Abstract
Objective
Prednisone is a first-line immunosuppressive treatment for myasthenia gravis (MG), whereas short-term and long-term adverse effects (AEs) are a limiting factor in its usage.
Method
The MG patient registry is a patient-driven, nation-wide database with patients of age ≥18 years, who were diagnosed with MG and live in the United States. Custom-designed “prednisone-steroid use and MG” survey was sent out to MG registry participants as part of semi-annual follow-up. Data were collected and analyzed for frequency.
Results
A total of 398 MG participants (21% response rate) completed the survey, including 173 men and 225 women. Among them, 298 reported current (174) or past (288) prednisone intake. Current prednisone dosage varied from 0.5 to 75 mg (median 10 mg, IQR 7–20), dosing frequency was daily in 132 (76%) and every other day in 31 (18%). Peak prednisone dose was commonly between 25 mg and 60 mg (Median 50 mg, IQR 25–60); however, doses more than 60 mg daily were reported in 59 (20%). Prednisone AEs were reported more commonly in women (95% vs 81%, p < 0.0001). Women reported more intolerable AEs (77% vs 50%, p < 0.00001) and less willingness to accept a dose increase (26% vs 44%, p = 0.03) compared with men.
Conclusions
Prednisone is commonly used in the treatment of MG, with highly variable dosages and dosing frequencies reflecting the absence of a standard guideline. Intolerable AEs were more commonly reported among women and was associated with unwillingness to accept a dose increase. Consensus guidelines and their validation are required to guide prednisone treatment for MG.
Prednisone has been shown to be effective in treating myasthenia gravis (MG) and is currently used as a first-line immunosuppressive therapy.1–6 The use of prednisone, however, is often limited because of the numerous short- and long-term adverse effects (AEs) associated with its glucocorticoid and mineralocorticoid activities. These AEs tend to increase with higher doses, more frequent dosing, and prolonged treatment period.7–13 The frequency of steroid AEs has been reported in up to 67% of treated populations2,3 and is likely underestimated. Clinicians are concerned of serious AEs such as osteoporosis-related fracture, aseptic necrosis, infection, and gastrointestinal bleeding. AEs that are clinically considered “benign” can still be disturbing from the patient's perspective and might lead to the request of dose reduction or poor compliance. Tapering the dose of prednisone while maintaining disease control has become a treatment goal in MG care and an important end point in MG clinical trials.14–16
Recent analyses from the MG patient registry showed that women were less likely to be on current prednisone treatment despite having worse disease severity ratings compared with men.17 We suspect that this observation is due to gender differences in the frequency or tolerability of steroid AEs; however, we found no studies that systemically evaluated this hypothesis in the MG population. Therefore, we designed a survey of MG registry participants regarding AEs and personal beliefs governing the use of prednisone to delineate potential gender effects on the perception of prednisone AEs.
Methods
The MG patient registry is a database managed by the Myasthenia Gravis Foundation of America and the coordinating center at the University of Alabama at Birmingham (UAB), with oversight by the UAB Institutional Review Board (IRB). Details of the registry, registry participants, mode of data collection, and collected participant-reported outcome measures were described in the previous study.17
We composed a Prednisone-Steroid use and MG Survey (Prednisone Survey), which included 11 questions asking the participants about the status of prednisone use, current and highest doses and frequencies, AEs experienced, and willingness to increase steroid dose for better disease control. The 33 items included in the AEs list were derived from MGTX treatment-associated symptoms and treatment-associated complications.14 Participants were asked to select AEs they experienced from taking steroid (prednisone) and select them once more if any of them were difficult to tolerate (appendix e-1, links.lww.com/NXI/A77). This survey was sent to the MG registry participants along with the semi-annual follow-up for those who enrolled before April 15, 2017.
Inclusion and exclusion criteria
Patients age ≥18 years who answered “Yes” to “Has your doctor diagnosed you with MG?”, resided in the United States, and completed the 9th semi-annual follow-up survey before November 29, 2017 were included.
Statistical analysis
Basic demographic, disease-related history and answers to the survey questions were compared between survey responders vs nonresponders, responder with prednisone use vs no prednisone use, male vs female responders, and responders with intolerable AEs vs no intolerable AEs. The Student t-test was used to compare continuous variables such as age, age at symptom onset, age at treatment onset, current dose, highest dose, treatment duration, MG quality of life 15 (MG-QOL15), and MG activity of daily living (MG-ADL) sum scores. Categorical variables such as sex, race, thymoma, thymectomy, intensive care unit (ICU) admission in the past, feeding tube in the past, current and past prednisone use, current use of immunosuppressant agents, current intravenous immunoglobulin (IVIG) treatment, and current plasma exchange (PLEX) treatment were summarized and compared using the Fisher exact test. A p value less than 0.05 was used for statistical significance without adjustments for multiple comparisons because of the exploratory nature of this article. SAS version 9.4 and programs from the R project version 3.3. 2 were used for statistical analysis.
Data availability statement
Data not provided in the article because of the space limitations will be made available in a trusted data repository or shared at the request of other investigators for purposes of replicating results.
Standard protocol approvals, registrations, and patient consents
General registry and each study and/or survey obtains approval by the UAB IRB, and consent for participation is believed to be obtained when each participant completes their survey.17
Results
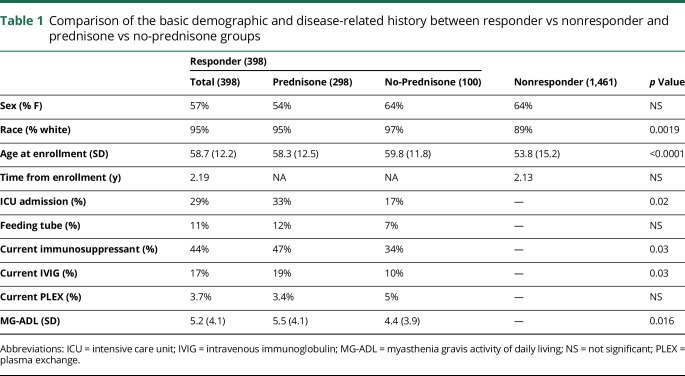
One thousand eight hundred fifty-nine MG patient registry enrollees received the 9th semi-annual follow-up and the Prednisone Survey irrespective of whether they had ever responded to a semi-annual update after registration. Among them, 398 participants responded to the 9th semi-annual follow-up survey and Prednisone Survey (21% response rate). When the demographics of survey responders were compared with nonresponders, survey responders were more likely to be white and older at enrollment. Among the 398 Prednisone Survey responders, 100 participants answered that they were never treated with prednisone and that the survey was ended (no-prednisone group). The remainder, 298 participants, who answered that they took prednisone (prednisone group) in the past or are currently taking prednisone completed the survey. Compared with the no-prednisone group, the prednisone group had higher (worse) MG-ADL score at enrollment, reported more frequent ICU admission, and was more likely to be receiving an immunosuppressant and IVIG treatment (table 1).
Table 1.
Comparison of the basic demographic and disease-related history between responder vs nonresponder and prednisone vs no-prednisone groups
Among the 298 participants in the prednisone group, 173 (58%) were currently receiving prednisone. Peak prednisone dose was reported most commonly between 25 and 60 mg (median 50 mg, IQR 25–60); however, doses more than 60 mg daily were reported in 59 (20%). Peak dosage was daily in 249 (83%) and every other day in 18 (6%). Current prednisone dosage varied from 0.5 to 75 mg (median 10 mg, IQR 7–20). Current prednisone dosing frequency was daily in 132 (76%) and every other day in 31 (18%).
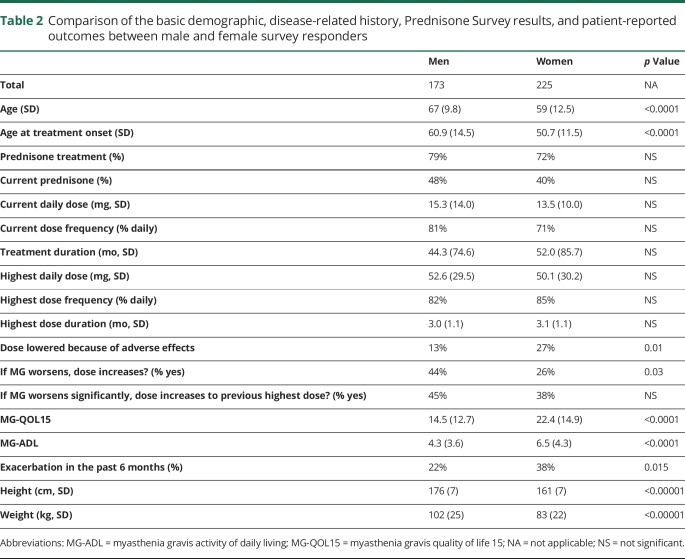
Among the survey responders who took prednisone, women were younger, younger at the age of initiation of steroid treatment, lower in weight, higher in MG-QOL15, and MG-ADL (worse) scores, and more likely to report an MG exacerbation in the past 6 months. The rate of treatment with prednisone, current prednisone treatment, current dose, highest dose, treatment duration, and dosing frequency were comparable between men and women. Women were more likely to answer that their steroid dose was lowered from the highest dose because of AEs compared with men. Women also answered “no” more frequently than men when asked “if your MG symptoms worsen, are you willing to try a dose of steroid (prednisone) higher than your current dose or to start steroid if not on it currently.?” This gender difference was not observed when asked “if your MG symptoms worsen significantly, are you willing to try your previous highest dose steroid (prednisone) or, if currently on your highest dose to increase it further?” (table 2).
Table 2.
Comparison of the basic demographic, disease-related history, Prednisone Survey results, and patient-reported outcomes between male and female survey responders
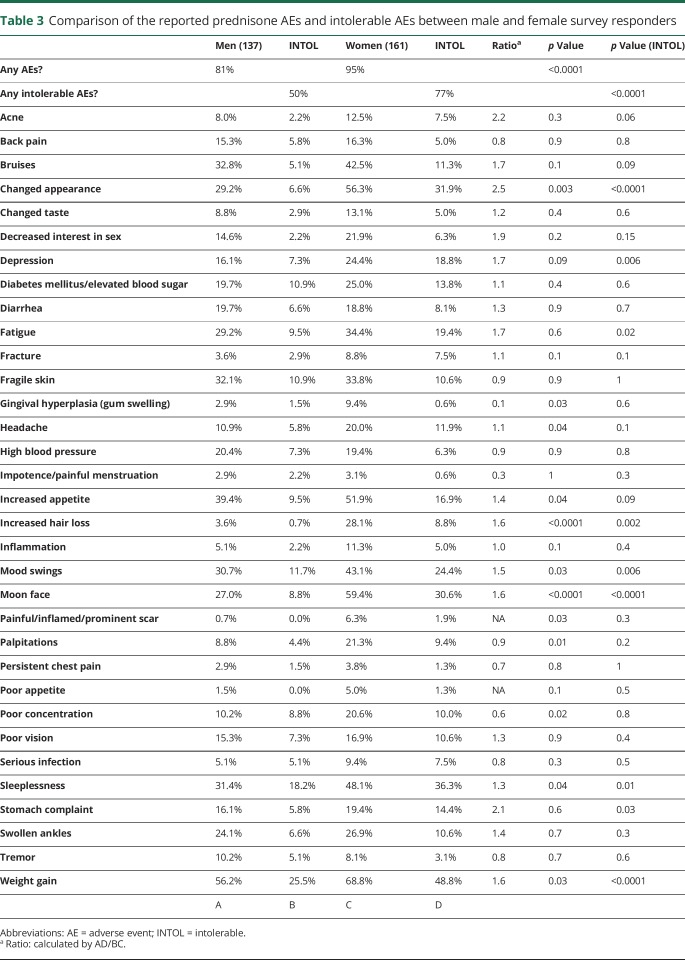
Women reported prednisone AEs and intolerable AEs more commonly compared with men (95% vs 81%, 77% vs 50%, respectively). Women reported weight gain, increased appetite, changed appearance, moon face, prominent scar, increased hair loss, gingival hyperplasia, mood swing, depression, fatigue, poor concentration, headache, sleeplessness, and palpitation more commonly than men. When asked about intolerable AEs, women reported more frequently than men including weight gain, changed appearance, moon face, depression, fatigue, mood swing, increased hair loss, sleeplessness, and stomach complaints (table 3).
Table 3.
Comparison of the reported prednisone AEs and intolerable AEs between male and female survey responders
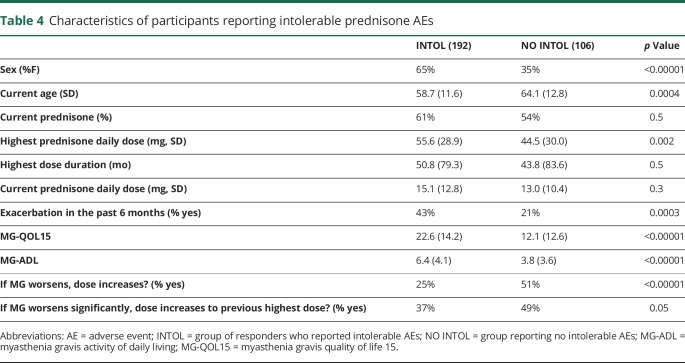
The group of responders who reported intolerable AEs (INTOL) were more likely to be women, younger in age, who took a higher peak dose of prednisone, and were less likely to say “yes” when asked to increase prednisone dose for worsened MG compared with the group reporting no intolerable AEs (NO INTOL) from prednisone. MG exacerbation in the past 6 months was more frequent in the group with intolerable AEs, and MG-QOL15 and MG-ADL sum scores were significantly higher (worse) compared with the no intolerable AE group (table 4).
Table 4.
Characteristics of participants reporting intolerable prednisone AEs
Discussion
In our study, majority of the participants took prednisone for the treatment of MG, whereas a quarter answered that they did not take prednisone. Those who did not take prednisone generally had less severe disease; however, a significant proportion of these participants had severe enough disease that required immunosuppression, IVIG, or PLEX. Among participants who took prednisone, the reported prednisone usage patterns were variable in terms of dosages and dosing frequencies. Comparable peak dosage between men and women suggests that the dosing in this population is not commonly based on ideal body weight. Daily dosing was the predominant dosing frequency, especially at the peak dose (84%), despite literature that supports the use of alternate-day dosing to decrease AEs.6,14
As expected, reported AEs were very common among patients with MG taking oral corticosteroid treatment, consistent with the previous reports.1–10,18 The efficacy of the corticosteroid medications such as prednisone rely on its pleiotropic effects on the glucocorticoid receptors through multiple signaling pathways, which inevitably evoke physiologic signaling along with its anti-inflammatory effect.19 Women in this study not only reported AEs more commonly but also perceived them as more intolerable compared with men. Consistent with this result, women more frequently reported that the dosage of prednisone had to be lowered because of AEs. Experiencing intolerable AEs was associated with a tendency to be resistant to a possible future dose increase if needed for an MG exacerbation, and this was more common in women.
A previous study that looked at symptom experience associated with chronic immunosuppressive treatment in heart transplant recipients and the result showed that clear gender difference exists.18 In the study, women reported adverse symptoms more frequently with a higher distress level, and the pattern of symptoms was different from men. Women also experienced more AEs in the MG patient registry, the MG population treated with long-term steroids. There are many potential factors that might explain this observation. Physiologically, women have lower height and weight compared with men. Considering that the mean highest and current dose of prednisone were comparable, women would generally be receiving a higher dose of prednisone on a per weight basis, which would be expected to be associated with more adverse events. Pharmacokinetics are different between men and women. Female sex and oral contraceptive use have been associated with lower prednisolone clearance and volume of distribution, increasing the area under the curve in healthy volunteers and kidney, lung, and heart transplant recipients.20 Women in the MG registry generally have more severe disease compared with men,17 which might have led to higher cumulative dosage exposure. The peak and current dosages and treatment durations were comparable between the sexes in our study; however, this may not precisely reflect the cumulative dosage of prednisone because of its highly variable titration and tapering courses.
AEs related to appearance and social interactions were significantly more likely to be intolerant in women compared with men, suggesting that the study result might have been affected by different perception of AEs between the 2 sexes. For example, altered appearance was 2.5 times more likely to be intolerable by women than men. Weight gain, acne, bruises, loss of sexual interest, depression, fatigue, increased hair loss, mood swing, moon face, weight gain, and stomach complaints all were more frequently noted as intolerable AEs in women. By contrast, factors that are neutral to social interaction such as palpitation, chest pain, poor vision, or infection were considered intolerable at similar frequencies between the sexes. This observation is not surprising because studies showed that women as a group maintain more social contacts, communicate more frequently, and strive to stay in the center of a social network.21,22 In the same context, women are more cognizant of their visual appearance as shown by the study of the social network using Facebook, a large social network service.22
Experiencing AEs is known to affect quality of life and can trigger medication noncompliance.23–26 The result from our study also demonstrates that having intolerable AEs might lead to resistance in prednisone dose increase when it is needed for MG treatment. Participants who reported intolerable AEs were more likely to be women, younger, had more severe disease, and were treated with higher peak dose of prednisone. These findings might simply indicate that those with more severe disease received higher dosages of prednisone and therefore developed intolerable AEs. Alternatively, initial high dose of prednisone challenge might have caused intolerable AEs and resistance to future prednisone treatment, leading to incomplete disease control. In the latter case, applying a strategy to avoid intolerable AEs might positively affect the patient's perception and compliance with prednisone, a potential target to improve the treatment outcome. We cannot make a cause and effect relationship based on this cross-sectional study, and a further prospective study is needed to further guide prednisone use in the treatment of MG.
Potential recall bias is one of the main limitations of our study. Most of our patients were treated with long-term prednisone, and it might be difficult to accurately remember and report various AEs they have experienced. Many of the patients were also treated with other medications including pyridostigmine, immunosuppressants, or received no MG-related medications, making it difficult for them to link certain symptoms to specific medications. We also acknowledge that there might be a gender bias in reporting, one way or another affecting the survey results. The responders of this particular survey were older and mostly white compared with nonresponders and may not reflect the whole MG patient registry population or patients with MG in the United States. The response rate for the semi-annual follow-up and Prednisone Survey was not high, reflecting the early evolution of the registry and not having a way to exclude participants who agree with registration but prefer not to participate in semi-annual updates. As pointed out in our previous study, data in the registry are entered by the participant and did not go through confirmation by a physician. Some of the information such as the dose or duration might not be precise, as it is relying on the sole memory of the individual participant. Nonetheless, we believe that the value of our study lies in the data collected to represent the perspective of patients without significant influence from the providers. Ultimately, the final decision whether or not to take the medicine is on the patient, not the treating physician.
In summary, subjective treatment-associated AEs are extremely common in patients taking prolonged oral corticosteroids such as prednisone, more frequent in women with higher tendency for intolerance. Experiencing intolerable AEs is linked to resistance in increasing the dose of prednisone when it is needed for the treatment of underlying disease. Consensus guidelines and their validation are required to guide prednisone treatment for MG.
Acknowledgment
The authors express their gratitude to the Myasthenia Gravis Foundation of America for their efforts in establishing and maintaining the Myasthenia Gravis Patient Registry.
Glossary
- AE
adverse effect
- ICU
intensive care unit
- IRB
institutional review board
- IVIG
IV immunoglobulin treatment
- MG
myasthenia gravis
- PLEX
current plasma exchange
- UAB
University of Alabama at Birmingham
Appendix 1. Author contributions
Study funding
No targeted funding reported.
Disclosure
I. Lee reports no disclosures. H.J. Kaminski served on the scientific advisory boards of the DSMB National Institutes of Health and NeuroNEXT Network; is on the editorial board of Experimental Neurology; holds a patent for targeted therapy of complement inhibitor to neuromuscular junction; receives publishing royalties from Springer; consulted for Akari Therapeutics, UCB, Alnylam, and RA Pharmaceuticals; received research support from Akari Therapeutics and the Muscular Dystrophy Association; and served as an expert witness. T. McPherson received research support from the Myasthenia Gravis Foundation of America. M. Feese received research support from the Myasthenia Gravis Foundation of America. G.R. Cutter served on the scientific advisory boards of AMO Pharmaceuticals, Apotek, Horizon, Modigenetech/Prolor, Merck, Merck/Pfizer, Onkobiologics, Neurim, Sanofi-Aventis, Reata Pharmaceuticals, Receptos/Celgene, Teva, NHLBI, and NICHD; is a statistical reviewer for the Am Journal of the Society of Nephrology; consulted for Atara Biotherapeutics, Argenix, Bioeq, GmBH, Consortium of MS Centers, Genzyme, Genentech, Innate Therapeutics, Klein-Buendel Incorporated, MedImmune, Medday, Novartis, Opexa Therapeutics, Roche, Savara Inc, Somahlution, Teva, Transparency Life Sciences, and TG Therapeutics; is president of Pythagoras Inc; received research support from the Myasthenia Gravis Foundation of America, NIH/NIAID, DOD, NIH/NHLBI, the Consortium of MS Centers, U.S. Department of Defense, NIH/NIDDK, BIH/Children's Hospital (Boston), Alabama Department of Commerce, NIH/NICHD, NIH/National Eye Institute, NIH/NINDS, PCORI, and NIH; and is president of the Consortium of MS Centers (only expenses covered). The MG Registry receives funding from the Myasthenia Gravis Foundation of America. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/NN.
References
- 1.Evoli A, Batocchi AP, Palmisani MT, Monaco ML, Tonali P. Long-term results of corticosteroid therapy in patients with myasthenia gravis. Eur Neurol 1992;32:37–43. [DOI] [PubMed] [Google Scholar]
- 2.Beekman R, Kuks JB, Oosterhuis HJ. Myasthenia gravis: diagnosis and follow-up of 100 consecutive patients. J Neurol 1997;244:112–118. [DOI] [PubMed] [Google Scholar]
- 3.Pascuzzi RM, Coslett HB, Johns TR. Long-term corticosteroid treatment of myasthenia gravis: report of 116 patients. Ann Neurol 1984;15:291–298. [DOI] [PubMed] [Google Scholar]
- 4.Sgirlanzoni A, Peluchetti D, Mantegazza R, Fiacchino F, Cornelio F. Myasthenia gravis: prolonged treatment with steroids. Neurology 1984;34:170–174. [DOI] [PubMed] [Google Scholar]
- 5.Benatar M, Mcdermott MP, Sanders DB et al. . Efficacy of prednisone for the treatment of ocular myasthenia (EPITOME): a randomized, controlled trial. Muscle Nerve 2016;53:363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howard FM, Duane DD, Lambert EH, Daube JR. Alternate-day prednisone: preliminary report of a double-blinded controlled study. Ann NY Acad Sci 1976;274:596–607. [DOI] [PubMed] [Google Scholar]
- 7.Ackerman GL, Nolan CM. Adrenocortical responsiveness after alternate-day corticosteroid therapy. N Engl J Med 1968;278:405–409. [DOI] [PubMed] [Google Scholar]
- 8.Soyka LF, Saxena KM. Alternate-day steroid therapy for nephrotic children. JAMA 1965;192:225–230. [DOI] [PubMed] [Google Scholar]
- 9.Hunder G, Sheps S, Allen G, Joyce JW. Daily and alternate day corticosteroid regimens in treatment of giant cell arteritis: comparison in a prospective study. Ann Intern Med 1975;82:613–618. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro G, Tattoni D, Vincent K, Pierson W, Bieman W. Growth, pulmonary and endocrine function in chronic asthma patients on daily and alternate-day adrencorticosteroid therapy. J Allergy Clin Immunol 1976;57:430–439. [DOI] [PubMed] [Google Scholar]
- 11.MacGregor R, Sheagren J, Lipsett M, Wolff S. Alternate-day prednisone thereapy—evaluation of delayed hypersensitivity responses, control of disease and steroid side effects. N Engl J Med 1969;280:1427–1433. [DOI] [PubMed] [Google Scholar]
- 12.Spratling L, Tenholder M, Underwood G, Feaster B, Requa R. Daily vs alternate day prednisone therapy for stage II sarcoidosis. Chest 1985;88:687–690. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Gonzalez J, Mireles-Zavala L, Rodriguez-Gutierrez R et al. . Hyperglycemia related to high-dose glucocorticoid use in noncritically ill patients. Diabetology Metab Syndr 2013;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolfe GI, Kaminski HJ, Aban IB, et al. . Randomized trial of thymectomy in myasthenia gravis. N Engl J Med 2016;375:511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanders DB, Hart IK, Mantegazza R, et al. . An international, phase III, randomized trial of mycophenolate mofetil in myasthenia gravis. Neurology 2008;71:400–406. [DOI] [PubMed] [Google Scholar]
- 16.Palace J, Newsom-Davis J, Lecky B. A randomized double-blinded trial of prednisolone alone or with azathioprine in myasthenia gravis. Myasthenia Gravis Study Group. Neurology 1998;50:1778–1783. [DOI] [PubMed] [Google Scholar]
- 17.Lee I, Kaminski HJ, Xin H, Cutter G. Gender and quality of life in myasthenia gravis patients from the myasthenia gravis foundation of America registry. Muscle Nerve Epub 2018 Feb 21. [DOI] [PubMed]
- 18.Moons P, De Geest S, Abraham I, Cleemput JV, Van Vanhaecke J. Symptom experience associated with maintenance immunosuppression after heart transplantation: patients' appraisal of side effects. Heart Lung 1998;27:315–325. [DOI] [PubMed] [Google Scholar]
- 19.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med 2005;353:1711–1723. [DOI] [PubMed] [Google Scholar]
- 20.Bergmann TK, Barraclough KA, Lee KJ, Staatz CE. Clinical pharmacokinetics and pharmacodynamics of prednisolone and prednisone in solid organ transplantation. Clin Pharmacokinet 2012;51:711–741. [DOI] [PubMed] [Google Scholar]
- 21.Psylla I, Sapiezynski P, Mones E, Lehmann S. The role of gender in social network organization. PLoS One 2017;12:e0189873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis K, Kaufman J, Gonzalez M, Wimmer A, Christakis N. Tastes, ties, and time: a new social network dataset using Facebook.com. Social Networks 2008;30:330–342. [Google Scholar]
- 23.Testa MA, Simonson DC. Assessment of quality-of-life outcomes. N Engl J Med 1996;334:35–40. [DOI] [PubMed] [Google Scholar]
- 24.Leventhal H, Diefenbach M, Leventhal EA. Illness cognition: using common sense to understand treatment adherence and affect cognition interactions. Cogn Ther Res 1992;16:143–163. [Google Scholar]
- 25.Didlake RH, Dreyfus K, Kerman RH, Van Buren CT, Kahan BD. Patient non-compliance: a major cause of late graft failure in cyclosporine-treated renal transplants. Transplant Proc 1988;20(suppl 3):63–69. [PubMed] [Google Scholar]
- 26.Schweitzer RT, Rovelli M, Palmeri D, Vossler E, Hull D, Bartus S. Non-compliance in organ transplant recipients. Transplantation 1990;49:374–377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data not provided in the article because of the space limitations will be made available in a trusted data repository or shared at the request of other investigators for purposes of replicating results.