallupi M., Zhou B., Smith L. C., Miranda P. O., George O., Janda K. D., An enzymatic advance in nicotine cessation therapy. Chem. Commun. (Camb) 54, 1686–1689 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes J. R., Gust S. W., Skoog K., Keenan R. M., Fenwick J. W., Symptoms of tobacco withdrawal. A replication and extension. Arch. Gen. Psychiatry 48, 52–59 (1991). [DOI] [PubMed] [Google Scholar]
- 21.Cosgrove K. P., Esterlis I., McKee S., Bois F., Alagille D., Tamagnan G. D., Seibyl J. P., Krishnan-Sarin S., Staley J. K., Beta2* nicotinic acetylcholine receptors modulate pain sensitivity in acutely abstinent tobacco smokers. Nicotine Tob. Res. 12, 535–539 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falcone M., Gold A. B., Wileyto E. P., Ray R., Ruparel K., Newberg A., Dubroff J., Logan J., Zubieta J.-K., Blendy J. A., Lerman C., μ-Opioid receptor availability in the amygdala is associated with smoking for negative affect relief. Psychopharmacology (Berl) 222, 701–708 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt B. L., Tambeli C. H., Gear R. W., Levine J. D., Nicotine withdrawal hyperalgesia and opioid-mediated analgesia depend on nicotine receptors in nucleus accumbens. Neuroscience 106, 129–136 (2001). [DOI] [PubMed] [Google Scholar]
- 24.Baiamonte B. A., Valenza M., Roltsch E. A., Whitaker A. M., Baynes B. B., Sabino V., Gilpin N. W., Nicotine dependence produces hyperalgesia: Role of corticotropin-releasing factor-1 receptors (CRF1Rs) in the central amygdala (CeA). Neuropharmacology 77, 217–223 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimbrough A., de Guglielmo G., Kononoff J., Kallupi M., Zorrilla E. P., George O., CRF1 receptor-dependent increases in irritability-like behavior during abstinence from chronic intermittent ethanol vapor exposure. Alcohol. Clin. Exp. Res. 41, 1886–1895 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolters A., de Wert G., van Schayck O. C., Horstman K., Vaccination against smoking: An annotated agenda for debate. A review of scientific journals, 2001–13. Addiction 109, 1268–1273 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Benowitz N. L., Henningfield J. E., Reducing the nicotine content to make cigarettes less addictive. Tob. Control 22 (suppl. 1), i14–i17 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benowitz N. L., Dains K. M., Hall S. M., Stewart S., Wilson M., Dempsey D., Jacob P. III, Smoking behavior and exposure to tobacco toxicants during 6 months of smoking progressively reduced nicotine content cigarettes. Cancer Epidemiol. Biomarkers Prev. 21, 761–769 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benowitz N. L., Nardone N., Dains K. M., Hall S. M., Stewart S., Dempsey D., Peyton J. III, Effect of reducing the nicotine content of cigarettes on cigarette smoking behavior and tobacco smoke toxicant exposure: 2-Year follow up. Addiction 110, 1667–1675 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abercrombie E. D., Keller R. W. Jr, Zigmond M. J., Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: Pharmacological and behavioral studies. Neuroscience 27, 897–904 (1988). [DOI] [PubMed] [Google Scholar]
- 31.Greenwald M. K., Lundahl L. H., Steinmiller C. L., Yohimbine increases opioid-seeking behavior in heroin-dependent, buprenorphine-maintained individuals. Psychopharmacology 225, 811–824 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grella S. L., Funk D., Coen K., Li Z., Lê A. D., Role of the kappa-opioid receptor system in stress-induced reinstatement of nicotine seeking in rats. Behav. Brain Res. 265, 188–197 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marinelli P. W., Funk D., Juzytsch W., Harding S., Rice K. C., Shaham Y., Lê A. D., The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology 195, 345–355 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Mantsch J. R., Baker D. A., Francis D. M., Katz E. S., Hoks M. A., Serge J. P., Stressor- and corticotropin releasing factor-induced reinstatement and active stress-related behavioral responses are augmented following long-access cocaine self-administration by rats. Psychopharmacology 195, 591–603 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.George O., Koob G. F., Vendruscolo L. F., Negative reinforcement via motivational withdrawal is the driving force behind the transition to addiction. Psychopharmacology 231, 3911–3917 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kippin T. E., Fuchs R. A., See R. E., Contributions of prolonged contingent and noncontingent cocaine exposure to enhanced reinstatement of cocaine seeking in rats. Psychopharmacology 187, 60–67 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Edwards S., Vendruscolo L. F., Schlosburg J. E., Misra K. K., Wee S., Park P. E., Schulteis G., Koob G. F., Development of mechanical hypersensitivity in rats during heroin and ethanol dependence: Alleviation by CRF1 receptor antagonism. Neuropharmacology 62, 1142–1151 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Guglielmo G., Kallupi M., Cole M. D., George O., Voluntary induction and maintenance of alcohol dependence in rats using alcohol vapor self-administration. Psychopharmacology 234, 2009–2018 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaplan S. R., Bach F. W., Pogrel J. W., Chung J. M., Yaksh T. L., Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 53, 55–63 (1994). [DOI] [PubMed] [Google Scholar]
- 40.Caine S. B., Koob G. F., Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science 260, 1814–1816 (1993). [DOI] [PubMed] [Google Scholar]


